Abstract
Leptin and its receptors have been identified as key regulators of body weight and energy homeostasis. A decrease in tissue sensitivity to leptin leads to the development of obesity and metabolic disorders, such as insulin resistance and dyslipidemia. Mechanisms underlying the development of leptin resistance include mutations in the genes encoding leptin and its receptors, as well as proteins involved in self-regulation of leptin synthesis and blood–brain barrier permeability. Leptin resistance encompasses a complex pathophysiological phenomenon with a number of potential research lines. In this review, we analyze the existing data on the methods used to diagnose leptin resistance.
Keywords: leptin, leptin resistance, soluble leptin receptor
Introduction
Leptin is an adipocyte-secreted hormone that regulates the appetite and represents a key factor in the development of obesity, a serious medical, social, and economic problem in modern society.1,2 More than 20 years ago, leptin and its receptors were identified as key regulators of body weight and energy homeostasis. A minor increase in leptin concentration reduces the appetite and leads to a decrease in body weight;3 however, in obesity, despite increased leptin concentration, the efficacy of the anorexic effect of leptin is decreased,1,3 with leptin resistance developing due to a defect in intracellular signaling associated with the leptin receptor or decreases in leptin transport across the blood–brain barrier (BBB).4 Clear criteria for defining leptin resistance and its diagnostic use have not been established. For in vivo studies, it is difficult to elucidate the molecular mechanisms underlying the development of leptin resistance. The majority of studies examining the effects of leptin resistance on various metabolic processes are performed using mice with a defect in the Ob-P leptin-receptor gene.5 This mutation is rare in the human population, which complicates research on leptin resistance and identification of possible diagnostic markers. Furthermore, when assessing leptin resistance, a number of questions should be answered, including whether the presence of high leptin concentration is sufficient to establish the diagnosis of leptin resistance, what threshold for the weakened response should be considered as leptin resistance, and whether leptin resistance should be defined according to a one-time increase in leptin concentration or to dynamic changes in the concentration over time.6,7 Research on the pleiotropic effects of leptin, including those involving the immune system, hemopoiesis, and angiogenesis, and the participation of leptin in the development of cardiovascular and autoimmune diseases and cancer, has renewed the interest in studying leptin, its receptors, and their possible diagnostic use.8
Leptin: history and perspectives
Leptin identification occurred in association with experiments on parabiotic animals. In 1950, Ingalls et al9 described a mutant strain of mice (ob−/ob−) characterized by severe obesity, a decrease in the rates of basic metabolism and thermogenesis, and low physical activity. Furthermore, in 1973, Coleman10 demonstrated that the crossing of ob−/ob− mice with wild-type mice led to normalization of body weight and basal metabolism in their offspring, suggesting that some genetic factors underlie the development of obesity together with the presence of a circulating “saturation” factor. Twenty years later, Zhang et al11 identified the ob gene responsible for the development of obesity in ob−/ob− mice. The product of the ob gene was subsequently named “leptin”, from a Greek word for “thin”. Administration of recombinant leptin protein into ob−/ob− and wild-type mice resulted in reduced adipose-tissue volume while maintaining lean mass.12–14
Because mice harboring a defective ob gene were associated with the development of severe obesity, subsequent research attempted to determine whether mutations in the human form of the gene were associated with obesity in humans. An analysis of the protein structure of leptin isolated from human subcutaneous fat cells was performed in 1995, revealing no differences in its structure between lean and overweight individuals;15 however, leptin mRNA levels were elevated in the obese subjects.16 Mutations in the OB gene in humans are extremely rare and result in development of hyperphagia, morbid obesity immediately after birth, and hypothalamic hypogonadism. A study demonstrated that experimental leptin-deficient rodents were protected from diseases associated with hyperleptinemia, whereas the introduction of leptin restored sensitivity and induced obesity.17 By contrast, in individuals with leptin deficiency, hyperinsulinemia, hyperglycemia, and hypothermia have not been detected, suggesting that obesity in humans is not associated with leptin-specific defects but rather with the insensitivity of leptin-target cells to such defects.17 Similarly, leptin administration to mice with a defective leptin receptor (genotype: db−/db−, associated with obesity and diabetes) did not affect their appetite or weight.18,19
Leptin receptors and signaling
Leptin receptors belong to the cytokine class 1 family and are encoded by the db gene in mice. Multiple splice variants of Ob-R mRNA encode at least six isoforms of leptin receptors (LepRa, LepRb, LepRc, LepRd, LepRe, and LepRf), which have a common leptin-binding domain but differ in their intracellular domains.20 The function of these leptin-receptor isoforms is not well understood, although studies suggest that truncated forms are involved in leptin transport. LepRe does not have a transmembrane domain and is the soluble LepR isoform, which allows LepRe to bind circulating leptin and inhibit leptin transfer. Only Lep-Rb (long isoform) contains the intracellular motif necessary for leptin-mediated activation of the JAK–STAT signaling pathway in the hypothalamus, which makes this isoform the primary cause of leptin-specific effects.20 LepRb is a typical class I cytokine receptor without the activity of an internal kinase. Instead, the binding of leptin to LepRb allows the recruitment and activation of JAK2, which undergoes autophosphorylation, as well as phosphorylation of three tyrosine residues in LepRb (Y985, Y1077, and Y1138). These phosphorylated residues interact with SH2-domain-containing signaling molecules, which bind to the LepRb–JAK2 complex to promote JAK2 phosphorylation of the signaling proteins and subsequent transmission of leptin-specific signals to second-order neurons located in the nucleus of the hypothalamus.21 Each of these phosphorylation sites induces a leptin-specific signaling pathway with different physiological functions. Phospho-Y985 activates signaling associated with SHP-2 and MAPK and mediates leptin-specific negative-feedback signals, with Shp2 mutations resulting in the development of early obesity in mice.22 Additionally, phospho-Y1077 activates STAT5 and mediates the reproductive effects of leptin. The absence of STAT5 in the central nervous system leads to hyperphagia and obesity, whereas the activation STAT5 in hypothalamic neurons suppresses hunger in mice,23 suggesting that the JAK2–STAT5 pathway is involved in preventing the development of obesity. Phospho-Y1138 activates STAT3 signaling and mediates the main effects of leptin on energy homeostasis and neuroendocrine functions, although this has little effect on reproduction.24 Additionally, leptin-induced phosphorylation of STAT3 has been used to identify relative changes in leptin sensitivity.25 STAT3 is subsequently phosphorylated by the LepRb–JAK2 complex, resulting in its dimerization and nuclear translocation where STAT3 dimers act as transcription factors to regulate the expression of target genes, including SOCS3.26 These findings support leptin-mediated signaling via the JAK2–STAT3 pathway to prevent obesity.
LepRb signaling is associated with two adapter molecules that serve as negative regulators of leptin signaling: SOCS3 and PTP1B. SOCS3 expression is enhanced by leptin-induced phospho-STAT3, and the SOCS3 protein binds to LepRb Y985 and JAK2 to block leptin transfer in classical inhibitory pathway of the feedback signal.27 Similarly, PTP1B mRNA levels are elevated following STAT3 nuclear translocation, whereas low levels of PTP1B result in increased leptin-related activity; however, the precise mechanisms involved in PTP1B interaction with LepRb remain unknown.28 Other genes indirectly regulated by LepRb activity include those encoding several hypothalamic neuropeptides, such as POMC, CART, AgRP, and NPY.29
Interactions between leptin and the hypothalamus
Leptin enters the brain through the mechanism of saturating transport, possibly mediated by transcytosis receptors across the BBB. Leptin is too large to cross the BBB by diffusion, and therefore, it requires to be transported via an adjustable, saturable transport system.30 Although the molecules associated with this leptin-transfer system remain unclear, it is believed that their action occurs independent of LepRb. Cerebral vessels express high levels of truncated forms of ObRa leptin receptors that bind leptin. A study suggested that these leptin receptors are located in the endothelium of capillaries, and that the vascular plexuses of the brain enable leptin transport from the blood to the interstitial tissue of the brain and finally to the cerebrospinal fluid through the BBB.31
Leptin activates neurons in the retrochiasmatic area and the lateral arcuate nucleus, which innervate sympathetic preganglionic neurons in the thoracic spinal cord and contain CARTs, ventral presumptive nuclei, medial preoptic nuclei, and dorsomedial, ventromedial, paraventricular, and lateral hypothalamic nuclei.32 Leptin targets in the arcuate, dorsomedial, ventromedial, and ventral pre-amminuclear nuclei are located in close proximity to the medial elevations, where leptin can reach neurons in the adjacent ventrobasal hypothalamus by diffusion. Additionally, the hormone can be transported to the brain via cerebrospinal fluid. ObRa, highly expressed in the choroid plexus, reportedly promotes the transport of leptins from the blood to the cerebrospinal fluid, where leptin concentration is ~100-fold lower than that in plasma, suggesting that cerebrospinal fluid is not the main source of leptin for brain targets.33 Leptin receptors located on the neurons of arcuate nuclei have been well studied. There are at least two types of neurons located in the arcuate zone of the hypothalamus, with the first type responsible for the synthesis of POMC and the second type responsible for the synthesis of AgRP and NPY. Moreover, these neurons harbor LepRb receptors.34 POMC synthesis is stimulated in POMC-producing neurons by leptin binding, which subsequently stimulates synthesis of anorexigenic (appetite-suppressant) neuropeptides and α-melanocytostimulating hormone, which reduces weight by binding and activating the receptors MC3R and MC4R.21 A previous study reported that structural alteration of the MC4R receptor and an insufficient number of MC3R receptors in mice result in leptin resistance and obesity, and that the leptin-specific modulation of the melanocortin system results in decreases in body weight.21 AgRP and NPY synthesis in the other neuron types induces appetite stimulation, with damage to these neurons responsible for hunger suppression in mice.24 Leptin inhibits NPY and AgRP synthesis; therefore, leptin-specific activity in the arcuate zone of the hypothalamus via LepRb receptors modulates the synthesis of neuropeptides that prevent the development of obesity.
Mechanisms of leptin resistance
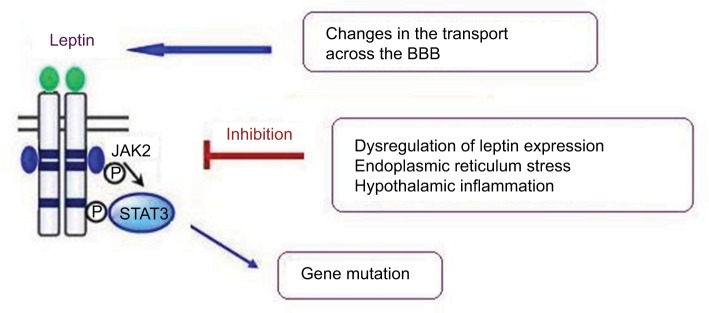
To date, several mechanisms have been identified as potentially underlying leptin resistance. These include a number of molecular and functional alterations characterized by structural changes to the molecule, its transport across the BBB, and the deterioration of leptin-receptor function and signaling (Figure 1).35,36
Figure 1.
Mechanisms of leptin resistance.
Note: Several mechanisms have been identified as potentially underlying leptin resistance, including a gene mutation resulting in structural changes in the leptin molecule, leptin transport across the BBB, and deterioration of leptin-receptor function accompanied by hypothalamic inflammation and endoplasmic reticulum stress.
Abbreviation: BBB, blood–brain barrier.
Genetic mutations
It is rarely possible to establish the hereditary nature of leptin resistance. Mutations in the OB and DBU genes in humans are extremely rare and cause hyperphagia, obesity soon after birth, and hypothalamic hypogonadism in homozygotes.37 Such mutations have only been described in three sisters and resulted in the replacement of guanine by an adenine at the splice-donor site of exon 16 and generation of a truncated leptin receptor lacking transmembrane and intracellular domains.38 Mutant receptors at high concentrations circulate and bind leptin. In addition to these consequences, dysfunctional secretion of thyrotropin and growth hormones can also occur.39 These findings indicate that mutations in the leptin gene and that of its receptor are not the main factors that induce the development of leptin resistance in the general population.
Altered leptin transport across the BBB
Leptin resistance can also be developed at the BBB, thereby allowing unregulated transport of leptin from the blood to the brain. Brain blood vessels express short forms of OBR, which bind leptin and transport it from blood to the interstitial tissue of the brain and into the cerebrospinal fluid.40,41 At serum leptin levels above the range of 25–30 ng/mL, the concentration of leptin in brain tissues and cerebrospinal fluid does not increase.42 This phenomenon likely plays a role in the development of leptin resistance and obesity, where excessive levels of leptin in the blood result in decreased BBB permeability.31 In the spinal fluid of obese individuals, decreased leptin concentrations were observed under these conditions.43
Regulation of leptin expression
Leptin concentrations and leptin-related effects are directly dependent upon the transcription of the OB gene; therefore, the factors affecting this can considerably affect the status of adipose tissue and the development of leptin resistance. A decrease in leptin levels in adipocytes might lead to increases in adipose-tissue volume until the required level of leptin is reached. In such cases, serum leptin remains at physiological levels, even when the individual is obviously obese. This phenomenon has been observed in Pima Indians, who display a predisposition to obesity associated with relatively low serum leptin levels.44
OB expression levels correlate with lipid content in cells and adipocyte size;29 however, the mechanism underlying the effects of cellular fat levels on leptin production remains unclear. Moreover, cultured adipocytes are capable of storing substantially lower amounts of fat as compared with their capacity in vivo, with this characteristic preventing a more complete understanding of the associated intracellular signaling pathway(s).45 Additionally, external stimuli, including hunger, overeating, and the circadian rhythm, modulate leptin expression, with nighttime leptin levels elevated by 30% on average.46
Furthermore, leptin itself plays an important role in the development of its resistance, with this phenomenon termed “leptin-induced leptin resistance”. Developing resistance to leptin increases the predisposition of patients to diet-induced obesity, which in turn contributes to a further increase in leptin levels and aggravation of existing leptin resistance in a vicious cycle.47 Additionally, hypothalamic inflammation, endoplasmic reticulum stress, and autophagy disorders are involved in the development of obesity-associated leptin resistance.48
The role of inflammation in the development of leptin resistance
Given the functional and anatomical relationship between adipocytes and lymphoid cells, it is likely that leptin affects the neuroendocrine and immune systems. Morphologically, the accumulation of lymphoid tissue, including lymph nodes, omentum, thymus, and bone marrow, is associated with adipose tissue. Fat deposits not only exhibit structural, metabolic, and heat-insulating functions but also provide a microenvironment conducive to supporting immune responses.49 In particular, lymphoid and adipose tissues interact through common mediators known as adipokines, molecules derived from adipocytes that connect metabolism and immune homeostasis (these molecules include leptin, adiponectin, chemokines, and other proinflammatory cytokines). Mattace Raso et al50 demonstrated that a high-fat diet induces low-grade inflammation in peripheral tissues (especially in adipose tissue and the liver), leading to an increase in inflammatory cytokines, such as IL-6 and tumor necrosis factor (TNF)-α.50
Leptin is also a proinflammatory cytokine belonging to the family of long-chain helical cytokines and structurally similar to IL-6, IL-12, IL-15, granulocyte colony-stimulating factor, oncostatin M, prolactin, and human growth hormone. Because of its dual nature as a hormone and cytokine, leptin ties the neuroendocrine system to the immune system. A number of studies consider leptin similar to C-reactive protein, IL-1, and IL-6 in its roles as an acute-phase inflammatory protein produced in high concentrations during sepsis and fever, as well as the due to the fact that its production can be induced by other inflammatory mediators, such as TNF-α and IL-1.51,52 However, other studies reported no increases in leptin concentration in people with acute experimental endotoxemia, neonatal sepsis, or HIV infections.53
Leptin-deficient (ob−/ob−) mice and people with congenital leptin deficiency present both metabolic and immune abnormalities, including abnormal cytokine secretion and thymus hypotrophy. Moreover, leptin deficiency in ob−/ob− mice is associated with immunosuppression and thymus atrophy, which represents a similar pattern observed with acute fasting.54 A sharp decrease in caloric intake causes a rapid decrease in serum leptin concentration accompanied by a decrease in the delayed-type hypersensitivity reaction and thymus atrophy, which are reversible with the introduction of leptin.55 Additionally, Farooqi et al56 reported congenital leptin deficiency in patients with elevated T cell levels; however, these data were refuted in subsequent studies.57 Moreover, people with congenital leptin deficiency have a much higher mortality rate from infection in childhood,58 whereas recombinant administration of leptin in children with congenital leptin deficiency normalizes the absolute number of naive CD4+/CD45RA+ T cells and almost restores the response to cytokine proliferation and release from lymphocytes.56 Furthermore, the effects of leptin on the adaptive immune response have been extensively studied in human CD4+ T cells, with reports indicating differential leptin-specific effects on the production and proliferation of cytokines by naive (CD45RA+), memory (CD45RO+), and CD4+ T cells (all expressing ObRb). Specifically, leptin promotes IL-2 proliferation and secretion by naive T cells but minimally affects the proliferation of memory cells.59
Despite observation of direct leptin-related effects on immune responses in vitro, its effect on in vivo immune responses remains unknown. T cells are sensitive to nutrient input, such as that by glucose,60 because they cannot process glycogen, and therefore, depend upon extracellular glucose supplies to meet their metabolic needs. Leptin contributes to the restoration of impaired T cell function caused by hunger via pathways associated with ERK1/ERK2 and PI3K-dependent signaling.60
In congenital immunity, leptin contributes to the activation and phagocytosis of monocytes/macrophages and the secretion of leukotriene B4, cyclooxygenase 2 (COX2), nitric oxide (NO), and proinflammatory cytokines.61 Products of the inducible form COX2, prostaglandin, eicosanoids, and NO are involved in the regulation of inflammation, chemotaxis, and the production of cytokines, and therefore, have a significant effect on the immune response. Additionally, leptin can induce neutrophil chemotaxis and the release of oxygen radicals, such as superoxide anion and hydrogen peroxide, which can be especially harmful to cells through their ability to denature proteins and damage membrane lipids via per-oxidation of unsaturated fatty acids. In human neutrophils, leptin mediates its effects through an indirect mechanism likely related to the release of TNF-α from monocytes.62 Additionally, leptin also affects the development and activation of natural killer cells in vitro and in vivo.63
Establishing criteria for diagnosing leptin resistance
There are currently no methods for effectively assessing leptin sensitivity in a clinical setting. Such sensitivity is reportedly related directly to obesity in general and adipose-tissue volume in particular. A typical obese patient can be characterized by increased leptin levels and excessive OB expression in adipose tissue.64,65 Therefore, many studies consider hyperleptinemia as a key marker of leptin resistance,66–68 and previous associations between abdominal obesity and leptin concentration have been identified as and explained by leptin resistance.69 However, no clear criteria have been established for the diagnosis of leptin resistance.
It is likely that every person shows an individual reaction to overeating or obesity, with this supported by findings of sexual dimorphism associated with concentrations of circulating leptin70 and decreases in OB expression levels with age.71 Additionally, the focus cannot be exclusively on serum concentrations of leptin, as these depend not only on OB expression levels but also on clearance of leptin protein and the presence and activity of its receptors.72
Based on these findings, many studies suggest that the key markers of leptin resistance can include leptin-receptor mRNA and protein levels, given that the basic metabolic effects of leptin depend primarily upon the number of available receptors. Obesity leads to a decrease in the expression of short and long isoforms of leptin receptors (OBRa and OBRb, respectively) in the hypothalamus, hepatocytes, adipose tissue, and muscles,73,74 and in vitro studies demonstrate that leptin-receptor levels are associated with leptin concentration, with subsequent introduction of exogenous leptin to cell cultures resulted in a decrease in leptin-receptor levels.75,76 Additionally, a decrease in the concentration of circulating leptin following prolonged fasting is accompanied by an increase in the expression of OBR.77 Therefore, definition of the free leptin index as describing the relationship between leptin levels and soluble OBR multiplied by 100 is gaining diagnostic value.78–80 However, although this index has been in use since 2003, the reference range has not been clearly established, which limits its wider application.
Jacquier et al81 developed a mathematical approach to the diagnosis of leptin resistance based on the assumption that leptin itself regulates the activity of its own receptors. The model was based on previous models that predicted the dynamics of body weight in rodents, whereas the new model includes the dynamics of leptin, leptin receptors, and the regulation of food intake, as well as body weight, with two stable equilibrium positions: normal body weight with leptin sensitivity and obesity with leptin resistance. Use of this model to analyze the physiological level of adipose tissue showed that constant leptin infusions potentiated a decrease in leptin sensitivity resulting from a decrease in receptor density and leading to increased food intake. The authors suggested a further improvement to the method that involved including the rate of leptin passage through the BBB.81
Leptin receptors are located in many tissues; however, it has not been elucidated which of these are involved in the development of leptin resistance.81 No correlation between basal levels of leptin in obesity and the expression of leptin receptors in skeletal muscles (specifically, in the muscles of the upper limbs) has been reported. By contrast, a negative relationship was found between the concentration of plasma leptin and OBRa levels and the expression of OBRb in the hypothalamus and liver of rodents.82,83 Additionally, the contribution of distinctly localized fatty tissue to leptin and leptin-receptor levels needs to be examined. In epicardial adipocytes, leptin-secretion levels were found to be higher relative to those of adipocytes from subcutaneous adipose tissue.84 Therefore, not only the number of leptin receptors but also their localization might contribute to the development of leptin resistance.
Conclusion
Despite its importance as a key factor underlying the development of metabolic disorders, leptin resistance requires further in-depth experimental and clinical study to elucidate its underlying mechanisms and formulate clear criteria for its diagnosis.
Acknowledgments
This study was supported by the Russian Science Foundation (grant number 17-75-20026).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Farr OM, Gavrieli A, Mantzoros CS. Leptin applications in 2015: what have we learned about leptin and obesity? Curr Opin Endocrinol Diabetes Obes. 2015;22(5):353–359. doi: 10.1097/MED.0000000000000184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jiang N, Sun R, Sun Q. Leptin signaling molecular actions and drug target in hepatocellular carcinoma. Drug Des Devel Ther. 2014;8:2295–2302. doi: 10.2147/DDDT.S69004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morioka T, Mori K, Motoyama K, Emoto M. Ectopic fat accumulation and glucose homeostasis: role of leptin in glucose and lipid metabolism and mass maintenance in skeletal muscle. In: Inaba M, editor. Musculoskeletal Disease Associated with Diabetes Mellitus. Tokyo: Springer Japan; 2016. pp. 201–213. [Google Scholar]
- 4.Banks WA, William A. Role of the blood-brain barrier in the evolution of feeding and cognition. Ann N Y Acad Sci. 2012;1264(1):13–19. doi: 10.1111/j.1749-6632.2012.06568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malli F, Papaioannou AI, Gourgoulianis KI, Daniil Z. The role of leptin in the respiratory system: an overview. Respir Res. 2010;11(1):152. doi: 10.1186/1465-9921-11-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saravanan G, Ponmurugan P, Deepa MA, Senthilkumar B. Anti-obesity action of gingerol: effect on lipid profile, insulin, leptin, amylase and lipase in male obese rats induced by a high-fat diet. J Sci Food Agric. 2014;94(14):2972–2977. doi: 10.1002/jsfa.6642. [DOI] [PubMed] [Google Scholar]
- 7.Paz-Filho G, Mastronardi CA, Licinio J. Leptin treatment: facts and expectations. Metabolism. 2015;64(1):146–156. doi: 10.1016/j.metabol.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 8.Barbarash O, Gruzdeva O, Uchasova E, et al. The role of adipose tissue and adipokines in the manifestation of type 2 diabetes in the long-term period following myocardial infarction. Diabetol Metab Syndr. 2016;8(1):24. doi: 10.1186/s13098-016-0136-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ingalls AM, Dickie MM, Snell GD. Obese, a new mutation in the house mouse. J Hered. 1950;41(12):317–318. doi: 10.1093/oxfordjournals.jhered.a106073. [DOI] [PubMed] [Google Scholar]
- 10.Coleman DL. Effects of parabiosis of obese with diabetes and normal mice. Diabetologia. 1973;9(4):294–298. doi: 10.1007/BF01221857. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 12.Szczesna M, Zieba DA. Phenomenon of leptin resistance in seasonal animals: the failure of leptin action in the brain. Domest Anim Endocrinol. 2015;52:60–70. doi: 10.1016/j.domaniend.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 13.Crujeiras AB, Carreira MC, Cabia B, Andrade S, Amil M, Casanueva FF. Leptin resistance in obesity: an epigenetic landscape. Life Sci. 2015;140:57–63. doi: 10.1016/j.lfs.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 14.Kosteli A, Sugaru E, Haemmerle G, et al. Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. J Clin Invest. 2010;120(10):3466–3479. doi: 10.1172/JCI42845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Considine RV, Considine EL, Williams CJ, et al. Evidence against either a premature stop codon or the absence of obese gene mRNA in human obesity. J Clin Invest. 1995;95(6):2986–2988. doi: 10.1172/JCI118007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miyoshi Y, Funahashi T, Tanaka S, et al. High expression of leptin receptor mRNA in breast cancer tissue predicts poor prognosis for patients with high, but not low, serum leptin levels. Int J Cancer. 2006;118(6):1414–1419. doi: 10.1002/ijc.21543. [DOI] [PubMed] [Google Scholar]
- 17.Kilpeläinen TO, Carli JF, Skowronski AA, et al. Genome-wide meta-analysis uncovers novel loci influencing circulating leptin levels. Nat Commun. 2016;7(1):10494. doi: 10.1038/ncomms10494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koch CE, Lowe C, Pretz D, Steger J, Williams LM, Tups A. High-fat diet induces leptin resistance in leptin-deficient mice. J Neuroendocrinol. 2014;26(2):58–67. doi: 10.1111/jne.12131. [DOI] [PubMed] [Google Scholar]
- 19.Farooqi IS, O’Rahilly S. 20 years of leptin: human disorders of leptin action. J Endocrinol. 2014;223(1):T63–T70. doi: 10.1530/JOE-14-0480. [DOI] [PubMed] [Google Scholar]
- 20.Wada N, Hirako S, Takenoya F, Kageyama H, Okabe M, Shioda S. Leptin and its receptors. J Chem Neuroanat. 2014;61–62:191–199. doi: 10.1016/j.jchemneu.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Zhou Y, Rui L. Leptin signaling and leptin resistance. Front Med. 2013;7(2):207–222. doi: 10.1007/s11684-013-0263-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Myers MG, Münzberg H, Leinninger GM, Leshan RL. The geometry of leptin action in the brain: more complicated than a simple ARC. Cell Metabolism. 2009;9(2):117–123. doi: 10.1016/j.cmet.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee J-Y, Muenzberg H, Gavrilova O, et al. Loss of cytokine-STAT5 signaling in the CNS and pituitary gland alters energy balance and leads to obesity. PLoS One. 2008;3(2):e1639. doi: 10.1371/journal.pone.0001639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Myers MG, Cowley MA, Münzberg H. Mechanisms of leptin action and leptin resistance. Annu Rev Physiol. 2008;70(1):537–556. doi: 10.1146/annurev.physiol.70.113006.100707. [DOI] [PubMed] [Google Scholar]
- 25.Münzberg H, Flier JS, Bjørbæk C. Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology. 2004;145(11):4880–4889. doi: 10.1210/en.2004-0726. [DOI] [PubMed] [Google Scholar]
- 26.Aw X, Ste-Marie L, Kaelin CB, Barsh GS. Inactivation of signal transducer and activator of transcription 3 in proopiomelanocortin (POMC) neurons causes decreased POMC expression, mild obesity, and defects in compensatory refeeding. Endocrinology. 2007;148(1):72–80. doi: 10.1210/en.2006-1119. [DOI] [PubMed] [Google Scholar]
- 27.Bjørbæk C, Lavery HJ, Bates SH, et al. SOCS3 mediates feedback inhibition of the leptin receptor via Tyr985. J Biol Chem. 2000;275(51):40649–40657. doi: 10.1074/jbc.M007577200. [DOI] [PubMed] [Google Scholar]
- 28.Zabolotny JM, Bence-Hanulec KK, Stricker-Krongrad A, et al. PTP1B regulates leptin signal transduction in vivo. Dev Cell. 2002;2(4):489–495. doi: 10.1016/s1534-5807(02)00148-x. [DOI] [PubMed] [Google Scholar]
- 29.Santoro A, Mattace Raso G, Meli R. Drug targeting of leptin resistance. Life Sci. 2015;140:64–74. doi: 10.1016/j.lfs.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 30.Maness LM, Banks WA, Kastin AJ. Persistence of blood-to-brain transport of leptin in obese leptin-deficient and leptin receptor-deficient mice. Brain Res. 2000;873(1):165–167. doi: 10.1016/s0006-8993(00)02520-8. [DOI] [PubMed] [Google Scholar]
- 31.Mantzoros CS. The role of leptin in human obesity and disease: a review of current evidence. Ann Intern Med. 1999;130(8):671–680. doi: 10.7326/0003-4819-130-8-199904200-00014. [DOI] [PubMed] [Google Scholar]
- 32.Scott MM, Lachey JL, Sternson SM, et al. Leptin targets in the mouse brain. J Comp Neurol. 2009;514(5):518–532. doi: 10.1002/cne.22025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwartz MW, Seeley RJ. The new biology of body weight regulation. J Am Diet Assoc. 1997;97(1):54–58. doi: 10.1016/S0002-8223(97)00018-7. [DOI] [PubMed] [Google Scholar]
- 34.Gautron L, Elmquist JK. Sixteen years and counting: an update on leptin in energy balance. J Clin Invest. 2011;121(6):2087–2093. doi: 10.1172/JCI45888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morris DL, Rui L. Recent advances in understanding leptin signaling and leptin resistance. Am J Physiol Endocrinol Metab. 2009;297(6):E1247–E1259. doi: 10.1152/ajpendo.00274.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morris A. Mechanisms of leptin resistance revealed. Nat Rev Endocrinol. 2018;14(11):628. doi: 10.1038/s41574-018-0091-4. [DOI] [PubMed] [Google Scholar]
- 37.Fei H, Okano HJ, Li C, et al. Anatomic localization of alternatively spliced leptin receptors (Ob-R) in mouse brain and other tissues. Proc Natl Acad Sci U S A. 1997;94(13):7001–7005. doi: 10.1073/pnas.94.13.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wabitsch M, Funcke JB, Lennerz B, et al. Biologically inactive leptin and early-onset extreme obesity. N Engl J Med. 2015;372(1):48–54. doi: 10.1056/NEJMoa1406653. [DOI] [PubMed] [Google Scholar]
- 39.Friedman J. 20 years of leptin: leptin at 20: an overview. J Endocrinol. 2014;223(1):T1–T8. doi: 10.1530/JOE-14-0405. [DOI] [PubMed] [Google Scholar]
- 40.Chumakova GA, Ott AV, Veselovskaya NG, Gritsenko OV, Shenkova NN. Pathogenetic mechanisms of leptin resistance. Russ J Cardiol. 2015;4(4):107–110. [Google Scholar]
- 41.Tartaglia LA, Dembski M, Weng X, et al. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995;83(7):1263–1271. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- 42.Holtkamp K, Hebebrand J, Mika C, Heer M, Heussen N, Herpertz-Dahlmann B. High serum leptin levels subsequent to weight gain predict renewed weight loss in patients with anorexia nervosa. Psychoneuroendocrinology. 2004;29(6):791–797. doi: 10.1016/S0306-4530(03)00143-4. [DOI] [PubMed] [Google Scholar]
- 43.Philbrick KA, Wong CP, Branscum AJ, Turner RT, Iwaniec UT. Leptin stimulates bone formation in ob/ob mice at doses having minimal impact on energy metabolism. J Endocrinol. 2017;232(3):461–474. doi: 10.1530/JOE-16-0484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mainardi M, Pizzorusso T, Maffei M. Environment, leptin sensitivity, and hypothalamic plasticity. Neural Plasticity. 2013;2013(6778):1–8. doi: 10.1155/2013/438072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller AA. dissertation. Accra: Northwestern University; 2013. Deconstructing Normal: Leptin Variation Across and Within Population. [Google Scholar]
- 46.Zhang Y, Ren J. Obesity. Switzerland: Springer International Publishing; 2016. Leptin and obesity; pp. 45–58. [Google Scholar]
- 47.Gonzalez-Carter D, Goode AE, Fiammengo R, Dunlop IE, Dexter DT, Porter AE. Inhibition of Leptin-ObR interaction does not prevent leptin translocation across a human blood-brain barrier model. . J Neuroendocrinol. 2016;28(6) doi: 10.1111/jne.12392. [DOI] [PubMed] [Google Scholar]
- 48.de Git KCG, Adan RAH. Leptin resistance in diet-induced obesity: the role of hypothalamic inflammation. Obes Rev. 2015;16(3):207–224. doi: 10.1111/obr.12243. [DOI] [PubMed] [Google Scholar]
- 49.Matarese G, La Cava A, Sanna V, et al. Balancing susceptibility to infection and autoimmunity: a role for leptin? Trends Immunol. 2002;23(4):182–187. doi: 10.1016/s1471-4906(02)02188-9. [DOI] [PubMed] [Google Scholar]
- 50.Mattace Raso G, Simeoli R, Russo R, et al. Effects of sodium butyrate and its synthetic amide derivative on liver inflammation and glucose tolerance in an animal model of steatosis induced by high fat diet. PLoS One. 2013;8(7):e68626. doi: 10.1371/journal.pone.0068626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395(6704):763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 52.Landman RE, Puder JJ, Xiao E, Freda PU, Ferin M, Wardlaw SL. Endotoxin stimulates leptin in the human and nonhuman primate. J Clin Endocrinol Metab. 2003;88(3):1285–1291. doi: 10.1210/jc.2002-021393. [DOI] [PubMed] [Google Scholar]
- 53.Koç E, Üstündag G, Aliefendioğlu D, Ergenekon E, Bideci A, Atalay Y. Scrum leptin levels and their relationship to tumor necrosis factor« and interleukin-6 in neonatal sepsis. J Pediatr Endocrinol Metab. 2003;16(9):1283–1287. doi: 10.1515/jpem.2003.16.9.1283. [DOI] [PubMed] [Google Scholar]
- 54.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 55.Lord GM, Matarese G, Howard JK, Lechler RI. The bioenergetics of the immune system. Science. 2001;292(5518):855–856. doi: 10.1126/science.292.5518.855. [DOI] [PubMed] [Google Scholar]
- 56.Farooqi IS, Matarese G, Lord GM, et al. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J Clin Invest. 2002;110(8):1093–1103. doi: 10.1172/JCI15693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fischer-Posovszky P, von Schnurbein J, Moepps B, et al. A new mis-sense mutation in the leptin gene causes mild obesity and hypogonadism without affecting T cell responsiveness. J Clin Endocrinol Metab. 2010;95(6):2836–2840. doi: 10.1210/jc.2009-2466. [DOI] [PubMed] [Google Scholar]
- 58.Ozata M, Ozdemir IC, Licinio J. Human leptin deficiency caused by a missense mutation: multiple endocrine defects, decreased sympathetic tone, and immune system dysfunction indicate new targets for leptin action, greater central than peripheral resistance to the effects of leptin, and spontaneous correction of leptin-mediated defects. J Clin Endocrinol Metab. 1999;84(10):3686–3695. doi: 10.1210/jcem.84.10.5999. [DOI] [PubMed] [Google Scholar]
- 59.Ducy P, Amling M, Takeda S, et al. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000;100:197–207. doi: 10.1016/s0092-8674(00)81558-5. [DOI] [PubMed] [Google Scholar]
- 60.Yarasheski KE, Zachwieja JJ, Horgan MM, Powderly WG, Santiago JV, Landt M. Serum leptin concentrations in human immunodeficiency virus-infected men with low adiposity. Metabolism. 1997;46(3):303–305. doi: 10.1016/s0026-0495(97)90258-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cava AL, Matarese G. The weight of leptin in immunity. Nate Rev Immunol. 2004;4(5):371–379. doi: 10.1038/nri1350. [DOI] [PubMed] [Google Scholar]
- 62.Caldefie-Chezet F, Poulin A, Vasson M-P. Leptin regulates functional capacities of polymorphonuclear neutrophils. Free Radic Res. 2003;37(8):809–814. doi: 10.1080/1071576031000097526. [DOI] [PubMed] [Google Scholar]
- 63.Zhao Y, Sun R, You L, Gao C, Tian Z. Expression of leptin receptors and response to leptin stimulation of human natural killer cell lines. Biochem Biophys Res Commun. 2003;300(2):247–252. doi: 10.1016/s0006-291x(02)02838-3. [DOI] [PubMed] [Google Scholar]
- 64.Mantzoros CS. The role of leptin in human obesity and disease: a review of current evidence. Ann Intern Med. 1999;130(8):671–680. doi: 10.7326/0003-4819-130-8-199904200-00014. [DOI] [PubMed] [Google Scholar]
- 65.Scarpace PJ, Zhang Y. Leptin resistance: a prediposing factor for diet-induced obesity. Am J Physiol Regul Integr Comp Physiol. 2009;296(3):R493–R500. doi: 10.1152/ajpregu.90669.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Posey KA, Clegg DJ, Printz RL, et al. Hypothalamic proinflammatory lipid accumulation, inflammation, and insulin resistance in rats fed a high-fat diet. Am J Physiol Endocrinol Metab. 2009;296(5):E1003–E1012. doi: 10.1152/ajpendo.90377.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zubiría MG, Vidal-Bravo J, Spinedi E, Giovambattista A. Relationship between impaired adipogenesis of retroperitoneal adipose tissue and hypertrophic obesity: role of endogenous glucocorticoid excess. J Cell Mol Med. 2014;18(8):1549–1561. doi: 10.1111/jcmm.12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rehman Khan A, Awan FR. Leptin resistance: a possible interface between obesity and pulmonary-related disorders. Int J Endocrinol Metab. 2016;14(1):e32586. doi: 10.5812/ijem.32586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Münzberg H, Heymsfield SB. Leptin, obesity, and leptin resistance. In: Dagogo-Jack S, editor. Leptin. Cham: Springer International Publishing; 2015. pp. 67–78. [Google Scholar]
- 70.Lönnqvist F, Arner P, Nordfors L, Schalling M. Overexpression of the obese (ob) gene in adipose tissue of human obese subjects. Nat Med. 1995;1(9):950–953. doi: 10.1038/nm0995-950. [DOI] [PubMed] [Google Scholar]
- 71.Silha J, Krsek M, Skrha J, Sucharda P, Nyomba BL, Murphy LJ. Plasma resistin, adiponectin and leptin levels in lean and obese subjects: correlations with insulin resistance. Eur J Endocrinol. 2003;149(4):331–335. doi: 10.1530/eje.0.1490331. [DOI] [PubMed] [Google Scholar]
- 72.Ravussin Y, LeDuc CA, Watanabe K, et al. Effects of chronic leptin infusion on subsequent body weight and composition in mice: can body weight set point be reset? Mol Metab. 2014;3(4):432–440. doi: 10.1016/j.molmet.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schaab M, Kratzsch J. The soluble leptin receptor. Best Pract Res Clin Endocrinol Metab. 2015;29(5):661–670. doi: 10.1016/j.beem.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 74.Groba C, Mayerl S, van Mullem AA, et al. Hypothyroidism compromises hypothalamic leptin signaling in mice. Mol Endocrinol. 2013;27(4):586–597. doi: 10.1210/me.2012-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nejati-Koshki K, Akbarzadeh A, Pourhasan-Moghaddam M, Abhari A, Dariushnejad H. Inhibition of leptin and leptin receptor gene expression by silibinincurcumin combination. Asian Pac J Cancer Prev. 2013;14(11):6595–6599. doi: 10.7314/apjcp.2013.14.11.6595. [DOI] [PubMed] [Google Scholar]
- 76.de Queiroz KB, Guimarães JB, Coimbra CC, et al. Endurance training increases leptin expression in the retroperitoneal adipose tissue of rats fed with a high-sugar diet. Lipids. 2014;49(1):85–96. doi: 10.1007/s11745-013-3854-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lana A, Valdés-Bécares A, Buño A, Rodríguez-Artalejo F, Lopez-Garcia E. Serum leptin concentration is associated with incident frailty in older adults. Aging Dis. 2017;8(2):240–249. doi: 10.14336/AD.2016.0819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang J, Scarpace PJ. The soluble leptin receptor neutralizes leptin-mediated STAT3 signalling and anorexic responses in vivo. Br J Pharmacol. 2009;158(2):475–482. doi: 10.1111/j.1476-5381.2009.00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Baranowska-Bik A, Bik W, Styczynska M, et al. Plasma leptin levels and free leptin index in women with Alzheimer’s disease. Neuropeptides. 2015;52:73–78. doi: 10.1016/j.npep.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 80.Rizk NM, Sharif E. Leptin as well as free leptin receptor is associated with polycystic ovary syndrome in young women. Int J Endocrinol. 2015;2015(1):1–10. doi: 10.1155/2015/927805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jacquier M, Soula HA, Crauste F. A mathematical model of leptin resistance. Math Biosci. 2015;267:10–23. doi: 10.1016/j.mbs.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 82.Murugesan D, Arunachalam T, Ramamurthy V, Subramanian S. Association of polymorphisms in leptin receptor gene with obesity and type 2 diabetes in the local population of Coimbatore. Indian J Hum Genet. 2010;16:72–77. doi: 10.4103/0971-6866.69350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Guerra B, Ponce-González JG, Morales-Alamo D, et al. Leptin signaling in skeletal muscle after bed rest in healthy humans. Eur J Appl Physiol. 2014;114(2):345–357. doi: 10.1007/s00421-013-2779-4. [DOI] [PubMed] [Google Scholar]
- 84.Gruzdeva O, Uchasova E, Dyleva Y, et al. Relationships between epicardial adipose tissue thickness and adipofibrokine indicator profiles post-myocardial infarction. Cardiovasc Diabetol. 2018;17(1):40. doi: 10.1186/s12933-018-0679-y. [DOI] [PMC free article] [PubMed] [Google Scholar]