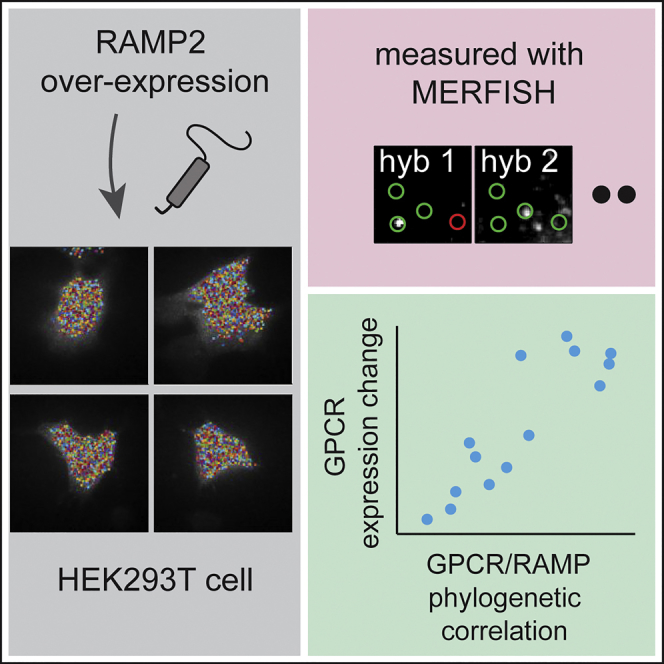
Summary
A recent phylogenetic analysis showed global co-evolution of G protein-coupled receptors (GPCRs) and receptor-activity-modifying proteins (RAMPs) suggesting global interactions between these two protein families. Experimental validation of these findings is challenging because in humans whereas there are only three genes encoding RAMPs, there are about 800 genes encoding GPCRs. Here, we report an experimental approach to evaluate GPCR-RAMP interactions. As a proof-of-concept experiment, we over-expressed RAMP2 in HEK293T cells and evaluated the effect on the transcriptional levels of 14 representative GPCRs that were selected based on the earlier phylogenetic analysis. We utilized a multiplexed error-correcting fluorescence in situ hybridization (MERFISH) method to detect message levels for individual GPCRs in single cells. The MERFISH results showed changes in GPCR message levels with RAMP2 over-expression in a concordant pattern that was predicted by the earlier phylogenetic analysis. These results provide additional evidence that GPCR-RAMP interactions are more widespread than previously appreciated and that these interactions have functional consequences.
Subject Areas: Biological Sciences, Cell Biology, Molecular Biology
Graphical Abstract
Highlights
-
•
A multiplexed fluorescence in situ hybridization (MERFISH) approach was used
-
•
GPCR and RAMP2 transcriptional levels were measured in single cells
-
•
Transcriptional levels were correlated as predicted by phylogenetic analysis
-
•
Results suggest that RAMPs and GPCRs globally interact with functional consequences
Biological Sciences; Cell Biology; Molecular Biology
Introduction
G protein-coupled receptors (GPCRs) participate in a wide range of basic molecular processes and are highly druggable therapeutic targets (Venkatakrishnan et al., 2013). Receptor-activity-modifying proteins (RAMPs) have been shown to interact with several GPCRs and affect their ligand specificity, cellular trafficking, and post-translational modification (McLatchie et al., 1998, Parameswaran and Spielman, 2006, Weston et al., 2015). For example, the calcitonin receptor-like receptor (CALCRL), arguably the most studied GPCR in this context, interacts with RAMP1 in a way that facilitates the receptor's transport to the plasma membrane (Parameswaran and Spielman, 2006) and determines its ligand specificity (Hay and Pioszak, 2016, Hay et al., 2006). Recently, we compared estimates of GPCR-RAMP co-evolution with those for established interacting protein pairs such as subunits of receptor complexes and endogenous protein ligands with their receptors. We found global co-evolution between GPCRs and RAMPs, suggesting that the interactions between the protein products of the two gene families are more widespread than previously presumed (Barbash et al., 2017).
Previous studies showed that GPCR activation modulates RNA stability (Tholanikunnel and Malbon, 1997) and general transcriptional programs (Lefkowitz and Shenoy, 2005) as well as specific transcriptional programs for the activated receptors (Sharp et al., 2013, Tsao et al., 2001). Based on these observations, we hypothesized that there might be some degree of correlation between RAMP and GPCR expression changes and that we could use such a correlation to validate our previous co-evolution analysis experimentally. Recent developments in highly multiplexed RNA profiling enable single-molecule measurements with error detection and correction in single cells (Chen et al., 2015). We modified this technique to enable detection of shorter genes when compared with the original MERFISH method (see Transparent Methods). We applied our modified MERFISH method to a subset of target genes in HEK293T cells over-expressing RAMP2 and proper controls for correlated detection of 14 GPCR messages. We compared the across-cells expression change data with the results from our previous global phylogenetic analysis. We conclude that the levels of GPCR-RAMP interactions determined by RNA expression and the interactions determined by co-evolution analyses correspond. The concordance of the phylogenetic and expression measurements gives further confidence in the “global GPCR-RAMP interaction” hypothesis. In summary, we establish a potential experimental approach to validate earlier phylogenetic results and give further support for GPCR-RAMP interactions based on RNA expression patterns in HEK293T cells.
Results
Designing and Characterizing MERFISH for GPCRs
The direct experimental testing of all the possible pairwise interactions among the three RAMPs and over 800 GPCRs is not currently feasible. However, we wanted to devise an experimental method to begin to validate the hypothesis that there exists a global interaction network between RAMPs and GPCRs. Therefore, we adopted the MERFISH method and applied it to test a selected tractable subset of GPCRs expressed in HEK293T cells. HEK293T cells are widely used for the pharmacological characterization of GPCR signaling pathways. Approximately 61 individual GPCRs are known to be expressed in HEK293 cells in culture (Atwood et al., 2011). We used several criteria to select 14 representative GPCRs (Table S1) for examination in the MERFISH assay: (1) homogeneous distribution across the phylogenetic measures (Barbash et al., 2017) to represent either high or low phylogenetic association with RAMP2, (2) known expression in HEK293T cells, and (3) satisfactory message length for hybridization of enough detecting probes (10 for each gene) to establish a sufficient signal-to-noise ratio.
We adopted and modified the MERFISH technique described earlier (Chen et al., 2015) to enable detection of shorter mRNA transcripts typical for GPCRs. The original MERFISH method used 96 probes per transcript, limiting the method in practice to genes of at least 3,000-nucleotide (nt) in length. As family A (rhodopsin-like) GPCRs are typically approximately 350 amino acids in length, their transcripts are about 1,050 nt long and are too short for standard MERFISH. Briefly, a set of 30-nt-long orthogonal DNA probes was designed with standard probe optimization methods (see “Probe Design and Synthesis” in Transparent Methods for criteria) for the chosen 14 GPCRs with 10 probes per GPCR gene. These probes have 30-nt-long unhybridizing flanking tails on both ends of the target region that hybridize with 30-nt-long fluorescent probes (see Transparent Methods, Figure 1A for hybridization scheme and Tables S1 and S2 and Data S1 for full probe sequences). The modification described also allowed for more efficient detection of a relatively low number of genes.
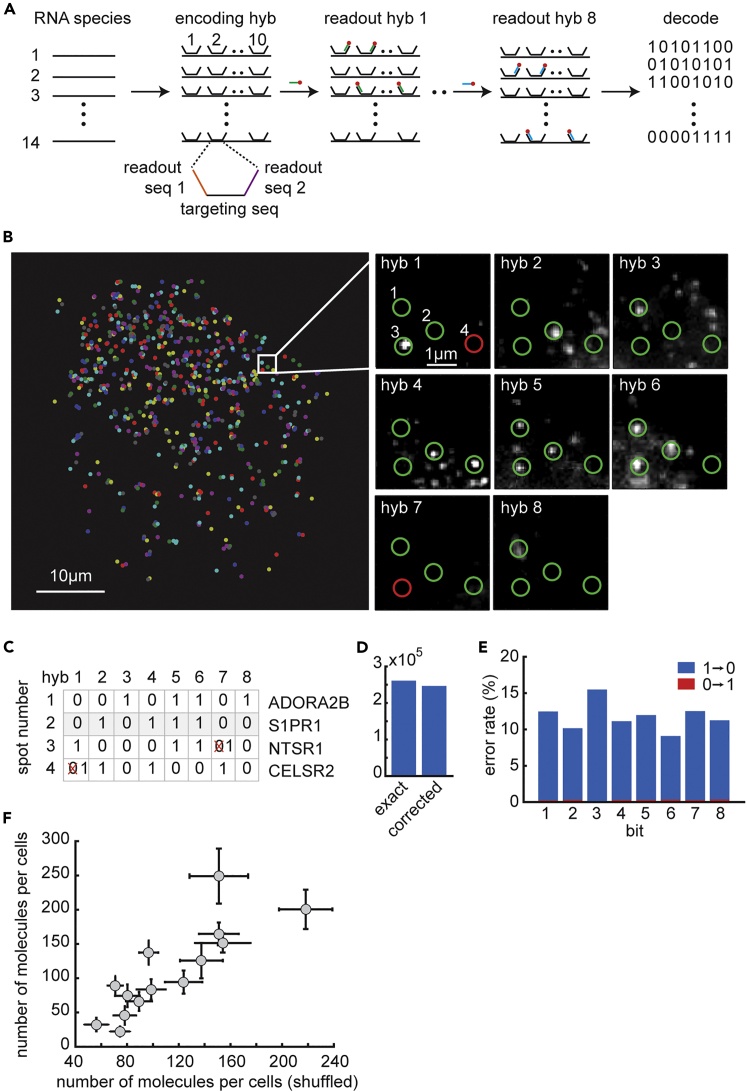
Figure 1.
Design and Characterization of a MERFISH Tailored for GPCRs
(A) Hybridization scheme, as explained in Transparent Methods. Briefly, 10 encoding probes per gene were hybridized followed by a series of eight hybridization rounds in which fluorescent probes were flowed across the sample, hybridized with the corresponding encoding probes, imaged, and then bleached. Last, genes were decoded by their built-in code words.
(B) All detected single molecules in an example cell are shown colored based on gene identity. Four localizations are shown in the enlarged subregion (white square) in which single molecule was detected based on the code book, with error correction, across the eight hybridization rounds. Green circles indicate signals that correspond to the expected bit in the code word of a gene, whereas red circles indicate corrected bits. This correction was enabled due to Hamming distance 4 between the code words, as explained in Transparent Methods.
(C) Summary of the detection results shown in (B) for each location and the corresponding decoded genes.
(D) The total number of RNAs decoded without (exact) and with (corrected) error correction.
(E) The error rate for each bit. The error rate is calculated as the fraction of measured bar codes that contain a given bit flip. Both 1-to-0 error rates (blue) and 0-to-1 error rates (red) are shown for each bit.
(F) Scatterplot for the number of molecules detected per cell for each of the 14 GPCRs in the original “code word” assignment (i.e., the order of hybridization rounds during imaging, see Transparent Methods) versus the shuffled version. Error bars are SEM across cells in each of the conditions. Pearson's correlation coefficient for the scatter plot is 0.83 and correlation p value <0.01.
The fluorescent probes in cultured cells were detected with a Nikon Ti-E inverted microscope in a series of hybridization and bleaching rounds that build up a series of detection and no-detection readouts for each location in the observed field. This series is later treated as a binary word of eight bits where 1 and 0 denote detection and no detection and each bit corresponds to a single hybridization round. See Figure S1A for an example of raw image data and Figure S1B for analysis of bleaching efficiency. Hybridization images for each cell field were registered based on signals from carboxylate-modified microspheres (see “Sample Preparation” in Transparent Methods and Figure S1C). Series signals were detected by identifying objects of higher intensity compared with their surroundings, followed by size filtration, clustering of identical neighboring objects, and a second round of size filtration (see “Image Analysis” in Transparent Methods). Next, these series were matched against the predicted pattern for each gene, while allowing for error detection and correction based on the probe library design (see “Probe Design and Synthesis” in Transparent Methods and Figures 1B and 1C). About one-half of RNA molecules were decoded without error correction, and about one-half were decoded with error correction (Figure 1D). The measured error rate (the event of a bit flip) across the hybridization rounds was 10%–15% (Figure 1E). Example cells overlaid with the 14 detected GPCRs are shown in Figure S2A. We performed a permutation version of the order of hybridizations (“shuffled” version) and measured similar expression levels, validating the library design, imaging, and analysis steps (Figure 1F). We also measured the averaged signal intensity due to sample autofluorescence and probe non-specific binding and found the value to be less than 5% of the averaged signal intensity in any of the hybridization rounds, which we considered satisfactory.
Over-expression of RAMP2 in HEK293T Cells
In our previous work (Barbash et al., 2017) we showed that RAMP1 and RAMP3 co-evolved with similar GPCRs and have structural resemblance at the extracellular region, which is required for transport of RAMP1-CALCRL to the plasma membrane. These observations suggest redundancy at the functional level. In other words, RAMP2, presumably, is distinct in its set of interacting GPCRs. Under over-expression of either RAMP1 or RAMP3, there would have likely been a compensation of one on the effect of the other. Therefore, it would have been difficult to expect a RAMP1- or RAMP3-specific effect on GPCRs' expression, and so difficult to predict that the pattern of GPCR expression change would correlate with the observed phylogenetic pattern. RAMP2 over-expression, on the other hand, does not suffer from this hurdle. For this reason, we designed and conducted RAMP2 over-expression experiments.
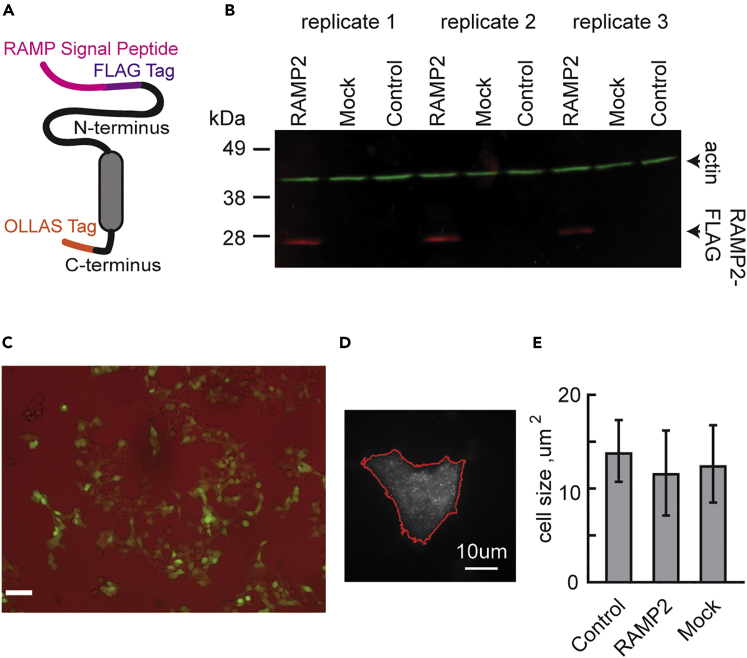
The steps described above were performed under three experimental conditions: non-transfected (control) HEK293T cells, cells transfected with a FLAG tag construct expressing RAMP2 (see Figure 2A for construct schematic), and cells transfected with an empty construct (mock) of the same vector backbone (pcDNA 3.1) as the one for the RAMP2. Three experimental replicates were done for each condition, and the number of cells analyzed in each one was 47 cells in control experiments, 37 cells for RAMP2 over-expression, and 53 cells for mock (see Transparent Methods for criteria for cells that were filtering out during imaging). RAMP2 expression across the three conditions is shown in Figure 2B. Transfection efficiency was estimated by HEK293T cells transfected with a pIRES2-EGFP vector (Figure 2C). No significant cell size difference was observed among the conditions tested (Figures 2D and 2E). We compared relative expression values of the 14 GPCRs with two available RNA-sequencing (RNA-seq) datasets (GSE53386 and GSE60559) of HEK293T and observed high correlations (Figure S2B).
Figure 2.
Over-expression of RAMP2 in HEK293T Cells
(A) HEK293T cells were transfected with a RAMP2 DNA construct that encoded a FLAG epitope tag on the N-terminal end and an OLLAS epitope tag on the C-terminal end. The entire native sequence of RAMP2 except for the initiator Met and predicted cleavable signal peptide was included between the epitope tags. The DNA construct was in a pcDNA3.1+ vector.
(B) Immunoblot of transfected HEK293T cells using antibodies against FLAG (red) and actin (green). RAMP2, cells transfected with RAMP2 DNA construct; MOCK, cells transfected with empty pcDNA3.1 plasmid; control, non-transfected cells.
(C) HEK293T cells transfected with a pIRES2-EGFP vector. Bright field is represented by red channel, and GFP detection is represented by green channel; 48% of the cells were GFP-positive 48 h post transfection. Scale bar, 1 mm.
(D) Borders of cells were traced with the Moore-Neighbor tracing algorithm, based on general localized intensities and their area calculated.
(E) Bar graph of cell area (mean, SEM across all cells in condition) shows no significant difference in cell size across conditions. One-Way-ANOVA p value >0.05.
GPCR-RAMP2 Co-expression Interaction in HEK293T Cells
These expression data were compared to the phylogenetic measures on a cell-by-cell basis and on a population level. On the population level, for each GPCR we calculated the absolute value of log2 for the fold change of expression between RAMP2 transfection and control, and similarly between mock transfection and control (Figure 3A). An absolute value of the log2 for the fold change was then plotted against the phylogenetic mammalian measures of the 14 GPCRs. Specifically, the absolute value was used because there was no reason to favor a prediction for either up- or down-regulation. We tested the most straightforward hypothesis that if a gene pair interacts, as estimated by phylogenetic analysis, then the expression of one member is likely to affect the expression of the other in either the upward or downward direction.
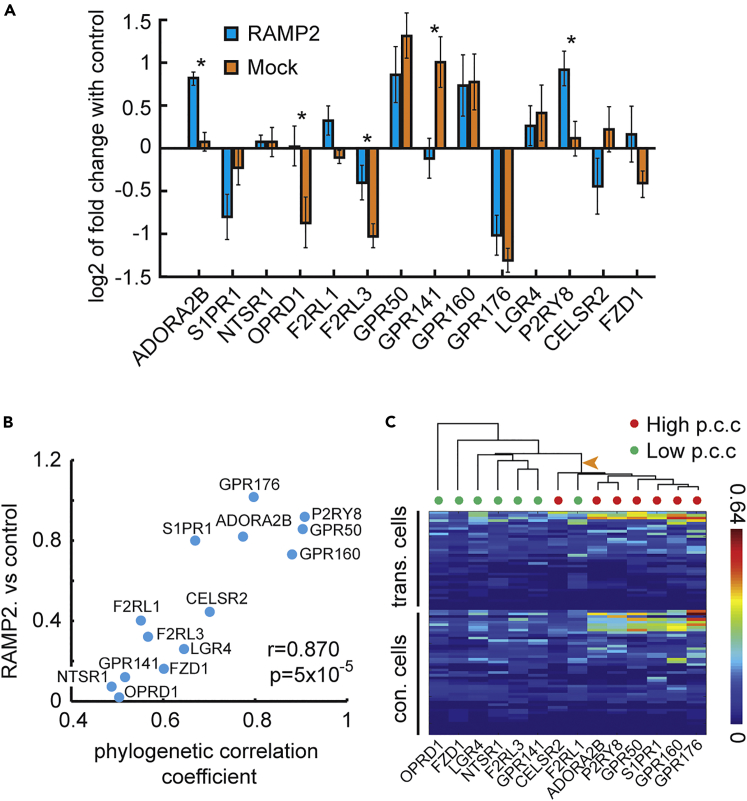
Figure 3.
GPCR-RAMP2 Co-expression Interaction in HEK293T Cells
(A) Bar graph (mean, SEM) of the log2 transformation of fold change of GPCR expression between HEK293T cells transfected with RAMP2 and control cells (blue) and between Mock transfection and control cells (orange) for 14 GPCRs detected with the MERFISH method. Asterisks denote significant t test p values corrected for multiple comparisons with Bonferroni.
(B) Scatterplot of log2 absolute value of fold change of GPCR expression between HEK293T cells transfected with RAMP2 and control cells (y axis) versus phylogenetic correlation coefficient between RAMP2 and each of the GPCRs (x axis). Pearson's r = 0.87 and p value = 5∗10-5, also shown in figure.
(C) Hierarchical clustering tree (dendrogram) based on GPCR expression distance (euclidean) between RAMP2 transfection cells and non-transfected control cells shows significant clustering of the top seven phylogenetically associated GPCRs (red circles) on the branch marked with an orange arrow (two-tailed Fisher's exact test for the clustering, p = 0.0047). Color bar represents the density of identified RNA molecules (number of molecules/pixel). p.c.c., phylogenetic correlation coefficient.
A significant correlation between mammalian phylogenetic measures and MERFISH expression was observed for the RAMP2 transfection, but not for the mock transfection (Figures 3B and S3A–S3C). On the cell-by-cell level, we first split the GPCR group for two subgroups equal in size (seven each) based on their phylogenetic correlation with RAMP2, which is equivalent to setting the phylogenetic correlation coefficient threshold at 0.65. Next, a dendrogram based on between-cells expression distances was built, and the members of the two subgroups were marked on it (Figure 3C). The dendrogram shows that the top RAMP2 phylogenetic-associated GPCRs changed in a similar manner, and independently from, the other group upon RAMP2 over-expression, which suggests a common effector. This expression-based clusterization was neither observed for mock versus control nor for mock versus RAMP2 over-expression (Figure S3D). Taken together, these data show measurable interaction for highly phylogenetically associated gene pairs.
Among the GPCRs tested, five showed a significant change of expression in MERFISH upon RAMP2 over-expression (Figure 3A). These were the class A GPCRs: ADORA2B, OPRD1, F2RL3, GPR141, and P2RY8. Based on our previous phylogenetic analysis, all five GPCRs have relatively high phylogenetic correlation coefficients with all three RAMPs. In addition, F2RL3, GPR141, and P2RY8 also have relatively high coexpression correlation coefficients with RAMP2 and RAMP3. Last, ADORA2B, OPRD1, F2RL3, and P2RY8 are expressed at high levels across human tissues, whereas GPR141 is expressed at moderate levels (Expression Atlas; https://www.ebi.ac.uk/gxa/home/).
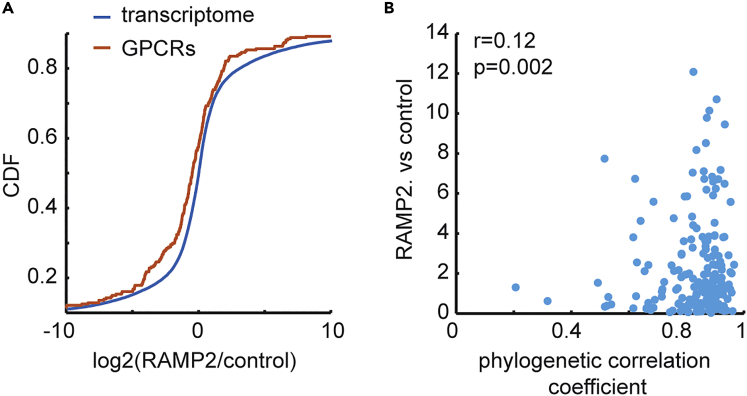
To examine GPCR expression change upon RAMP2 over-expression for more GPCRs, we performed whole-exome expression profiling by RNA-seq for the same three experimental conditions as before (see Methods for details). We found a significant global shift of GPCR expression toward down-regulation compared with the rest of the transcriptome (Figure 4B and S4). A comparable expression shift was not observed for mock transfection (Figure S5A). Next, we examined the concordance of global GPCR expression as measured by RNA sequencing with the phylogenetic correlation coefficient. We observed statistically significant correlation among the two measures, albeit of a relatively low value of Pearson's r. These observations, taken together, strengthen our initial hypothesis of general GPCR-RAMP interaction. Specifically, when comparing the expression change of the 14 MERFISH-examined GPCRs upon RAMP2 over-expression as measured by RNA-seq and by the MERFISH method, we did not see any significant correlation among the two (Figure S5B, see Discussion).
Figure 4.
Global GPCR Expression Change upon RAMP2 Over-Expression
(A) Cumulative Distribution Function (CDF) of log2 of fold change between the average across three samples of RAMP2 over-expression and three control samples. CDF is shown separately for the whole transcriptome (red) and for all GPCR transcripts (red). Kolmogorov-Smirnov p value for difference between distributions: 0.048.
(B) Scatterplot of log2 absolute value of fold change of all GPCR expression, based on RNA-seq-derived Fragments Per Kilobase Million (FPKM) values, between HEK293T cells transfected with RAMP2 and control cells (y axis) versus phylogenetic correlation coefficient between RAMP2 and each of the GPCRs (x axis). Pearson's r = 0.12 and p value = 0.002, also shown in figure.
Discussion
In this study, we present supportive evidence for GPCR-RAMP interactions based on RNA expression patterns in HEK293T cells. We adapted a recently reported multiplexed error-robust fluorescence in situ hybridization (MERFISH) approach and used it to count the number of candidate GPCR RNA transcripts per cell. The single-molecule technique that we utilized was based on the one presented by Chen et al. (Chen et al., 2015) and was modified to better suit efficient detection of a smaller number of genes and with fewer hybridization rounds. Chen et al. used 16 hybridization rounds to resolve 140 genes with error detection and correction, whereas we were able to resolve 14 genes with 8 hybridization rounds. Our adaptation to the multiplexed in situ hybridization protocol adds flexibility and shows that experiments can be designed in different ways to target greater or fewer numbers of molecules depending on the research question while minimizing the number of imaging rounds and the technical burden of synthesizing large probe libraries. Although our method is relatively simplified compared with Chen et al., we were able to reliably detect single genes with a lower number of fluorescent probes. Other studies have reported results using even fewer than 10 encoding probes (Batish et al., 2011, Buxbaum et al., 2014, Lubeck and Cai, 2012). The robust detection efficiency we observed can be attributed, at least in part, to the probe synthesis method we used. Unlike the bulk probe synthesis utilized by others (Chen et al., 2015), we used an individual, probe-by-probe synthesis, including a separate quality control step for each probe (see “Probe Design and Synthesis” in Transparent Methods). Our probe synthesis protocol also allowed us to control the concentration of each different probe, which is not generally possible with bulk synthesis.
The MERFISH results were consistent with the results obtained from our previous phylogenetic analysis (Barbash et al., 2017). As co-evolution of gene pairs could be an indicator for gene interaction, we expected to observe a change in the expression for GPCR transcripts upon RAMP2 over-expression in cases in which the GPCR co-evolved with RAMP2. Based on the previous literature on the mechanisms by which RAMP2 affects GPCRs (McLatchie et al., 1998, Parameswaran and Spielman, 2006), a change in GPCR expression is likely to be driven by a direct effect of RAMP2 on GPCR signaling or trafficking, which in turn could affect synthesis levels via feedback regulation, as was shown before (Black et al., 2016, Lefkowitz and Shenoy, 2005, Tsao et al., 2001). Indeed, we observed a correlation between expression effects on GPCRs upon over-expression of RAMP2 and the degree of co-evolution. The feedback regulation, together with the fact that we measured the association of phylogenetic patterns with expression, are probably the reasons for the relatively subtle effect we observed in the RNA in situ hybridization experiments (Figure 3). Nevertheless, the expression data generally support the approach of utilizing phylogenetic data to estimate gene pair interaction and specifically support GPCR-RAMP2 interactions for the receptors tested.
It is worth noting that some GPCRs showed larger expression changes in our mock transfection rather than RAMP2 transfection experiments. One possible explanation for this observation could be an opposing effect of RAMP2 and mock transfections. For example, if the expression of a given GPCR was up-regulated under the existence of a transfection construct, and the effect of RAMP2 over-expression on the GPCR would be to down-regulate it, the outcome could be larger expression change for the mock condition.
The five GPCRs that show a significant change of expression in MERFISH upon RAMP2 over-expression are involved in a variety of human diseases. ADORA2B is an adenosine GPCR, activating adenylatecyclase in the presence of a ligand. ADORA2B modulates inflammation, and as such is involved in inflammatory diseases (Hasko et al., 2008). OPRD1 is an opioid receptor, which reacts with the endogenous ligand enkephalin, is expressed in particularly high levels in the basal ganglia and neocortical regions of the brain (Peppin and Raffa, 2015), and is involved in major depressive disorder (Kempton et al., 2011). F2RL3 codes for coagulation factor II (thrombin) receptor-like 3, activated by proteases and is involved in cardiovascular disease (Leger et al., 2006). GPR141 has some evidence for involvement in autism spectrum disorders (Mitra et al., 2017). P2RY8 codes for P2Y purinoceptor 8 activated by adenosine and uridine nucleotides and is associated with diffuse large B cell lymphoma (Lohr et al., 2012). No previous receptor-RAMP interaction was reported before for any of these GPCRs, and further study is warranted to delineate the nature of this interaction and determine its involvement in the above-mentioned diseases.
We also examined our hypothesis of global GPCR-RAMP expression interaction using an independent method for expression profiling, i.e., RNA-seq. We observed a global shift in GPCR expression upon RAMP2 over-expression and global correlation with our previously reported phylogenetic measures. Specifically, comparing the MERFISH and RNA-seq methods, across the 14 GPCRs that overlap the two methods, we did not find significant expression correlation. This lack of correlation could be due to different sources of technical noise in those methods. For example, whereas MERFISH is a single-cell, single-molecule method, RNA-seq is a whole-tissue method. In addition, each of the methods has intrinsic biases as to the transcripts that are more easily detected by it. For example, MERFISH strongly depends on RNA linearity, whereas RNA-seq strongly depends on the GC content. Importantly, however, although the two methods do not correlate on the specific set of 14 GPCRs, they both point at the same conclusion: that RAMP2 over-expression leads to a detectable change in GPCR population and that this change is in concordance with phylogenetic measures of GPCR-RAMP co-evolution.
We interpret the results of our over-expression experiments as supportive evidence for a global GPCR-RAMP interaction. It is worth mentioning that an alternative explanation could contribute to the observed results. Previous studies showed that over-expression of transmembrane proteins could indirectly change the mRNA levels of other transmembrane proteins (Buskirk and Green, 2017, Hollien and Weissman, 2006) with the following presumable mechanism. Typically, ribosomes translate the mRNA of transmembrane proteins until encountering the membrane translocation signal (signal sequence), which results in the recruitment of the signal recognition particle (SRP). The ribosome then docks to the translocon in the endoplasmic reticulum following by dissociation of the SRP before any further translation of the polypeptide can occur. Introducing high levels of a signal-sequence-harboring mRNA (e.g., a RAMP mRNA) can lead to competition on the limited number of translocons. This competition could lead to stalled translation, and as a result, to mRNA degradation of transmembrane proteins.
In summary, our results based on RNA expression analysis give further evidence for the existence of a global GPCR-RAMP interaction map. The correlation of message levels for GPCRs and RAMPs supports the hypothesis that GPCR-RAMP interactions are widespread and have functional consequences. As GPCR signaling is a fundamental mechanism across animal organisms, tissues, and cell types, further detailing of the GPCR-RAMP interaction map is warranted.
Limitations of the Study
We carried out the MERFISH analysis to evaluate the effect of RAMP2 over-expression on a limited number of 14 candidate GPCRs. Our analysis in the current study is limited almost entirely to the RNA level, which indirectly supports functional interactions at the protein level. However, future studies will be required to validate candidate GPCR-RAMP interactions and assess their functional consequences.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We acknowledge the generous support of the Rothschild post-doctoral fellowship (S.B.), the BengtWinblad’s Foundation for Young Scientists, the Margareta af Ugglas Foundation, the Gun och Bertil Stohnes Foundation (T.P.), the Nicholson Short-Term Exchange fellowship (E.L.), the Robertson Therapeutic Development Fund, the Crowley Family Fund, and the Danica Foundation (T.H.). We also acknowledge the Rockefeller Bio-Imaging Resource Center (BIRC) and particularly Dr. Kaye Thomas for technical support and guidance.
Author Contributions
S.B., T.P.S., T.H., E.L., and T.P. designed experiments and analyses; S.B., M.A.K., and T.P. performed experiments; S.B. performed computational analyses; and S.B., T.P.S., T.H., E.L., and T.P. wrote the paper.
Declaration of Interests
The authors have no financial interests to declare.
Published: January 25, 2019
Footnotes
Supplemental Information includes Transparent Methods, five figures, two tables, and one data file and can be found with this article online at https://doi.org/10.1016/j.isci.2018.12.024.
Contributor Information
Thomas Huber, Email: hubert@rockefeller.edu.
Thomas P. Sakmar, Email: sakmar@rockefeller.edu.
Data and Software Availability
The accession number for the RNA-seq data reported in this paper is Gene Expression Omnibus: GSE122633.
Supplemental Information
Shown are the 10 probes used for each of the 14 GPCRs. Upper case denotes flanking tail parts, and lower case denotes RNA-targeting region. Attached as a separate excel file.
References
- Atwood B.K., Lopez J., Wager-Miller J., Mackie K., Straiker A. Expression of G protein-coupled receptors and related proteins in HEK293, AtT20, BV2, and N18 cell lines as revealed by microarray analysis. BMC Genomics. 2011;12:14. doi: 10.1186/1471-2164-12-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbash S., Lorenzen E., Persson T., Huber T., Sakmar T.P. GPCRs globally coevolved with receptor activity-modifying proteins, RAMPs. Proc. Natl. Acad. Sci. U S A. 2017;114:12015–12020. doi: 10.1073/pnas.1713074114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batish M., Raj A., Tyagi S. Single molecule imaging of RNA in situ. Methods Mol. Biol. 2011;714:3–13. doi: 10.1007/978-1-61779-005-8_1. [DOI] [PubMed] [Google Scholar]
- Black J.B., Premont R.T., Daaka Y. Feedback regulation of G protein-coupled receptor signaling by GRKs and arrestins. Semin.Cell Dev. Biol. 2016;50:95–104. doi: 10.1016/j.semcdb.2015.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buskirk A.R., Green R. Ribosome pausing, arrest and rescue in bacteria and eukaryotes. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017;372:20160183. doi: 10.1098/rstb.2016.0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxbaum A.R., Wu B., Singer R.H. Single beta-actin mRNA detection in neurons reveals a mechanism for regulating its translatability. Science. 2014;343:419–422. doi: 10.1126/science.1242939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K.H., Boettinger A.N., Moffitt J.R., Wang S., Zhuang X. RNA imaging.Spatially resolved, highly multiplexed RNA profiling in single cells. Science. 2015;348:aaa6090. doi: 10.1126/science.aaa6090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasko G., Linden J., Cronstein B., Pacher P. Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat. Rev. Drug Discov. 2008;7:759–770. doi: 10.1038/nrd2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay D.L., Pioszak A.A. Receptor activity-modifying proteins (RAMPs): new insights and roles. Annu. Rev. Pharmacol. Toxicol. 2016;56:469–487. doi: 10.1146/annurev-pharmtox-010715-103120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay D.L., Poyner D.R., Sexton P.M. GPCR modulation by RAMPs. Pharmacol.Ther. 2006;109:173–197. doi: 10.1016/j.pharmthera.2005.06.015. [DOI] [PubMed] [Google Scholar]
- Hollien J., Weissman J.S. Decay of endoplasmic reticulum-localized mRNAs during the unfolded protein response. Science. 2006;313:104–107. doi: 10.1126/science.1129631. [DOI] [PubMed] [Google Scholar]
- Kempton M.J., Salvador Z., Munafo M.R., Geddes J.R., Simmons A., Frangou S., Williams S.C. Structural neuroimaging studies in major depressive disorder.Meta-analysis and comparison with bipolar disorder. Arch. Gen. Psychiatry. 2011;68:675–690. doi: 10.1001/archgenpsychiatry.2011.60. [DOI] [PubMed] [Google Scholar]
- Lefkowitz R.J., Shenoy S.K. Transduction of receptor signals by beta-arrestins. Science. 2005;308:512–517. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- Leger A.J., Covic L., Kuliopulos A. Protease-activated receptors in cardiovascular diseases. Circulation. 2006;114:1070–1077. doi: 10.1161/CIRCULATIONAHA.105.574830. [DOI] [PubMed] [Google Scholar]
- Lohr J.G., Stojanov P., Lawrence M.S., Auclair D., Chapuy B., Sougnex C., Cruz-Gordillo P., Knoechel B., Asmann Y.W., Slager S.L. Discovery and prioritization of somatic mutations in diffuse large B-cell lymphoma (DLBCL) by whole-exome sequencing. Proc. Natl. Acad. Sci. U S A. 2012;109:3879–3884. doi: 10.1073/pnas.1121343109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubeck E., Cai L. Single-cell systems biology by super-resolution imaging and combinatorial labeling. Nat. Methods. 2012;9:743–748. doi: 10.1038/nmeth.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLatchie L.M., Fraser N.J., Main M.J., Wise A., Brown J., Thompson N., Solari R., Lee M.G., Foord S.M. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature. 1998;393:333–339. doi: 10.1038/30666. [DOI] [PubMed] [Google Scholar]
- Mitra I., Lavillaureix A., Yeh E., Traglia M., Tsang K., Bearden C.E., Rauen K.A., Weiss L.A. Reverse pathway genetic approach identifies epistasis in autism spectrum disorders. PLoS Genet. 2017;13:e1006516. doi: 10.1371/journal.pgen.1006516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parameswaran N., Spielman W.S. RAMPs: the past, present and future. Trends.Biochem.Sci. 2006;31:631–638. doi: 10.1016/j.tibs.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Peppin J.F., Raffa R.B. Delta opioid agonists: a concise update on potential therapeutic applications. J. Clin. Pharm. Ther. 2015;40:155–166. doi: 10.1111/jcpt.12244. [DOI] [PubMed] [Google Scholar]
- Sharp S.I., Hu Y., Weymer J.F., Rizig M., McQuillan A., Hunt S.P., Gurling H.M. The effect of clozapine on mRNA expression for genes encoding G protein-coupled receptors and the protein components of clathrin-mediated endocytosis. Psychiatr.Genet. 2013;23:153–162. doi: 10.1097/YPG.0b013e32835fe51d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tholanikunnel B.G., Malbon C.C. A 20-nucleotide (A + U)-rich element of beta2-adrenergic receptor (beta2AR) mRNA mediates binding to beta2AR-binding protein and is obligate for agonist-induced destabilization of receptor mRNA. J. Biol. Chem. 1997;272:11471–11478. doi: 10.1074/jbc.272.17.11471. [DOI] [PubMed] [Google Scholar]
- Tsao P., Cao T., Von Zastrow M. Role of endocytosis in mediating downregulation of G-protein-coupled receptors. Trends.Pharmacol. Sci. 2001;22:91–96. doi: 10.1016/s0165-6147(00)01620-5. [DOI] [PubMed] [Google Scholar]
- Venkatakrishnan A.J., Depui X., Lebon G., Tate C.G., Schertler G.F., Babu M.M. Molecular signatures of G-protein-coupled receptors. Nature. 2013;494:185–194. doi: 10.1038/nature11896. [DOI] [PubMed] [Google Scholar]
- Weston C., Lu J., Li N., Barkan K., Richards G.O., Roberts D.J., Skerry T.M., Poyner D., Pardamwar M., Reynolds C.A. Modulation of glucagon receptor pharmacology by receptor activity-modifying protein-2 (RAMP2) J. Biol. Chem. 2015;290:23009–23022. doi: 10.1074/jbc.M114.624601. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Shown are the 10 probes used for each of the 14 GPCRs. Upper case denotes flanking tail parts, and lower case denotes RNA-targeting region. Attached as a separate excel file.