Abstract
Patient: Female, 32
Final Diagnosis: Progressive multifocal leukoencephalopathy
Symptoms: Progressive behavioral changes • seizures
Medication: —
Clinical Procedure: Management
Specialty: Neurology
Objective:
Rare disease
Background:
Progressive multifocal leukoencephalopathy (PML) is a serious opportunistic infectious disease with high morbidity and mortality. Its incidence in multiple sclerosis (MS) patients has risen since the introduction of disease modifying drugs. In the absence of a specific treatment, the outcome depends heavily on early diagnosis, which illustrates the importance of the role of characteristic brain magnetic resonance imaging (MRI). However, when relying mainly on MRI, the diagnosis of cases with atypical radiological changes may be missed or delayed.
Case Report:
A 32-year-old female diagnosed with elapsing remitting MS in 2009 was started on interferon-beta-1b that was escalated to natalizumab due to progression of the disease. Later, she was shifted to fingolimod as testing for John Cunningham polyoma virus (JCV) antibodies was positive. Three years later, she presented with a 3-week history of progressive walking impairment associated with twitching of her facial muscles and abnormal sensation all over her body that was associated with left hemi-paresis and sensory changes, in addition to truncal ataxia, which was treated with steroids as a relapse of MS. However, the patient continued to deteriorate and developed significant cognitive and behavioral changes. In view of this clinical picture, the diagnosis of PML was raised in spite of her atypical brain MRI features. Treatment with fingolimod was stopped and a sample of her cerebrospinal fluid was sent for JCV DNA analysis, which came back positive at 11 copies/mL. Treatment with mirtazepine and mefloquine was started, but the patient deteriorated further, and MRI showed severe changes consistent with immune reconstitution inflammatory syndrome. Intravenous steroids and intravenous immunoglobulin were given, and within a few weeks, the patient was stabilized and started to gradually improve.
Conclusions:
In patients at risk for developing PML who present with typical clinical features, testing for JCV DNA is recommended even in the absence of typical radiological findings in order to prevent any delay in the diagnosis.
MeSH Keywords: JC Virus; Leukoencephalopathy, Progressive Multifocal; Magnetic Resonance Imaging; Multiple Sclerosis
Background
Progressive multifocal leukoencephalopathy (PML) is a potentially devastating and fatal demyelinating infectious disease caused by reactivation of the opportunistic John Cunningham polyoma virus (JCV) in immune compromised patients [1]. Recently, many multiple sclerosis (MS) disease modifying drugs (DMDs) have been implicated, including natalizumab and to a lesser degree dimethyl fumarate and fingolimod, while alemtuzumab, mitoxantrone, rituximab, and teriflunomide are also considered to have a potential risk for causing PML [2]. The risk associated with new DMDs, such as ocrelizumab, is still unknown.
PML usually manifests with typical clinical and radiological features [3–5]. Diagnosis is confirmed either by brain biopsy with characteristic neuropathological features or by detecting JCV DNA in cerebrospinal fluid (CSF) [6,7]. However, atypical clinical or radiological features have been frequently reported in the literature, especially in the early stages of PML [8–13]. In such instances, the diagnosis will most likely be delayed unless a high level of suspicion exists. This case report provides illustrates the difficulties encountered in reaching the diagnosis of PML in the absence of characteristic magnetic resonance imaging (MRI) changes.
Case Report
A 32-year-old female diagnosed with relapsing remitting MS in 2009 was started on interferon-beta-1b, and then escalated to natalizumab in December 2010 due to ongoing MS disease activity in terms of relapses and increasing MRI lesion load. In June 2012, she was shifted to fingolimod treatment after moving to a remote area where natalizumab was not available and her serum JCV antibodies were positive with an index of 0.57. During the next 3 years, she was generally stable on regular follow-up visits. In June 2015, her expanded disability status scale (EDSS) was 1.5 due to mild sensory symptoms and bladder dysfunction. Brain MRI showed no new lesions and her lymphocytes count was 700 cells/mL.
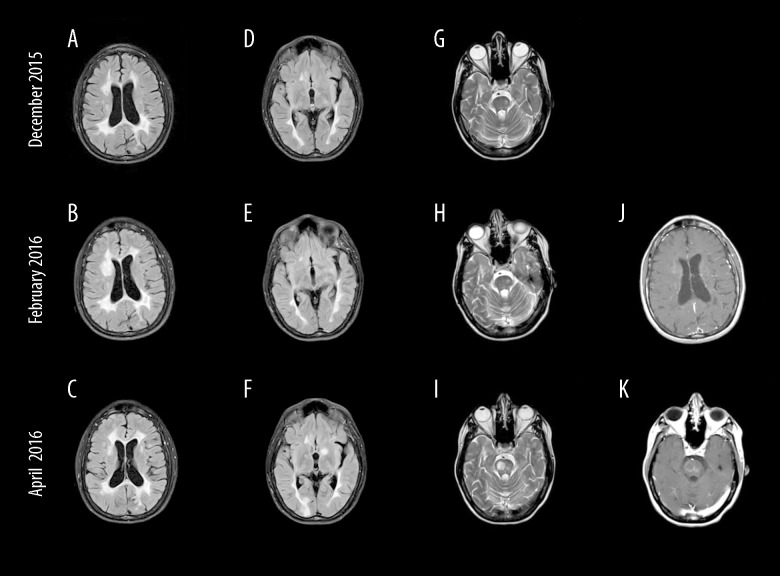
In November 2015, she presented with a 3-week history of progressive walking impairment, associated with twitching of her facial muscles and abnormal sensation all over her body. On examination, she had mild left hemi-paresis and hemi-sensory changes, mild truncal ataxia and bilaterally exaggerated deep tendon reflexes. Brain MRI showed minimal new changes (Figure 1A, 1D, 1G), and her lymphocyte count was 400 cells/mL. Assuming that she sustained a relapse, 5 sessions of 1 g intravenous (IV) methylprednisolone (MP) were given. However, on follow-up visit a month later her walking was found to be worse. In addition, she had a generalized tonic clonic seizure and was started on carbamazepine. Her husband reported that she became more irritable, having difficulty with sleep, was forgetful with less comprehensible speech. She was losing her temper easily with her 8-year-old daughter and became less tolerant, which was contrary to her caring and calm nature. On examination, the patient exhibited obvious cognitive impairment with short attention span and significant working memory deficit. Her mini-mental state examination (MMSE) score was 25. She also had mild motor dysphasia and dysarthria, severe asymmetric bilateral pyramidal weakness of 4/5 on the MRC scale, worse in the left side with truncal ataxia and an EDSS of 4.5.
Figure 1.
Serial magnetic resonance imaging of the brain at different times (2 months apart) during the course of the disease. (A–F) Fluid attenuation inversion recovery images; (G–I) T2-weighted spin-echo images; and (J, K) contrast-enhanced T1-weighted spin-echo images. Mass-like lesion developed in the right frontal periventricular white matter (B) and disappeared 2 months later (C), and showed only faint enhancement (J). On follow-up examination, 3 lesions had developed: 1 in the left basal ganglia (F) and 2 in the pons (I). The right pontine lesion showed only a peripheral enhancement, while the smaller left pontine lesion showed solid faint enhancement (K).
In view of this clinical presentation with subacute progressive focal neurological deficits along with her history of a seizure and current significant behavioral and cognitive changes, the diagnosis of PML was considered and fingolimod was stopped in January 2016. In spite of the opinion of neuroradiology colleagues on the non-supportive MRI changes, CSF was sent in February 2016 for JCV DNA analysis (Unilabs, Copenhagen, Denmark). PCR for JCV was positive with 11 copies/mL. On confirmation of PML diagnosis, mirtazepine 30 mg twice daily and mefloquine 250 mg once weekly were started. There was no significant clinical improvement over the next few weeks, and, almost 5 weeks after stopping fingolimod, she was read-mitted with acute hemiparesis, unable to walk unaided with an EDSS of 6.5. Brain MRI revealed numerous, even mass-like new enhancing lesions, consistent with a severe MS rebound (Figure 1B, 1E, 1H, 1J). Mefloquine was stopped and mirtazepine reduced to 30 mg at night. She was given 2 consecutive courses of 5 sessions of 1 g IV MP followed 2 weeks later by a course of 30 g daily for 5 days of IV immunoglobulins (IVIG). However, her condition deteriorated further, and an MRI in April 2016 showed even more new enhancing lesions sparing cortical areas (Figure 1C, 1F, 1I, 1K), and her lymphocyte count was 1200 cells/mL. In view of these results, immune reconstitution inflammatory syndrome (IRIS) was considered and a further 5 sessions of IV MP were given, followed by another course of IVIG Shortly afterwards her clinical state stabilized, and she started to slowly show some improvement. In August 2016, she had already made significant recovery as she received her first dose of rituximab. She was able to walk unaided with an EDSS of 5. She was followed regularly in the clinic and did not have any relapse afterwards. On her last visit in August 2018, she received her fifth dose of rituximab, and her EDSS was 4.
Discussion
MRI plays an important role in differentiating MS from PML [4,5]. In PML, lesions tend to be subcortical, restricting on diffusion-weighted images (DWI), and show characteristic punctate pattern of enhancement. Periventricular location on the other hand favors MS lesions. In our patient, the new lesions involved the periventricular white matter, basal ganglia, and pons. Such findings were not noted previously in our patient, thus our initial suspicion of PML was strongly questioned. However, similar cases have been reported in the literature where MRI, especially in the early stages, showed a variety of atypical changes including small, non-confluent and enhancing lesions [10–13]. Cases of PML with even minimal brain MRI changes have also been reported [14,15]. In such instances, MRI lesions can easily be misinterpreted as MS-related, therefore delaying the diagnosis and leading to the unwarranted use of high dose steroids with probable detrimental effect [16–18]. It is worth noting that small and enhancing PML lesions have been noted to occur earlier in the disease and associated with a better outcome [11,19].
Although MRI is a main criterion in the diagnosis, it is not considered mandatory [7]. According to the American Academy of Neurology criteria, PML is diagnosed when typical clinical presentation and characteristic MRI findings are associated with a positive JCV PCR in the CSF. However, when either of these features is missing, as it was the case in our present patient, diagnosis of PML can be made, though with a lesser certainty, and is labelled as probable.
PML may present with a multitude of clinical features, but cognitive and behavioral changes associated with focal neurological symptoms such as dysphasia or dysarthria, hemianopsia, and progressive weakness are the commonest and most typical [3]. Our patient conformed well to this picture as she presented with subacute progressive cognitive dysfunction associated with dysarthria and hemiparesis. This non-remitting clinical presentation, progressing over many weeks, was more typical of PML and highly atypical of a MS relapse [20]. Our patient even had seizures, which are uncommon in MS, but well recognized in PML, occurring in up to 18% of cases [21].
Furthermore, our patient was already at risk of developing PML. She had positive JCV serum antibodies and was on fingolimod for more than 3 years after stopping natalizumab. Fingolimod-related PML is rare, and in a recent review, Berger et al. estimated the risk at 0.069 per 1000 [22].
The absence of PML characteristic brain MRI changes, but the typical clinical picture of suspected PML urged verification of diagnosis by CSF analysis for JCV [15]. It is worth noting that JCV PCR is a rapid, reproducible, and easy to perform test [6]. It is highly specific at 95–100%, with a sensitivity of about 70% [23,24]. JCV PCR was positive in our patient, but copy count was low. Although this was explained by the testing laboratory to be due to the low sample volume, Dong-Si et al. associated low copy count with the early stages of the disease, and also with a better prognosis [25].
Prognosis of PML used to be very poor, with mortality reaching 90% in acquired immune deficiency syndrome (AIDS) patients, diminishing to about 50% mortality rate after the introduction of anti-retroviral therapy [26,27]. PML associated with natalizumab is much different, but still associated with significant morbidity with an excess of 20% mortality rate [28]. This relatively better prognosis is related mainly to the ability to restore immune surveillance by withholding the immune suppressing drug. In the absence of specific treatments for PML, early diagnosis is evidently the most important factor in improving the outcome and depends highly on clinical vigilance in high-risk patients. Recommendations on risk minimization of PML by early diagnosis include more frequent serum JCV antibody assessments and brain MRI, and earlier CSF analysis for JCV PCR [15,19,29]. Similar recommendations are expected in cases of PML associated with other drugs including fingolimod.
Our patient was labelled according to American Academy of Neurology (AAN) criteria as probable PML, which is advised to be managed similar to definite PML cases [5]. However, in patients with possible PML, the decision on management is left to the attending neurologist. Our patient received a combination of mirtazepine and mefloquine, though their effectiveness together with few other agents is doubtful [30–34]. Results for maraviroc and interleukin-7 are promising but still inconclusive, and neither were locally available [35–38].
It is worth noting finally, that this may well be a case of PML-IRIS. The patient, after 5 weeks of fingolimod treatment withdrawal underwent severe clinical deterioration associated with excessive new MRI enhancing lesions. In the setting of PML and the rapid normalization of lymphocyte count, these features are consistent with IRIS [39]. Fingolimod-related PML-IRIS is very rare, and most cases are linked to the prior use of natalizumab [40]. To our knowledge, there is only one report of PML-IRIS linked to the use of fingolimod alone [41]. Nishiyama et al. suggested that this rarity may be related to the fact that immune reconstitution occurs relatively slowly after fingolimod withdrawal compared with the rapid immune system recovery after the urgent removal of natalizumab.
Conclusions
In summary, when the diagnosis of PML is suspected in the presence of typical clinical symptoms but is not supported by radiological findings, patients should be given the benefit of the doubt by requesting JCV PCR. Waiting for a characteristic MRI in such instances may result in delaying the diagnosis with probable detrimental effect to the patient.
References:
- 1.Martinez AJ, Sell M, Mitrovics T, et al. The neuropathology and epidemiology of AIDS. A Berlin experience. A review of 200 cases. Pathol Res Pract. 1995;191:427–43. doi: 10.1016/S0344-0338(11)80730-2. [DOI] [PubMed] [Google Scholar]
- 2.Berger JR. Classifying PML risk with disease modifying therapies. Mult Scler Relat Disord. 2017;12:59–63. doi: 10.1016/j.msard.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 3.Dworkin MS. A review of progressive multifocal leukoencephalopathy in persons with and without AIDS. Curr Clin Top Infect Dis. 2002;22:181–95. [PubMed] [Google Scholar]
- 4.Hodel J, Outteryck O, Dubron C, et al. Aymptomatic progressive multifocal leukoencephalopathy associated with natalizumab. Diagnostic precision with MR imaging. Radiology. 2016;278(3):863–72. doi: 10.1148/radiol.2015150673. [DOI] [PubMed] [Google Scholar]
- 5.Wijburg MT, Witte BI, Vennegoor A, et al. MRI criteria differentiating asymptomatic PML from new MS lesions during natalizumab pharmacovigilance. J Neurol Neurosurg Psychiatry. 2016;87:1138–45. doi: 10.1136/jnnp-2016-313772. [DOI] [PubMed] [Google Scholar]
- 6.Hammarin L, Bogdanovic G, Svedhem V, et al. Analysis of PCR as a tool for detection of JC Virus DNA in cerebrospinal fluid for diagnosis of progressive multifocal leukoencephalopathy. J Clinical Microbiol. 1996;34:2929–32. doi: 10.1128/jcm.34.12.2929-2932.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berger JR, Aksamit AJ, Clifford DB, et al. PML diagnostic criteria: Consensus statement from the AAN neuroinfectious disease section. Neurology. 2013;80:1430–38. doi: 10.1212/WNL.0b013e31828c2fa1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carruthers RL, Berger J. Progressive multifocal leukoencephalopathy and JC Virus-related disease in modern neurology practice. Mult Scler Relat Disord. 2014;3:419–30. doi: 10.1016/j.msard.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 9.Dong-Si T, Richman S, Wattjes MP, et al. Outcome and survival of asymptomatic PML in natalizumab-treated MS patients. Ann Clin Transl Neurol. 2014;1:755–64. doi: 10.1002/acn3.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ng S, Tse VCK, Rubinstein J, et al. Progressive multifocal leukoencephalopathy: Unusual MR findings. J Comput Assist Tomogr. 1995;19:302–5. doi: 10.1097/00004728-199503000-00025. [DOI] [PubMed] [Google Scholar]
- 11.Lima MA, Hanto DW, Curry MP, et al. Atypical radiological presentation of progressive multifocal leukoencephalopathy following liver transplantation. J Neurovirol. 2005;11:46–50. doi: 10.1080/13550280590900742. [DOI] [PubMed] [Google Scholar]
- 12.Wattjes MP, Richert ND, Killestein J, et al. The chameleon of neuroinflammation: magnetic resonance imaging characteristics of natalizumab-associated progressive multifocal leukoencephalopathy. Mult Scler. 2013;19:1826–40. doi: 10.1177/1352458513510224. [DOI] [PubMed] [Google Scholar]
- 13.Piola M, Di Palma P, Mascoli N, et al. Atypical MRI features at early onset natalizumab-associated progressive multifocal leukoencephalopathy: A case report. J Neurol Sciences. 2014;340:213–14. doi: 10.1016/j.jns.2014.02.037. [DOI] [PubMed] [Google Scholar]
- 14.Di Giambenedetto S, Vago G, Pompucci A, et al. Fatal inflammatory AIDS-associated PML with high CD4 counts on HAART: A new clinical entity. Neurology. 2004;63:2452–53. doi: 10.1212/01.wnl.0000148585.41802.6c. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Kirby JE, Qian Q. Effective use of JC virus PCR for diagnosis of progressive multifocal leukoencephalopathy. J Med Microbiol. 2009;58:253–55. doi: 10.1099/jmm.0.004432-0. [DOI] [PubMed] [Google Scholar]
- 16.Wenning W, Haghikia A, Laubenberger J, et al. Treatment of progressive multifocal leukoencephalopathy associated with natalizumab. N Engl J Med. 2009;361:1075–80. doi: 10.1056/NEJMoa0810257. [DOI] [PubMed] [Google Scholar]
- 17.Kuhle J, Gosert R, Bühler R, et al. Management and outcome of CSF-JC virus PCR-negative PML in a natalizumab-treated patient with MS. Neurology. 2011;77:2010–16. doi: 10.1212/WNL.0b013e31823b9b27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boster AL, Nicholas JA, Nicholas JA, et al. Lessons learned from fatal progressive multifocal leukoencephalopathy in a patient with multiple sclerosis treated with natalizumab. JAMA Neurol. 2013;70:398–402. doi: 10.1001/jamaneurol.2013.1960. [DOI] [PubMed] [Google Scholar]
- 19.Phan-Ba R, Belachew S, Outteryck O. The earlier, the smaller, the better for natalizumab-associated PML: In MRI vigilance veritas. Neurology. 2012;79:1067–69. doi: 10.1212/WNL.0b013e31826846b4. [DOI] [PubMed] [Google Scholar]
- 20.Boster A, Hreha S, Berger JR, et al. Progressive multifocal leukoencephalopathy and relapsing-remitting multiple sclerosis: A comparative study. Arch Neurol. 2009;66:593–99. doi: 10.1001/archneurol.2009.31. [DOI] [PubMed] [Google Scholar]
- 21.Lima MA, Drislane FW, Koralnik IJ. Seizures and their outcome in progressive multifocal leukoencephalopathy. Neurology. 2006;66:262–64. doi: 10.1212/01.wnl.0000194227.16696.11. [DOI] [PubMed] [Google Scholar]
- 22.Berger JR, Cree BA, Greenberg B, et al. Progressive multifocal leukoencephalopathy after fingolimod treatment. Neurology. 2018;90(20):1815–21. doi: 10.1212/WNL.0000000000005529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bossolasco S, Calori G, Moretti F, et al. Prognostic significance of JC virus DNA levels in cerebrospinal fluid of patients with HIV-associated progressive multifocal leukoencephalopathy. Clin Infect Dis. 2005;40:738–44. doi: 10.1086/427698. [DOI] [PubMed] [Google Scholar]
- 24.Iacobaeus E, Ryschkewitsch C, Gravell M, et al. Analysis of cerebrospinal fluid and cerebrospinal fluid cells from patients with multiple sclerosis for detection of JC virus DNA. Mult Scler. 2009;15:28–35. doi: 10.1177/1352458508096870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dong-Si T, Gheuens S, Gangadharan A, et al. Predictors of survival and functional outcomes in natalizumab-associated progressive multifocal leukoencephalopathy. J NeuroVirology. 2015;21:637–44. doi: 10.1007/s13365-015-0316-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Christensen KL, Holman RC, Hammett TA, et al. Progressive multifocal leukoencephalopathy deaths in the USA, 1979–2005. Neuroepidemiology. 2010;35:178–84. doi: 10.1159/000311014. [DOI] [PubMed] [Google Scholar]
- 27.Lima MA, Bernal-Cano F, Clifford DB, et al. Clinical outcome of long-term survivors of progressive multifocal leukoencephalopathy. J Neurol Neurosurg Psychiatry. 2010;81:1288–91. doi: 10.1136/jnnp.2009.179002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.D’Amico E, Zanghi A, Leone C, et al. Treatment-related progressive multifocal leukoencephalopathy in multiple sclerosis: A comprehensive review of current evidence and future needs. Drug Saf. 2016;39:1163–74. doi: 10.1007/s40264-016-0461-6. [DOI] [PubMed] [Google Scholar]
- 29.EMA Press release. New recommendations to minimise risks of the rare brain infection PML a type of skin cancer with Gilenya. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Press_release/2015/12/WC500199041.pdf.
- 30.Cettomi D, McArthur JC. Mirtazapine use in human immunodeficiency virus-infected patients with progressive multifocal leukoencephalopathy. Arch Neurol. 2009;66:255–58. doi: 10.1001/archneurol.2008.557. [DOI] [PubMed] [Google Scholar]
- 31.Brickelmaier M, Lugovskoy A, Kartikeyan R, et al. Identification and characterization of mefloquine efficacy against JC virus in vitro. Antimicrob Agents Chemother. 2009;53:1840–49. doi: 10.1128/AAC.01614-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clifford DB, Nath A, Cinque P, et al. A study of mefloquine treatment for progressive multifocal leukoencephalopathy: Result and exploration of predictors of PML outcome. J Neurovirol. 2013;19:351–58. doi: 10.1007/s13365-013-0173-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hall CD, Dafni U, Simpson D, et al. Failure of cytarabine in progressive multifocal leukoencephalopathy associated with human immunodeficiency virus infection. AIDS Clinical Trials Group 243 Team. N Engl J Med. 1998;338:1345–51. doi: 10.1056/NEJM199805073381903. [DOI] [PubMed] [Google Scholar]
- 34.De Luca A, Ammassari A, Pezzotti P, et al. Cidofovir in addition to antiretroviral treatment is not effective for AIDS-associated progressive multifocal leukoencephalopathy: A multicohort analysis. AIDS. 2008;22:1759–67. doi: 10.1097/QAD.0b013e32830a5043. [DOI] [PubMed] [Google Scholar]
- 35.Giacomini PS, Rozenberg A, Metz I, et al. Maraviroc and JC virus-associated immune reconstitution inflammatory syndrome. N Engl J Med. 2014;370:486–88. doi: 10.1056/NEJMc1304828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Middel A, Arends JE, van Lelyveld SF, et al. Clinical and immunologic effects of maraviroc in progressive multifocal leukoencephalopathy. Neurology. 2015;85:104–6. doi: 10.1212/WNL.0000000000001713. [DOI] [PubMed] [Google Scholar]
- 37.Bsteh G, Auer M, Iglseder S, et al. Severe early natalizumab-associated PML in MS: Effective control of PML-IRIS with maraviroc. Neurol Neuroimmunol Neuroinflamm. 2017;4(2):e323. doi: 10.1212/NXI.0000000000000323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alstadhaug KB, Croughs T, Henriksen S, et al. Treatment of progressive multifocal leukoencephalopathy with interleukin 7. JAMA Neurol. 2014;71:1030–35. doi: 10.1001/jamaneurol.2014.825. [DOI] [PubMed] [Google Scholar]
- 39.Wattjes MP, Wijburg MT, Vennegoor A, et al. MRI characteristics of early PML-IRIS after natalizumab treatment in patients with MS. J Neurol Neurosurg Psychiatry. 2016;87(8):879–84. doi: 10.1136/jnnp-2015-311411. [DOI] [PubMed] [Google Scholar]
- 40.Yoshi F, Moriya M, Ohnuki T, et al. Neurological safety of fingolimod: An updated review. Clin Exp Neuroimmunol. 2017;8(3):233–42. doi: 10.1111/cen3.12397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nishiyama S, Misu T, Shishido-Hara Y, et al. Fingolimod-associated PML with mild IRIS in MS. A clinicopathological study. Neurology Neuroimmn Neuroinflamm. 2018;5:e415. doi: 10.1212/NXI.0000000000000415. [DOI] [PMC free article] [PubMed] [Google Scholar]