Abstract
The appropriate timing of the onset of labor is critical to a successful pregnancy, with potentially devastating consequences resulting to both the mother and child with the onset of preterm labor. In this study, we tested the central hypothesis that progesterone maintains uterine quiescence through regulation of active uterine caspase 3. Using the mouse as our model system, we examined, by Western blot analysis, levels of active caspase 3 and its association with the degradation of uterine contractile proteins during pregnancy. Our data demonstrate that caspase 3-specific cleavage fragments of uterine myocyte contractile proteins are elevated in late gestation. Prior to the onset of labor, active caspase 3 levels and fragmentation of the uterine myocyte contractile proteins decline. We postulate that uterine caspase 3 acts as an anticontractile agent maintaining uterine quiescence through degradation of uterine contractile proteins during late pregnancy. We propose that decreased progesterone action during the final days of pregnancy controls the timing of the onset of uterine contractions by removing the anticontractile action of the apoptotic protein caspase 3 locally in the pregnant myometrium.
Keywords: actin, apoptosis, caspase 3, myometrium, parturition, pregnancy, progesterone, uterus
Introduction
In preparation for the onset of labor, the pregnant uterus undergoes a remarkable transition, from a state of relative quiescence to that of an active contractile unit. Priming of the pregnant uterus for contraction is associated with increased oxytocin receptors [1, 2], increased gap junctions, and cervical ripening [3]. In most mammalian species, but not the human, a precipitous decline in circulating levels of progesterone (P4) heralds the onset of labor [4]. Recent evidence suggests that P4 administration can prevent the onset of premature labor in women with a history of very high-risk pregnancies [5]. In the human and the mouse, a decrease in uterine progesterone receptor (PR) action has also been associated with the onset of labor [6–11]. In women at term, treatment with the PR antagonist, RU-486, caused the onset of spontaneous labor [2]. Taken together, these data indicate that uterine quiescence can be maintained by increased circulating P4 levels and increased PR action in both pregnant women and mice.
To date, the role played by activated caspase 3 in the pregnant uterus is unknown; however, the functional consequences of caspase 3 activation have been well documented in cardiac [12], skeletal [13], and smooth muscle [14]. In these studies, the conversion of pro-caspase 3 to active caspase 3 has consistently been correlated with reduced myocyte contractile ability. The contractile architecture of the cell has been isolated as a target of caspase 3 protease action. Many components of the contractile apparatus, such as α-actin, α-actinin, and myosin heavy and light 1/2 chain, have specific caspase recognition sites that mediate a caspase-regulated proteolysis [12]. Elevated active caspase 3 levels have previously been found in the pregnant mouse [15], rat [16], and human uterus [15]. In elegant studies by Shynlova et al. [16], high caspase 3 levels were demonstrated in the pregnant rat uterus at midgestation, and were observed to decline at term. A similar pattern of active caspase 3 levels was observed in human fetal membranes at mid- and late gestation [17].
In this study, we examined the role that P4 plays in regulating the levels of, and actions mediated by, uterine caspase 3. We sought to identify the consequences of the protease actions of active caspase 3 on components of the uterine contractile architecture, and to determine the molecular mechanisms that regulate active caspase 3 depletion from the pregnant uterus at term.
Materials and Methods
Mouse Studies
All animal studies were approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh. Timed-pregnant female ICR (an outbred Institute for Cancer Research strain) mice (8 wk old) were obtained from the Jackson Laboratory; 50% of the mice were implanted with 10-mg time-release P4 pellets (Innovative Research of America) on Embryonic Day (E) 11. The P4 pellets were designed to release 1.25 mg P4 each day over an 8-day period. To implant the 8-day, time-release P4 pellet, mice were lightly anesthetized with halothane, and a small incision was made at the nape of the neck before pellet implantation, which was performed using 8 mM trocar supplied by the hormone pellet manufacturer (Innovative Research of America). The incision was sutured after pellet implantation, and all mice tolerated the procedure without complication. Uterine tissues (n = 3 for each gestational time point) were harvested at 1000 h from pregnant mice treated with and without P4 at E12–E19. Control and P4-treated mice that delivered after 1000 h on E19 were termed E19IL, and were killed upon delivery of the first four pups. The P4-treated animals did not display a delay in the onset of labor, as they underwent a decline in circulating P4 levels similar to that seen at term in the pregnant control animals. The P4 treatment was designed so that the 8-day time release of P4 concluded on E18, so that serum P4 would decline and not affect the timing of birth. All animals delivered prior to E19.5 (1600 h). The animal studies were performed in this manner in order to assess the effect of progesterone in vivo on uterine caspase 3 activity without the complications of excessive uterine stretch as a result of delayed labor. The uterine horn was cleared of all embryonic material and maternal decidua. The remaining whole uterine tissue was washed in 1× PBS and flash frozen for subsequent protein and mRNA analysis.
Radioimmunoassay
Circulating serum samples of maternal progesterone during pregnancy were measured by collecting blood from control (n = 3) and P4-treated (n = 3) pregnant mice at E18–E19 in labor (19IL). Radioimmunoassay (RIA) was performed by incubating serum samples in anti-analyte antibody-coated tubes in the presence of 125I-labeled progesterone according to the Coat-A-Count assay instructions provided by the manufacturer (Diagnostic Products Corp.). The tubes were incubated at room temperature for 3 h, the supernatants decanted, and the remaining radioactivity in each tube counted for 1 min. The limit of the sensitivity was 0.02 ng/ml for P4 measurements. The intraassay coefficient of variation was 6.4%.
First-Strand cDNA Synthesis and Quantitative PCR
Frozen uterine tissue specimens (n = 3 for each gestational time point) were pulverized and homogenized in Trizol (Invitrogen, Carlsbad, CA). Total RNA was isolated and reverse transcribed to cDNA by a two-step method, followed by quantitative PCR (Q-PCR), as described previously in detail [6]. The ABI PRISM Sequence Detection System 7900HT (Perkin-Elmer, Applied Biosystems, Waltham, MA) was used to monitor the increase of reporter fluorescence following PCR. Reporter signal was normalized to the emission of a passive reference. PCR amplification was carried out using a Sybergreen PCR Master Mix (Applied Biosystems). Primer Express software (Applied Biosystems) was used to design gene-specific primers with a preferable amplicon size of 50–150 bp as follows: mouse smooth muscle α-actin (Acta2) primers (forward, 5′-ggctgttttcccatccatcg-3′; reverse, 5′-cccattccaaccattactccctg-3′), mouse smooth muscle γ-actin (Actg2) primers (forward, 5′-tttaccaccactgctgagaggg-3′; reverse, 5′-tcaaaatccagggcaacatagc-3′), mouse pro-caspase 3 primers (forward, 5′-tctgactggaaagccgaaactc-3′; reverse, 5′-tcccactgtctgtctcaatgccac-3′), and mouse acidic ribosomal phosphoprotein primers (Rplp0) (forward, 5′-acctccttcttccaggcttt-3′; reverse, 5′-cccaccttgtctccagtcttt-3′), which was used as a housekeeping reference gene [18]. We have found Rplp0 levels to be a suitable housekeeping gene for the pregnant mouse uterus, as its expression remains stable across gestation. Each uterine sample (n = 3 for each gestational time point) was assayed in triplicate. ACTA2 and ACTG2 shall hereafter be referred to as mouse smooth muscle α-actin and mouse smooth muscle γ-actin, respectively.
Subcellular Fractionation
Cytosolic extracts were prepared from frozen uterine tissue as described previously [6, 19]. Described briefly here, we pulverized the myometrial tissue in liquid nitrogen, and homogenized in ice-cold buffer containing 10 mM Hepes (pH 7.5), 10 mM MgCl2, 5 mM KCL, and 0.1% Triton X-100. The homogenate was centrifuged at 5000 rpm for 10 min at 4°C, and the supernatant retained as the cytoplasmic fraction.
Immunoblotting
Aliquots of cytoplasmic proteins, quantified by colorimetric BCA Protein Assay (Promega, Madison, WI) were separated in gradient polyacrylamide gels (Invitrogen) and transferred onto Hybond-P (Amersham Pharmacia, Piscataway, NJ). Blots were probed using primary antibodies against calponin (1:250 dilution; EP798Y; Novus Biologicals, Littleton, CO), anti-caspase 3 cleaved form (1:1000 dilution; AB3623; Chemicon, Billerica, MA), smooth muscle α-actin (1:1000 dilution; CBL171; Chemicon), and smooth muscle γ-actin (1:1000 dilution; ACTG2; Novus Biologicals). Smooth muscle γ-actin is a specific actin found only in smooth muscle. We utilized smooth muscle γ-actin in this study to identify caspase 3 action in the smooth muscle compartment of the uterus.
Caspase 3 cleavage of both smooth muscle α- and γ-actin results in the release of a 17-kDa and a 25-kDa fragment as a result of a predicted caspase 3-specific consensus site, DXXD [20], found between 156 amino acid (aa) and 158 aa in both smooth muscle α- and γ-actin. Antibodies raised against the N terminus of both smooth muscle α- and γ-actin identify the 17-kDa fragment and the full-length 42-kDa parent actin. Primary antibodies were diluted in 5% milk-1× TBS-T buffer, followed by incubation with horseradish peroxidase-conjugated secondary antibodies diluted in 5% milk-1× TBS-T buffer. Immunoreactive bands were visualized using an ECL detection system (Amersham Pharmacia).
Determination of Putative P4 Response Elements on the Caspase 3 Promoter
The sequence for mouse caspase 3 gene was downloaded from NCBI (NC_000074.5 reference assembly [C57BL/6J]); 2 kb of sequence 5′ of the 5′ untranslated region was added in Genbank prior to download of the genbank file and searched against known nucleic acid subsequences for P4 response elements (PREs) [21] using Macvector (Cary, NC). This search was also used for human caspase 3 using reference assembly NC_000004.10.
Determination of Caspase 3 Activity in the Pregnant Mouse Uterus
Uterine samples were collected from three different gestational series of pregnant mice, ranging from E12 to E19, with or without progesterone treatment. Uterine cell lysates were extracted and protein concentration was measured by BCA protein kit (Pierce, Rockford, IL). Caspase 3 activity was detected using caspase 3 colorimetric detection kit (Assay Designs, Ann Arbor, MI) by measuring the absorbance of free p-nitroaniline (pNA) generated by cleavage of Ac-DEVD-pNA as a colorimetric substrate according to the manufacturer's instructions. Briefly, 4 μg of protein for each uterine cell lysate in triplicate in a 96-well plate were incubated with 75 μl of caspase 3 substrate for 3 h at 37°C. Then, the caspase 3 activity was measured by spectrophotometer (Wallac Victor, Perkin-Elmer) at 405 nm absorbance. Activity was expressed as U/μg cytoplasmic protein. One unit of caspase 3 activity corresponds to the amount of enzyme needed to convert one picomole of substrate per minute at 37°C.
Statistical Analysis
NIH Image quant was used to identify immunoreactive protein band intensity from our Western blot analysis. The relative optical density was obtained by normalizing immunoreactive protein band intensity of active caspase 3 and smooth muscle α- and γ-actin to calponin. Amplified mRNAs identified by Q-PCR were normalized to a housekeeping gene, acidic ribosomal phosphoprotein (Rplp0) [17]. Statistical analysis was performed in Excel 4.0 (Microsoft, Seattle, WA) to identify the mean and SEM. Statistical significance was verified using two-tailed Student t-tests in Excel 4.0. All data are representative of at least three individual experiments performed in triplicate.
Results
Activated Caspase 3 Levels in the Pregnant Mouse Uterus
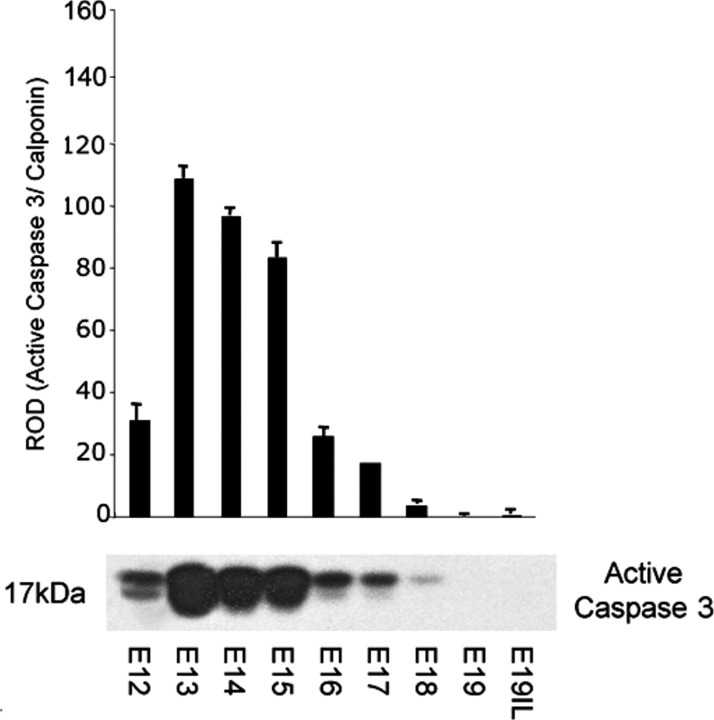
Cytoplasmic extracts were isolated from the pregnant uteri of mice from Day 12 of pregnancy (E12) to E19, and uteri from mice in labor (E19IL). Western blots were performed using an antibody that recognizes only the cleaved active form of caspase 3 at 17 kDa. As shown in Figure 1, abundant active caspase 3 was observed in the pregnant mouse uterus from E12 to E17, with the highest levels of active caspase 3 detectable on E13, with an observed decline at E15. As term approached E19, active caspase 3 levels declined to undetectable levels.
Fig. 1.
The effect of gestational age on the presence of active caspase 3 proteins in the pregnant mouse uterus. A representative Western blot of active caspase 3 is shown. Active caspase 3 levels were normalized to calponin. Data are presented as mean ± SEM. ROD, relative optical density.
Caspase 3 Cleavage of Smooth Muscle α- and γ-Actin in the Pregnant Mouse Uterus
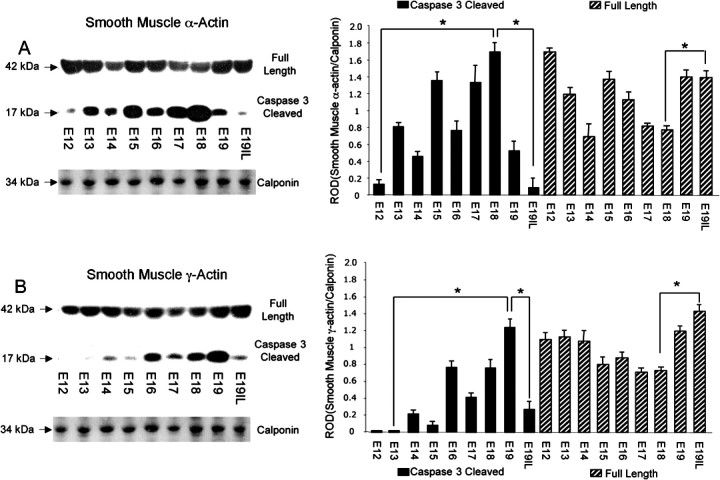
Western blot analysis was performed on cytoplasmic extracts isolated from uteri of pregnant mice from E13 to E19IL using antibodies that detect both full-length and caspase 3-specific cleavage fragments of smooth muscle α- and γ-actin (n = 3–4 for each gestational time point). As can be seen in Figure 2, A and B, both smooth muscle α- and γ-actin displayed elevated levels of caspase 3-specific 17 kDa cleaved fragments, resulting in decreased levels of full-length parent actins at E18. With the onset of labor (E19IL), smooth muscle α- and γ-actin caspase 3-cleaved products were clearly diminished, and restoration of the levels of full-length parent actins occurred. An approximate 2-fold increase between E18 and E19IL of the full-length smooth muscle α- and γ-actins was also observed (P < 0.005). We have highlighted the observed 13-fold increase between E12 and E18 and the 20-fold decrease between E18 and E19IL in smooth muscle α-actin-cleaved fragments (Fig. 2A). We have also highlighted the 70-fold increase in caspase 3-cleaved smooth muscle γ-actin between E13 and E19, and the 5-fold decrease observed between E19 and E19IL (Fig. 2B).
Fig. 2.
The effect of gestational age on the levels of full-length versus caspase 3-cleaved (A) smooth muscle α-actin and (B) smooth muscle γ-actin in the pregnant mouse uterus. A representative Western blot of the full-length smooth muscle α- and γ-actins and their caspase 3-cleaved fragments are shown. Smooth muscle α- and γ-actin full length and caspase 3-cleaved levels were normalized to calponin. Data are presented as mean ± SEM. *ROD for cleaved and full-length smooth muscle α- and γ-actin is significantly different (P < 0.005).
Smooth Muscle α- and γ-Actin mRNA Levels Are Not Elevated at Term
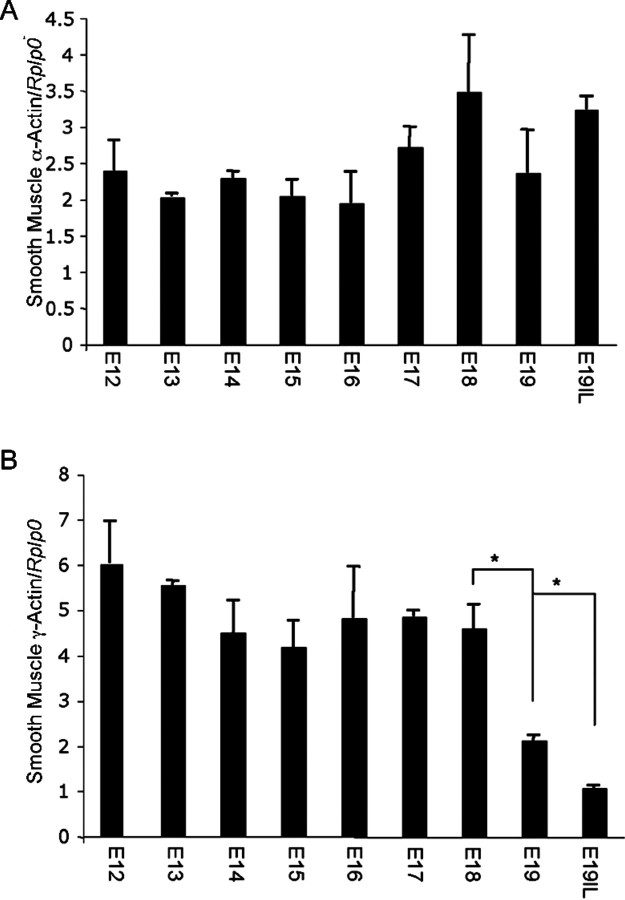
Messenger RNA from uteri of three different gestational series of mice was analyzed for uterine smooth muscle α- and γ-actin using oligonucleotides that primed with equal efficiency. Real-time Q-PCR analysis did not show any significant changes in uterine smooth muscle α-actin mRNA levels from E12 to E19IL; however, smooth muscle γ-actin mRNA levels decreased at term (Fig. 3, A and B). These data demonstrate that smooth muscle α- and γ-actin mRNA levels are not elevated at term, and suggest that increases observed in smooth muscle α- and γ-actin proteins at term may, in part, be a result of posttranslational modification. Levels of smooth muscle α- and γ-actin mRNA were normalized to Rplp0.
Fig. 3.
The effect of gestational age on the mRNA expression levels of (A) smooth muscle α-actin and (B) smooth muscle γ-actin in the pregnant mouse uterus. Data are presented as mean ± SEM. *Significantly different (P < 0.005).
Exogenous P4 Treatment Elevates Active Caspase 3 Levels in the Pregnant Mouse Uterus Across Gestation
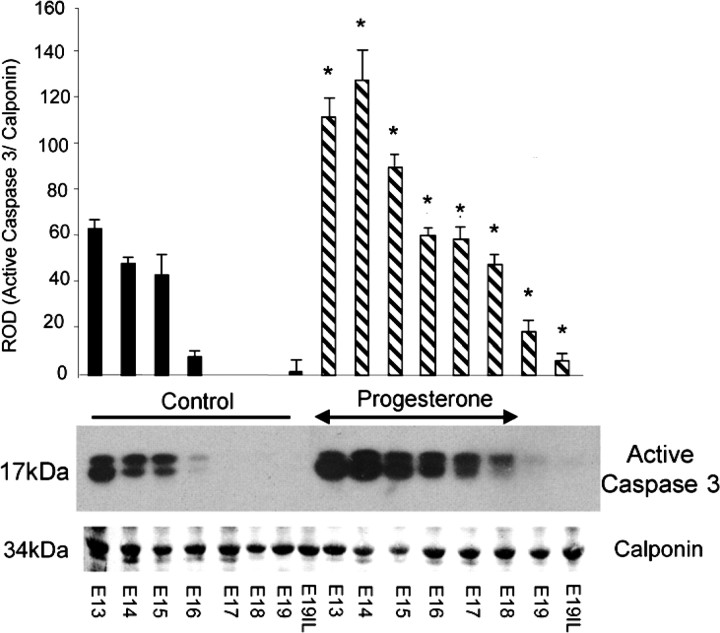
Elevated levels of active caspase 3 were observed in P4-treated mice compared with untreated mice, as determined by Western blot analysis of cytoplasmic extracts of uterine tissue isolated from pregnant mice across gestation, treated with (E11–E18) and without P4 (Fig. 4). A 2-fold elevation in active uterine caspase 3 levels was observed in P4-treated mice as compared with untreated mice at E13–E15. A 6-fold induction of active caspase 3 levels was observed on E16 in the P4-treated mice as compared with controls. Prolonged periods of elevated active caspase 3 levels were observed at E17–E19 in the P4-treated mice in contrast to the untreated animals, where caspase 3 levels declined to undetectable levels. Elevated serum P4 levels at E18, followed by a decline at E19–E19IL in the P4-treated animals, were confirmed by RIA. Control mice displayed P4 levels of 25 ± 4.2 ng/ml at E18, 10.3 ± 1.4 ng/ml at E19, and 8 ± 3.2 ng/ml at E19IL. P4-treated mice displayed P4 levels at 101.3 ± 20 ng/ml at E18. However, by E19, P4 levels had fallen to 10.4 ± 2.3 ng/ml, and, at E19IL, P4 levels had further declined to 4.3 ± 0.8 ng/ml. The observed decline in circulating progesterone levels in the progesterone-treated animals at E19 and E19IL was expected, as our P4 pellet was designed to release hormone for 8 days in order to permit a decline in circulating P4 levels after E18 similar to that seen at term in the pregnant control animals. We executed our animal studies in this manner to determine the in vivo effects of elevated P4 on caspase 3 activity and expression, independent of excessive stretch associated with a P4-mediated delay in the onset of labor, which may modify or interfere with uterine caspase 3 signaling.
Fig. 4.
The effect of exogenous progesterone treatment (which was halted on E18, as indicated by arrowheads) on the gestational profile of active caspase 3 proteins in the pregnant mouse uterus is shown by representative Western blots. Active caspase 3 levels were normalized to calponin. Data are presented as mean ± SEM (n = 3–4 at each time point). *Progesterone-treated samples significantly different from control samples (P < 0.05).
P4 Treatment Elevated Caspase 3 Activity in the Pregnant Mouse Uterus
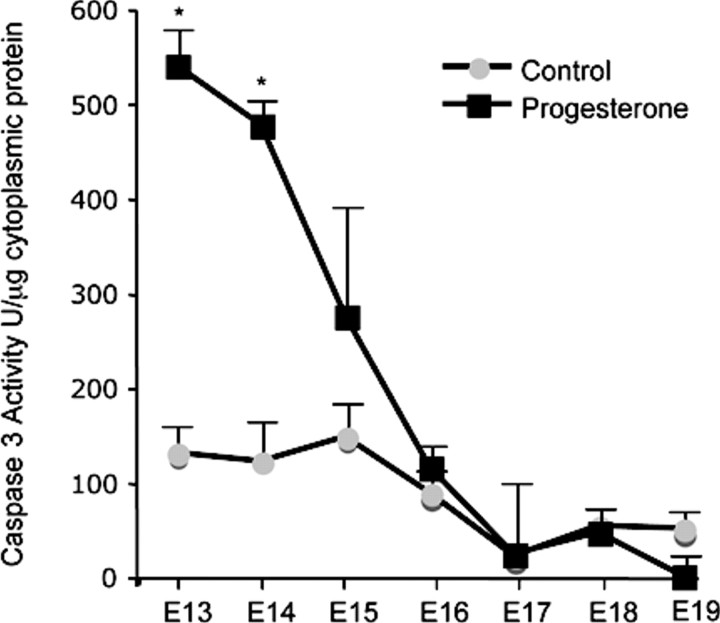
The effect of exogenous P4 treatment on the gestational profile of caspase 3 activity in the pregnant mouse uterus is shown in Figure 5. Cytosolic preparations of mouse uterus isolated from a gestational series of pregnant mice (control and P4 treated) were evaluated for caspase 3 activity by colorimetric analysis. P4 treatment increased caspase 3 activity levels over 5-fold on E13 and 4-fold on E14. However, similar to the pro-caspase mRNA data, caspase 3 activity levels declined toward term.
Fig. 5.
The effect of exogenous progesterone treatment on the gestational profile of uterine caspase 3 activity is shown. Caspase 3 activity was normalized to protein concentration. Data are presented as mean ± SEM. *Progesterone-treated samples significantly different from control samples (P < 0.05).
Elevated Active Uterine Caspase 3 Levels in the P4-Treated Mice Results in Increased Caspase 3 Cleavage of Smooth Muscle α- and γ-Actins
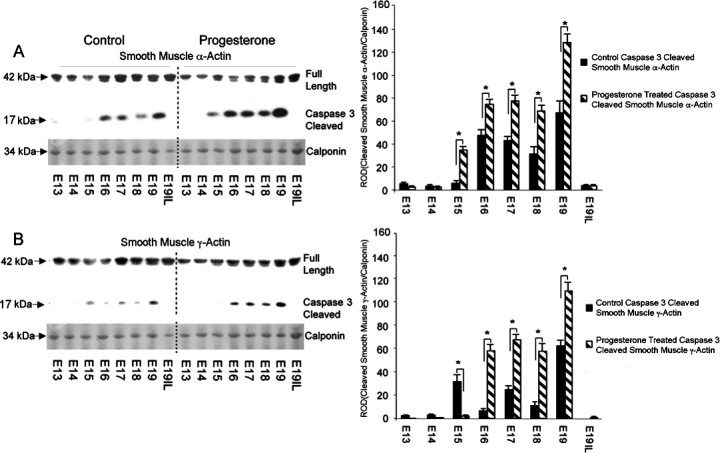
Western blot analysis of uterine tissue from pregnant mice treated with and without P4 was performed using N-terminal antibodies that detect both the full-length and caspase 3-specific smooth muscle α- and γ-actin 17-kDa cleavage products that result from exposure to active caspase 3. Figure 6A demonstrates elevated levels of smooth muscle α- and γ-actin caspase 3-specific cleavage fragments in the P4-treated animals as compared with the control animals. Figure 6B represents the appearance of caspase 3 cleaved smooth muscle α- and γ-actin fragments in the P4-treated animals as compared to the control animals. Elevation in the appearance of caspase 3 cleaved smooth muscle α-actin fragments was observed at E15–19 in the P4-treated animals as compared to control animals, ranging from 4-fold on E15, 1.5-fold on E16, and 2-fold on E17–E19. Increased levels of cleaved smooth muscle γ-actin fragments were also observed in P4-treated animals as compared with control animals, ranging from a 7-fold increase in E16 to 2-fold on E17, a 3-fold increase on E18, and a 1.5-fold increase on E19. In each set of animals tested, as observed in Figure 6A, cleavage of the actin protein was abruptly halted before the onset of labor.
Fig. 6.
The effect of exogenous progesterone treatment on the gestational profile of full-length versus caspase 3-cleaved (A) smooth muscle α-actin and (B) smooth muscle γ-actin in the pregnant mouse uterus. Representative Western blots of the full length smooth muscle α- and γ-actins and their caspase 3-cleaved fragments are shown. The levels of smooth muscle α- and γ-actin were normalized to calponin. Data are presented as mean ± SD ROD for smooth muscle α- and γ-actin caspase 3-cleaved fragments. *Control samples significantly different from progesterone-treated samples (P < 0.05).
Pro-Caspase 3 Promoter Contains PREs
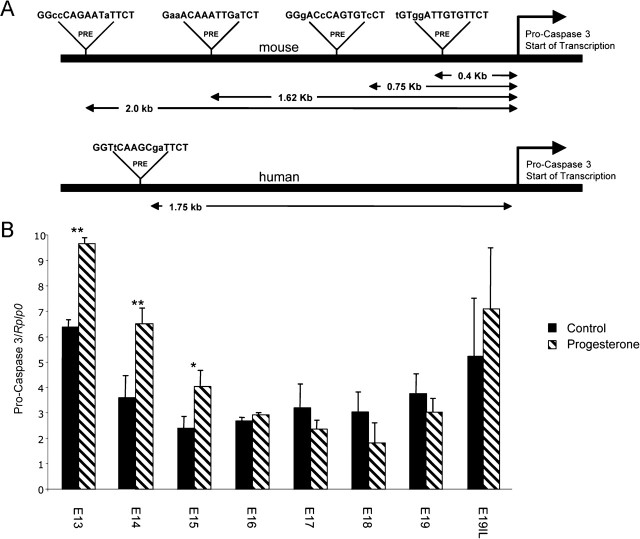
To further characterize the mechanisms controlling uterine caspase 3, we analyzed the pro-caspase 3 promoter for PREs. Four putative PREs were observed on the mouse pro-caspase 3 promoter at 2.0, 1.62, 0.75, and 0.4 kb upstream of the pro-caspase 3 start site of transcription. One putative PRE was observed in the human pro-caspase 3 promoter 1.75 kb upstream of the pro-caspase 3 start site of transcription (Fig. 7A).
Fig. 7.
A) Analysis of the pro-caspase 3 promoter identified four putative PREs and one putative PRE in the mouse and human, respectively. B) The effect of exogenous progesterone treatment on the gestational profile of pro-caspase 3 mRNA in the pregnant mouse uterus is shown. Data are presented as mean ± SEM. Data labeled with asterisks are significantly different (**P < 0.005; *P < 0.025).
Exogenous P4 Treatment Across Gestation Elevated mRNA Levels of Pro-Caspase 3 in the Pregnant Mouse Uterus
Messenger RNA from uteri of three different gestational series of mice was analyzed for the precursor of caspase 3, pro-caspase 3, using oligonucleotides that primed with equal efficiency. Q-PCR analysis demonstrated significant increases in uterine pro-caspase 3 mRNA levels from E13 to E15 (Fig. 7B) in response to elevated P4 levels.
Discussion
This study is consistent with the hypothesis that the active form of caspase 3 is regulated by circulating levels of P4, and may play a role in the cleavage of the smooth muscle α- and γ-actin components of the mouse uterine contractile architecture during late pregnancy. We propose that elevated levels of activated caspase 3 (Fig. 1), which are present in the pregnant mouse uterus from E12 to E18, result in increased levels of both smooth muscle α- and γ-actin cleavage. At term, and with the onset of labor, cleavage products of the actin proteins recede, and there is restoration of the full-length parent actins (Fig. 2). We propose that disruption of the uterine contractile apparatus may dampen the contractile ability of the nonlaboring uterus. Our data also predict that active caspase 3 would render the uterine myocyte less likely to contract at earlier gestational time points. Our study identified that circulating levels of P4 during pregnancy regulate the levels of activated caspase 3 found in the gravid uterus (Figs. 4 and 5). Accompanying the P4-mediated increase in active uterine caspase 3, we also observed an increase in the levels of smooth muscle α- and γ-actin fragmentation (Fig. 6). These data suggest a possible mechanism by which exogenous P4 delays the onset of labor in the mouse [4] and human [5]. Elevated levels of P4 may maintain components of the uterine contractile architecture in a prolonged degraded state through increased active caspase 3, thereby reducing the contractile potential of the uterine myocyte. Analysis of the pro-caspase 3 promoter identified putative PREs present in the mouse and human (Fig. 7A). However, our mRNA data demonstrate increased pro-caspase 3 levels in response to P4 isolated to the earlier gestational time points, E13–E15 (Fig. 7). This suggests that the precursor to active caspase 3 levels may be a limiting factor, and may indicate why active caspase 3 levels, although elevated in the presence of P4, continue to decline toward term (Figs. 4 and 5).
Recent studies have demonstrated that the contractile state of cardiac, skeletal, and smooth muscle types is controlled by caspase 3 activity [12–14]. A function of caspase 3 is to induce the breakdown of myofibrillar proteins, such as α-actin, α-actinin, tropomyosin c, resulting in impaired force and contractile dysfunction [12]. Cleavage of the actin family members by caspase 3 is a result of a conserved caspase 3-specific cleavage site [20]. Previous studies demonstrate that the contractile force of laboring human myometrium at term is greater than nonlaboring myometrium at term [22], which is, in turn, greater than that of nonpregnant myometrium [23]. Similar studies in the rat also document increased contractile potential between laboring and nonlaboring myometrial samples [24], and between nonpregnant and pregnant term uteri [25]. One report found no differences observed in the contractile potential between nonpregnant and pregnant myometrium [26].
The appearance of caspase 3-specific cleaved smooth muscle α- and γ-actins intensifies at E18–E19 (Figs. 2 and 6). We hypothesize that this represents an accumulation of actin degradation products that result from the elevated levels of caspase 3 activity present at earlier gestational time points. These findings are consistent with previous studies on caspase 3-mediated cleavage of α-spectrin (another caspase 3 target gene), where caspase 3 cleavage products were not seen until later time points [27, 28]. Citron et al. [29] describe a timeline of events in which pro-caspase 3 expression is induced 1–8 h after injury, and caspase 3 activity is evident within 24 h. Seventy-two hours after the appearance of caspase 3 activity cleavage products of caspase 3 substrates begin to appear. The disappearance of the caspase 3-specific cleaved smooth muscle α- and γ-actins and restoration of the full-length smooth muscle α- and γ-actins at term (Figs. 2 and 6) suggest a possible mechanism of actin reassembly. We suggest that restoration of the full-length actins in the pregnant uterus on the day of labor may be indicative of a reorganized, intact contractile architecture, and signals the onset of increased uterine responsiveness. The mechanism whereby smooth muscle α- and γ-actin levels reconstitute in the pregnant uterus at term is currently unknown; however, our RNA data clearly demonstrate no significant changes in smooth muscle α-actin mRNA levels across gestation, and a decline in smooth muscle γ-actin mRNA levels on the day of labor (Fig. 3). These data correlate with the RNA data previously reported in the pregnant rat uterus [30]. Reorganization of the γ-actin network has previously been described in the pregnant rat uterus. Cytoplasmic γ-actin transforms into cortical staining during the final days of pregnancy, and reverts back to cytoplasmic staining at term [30]. This phenomenon has been described by other investigators to be a result of the breakdown of actin stress fibers in the cell [31]. Reorganization of the actin network under the plasma membrane occurs upon cleavage of the actin contractile apparatus [32].
Our data suggest that the effects of activated caspase 3 in the pregnant uterus may not be permanent. Indeed, the reversibility of the anticontractile caspase 3 effects has clearly been demonstrated in the rabbit and rat models of heart failure, where blocking caspase activity improved the contractile function in the failing myocardium [33, 34]. Activation of caspase 3 is usually associated with apoptotic episodes [35–37], and should conclude in cell death. However, in the failing heart, it has been shown, surprisingly, that cardiomyocytes displaying cytochrome-c release and caspase 3 activation demonstrate normal nuclei, and remain unaffected by the apoptotic process [38]. In both the pregnant rat uterus and human fetal membranes, high levels of activated caspase 3 in the second trimester of pregnancy were demonstrated, with no evidence of wide-scale apoptosis [16, 17]. We hypothesize that, similarly, in the pregnant mouse uterus, elevated caspase 3 activity is not associated with uterine apoptotic episodes, but acts as a regulator of the uterine contractile architecture by promoting cleavage of the uterine smooth muscle α- and γ-actins during pregnancy. We suggest that elevated progesterone levels during pregnancy may maintain high levels of caspase 3 activity in the pregnant mouse uterus, and that the withdrawal of P4 during the final days of pregnancy may regulate the removal of uterine caspase 3 as term approaches. We propose that decreased levels of uterine caspase 3 activity observed at term permits the reversal of active caspase 3 anticontractile action, resulting in a pregnant uterus that is more susceptible to contraction in response to external and internal stimuli.
Acknowledgment
We would like to thank Professor Anthony Zeleznik for critical reading of this manuscript.
References
- 1. Fuchs AR, Fuchs F, Husslein P, Soloff MS. Oxytocin receptors in the human uterus during pregnancy and parturition. Am J Obstet Gynecol 1984; 150:734–741. [DOI] [PubMed] [Google Scholar]
- 2. Frydman R, Lelaidier C, Baton-Saint-Mleux C, Fernandez H, Vial M, Bourget P. Labor induction in women at term with mifepristone (RU 486): a double-blind, randomized, placebo-controlled study. Obstet Gynecol 1992; 80:972–975. [PubMed] [Google Scholar]
- 3. Garfield RE, Sims SM, Kannan MS, Daniel EE. Possible role of gap junctions in activation of myometrium during parturition. Am J Physiol 1978; 235:C168–C179. [DOI] [PubMed] [Google Scholar]
- 4. Challis JRG, Matthews SG, Gibb W, Lye SJ. Endocrine and paracrine regulation of birth at term and preterm. Endocr Rev 2000; 21:514–550. [DOI] [PubMed] [Google Scholar]
- 5. Meis PJ. 17 Hydroxyprogesterone for the prevention of preterm delivery. Obstet Gynecol 2005; 105:1128–1135. [DOI] [PubMed] [Google Scholar]
- 6. Condon JC, Hardy DB, Kovaric K, Mendelson CR. Up-regulation of the progesterone receptor (PR)-C isoform in laboring myometrium by activation of nuclear factor-kappaB may contribute to the onset of labor through inhibition of PR function. Mol Endocrinol 2006; 20:764–775. [DOI] [PubMed] [Google Scholar]
- 7. Condon JC, Jeyasuria P, Faust JM, Wilson JW, Mendelson CR. A decline in the levels of progesterone receptor coactivators in the pregnant uterus at term may antagonize progesterone receptor function and contribute to the initiation of parturition. Proc Natl Acad Sci U S A 2003; 100:9518–9523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mesiano S, Chan EC, Fitter JT, Kwek K, Yeo G, Smith R. Progesterone withdrawal and estrogen activation in human parturition are coordinated by progesterone receptor A expression in the myometrium. J Clin Endocrinol Metab 2002; 87:2924–2930. [DOI] [PubMed] [Google Scholar]
- 9. Pieber D, Allport VC, Hills F, Johnson M, Bennett PR. Interactions between progesterone receptor isoforms in myometrial cells in human labour. Mol Hum Reprod 2001; 7:875–879. [DOI] [PubMed] [Google Scholar]
- 10. Mahendroo MS, Porter A, Russell DW, Word RA. The parturition defect in steroid 5alpha-reductase type 1 knockout mice is due to impaired cervical ripening. Mol Endocrinol 1999; 13:981–992. [DOI] [PubMed] [Google Scholar]
- 11. Dudley DJ, Branch DW, Edwin SS, Mitchell MD. Induction of preterm birth in mice by RU486. Biol Reprod 1996; 55:992–995. [DOI] [PubMed] [Google Scholar]
- 12. Communal C, Sumandea M, de Tombe P, Narula J, Solaro RJ, Hajjar RJ. Functional consequences of caspase activation in cardiac myocytes. Proc Natl Acad Sci U S A 2002; 99:6252–6256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Supinski GS, Callahan LA. Caspase activation contributes to endotoxin-induced diaphragm weakness. J Appl Physiol 2006; 100:1770–1777. [DOI] [PubMed] [Google Scholar]
- 14. Hong SK, Son H, Kim SW, Oh SJ, Choi H. Effect of glycine on recovery of bladder smooth muscle contractility after acute urinary retention in rats. BJU Int 2005; 96:1403–1408. [DOI] [PubMed] [Google Scholar]
- 15. Joswig A, Gabriel HD, Kibschull M, Winterhager E. Apoptosis in uterine epithelium and decidua in response to implantation: evidence for two different pathways. Reprod Biol Endocrinol 2003; 1:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shynlova O, Oldenhof A, Dorogin A, Xu Q, Mu J, Nashman N, Lye SJ. Myometrial apoptosis: activation of the caspase cascade in the pregnant rat myometrium at midgestation. Biol Reprod 2006; 74:839–849. [DOI] [PubMed] [Google Scholar]
- 17. Runic R, Lockwood CJ, LaChapelle L, Dipasquale B, Demopoulos RI, Kumar A, Guller S. Apoptosis and Fas expression in human fetal membranes. J Clin Endocrinol Metab 1998; 83:660–666. [DOI] [PubMed] [Google Scholar]
- 18. van Wijngaarden P, Brereton HM, Coster DJ, Williams KA. Stability of housekeeping gene expression in the rat retina during exposure to cyclic hyperoxia. Mol Vis 2007; 13:1508–1515. [PubMed] [Google Scholar]
- 19. Blough E, Dineen B, Esser K. Extraction of nuclear proteins from striated muscle tissue. Biotechniques 1999; 26:202–204, 206. [DOI] [PubMed] [Google Scholar]
- 20. Mashima T, Naito M, Tsuruo T. Caspase-mediated cleavage of cytoskeletal actin plays a positive role in the process of morphological apoptosis. Oncogene 1999; 18:2423–2430. [DOI] [PubMed] [Google Scholar]
- 21. Evans RM. The steroid and thyroid hormone receptor superfamily. Science 1988; 240:889–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jie Z, Kendrick A, Quenby S, Wray S. Contractility and calcium signaling of human myometrium are profoundly affected by cholesterol manipulation: implications for labor? Reprod Sci 2007; 14:456–466. [DOI] [PubMed] [Google Scholar]
- 23. Izumi H, Ichihara J, Uchiumi Y, Shirakawa K. Gestational changes in mechanical properties of skinned muscle tissues of human myometrium. Am J Obstet Gynecol 1990; 163:638–647. [DOI] [PubMed] [Google Scholar]
- 24. Izumi H, Byam-Smith M, Garfield RE. Gestational changes in oxytocin- and endothelin-1-induced contractility of pregnant rat myometrium. Eur J Pharmacol 1995; 278:187–194. [DOI] [PubMed] [Google Scholar]
- 25. Kim BK, Ozaki H, Hori M, Takahashi K, Karaki H. Increased contractility of rat uterine smooth muscle at the end of pregnancy. Comp Biochem Physiol A Mol Integr Physiol 1998; 121:165–173. [DOI] [PubMed] [Google Scholar]
- 26. Word RA, Stull JT, Casey ML, Kamm KE. Contractile elements and myosin light chain phosphorylation in myometrial tissue from nonpregnant and pregnant women. J Clin Invest 1993; 92:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stone JR, Okonkwo DO, Singleton RH, Mutlu LK, Helm GA, Povlishock JT. Caspase 3-mediated cleavage of amyloid precursor protein and formation of amyloid Beta peptide in traumatic axonal injury. J Neurotrauma 2002; 19:601–614. [DOI] [PubMed] [Google Scholar]
- 28. Buki A, Okonkwo DO, Wang KK, Povlishock JT. Cytochrome c release and caspase activation in traumatic axonal injury. J Neurosci 2000; 20:2825–2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Citron BA, Arnold PM, Sebastian C, Qin F, Malladi S, Ameenuddin S, Landis ME, Festoff BW. Rapid upregulation of caspase 3 in rat spinal cord after injury: mRNA, protein, and cellular localization correlates with apoptotic cell death. Exp Neurol 2000; 166:213–226. [DOI] [PubMed] [Google Scholar]
- 30. Shynlova O, Tsui P, Dorogin A, Chow M, Lye SJ. Expression and localization of alpha-smooth muscle and gamma-actins in the pregnant rat myometrium. Biol Reprod 2005; 73:773–780. [DOI] [PubMed] [Google Scholar]
- 31. van de Water B, Tijdens IB, Verbrugge A, Huigsloot M, Dihal AA, Stevens JL, Jaken S, Mulder GJ. Cleavage of the actin-capping protein alpha-adducin at Asp-Asp-Ser-Asp633-Ala by caspase 3 is preceded by its phosphorylation on serine 726 in cisplatin-induced apoptosis of renal epithelial cells. J Biol Chem 2000; 275:25805–25813. [DOI] [PubMed] [Google Scholar]
- 32. Coutant KD, Corvaia N, Ryder NS. Bradykinin induces actin reorganization and enhances cell motility in HaCaT keratinocytes. Biochem Biophys Res Commun 1997; 237:257–261. [DOI] [PubMed] [Google Scholar]
- 33. Laugwitz KL, Moretti A, Weig HJ, Gillitzer A, Pinkernell K, Ott T, Pragst I, Stadele C, Seyfarth M, Schomig A, Ungerer M. Blocking caspase-activated apoptosis improves contractility in failing myocardium. Hum Gene Ther 2001; 12:2051–2063. [DOI] [PubMed] [Google Scholar]
- 34. Ruetten H, Badorff C, Ihling C, Zeiher AM, Dimmeler S. Inhibition of caspase 3 improves contractile recovery of stunned myocardium, independent of apoptosis-inhibitory effects. J Am Coll Cardiol 2001; 38:2063–2070. [DOI] [PubMed] [Google Scholar]
- 35. Lauber K, Bohn E, Krober SM, Xiao YJ, Blumenthal SG, Lindemann RK, Marini P, Wiedig C, Zobywalski A, Baksh S, Xu Y, Autenrieth IB et al. Apoptotic cells induce migration of phagocytes via caspase 3-mediated release of a lipid attraction signal. Cell 2003; 113:717–730. [DOI] [PubMed] [Google Scholar]
- 36. Lauber K, Blumenthal SG, Waibel M, Wesselborg S. Clearance of apoptotic cells: getting rid of the corpses. Mol Cell 2004; 14:277–287. [DOI] [PubMed] [Google Scholar]
- 37. Cohen GM. Caspases: the executioners of apoptosis. Biochem J 1997; 326(pt 1):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Narula J, Haider N, Arbustini E, Chandrashekhar Y. Mechanisms of disease: apoptosis in heart failure—seeing hope in death. Nat Clin Pract Cardiovasc Med 2006; 3:681–688. [DOI] [PubMed] [Google Scholar]