Abstract
Introduction
Retroperitoneal sarcoma (RPS) is a rare disease accounting for 0.1%–0.2% of all malignancies. Management of RPS is complex and requires multidisciplinary, tailored treatment strategies at all stages, but especially in the context of metastatic or multifocal recurrent disease. Due to the rarity and heterogeneity of this family of diseases, the literature to guide management is limited.
Methods
The Trans-Atlantic Retroperitoneal Sarcoma Working Group (TARPSWG) is an international collaboration of sarcoma experts from all disciplines convened in an effort to overcome these limitations. The TARPSWG has compiled the available evidence surrounding metastatic and multifocally recurrent RPS along with expert opinion in an iterative process to generate a consensus document regarding the complex management of this disease. The objective of this document is to guide sarcoma specialists from all disciplines in the diagnosis and treatment of multifocal recurrent or metastatic RPS.
Results
All aspects of patient assessment, diagnostic processes, local and systemic treatments, and palliation are reviewed in this document, and consensus recommendations provided accordingly. Recommendations were guided by available evidence, in conjunction with expert opinion where evidence was lacking.
Conclusions
This consensus document combines the available literature regarding the management of multifocally recurrent or metastastic RPS with the practical expertise of high-volume sarcoma centers from multiple countries. It is designed as a tool for decision making in the complex multidisciplinary management of this condition and is expected to standardize management across centers, thereby ensuring that patients receive the highest quality care.
Keywords: sarcoma, retroperitoneal sarcoma, recurrence, metastasis, surgery, chemotherapy, outcome
Key Message
Despite multimodal treatment, outcomes for metastatic RPS are poor with median overall survival of 16 months. Due to the paucity of high level evidence, their management is heavily nuanced by the experience and expertise of high volume sarcoma specialists. The importance of an experienced multidisciplinary team underscores the need for referral of these patients to specialist centers.
Introduction
Retroperitoneal sarcomas (RPS) represent a heterogeneous group of rare malignancies with an overall expected incidence of 0.5–1/100 000. Patterns of recurrence vary by histologic subtype, with biologic behavior spanning a broad spectrum from no metastatic potential to a propensity for local recurrence to predominantly distant relapse [1]. Metastatic RPS includes both systemic disease, with lung and liver being the most common sites of distant failure, and multifocal intra-abdominal disease, or sarcomatosis. Despite multimodal treatment, outcomes for metastatic RPS are poor with median overall survival of 16 months [2] and a dismal 5% 5-year survival [3]. Nonetheless, the possibility of long-term survival or even cure remains. Therefore, each case must be considered individually by a multidisciplinary team of sarcoma specialists in order to tailor an appropriate treatment strategy, taking into account a variety of disease- and patient-specific factors.
The existing literature regarding the multidisciplinary management of RPS is limited by the rarity and heterogeneity of this disease, as well as the evolution in histologic classification over time. Investigation of optimal management strategies in a prospective, randomized fashion is constrained by the low incidence of RPS overall, and the applicability of available data to specific histologic subtypes is unclear. The Trans-Atlantic RPS Working Group (TARPSWG) was established in 2013 in an effort to address these challenges, bringing together high-volume sarcoma centers to generate a combined experience of the multidisciplinary management of RPS and to establish consensus regarding various aspects of the approach to this family of diseases. From an original 8 institutions, membership has now expanded across Europe and North America to 35 institutions and consensus documents have been published concerning the management of primary and locally recurrent RPS [4, 5]. The current work addresses the management of metastatic RPS and represents a collation of published literature and expert opinion. The objective of this document is to guide sarcoma specialists, including surgeons, medical and radiation oncologists, pathologists, and radiologists in the diagnosis and treatment of metastatic RPS. Due to the paucity of high-level evidence in this domain, the management of metastatic RPS is heavily nuanced by the experience and expertise of high-volume sarcoma specialists. For this reason, a consensus document reflecting the current practices and recommendations of these opinion leaders and the evidence underpinning them is of great value. Of note, these recommendations were developed with a unanimous consensus. This is reflected by the level of recommendations, which is often high even in the absence of strong data. The importance of an experienced multidisciplinary team for the treatment of metastatic RPS underscores the need for referral of these patients to specialist centers.
Methods
A comprehensive literature search of the PubMed database was carried out encompassing the topics of metastasectomy, ablative therapies, and systemic therapies for soft tissue sarcoma (STS). On the basis of available evidence, a series of best practice recommendations was generated. The first version of the document was drafted and circulated in advance of the 2016 Connective Tissue Oncology Society Annual Meeting in Lisbon, Portugal where it was discussed at the meeting of the TARPSWG. The document was revised in the following months and the second iteration debated at the 2017 Society of Surgical Oncology Annual Meeting in Seattle, Washington. Once informal consensus was achieved, the final version was circulated for approval by all group members. To further validate the document, the ‘Appraisal of Guidelines for Research and Evaluation II instrument’ (AGREE II) was employed [6, 7]. The overall scores of the different domains after review by four independent experts are shown in the supplementary Table S1, available at Annals of Oncology online.
The recommendations that follow apply to select subtypes of RPS in the metastatic setting (Table 1).
Table 1.
Subtypes of retroperitoneal sarcoma included and excluded from consideration in this consensus document
| Included | Excluded |
|---|---|
|
|
Results
Principles of recommended practice from diagnosis to follow-up are summarized in 43 statements. Each statement has been attributed a level of evidence according to the scale reported in Table 2.
Table 2.
Level of evidence and grade of recommendation adapted from the Infectious Diseases Society of America–United States Public Health Service Grading System
| I | Evidence from at least one large randomized control trial of good methodologic quality (low potential for bias) or meta-analyses of wellconducted randomized trials without heterogeneity |
| II | Small randomized trials or large randomized trials with a suspicion of bias (lower methodologic quality) or meta-analyses of such trials or of trials with demonstrated heterogeneity |
| III | Prospective cohort studies |
| IV | Retrospective cohort studies or case–control studies |
| V | Studies without control group, case reports, experts opinions |
| A | Strong evidence for efficacy with a substantial clinical benefit, strongly recommended |
| B | Strong or moderate evidence for efficacy but with a limited clinical benefit, generally recommended |
| C | Insufficient evidence for efficacy or benefit does not outweigh the risk or the disadvantages (adverse events, costs,), optional |
| D | Moderate evidence against efficacy or for adverse outcome, generally not recommended |
| E | Strong evidence against efficacy or for adverse outcome, never recommended |
1. Patients with metastatic RPS should be evaluated in specialized sarcoma centers by multidisciplinary teams with expertise and experience in the full range of treatments of this complex and rare family of diseases. If appropriate, care may then be administered at local centers to minimize patient inconvenience. (VA)
Pretreatment assessment
Clinical history and prior treatment
Details regarding the patient’s clinical history and treatment(s) of RPS to date should be procured and reviewed, with particular attention to time course and response to therapy.
2. Operative reports from all prior procedures should be obtained and details of resection(s) undertaken to date understood. (VA)
3. If systemic therapy was previously administered, details regarding agent(s), dose (including cumulative dose for anthracyclines), regimen, toxicities, and response to treatment should be ascertained. (VA)
4. If radiotherapy was previously administered, the dose, regimen, volumes, and boundaries of the radiation field should be determined. (VA)
Imaging
Suspected metastases should be characterized using appropriate imaging modalities for the anatomic location(s) in question.
5. Imaging from the time of initial presentation and diagnosis to completion of treatment as well as postoperative baseline, if applicable, should be reviewed. (VB)
6. Computed tomography (CT) is the standard imaging modality for staging of the chest [4, 5, 8]. (IVA)
7. Contrast-enhanced CT of the abdomen and pelvis is the preferred imaging modality for intra-abdominal/retroperitoneal disease [8]. (IVA)
8. If the anatomic relationship of metastases to specific neurovascular structures requires clarification in order to initiate treatment, magnetic resonance imaging (MRI) can be a useful adjunct [9]. (IVB)
9. Suspected liver metastases can be further imaged with triphasic CT, contrast-enhanced MRI, or targeted liver ultrasound if the nature of the liver lesions is in question or if precise determination of burden of disease is necessary for initiation of treatment (e.g. consideration of resection/local therapies) [10]. (IVB)
10. Additional imaging is recommended only as clinically indicated:
Suspected brain and soft tissue metastases are best characterized with MRI. (VA)
Suspected bone metastases can be investigated with bone scan or [18]FDG-PET, but routine bone imaging is unnecessary, with the exception of solitary fibrous tumor (SFT) which entails a higher likelihood of bone metastases than other sarcoma subtypes [11]. (VA)
[18]FDG-PET may be helpful in differentiating metastatic disease from benign processes if other imaging modalities are equivocal, or to evaluate treatment response [12, 13]. (IVB)
Pathology
11. Pathology review of the primary tumor should be carried out, including those cases where the original diagnosis was made at a sarcoma center, as variability among institutions and expert sarcoma pathologists is not uncommon. (VA) The diagnosis of histologic subtypes marked by specific chromosomal alterations should be confirmed by molecular genetic testing [14]. (IVA)
12. Lung and liver lesions with a radiographic appearance consistent with metastatic disease in the context of a biopsy-proven primary RPS do not necessarily require tissue diagnosis. However, lesions with radiographic features atypical for STS metastases, in unusual anatomic locations, or those occurring in the context of a known second malignancy or a hereditary syndrome (e.g. Li–Fraumeni) should be sampled before treatment. If ablative therapies [i.e. radiotherapy, radiofrequency ablation (RFA), etc.] are planned where tissue destruction will preclude pathologic diagnosis, biopsy should be considered before initiating treatment. Tissue sampling can also be undertaken for the purposes of enrolment in clinical trials, tissue banking for research, and potential future use for personalizing treatment. (VB)
13. For subcutaneous or soft tissue lesions suspicious for metastases, core needle or open biopsy can be carried out. (VB)
14. Intra-abdominal/retroperitoneal masses in keeping with multifocal recurrence or metastases do not require confirmation with tissue sampling if they are widespread and the pattern and distribution are radiographically consistent with metastases. However, biopsy should be considered to rule out alternative pathology (e.g. fibromatosis) when the radiographic appearance is less characteristic. Solitary lesions contralateral to the primary site should be confirmed with tissue diagnosis if feasible as these have a broad differential diagnosis (e.g. lymphoma, germ-cell tumor, schwannoma, paraganglioma, gastrointestinal stromal tumor (GIST), metastasis from another primary). (VB)
15. Biopsies of retroperitoneal and abdominal masses should be obtained under image guidance, ideally without transgressing the peritoneal cavity, and should be carried out by expert radiologists with experience in soft tissue neoplasms. (VA)
16. To ensure adequate tissue sampling, a minimum of four large gauge cores (14G–16G) is advised [15]. There is no role for fine-needle aspiration biopsy in initial diagnostic evaluation for connective tissue neoplasms; however, recurrences of known sarcomas can be accurately detected using fine-needle aspiration. (IVA)
Patient evaluation
17. Patients should be evaluated with respect to the nature and severity of symptoms as well as performance status in order to guide decisions regarding appropriate treatment modalities and sequencing thereof. This should include a comprehensive assessment of comorbidities and conditions affecting candidacy for multimodal treatment (e.g. geriatric comorbidity indices [16]), nutritional and functional status, physiologic sequelae of prior treatment (e.g. cardiomyopathy, impaired kidney function), and pain or other disability resulting from metastatic disease. (IVA)
18. Consideration for metastasectomy must take into account the likelihood of achieving a macroscopically complete resection as well as the number and extent of prior operations and complications thereof in order to estimate operative risk and counsel patients regarding anticipated morbidity. (VA)
19. All newly referred patients and patients with a first presentation of metastatic sarcoma should be presented at a multidisciplinary tumor board with sarcoma specialists from surgical, medical, and radiation oncology, radiology, and pathology. Patients relapsing or progressing after treatment of metastases should be re-reviewed at a multidisciplinary tumor board, as should patients exhibiting a favorable response to treatment who might be eligible for resection. A tailored treatment strategy should be formulated on an individual basis taking into consideration disease biology, patient performance status, likelihood of disease control or symptom relief with eligible treatment modalities and risks thereof, as well as patient preference and goals of care. (VA)
20. Patients with suspected synchronous metastases based on equivocal lesions on imaging should not be precluded from appropriate treatment of their primary tumor, but short-term follow-up imaging should be obtained in an attempt to clarify tumor stage. (VA)
21. Early involvement of palliative care specialists is encouraged for symptom management and coordination of services with a view to maintaining active treatment and supportive care within an outpatient environment for as long as possible[17]. (IA)
22. An understanding of patient goals of care should be established before initiation of therapy. (VB)
Treatment
Local therapies
It is widely believed that the best possibility for long-term survival with metastatic RPS involves complete extirpation of disease, with metastasectomy considered the preferred treatment strategy for resectable oligometastatic disease in appropriately selected patients. In recent years, other local therapies such as RFA and stereotactic body radiotherapy have been shown to achieve similar rates of disease control for hepatic and pulmonary metastases and are thus considered acceptable alternatives [18–28]. Microwave ablation has supplanted RFA as the preferred ablative modality in many centers, based on evidence from other disease sites. These less invasive treatment modalities may offer the benefit of lower complication rates, shortened disruption of systemic treatment, and expanded application to patients deemed unsuitable for major operative intervention. In addition, they can be combined with surgery to achieve complete disease eradication [19, 29, 30]. Although multiple retrospective series demonstrate prolonged survival in patients who have undergone pulmonary or hepatic metastasectomy [18, 30–45] (Tables 3 and 4), there is no level 1 evidence to show that any apparent benefit of either metastasectomy or ablative therapies is due to the treatment itself rather than a consistent selection of patients with favorable disease biology. The available literature is further limited by the inclusion of both bone and soft tissue sarcomas, as well as STS of extremity/trunk and retroperitoneal origins, and in the case of hepatic metastasectomy, by the inclusion of metastatic GIST and visceral sarcomas. Prognostic factors consistently shown to be associated with improved overall survival after metastasectomy include a prolonged disease-free interval between treatment of the primary tumor and detection of metastases, and complete resection of all metastatic disease [26, 30–32, 35–41, 46–49]. Resection of synchronous metastases has not been associated with improved survival and thus metastasectomy is typically restricted to the setting of metachronous disease [48, 50].
Table 3.
Overview of the literature for pulmonary metastasectomy for soft tissue sarcoma
| Reference | Institution | N | 5y OS (%) | Median OS (mo) | Proportion RPS (%) |
|---|---|---|---|---|---|
| Roth 1985 [98] | NCI, Bethesda, USA | 67 | 15 (est, for DFI>12 mo) | 30 (est, for DFI>12 mo) | N/A |
| Jablons 1989 [99] | NIH, Bethesda, USA | 63 | – | – | N/A |
| Lanza 1991 [100] | MDACC, Houston, USA | 24 | 18.5 | 22 | 15 |
| Casson 1992 [52] | MDACC, Houston, USA | 65 | 25.8 | 25 | N/A |
| Verazin 1992 [40] | RPCI, Buffalo, USA | 61 | 22 | 21 | N/A |
| Gadd 1993 [101] | MSCKK, New York, USA | 135 | 18 | 19 | 0 |
| Mentzer 1993 [102] | BWH, Boston, USA | 34 | 21 (4YS) | 26 | N/A |
| Saltzman 1993 [103] | UMH, Minnesota, USA | 23 | 71 | – | N/A |
| Ueda 1993 [104] | Osaka, Japan | 23 | 24.8 | – | N/A |
| Van Geel 1994 [105] | Rotterdam, Netherlands | 9 | – | – | N/A |
| Choong 1995 [106] | Mayo clinic, Rochester, USA | 214 | 40 | – | 0 |
| Van Geel 1996 [38] | Multi-institutional | 255 | 38 | – | 5.2 |
| Pastorino 1997 [37] | Multi-institutional | 1917 | 31 | – | N/A |
| Billingsley 1999 [39] | MSKCC, New York, USA | 138 | 46 (3YS) | 33 | N/A (9% of all sarcoma patients in used database) |
| Weiser 2000 [36] | MSKCC, New York, USA | 86 (repeated resections) | 36 | 42.8 | N/A |
| Canter 2007 [35] | MSKCC, New York, USA | 138 | 29 (DSS) | 30 | N/A |
| Rehders 2007 [30] | Hamburg, Germany | 61 | 25 | 33 | N/A |
| Liebl 2007 [107] | Hamburg, Germany | 42 | 40.5 | 66 | N/A |
| Chen 2009 [108] | Kyoto, Japan | 23 | 43 | 24 (est) | 9 |
| Smith 2009 [34] | RPCI, Buffalo, USA | 94 | 18 | 16 | 6 |
| Blackmon 2009 [33] | MDACC, Houston, USA | 147 | 26 | 35.5 | N/A |
| Stephens 2011 [32] | MDACC, Houston, USA | 81 (after chemotherapy) | 32 | 35.5 | N/A (44% non-extremity) |
| Nakamura 2011 [109] | Mie, Japan | 45 | 10.6 | N/A | N/A |
| Casiraghi 2011 [110] | Milan, Italy | 80 | 39 | N/A | N/A |
| Predina 2011 [111] | JKCC, Philadelphia, USA | 48 | 52 | 20.4 (DFS) | N/A |
| Hornbech 2011 [112] | Copenhagen, Denmark | 32 | 21.7 | 25.5 | N/A |
| Kim 2011 [113] | MGH, Boston, USA | 62 | 50.1 (including bone sarcoma) | 25 (including bone sarcoma) | N/A |
| Burt 2011 [31] | BWH, Boston, USA | 82 | 52 (LMS) | 70 (LMS) | 8.5 |
| Treasure 2012 [114] | Thames Registry, UK | 6256 | 15 | N/A | N/A |
| Schur 2014 [43] | Vienna, Austria | 46 | 32 | 45.3 | 19.6 (abdomen/pelvis) |
| Dossett 2015 [44] | Moffitt Cancer Center, Tampa, USA | 120 | – | 48 | N/A |
| Lin 2015 [41] | UCLA, Los Angeles, USA | 108 | 61 (LMS), 17 (LPS) | 35.4 | 6.5 |
| Chudgar 2017 [45] | MSKCC, New York, USA | 539 | 34% | 33.2 | 12 (RP/abdomen/pelvis) |
GISTs and bone sarcomas were excluded when possible.
OS, overall survival; RPS, retroperitoneal sarcoma; N/A, not available.
Table 4.
Overview of the literature for hepatic metastasectomy for soft tissue sarcoma
| Reference | Institution | N | 5y OS (%) | Median OS (mo) | Proportion RPS (%) |
|---|---|---|---|---|---|
| Harrison 1997 [115] | MSKCC, New York, USA | 27 | 26 | 31 | 22 |
| Chen 1998 [116] | Johns Hopkins, Baltimore, USA | 11 | – | 39 | 45 |
| Lang 2000 [117] | Hannover, Germany | 26 | 20 (after R0-resection) | 32 (after R0 resection) | 19 |
| DeMatteo 2001 [46] | MSKCC, New York, USA | 22 | 30 | 39 | N/A |
| Ercolani 2005 [118] | Bologna, Italy | 10 | 36 | 44 | N/A |
| Yedibela 2005 [119] | Erlangen, Germany | 15 | – | – | N/A |
| Adam 2006 [120] | Multi-institutional | 125 | 31 | 32 | N/A |
| Pawlik 2006 [121] | MDACC, Houston, USA | 53 | 27 | 47 | 42 (abdomen/RP) |
| Rehders 2009 [30] | Dusseldorf, Germany | 27 | 49 | 44 | 30 (RS/pelvis) |
| Marudanayagam 2011 [122] | Birmingham, UK | 36 | 31.8 | 24 | 5.5 |
| Groeschl 2012 [123] | Multi-institutional (four centers) | 98 (excluding GIST) | 32 | 72 | N/A |
| Brudvik 2015 [26] | MDACC, Houston, USA | 47 LMS, 50 other subtypes | 48.4 LMS | 42.1 LMS | 21 (LMS) |
| 44.9 other subtypes | 45.5 other subtypes |
GISTs and bone sarcomas were excluded when possible.
OS, overall survival; RPS, retroperitoneal sarcoma; N/A, not available.
23. Following the diagnosis of potentially resectable metastatic disease, a period of observation without any therapy may be considered to establish disease biology, provided the resectability of existing disease is unlikely to be compromised by a planned delay. (VB)
24. Patients being considered for metastasectomy should, in general, meet the following criteria: [51] (IVA)
The primary tumor should be completely resected.
All metastatic disease should be completely resectable or controllable with local ablative therapies, unless palliative resection is being considered for symptom relief or control of progressing foci.
The patient should have a suitable performance status and the planned procedure should entail acceptable anticipated morbidity for the individual patient.
25. Selection of patients with favorable tumor biology requires consideration of prognostic features including: (IVA)
disease-free interval of 12 months or longer [32, 35–41, 45, 53, 54];
confirmed response to or prolonged stable disease (≥6 months) on systemic therapy [32].
26. Minimally invasive approaches to metastasectomy may be safely undertaken provided both the surgeon and the treating center have appropriate expertise and experience with these techniques [45, 55, 56]. (IVB)
Pulmonary metastases
27. When considering definitive treatment of pulmonary metastases, the possibility of extrapulmonary metastatic disease should be investigated using CT of the abdomen and either bone scan or [18]FDG-PET [57]. (IVA)
28. In selected patients, the presence of concomitant pulmonary and extrapulmonary metastases is not an absolute contraindication to curative treatment, and occasionally prolonged survival can be achieved with complete extirpation of multiorgan disease [33]. (IVB)
29. Lung function should be optimized in advance of pulmonary metastasectomy. Patients should have preoperative pulmonary function tests before planned extensive resection [58] and should achieve complete smoking cessation at least 3 weeks in advance of pulmonary metastasectomy [42]. (IVA)
30. Patients with compromised pulmonary function may be candidates for local ablative therapies as these result in less tissue destruction than resection [18, 19, 21, 22, 24, 28]. (IVB)
Hepatic metastases
31. For patients with large-volume liver metastatic disease and limited extrahepatic disease, liver-directed therapies such as transarterial embolization and transarterial chemoembolization may be considered. The evidence for these techniques in metastatic sarcoma is limited [59, 60], but their documented efficacy in other malignancies has prompted some centers to extrapolate their use to this setting. (IVC)
Intra-abdominal metastases
32. The role of surgery for multifocal intra-abdominal metastases is limited to palliative intervention as dictated by symptoms (e.g. intestinal obstruction, pain control). Incomplete resection confers no survival benefit and can lead to significant morbidity [5]. (IVB)
33. The role of hyperthermic intraperitoneal chemotherapy in sarcomatosis has been investigated, with no evidence of benefit [2] [61–67]. (IIIB)
Recurrent metastases
34. Surveillance with imaging every 3–6 months is warranted after resection of metastatic disease, as many patients will develop recurrent metastases and some will be candidates for further local or systemic treatment [68–70]. (IVA)
35. Local ablative therapies should be considered in the treatment of recurrent metastatic disease, taking into account the likelihood of disease control and the anticipated morbidity of these modalities compared with repeat resection. (VB)
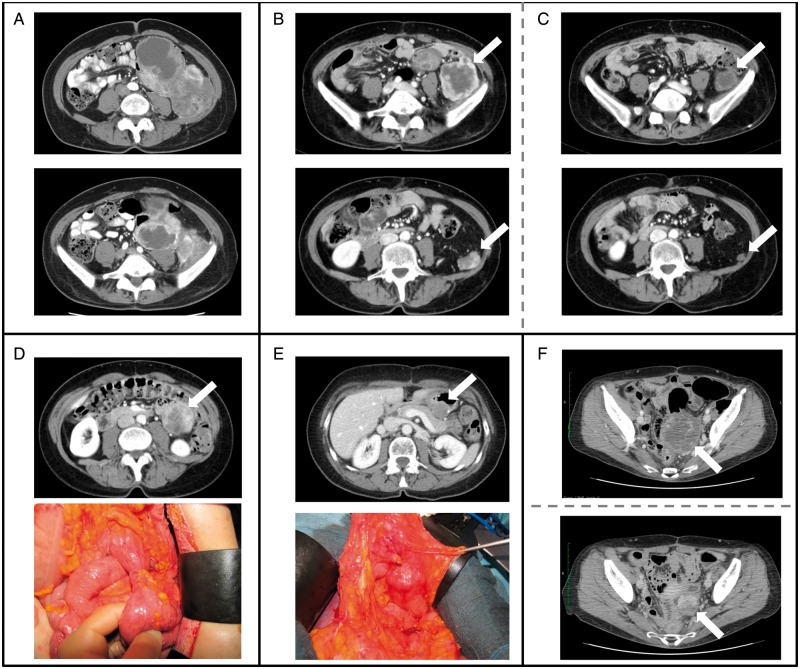
36. Repeated metastasectomy for recurrent metastatic disease may be appropriate in patients with evidence of favorable tumor biology (Figure 1). High-grade histology, high tumor volume, and short disease-free interval (i.e. <1 year) are associated with poor outcomes after re-resection and should discourage further surgery [31, 36]. (IVB)
Figure 1.
A 59 year-old patient presented with a recurrent multifocal grade 3 dedifferentiated liposarcoma originating from the left retroperitoneum (B). The primary tumor (A) had been resected 6 months prior in a peripheral hospital with microscopically positive margins. At recurrence (B), the patient presented with ipsilateral retroperitoneal nodules (arrows), the largest in the iliac muscle, and limited intraperitoneal nodules (maximum diameter 3cm). She was treated with epirubicine + ifosfamide (5 cycles) with major response (C) at all tumor sites. She then underwent complete surgical resection of multiple peritoneal nodules, en bloc with a 20cm tract of small bowel, and the left retroperitoneal masses. After surgery, two additional cycles of epirubicine and ifosfamide were administered. Twenty months later the patient was diagnosed with a second intraperitoneal recurrence (D). She was treated with 6 cycles of high-dose ifosfamide with dimensional response and then with surgery (D). The single abdominal nodule was resected en bloc with a small bowel loop and a small wedge of stomach. One year later (E) she developed a third recurrence on the stomach that was treated with surgery (partial gastrectomy). Four months later the patient developed a pelvic recurrence that was treated with high-dose ifosfamide for 2 cycles with progression (F, upper figure), then with Trabectedin for 6 cycles with dimensional response (F, lower figure) and then with surgery (excision of the pevic mass en bloc with sigmoid colon, uterus and adnexa). The patient died two years later due to other causes. Arrows, tumor; Dotted line, pre/post-operative imaging; Continuous line, oncological event.
Palliation
37. Radiotherapy can be used for palliation of symptoms, in particular for pain, dyspnea due to post-obstructive atelectasis or pneumonia, and symptoms of spinal compression. (VA)
Systemic therapy
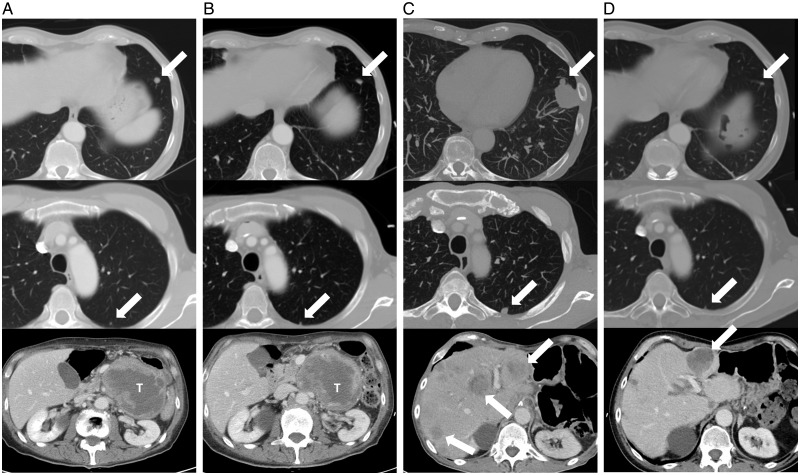
Systemic therapy is the preferred first-line approach in patients presenting with synchronous primary and metastatic disease or when complete extirpation of metastatic disease is not possible with surgery or other local techniques [71–73]. In the event of a favorable response to systemic therapy, defined as either clear regression or stable disease over 6 months, these patients may eventually be considered for resection (Figure 2). First-line systemic therapy may also be considered in patients with resectable metastatic disease in order to observe disease biology and determine appropriateness of aggressive local therapy. In the setting of clearly unresectable metastatic disease, the goal of systemic treatment should be maximal prolongation of an acceptable quality of life, balancing the potential benefits of systemic treatment against expected toxicity.
Figure 2.
A 66 year-old patient presented with a primary localized 10cm grade 2 leiomyosarcoma of the left retroperitoneum and was scheduled for surgery. Preoperative triphasic CT scan (A) performed one month after initial imaging revealed two synchronous pulmonary metastases (12mm in the left lower lobe and 4mm in the left upper lobe, arrows) and progression of the abdominal mass (10cm to 12cm, T). The patient was treated with upfront Adriamycin + Dacarbazine (DTIC). After two courses the abdominal mass and major lung nodule were stable while the minor lung nodule showed mild progression (4mm to 6mm). The chemotherapy regimen was altered to Gemcitabine 900 mg/m2 + DTIC 750 mg/m2 and after 5 courses the CT scan (B) showed partial response of the lung nodules (12mm to 8mm, 6mm to 3mm, arrows) and stable disease of the primary tumor. The patient then underwent resection of the primary tumor en bloc with the pancreatic tail, spleen, duodenojejunal flexure and part of the portal vein. Two months after surgery the CT scan (C) showed dimensional and numerical progression of the lung nodules and appearance of bilateral liver metastases. Gemcitabine + Vinorelbine were started with major response in the lungs and partial response in the liver. Eighteen months after the first course of Gemcitabine (D) the lung disease burden was limited and, due to the progression of a single liver nodule, the patient was treated with transarterial chemoembolization with Adriamycin. Twenty-seven months after disease onset the patient is alive with limited disease in the lungs and stable liver metastases.
Although the evidence for individual histologic subtypes is limited, a number of prospective, randomized trials are available to guide treatment [74] (Table 5). Additional potential therapeutic agents are currently under investigation in pre-clinical and early clinical trials. The following recommendations are based on currently approved therapies for metastatic sarcoma, often guided by histologies and molecular alterations.
Table 5.
Randomized trials investigating the use of systemic therapy in the treatment of locally advanced or metastatic soft tissue sarcoma
| Trial | Study design | N | Objective response rate | Progression-free survival | Overall Survival | Conclusions |
|---|---|---|---|---|---|---|
| Maki 2007 [92] | Phase II: Gemcitabine + docetaxel versus gemcitabine | 122 | 16% versus 8% | 6.2 versus 3 mo | 18 versus 12 mo | Combination superior but has increased toxicity (prohibitive of long-term use) |
| Lorigan 2007 [84] | Phase III: Two investigational schedules of ifosfamide versus doxorubicin | 326 |
|
|
|
Increased toxicity with ifosfamide with no benefit over doxorubicin alone |
| Garcia-Del-Muro 2011 [94] | Phase II: Gemcitabine + dacarbazine versus gemcitabine | 113 |
|
|
|
Combination superior and well tolerated, no increased toxicity |
| PALETTE van der Graaf 2012 [90] | Phase III: Pazopanib versus placebo in non-adipocytic STS | 369 | 6% versus 0% |
|
|
Superior disease control with pazopanib, acceptable toxicity |
| TAXOGEM Pautier 2012 [93] | Phase II: Gemcitabine + docetaxel versus gemcitabine in LMS | 44 (non-uterine LMS only) | 5% versus 14% |
|
|
Increased toxicity with combination with no difference in disease control |
| EORTC 62012 Judson 2014 [81] | Phase III: Doxorubicin + ifosfamide versus doxorubicin | 455 |
|
|
|
No benefit to combination for palliation of advanced STS unless the goal of treatment is tumor shrinkage |
| GeDDiS Seddon 2015 [79] | Phase III: Gemcitabine + docetaxel versus doxorubicin | 257 | N/A |
|
|
Increased toxicity with combination with no difference in disease control |
| Demetri 2016 [87] | Phase III: Trabectedin versus dacarbazine in LPS and LMS | 518 |
|
4.2 versus 1.5 moP <0.0001 |
|
Superior disease control with trabectedin. Led to FDA approval |
| Ryan 2016 [77] | Phase III: Doxorubicin + palifosfamide versus doxorubicin | 447 | 28.3% versus 19.9% | 6.0 versus 5.2 moP=0.19 |
|
Increased toxicity with combination with no difference in disease control |
| Tap 2016 [80] | Phase II: Doxorubicin + olaratumab versus doxorubicin | 133 |
|
|
|
Highly significant 11.8-mo survival benefit with olaratumab |
| Schoffski 2016 [89] | Phase III: Eribulin versus dacarbazine in LPS and LMS | 452 |
|
|
|
Survival benefit with eribulin in LPS and LMS |
STS, soft tissue sarcoma; LPS, liposarcoma; LMS, leiomyosarcoma; NS, not significant; mo, months.
38. For patients with indolent or limited disease, an active surveillance policy can be adopted. (VB)
39. Given the poor outcomes and limited options available to patients with metastatic RPS, inclusion in clinical trials is encouraged. Referral to appropriate academic centers for this purpose is recommended. (VA)
40. For first-line systemic treatment in the palliative setting, an anthracycline-based regimen (doxorubicin or epirubicin) either alone or in combination is recommended [75–79]. (IA) The combination of doxorubicin and olaratumab was shown to be superior in a randomized phase II trial and should be considered for patients with doxorubicin-sensitive histologies [80]. (IIB)
41. If the goal of treatment is tumor downsizing, either for symptomatic relief or possible resection, a combination of doxorubicin with other agents, including high-dose ifosfamide or dacarbazine (DTIC), can be considered [75, 76, 81]. (IA) The combination of doxorubicin and DTIC is preferred in leiomyosarcoma (LMS) and SFT, as ifosfamide may have limited activity in these subtypes [82, 83]. (VA)
42. A multicenter trial randomizing patients with metastatic STS to receive either doxorubicin or gemcitabine/docetaxel in the first-line setting has reported equivalent efficacy but more toxicity with the combination [79]. (IA)
43. There is no clear agent of choice for second-, third- and higher-line treatment, or in the event that anthracycline-based therapy is contraindicated, but the following agents can be considered based on histologic subtype:
Single-agent ifosfamide can be used for selected subtypes [84]. An infusional schedule of ifosfamide (1 g/m2 for 14 days followed by 14 days off) may be particularly effective for dedifferentiated liposarcoma (LPS), synovial sarcoma, and malignant peripheral nerve sheath tumor [85]. For synovial sarcoma in particular, high-dose (>10 g/m2) ifosfamide can be effective [86]. (IB)
Trabectedin can be considered in sensitive histologies, such as LMS and LPS [87, 88]. (IB)
Eribulin has been shown to confer a survival advantage over treatment with DTIC in advanced pre-treated liposarcoma [89]. (IB)
For non-LPS, pazopanib can be considered based on the results of a randomized placebo-controlled trial in pre-treated STS [90]. (IB)
Gemcitabine can be used alone or in combination with docetaxel or DTIC for all subtypes, but especially LMS and undifferentiated pleomorphic sarcoma [79, 91–94]. (IB)
DTIC can be used alone or in combination with anthracyclines for LMS and SFT [72, 92, 95]. (IB)
Antiangiogenics, such as sunitinib, pazopanib, or temozolomide, can be considered for SFT [95]. (IVB)
Sirolimus and other mTOR inhibitors can be considered in PEComa [96]. (VB)
Crizotinib and other ALK inhibitors can be considered for inflammatory myofibroblastic tumor, although they are not yet approved for this application [97]. (VB)
Conclusions
The probability of cure in the context of metastatic RPS is low, but long-term survival has been achieved with metastasectomy in carefully selected patients, often as part of a multimodal treatment strategy. The literature surrounding the management of metastatic RPS is limited in multiple respects. Although level 1 evidence exists for the selection of systemic therapies, and does suggest a limited survival benefit, these trials include sarcomas arising from a variety of primary sites as well as multiple histologic subtypes with widely variable tumor biology. The data in support of metastasectomy consist largely of retrospective, single-institution case series with relatively small numbers, and these are similarly limited in their generalizability due to inclusion of multiple histologic subtypes and anatomic sites of origin. Published reports for local therapies such as RFA and stereotactic body radiotherapy in the treatment of oligometastatic disease demonstrate good efficacy; however, literature with respect to RPS is scant and further work is needed to clarify the role of non-surgical local management.
This consensus document is intended to add to the limited available literature the practical expertise of multiple high-volume sarcoma centers and to serve as a tool for decision making in the complex, multidisciplinary management of this family of diseases. Implementation of the recommendations contained herein may be limited by lack of approval or availability of the described treatment modalities in certain jurisdictions or centers. Referral to specialist centers is strongly encouraged to ensure that patients have access to the full armamentarium of therapeutic options, including experimental therapies.
A prospective registry has been established to improve the quality of evidence going forward and to afford a better understanding of metastatic RPS in order to optimize the complementarity of survival and quality of life. This registry may also allow for investigation of adherence to the recommendations put forth here.
Funding
None declared.
Disclosure
The authors and the members of the TARPSWG have declared no conflicts of interest.
Supplementary Material
Appendix: Trans-Atlantic Retroperitoneal Sarcoma Group Collaborators
| 1. | Jan | Ahlen | Karolinska University Hospital | Stockholm | Sweden |
| 2. | Nita | Ahuja | Johns Hopkins Hospital | Baltimore | USA |
| 3. | Markus | Albertsmeier | University of Munich | Munich | Germany |
| 4. | Waddah B. | Al-Refaie | Georgetown University Medical Center | Washington, DC | USA |
| 5. | Robert | Andtbacka | Huntsman Cancer Institute | Salt Lake City | USA |
| 6. | Martin | Angele | University of Munich | Munich | Germany |
| 7. | Sanjay P | Bagaria | Mayo Clinic | Jacksonville | USA |
| 8. | Elizabeth | Baldini | Dana Farber Cancer Institute | Boston | USA |
| 9. | Francesco | Barretta | Fondazione IRCCS Istituto Nazionale dei Tumori | Milan | Italy |
| 10. | Georgia | Beasley | Ohio State University | Columbus | USA |
| 11. | Jean-Yves | Blay | Centre Leon Berard | Lyon | France |
| 12. | Dan G. | Blazer III | Duke Cancer Center | Durham | USA |
| 13. | Sylvie | Bonvalot | Institut Curie | Paris | France |
| 14. | Sally | Burtenshaw | Princess Margaret Cancer Center | Toronto | Canada |
| 15. | Dario | Callegaro | Fondazione IRCCS Istituto Nazionale dei Tumori | Milan | Italy |
| 16. | Robert | Canter | UC Davis | Sacramento | USA |
| 17. | Kenneth | Cardona | Emory University Hospital Midtown Campus | Atlanta | USA |
| 18. | Paolo G. | Casali | Fondazione IRCCS Istituto Nazionale dei Tumori | Milan | Italy |
| 19. | Charles | Catton | Princess Margaret Cancer Center | Toronto | Canada |
| 20. | Yoon-La | Choi | Samsung Medical Center | Seoul | South Korea |
| 21. | Chiara | Colombo | Fondazione IRCCS Istituto Nazionale dei Tumori | Milan | Italy |
| 22. | Antonino | De Paoli | Centro di Riferimento Oncologico | Aviano | Italy |
| 23. | Angelo P. | Dei Tos | Treviso Hospital | Treviso | Italy |
| 24. | Tom | Delaney | Massachusset General Hospital | Boston | USA |
| 25. | Anant | Desai | Queen Elizabeth Hospital Birmingham | Birmingham | UK |
| 26. | Brendan | Dickson | Mount Sinai Hospital | Toronto | Canada |
| 27. | Francoise | Ducimitiere | Centre Leon Berard | Lyon | France |
| 28. | Fritz C | Eilber | UCLA | Los Angeles | USA |
| 29. | Darja | Erzen | Institute of Oncology Ljubljana | Ljubljana | Slovenia |
| 30. | Juan Angel | Fernandez | Hospital Clínico Universitario Virgen de La Arrixaca | Murcia | Spain |
| 31. | Marco | Fiore | Fondazione IRCCS Istituto Nazionale dei Tumori | Milan | Italy |
| 32. | Chris | Fletcher | Dana Farber Cancer Institute | Boston | USA |
| 33. | Samuel | Ford | Queen Elizabeth Hospital Birmingham | Birmingham | UK |
| 34. | Annamaria | Frezza | Fondazione IRCCS Istituto Nazionale dei Tumori | Milan | Italy |
| 35. | AJ Hans | Gelderblom | LUMC | Leiden | The Netherlands |
| 36. | Maikim | Gervais | Hôpital Maisonneuve-Rosemont | Montreal | Canada |
| 37. | Rebecca | Gladdy | Mount Sinai Hospital | Toronto | Canada |
| 38. | Ricardo | Gonzalez | Moffitt Cancer Center | Tampa | USA |
| 39. | Giovanni | Grignani | Candiolo Cancer Institute | Candiolo | Italy |
| 40. | Valerie | Grignol | Ohio State University | Columbus | USA |
| 41. | Alessandro | Gronchi | Fondazione IRCCS Istituto Nazionale dei Tumori | Milan | Italy |
| 42. | David | Gyorki | Peter McCallum Cancer Center | Melbourne | Australia |
| 43. | Rick | Haas | Antoni van Leeuwenhoek Nederland Kanker Institut | Amsterdam | The Netherlands |
| 44. | Trevor | Hamilton | Vancouver General Hospital | Vancouver | Canada |
| 45. | Wolfgang | Hartmann | University Muenster | Muenster | Germany |
| 46. | Andrew | Hayes | Royal Marsden Hospital | London | UK |
| 47. | Thomas | Henzler | University Medical Center Mannheim | Mannheim | Germany |
| 48. | Peter | Hohenberger | Mannheim University Medical Center | Mannheim | Germany |
| 49. | Antoine | Italiano | Institut Bergonié | Bordeaux | France |
| 50. | Jens | Jakob | Mannheim University Medical Center | Mannheim | Germany |
| 51. | Robin L | Jones | Royal Marsden Hospital | London | UK |
| 52. | John | Kane | Roswell Park Cancer | Buffalo | USA |
| 53. | Bernd | Kasper | Mannheim University Medical Center | Mannheim | Germany |
| 54. | Steven C. | Katz | Roger William Medical Center | Providence | USA |
| 55. | David Guy | Kirsch | Duke Cancer Institute | Durham | USA |
| 56. | Guy | Lahat | Tel Aviv Sourasky Medical Center | Tel Aviv | Israel |
| 57. | Kyo Won | Lee | Samsung Medical Center | Seoul | South Korea |
| 58. | Christina | Lynn Roland | MD Anderson Cancer Center | Houston | USA |
| 59. | Andrea | MacNeill | Vancouver General Hospital | Vancouver | Canada |
| 60. | Roberta | Maestro | Centro di Riferimento Oncologico | Aviano | Italy |
| 61. | Robert | Maki | Monter Cancer Center | Lake Success | USA |
| 62. | Gary | Mann | MD Anderson Cancer Center | Houston | USA |
| 63. | Pierre | Meeus | Centre Leon Berard | Lyon | France |
| 64. | Christina | Messiou | Royal Marsden Hospital | London | UK |
| 65. | Aisha | Miah | Royal Marsden Hospital | London | UK |
| 66. | Rosalba | Miceli | Fondazione IRCCS Istituto Nazionale dei Tumori | Milan | Italy |
| 67. | Augusto | Moreira | CUF Porto Hospital | Porto | Portugal |
| 68. | John T | Mullen | Massachusset General Hospital | Boston | USA |
| 69. | Wasif | Nabil | Mayo Clinic | Phoenix | USA |
| 70. | Carolyne | Nessim | The Ottawa Hospital | Ottawa | Canada |
| 71. | Marko | Novak | Institute of Oncology Ljubljana | Ljubljana | Slovenia |
| 72. | Vicente | Olivares Ripoll | Hospital Clínico Universitario Virgen de La Arrixaca | Murcia | Spain |
| 73. | Elena | Palassini | Fondazione IRCCS Istituto Nazionale dei Tumori | Milan | Italy |
| 74. | Sandro | Pasquali | Fondazione IRCCS Istituto Nazionale dei Tumori | Milan | Italy |
| 75. | Shreyaskumar | Patel | MD Anderson Cancer Center | Houston | USA |
| 76. | Elisabetta | Pennacchioli | Istituto Europeo di Oncologia | Milan | Italy |
| 77. | Venu G | Pillarisetty | University of Washington | Seattle | USA |
| 78. | Raphael E | Pollock | Ohio State University | Columbus | USA |
| 79. | Bibianna | Purgina | The Ottawa Hospital | Ottawa | Canada |
| 80. | Vittorio | Quagliuolo | Istituto Clinico Humanitas | Milan | Italy |
| 81. | Stefano | Radaelli | Fondazione IRCCS Istituto Nazionale dei Tumori | Milan | Italy |
| 82. | Marco | Rastrelli | Istituto Oncologico Veneto | Padova | Italy |
| 83. | Chandrajit P | Raut | Brigham and Women’s Hospital/Dana-Farber Cancer Institute | Boston | USA |
| 84. | Salvatore L | Renne | Fondazione IRCCS Istituto Nazionale dei Tumori | Milan | Italy |
| 85. | Paul | Ridgway | Tallaght Hospital | Dublin | Ireland |
| 86. | Piotr | Rutkowski | Maria Sklodowska-Curie Memorial Cancer Center and Institute of Oncology | Warsaw | Poland |
| 87. | Sergio | Sandrucci | Città della Salute e della Scienza | Turin | Italy |
| 88. | Roberta | Sanfilippo | Fondazione IRCCS Istituto Nazionale dei Tumori | Milan | Italy |
| 89. | Paul | Sargos | Institut Bergonié | Lyon | France |
| 90. | Marta | Sbaraglia | Treviso Hospital | Treviso | Italy |
| 91. | Yvonne | Schrage | LUMC | Leiden | The Netherlands |
| 92. | Jason | Sicklick | UC San Diego | San Diego | USA |
| 93. | Myles | Smith | Royal Marsden Hospital | London | UK |
| 94. | Silvia | Stacchiotti | Fondazione IRCCS Istituto Nazionale dei Tumori | Milan | Italy |
| 95. | Eberhard | Stoeckle | Institut Bergonié | Bordeaux | France |
| 96. | Dirk C | Strauss | Royal Marsden Hospital | London | UK |
| 97. | Kim | Sung Joo | Samsung Medical Center | Seoul | South Korea |
| 98. | Carol J | Swallow | Mount Sinai Hospital/Princess Margaret Cancer Center | Toronto | Canada |
| 99. | William D | Tap | Memorial Sloan Kettering Cancer Center | New York | USA |
| 100. | William W | Tseng | University South California | Los Angeles | USA |
| 101. | Frits | Van Coevorden | Antoni van Leeuwenhoek Nederland Kanker Institute | Amsterdam | The Netherlands |
| 102. | Winette | Van der Graaf | Royal Marsden Hospital | London | UK |
| 103. | Winan | Van Houdt | Royal Marsden Hospital | London | UK |
| 104. | Andrew | Wagner | Dana Farber Cancer Institute | Boston | USA |
| 105. | Eva | Wardelmann | University Muenster | Muenster | Germany |
| 106. | Branko | Zakotnik | Institute of Oncology Ljubljana | Ljubljana | Slovenia |
Contributor Information
Trans-Atlantic Retroperitoneal Sarcoma Working Group (TARPSWG):
Jan Ahlen, Nita Ahuja, Markus Albertsmeier, Waddah B Al-Refaie, Robert Andtbacka, Martin Angele, Sanjay P Bagaria, Elizabeth Baldini, Francesco Barretta, Georgia Beasley, Jean-Yves Blay, Dan G Blazer, III, Sylvie Bonvalot, Sally Burtenshaw, Dario Callegaro, Robert Canter, Kenneth Cardona, Paolo G Casali, Charles Catton, Yoon-La Choi, Chiara Colombo, Antonino De Paoli, Angelo P Dei Tos, Tom Delaney, Anant Desai, Brendan Dickson, Francoise Ducimitiere, Fritz C Eilber, Darja Erzen, Juan Angel Fernandez, Marco Fiore, Chris Fletcher, Samuel Ford, Annamaria Frezza, A J Hans Gelderblom, Maikim Gervais, Rebecca Gladdy, Ricardo Gonzalez, Giovanni Grignani, Valerie Grignol, Alessandro Gronchi, David Gyorki, Rick Haas, Trevor Hamilton, Wolfgang Hartmann, Andrew Hayes, Thomas Henzler, Peter Hohenberger, Antoine Italiano, Jens Jakob, Robin L Jones, John Kane, Bernd Kasper, Steven C Katz, David Guy Kirsch, Guy Lahat, Kyo Won Lee, Christina Lynn Roland, Andrea MacNeill, Roberta Maestro, Robert Maki, Gary Mann, Pierre Meeus, Christina Messiou, Aisha Miah, Rosalba Miceli, Augusto Moreira, John T Mullen, Wasif Nabil, Carolyne Nessim, Marko Novak, Vicente Olivares Ripoll, Elena Palassini, Sandro Pasquali, Shreyaskumar Patel, Elisabetta Pennacchioli, Venu G Pillarisetty, Raphael E Pollock, Bibianna Purgina, Vittorio Quagliuolo, Stefano Radaelli, Marco Rastrelli, Chandrajit P Raut, Salvatore L Renne, Paul Ridgway, Piotr Rutkowski, Sergio Sandrucci, Roberta Sanfilippo, Paul Sargos, Marta Sbaraglia, Yvonne Schrage, Jason Sicklick, Myles Smith, Silvia Stacchiotti, Eberhard Stoeckle, Dirk C Strauss, Kim Sung Joo, Carol J Swallow, William D Tap, William W Tseng, Frits Van Coevorden, Winette Van der Graaf, Winan Van Houdt, Andrew Wagner, Eva Wardelmann, and Branko Zakotnik
References
- 1. Gronchi A, Strauss DC, Miceli R. et al. Variability in patterns of recurrence after resection of primary retroperitoneal sarcoma (RPS): a report on 1007 patients from the multi-institutional collaborative RPS Working Group. Ann Surg 2016; 263(5): 1002–1009. [DOI] [PubMed] [Google Scholar]
- 2. Toulmonde M, Bonvalot S, Ray-Coquard I. et al. Retroperitoneal sarcomas: patterns of care in advanced stages, prognostic factors and focus on main histological subtypes: a multicenter analysis of the French Sarcoma Group. Ann Oncol 2014; 25(3): 730–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Blay JY, van Glabbeke M, Verweij J. et al. Advanced soft-tissue sarcoma: a disease that is potentially curable for a subset of patients treated with chemotherapy. Eur J Cancer 2003; 39(1): 64–69. [DOI] [PubMed] [Google Scholar]
- 4. Trans-Atlantic RPSWG. Management of primary retroperitoneal sarcoma (RPS) in the adult: a consensus approach from the Trans-Atlantic RPS Working Group. Ann Surg Oncol 2015; 22(1): 256–263. [DOI] [PubMed] [Google Scholar]
- 5. Trans-Atlantic RPSWG. Management of recurrent retroperitoneal sarcoma (RPS) in the adult: a consensus approach from the Trans-Atlantic RPS Working Group. Ann Surg Oncol 2016; 23(11): 3531–3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Collaboration A. Development and validation of an international appraisal instrument for assessing the quality of clinical practice guidelines: the AGREE project. Qual Saf Health Care 2003; 12(1): 18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brouwers MC, Kho ME, Browman GP. et al. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ 2010; 182(18): E839–E842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Messiou C, Moskovic E, Vanel D. et al. Primary retroperitoneal soft tissue sarcoma: imaging appearances, pitfalls and diagnostic algorithm. Eur J Surg Oncol 2017; 43(7): 1191–1198. [DOI] [PubMed] [Google Scholar]
- 9. Shiraev T, Pasricha SS, Choong P. et al. Retroperitoneal sarcomas: a review of disease spectrum, radiological features, characterisation and management. J Med Imaging Radiat Oncol 2013; 57(6): 687–700. [DOI] [PubMed] [Google Scholar]
- 10. Lamba R, Fananapazir G, Corwin MT, Khatri VP.. Diagnostic imaging of hepatic lesions in adults. Surg Oncol Clin N Am 2014; 23(4): 789–820. [DOI] [PubMed] [Google Scholar]
- 11. Colia V, Provenzano S, Hindi N. et al. Systemic therapy for selected skull base sarcomas: chondrosarcoma, chordoma, giant cell tumour and solitary fibrous tumour/hemangiopericytoma. Rep Pract Oncol Radiother 2016; 21(4): 361–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Niccoli-Asabella A, Notaristefano A, Rubini D. et al. 18F-FDG PET/CT in suspected recurrences of epithelial malignant pleural mesothelioma in asbestos-fibers-exposed patients (comparison to standard diagnostic follow-up). Clin Imaging 2013; 37(6): 1098–1103. [DOI] [PubMed] [Google Scholar]
- 13. Alford S, Choong P, Chander S. et al. Value of PET scan in patients with retroperitoneal sarcoma treated with preoperative radiotherapy. Eur J Surg Oncol 2012; 38(2): 176–180. [DOI] [PubMed] [Google Scholar]
- 14. Italiano A, Di Mauro I, Rapp J. et al. Clinical effect of molecular methods in sarcoma diagnosis (GENSARC): a prospective, multicentre, observational study. Lancet Oncol 2016; 17(4): 532–538. [DOI] [PubMed] [Google Scholar]
- 15. Wu JS, Goldsmith JD, Horwich PJ. et al. Bone and soft-tissue lesions: what factors affect diagnostic yield of image-guided core-needle biopsy? Radiology 2008; 248(3): 962–970. [DOI] [PubMed] [Google Scholar]
- 16. Charlson ME, Pompei P, Ales KL, MacKenzie CR.. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40(5): 373–383. [DOI] [PubMed] [Google Scholar]
- 17. Temel JS, Greer JA, Muzikansky A. et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med 2010; 363(8): 733–742. [DOI] [PubMed] [Google Scholar]
- 18. Nakamura T, Matsumine A, Yamakado K. et al. Lung radiofrequency ablation in patients with pulmonary metastases from musculoskeletal sarcomas [corrected]. Cancer 2009; 115(16): 3774–3781. [DOI] [PubMed] [Google Scholar]
- 19. Palussiere J, Marcet B, Descat E. et al. Lung tumors treated with percutaneous radiofrequency ablation: computed tomography imaging follow-up. Cardiovasc Intervent Radiol 2011; 34(5): 989–997. [DOI] [PubMed] [Google Scholar]
- 20. Dhakal S, Corbin KS, Milano MT. et al. Stereotactic body radiotherapy for pulmonary metastases from soft-tissue sarcomas: excellent local lesion control and improved patient survival. Int J Radiat Oncol Biol Phys 2012; 82(2): 940–945. [DOI] [PubMed] [Google Scholar]
- 21. Koelblinger C, Strauss S, Gillams A.. Outcome after radiofrequency ablation of sarcoma lung metastases. Cardiovasc Intervent Radiol 2014; 37(1): 147–153. [DOI] [PubMed] [Google Scholar]
- 22. Falk AT, Moureau-Zabotto L, Ouali M. et al. Effect on survival of local ablative treatment of metastases from sarcomas: a study of the French sarcoma group. Clin Oncol 2015; 27(1): 48–55. [DOI] [PubMed] [Google Scholar]
- 23. Savina M, Le Cesne A, Blay JY. et al. Patterns of care and outcomes of patients with METAstatic soft tissue SARComa (STS) in a real-life setting: the METASARC observational study. BMC Med 2017; 15(1): 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Frakulli R, Salvi F, Balestrini D. et al. Stereotactic radiotherapy in the treatment of lung metastases from bone and soft-tissue sarcomas. Anticancer Res 2015; 35(10): 5581–5586. [PubMed] [Google Scholar]
- 25. Navarria P, Ascolese AM, Cozzi L. et al. Stereotactic body radiation therapy for lung metastases from soft tissue sarcoma. Eur J Cancer 2015; 51(5): 668–674. [DOI] [PubMed] [Google Scholar]
- 26. Brudvik KW, Patel SH, Roland CL. et al. Survival after resection of gastrointestinal stromal tumor and sarcoma liver metastases in 146 patients. J Gastrointest Surg 2015; 19(8): 1476–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jones RL, McCall J, Adam A. et al. Radiofrequency ablation is a feasible therapeutic option in the multi modality management of sarcoma. Eur J Surg Oncol 2010; 36(5): 477–482. [DOI] [PubMed] [Google Scholar]
- 28. Baumann BC, Nagda SN, Kolker JD. et al. Efficacy and safety of stereotactic body radiation therapy for the treatment of pulmonary metastases from sarcoma: a potential alternative to resection. J Surg Oncol 2016; 114(1): 65–69. [DOI] [PubMed] [Google Scholar]
- 29. Pawlik TM, Vauthey JN, Abdalla EK. et al. Results of a single-center experience with resection and ablation for sarcoma metastatic to the liver. Arch Surg 2006; 141(6): 537–543; discussion 543–534. [DOI] [PubMed] [Google Scholar]
- 30. Rehders A, Stoecklein NH, Poremba C. et al. Reexcision of soft tissue sarcoma: sufficient local control but increased rate of metastasis. World J Surg 2009; 33(12): 2599–2605. [DOI] [PubMed] [Google Scholar]
- 31. Burt BM, Ocejo S, Mery CM. et al. Repeated and aggressive pulmonary resections for leiomyosarcoma metastases extends survival. Ann Thorac Surg 2011; 92(4): 1202–1207. [DOI] [PubMed] [Google Scholar]
- 32. Stephens EH, Blackmon SH, Correa AM. et al. Progression after chemotherapy is a novel predictor of poor outcomes after pulmonary metastasectomy in sarcoma patients. J Am Coll Surg 2011; 212(5): 821–826. [DOI] [PubMed] [Google Scholar]
- 33. Blackmon SH, Rice DC, Correa AM. et al. Management of primary pulmonary artery sarcomas. Ann Thorac Surg 2009; 87(3): 977–984. [DOI] [PubMed] [Google Scholar]
- 34. Smith R, Pak Y, Kraybill W, Kane JM III. Factors associated with actual long-term survival following soft tissue sarcoma pulmonary metastasectomy. Eur J Surg Oncol 2009; 35(4): 356–361. [DOI] [PubMed] [Google Scholar]
- 35. Canter RJ, Qin LX, Downey RJ. et al. Perioperative chemotherapy in patients undergoing pulmonary resection for metastatic soft-tissue sarcoma of the extremity: a retrospective analysis. Cancer 2007; 110(9): 2050–2060. [DOI] [PubMed] [Google Scholar]
- 36. Weiser MR, Downey RJ, Leung DH, Brennan MF.. Repeat resection of pulmonary metastases in patients with soft-tissue sarcoma. J Am Coll Surg 2000; 191(2): 184–190; discussion 190–191. [DOI] [PubMed] [Google Scholar]
- 37. Pastorino U. Lung metastasectomy: why, when, how. Crit Rev Oncol Hematol 1997; 26(3): 137–145. [DOI] [PubMed] [Google Scholar]
- 38. van Geel AN, Pastorino U, Jauch KW. et al. Surgical treatment of lung metastases: the European Organization for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group study of 255 patients. Cancer 1996; 77(4): 675–682. [DOI] [PubMed] [Google Scholar]
- 39. Billingsley KG, Burt ME, Jara E. et al. Pulmonary metastases from soft tissue sarcoma: analysis of patterns of diseases and postmetastasis survival. Ann Surg 1999; 229(5): 602–610; discussion 610–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Verazin GT, Warneke JA, Driscoll DL. et al. Resection of lung metastases from soft-tissue sarcomas. A multivariate analysis. Arch Surg 1992; 127(12): 1407–1411. [DOI] [PubMed] [Google Scholar]
- 41. Lin AY, Kotova S, Yanagawa J. et al. Risk stratification of patients undergoing pulmonary metastasectomy for soft tissue and bone sarcomas. J Thorac Cardiovasc Surg 2015; 149(1): 85–92. [DOI] [PubMed] [Google Scholar]
- 42. Ceppa DP. Results of pulmonary resection: sarcoma and germ cell tumors. Thorac Surg Clin 2016; 26(1): 49–54. [DOI] [PubMed] [Google Scholar]
- 43. Schur S, Hoetzenecker K, Lamm W. et al. Pulmonary metastasectomy for soft tissue sarcoma–report from a dual institution experience at the Medical University of Vienna. Eur J Cancer 2014; 50(13): 2289–2297. [DOI] [PubMed] [Google Scholar]
- 44. Dossett LA, Toloza EM, Fontaine J. et al. Outcomes and clinical predictors of improved survival in a patients undergoing pulmonary metastasectomy for sarcoma. J Surg Oncol 2015; 112(1): 103–106. [DOI] [PubMed] [Google Scholar]
- 45. Chudgar NP, Brennan MF, Munhoz RR. et al. Pulmonary metastasectomy with therapeutic intent for soft-tissue sarcoma. J Thorac Cardiovasc Surg 2017; 154(1): 319–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. DeMatteo RP, Shah A, Fong Y. et al. Results of hepatic resection for sarcoma metastatic to liver. Ann Surg 2001; 234(4): 540–547; discussion 547–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Choong P, Pritchard D, Sim F. et al. Long-term survival in high-grade soft-tissue sarcoma—prognostic factors in synovial sarcoma. Int J Oncol 1995; 7(1): 161–169. [DOI] [PubMed] [Google Scholar]
- 48. Kane JM, Finley JW, Driscoll D. et al. The treatment and outcome of patients with soft tissue sarcomas and synchronous metastases. Sarcoma 2002; 6(2): 69–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nakamura T, Matsumine A, Takao M. et al. Impact of tumor volume doubling time on post-metastatic survival in bone or soft-tissue sarcoma patients treated with metastasectomy and/or radiofrequency ablation of the lung. Onco Targets Ther 2017; 10: 559–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ferguson PC, Deheshi BM, Chung P. et al. Soft tissue sarcoma presenting with metastatic disease: outcome with primary surgical resection. Cancer 2011; 117(2): 372–379. [DOI] [PubMed] [Google Scholar]
- 51. Abdalla EK, Pisters PW.. Metastasectomy for limited metastases from soft tissue sarcoma. Curr Treat Options Oncol 2002; 3(6): 497–505. [DOI] [PubMed] [Google Scholar]
- 52. Casson AG, Putnam JB, Natarajan G. et al. Five-year survival after pulmonary metastasectomy for adult soft tissue sarcoma. Cancer 1992; 69(3): 662–668. [DOI] [PubMed] [Google Scholar]
- 53. Choong PF, Gustafson P, Rydholm A.. Size and timing of local recurrence predicts metastasis in soft tissue sarcoma. Growth rate index retrospectively analyzed in 134 patients. Acta Orthop Scand 1995; 66(2): 147–152. [DOI] [PubMed] [Google Scholar]
- 54. Smith R, Demmy TL.. Pulmonary metastasectomy for soft tissue sarcoma. Surg Oncol Clin N Am 2012; 21(2): 269–286. [DOI] [PubMed] [Google Scholar]
- 55. Molnar TF, Gebitekin C, Turna A.. What are the considerations in the surgical approach in pulmonary metastasectomy? J Thorac Oncol 2010; 5(6): S140–S144. [DOI] [PubMed] [Google Scholar]
- 56. Zheng Y, Fernando HC.. Surgical and nonresectional therapies for pulmonary metastasis. Surg Clin N Am 2010; 90(5): 1041–1051. [DOI] [PubMed] [Google Scholar]
- 57. Group ESESNW. Soft tissue and visceral sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014; 25(Suppl 3): iii102–iii112. [DOI] [PubMed] [Google Scholar]
- 58. Keung EZ, Fairweather M, Raut CP.. Surgical management of metastatic disease. Surg Clin North Am 2016; 96(5): 1175–1192. [DOI] [PubMed] [Google Scholar]
- 59. Maluccio MA, Covey AM, Schubert J. et al. Treatment of metastatic sarcoma to the liver with bland embolization. Cancer 2006; 107(7): 1617–1623. [DOI] [PubMed] [Google Scholar]
- 60. Chapiro J, Duran R, Lin M. et al. Transarterial chemoembolization in soft-tissue sarcoma metastases to the liver—the use of imaging biomarkers as predictors of patient survival. Eur J Radiol 2015; 84(3): 424–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Baratti D, Pennacchioli E, Kusamura S. et al. Peritoneal sarcomatosis: is there a subset of patients who may benefit from cytoreductive surgery and hyperthermic intraperitoneal chemotherapy? Ann Surg Oncol 2010; 17(12): 3220–3228. [DOI] [PubMed] [Google Scholar]
- 62. Colombo C, Baratti D, Kusamura S. et al. The role of hyperthermic intraperitoneal chemotherapy (HIPEC) and isolated perfusion (ILP) interventions in sarcoma. J Surg Oncol 2015; 111(5): 570–579. [DOI] [PubMed] [Google Scholar]
- 63. Honore C, Amroun K, Vilcot L. et al. Abdominal desmoplastic small round cell tumor: multimodal treatment combining chemotherapy, surgery, and radiotherapy is the best option. Ann Surg Oncol 2015; 22(4): 1073–1079. [DOI] [PubMed] [Google Scholar]
- 64. Randle RW, Swett KR, Swords DS. et al. Efficacy of cytoreductive surgery with hyperthermic intraperitoneal chemotherapy in the management of malignant ascites. Ann Surg Oncol 2014; 21(5): 1474–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Salti GI, Ailabouni L, Undevia S.. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for the treatment of peritoneal sarcomatosis. Ann Surg Oncol 2012; 19(5): 1410–1415. [DOI] [PubMed] [Google Scholar]
- 66. Bonvalot S, Cavalcanti A, Le Pechoux C. et al. Randomized trial of cytoreduction followed by intraperitoneal chemotherapy versus cytoreduction alone in patients with peritoneal sarcomatosis. Eur J Surg Oncol 2005; 31(8): 917–923. [DOI] [PubMed] [Google Scholar]
- 67. Sommariva A, Pasquali S, Del Fiore P. et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in patients with peritoneal sarcomatosis: long-term outcome from a single institution experience. Anticancer Res 2013; 33(9): 3989–3994. [PubMed] [Google Scholar]
- 68. Kandioler D, Kromer E, Tuchler H. et al. Long-term results after repeated surgical removal of pulmonary metastases. Ann Thorac Surg 1998; 65(4): 909–912. [DOI] [PubMed] [Google Scholar]
- 69. Jaklitsch MT, Mery CM, Lukanich JM. et al. Sequential thoracic metastasectomy prolongs survival by re-establishing local control within the chest. J Thorac Cardiovasc Surg 2001; 121(4): 657–667. [DOI] [PubMed] [Google Scholar]
- 70. Pogrebniak HW, Roth JA, Steinberg SM. et al. Reoperative pulmonary resection in patients with metastatic soft tissue sarcoma. Ann Thorac Surg 1991; 52(2): 197–203. [DOI] [PubMed] [Google Scholar]
- 71. Cardona K, Williams R, Movva S.. Multimodality therapy for advanced or metastatic sarcoma. Curr Probl Cancer 2013; 37(2): 74–86. [DOI] [PubMed] [Google Scholar]
- 72. Garcia del Muro X, de Alava E, Artigas V. et al. Clinical practice guidelines for the diagnosis and treatment of patients with soft tissue sarcoma by the Spanish group for research in sarcomas (GEIS). Cancer Chemother Pharmacol 2016; 77(1): 133–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. van Houdt WJ, Zaidi S, Messiou C. et al. Treatment of retroperitoneal sarcoma: current standards and new developments. Curr Opin Oncol 2017; 29(4): 260–267. [DOI] [PubMed] [Google Scholar]
- 74. Macneill AJ, Gupta AA, Swallow CJ.. Randomized clinical trials in soft tissue sarcoma. Surg Oncol Clin N Am 2017; 26(4): 531–544. [DOI] [PubMed] [Google Scholar]
- 75. Wagner MJ, Amodu LI, Duh MS. et al. A retrospective chart review of drug treatment patterns and clinical outcomes among patients with metastatic or recurrent soft tissue sarcoma refractory to one or more prior chemotherapy treatments. BMC Cancer 2015; 15: 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Leahy M, Garcia Del Muro X, Reichardt P. et al. Chemotherapy treatment patterns and clinical outcomes in patients with metastatic soft tissue sarcoma. The SArcoma treatment and Burden of Illness in North America and Europe (SABINE) study. Ann Oncol 2012; 23(10): 2763–2770. [DOI] [PubMed] [Google Scholar]
- 77. Ryan CW, Merimsky O, Agulnik M. et al. PICASSO III: a phase III, placebo-controlled study of doxorubicin with or without palifosfamide in patients with metastatic soft tissue sarcoma. J Clin Oncol 2016; 34(32): 3898–3905. [DOI] [PubMed] [Google Scholar]
- 78. Judson I, Radford JA, Harris M. et al. Randomised phase II trial of pegylated liposomal doxorubicin (DOXIL/CAELYX) versus doxorubicin in the treatment of advanced or metastatic soft tissue sarcoma: a study by the EORTC Soft Tissue and Bone Sarcoma Group. Eur J Cancer 2001; 37(7): 870–877. [DOI] [PubMed] [Google Scholar]
- 79. Seddon B, Scurr M, Jones RL. et al. A phase II trial to assess the activity of gemcitabine and docetaxel as first line chemotherapy treatment in patients with unresectable leiomyosarcoma. Clin Sarcoma Res 2015; 5(1): 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Tap WD, Jones RL, Van Tine BA. et al. Olaratumab and doxorubicin versus doxorubicin alone for treatment of soft-tissue sarcoma: an open-label phase 1b and randomised phase 2 trial. Lancet 2016; 388(10043): 488–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Judson I, Verweij J, Gelderblom H. et al. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: a randomised controlled phase 3 trial. Lancet Oncol 2014; 15(4): 415–423. [DOI] [PubMed] [Google Scholar]
- 82. Sleijfer S, Ouali M, van Glabbeke M. et al. Prognostic and predictive factors for outcome to first-line ifosfamide-containing chemotherapy for adult patients with advanced soft tissue sarcomas: an exploratory, retrospective analysis on large series from the European Organization for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group (EORTC-STBSG). Eur J Cancer 2010; 46(1): 72–83. [DOI] [PubMed] [Google Scholar]
- 83. Stacchiotti S, Libertini M, Negri T. et al. Response to chemotherapy of solitary fibrous tumour: a retrospective study. Eur J Cancer 2013; 49(10): 2376–2383. [DOI] [PubMed] [Google Scholar]
- 84. Lorigan P, Verweij J, Papai Z. et al. Phase III trial of two investigational schedules of ifosfamide compared with standard-dose doxorubicin in advanced or metastatic soft tissue sarcoma: a European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Study. J Clin Oncol 2007; 25(21): 3144–3150. [DOI] [PubMed] [Google Scholar]
- 85. Martin-Liberal J, Alam S, Constantinidou A. et al. Clinical activity and tolerability of a 14-day infusional Ifosfamide schedule in soft-tissue sarcoma. Sarcoma 2013; 2013: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Nielsen OS, Judson I, van Hoesel Q. et al. Effect of high-dose ifosfamide in advanced soft tissue sarcomas. A multicentre phase II study of the EORTC Soft Tissue and Bone Sarcoma Group. Eur J Cancer 2000; 36(1): 61–67. [DOI] [PubMed] [Google Scholar]
- 87. Demetri GD, von Mehren M, Jones RL. et al. Efficacy and safety of trabectedin or dacarbazine for metastatic liposarcoma or leiomyosarcoma after failure of conventional chemotherapy: results of a phase III randomized multicenter clinical trial. J Clin Oncol 2016; 34(8): 786–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Le Cesne A, Blay JY, Judson I. et al. Phase II study of ET-743 in advanced soft tissue sarcomas: a European Organisation for the Research and Treatment of Cancer (EORTC) soft tissue and bone sarcoma group trial. J Clin Oncol 2005; 23(3): 576–584. [DOI] [PubMed] [Google Scholar]
- 89. Schoffski P, Chawla S, Maki RG. et al. Eribulin versus dacarbazine in previously treated patients with advanced liposarcoma or leiomyosarcoma: a randomised, open-label, multicentre, phase 3 trial. Lancet 2016; 387(10028): 1629–1637. [DOI] [PubMed] [Google Scholar]
- 90. van der Graaf WT, Blay JY, Chawla SP. et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2012; 379(9829): 1879–1886. [DOI] [PubMed] [Google Scholar]
- 91. Patel SR, Gandhi V, Jenkins J. et al. Phase II clinical investigation of gemcitabine in advanced soft tissue sarcomas and window evaluation of dose rate on gemcitabine triphosphate accumulation. J Clin Oncol 2001; 19(15): 3483–3489. [DOI] [PubMed] [Google Scholar]
- 92. Maki RG, Wathen JK, Patel SR. et al. Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: results of sarcoma alliance for research through collaboration study 002 [corrected]. J Clin Oncol 2007; 25(19): 2755–2763. [DOI] [PubMed] [Google Scholar]
- 93. Pautier P, Floquet A, Penel N. et al. Randomized multicenter and stratified phase II study of gemcitabine alone versus gemcitabine and docetaxel in patients with metastatic or relapsed leiomyosarcomas: a Federation Nationale des Centres de Lutte Contre le Cancer (FNCLCC) French Sarcoma Group Study (TAXOGEM study). Oncologist 2012; 17(9): 1213–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Garcia-Del-Muro X, Lopez-Pousa A, Maurel J. et al. Randomized phase II study comparing gemcitabine plus dacarbazine versus dacarbazine alone in patients with previously treated soft tissue sarcoma: a Spanish Group for Research on Sarcomas study. J Clin Oncol 2011; 29(18): 2528–2533. [DOI] [PubMed] [Google Scholar]
- 95. Stacchiotti S, Tortoreto M, Bozzi F. et al. Dacarbazine in solitary fibrous tumor: a case series analysis and preclinical evidence vis-a-vis temozolomide and antiangiogenics. Clin Cancer Res 2013; 19(18): 5192–5201. [DOI] [PubMed] [Google Scholar]
- 96. Wagner AJ, Malinowska-Kolodziej I, Morgan JA. et al. Clinical activity of mTOR inhibition with sirolimus in malignant perivascular epithelioid cell tumors: targeting the pathogenic activation of mTORC1 in tumors. J Clin Oncol 2010; 28(5): 835–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Butrynski JE, D'Adamo DR, Hornick JL. et al. Crizotinib in ALK-rearranged inflammatory myofibroblastic tumor. N Engl J Med 2010; 363(18): 1727–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Roth JA, Putnam JB Jr, Wesley MN. et al. Differing determinants of prognosis following resection of pulmonary metastases from osteogenic and soft tissue sarcoma patients. Cancer 1985; 55(6): 1361–1366. [DOI] [PubMed] [Google Scholar]
- 99. Jablons D, Steinberg SM, Roth J. et al. Metastasectomy for soft tissue sarcoma. Further evidence for efficacy and prognostic indicators. J Thorac Cardiovasc Surg 1989; 97(5): 695–705. [PubMed] [Google Scholar]
- 100. Lanza LA, Putnam JB Jr, Benjamin RS. et al. Response to chemotherapy does not predict survival after resection of sarcomatous pulmonary metastases. Ann Thorac Surg 1991; 51(2): 219–224. [DOI] [PubMed] [Google Scholar]
- 101. Gadd MA, Casper ES, Woodruff JM. et al. Development and treatment of pulmonary metastases in adult patients with extremity soft tissue sarcoma. Ann Surg 1993; 218(6): 705–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Mentzer SJ, Antman KH, Attinger C. et al. Selected benefits of thoracotomy and chemotherapy for sarcoma metastatic to the lung. J Surg Oncol 1993; 53(1): 54–59. [DOI] [PubMed] [Google Scholar]
- 103. Saltzman DA, Snyder CL, Ferrell KL. et al. Aggressive metastasectomy for pulmonic sarcomatous metastases: a follow-up study. Am J Surg 1993; 166(5): 543–547. [DOI] [PubMed] [Google Scholar]
- 104. Ueda T, Uchida A, Kodama K. et al. Aggressive pulmonary metastasectomy for soft tissue sarcomas. Cancer 1993; 72(6): 1919–1925. [DOI] [PubMed] [Google Scholar]
- 105. van Geel AN, Hoekstra HJ, van Coevorden F. et al. Repeated resection of recurrent pulmonary metastatic soft tissue sarcoma. Eur J Surg Oncol 1994; 20(4): 436–440. [PubMed] [Google Scholar]
- 106. Choong PF, Pritchard DJ, Rock MG. et al. Survival after pulmonary metastasectomy in soft tissue sarcoma. Prognostic factors in 214 patients. Acta Orthop Scand 1995; 66(6): 561–568. [DOI] [PubMed] [Google Scholar]
- 107. Liebl LS, Elson F, Quaas A. et al. Value of repeat resection for survival in pulmonary metastases from soft tissue sarcoma. Anticancer Res 2007; 27(4C): 2897–2902. [PubMed] [Google Scholar]
- 108. Chen F, Fujinaga T, Sato K. et al. Significance of tumor recurrence before pulmonary metastasis in pulmonary metastasectomy for soft tissue sarcoma. Eur J Surg Oncol 2009; 35(6): 660–665. [DOI] [PubMed] [Google Scholar]
- 109. Nakamura T, Matsumine A, Matsubara T. et al. Clinical impact of the tumor volume doubling time on sarcoma patients with lung metastases. Clin Exp Metastasis 2011; 28(8): 819–825. [DOI] [PubMed] [Google Scholar]
- 110. Casiraghi M, De Pas T, Maisonneuve P. et al. A 10-year single-center experience on 708 lung metastasectomies: the evidence of the "international registry of lung metastases". J Thorac Oncol 2011; 6(8): 1373–1378. [DOI] [PubMed] [Google Scholar]
- 111. Predina JD, Puc MM, Bergey MR. et al. Improved survival after pulmonary metastasectomy for soft tissue sarcoma. J Thorac Oncol 2011; 6(5): 913–919. [DOI] [PubMed] [Google Scholar]
- 112. Hornbech K, Ravn J, Steinbrüchel DA.. Outcome after pulmonary metastasectomy: analysis of 5 years consecutive surgical resections 2002-2006. J Thorac Oncol 2011; 6(10): 1733–1740. [DOI] [PubMed] [Google Scholar]
- 113. Kim S, Ott HC, Wright CD. et al. Pulmonary resection of metastatic sarcoma: prognostic factors associated with improved outcomes. Ann Thorac Surg 2011; 92(5): 1780–1786. [DOI] [PubMed] [Google Scholar]
- 114. Treasure T, Fiorentino F, Scarci M. et al. Pulmonary metastasectomy for sarcoma: a systematic review of reported outcomes in the context of Thames Cancer Registry data. BMJ Open 2012; 2(5). pii: e001736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Harrison LE, Brennan MF, Newman E. et al. Hepatic resection for noncolorectal, nonneuroendocrine metastases: a fifteen-year experience with ninety-six patients. Surgery 1997; 121(6): 625–632. [DOI] [PubMed] [Google Scholar]
- 116. Chen H, Pruitt A, Nicol TL. et al. Complete hepatic resection of metastases from leiomyosarcoma prolongs survival. J Gastrointest Surg 1998; 2(2): 151–155. [DOI] [PubMed] [Google Scholar]
- 117. Lang H, Nussbaum KT, Kaudel P. et al. Hepatic metastases from leiomyosarcoma: a single-center experience with 34 liver resections during a 15-year period. Ann Surg 2000; 231(4): 500–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Ercolani G, Grazi GL, Ravaioli M. et al. The role of liver resections for noncolorectal, nonneuroendocrine metastases: experience with 142 observed cases. Ann Surg Oncol 2005; 12(6): 459–466. [DOI] [PubMed] [Google Scholar]
- 119. Yedibela S, Gohl J, Graz V. et al. Changes in indication and results after resection of hepatic metastases from noncolorectal primary tumors: a single-institutional review. Ann Surg Oncol 2005; 12(10): 778–785. [DOI] [PubMed] [Google Scholar]
- 120. Adam R, Chiche L, Aloia T. et al. Hepatic resection for noncolorectal nonendocrine liver metastases: analysis of 1,452 patients and development of a prognostic model. Ann Surg 2006; 244(4): 524–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Pawlik TM, Vauthey JN, Abdalla EK. et al. Results of a single-center experience with resection and ablation for sarcoma metastatic to the liver. Arch Surg 2006; 141(6): 537–543. [DOI] [PubMed] [Google Scholar]
- 122. Marudanayagam R, Sandhu B, Perera MT. et al. Liver resection for metastatic soft tissue sarcoma: an analysis of prognostic factors. Eur J Surg Oncol 2011; 37(1): 87–92. [DOI] [PubMed] [Google Scholar]
- 123. Groeschl RT, Nachmany I, Steel JL. et al. Hepatectomy for noncolorectal non-neuroendocrine metastatic cancer: a multi-institutional analysis. J Am Coll Surg 2012; 214(5): 769–777. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.