Abstract
Background
Pembrolizumab improved survival as first- and second-line therapy compared with chemotherapy in patients with highly programmed death ligand 1 (PD-L1) expressing advanced non-small cell lung cancer (NSCLC). We report the long-term safety and clinical activity of pembrolizumab as first-line therapy for patients with advanced NSCLC and the correlation between PD-L1 expression and efficacy.
Patients and methods
In the open-label phase 1b KEYNOTE-001 trial, treatment-naive patients with advanced NSCLC whose tumors expressed PD-L1 (≥1% staining, assessed using a prototype assay) were randomly assigned to intravenous pembrolizumab 2 or 10 mg/kg every 3 (Q3W) or 2 (Q2W) weeks. Response was assessed per central RECIST v1.1 every 9 weeks in all patients who received ≥1 pembrolizumab dose. Using pre-treatment tumor tissue, a clinical assay quantified the percentage of tumor cells expressing PD-L1 as tumor proportion score (TPS).
Results
Between 1 March 2013 and 18 September 2015, 101 patients received pembrolizumab 2 mg/kg Q3W (n = 6), 10 mg/kg Q3W (n = 49), or 10 mg/kg Q2W (n = 46). Of these, 27 (26.7%) had TPS ≥50%, 52 (51.5%) had TPS 1%–49%, and 12 (11.9%) had TPS <1%. The objective response rate (ORR) was 27% (27/101, 95% CI 18–37) and median overall survival was 22.1 months (95% CI 17.1–27.2). In patients with PD-L1 TPS ≥50%, ORR, 12-month PFS, and 12-month OS were higher [14/27 (51.9%; 95% CI 32%–71%), 54%, and 85%, respectively] than the overall population [27/101 (26.7%; 95% CI 18.4%–36.5%), 35%, 71%]. Pembrolizumab was well tolerated, with only 12 (11.9%) patients experiencing grade 3/4 treatment-related adverse events and no treatment-related deaths.
Conclusions
Pembrolizumab provides promising long-term OS benefit with a manageable safety profile for PD-L1-expressing treatment-naive advanced NSCLC, with greatest efficacy observed in patients with TPS ≥50%.
Clinical trial name and number
KEYNOTE-001 (ClinicalTrials.gov, NCT01295827).
Keywords: pembrolizumab, non-small cell lung cancer, immunotherapy, anti-PD-1
Introduction
Current standard first-line treatment of advanced non-small cell lung cancer (NSCLC) without driver mutations is platinum-based chemotherapy [1], yet its benefit is minimal [2]. There is an urgent need for improved first-line therapies. Programmed death 1 (PD-1), an immune checkpoint receptor, is primarily expressed on activated T and B cells [3]. Some tumors exploit the PD-1 pathway by constitutively expressing programmed death ligand 1 (PD-L1) or adaptively upregulating PD-L1 expression to evade immune attack and allowing neoplastic growth [3]. The PD-1 pathway is therefore a target for cancer immunotherapy.
Pembrolizumab is a high-affinity, humanized, IgG4, highly selective monoclonal antibody against PD-1 that has shown important clinical activity in multiple tumor types [4]. Previously, in a pooled dataset of treatment-naive and previously treated patients with advanced NSCLC enrolled in the large phase 1 KEYNOTE-001 study, we showed that pembrolizumab provided an objective response rate (ORR) of 19.4%, with a median duration of response of 12.5 months and a median overall survival (OS) of 12.0 months [5]. Among all patients with membranous PD-L1 expression on ≥50% of tumor cells [tumor proportion score (TPS) ≥50%] who were treated with pembrolizumab, ORR was 45.2%, and median OS was not reached. Based on this study, pembrolizumab was approved in the United States as therapy for patients with PD-L1-expressing NSCLC who had progressed on platinum-containing chemotherapy or on epidermal growth factor receptor (EGFR) or anaplastic lymphoma kinase (ALK) inhibitor medicine if they had an EGFR mutation or ALK translocation. Here, we report the updated safety and efficacy of first-line pembrolizumab therapy and the correlation between PD-L1 expression and clinical activity in treatment-naive patients with advanced NSCLC enrolled in KEYNOTE-001 (ClinicalTrials.gov, NCT01295827).
Methods
Patients and study design
KEYNOTE-001 was an international, randomized, open-label, phase 1 study designed to assess the efficacy and safety of pembrolizumab in patients with advanced solid tumors. The study design and methods have been published previously [5]. For treatment-naive cohorts, patients were required to have histologically or cytologically confirmed stage IV NSCLC, age ≥18 years, wild-type EGFR and negative ALK translocation status (not required for the first 11 patients enrolled under an earlier protocol version), measurable disease per investigator-assessed immune-related response criteria (irRC), an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, positive tumor expression of PD-L1 assessed by a prototype assay, and completion of adjuvant therapy >1 year before recurrent/metastatic disease. A new tumor sample for assessment of PD-L1 expression was required for all randomized patients.
The study protocol and its amendments were approved by the appropriate institutional review boards or ethics committees, and the study was conducted in accordance with the protocol, good clinical practice guidelines, and the provisions outlined in the Declaration of Helsinki. All patients provided written informed consent.
Treatments and assessments
The first 11 patients enrolled were randomly assigned 1:1 to pembrolizumab 2 mg/kg Q3W or 10 mg/kg Q3W. Following a protocol amendment, subsequent patients (n = 90) were randomly assigned 1:1 to receive pembrolizumab 10 mg/kg every 2 weeks (Q2W) or 3 weeks (Q3W).
Treatment was continued until disease progression per investigator-assessed irRC, unacceptable toxicity, physician decision, or patient withdrawal. Tumor imaging was done every 9 weeks and reviewed centrally. Response was assessed per RECIST v1.1 by independent central review (primary end point for efficacy) and per irRC by investigator (primary end point for clinical decision-making). Adverse events (AEs) were collected throughout the study and for 30 days thereafter (90 days for serious AEs) and graded per National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0; pre-specified events of clinical interest with an immune-mediated mechanism of action were also reported.
The primary efficacy end point was ORR. Secondary end points included duration of response, disease control rate [defined as complete response + partial response + stable disease + non-complete response/non-progressive disease (defined as patients without measurable disease per central review at baseline who did not experience complete response or disease progression)], progression-free survival (PFS), OS, and relationship between PD-L1 expression and antitumor activity.
PD-L1 expression
As previously described [5], PD-L1 expression for enrollment was assessed with a prototype immunohistochemistry assay (QualTek Molecular Laboratories, Goleta, CA) and the relationship between PD-L1 expression and efficacy was investigated using a clinical trial immunohistochemistry assay (early version of the PD-L1 22C3 IHC pharmDx assay, Dako North America, Carpinteria, CA). Both assays used the murine 22C3 anti-human PD-L1 antibody (Merck & Co., Inc., Kenilworth, NJ). PD-L1 positivity was defined as membrane staining on ≥1% of cells within tumor nests, including both neoplastic cells and intercalated mononuclear inflammatory cells, or a distinctive pattern of staining caused by mononuclear inflammatory cells infiltrating the stroma, forming a banding pattern adjacent to tumor nests [6]. Tumors were categorized based on TPS, the percentage of tumor cells demonstrating membranous PD-L1 staining [7].
Statistical analysis
Analyses of ORR, PFS, OS, and safety were carried out in the as-treated population (i.e. all patients who received ≥1 pembrolizumab dose). The exact binomial method was used to estimate ORR and its corresponding 95% CI. Duration of response, PFS, and OS were estimated using the Kaplan–Meier method. SAS, version 9.3 (SAS, Cary, NC), was used for all analyses. The current report is based on a database lock date of 18 September 2015. This trial is registered with ClinicalTrials.gov, NCT01295827.
Results
Between 1 March 2013 and 26 March 2014, 101 patients with treatment-naive advanced NSCLC enrolled and were randomly assigned to receive pembrolizumab 2 mg/kg Q3W (n = 6), 10 mg/kg Q3W (n = 49), or 10 mg/kg Q2W (n = 46) (supplementary Figure S1, available at Annals of Oncology online). Of the 211 initial patients whose tumor samples were screened for PD-L1 expression using the prototype assay, 195 (92.4%) had an evaluable sample, and 145 (74.3%) had PD-L1-positive tumors based on the prototype assay. The relationship between PD-L1 expression and efficacy was subsequently investigated using the clinical trial immunohistochemistry assay. Of the 101 (69.7%) patients with PD-L1–positive tumors per prototype assay subsequently enrolled in the study (supplementary Figure S1, available at Annals of Oncology online), 27 (26.7%) had TPS ≥50%, 52 (51.4%) had TPS 1%–49%, and 12 (11.9%) had TPS <1% based on the clinical trial assay; the PD-L1 status of the remaining 10 patients was not evaluable using the clinical trial assay (insufficient/no tumor sample, n = 7; samples not sent, n = 2; unacceptable specimen, n = 1). The distribution of staining was similar in the 44 patients with PD-L1-positive tumors who did not enroll (36.4%, 40.9%, and 13.6%, respectively). Patient characteristics were similar across PD-L1 expression levels; only 1 (4%) patient in the TPS ≥50% group had squamous histology (Table 1). As of the analysis cut-off date of 18 September 2015, median follow-up duration was 22.2 months (range 17.8–30.5); 36 (35.6%) patients were alive without new anticancer therapy, and 13 (13%) were still receiving pembrolizumab. Of the 39 patients who discontinued because of disease progression, 21 (53.8%) received subsequent lines of therapies.
Table 1.
Baseline characteristics in the intent-to-treat population
| Characteristic | Overall (N = 101) |
|---|---|
| Age, years | |
| Median | 68.0 |
| Range | 39 –93 |
| Sex, n (%) | |
| Male | 60 (59) |
| Female | 41 (41) |
| ECOG performance status,an (%) | |
| 0 | 44 (44) |
| 1 | 57 (56) |
| Race, n (%) | |
| White | 91 (90) |
| Asian | 8 (8) |
| Black or African American | 1 (1) |
| Other | 1 (1) |
| Histology, n (%) | |
| Adenosquamous | 2 (2) |
| Non-squamous | 79 (78) |
| Adenocarcinoma, n | 73 |
| Large cell, n | 4 |
| Epidermoid, n | 1 |
| Pleomorphic carcinoma, n | 1 |
| Squamous | 19 (19) |
| Unknown | 1 (1) |
| Smoking history, n (%) | |
| Former/current | 90 (89) |
| Never | 11 (11) |
| EGFR mutation, n (%) | |
| Yes | 3 (3) |
| No | 95 (94) |
| Unknown | 3 (3) |
| PD-L1 expression, n (%) | |
| TPS ≥50% | 27 (26.7) |
| TPS 1%–49% | 52 (51.4) |
| TPS <1% | 12 (11.9) |
| Not evaluableb | 10 (9.9) |
ECOG, Eastern Cooperative Oncology Group performance status; TPS, tumor proportion score.
An ECOG performance status of 0 indicates that the patient is fully active and 1, that the patient is ambulatory and able to carry out work of a light or sedentary nature but is restricted in physically strenuous activity.
Insufficient/no tumor sample, n = 7; samples not sent, n = 2; unacceptable specimen, n = 1.
The confirmed ORR per RECIST v1.1 by independent central review was 26.7% (n = 27; 95% CI 18.4%–36.5%) in all patients who received ≥1 pembrolizumab dose (N = 101; supplementary Table S1, available at Annals of Oncology online). Best objective response was partial response in 14 [51.9% (95% CI 31.9%–71.3%)] patients with TPS ≥50% (n = 27), in 9 [17.3% (95% CI 8.2%–30.3%)] patients with TPS 1%–49% (n = 52), and in 1 [8.3% (95% CI 0.2%–38.5%)] patient with TPS <1% (n = 12). One of the three patients with EGFR mutation had a partial response of 10.4 months’ duration. There was no effect of dosing schedule on antitumor activity (supplementary Table S1, available at Annals of Oncology online). ORR in subgroups by PD-L1 strata are shown in supplementary Table S2, available at Annals of Oncology online.
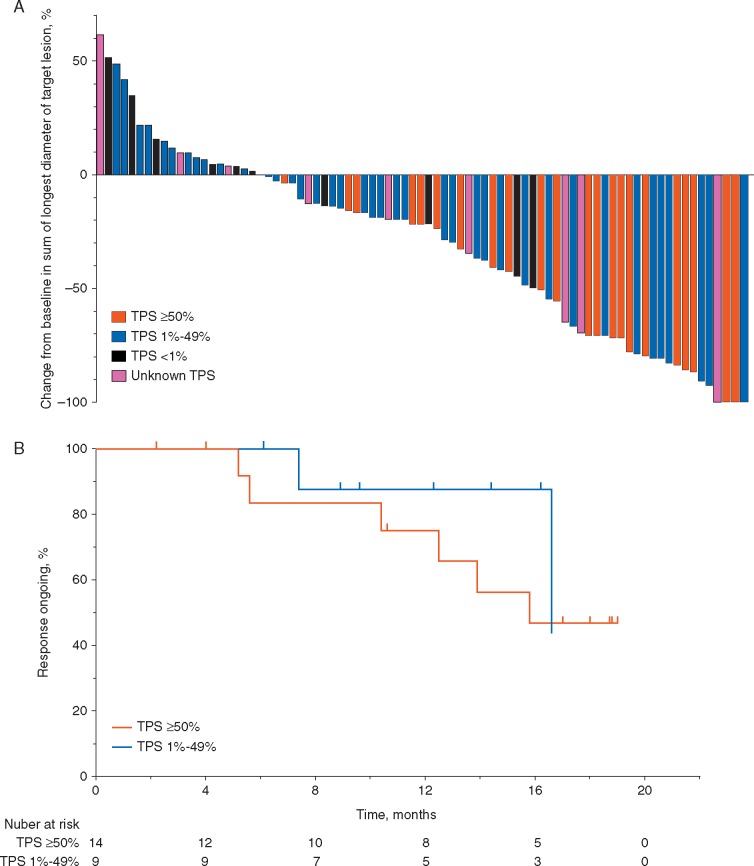
Disease control rate was 67.3% (n = 68 of 101; 95% CI 57.3%–76.3%) in the overall population, and was similar across subgroups and irrespective of PD-L1 status (supplementary Table S2, available at Annals of Oncology online). A decrease from baseline in the size of target lesions was observed in 60 of the 81 evaluable patients (74.0%) overall; in 22 of the 23 patients (95.6%) with a TPS ≥50%; in 28 of the 39 patients (71.8%) with TPS 1%–49%; and in 4 of the 10 patients (40.0%) with a TPS <1% (Figure 1A). Tumor shrinkage was observed in patients regardless of histology.
Figure 1.
Efficacy of pembrolizumab. (A) Maximum percentage change from baseline in the sum of the longest diameters of target lesions per RECIST v1.1 by independent central review in patients with measurable disease at baseline. (B) Kaplan–Meier estimate of duration of response per RECIST v1.1 by independent central review in responding patients.*RECIST v1.1, Response Evaluation Criteria in Solid Tumors (version 1.1); TPS, tumor proportion score. *TPS <1% data is not shown as there was only one patient with duration of response; as of the cut-off date, duration of response has been 17.5 months in this patient.
Median time to response in the overall population was 2.2 months (range 1.7–10.6). The median duration of response was 19.0 months (range 2.1+ to 19.0), with no evident impact of PD-L1 expression level on response duration (Figure 1B). Response was ongoing (alive, with no progression) in 17 of the 27 patients (63.0%); of these 27 patients, 14 (51.9%) had response by first assessment following treatment initiation. Of the 56 patients who had investigator-assessed disease progression per irRC, 32 continued on treatment beyond initial irRC progression, with a median duration of 24 days (range 2–592). At data cut-off, 3 were still on treatment.
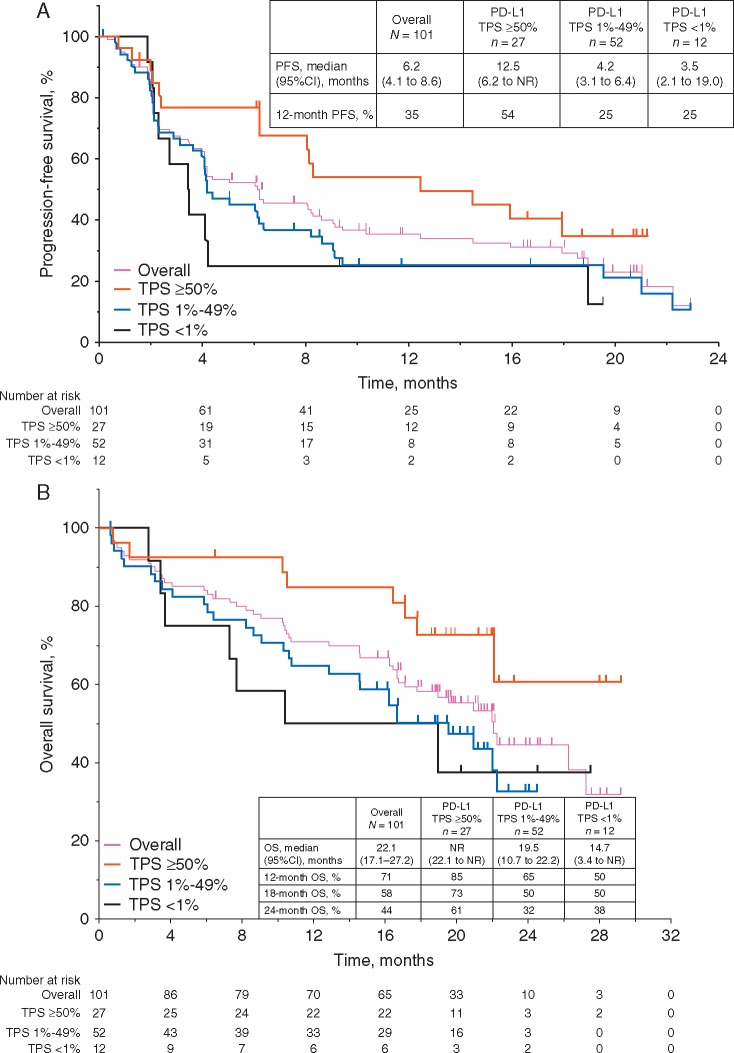
Median PFS was 6.2 months (95% CI 4.1–8.6) in the overall population, with a 12-month PFS rate of 35% (Figure 2A). Among patients with TPS ≥50%, the median PFS was 12.5 months (95% CI 6.2 to not reached; Figure 2A) and 12-month PFS rate was 54%. Median OS was 22.1 months in the overall population (95% CI 17.1–27.2). In the TPS ≥50% group, median OS was not reached (95% CI 22.1 to not reached) (Figure 2B).
Figure 2.
Kaplan–Meier estimates of PFS per RECIST v1.1 by independent central review (A) and OS (B) by PD-L1 expression level. OS, overall survival; PFS, progression-free survival; RECIST v1.1, Response Evaluation Criteria in Solid Tumours (version 1.1); TPS, tumor proportion score.
Any-grade treatment-related AEs occurred in 86 (85.1%) patients (Table 2), most commonly fatigue (n = 28; 27.7%), pruritus (n = 15; 14.9%), hypothyroidism (n = 14; 13.9%), rash (n = 14; 13.9%), arthralgia (n = 12; 11.9%), and nausea (n = 12; 11.9%). Grade 3/4 treatment-related AEs occurred in 12 (11.9%) patients (Table 3). Overall, 22 (21.8%) patients experienced immune-mediated AEs regardless of attribution to treatment by investigators, the most common of which was hypothyroidism in 15 (15%) patients. All were of grade 1 or 2 severity, except for 1 case each of grade 3 pneumonitis, grade 3 hypophysitis, grade 3 colitis, and grade 3 dermatitis. Median onset of hypothyroidism was 129 days (range 21–232). The colitis resolved after treatment with steroids (24 days), and treatment was resumed with no recurrence of toxicity. Six (6%) patients discontinued for treatment-related events (n = 2 for pneumonitis, n = 1 for hyperkeratosis, n = 1 for neuralgia, n = 1 for bronchospasm, and n = 1 for renal failure). There were no treatment-related deaths.
Table 2.
Any-grade treatment-related adverse events in ≥5% of the as-treated population
| n (%) | Overall (N = 101) | 2 mg/kg Q3W (n = 6) | 10 mg/kg Q3W (n = 49) | 10 mg/kg Q2W (n = 46) |
|---|---|---|---|---|
| Any | 86 (85.1) | 5 (83.3) | 42 (85.7) | 39 (84.8) |
| Fatigue | 28 (27.7) | 0 | 20 (40.8) | 8 (17.4) |
| Pruritus | 15 (14.9) | 1 (16.7) | 6 (12.2) | 8 (17.4) |
| Hypothyroidism | 14 (13.9) | 1 (16.7) | 7 (14.3) | 6 (13.0) |
| Rash | 14 (13.9) | 0 | 6 (12.2) | 8 (17.4) |
| Arthralgia | 12 (11.9) | 0 | 5 (10.2) | 7 (15.2) |
| Nausea | 12 (11.9) | 0 | 8 (16.3) | 4 (8.7) |
| Dyspnea | 9 (8.9) | 1 (16.7) | 4 (8.2) | 4 (8.7) |
| Diarrhea | 8 (7.9) | 0 | 6 (12.2) | 2 (4.3) |
| Asthenia | 8 (7.9) | 0 | 3 (6.1) | 5 (10.9) |
| Decreased appetite | 7 (6.9) | 0 | 5 (10.2) | 2 (4.3) |
| Aspartate aminotransferase increased | 6 (5.9) | 0 | 5 (10.2) | 1 (2.2) |
| Blood thyroid stimulating hormone increased | 5 (5.0) | 0 | 3 (6.1) | 2 (4.3) |
| Constipation | 5 (5.0) | 0 | 2 (4.1) | 3 (6.5) |
| Myalgia | 5 (5.0) | 0 | 1 (2.0) | 4 (8.7) |
| Weight decreased | 5 (5.0) | 1 (16.7) | 4 (8.2) | 0 |
Table 3.
Grade 3/4 treatment-related adverse events in the as-treated population and immune-related adverse events
| Grade 3/4 TRAE, n (%) | Overall (N = 101) | 2 mg/kg Q3W (n = 6) | 10 mg/kg Q3W (n = 49) | 10 mg/kg Q2W (n = 46) |
|---|---|---|---|---|
| Any | 12 (11.9) | 0 (0) | 9 (18.4) | 3 (6.5) |
| Hypertension | 2 (2.0) | 0 (0) | 2 (4.1) | 0 (0) |
| Blood creatinine phosphokinase increased | 1 (1.0) | 0 (0) | 0 (0) | 1 (2.2) |
| Bronchospasm | 1 (1.0) | 0 (0) | 0 (0) | 1 (2.2) |
| Colitis | 1 (1.0) | 0 (0) | 1 (2.0) | 0 (0) |
| Dehydration | 1 (1.0) | 0 (0) | 1 (2.0) | 0 (0) |
| Dyspnea | 1 (1.0) | 0 (0) | 1 (2.0) | 0 (0) |
| Hyperkeratosis | 1 (1.0) | 0 (0) | 1 (2.0) | 0 (0) |
| Hypophosphatemia | 1 (1.0) | 0 (0) | 1 (2.0) | 0 (0) |
| Hypophysitis | 1 (1.0) | 0 (0) | 1 (2.0) | 0 (0) |
| Interstitial lung disease | 1 (1.0) | 0 (0) | 0 (0) | 1 (2.2) |
| Lichenoid keratosis | 1 (1.0) | 0 (0) | 1 (2.0) | 0 (0) |
| Pericardial effusion | 1 (1.0) | 0 (0) | 1 (2.0) | 0 (0) |
| Pleural effusion | 1 (1.0) | 0 (0) | 1 (2.0) | 0 (0) |
| Pneumonitis | 1 (1.0) | 0 (0) | 1 (2.0) | 0 |
| Pulmonary embolism | 1 (1.0) | 0 (0) | 1 (2.0) | 0 (0) |
| Vasculitis | 1 (1.0) | 0 (0) | 1 (2.0) | 0 (0) |
| Weight decreased | 1 (1.0) | 0 (0) | 1 (2.0) | 0 (0) |
| Immune-related AE, n (%) | ||||
| Any | 22 (21.8) | 1 (16.7) | 12 (24.5) | 9 (19.6) |
| Adrenal insufficiency | 1 (1.0) | 0 | 1 (2.0) | 0 |
| Hypophysitis | 1 (1.0) | 0 | 1 (2.0) | 0 |
| Hypothyroidism | 15 (14.9) | 1 (16.7) | 8 (16.3) | 6 (13.0) |
| Myxedema | 1 (1.0) | 0 | 1 (2.0) | 0 |
| Colitis | 1 (1.0) | 0 | 1 (2.0) | 0 |
| Myasthenic syndrome | 1 (1.0) | 0 | 1 (2.0) | 0 |
| Interstitial lung disease | 1 (1.0) | 0 | 0 | 1 (2.2) |
| Pneumonitis | 3 (3.0) | 0 | 2 (4.1) | 1 (2.2) |
| Dermatitis | 1 (1.0) | 0 | 0 | 1 (2.2) |
There were no grade 5 treatment-related adverse events.
Discussion
In this cohort of 101 patients with treatment-naive metastatic NSCLC treated with pembrolizumab, while the ORR of 27% was comparable with first-line chemotherapy, the impressive median OS of 22.1 months clearly exceeded the expected survival from first-line chemotherapy for patients without driver mutations. Most reported NSCLC studies suggest that high PD-L1 expression could identify an enriched population with higher probability of benefit from anti-PD-1 and anti-PD-L1 agents [5, 8–11]. Similarly, in this study, higher response and survival rates were observed in patients with PD-L1 TPS ≥50%, with a response rate of 52%, 12-month survival rate of 85%, and 18-month survival rate of 72.7%. The response rate to pembrolizumab increased with increasing expression of PD-L1, although patients who responded to pembrolizumab had durable responses irrespective of PD-L1 status, with median duration of response of 19.0 months (range 2.1+ to 19.0).
The recently published phase 3 study (KEYNOTE-024; ClinicalTrials.gov, NCT02142738) confirms the significantly improved progression-free survival [hazard ratio (HR) 0.50 (95% CI, 0.37–0.68); P < 0.001] in treatment-naive patients with PD-L1 expression TPS ≥50% receiving monotherapy pembrolizumab compared with platinum-based chemotherapy [12]. An OS benefit [HR 0.60 (95% CI 0.41–0.89); P = 0.005] was also evident despite a 50% crossover rate to receive an anti-PD-1 upon disease progression in the chemotherapy arm [12]. In contrast, there was no survival difference in patients receiving nivolumab or chemotherapy in the phase 3 CheckMate 026 study (ClinicalTrials.gov, NCT02041533), which used a much lower PD-L1 cutpoint of 5% (PFS HR 1.19; OS HR 1.08) [13]. Unlike the second-line setting in which docetaxel only provides modest benefit with a low response rate, a more enriched population with high PD-L1 expression would likely be required in the first-line setting to surpass the proven benefit from platinum-based doublet chemotherapy. Interestingly, the first-line nivolumab study (CheckMate 012, ClinicalTrials.gov, NCT01454102) had used a higher PD-L1 cutpoint of ≥50% and showed a response rate of 50% and 12-month OS rate of 83%, similar to our study [14]. However, this was not duplicated in subgroup analyses of the CheckMate 026 data using a PD-L1 cutpoint of ≥50% (PFS HR 1.07; OS HR 0.90) [13]. In addition, a response rate of only 26% was reported in patients with highest levels of PD-L1 expression treated with first-line atezolizumab [15].
The differential impact of PD-L1 expression as a predictor of response to PD-1 blockade may be explained by differences in the PD-L1 assays, antibodies, expression scoring method, and definition of PD-L1 positivity used in each of these studies [16]. In this first-line study, ORR was higher in patients with TPS ≥50% in non-squamous cell type; conclusions cannot be drawn for those with squamous histology, given that only one patient enrolled. In the KEYNOTE-024 study, patients with highly PD-L1 expressing squamous and non-squamous histology demonstrated superior progression-free survival over chemotherapy with a HR of 0.35 (95% CI 0.17–0.71) and 0.55 (95% CI 0.39–0.76), respectively [12]. The impact of PD-L1 status on histologic types in the first-line setting will be further evaluated in the large phase 3 KEYNOTE-042 study (ClinicalTrials.gov, NCT02220894), in which patients with PD-L1-expressing (TPS ≥1%) NSCLC are randomly assigned to receive either pembrolizumab or platinum-based doublet chemotherapy.
Similar to results from the entire population of the phase 1 KEYNOTE-001 study [17] and the randomized phase 3 KEYNOTE-010 study (NCT01905657) [18], no significant exposure dependency on efficacy or safety was identified for pembrolizumab across doses of 2–10 mg/kg. Thus, a flat dose of 200 mg Q3W was used in subsequent phase 3 first-line studies in NSCLC. An every 3-week dosing schedule enables less frequent patient visits compared with Q2W dosing, along with shorter chair time, particularly in comparison with first-line chemotherapy, thus allowing more patients to be treated promptly. Optimal duration of treatment is not yet determined.
Pembrolizumab monotherapy was generally well tolerated in the first-line treatment of NSCLC with low grade 3/4 toxicity and without any treatment-related death. Three of five patients with grade 3/4 immune-mediated AEs were able to resume treatment following appropriate management. Additionally, immune-mediated hypothyroidism, while approximately two times that reported previously [5], was manageable. The phase 3 KEYNOTE-024 study showed similarly manageable toxicity profile from pembrolizumab in treatment-naive patients with TPS ≥50% NSCLC [12].
First-line combination chemotherapy with pembrolizumab showed encouraging results with higher response rate and superior progression-free survival over platinum-based chemotherapy in a randomized phase 2 study for advanced NSCLC [19]. Randomized phase 3 studies will further explore combinations of pembrolizumab with chemotherapy (KEYNOTE-189, NCT02578680, and KEYNOTE-407, NCT02775435) to better understand how PD-L1 expression determines outcomes in these settings and whether survival benefits can be observed irrespective of PD-L1 expression. Furthermore, evaluation of other potential biomarkers including mutational load [20], immune gene expression signatures [21], DNA mismatch repair defect [22], other ligands to immune checkpoint inhibitors [23], and immune competency with pre-existing CD8+ T lymphocytes in the tumor microenvironment [24] is ongoing to better define predictive markers of immunotherapy.
In conclusion, these data demonstrated durable responses with promising long-term survival and a manageable safety profile with first-line pembrolizumab therapy for metastatic NSCLC, in particular in patients with TPS ≥50%. These findings surpass survival anticipated with standard chemotherapy and together with KEYNOTE-024 data, pembrolizumab has emerged as a first-line treatment option for high PD-L1-expressing advanced NSCLC.
Acknowledgments
The authors have contributed to the acquisition and interpretation of the data and drafting the manuscript; they approved the final version for submission and accept responsibility for all aspects of the manuscript.
Funding
This study was supported by Merck & Co., Inc., Kenilworth, NJ. Editorial assistance was provided by Tricia Brown and Jennifer M. Kulak, PhD (ApotheCom, Yardley, PA) and was funded by Merck & Co., Inc. No grant numbers apply.
Disclosure
RH: advisory board member for Merck Sharp & Dohme Corp., AstraZeneca, Novartis, and Pfizer; speaker honorarium from Merck Sharp & Dohme Corp., AstraZeneca, Bristol-Myers Squibb, and Boehringer-Ingelheim. EBG: research grants to institution from Merck & Co., Inc., AstraZeneca, Bristol-Myers Squibb, Genentech, Novartis, Eli Lilly, Pfizer, and Boehringer-Ingelheim. JWG: research grants from AstraZeneca, MedImmune, and Bristol-Myers Squibb. NBL: research grants to institution from Novartis Canada; CME travel funding from AstraZeneca; CME honoraria from Merck & Co., Inc.; and personal fees from Pfizer. MDH: personal fees from Merck & Co., Inc., Genentech, Bristol-Myers Squibb, AstraZeneca, Third Rock Ventures, Alexion Pharmaceuticals, Inovio Pharmaceuticals, and Blueprint Medicines. AP: research grants to institution from Merck & Co., Inc. LG: advisory board member for Merck & Co., Inc., AstraZeneca, AbbVie, Pfizer, Genentech/Roche; research grant from Bristol-Myers Squibb. JPE: educational honoraria from Merck & Co., Inc. M-JA: no conflicts of interest. LH: personal fees from Merck & Co., Inc., Genentech, and AbbVie; unpaid consulting for Boehringer Ingelheim, Bristol-Myers Squibb, and Xcovery. EF: consulting/advisory for Eli Lilly, Pfizer, Roche, Boehringer Ingelheim, Bristol-Myers Squibb, Merck Sharp & Dohme Corp., Novartis, and Celgene. EC: no conflicts of interest. RR: full-time employment with Merck & Co., Inc.; stock ownership of Merck & Co., Inc. GML: full-time employment with Merck & Co., Inc.; stock ownership of Merck & Co., Inc. JZ: full-time employment with Merck & Co., Inc. KE: full-time employment with Merck & Co., Inc.; stock ownership of Merck & Co., Inc., Bayer AG, and Johnson and Johnson; spousal employment and stock options with Celgene. CR: full-time employment with Dako; stock ownership of Dako. NAR: advisory boards for Merck & Co., Inc., Novartis, Roche, Pfizer, Lilly, and AstraZeneca; and stocks for Gritstone Oncology.
Supplementary Material
Footnotes
Present address: Laura and Isaac Perlmutter Cancer Center, NYU Langone Medical Center, New York, USA
References
- 1. NCCN Clinical Practice Guidelines in Oncology: Non-small cell lung cancer—v4.2016; http://www.nccn.org/professionals/physician_gls/f_guidelines.asp (7 October 2016, date last accessed).
- 2. Schiller JH, Harrington D, Belani CP. et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 2002; 346: 92–98. [DOI] [PubMed] [Google Scholar]
- 3. Blank C, Mackensen A.. Contribution of the PD-L1/PD-1 pathway to T-cell exhaustion: an update on implications for chronic infections and tumor evasion. Cancer Immunol Immunother 2007; 56: 739–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Khoja L, Butler MO, Kang SP. et al. Pembrolizumab. J Immunother Cancer 2015; 3: 36.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Garon EB, Rizvi NA, Hui R. et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015; 372: 2018–2028. [DOI] [PubMed] [Google Scholar]
- 6. Dolled-Filhart M, Locke D, Murphy T. et al. Development of a prototype immunohistochemistry assay to measure programmed death ligand-1 expression in tumor tissue. Arch Pathol Lab Med 2016; 140: 1259–1266. [DOI] [PubMed] [Google Scholar]
- 7. Dolled-Filhart M, Roach C, Toland G. et al. Development of a companion diagnostic for pembrolizumab in non-small cell lung cancer using immunohistochemistry for programmed death ligand-1. Arch Pathol Lab Med 2016; 140: 1243–1249. [DOI] [PubMed] [Google Scholar]
- 8. Borghaei H, Paz-Ares L, Horn L. et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015; 373: 1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barlesi F, Von Pawel J, Kowalski D. et al. Primary analysis from OAK, a randomized phase III study comparing atezolizumab with docetaxel in 2L/3L NSCLC. Presented at European Society for Medical Oncology (ESMO) 2016 Congress; October 7–11, 2016; Copenhagen, Denmark. Abstract LBA44_PR.
- 10. Fehrenbacher L, Spira A, Ballinger M. et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016; 387: 1837–1846. [DOI] [PubMed] [Google Scholar]
- 11. Rizvi NA, Hellmann MD, Brahmer JR. et al. Nivolumab in combination with platinum-based doublet chemotherapy for first-line treatment of advanced non-small-cell lung cancer. J Clin Oncol 2016; 34: 2969–2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Reck M, Rodriguez-Abreu D, Robinson AG. et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 2016; 375: 1823–1833. [DOI] [PubMed] [Google Scholar]
- 13. Socinski M, Horn L, Steins M. et al. CheckMate 026: a phase 3 trial of nivolumab vs investigator's choice (IC) of platinum-based doublet chemotherapy (PT-DC) as first-line therapy for stage IV/recurrent programmed death ligand 1 (PD-L1)-positive NSCLC. Presented at European Society for Medical Oncology (ESMO) 2016 Congress; October 7–11, 2016; Copenhagen, Denmark. Abstract LBA7_PR.
- 14. Gettinger S, Rizvi NA, Chow LQ. et al. Nivolumab monotherapy for first-line treatment of advanced non-small-cell lung cancer. J Clin Oncol 2016; 34: 2980–2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Besse B, Johnson ML, Janne PA. et al. Phase II single arm trial (BIRCH) of atezolizumab as first line or subsequent therapy for locally advanced or metastatic PD-L1 selected NSCLC. Presented at European Cancer Congress 2015; September 25–29, 2015, Vienna, Austria. Abstract 16LBA.
- 16. Hirsch FR, Averbuch S, Emami T. et al. The Blueprint Project: harmonizing companion diagnostics across a class of targeted therapies. Presented at: American Association for Cancer Research Annual Meeting 2016; April 16–20, 2016, New Orleans, LA.
- 17. Chatterjee M, Turner DC, Felip E. et al. Systematic evaluation of pembrolizumab dosing in patients with advanced non-small-cell lung cancer. Ann Oncol 2016; 27: 1291–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Herbst RS, Baas P, Kim DW. et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016; 387: 1540–1550. [DOI] [PubMed] [Google Scholar]
- 19. Langer CL, Gadgeel SM, Borghaei H. et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol 2016; 17: 1497–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rizvi NA, Hellmann MD, Snyder A. et al. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015; 348: 124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen PL, Roh W, Reuben A. et al. Analysis of immune signatures in longitudinal tumor samples yields insight into biomarkers of response and mechanisms of resistance to immune checkpoint blockade. Cancer Discov 2016; 6: 827–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Le DT, Uram JN, Wang H. et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 2015; 372: 2509–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Herbst RS, Soria JC, Kowanetz M. et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014; 515: 563–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tumeh PC, Harview CL, Yearly JH. et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014; 515: 568–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.