Summary
Long, non-coding RNAs (lncRNAs) are involved in the regulation of many cellular processes. The lncRNA IFNG-AS1 was found to strongly influence the responses to several pathogens in mice by increasing interferon gamma (IFNγ) secretion. Studies have looked at IFNG-AS1 in T cells, yet IFNG-AS1 function in natural killer cells (NKs), an important source of IFNγ, remains unknown. Here, we show a previously undescribed sequence of IFNG-AS1 and report that it may be more abundant in cells than previously thought. Using primary human NKs and an NK line with IFNG-AS1 overexpression, we show that IFNG-AS1 is quickly induced upon NK cell activation, and that IFNG-AS1 overexpression leads to increased IFNγ secretion. Taken together, our work expands IFNG-AS1's activity to the innate arm of the type I immune response, helping to explain its notable effect in animal models of disease.
Subject Areas: Molecular Biology, Molecular Mechanism of Gene Regulation, Immunology, Immune Response
Graphical Abstract
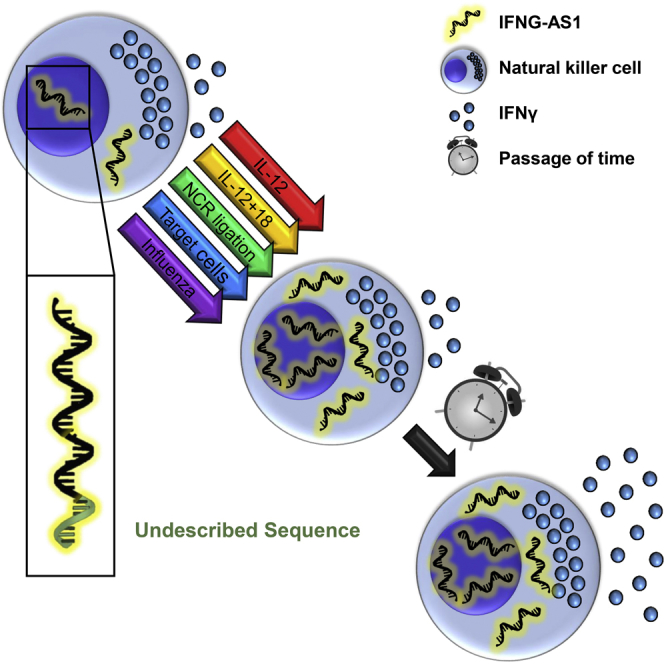
Highlights
-
•
Natural killer cells (NKs) express a previously undescribed transcript of IFNG-AS1
-
•
Upon activation, NKs upregulate IFNG-AS1 along with later IFNγ expression
-
•
Overexpression of IFNG-AS1 in an NK line augments IFNγ expression and secretion
-
•
IFNG-AS1 influences innate immunity, suggesting a general role in the IFNγ response
Molecular Biology; Molecular Mechanism of Gene Regulation; Immunology; Immune Response
Introduction
The central dogma of molecular biology states that information travels from DNA to RNA to protein. Recently, a large body of work has shown that many species of RNA have roles other than shuttling information and are fully functional on their own. This includes the long, non-coding RNAs (lncRNAs).
One lncRNA that has been shown to be particularly important is IFNG-AS1, also known as TMEVPG1 or NeST. It was originally found as a candidate to explain differences in murine response to Theiler's virus (Vigneau et al., 2001). Later works showed that IFNG-AS1 regulates interferon gamma (abbreviated IFNG for the RNA transcript and IFNγ for the protein) (Vigneau et al., 2003) and proved that IFNG-AS1 controls the response to Theiler's virus and Salmonella (Gomez et al., 2013). IFNG-AS1 dysregulation was found in a number of human diseases, and IFNG-AS1 levels correlate with clinical markers in some diseases (Li et al., 2016, Padua et al., 2016, Peng et al., 2015, Wang et al., 2016). This is consistent with the knowledge that IFNγ, which IFNG-AS1 regulates, is the pro-inflammatory end product of the type I immune response (Eberl, 2016) and with clinical findings that defects in the IFNγ pathway lead to increased susceptibility to a wide variety of diseases (Averbuch et al., 2011, Filipe-Santos et al., 2006).
IFNG-AS1 has also been studied on a molecular level. Significant work by Collier et al. and Spurlock et al. has characterized regulatory features of IFNG-AS1, including transcription factors important for IFNG-AS1 function and specificity to Th1 cells among the T cell lineages (Collier et al., 2012, Collier et al., 2014, Spurlock et al., 2015, Spurlock et al., 2017). Their study and that of others indeed focuses on Th1 cells, an important source of IFNγ. There is little data, however, on the function of IFNG-AS1 in another IFNγ producer, natural killer cells (NKs).
NKs are part of the innate immune system and are a subset of the type I innate lymphoid cells (Spits et al., 2016, Vivier et al., 2008). They were named for their ability to kill cancer cells without prior exposure to antigen, but have since been shown to recognize a wide variety of threats (Bar-On et al., 2017, Gur et al., 2015, Vitenshtein et al., 2016), including Theiler's virus itself (Paya et al., 1989). Accordingly, defects in human NKs cause serious immune deficiency (Orange, 2013).
NKs are also important secretors of various cytokines and chemokines. Several studies have suggested that NKs not only secrete IFNγ earlier than T cells but also play crucial regulatory roles, including activating the T cell phase of type I immunity (Heremans et al., 1994, Mocikat et al., 2003, Orange et al., 1995, Waggoner et al., 2016, Yang et al., 1997). Furthermore, NKs also express the transcription factors shown to be involved in IFNG-AS1 function (Miyagi et al., 2007, Paolini et al., 2015, Spits et al., 2016). Given the importance of NKs in the systemic IFNγ response, we set out to determine what, if any, role IFNG-AS1 plays in human NKs.
Results
IFNG-AS1 Is Expressed in Activated Human Natural Killer Cells
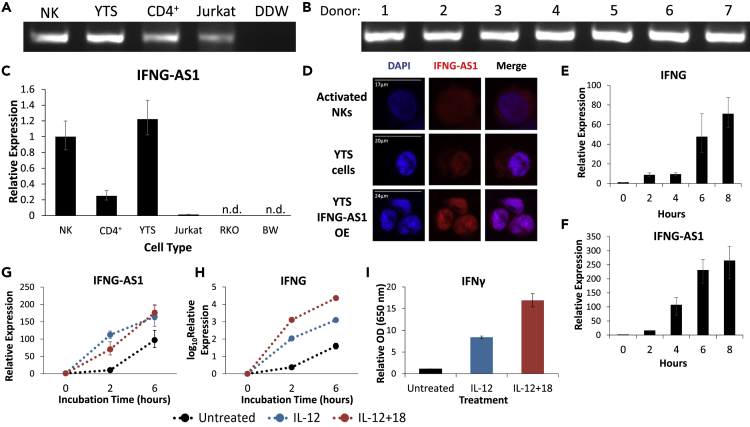
We first determined the presence of IFNG-AS1 in NK cells by PCR. Using a sequence in exon 5 of IFNG-AS1, we found expression in cDNA prepared from bulk (activated) NKs, bulk CD4+ T cells, an NK cell line, and a T cell line (YTS and Jurkat, respectively, Figure 1A). To confirm that IFNG-AS1 expression in NKs is not donor specific, we analyzed NK cells derived from seven healthy donors and observed expression in all of them (Figure 1B).
Figure 1.
IFNG-AS1 Is Expressed in Human NK Cells
(A) PCR demonstrating expression of IFNG-AS1 in bulk (activated) NK and CD4+ T cells derived from healthy donors, as well as NK and T cell lines (YTS and Jurkat, respectively). Water (DDW) was used as a negative control.
(B) IFNG-AS1 expression in NK cells derived from seven different healthy donors. (A) and (B) were performed simultaneously, with the same negative control (DDW).
(C) Quantitative PCR (qPCR) of IFNG-AS1 expression in NK and CD4+ cells derived from healthy donors, as well as NK and T cell lines (YTS and Jurkat, respectively). The non-hematopoetic cell line RKO and the non-human cell line BW are negative controls. Representative of at least two independent experiments. All graphs are shown as mean ± standard error of the mean.
(D) Fluorescence in situ hybridization (FISH) targeting IFNG-AS1 in the indicated cell types. DAPI is used as a counterstain. Scale bars shown are for a given row of images.
(E and F) NK cells derived from healthy donors were incubated in the presence of cycloheximide (CHX) for the indicated times. Expression levels of (E) IFNG and (F) IFNG-AS1 are shown. Representative of at least three independent experiments.
(G–I) NK cells derived from healthy donors were incubated in the presence of the indicated cytokines for the indicated times. Expression levels of (G) IFNG-AS1 and (H) IFNG are shown. Given its exponential rise, the log10 value of IFNG expression is shown. Protein secretion at 6 h is shown in (I). Representative of at least two independent experiments. n.d., not detected; OE, overexpression.
To assess IFNG-AS1 abundance, we used quantitative real-time PCR, with the colon carcinoma line RKO and the mouse thymoma line BW as negative controls (Figure 1C). Consistent with the band strength in Figure 1A, NK and YTS cells both express IFNG-AS1 at higher levels than do CD4+ T cells, and the expression of IFNG-AS1 in Jurkat cells is minimal.
Intrinsic Regulation of IFNG-AS1 in NKs
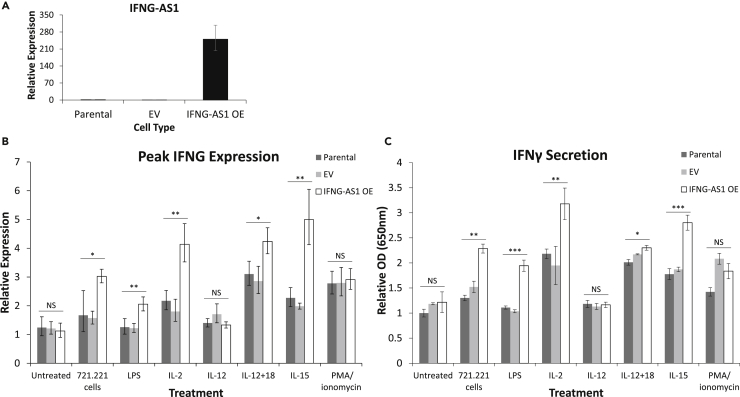
An important form of lncRNA regulation involves cellular localization. Like proteins, lncRNAs can localize to a specific organelle, with a basic division between nuclear and cytoplasmic lncRNAs. Using RNA fluorescence in situ hybridization (FISH) we found IFNG-AS1 expression in the cytoplasm and nucleus of NKs (Figure 1D, top row). In YTS cells, IFNG-AS1 was also expressed in both compartments; however, we observed clear nuclear enrichment (Figure 1D, middle row). To confirm specificity, we performed RNA FISH on YTS cells overexpressing IFNG-AS1 and found increased fluorescence (Figure 1D, bottom row; overexpression shown in Figure 4A).
Figure 4.
Induction of IFNγ in IFNG-AS1 Overexpression
(A) Expression of IFNG-AS1 in parental YTS cells and YTS cells transfected with empty vector or IFNG-AS1. Representative of five independent experiments.
(B) Peak IFNG expression as measured by qPCR at several time points with the indicated treatments. Expression is normalized to an independent sample of untreated parental YTS cells. Representative of at least three independent experiments.
(C) IFNγ secretion after 24 h of a variety of activating treatments, as measured by ELISA. Representative of at least three independent experiments.
EV, empty vector; OE, overexpression. *p < 0.05, **p < 0.005, ***p < 0.0005.
IFNγ secretion is regulated at several stages, including at the post-transcriptional level. RNA-binding proteins actively downregulate IFNG transcripts by binding AU-rich elements (Ogilvie et al., 2009). Given that IFNG-AS1 is a globally AU-rich transcript (GC content = 38.45%), we wondered whether it shared this form of regulation with IFNG. Previous reports have used cycloheximide (CHX), a global translation inhibitor, to allow accumulation of IFNG transcripts (Lebendiker et al., 1987), so we applied the same treatment to NK cells. Using quantitative real-time PCR we indeed found that IFNG-AS1, like IFNG, accumulates in NKs after CHX treatment (Figures 1E and 1F).
Several cytokines can also stimulate NKs to secrete large amounts of IFNγ, notably interleukin (IL)-12 and the combination of IL-12 and IL-18 (Lusty et al., 2017, Miyake et al., 2010). We wished to check if such strong induction of IFNγ is associated with an increase in IFNG-AS1 expression. Treatment with these cytokines indeed upregulated IFNG-AS1 (Figure 1G), along with the expected induction of IFNγ at both RNA and protein levels (Figures 1H and 1I).
Sequence of IFNG-AS1 Expressed in NKs
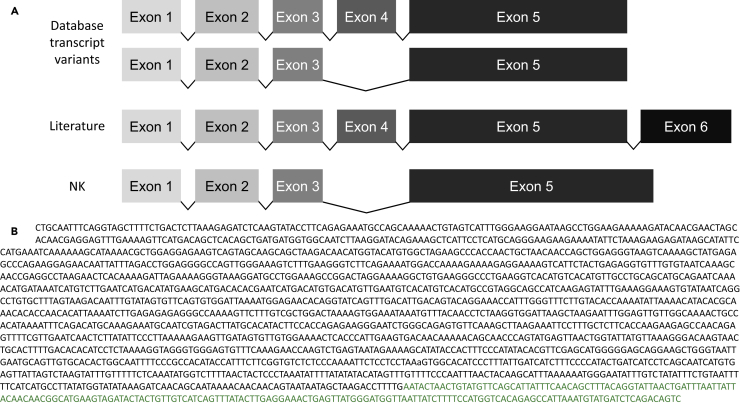
As lncRNA function is influenced by its sequence and structure, we wished to ensure that we had the complete sequence of IFNG-AS1. Public databases provide two transcript variants for human IFNG-AS1. The first contains five exons, and the second lacks exon 4. This, however, contradicts previous reports, which refer to six exons (Vigneau et al., 2003) (Figure 2A). To determine the sequence expressed in NKs, we performed a 3′ rapid amplification of cDNA ends (RACE). Using primers taken from exon 5 and those designed for RACE, we used nested PCR to lift the 3′ end of the gene from the cDNA of NK cells. Sequencing of our final product successfully recovered the poly(A) tail and the attached adaptor, but matched neither available data source.
Figure 2.
NK Cells Express a Novel Sequence of IFNG-AS1
(A) Publicly available databases contain two transcript variants of IFNG-AS1, which differ in the presence of exon 4. The literature, however, references a sixth exon. 3′ RACE on cDNA derived from NKs from three distinct human donors found transcript variant 2 (absence of exon 4) and a previously unannotated length of exon 5, but no evidence of a sixth exon.
(B) The sequence of IFNG-AS1 in NK cells. Nucleotides in green are not found in publicly available databases.
The majority of our sequencing of IFNG-AS1 in NKs matched database sequences, whereas the 3′ end was previously unreported. We found no evidence of a sixth exon, although exon 5 is approximately 200 bp longer than database sequences (Figure 2A). We found this sequence in three healthy donors, as well as in YTS cells. Furthermore, we confirmed that the genomic region downstream to the end of our sequence is not A-rich, making off-target priming by oligo-(dT) in cDNA preparation highly unlikely. The sequence of IFNG-AS1 in NKs is shown in Figure 2B.
Triggering of the Natural Cytotoxicity Receptors Induces IFNG-AS1 and IFNG Expression
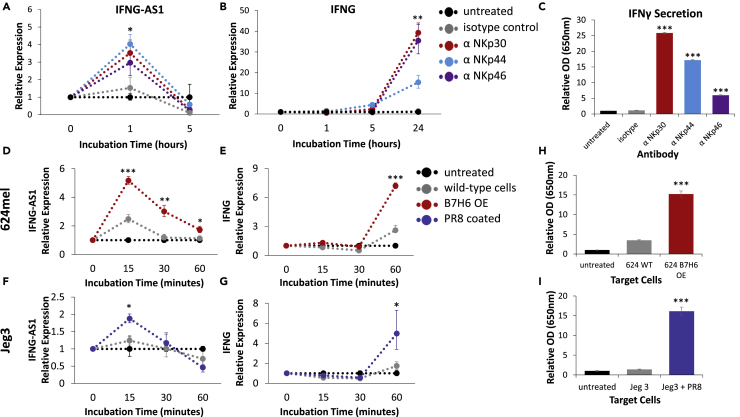
We then tested whether activation of various NK receptors affects IFNG-AS1 expression using a plate-bound antibody assay (PBA, please see the Transparent Methods for more information). We examined the three members of the natural cytotoxicity receptor (NCR) family—NKp30, NKp44, and NKp46—which are potent activators of NKs. Interestingly, we found that IFNG-AS1 is upregulated very quickly—1 h after activation (Figure 3A). By 5 h, IFNG-AS1 seems to be repressed (Figure 3A), and at 24 h it largely returns to baseline (data not shown). We noted that the kinetics were consistent with IFNG-AS1 being an enhancer, as its upregulation precedes that of IFNG (Figure 3B). In addition, stimulation of these receptors led to increased IFNγ secretion (Figure 3C).
Figure 3.
IFNG-AS1 Induction in Activated Human NK Cells
(A–C) NK cells were incubated in plates pre-bound with the indicated antibodies for the indicated times. Expression levels of (A) IFNG-AS1 and (B) IFNG are shown, as determined by qPCR. IFNγ secretion after 24 h is shown in (C). Representative of three independent experiments.
(D–G) NK cells were incubated with the indicated cells for the indicated times. Expression levels of (D and F) IFNG-AS1 and (E and G) IFNG are shown. The cell line used is shown to the left of each row of graphs. Representative of at least three independent experiments.
(H and I) IFNγ secretion by NKs incubated with the indicated cells for 5 h. Representative of at least three independent experiments. OE, overexpression. *p < 0.05, **p < 0.005, ***p < 0.0005 as compared to the the untreated and isotype/ wild-type samples of the given timepoint.
We next wanted to examine whether IFNG-AS1 and IFNG are similarly induced upon interaction of the NCRs with their cellular ligands (Figures 3D–3I). To assess NKp30 activation, we used the melanoma line 624mel overexpressing the NKp30 ligand B7H6 (Figures 3D, 3E, and S1A). For NKp44 and NKp46 we used the choriocarcinoma line Jeg3 preincubated with the PR8 strain of influenza, which expresses hemagglutinin—a ligand for both NKp44 and NKp46 (Arnon et al., 2001, Mandelboim et al., 2001) (Figures 3F, 3G, and S1B). We normalized IFNG-AS1 and IFNG expression levels using NKp44 and 2B4 (expressed only by the NKs) along with the housekeeping gene GAPDH, with the untreated NKs serving as a pure reference sample.
The kinetics was much faster in the cell-based models than in the PBA, and 15-, 30-, and 60-min time points are shown. Similar to the PBA, we saw quick induction and repression of IFNG-AS1 followed by induction of IFNG in the NCR-activated samples (Figures 3D–3G). As with the PBA, the increases in RNA levels were accompanied by increased IFNγ secretion (Figures 3H and 3I).
IFNG-AS1 Overexpression Enhances IFNγ Production
To isolate IFNG-AS1's effect, we overexpressed IFNG-AS1 in YTS cells using lentiviral transduction (Figure 4A). YTS cells do not express any of the NCRs in a functional manner, but instead are stimulated via the co-activating receptor 2B4 (Elias et al., 2014). As YTS cells also express 2B4's ligand CD48 (Figure S1C), the cells have a baseline level of activation and secrete IFNγ under normal growth conditions.
YTS cells can be further activated by a variety of methods. We activated the IFNG-AS1-overexpressing YTS cells using Toll-like receptor (TLR) ligation (lipopolysaccharide [LPS]), stimulatory cytokines (IL-2, IL-12, IL-12 + IL-18, and IL-15), non-specific lymphocyte activation (phorbol 12-myristate 13-acetate [PMA] and ionomycin), and target-mediated activation (721.221 cells, which express high levels of CD48 [Figure S1D]). As the treatments have different kinetics, we show peak IFNG RNA levels (Figure 4B) and cumulative IFNγ secretion after 24 h of incubation (Figure 4C). Representative time course graphs of IFNG expression are shown in Figure S2. For concentrations of treatments, please see the Transparent Methods.
As seen in Figures 4B and 4C, some forms of activation (721.221 cells, IL-2, and IL-15) activated all cells but caused significantly more IFNγ production in the cells overexpressing IFNG-AS1. IL-12 alone did not seem to stimulate YTS cells, whereas the addition of IL-18 caused increases in all cells, but significantly more in the context of IFNG-AS1 overexpression. PMA/ionomycin increased production in all cells but showed no effect of IFNG-AS1 overexpression. Finally, LPS seemed to effectively activate only the cells overexpressing IFNG-AS1. These increases indicate that IFNG-AS1 is a functional regulator of IFNγ in NKs.
Discussion
Here we have shown that the lncRNA IFNG-AS1 is not only relatively abundant in NKs but also enhances IFNγ secretion in these cells. Many of the facts about IFNG-AS1 in T cells hold true in NKs. In both cells, for example, IFNG-AS1 is upregulated upon cellular activation and contributes to increased IFNγ production. The models we used also add information not previously shown in T cells.
First and foremost, we provide a previously undescribed sequence of IFNG-AS1 in humans. The extra nucleotides described here may influence 3D structure, and therefore function. IFNG-AS1 in NKs is indeed spliced and polyadenylated, consistent with its function as an enhancer RNA (Gil and Ulitsky, 2018), and this sequence may alter processing. Second, whereas NKs express IFNG-AS1 at higher levels than do T cells (Figure 1C), RNA FISH shows even more expression than would be expected based on previous quantification. Almost all cells examined expressed IFNG-AS1, although we did find a few cells with no fluorescence. This may be consistent with higher copy numbers of IFNG-AS1 found by Gomez et al. when T cells were activated. We did, however, clearly see cytoplasmic expression, whereas previous work suggested nuclear restriction (Gomez et al., 2013). This, too, may be a by-product of basal levels of activation found in cultured NK cells, which could provide hints of IFNG-AS1's mechanism of action.
Vigneau et al. originally observed the expression of IFNG-AS1 or IFNG, yet not both at any one time, and hypothesized that IFNG-AS1 inhibits IFNG. Subsequent works have shown that the opposite is true, and our use of time course assays may resolve the contradiction. We show that IFNG-AS1 and IFNG transcripts are simply upregulated at different time points following activation, and that expression largely does not overlap.
In addition, whereas other studies use PMA/ionomycin or anti-CD3 antibodies, we describe a variety of signals that activate IFNG via IFNG-AS1. This is similar to earlier works on IFNG itself (Billiau and Matthys, 2009). IL-2, IL-12, IL-15, and IL-18 are important activators of NKs, specifically with regard to IFNγ production (Carson et al., 1995, Handa et al., 1983, Schoenborn and Wilson, 2007). NKs also respond to TLR ligation (Vivier et al., 2008), and to specific receptors, especially the NCRs (Mandelboim et al., 1998). Broadening our pool of signals may help in expanding IFNG-AS1 study to other cell types and experimental models.
IFNG-AS1 was originally found due to its role in the murine response to Theiler's virus, and the initial focus was on the T cells in this process. Paya et al. show that NKs are crucial in the response to Theiler's virus, and our findings may help link these works. Several studies have suggested that IFNγ produced by the innate immune system must prime the T cell response, with NKs playing a central part in this process (Guo et al., 2018, Heremans et al., 1994). NKs integrate a variety of signals received through their activating and inhibitory receptors (Kruse et al., 2014, Vivier et al., 2008). The first steps of this pathway are fairly well known, including, for example, the signaling molecules DAP12, ZAP70, and Syk (Jelencic et al., 2018, Pugh et al., 2018), but the later stages are less understood. IFNG-AS1 can now join the web of intracellular signaling at this phase of the immune response.
Although IFNG-AS1 specificity to Th1 cells has been suggested, we look beyond the T cell populations and find an additional set of models in which IFNG-AS1 plays an important role. This can pave the way to study of IFNG-AS1's significance in various disease models, and help us understand the dysregulation found in clinical works. Our findings also underscore the importance of IFNG-AS1 on a systemic level. The notable phenotypic differences that originally led to IFNG-AS1's discovery imply a central role. Here we help explain that role by demonstrating IFNG-AS1's activity in the innate immune system, suggesting that IFNG-AS1 is a general regulator of the type I immune response.
Limitations of the Study
As our work focuses on human cells, it is limited by the usual problems associated with ex vivo study, namely, the reductionism inherent to these methods. The human cells we use are themselves activated, a state that reflects only our best approximation of the actual state of these cells in action. Finally, as genetic alteration in primary human NKs is an extremely inefficient process, we used an NK cell line, again an approximation of NK cells themselves.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This work was supported by the Israel Science Foundation (Moked grant), the GIF Foundation, the ICRF professorship grant, the ISF Israel-China grant, and the Ministry of Science and Technology grant (all to O.M.).
Author Contributions
N.S. designed and performed experiments, analyzed results, and wrote the paper. O.B., D.S., A.D.-C., E.S., I.K., M.H., A.R., M.G., and R.Y. assisted in designing and performing experiments. P.T., A.O., and Y.C.-A. provided critical reagents. O.M. designed experiments, wrote the paper, and supervised the project.
Declaration of Interests
The authors declare no competing interests.
Published: January 25, 2019
Footnotes
Supplemental Information includes Transparent Methods and two figures and can be found with this article online at https://doi.org/10.1016/j.isci.2018.12.034.
Data and Software Availability
The sequence for the isoform of IFNG-AS1 shown in Figure 2B is also available as GenBank: MK296539.
Supplemental Information
References
- Arnon T.I., Lev M., Katz G., Chernobrov Y., Porgador A., Mandelboim O. Recognition of viral hemagglutinins by NKp44 but not by NKp30. Eur. J. Immunol. 2001;31:2680–2689. doi: 10.1002/1521-4141(200109)31:9<2680::aid-immu2680>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Averbuch D., Chapgier A., Boisson-Dupuis S., Casanova J.-L., Engelhard D. The clinical spectrum of patients with deficiency of signal transducer and activator of transcription-1. Pediatr. Infect. Dis. J. 2011;30:352–355. doi: 10.1097/INF.0b013e3181fdff4a. [DOI] [PubMed] [Google Scholar]
- Bar-On Y., Charpak-Amikam Y., Glasner A., Isaacson B., Duev-Cohen A., Tsukerman P., Varvak A., Mandelboim M., Mandelboim O. NKp46 recognizes the sigma1 protein of reovirus: implications for reovirus-based cancer therapy. J. Virol. 2017;91 doi: 10.1128/JVI.01045-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billiau A., Matthys P. Interferon-gamma: a historical perspective. Cytokine Growth Factor Rev. 2009;20:97–113. doi: 10.1016/j.cytogfr.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Carson W.E., Ross M.E., Baiocchi R.A., Marien M.J., Boiani N., Grabstein K., Caligiuri M.A. Endogenous production of interleukin 15 by activated human monocytes is critical for optimal production of interferon-gamma by natural killer cells in vitro. J. Clin. Invest. 1995;96:2578–2582. doi: 10.1172/JCI118321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier S.P., Collins P.L., Williams C.L., Boothby M.R., Aune T.M. Cutting edge: influence of Tmevpg1, a long intergenic noncoding RNA, on the expression of Ifng by Th1 cells. J. Immunol. 2012;189:2084–2088. doi: 10.4049/jimmunol.1200774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier S.P., Henderson M.A., Tossberg J.T., Aune T.M. Regulation of the Th1 genomic locus from Ifng through Tmevpg1 by T-bet. J. Immunol. 2014;193:3959–3965. doi: 10.4049/jimmunol.1401099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberl G. Immunity by equilibrium. Nat. Rev. Immunol. 2016;16:524–532. doi: 10.1038/nri.2016.75. [DOI] [PubMed] [Google Scholar]
- Elias S., Yamin R., Golomb L., Tsukerman P., Stanietsky-Kaynan N., Ben-Yehuda D., Mandelboim O. Immune evasion by oncogenic proteins of acute myeloid leukemia. Blood. 2014;123:1535–1543. doi: 10.1182/blood-2013-09-526590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipe-Santos O., Bustamante J., Chapgier A., Vogt G., de Beaucoudrey L., Feinberg J., Jouanguy E., Boisson-Dupuis S., Fieschi C., Picard C. Inborn errors of IL-12/23- and IFN-gamma-mediated immunity: molecular, cellular, and clinical features. Semin. Immunol. 2006;18:347–361. doi: 10.1016/j.smim.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Gil N., Ulitsky I. Production of spliced long noncoding RNAs specifies regions with increased enhancer activity. Cell Syst. 2018;7 doi: 10.1016/j.cels.2018.10.009. 537–547.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez J.A., Wapinski O.L., Yang Y.W., Bureau J.-F., Gopinath S., Monack D.M., Chang H.Y., Brahic M., Kirkegaard K. The NeST long ncRNA controls microbial susceptibility and epigenetic activation of the interferon-gamma locus. Cell. 2013;152:743–754. doi: 10.1016/j.cell.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y., Patil N.K., Luan L., Bohannon J.K., Sherwood E.R. The biology of natural killer cells during sepsis. Immunology. 2018;153:190–202. doi: 10.1111/imm.12854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur C., Ibrahim Y., Isaacson B., Yamin R., Abed J., Gamliel M., Enk J., Bar-On Y., Stanietsky-Kaynan N., Coppenhagen-Glazer S. Binding of the Fap2 protein of Fusobacterium nucleatum to human inhibitory receptor TIGIT protects tumors from immune cell attack. Immunity. 2015;42:344–355. doi: 10.1016/j.immuni.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa K., Suzuki R., Matsui H., Shimizu Y., Kumagai K. Natural killer (NK) cells as a responder to interleukin 2 (IL 2). II. IL 2-induced interferon gamma production. J. Immunol. 1983;130:988–992. [PubMed] [Google Scholar]
- Heremans H., Dillen C., van Damme J., Billiau A. Essential role for natural killer cells in the lethal lipopolysaccharide-induced Shwartzman-like reaction in mice. Eur. J. Immunol. 1994;24:1155–1160. doi: 10.1002/eji.1830240522. [DOI] [PubMed] [Google Scholar]
- Jelencic V., Sestan M., Kavazovic I., Lenartic M., Marinovic S., Holmes T.D., Prchal-Murphy M., Lisnic B., Sexl V., Bryceson Y.T. NK cell receptor NKG2D sets activation threshold for the NCR1 receptor early in NK cell development. Nat. Immunol. 2018;19:1083–1092. doi: 10.1038/s41590-018-0209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse P.H., Matta J., Ugolini S., Vivier E. Natural cytotoxicity receptors and their ligands. Immunol. Cell Biol. 2014;92:221–229. doi: 10.1038/icb.2013.98. [DOI] [PubMed] [Google Scholar]
- Lebendiker M.A., Tal C., Sayar D., Pilo S., Eilon A., Banai Y., Kaempfer R. Superinduction of the human gene encoding immune interferon. EMBO J. 1987;6:585–589. doi: 10.1002/j.1460-2075.1987.tb04794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Hao Y., Zhang D., Fu R., Liu W., Zhang X., Xue F., Yang R. Aberrant expression of long noncoding RNA TMEVPG1 in patients with primary immune thrombocytopenia. Autoimmunity. 2016;49:496–502. doi: 10.3109/08916934.2016.1167192. [DOI] [PubMed] [Google Scholar]
- Lusty E., Poznanski S.M., Kwofie K., Mandur T.S., Lee D.A., Richards C.D., Ashkar A.A. IL-18/IL-15/IL-12 synergy induces elevated and prolonged IFN-gamma production by ex vivo expanded NK cells which is not due to enhanced STAT4 activation. Mol. Immunol. 2017;88:138–147. doi: 10.1016/j.molimm.2017.06.025. [DOI] [PubMed] [Google Scholar]
- Mandelboim O., Kent S., Davis D.M., Wilson S.B., Okazaki T., Jackson R., Hafler D., Strominger J.L. Natural killer activating receptors trigger interferon gamma secretion from T cells and natural killer cells. Proc. Natl. Acad. Sci. U S A. 1998;95:3798–3803. doi: 10.1073/pnas.95.7.3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelboim O., Lieberman N., Lev M., Paul L., Arnon T.I., Bushkin Y., Davis D.M., Strominger J.L., Yewdell J.W., Porgador A. Recognition of haemagglutinins on virus-infected cells by NKp46 activates lysis by human NK cells. Nature. 2001;409:1055–1060. doi: 10.1038/35059110. [DOI] [PubMed] [Google Scholar]
- Miyagi T., Gil M.P., Wang X., Louten J., Chu W.-M., Biron C.A. High basal STAT4 balanced by STAT1 induction to control type 1 interferon effects in natural killer cells. J. Exp. Med. 2007;204:2383–2396. doi: 10.1084/jem.20070401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake T., Satoh T., Kato H., Matsushita K., Kumagai Y., Vandenbon A., Tani T., Muta T., Akira S., Takeuchi O. IkappaBzeta is essential for natural killer cell activation in response to IL-12 and IL-18. Proc. Natl. Acad. Sci. U S A. 2010;107:17680–17685. doi: 10.1073/pnas.1012977107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocikat R., Braumuller H., Gumy A., Egeter O., Ziegler H., Reusch U., Bubeck A., Louis J., Mailhammer R., Riethmuller G. Natural killer cells activated by MHC class I(low) targets prime dendritic cells to induce protective CD8 T cell responses. Immunity. 2003;19:561–569. doi: 10.1016/s1074-7613(03)00264-4. [DOI] [PubMed] [Google Scholar]
- Ogilvie R.L., Sternjohn J.R., Rattenbacher B., Vlasova I.A., Williams D.A., Hau H.H., Blackshear P.J., Bohjanen P.R. Tristetraprolin mediates interferon-gamma mRNA decay. J. Biol. Chem. 2009;284:11216–11223. doi: 10.1074/jbc.M901229200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orange J.S. Natural killer cell deficiency. J. Allergy Clin. Immunol. 2013;132:515–525. doi: 10.1016/j.jaci.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orange J.S., Wang B., Terhorst C., Biron C.A. Requirement for natural killer cell-produced interferon gamma in defense against murine cytomegalovirus infection and enhancement of this defense pathway by interleukin 12 administration. J. Exp. Med. 1995;182:1045–1056. doi: 10.1084/jem.182.4.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padua D., Mahurkar-Joshi S., Law I.K.M., Polytarchou C., Vu J.P., Pisegna J.R., Shih D., Iliopoulos D., Pothoulakis C. A long noncoding RNA signature for ulcerative colitis identifies IFNG-AS1 as an enhancer of inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2016;311:G446–G457. doi: 10.1152/ajpgi.00212.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolini R., Bernardini G., Molfetta R., Santoni A. NK cells and interferons. Cytokine Growth Factor Rev. 2015;26:113–120. doi: 10.1016/j.cytogfr.2014.11.003. [DOI] [PubMed] [Google Scholar]
- Paya C.V., Patick A.K., Leibson P.J., Rodriguez M. Role of natural killer cells as immune effectors in encephalitis and demyelination induced by Theiler’s virus. J. Immunol. 1989;143:95–102. [PubMed] [Google Scholar]
- Peng H., Liu Y., Tian J., Ma J., Tang X., Rui K., Tian X., Mao C., Lu L., Xu H. The long noncoding RNA IFNG-AS1 promotes T helper type 1 cells response in patients with Hashimoto’s thyroiditis. Sci. Rep. 2015;5:17702. doi: 10.1038/srep17702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh J.L., Nemat-Gorgani N., Norman P.J., Guethlein L.A., Parham P. Human NK cells downregulate Zap70 and Syk in response to prolonged activation or DNA damage. J. Immunol. 2018;200:1146–1158. doi: 10.4049/jimmunol.1700542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenborn J.R., Wilson C.B. Regulation of interferon-gamma during innate and adaptive immune responses. Adv. Immunol. 2007;96:41–101. doi: 10.1016/S0065-2776(07)96002-2. [DOI] [PubMed] [Google Scholar]
- Spits H., Bernink J.H., Lanier L. NK cells and type 1 innate lymphoid cells: partners in host defense. Nat. Immunol. 2016;17:758–764. doi: 10.1038/ni.3482. [DOI] [PubMed] [Google Scholar]
- Spurlock C.F., 3rd, Tossberg J.T., Guo Y., Collier S.P., Crooke P.S., 3rd, Aune T.M. Expression and functions of long noncoding RNAs during human T helper cell differentiation. Nat. Commun. 2015;6:6932. doi: 10.1038/ncomms7932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurlock C.F., 3rd, Shaginurova G., Tossberg J.T., Hester J.D., Chapman N., Guo Y., Crooke P.S., 3rd, Aune T.M. Profiles of long noncoding RNAs in human naive and memory T cells. J. Immunol. 2017;199:547–558. doi: 10.4049/jimmunol.1700232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigneau S., Levillayer F., Crespeau H., Cattolico L., Caudron B., Bihl F., Robert C., Brahic M., Weissenbach J., Bureau J.F. Homology between a 173-kb region from mouse chromosome 10, telomeric to the Ifng locus, and human chromosome 12q15. Genomics. 2001;78:206–213. doi: 10.1006/geno.2001.6656. [DOI] [PubMed] [Google Scholar]
- Vigneau S., Rohrlich P.-S., Brahic M., Bureau J.-F. Tmevpg1, a candidate gene for the control of Theiler’s virus persistence, could be implicated in the regulation of gamma interferon. J. Virol. 2003;77:5632–5638. doi: 10.1128/JVI.77.10.5632-5638.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitenshtein A., Charpak-Amikam Y., Yamin R., Bauman Y., Isaacson B., Stein N., Berhani O., Dassa L., Gamliel M., Gur C. NK cell recognition of Candida glabrata through binding of NKp46 and NCR1 to fungal ligands Epa1, Epa6, and Epa7. Cell Host Microbe. 2016;20:527–534. doi: 10.1016/j.chom.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivier E., Tomasello E., Baratin M., Walzer T., Ugolini S. Functions of natural killer cells. Nat. Immunol. 2008;9:503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- Waggoner S.N., Reighard S.D., Gyurova I.E., Cranert S.A., Mahl S.E., Karmele E.P., McNally J.P., Moran M.T., Brooks T.R., Yaqoob F. Roles of natural killer cells in antiviral immunity. Curr. Opin. Virol. 2016;16:15–23. doi: 10.1016/j.coviro.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Peng H., Tian J., Ma J., Tang X., Rui K., Tian X., Wang Y., Chen J., Lu L. Upregulation of long noncoding RNA TMEVPG1 enhances T helper type 1 cell response in patients with Sjogren syndrome. Immunol. Res. 2016;64:489–496. doi: 10.1007/s12026-015-8715-4. [DOI] [PubMed] [Google Scholar]
- Yang J., Kawamura I., Mitsuyama M. Requirement of the initial production of gamma interferon in the generation of protective immunity of mice against Listeria monocytogenes. Infect. Immun. 1997;65:72–77. doi: 10.1128/iai.65.1.72-77.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.