Abstract
Background
Colon cancer is one of the most common cancers and it is the fourth leading cause of cancer-related deaths worldwide. YAP can promote cell proliferation and inhibit apoptosis, leading to loss of cell contact inhibition and promoting malignant cell transformation.
Material/Methods
In this study we analyzed the effects of different curcumin concentrations on the proliferation of colon cancer cells using MTT and colony formation assays. Western blot detection was performed to confirm the YAP, LC3-II, and P62 expression.
Results
Curcumin inhibited proliferation and promoted colon cancer cell autophagy. In addition, Western blot results indicated that curcumin suppressed YAP expression in colon cancer cells. To assess the mechanism, we treated the cell lines with curcumin and assessed YAP overexpression and YAP knockdown. The results revealed that curcumin inhibits the proliferation and promotes autophagy of these cell lines. Western blot results showed that curcumin reversed the effect of YAP in colon cancer cells.
Conclusions
Our results suggest that YAP has great promise for treatment of colon cancer and that it might be a potential diagnostic marker for colon cancer.
MeSH Keywords: Autophagy, Colonic Neoplasms, Curcumin
Background
Cancer is the second leading cause of death worldwide. Colon cancer is one of the most common cancers and is the fourth leading cause of all cancer-related deaths in the world. The incidence of colon cancer in males and females is ranked third and second, respectively, and the incidence of colon cancer in young people has gradually increased [1]. In China, the incidence of colon cancer is increasing each year as the size and age of the population increases [2]. Currently, the treatments for colon cancer are mainly surgical resection, adjuvant chemotherapy, and radiation therapy. Radiation therapy and chemotherapy for patients with colon cancer have obvious adverse effects [3], seriously affecting the quality of life of patients. Therefore, finding effective drugs with few adverse effects is of major importance.
Recent studies have found that the Hippo signaling pathway plays an important role in maintaining the size of organs and in tumor development [4]. YAP was first described by Sudol [5], who revealed that its higher expression levels and abnormal activity can have carcinogenic potential. YAP can promote cell proliferation and inhibit apoptosis, leading to a loss of cell contact inhibition and promoting malignant cell transformation. The literature has suggested that YAP protein expression in gastric cancer and cancer of the liver [6] was excessive and had enhanced activity. Curcumin, which is a natural phenolic antioxidant, has many pharmacological effects, such as antioxidant, anti-inflammatory, antitumor, and anti-Alzheimer’s disease properties, and it is used in the treatment of rheumatism and other diseases [7]. The National Cancer Institute in the United States has listed curcumin as a third-generation cancer drug. Studies have shown that the anti-apoptotic effect of curcumin is associated with inhibiting tumor cell autophagy, which can induce autophagy in leukemia K562 cells glioma cells [8].
In the present study, we explored whether YAP was a target of curcumin in colon cancer cells. We examined whether curcumin affected YAP. In this study, we showed that curcumin inhibited cell proliferation and induced autophagy in colon cancer cells. We also found that YAP was down-regulated after curcumin treatment. In addition, when YAP was over-expressed, autophagy was inhibited, and it was activated by a decrease in YAP expression. Our study shows that curcumin may be an effective treatment because of its potential ability to suppress YAP and reverse autophagy in colon cancer.
Material and Methods
Cell lines and cell culture
The colon cancer cell lines HCT116 and SW620 were purchased from the American Tissue Culture Collection (ATCC; Manassas, VA, USA). HCT116 cells were cultured in McCoy’ 5A containing 10% fetal bovine serum (FBS; HyClone Laboratories, Inc., Logan, UT, USA), and 100 U/ml penicillin (Amresco, Cleveland, OH, USA), 100 mg/ml streptomycin (Amresco, Cleveland, OH, USA). SW620 cells were cultured in L-15 (HyClone Laboratories, Inc., Logan, UT, USA) containing 10% fetal bovine serum, 100 U/ml penicillin, and 100 mg/ml streptomycin. All cells were incubated in an atmosphere with 5% CO2 at 37°C.
Cell proliferation assay (MTT assay)
Cells were cultured in 96-well plates (8×103 cells in every well). Increasing doses of curcumin (0, 2, 4, 8, and 16 μM) were added to each well for the indicated durations. We added curcumin to each well after 24, 48, and 72 h and then MTT solution (10 mg/ml) was added. Cells were incubated for 2 h at 37°C. We added 100 μl of DMSO to each well. The optical density of each well was measured at 490 nm.
Colony formation assay
These cell lines were seeded in 6-well plates (103 cells/well) and treated with curcumin. After 15 days, cells were stained with 0.2% crystal violet and the visible colonies were counted.
Stable cell line generation
The YAP overexpression cell lines, YAP knockdown cell lines, and control cell lines were established by infecting with lentivirus packing YAP expression vector, YAP shRNA expression vector, and control vector, respectively (GenePharma, Shanghai, China). Target cells were infected with lentivirus for 48 h, then selected with puromycin (Santa Cruz) for 3 weeks.
Western blot
The soluble proteins were extracted from the cell lysate in RIPA buffer (50 mM Tris, pH 8.0, 150 mM NaCl, 0.5% deoxycholate, 0.1% SDS, 1 mM sodium vanadate, 1 mM PMSF) (Beyotime, Shanghai, China). Lysates were normalized for total protein (25 μg) and then loaded on 10%–15% sodium sulfate polyacrylamide gel. After electrophoresis, the proteins were transferred to a PVDF membrane (Millipore, Kenilworth, NJ, USA), followed by blocking with 5% skim milk. The membrane was incubated with primary antibodies overnight at 4°C and rinsed with Tris-buffered saline with Tween 20. The primary antibodies used were: anti-GAPDH (1: 2000 dilution; catalog no. AF0006) (Beyotime), anti-LC3 (1: 1000 dilution; catalog no. 3868T) (CST), anti-P62 (1: 2000 dilution; catalog no. 5114T) (CST), and anti-YAP (1: 1000 dilution; catalog no. 14074) (CST).
Statistical analysis
All statistical data were analyzed using SPSS 19.0 software (SPSS, Chicago, USA). P-values <0.05 was considered statistically significant. All experiments were repeated 3 times.
Results
The cytotoxic effects of curcumin on HCT116 and SW620 cells
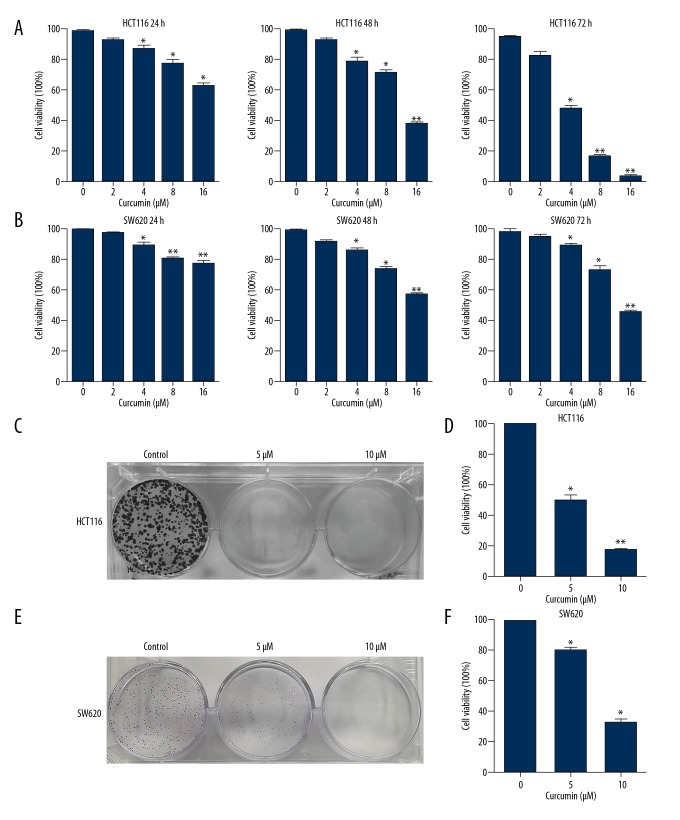
Curcumin is natural plant extract that can inhibit cancer cell growth [9]. Figure 1 shows the chemical structure of curcumin. The MTT assay was used on HCT116 and SW620 cells, and we found that curcumin had moderate cytotoxicity to HCT116 and SW620 cells. The results showed a time- and dose-dependent suppression with curcumin in colon cancer cells (Figure 2A, 2B). In the following study, the colony formation assay showed that curcumin significantly decreased the colony formation (Figure 2C, 2E). Quantification of colony formation is shown in Figure 2D, 2F. Our data suggest that curcumin has cytotoxic effects on HCT116 and SW620 cells.
Figure 1.
Chemical structure of curcumin.
Figure 2.
Curcumin inhibited colon cells growth. (A) The inhibitory effects of curcumin in HCT116 cells, detected by MTT assay after treatment with curcumin for 24, 48, and 72 h. (B) The inhibitory effects of curcumin in SW620 cells, detected by MTT assay after treatment with curcumin for 24, 48, and 72 h. (C) The colony formation assay of HCT116 treated with curcumin at indicated concentrations. (D) The number of colonies was counted. (E) The colony formation assay of SW620 treated with curcumin at indicated concentrations. (F) The number of colonies was counted. * P<0.05, ** P<0.01, compared to the control groups, DMSO treatment group.
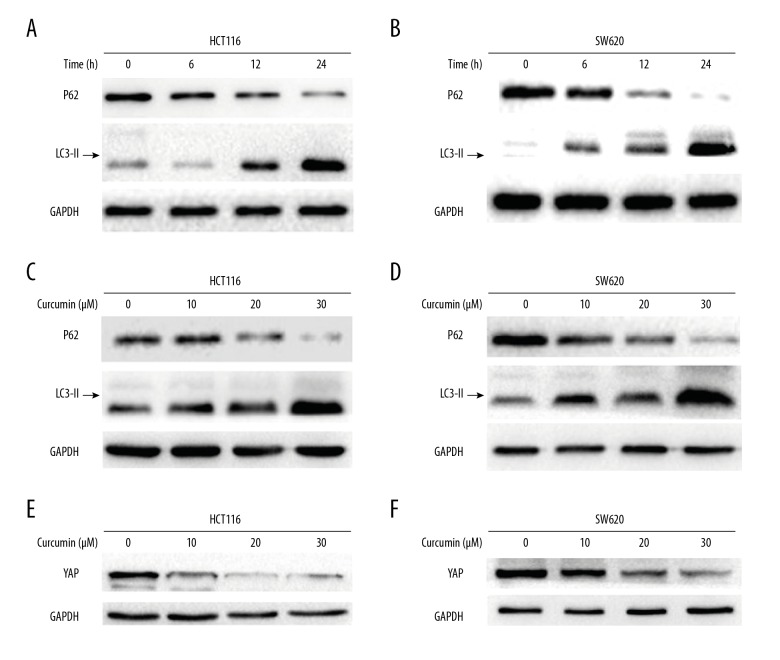
Curcumin induced autophagy and decreased YAP expression in colon cancer cells
Autophagy is the lysosomal degradation process of cytoplasmic components under stress conditions. Evidence had shown that curcumin can inhibit cell growth due to the increase of autophagy. When autophagy is activated, LC3 is cut on the C-terminal and the LC3 protein is produced and then transferred to autophagosomes [10]. In the investigation of whether curcumin triggered autophagy in colon cancer cells, we treated HCT116 and SW620 cells with curcumin in different doses and for different times. The expression of LC3-II was assessed with Western blot analysis (Figure 3A–3D). The degradation of sequestosome1 (SQSTM1/P62) was assumed to be an accurate indicator of autophagy. Thus, the expression of P62 within curcumin-exposed colon cancer cells was investigated. Curcumin-induced P62 degradation was observed to be dose- and time-dependent (Figure 3A–3D). Taken together, these results indicate that curcumin can induce autophagy in colon cancer cells.
Figure 3.
Curcumin induced autophagy and decreased YAP expression in colon cancer cells. (A) HCT116 cells were treated with 30 μM curcumin for 6, 12, and 24 h. Expressions of LC3-II and P62 were detected by Western blot assay. (B) SW620 cells were treated with 30 μM curcumin for 6, 12, and 24 h. Expressions of LC3-II and P62 were detected by Western blot assay. (C) HCT116 cells were treated with curcumin at increasing concentration for 24 h, and expressions of LC3-II and P62 were detected by Western blot assay. (D) SW620 cells were treated with curcumin at increasing concentrations for 24 h, and expressions of LC3-II and P62 were detected by Western blot assay. (E) The protein expression of YAP was inhibited by curcumin in HCT116 cells. (F) The protein expression of YAP was inhibited by curcumin in SW620 cells.
It was documented that YAP affects tumorigenesis. Therefore, in order to explore the potential mechanism of curcumin cytotoxicity in colon cancer cells, we focused on changes in YAP. The YAP expression in HCT116 and SW620 cells was down-regulated in a dose-dependent manner by treatment with curcumin (Figure 3E, 3F).
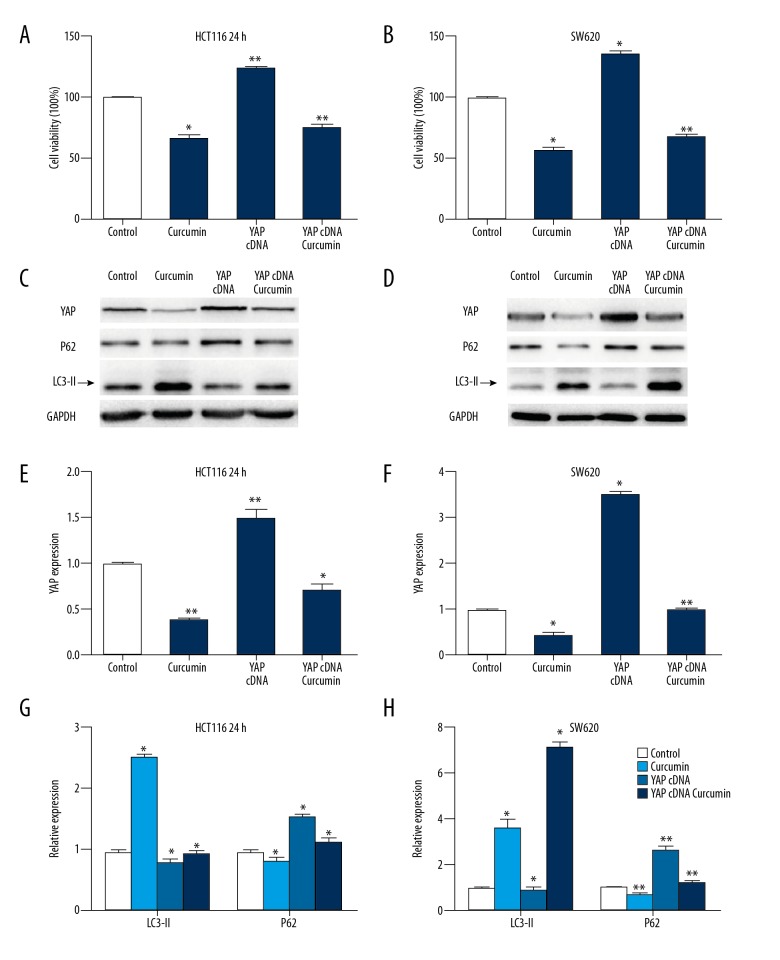
Curcumin reverses the effect of over-expressed YAP on colon cancer cells
To examine whether curcumin plays a role in suppressing HCT116 cells and SW620 cells propagation, we transfected them with YAP-specific cDNA. These HCT116 cells and SW620 cells lines were treated with curcumin in increasing doses after 24 h, and were treated with the same dose after 0, 6, 12, and 24 h (Figure 4A, 4B). The results indicated that overexpression of YAP promoted colon cancer cell growth and reversed curcumin-induced cell growth inhibition to a certain extent. Moreover, as shown in Figures 4C and 4D, YAP overexpression significantly reduced the percentage of autophagic cells in the HCT116 cell and SW620 cell population. An obvious increase in LC3II and reduction in p62 were observed (Figure 4C, 4D). Western blot analysis of YAP, LC3-II and P62 was shown in Figure 4E–4H. These results indicate that the anti-cancer activity of curcumin is partly due to the inactivation of YAP.
Figure 4.
Overexpression of YAP promoted cell proliferation and restrained cell autophagy. (A) The effect of YAP overexpression in combination with curcumin treatment on HCT116 cells growth was detected by MTT assay. (B) The effect of YAP overexpression in combination with curcumin treatment on SW620 cells growth was detected by MTT assay. (C) The expressions of YAP and LC3-II and P62 were measured in YAP cDNA-transfected colon cells treated with curcumin (HCT116). (D) The expressions of YAP and LC3-II and P62 were measured in YAP cDNA-transfected colon cells treated with curcumin (SW620). (E) The Western blot densities were normalized to the density of GAPDH in each lane in HCT116 cells. (F) The Western blot densities were normalized to the density of GAPDH in each lane in SW620 cells. (G) The Western blot densities were normalized to the density of GAPDH in each lane in HCT116 cells. (H) The Western blot densities were normalized to the density of GAPDH in each lane in SW620 cells. * P<0.05, **P<0.01, compared with control.
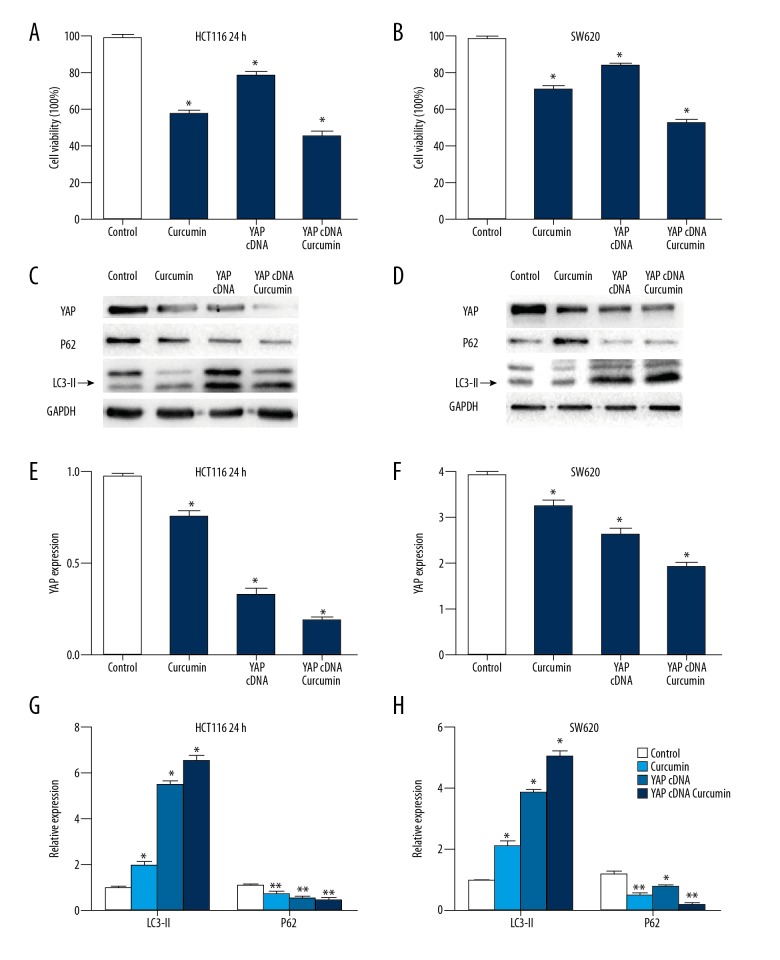
Knockdown of YAP sensitized colon cancer cells to curcumin
We sought to determine whether cellular sensitivity to the cytotoxicity of curcumin was related to the adjustment of YAP; thus, viability assays were performed with the HCT116 and SW620 cells. These cell lines were transfected with YAP-specific siRNA. As a result, we found that down-regulation of YAP evidently inhibited cell proliferation (Figures 5A, 5B). Furthermore, we found increased expression of LC3-II and decreased expression of P62 (Figure 5C, 5D). Western blot analysis of YAP, LC3-II and P62 was shown in Figure 5E–5H. Therefore, YAP siRNA and curcumin have a stronger induction of autophagy than curcumin or siRNA transfection alone. In general, these results suggest that the synergistic effects of curcumin and YAP-specific siRNA can promote the autophagy induced by curcumin in the 2 colon cancer cell lines, and make the colon cancer cells more susceptible to curcumin.
Figure 5.
Knockdown of YAP inhibited cells proliferation and promoted cell autophagy in colon cells. (A) The effects of down-regulated YAP in combination with curcumin treatment on HCT116 cells growth were detected by MTT assay. (B) The effect of down-regulated YAP in combination with curcumin treatment on SW620 cells growth was detected by MTT assay. (C) The expressions of YAP and LC3-II and P62 was measured in YAP siRNA-transfected colon cells treated with curcumin (HCT116). (D) The expressions of YAP and LC3-II and P62 were measured in YAP siRNA-transfected colon cells treated with curcumin (SW620). (E) The Western blot densities were normalized to the density of GAPDH in each lane in HCT116 cells. (F) The Western blot densities were normalized to the density of GAPDH in each lane in SW620 cells. (G) The Western blot densities were normalized to the density of GAPDH in each lane in HCT116 cells. (H) The Western blot densities were normalized to the density of GAPDH in each lane in SW620 cells. * P<0.05, ** P<0.01, compared with control.
Discussion
In this work, we confirmed the cytotoxic impact of curcumin on colon cancer cells, showing that the proliferation of HCT116 and SW620 cells was significantly suppressed and that cell autophagy was promoted by curcumin in a dose-dependent manner. Furthermore, the expression of YAP was markedly decreased in colon cancer cells treated with curcumin. We found that cell autophagy was correlated with changes in YAP, suggesting that YAP and autophagy might be related to the cytotoxic effects of curcumin on colon cancer cells.
The Hippo signaling pathway was described in 1995 when Drosophila WTS mutations were observed to cause an overgrowth phenotype in the eyes and wings of fruit flies [11]. YAP is the main downstream effector of the Hippo signaling pathway in mammals and in knockout mice. YAP can also inhibit the excessive proliferation caused by the absence of mst1/2 or other upstream signals [12]. The decrease in YAP activity is associated with the autophagy and differentiation of cells [13]. In some solid tumor cells, activation of YAP in the nucleus is necessary for the development of cancer [14]. Therefore, YAP has become a new target for cancer therapy.
Autophagy is a conservative cellular defense mechanism and a form of programmed cell death [15]. During autophagy, LC3-II plays an indispensable role in the formation of autophagosomes. Sequestosome1 (p62/SQSTM1) is a multidomain protein that interacts with the autophagy machinery as a key adaptor of target cargo. Our present study illustrated that curcumin activated autophagy by changing the expression of LC3-II and P62, and we also identified that the HCT116/SW620 cells treated with curcumin could reverse autophagy via upregulation of YAP.
It was reported that curcumin can inhibit the growth of a wide variety of tumor cell lines, as well as preventing chemical- and radiation-induced gastric cancer [16], colorectal cancer [17], pancreatic cancer [18], bladder cancer cells [19] and other cancers [20]. In recent years, researchers have been studying the anti-apoptotic effect of curcumin by inhibiting the invasion of tumor cells. It has been a topic of great interest for researchers to study autophagy. Studies have shown that curcumin can induce autophagy in K562 cells [21] and brain glioma cells [8]. Curcumin inhibited cell growth via downregulating YAP in pancreatic carcinoma cells [18]. Compared to other cytotoxic drugs, curcumin is the least toxic and is safe in human clinical trials. Hence, curcumin inhibits YAP and activates autophagy, providing a promising therapeutic strategy for the treatment of colon cancer patients. Further studies are needed to determine the specific mechanisms by which curcumin plays an anti-cancer role by inhibiting YAP and activating autophagy signaling pathways in colon cancer cells.
Conclusions
Our study revealed that curcumin inhibited the growth colon cancer cells at different concentrations and different times. Furthermore, curcumin was found to suppress the expression of YAP and can reverse the effect of YAP on colon cancer cells. We conclude that YAP has potential for use in treating colon cancer and that it might be a useful diagnostic marker for colon cancer.
Footnotes
Source of support: This study was supported by the National Natural Technology Foundation of China (No. 81428016) and the Key Program for Science and Technology Development of Beijing (No. Z151100003915073)
References
- 1.Torre LA, Siegel RL, Ward EM, et al. Global Cancer Incidence and Mortality Rates and Trends – An Update. Cancer Epidemiol Biomarker Prev. 2016;25(1):16–27. doi: 10.1158/1055-9965.EPI-15-0578. [DOI] [PubMed] [Google Scholar]
- 2.Boehmer U, Miao X, Maxwell NI, et al. Sexual minority population density and incidence of lung, colorectal and female breast cancer in California. BMJ Open. 2014;4(3):e004461. doi: 10.1136/bmjopen-2013-004461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carvalho C, Glynne-Jones R. Challenges behind proving efficacy of adjuvant chemotherapy after preoperative chemoradiation for rectal cancer. Lancet Oncol. 2017;18(6):e354–63. doi: 10.1016/S1470-2045(17)30346-7. [DOI] [PubMed] [Google Scholar]
- 4.Wang SP, Wang LH. Disease implication of hyper-Hippo signalling. Open Biol. 2016;6(10) doi: 10.1098/rsob.160119. pii: 160119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sudol M. Yes-associated protein (YAP65) is a proline-rich phosphoprotein that binds to the SH3 domain of the Yes proto-oncogene product. Oncogene. 1994;9(8):2145–52. [PubMed] [Google Scholar]
- 6.Wang YP, Tang DX. Expression of Yes-associated protein in liver cancer and its correlation with clinicopathological features and prognosis of liver cancer patients. Int J Clin Exp Med. 2015;8(1):1080–86. [PMC free article] [PubMed] [Google Scholar]
- 7.Hewlings SJ, Kalman DS. Curcumin: A review of its’ effects on human health. Foods. 2017;6(10) doi: 10.3390/foods6100092. pii: E92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhuang W, Long L, Zheng B, et al. Curcumin promotes differentiation of glioma-initiating cells by inducing autophagy. Cancer Sci. 2012;103(4):684–90. doi: 10.1111/j.1349-7006.2011.02198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Commandeur JN, Vermeulen NP. Cytotoxicity and cytoprotective activities of natural compounds. The case of curcumin. Xenobiotica. 1996;26(7):667–80. doi: 10.3109/00498259609046741. [DOI] [PubMed] [Google Scholar]
- 10.Justice RW, Zilian O, Woods DF, et al. The Drosophila tumor suppressor gene warts encodes a homolog of human myotonic dystrophy kinase and is required for the control of cell shape and proliferation. Genes Dev. 1995;9(5):534–46. doi: 10.1101/gad.9.5.534. [DOI] [PubMed] [Google Scholar]
- 11.Xu T, Wang W, Zhang S, et al. Identifying tumor suppressors in genetic mosaics: The Drosophila lats gene encodes a putative protein kinase. Development. 1995;121(4):1053–63. doi: 10.1242/dev.121.4.1053. [DOI] [PubMed] [Google Scholar]
- 12.Zhou D, Zhang Y, Wu H, et al. Mst1 and Mst2 protein kinases restrain intestinal stem cell proliferation and colonic tumorigenesis by inhibition of Yes-associated protein (Yap) overabundance. Proc Natl Acad Sci USA. 2011;108(49):E1312–20. doi: 10.1073/pnas.1110428108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Z, Zeng W, Wang S, et al. A potential role for the Hippo pathway protein, YAP, in controlling proliferation, cell cycle progression, and autophagy in BCPAP and KI thyroid papillary carcinoma cells. Am J Transl Res. 2017;9(7):3212–23. [PMC free article] [PubMed] [Google Scholar]
- 14.Moroishi T, Hansen CG, Guan KL. The emerging roles of YAP and TAZ in cancer. Nat Rev Cancer. 2015;15(2):73–79. doi: 10.1038/nrc3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petherick KJ, Williams AC, Lane JD, et al. Autolysosomal beta-catenin degradation regulates Wnt-autophagy-p62 crosstalk. EMBO J. 2013;32(13):1903–16. doi: 10.1038/emboj.2013.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang F, Yao Y, Wu J, et al. Curcumin inhibits gastric cancer-derived mesenchymal stem cells mediated angiogenesis by regulating NF-kappaB/VEGF signaling. Am J Transl Res. 2017;9(12):5538–47. [PMC free article] [PubMed] [Google Scholar]
- 17.Jalili-Nik M, Soltani A, Moussavi S, et al. Current status and future prospective of Curcumin as a potential therapeutic agent in the treatment of colorectal cancer. J Cell Physiol. 2018;233(9):6337–45. doi: 10.1002/jcp.26368. [DOI] [PubMed] [Google Scholar]
- 18.Zhou X, Su J, Feng S, et al. Antitumor activity of curcumin is involved in down-regulation of YAP/TAZ expression in pancreatic cancer cells. Oncotarget. 2016;7(48):79076–88. doi: 10.18632/oncotarget.12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao Y, Shi Q, Xu S, et al. Curcumin promotes KLF5 proteasome degradation through downregulating YAP/TAZ in bladder cancer cells. Int J Mol Sci. 2014;15(9):15173–87. doi: 10.3390/ijms150915173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu X, Duan C, Ji J, et al. Cucurbitacin B induces autophagy and apoptosis by suppressing CIP2A/PP2A/mTORC1 signaling axis in human cisplatin resistant gastric cancer cells. Oncol Rep. 2017;38(1):271–78. doi: 10.3892/or.2017.5648. [DOI] [PubMed] [Google Scholar]
- 21.Jia YL, Li J, Qin ZH, et al. Autophagic and apoptotic mechanisms of curcumin-induced death in K562 cells. J Asian Nat Prod Res. 2009;11(11):918–28. doi: 10.1080/10286020903264077. [DOI] [PubMed] [Google Scholar]