Abstract
Background
In the clinic, how to stratify renal cell carcinoma (RCC) patients with different risks and to accurately predict their prognostic outcome remains a crucial issue. In this study, we assessed the expression and prognostic value of gankyrin in RCC patients.
Methods
The expression of gankyrin was examined in public databases and validated in specimens from two independent centers. The clinical practice and disease correlation of gankyrin in RCC were evaluated in RCC patients, various cell lines and an orthotopic RCC model.
Findings
Upregulation of gankyrin expression in RCC was corroborated in two independent cohorts. High gankyrin expression positively associated with disease progression and metastasis of RCC patients. A positive correlation between gankyrin and sunitinib-resistance was also observed in RCC cell lines and in an orthotopic RCC model. Kaplan-Meier analysis revealed that patients with higher gankyrin expression presented worse prognosis of RCC patients in the two cohorts. Gankyrin served as an independent prognostic factor for RCC patients even after multivariable adjustment by clinical variables. Time-dependent AUC and Harrell's c-index analysis presented that the incorporation of the gankyrin classifier into the current clinical prognostic parameters such as TNM stage, Fuhrman nuclear grade or SSIGN score achieved a greater accuracy than without it in predicting prognosis of RCC patients. All results were confirmed in randomized training and validation sets from the two patient cohorts.
Interpretation
Gankyrin can serve as a reliable biomarker for disease progression and for prognosis of RCC patients. Combining gankyrin with the current clinical parameters may help patient management.
Fund
National Natural Science Foundation of China (No. 81773154, 81772747 and 81301861), Medical Discipline Construction Project of Pudong New Area Commission of Health and Family Planning (PWYgf2018-03), the Shanghai Medical Guidance (Chinese and Western Medicine) Science and Technology Support Project (No. 17411960200), Outstanding Leaders Training Program of Pudong Health Bureau of Shanghai (No. PWR12016-05)
Keywords: Renal cell carcinoma, Gankyrin, Prognosis, Disease progression
Research in context.
Evidence before this study
Current treatment modalities including targeted therapy or immunotherapy for advanced or metastatic RCC patients exhibit limited effects, and no clinically usable markers are available to differentiate different subgroups of RCC patients for a particular therapy and to accurately assess the prognosis and recurrence of the patients after treatments. In this context, many studies reported that gankyrin is over-expressed in various types of tumors and high expression predicts tumor progression and poor prognosis of patients.
Added value of this study
However, the expression and prognostic value of gankyrin in RCC patients have not been explored.
Implications of all the available evidence
Gankyrin is up-regulated in human RCC and high gankyrin expression is positively associated with progression, metastasis and sunitinib-resistance of RCC. Gankyrin is an independent risk factor for predicting prognosis of RCC patients even through multivariable adjustment by clinical variables. Furthermore, the incorporation of gankyrin classifier into the current clinical parameters (TNM stage, Fuhrman nuclear grade, SSIGN) confers more accurate evaluation in predicting prognosis of RCC patients. These assessments were confirmed by two independent cohorts or randomized two sets of the merged patients. Therefore, gankyrin can potentially serve as an indicator of disease progression and prognosis of patients with RCC.
Alt-text: Unlabelled Box
1. Introduction
Renal cell carcinoma (RCC) is one of the most common cancers in the US with an estimated 65,340 new cases and nearly 15,000 mortalities in 2018 [1]. Most RCC patients gain localized tumors which are generally treated with surgical operation, while the remaining patients harbor metastatic RCC or relapse after local therapy and need to receive systemic treatment with targeted agents and/or inhibitors of immune checkpoint regulators [2]. But these treatment regimens have limited effects on advanced or metastatic RCC patients [2]. Increasing evidence suggests that due to tumor heterogeneity and individual difference of patients, the choice and decision of therapy should become more personalized [3]. Yet, no clinical markers are currently available to distinguish different subgroups of RCC patients for select therapies and to accurately gauge the prognosis and recurrence of the patients after treatments [4].
In the clinic, risk stratification and treatment decisions on RCC have largely depended on traditional clinicopathological parameters. TNM is one of the strongest prognostic factors in predicting the clinical outcome of patients with RCC [5]. Another stage-scoring system named SSIGN (Stage Size Grade and Necrosis) has been reported to classify patients with RCC into low, intermediate and high-risk subgroup according to stage, tumor size, nuclear grade, and the presence of tumor necrosis [6]. In addition, Fuhrman grade is the most widely accepted histologic grading system in RCC [7]. Moreover, molecular markers including oncogene, tumor suppressor, long non-coding RNA (LncRNA), microRNA, circRNA, etc. have also been used to predict clinical outcomes of RCC patients [[8], [9], [10], [11]]. However, none of these factors have been used as the basis for therapy approval and they are difficult to apply to precision medicine, which calls for novel and reliable markers for predicting clinical outcomes of RCC patients.
Gankyrin, encoded by the gene PSMD10, has been described as an oncoprotein that is a component of the 19S regulatory cap of the proteasome and plays crucial roles in proliferation, invasion and metastasis of various tumors [12,13]. Moreover, many studies show that gankyrin is over-expressed in various types of tumors and high expression predicts tumor progression and poor prognosis of patients [14,15]. For example, gankyrin expression was positively associated with histological differentiation, TNM stage, and metastasis of cholangiocarcinoma, and gankyrin was an independent prognostic indicator for overall survival [16]. These data highlight gankyrin as a potential biomarker in different cancers, but the clinical significance and biological function of gankyrin in renal cancer have not been explored.
In this study, we demonstrate that gankyrin is up-regulated in human RCC and gankyrin expression is positively associated with progression, metastasis and sunitinib-resistance of RCC. Moreover, we confirm gankyrin as an independent risk factor for predicting prognosis of RCC patients even after multivariable adjustment by clinical variables. Further, the incorporation of gankyrin classifier into the current clinical parameters or scores (TNM stage, SSIGN, Fuhrman nuclear grade) achieves more accurate evaluation in predicting prognosis of RCC patients. Therefore, gankyrin serves as a novel and excellent indicator for evaluating disease progression and prognosis of patients with RCC.
2. Materials and methods
2.1. Public databases collection
TCGA data were downloaded using FirebrowseR package. We selected KIRC dataset from all TCGA cohorts, and filter out all patient barcodes and gene mRNA expression profiles and clinical data with tumor and matched controls. 72 RCC patients were considered finally for differential expression analysis. Interested gene expression data sets were queried in GEO database (https://www.ncbi.nlm.nih.gov/geo/) using key words, such as “kidney cancer” and “human” using either RNA micro arrays or RNA high-throughput sequencing platform. Then, for each dataset, we normalized gene expression profiles using quantile method via IntClust package in R (3.5.1). Differential expressed genes were analyzed using limma package. Metafor package was used to perform meta-analysis for total 5 datasets using Mixed effect models. A set of microarray data were analyzed with the Oncomine (http://www.oncomine.org) databases. The data were filtered by “PSMD10”, “RCC and normal tissues” and the data type was defined as mRNA. Then 10 datasets were acquired, and a meta-analysis was carried out to compare the level of the Gankyrin (PSMD10) between the RCC and adjacent non-tumor renal tissues.
2.2. Patients and specimens
Two independent cohorts, cohort 1 (n = 112, Changhai hospital, Shanghai, China) and cohort 2 (n = 135, Fudan University Shanghai Cancer Center, Shanghai, China), of RCC patients who were pathologically diagnosed between 2012 and 2013 were recruited in this study. The present study was followed the reporting recommendations for tumor marker prognostic studies (REMARK) [17], and was approved by the institutional ethical review boards from all hospitals, and written informed consent was obtained from all patients. The clinical characteristics of the two independent sets are summarized in Table 1 and Supplementary Table S1. The patients were also merged and randomly divided by 1:1 ratio into a training (n = 124) and a validation set (n = 123) (Table 2 and Supplementary Table S2). Pathologic specimens were evaluated by a surgical pathologist (GJH), with stage and grade determined according to the 2010 American Joint Committee on Cancer guidelines and Fuhrman grading system, respectively. The clinical data of these RCC patients at diagnosis included age, gender, Fuhrman nuclear grade, TNM stage, and SSIGN score [18]. The primary outcomes were overall and progression-free survival. Overall survival is the duration of follow-up from surgery to the date of death or last clinic visit, and progression-free survival is the duration of follow-up from surgery to the data of disease progression identified by MRI, CT or ECT, or last clinic visit.
Table 1.
Clinicopathologic characteristics of RCC patients by gankyrin expression in cohort 1 (n = 112).
| Characteristic | Gankyrin |
Sum (112) | P value | |
|---|---|---|---|---|
| Low expression(n = 76) | High expression(n = 36) | |||
| Age | 0.848 | |||
| <60 | 45 | 22 | 67 | |
| ≥60 | 31 | 14 | 45 | |
| Gender | 0.898 | |||
| Male | 54 | 26 | 80 | |
| Female | 22 | 10 | 32 | |
| Fuhrman | 0.061 | |||
| 1 | 16 | 2 | 18 | |
| 2 | 38 | 18 | 56 | |
| 3 | 18 | 10 | 28 | |
| 4 | 4 | 6 | 10 | |
| TNM stage | 0.001 | |||
| 1–2 | 71 | 25 | 96 | |
| 3 | 5 | 11 | 16 | |
| SSIGN | <0.001 | |||
| 1–4 | 76 | 25 | 101 | |
| ≥5 | 0 | 11 | 11 | |
| Overall survival | <0.001 | |||
| − | 73 | 24 | 97 | |
| + | 3 | 12 | 15 | |
| Progression free survival | 0.001 | |||
| − | 73 | 27 | 100 | |
| + | 3 | 9 | 12 | |
Statistical significance was calculated by chi-square test or fisher's exact test for categorical/binary measures and ANOVA for continuous measures.
Table 2.
Clinicopathologic characteristics of RCC patients by gankyrin expression in training data set (n = 124).
| Characteristic | Gankyrin |
Sum (124) | P value | |
|---|---|---|---|---|
| Low expression(n = 83) | High expression(n = 41) | |||
| Age | 0.114 | |||
| <60 | 37 | 24 | 61 | |
| ≥60 | 46 | 17 | 63 | |
| Gender | 0.182 | |||
| Male | 64 | 27 | 91 | |
| Female | 19 | 14 | 33 | |
| Fuhrman | 0.002 | |||
| 1 | 12 | 1 | 13 | |
| 2 | 48 | 15 | 63 | |
| 3 | 21 | 21 | 42 | |
| 4 | 2 | 4 | 6 | |
| TNM stage | 0.021 | |||
| 1–2 | 78 | 33 | 111 | |
| 3 | 5 | 8 | 13 | |
| SSIGN | <0.001 | |||
| 1–4 | 83 | 31 | 114 | |
| ≥5 | 0 | 10 | 10 | |
| Overall survival | <0.001 | |||
| − | 79 | 26 | 105 | |
| + | 4 | 15 | 19 | |
| Progression free survival | <0.001 | |||
| − | 80 | 24 | 104 | |
| + | 3 | 17 | 20 | |
Statistical significance was calculated by chi-square test or fisher's exact test for categorical/binary measures.
2.3. Real-time polymerase chain reaction (Real-time PCR)
Total RNAs of RCC specimens or cell lines were extracted with RNAiso Plus (Takara, Kusatsu, Shiga Prefecture, Japan) and their corresponding cDNAs were synthesized using a PrimeScript One Step RT reagent Kit (Takara). Real-time PCR was conducted with SYBR Green Real-Time PCR Master Mix (QPK201, Toyobo, Osaka, Kansai, Japan) on an ABI PRISM 7300HT Sequence Detection System. All results were normalized to the expression of β-actin. Fold change relative to the mean value was determined by 2−△△Ct. The primer sequences: PSMD10 (forward primer, 5′-AACTGACCAGGACAGCAGAACT-3′, and reverse primer, 5′-ACAGCATTCA.
CTTGAGCACCTT-3′), ACTIN (forward primer, 5′-CCCTGGCACCCAGCAC-3′, and reverse primer, 5′-GCCGATCCACACGGAG-3′).
2.4. Western blot
Western blot was performed as we previously reported [19]. The following primary antibodies were used: rabbit anti-gankyrin antibody (Abcam, USA), rabbit anti-GAPDH antibody (Cell Signaling Technology, USA). The secondary antibodies were goat anti-rabbit IgG-HRP-linked antibody from Cell Signaling Technology (Danvers, MA, USA).
2.5. Immunohistochemistry (IHC)
IHC was performed as we previously described [19], and the following primary antibodies were used: rabbit anti-gankyrin antibody (Abcam). Cases were scored semiquantitatively as negative, weakly positive, moderately positive, or strongly positive along with the percentage of positive cells, after which an H-score was generated as a score of 0–3 intensity multiplied by the percentage of positive cells (range 0–300) [20]. And The H score of each patient was calculated by two independent and experienced pathologists in double blind way.
2.6. Cell culture
RCC cell lines used in this study were obtained from the Cell Bank of the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China) in 2017. HK-2 cells were maintained in DMEM, High Glucose medium (Gibco, Waltham, MA, USA). A498 and ACHN cells were cultured in Minimum Essential Medium (Gibco). 786-O cells were maintained in RPMI-1640 medium (Gibco). Caki-1cells were maintained in McCoy's 5A Medium (Gibco). The culture media of all cell lines were supplemented with fetal bovine serum (FBS, 10%, Gibco), 1% penicillin/streptomycin (Gibco). RCC cell lines were cultured at 37 °C in 5% Co2. Sunitinib-resistant 786-O and 769-p cell lines (786-O-SR and 769-p-SR) were continuously exposed to increasing dose of sunitinib (Selleck, USA) for about 12 weeks. The starting dose was 5 μM and this was increased to 10 μM after 4 weeks, to 15 μM after another 4 weeks and continued at 15 μM for the last 4 weeks. The established resistant 786-O and 769-P cell lines were then maintained in DMEM medium with 10% (v/v) FBS and 10 μM sunitinib.
The cell lines in this study were authenticated by short tandem repeat (STR) profiling and detected for mycoplasma contamination using a Mycoplasma Detection Kit (Selleck Chemicals), and the most recent tests were conducted in September 2018. All cell lines used in this study were cultured within 40 passages.
2.7. Gene knockdown
The 786-O cells were cultured in 6-well plates, inoculated at a density of 5 × 104 cells/ml, and transfected with the shRNAs expressing lentivirus (sh-gankyrin) or control lentivirus at a multiplicity of infection (MOI) of 45. After 72 h transfection, they were observed and photographed under microscope. The sequences for shRNA were presented in Supplementary Table S1.
2.8. Cell proliferation assays
The proliferation of RCC cells under the indicated conditions was evaluated using a CCK-8 kit (Dojindo, Kumamoto, Kyushu, Japan), which was described in our previous study [19]. The proliferation rates are presented as a proportion of the control value, which was obtained from the treatment-free groups.
2.9. Animal experiments and in vivo imaging
All experimental animal procedures were approved by the Animal Care and Use Committee of the Second Military Medical University (Shanghai, China). 786-O cells were transfected with the luciferase reporter gene, which were applied in subcapsular RCC formation assays. Tumor growth was monitored weekly by live-animal bioluminescence optical imaging using an IVIS Lumina II imaging system (PerkinElmer, Hopkinton, MA, USA) after intraperitoneal injection of D-luciferin (150 mg/kg) (Gold Biotech, USA) in 100 μl of DPBS.
For renal subcapsular tumor cell implantation, briefly, 6-wk-old male Nod-scid mice (six per treatment group) were anesthetized and placed in the left lateral decubitus position. A vertical incision was made in the right flank through the skin and peritoneum, exposing the lateral aspect of the kidney. The kidney was lifted gently and stabilized. 20 μl of the Matrigel/medium (1:1) suspension containing 1 × 106 786-O or 786-O-SR-luc cells from indicated groups were inoculated under the renal capsule using a 24-gauge needle inserted from the lower pole of the kidney. Mice were divided into 4 groups 21 days after tumor implantation. Mice in treatment groups were treated with sunitinib (40 mg/kg) 5 days on, 2 days off by oral gavage, while the control group were treated with vehicle. The mice were sacrificed 10 weeks after injection, and tumors were removed and fixed in 10% buffered formalin solution for further experiments.
2.10. Statistical analysis
Numerical data were expressed as the mean ± S.D. Statistical differences between variables were analyzed by two-tailed Student's t-test. Survival curves were plotted using the Kaplan-Meier method and compared via log-rank analysis. Variables with p values < 0.1 on the univariate analysis were included in multivariate Cox proportional hazards analysis. Difference was considered significant at P < 0.05. Time-dependent receiver operating characteristics (ROC) analysis was used to determine the cut-off value of H-score of gankyrin with “survival ROC” package, R software 3.4.4. Time-dependent area under the receiver operating characteristic curve (AUC) was computed with the “time ROC” package. Prognostic accuracy of the gankyrin classifier and other prognostic indicators was performed by Harrell's concordance index (c-index). All experiments were performed independently at least three times. All the analyses were performed using the GraphPad Prism 5 (GraphPad Software, Inc.), SPSS 21.0 (IBM Corporation, Armonk, New York, USA) software and R-project for statistical computing (version 3.4.4).
3. Results
3.1. Gankyrin is up-regulated in renal cell carcinoma (RCC)
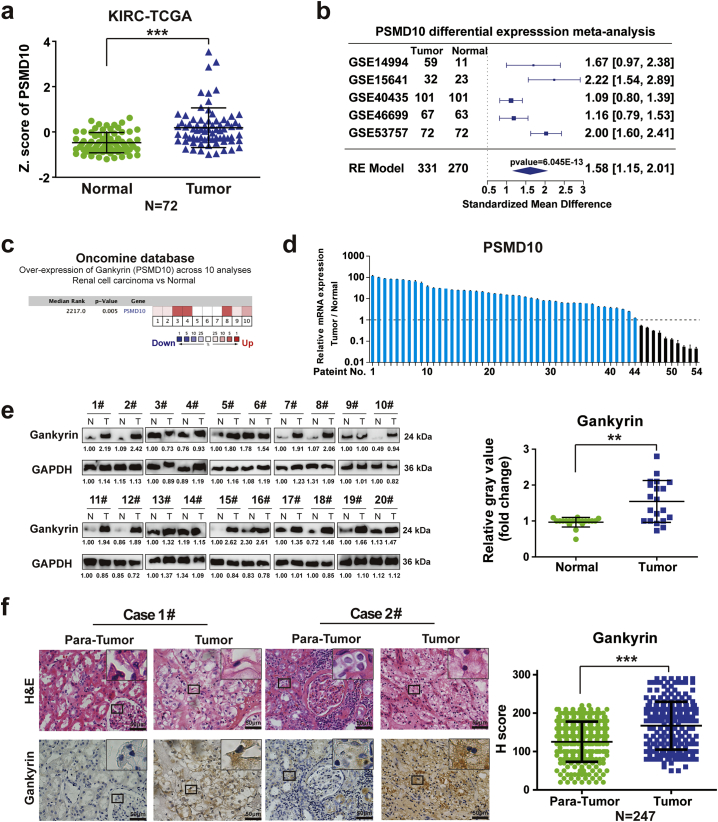
Though gankyrin serves as a helpful indicator for predicting disease progression and poor prognosis of patients with certain malignant tumors [21], the expression and clinical significance of gankyrin in RCC patients have not been established. We first examined the expression of gankyrin in specimens from RCC patients. Available databases from The Cancer Genome Atlas (TCGA), Gene Expression Omnibus (GEO) and ONCOMINE were analyzed, which all showed that higher mRNA expression of gankyrin (PSMD10) was observed in RCC specimens compared with adjacent non-tumor renal tissues (Fig. 1a–c). To validate this finding, real-time PCR and Western blot were employed to assess gankyrin expression in paired RCC specimens and their adjacent renal tissues from patients. Real-time PCR showed that most RCC specimens exhibited higher mRNA expression of gankyrin than that in normal tissues (43/54) (Fig. 1d). Secondly, the specificity of the anti-gankyrin antibody was examined (Supplementary Fig. S1a–c) and Western blot analysis revealed increased protein expression of gankyrin in most RCC specimens (16/20) in contrast to matched normal tissues (Fig. 1e). Furthermore, immunohistochemistry (IHC) was performed in tissue microarrays from RCC patients (n = 247) in two independent clinical centers to detect the expression of gankyrin. Consistent with the above results, most RCC specimens exhibited higher gankyrin expression compared with adjacent tissues (201/247) (Fig. 1f). Taken together, gankyrin is commonly up-regulated in human RCC specimens.
Fig. 1.
Gankyrin is up-regulated in renal cancer carcinoma (RCC). a, Expression of gankyrin (PSMD10) in human RCC tissues and normal renal tissues was analyzed using the TCGA database (kidney renal clear-cell carcinoma, KIRC). All data were divided into RCC and normal samples (n = 72). The z-score was calculated to compare the expression differences that is shown by box plots (***p < 0.001; Wilcoxon test), Values represented as the mean ± SEM. b, Forest plot of meta-analysis for gankyrin (PSMD10) was performed, and integrated statistics using random-effects model, Hedges estimator, was computed. Total 5 datasets were utilized to perform meta-analysis, with 331 tumors and 270 normal controls. c, The relative mRNA expression of gankyrin (PSMD10) in RCC specimens compared with adjacent non-tumor renal tissues, of which the data were obtained from ten independent studies published in ONCOMINE database. d, Real-time PCR was employed to detect the expression of gankyrin in paired tumor specimens and their adjacent renal tissues from RCC patients (n = 54). e, The protein expression of gankyrin was examined in matched RCC specimens and their adjacent renal tissues by Western blot analysis (n = 20) (**p < 0.01; Wilcoxon test), values represented as the mean ± SD. f, Representative hematoxylin and eosin (H&E) staining and immunohistochemistry (IHC) staining for gankyrin in RCC tissues and the matched adjacent tissues have been presented (n = 247; scale bar = 50 μm). The expression of gankyrin was evaluated by H-score, which has been listed in Materials and methods (p value: Wilcoxon test), values represented as the mean ± SD. All p values are defined as: *p < 0.05, **p < 0.01 and ***p < 0.001.
3.2. Gankyrin expression positively associates with disease progression, metastasis and sunitinib-resistance of RCC patients
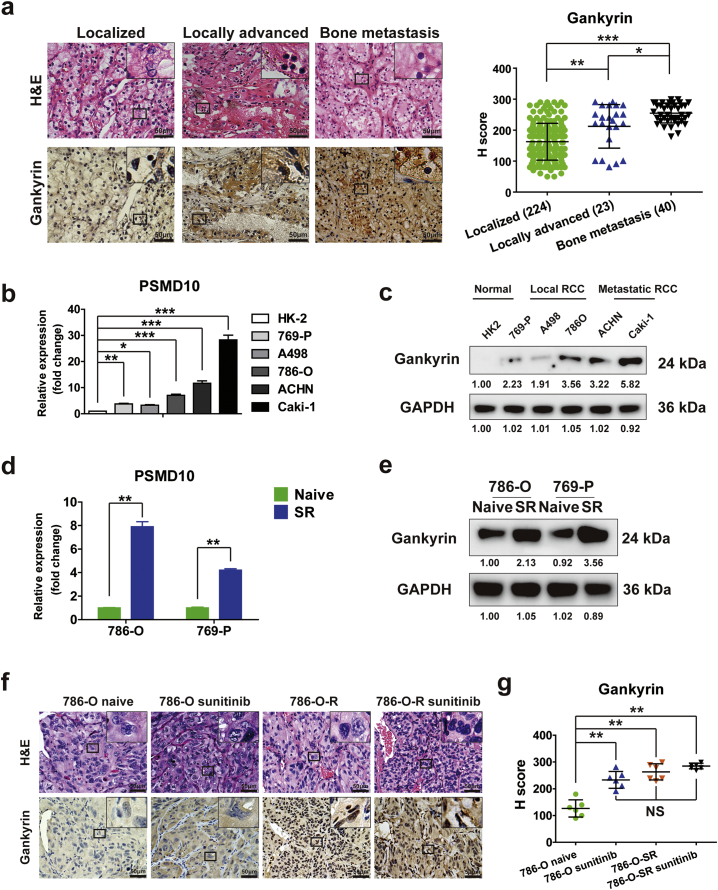
We postulated that gankyrin might be positively associated with progression and metastasis of RCC. We performed IHC in tissue microarrays from RCC patients (n = 247) and found that gankyrin expression was higher in specimens from locally advanced RCC or RCC with bone metastasis compared to localized RCC (Fig. 2a). Similarly, analyses in a normal renal cell line (HK-2) and various types of RCC cell lines demonstrated lower mRNA and protein expression of gankyrin in HK-2 and local RCC cell lines 769-P, A498, and 786-O, and higher expression of gankyrin in the metastatic cell lines such as ACHN and Caki-1 (Fig. 2b–c). Thus, there is a positive correlation between gankyrin expression and progression or metastasis of RCC.
Fig. 2.
Gankyrin expression positively associates with disease progression, metastasis and sunitinib-resistance of RCC patients. (a) Representative H&E and IHC staining for gankyrin in RCC tissues ranked by different disease stage as localized, locally advanced, bone metastasis (scale bar = 50 μm). And the expression of gankyrin was evaluated by H-score (P value: Wilcoxon test), values represented as the mean ± SD. b–c, The mRNA (b) and protein (c) expressions of gankyrin in a normal renal cell line (HK-2) and various types of RCC cell lines (769-P, A498, 786-O, ACHN and Caki-1) were respectively detected by real-time PCR (b), the PSMD10 expression was normalized to β-actin (ΔCt) and compared with the expression level in HK2 (ΔΔCt), fold change compared to HK2 is presented (P value: Wilcoxon test), values represented as the mean ± SD and Western blot (c). d–e, Real-time PCR (d) and Western blot (e) were used to examine the mRNA (d) (P value: Wilcoxon test), values represented as the mean ± SD, and protein (e) expressions of gankyrin in sunitinib-resistant 786-O (786-O-SR) and sunitinib-resistant A498 (A498-SR) cells. f–g, 786-O and 786-O-SR with stable luciferase expression were injected into the subcapsular kidney of mice and tumor growth was monitored using an in vivo imaging system. After 21 days, the mice were divided into four groups: (i) naïve 786-O group; (ii) 786-O group intraperitoneally injected with sunitinib (40 mg/kg); (iii) naïve 786-O-SR group; (ii) 786-O-SR group intraperitoneally injected with sunitinib (40 mg/kg). (f) The images of luciferase intensity and representative H&E and IHC staining for gankyrin in orthotopic tumors from mice in different groups were presented (scale bar = 50 μm). (g) The expression of gankyrin was evaluated by H-score (P value: Wilcoxon test), values represented as the mean ± SD. All p values are defined as: *p < 0.05, **p < 0.01 and ***p < 0.001.
Given that drug-resistance is a common problem in targeted therapies for RCC and a critical cause of poor prognosis, we sought to determine the association of gankyrin expression with sunitinib-resistance of RCC. First, sunitinib-resistant RCC cell lines 786-O-SR and 769-p–SR were established after treatment by sunitinib for three months, which was validated by CCK-8 proliferation assays showing that the growth of 786-O-SR or 769-p–SR was not inhibited under sunitinib treatment (Supplementary Fig. S2a–b). As shown in Fig. 2d-e by real-time PCR and Western blot assays, 786-O-SR or 769-p–SR cells exhibited higher gankyrin expression than that in naïve 786-O or 769-p cells. Moreover, an orthotopic RCC model was established using luciferase-expressing 786-O or 786-O-SR cells injected into the subcapsular kidney of NOD/SCID mice. After 21 days post-injection, all mice in the two groups were respectively randomly divided into two groups which were treated without or with sunitinib. As shown in Fig. 2f and Supplementary Fig. S2c, sunitinib-treated 786-O group exhibited the inhibition of tumor growth, but there was no statistical difference in tumor growth between sunitinib-treated 786-O-SR group and naïve 786-O-SR group, indicating the sunitinib-resistance of 786-O-SR in vivo. IHC staining showed that higher gankyrin expression was observed in sunitinib-treated 786-O-SR group and 786-O group in contrast to naïve 786-O group (Fig. 2g). These results indicate that up-regulated gankyrin is predictive of progression, metastasis and sunitinib-resistance of RCC.
3.3. High gankyrin expression predicts unfavorable clinicopathological characteristics and poor prognosis of RCC patients
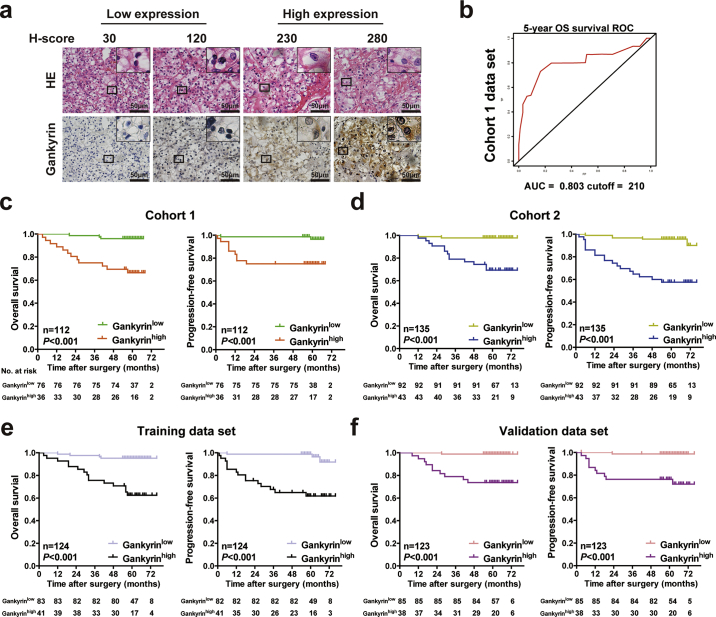
To further validate comprehensively the correlation between gankyrin expression and clinicopathological characteristics or prognosis of RCC patients, two independent cohorts of RCC patients from two independent clinical centers were recruited, and the clinicopathological characteristics of the patients are listed in Tables 1 and Supplementary Table S2. The study design is shown in Supplementary Fig. S2d. IHC was applied in tissue microarrays to examine the tissue expression of gankyrin in the two RCC cohorts (Fig. 3a), and gankyrin expression was determined by the H-score (listed in Materials and methods). The optimal cut-off values for dividing patients into the low and high gankyrin expression groups (Gankyrinhigh and Gankyrinlow) were determined by time-dependent ROC analysis, which indicated that the best cut-off value was 210 (H score) with an area under the curve (AUC) of 0.803 using 5-year overall survival (OS) as the end point in cohort 1 (n = 112) (Fig. 3b). As expected, high expression of gankyrin indicated advanced TNM stage and higher SSIGN score (Table 1). Then Kaplan-Meier survival analysis was applied to compare OS and progression-free survival (PFS) between Gankyrinhigh and Gankyrinlow subgroups of RCC patients, and Gankyrinhigh subgroup presented worse OS (p < 0.001) and PFS (p = 0.001), compared with that of Gankyrinlow subgroup (Fig. 3c). In addition, another cohort (cohort 2; n = 135) was employed as a validation set using the cut-off value (H-score = 210) derived from cohort 1. As expected, high expression of gankyrin indicated higher Fuhrman nuclear grade, advanced TNM stage, and higher SSIGN score (Supplementary Table S2). Kaplan-Meier analysis also revealed that patients with higher gankyrin expression exhibited markedly worse OS (p < 0.001) and PFS (p < 0.001) than their counterparts (Fig. 3d).
Fig. 3.
High gankyrin expression in specimens predicts unfavorable clinicopathological characteristics and poor prognosis of RCC patients. a, Representative H&E and IHC staining of gankyrin in tissues from RCC patients are shown (scale bar = 50 μm). According to the expression level of gankyrin (H-score) in specimens, RCC patients were divided into two groups: a gankyrinlow group (H-score values of 0–210) and a gankyrinhigh group (IRS values of 211–300). b, A time-dependent receiver operating characteristics (ROC) analysis was performed to determine the optimum cut-off value of the gankyrin (H-score) to predict 5-year overall survival (OS) in cohort 1 (n = 112). c-f, Kaplan-Meier curves for OS (c) and progression-free survival (PFS) (d) of RCC patients were analyzed according to OV6 expression in cohort 1 (n = 112; c), cohort 2 (n = 135; d), training set (n = 124; e) or validation set (n = 123; f) (P value: log rank test). All p values are defined as: *p < 0.05, **p < 0.01 and ***p < 0.001.
We then merged the RCC patients in the above two cohorts, and randomly divided them into the training set (n = 124) and the validation set (n = 123) at a 1:1 ratio (Table 2; Supplementary Table S3). Consistent with the best cut-off derived from cohort 1, the best cut-off value of H-score was also 210, with an AUC of 0.884 using 5-year OS as the end point in the training set (Supplementary Fig. S2e). As expected, high gankyrin expression in RCC samples was positively associated with higher Fuhrman nuclear grade, advanced TNM stage, and higher SSIGN score in both the randomized training and validation sets (Table 2; Supplementary Table S3). More importantly, Kaplan-Meier analysis revealed that Gankyrinhigh subgroup exhibited markedly poorer OS (p < 0.001 in both the training and validation sets) and PFS (p < 0.001 in both the training and validation sets) than the Gankyrinlow subgroup (Fig. 3e–f). Thus, gankyrin expression is a helpful indicator for clinicopathological characteristics and prognosis of RCC patients.
3.4. Incorporation of gankyrin classifier into the current clinical prognostic parameters better predicts prognosis of RCC patients
To further appraise the prognostic value of gankyrin in RCC patients, univariate and multivariate Cox regression analyses (including gankyrin, general clinical parameters and the existing clinical staging system such as Fuhrman nuclear grade, SSIGN and TNM stage) were used to determine if gankyrin expression could be an independent risk factor for predicting OS or PFS of RCC patients. Even after multivariable adjustment by clinical variables, gankyrin or TNM stage respectively appeared to be an independent risk factor not only in cohort 1 and cohort 2 (Table 3; Supplementary Table S4), but also in the two randomized sets (Supplementary Tables S5–6).
Table 3.
Univariate and multivariate Cox regression analysis of gankyrin expression classifier and clinical characteristics with Overall Survival and Progression Free Survival in cohort 1(n = 112).
| Overall survival |
Progression free survival |
|||||||
|---|---|---|---|---|---|---|---|---|
| Characteristics | Univariate |
Multivariate |
Univariate |
Multivariate |
||||
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Age (<60 y vs ≥ 60 y) | 1.764 (0.639–4.865) | 0.273 | 1.533 (0.494–4.755) | 0.460 | ||||
| Gender (Male vs Female) | 0.888 (0.283–2.790) | 0.839 | 0.814 (0.220–3.009) | 0.758 | ||||
| Fuhrman (1–2 vs 3–4) | 3.047 (1.084–8.561) | 0.035 | 0.698 (0.170–2.867) | 0.618 | 2.781 (0.882–8.766) | 0.081 | ||
| TNM stage (1–2 vs 3–4) | 12.455 (4.414-35.146) | <0.001 | 5.595 (1.452–21.561) | 0.012 | 15.905 (4.773–52.994) | <0.001 | 9.582 (2.317–39.620) | 0.002 |
| SSIGN (1–4 vs ≥ 5) | 12.809 (4.602–35.655) | <0.001 | 2.425 (0.550–10.700) | 0.242 | 8.107 (2.549–25.781) | <0.001 | 1.150 (0.279–4.733) | 0.847 |
| Gankyrin expression (Low vs High) | 10.030 (2.828–35.571) | <0.001 | 4.396 (1.029–18.777) | 0.046 | 6.809 (1.842–25.167) | 0.004 | 6.024 (1.606–22.594) | 0.039 |
Since many studies have shown that combined biomarkers are able to improve the prognostic accuracy for RCC patients than a single biomarker [22], we further investigated whether the incorporation of gankyrin into the current clinical staging systems or indicators could better predict the prognostic accuracy for OS or PFS of RCC patients. By using time-dependent AUC analysis, the incorporation into TNM stage showed higher AUC than that of gankyrin or TNM stage alone in cohort 1 and cohort 2, also in the randomized training and validation sets. These results demonstrate that combining gankyrin and TNM stage yields better prognostic accuracy in assessing prognosis of RCC patients than the two indicators individually. Moreover, the incorporation of gankyrin into the existing clinical parameters such as Fuhrman, SSIGN or TNM stage were compared with the indicator alone by Harrell's c-index analysis in predicting prognosis of RCC patients. As shown in Table 4 and Supplementary Table S7, c-index value of gankyrin combined with Fuhrman, SSIGN or TNM stage for OS or PFS of RCC patients in both the two independent cohorts was respectively higher than gankyrin or other indicators alone, which was also confirmed in the randomized training and validation sets. Thus, improved prognostic accuracy could be accomplished by combining gankyrin expression with the existing clinical staging systems in predicting survival of RCC patients.
Table 4.
C-index analysis of the prognostic accuracy of gankyrin classifier and other variables for Overall Survival and Progression Free Survival in cohort 1 and 2.
| Overall survival |
Progression free survival |
|||
|---|---|---|---|---|
| Characteristics | Cohort 1 (n = 112) | Cohort 2 (n = 135) | Cohort 1 (n = 112) | Cohort 2 (n = 135) |
| Age | 0.5682 (0.4431–0.6932) | 0.5582 (0.4320–0.6844) | 0.5515 (0.4184–0.6846) | 0.5604 (0.4330–0.6878) |
| Gender | 0.5133 (0.3970–0.6295) | 0.5019 (0.3904–0.6133) | 0.5519 (0.4281–0.6756) | 0.5067 (0.3952–0.6181) |
| Fuhrman | 0.6309 (0.5105–0.7513) | 0.7267 (0.6001–0.8534) | 0.6192 (0.4910–0.7474) | 0.7304 (0.6029–0.8580) |
| TNM stage | 0.7188 (0.6398–0.7978) | 0.6826 (0.6362–0.7290) | 0.7195 (0.6359–0.8032) | 0.6927 (0.6474–0.7379) |
| Gankyrin | 0.7602 (0.6444–0.8760) | 0.7868 (0.6699–0.9037) | 0.7898 (0.6650–0.9147) | 0.7954 (0.6781–0.9126) |
| SSIGN | 0.7036 (0.6397–0.7676) | 0.6773 (0.6238–0.7308) | 0.7316 (0.6574–0.8057) | 0.6863 (0.6349–0.7376) |
| Gankyrin + Fuhrman | 0.7880 (0.6513–0.9247) | 0.8235 (0.6865–0.9605) | 0.8053 (0.6595–0.9510) | 0.8321 (0.6941–0.9701) |
| Gankyrin + SSIGN | 0.8019 (0.6849–0.9189) | 0.8285 (0.7098–0.9473) | 0.8357 (0.7092–0.9622) | 0.8414 (0.7223–0.9605) |
| Gankyrin + TNM stage | 0.8368 (0.7144–0.9591) | 0.8445 (0.7267–0.9623) | 0.8748 (0.7441–1.0000) | 0.8590 (0.7406–0.9773) |
4. Discussion
Though surgical operation is an effective treatment for localized RCC, systemic therapy serves as the mainstay of treatment for patients who relapse after surgery or who have advanced or metastatic RCC [3]. Great progress has been made from the non-specific immunotherapy to current targeted therapy (against VEGF, PDGF, or other targets such as mTOR or MET and AXL tyrosine-protein kinase receptors) and to the use of immune-checkpoint inhibitors [22]. However, a serious clinical hurdle remains about how to accurately distinguish the subgroups of RCC patients and choose the appropriate treatment for them to obtain the best possible therapeutic benefit [2]. Many studies have contributed to the search for potential indicators for predicting the clinical outcomes for RCC patients, which might help to make more appropriate treatment selection for individual patients [[23], [24], [25], [26]]. In the present study, we for the first time demonstrate that the expression of gankyrin is positively associated with disease progression, metastasis, sunitinib-resistance and poor prognosis of RCC patients. Moreover, gankyrin expression is an independent risk factor for overall survival and progression-free survival of RCC patients.
An accumulating number of studies indicate that combined molecular biomarkers with clinicopathological features or scores are able to improve the prognostic accuracy for RCC patients than a single biomarker [22]. The University of California Los Angeles (UCLA) Integrated Staging System (UISS) and SSIGN score are integrated with clinical TNM stage and Fuhrman nuclear grade, which could accurately screen out patients with high-risk for adjuvant studies [27]. Though there are numerous reports of gankyrin as a potential biomarker for predicting prognostic outcome of patients with various tumors [21], the combination of gankyrin with the current clinical parameters or indicators had not been attempted for the prognostic value for RCC patients. In the present study, we further incorporated gankyrin into the clinical parameters such as Fuhrman nuclear grade, TNM or SSIGN staging score, which resulted in improved prognostic superiority and accuracy in OS and PFS of RCC patients, compared to using gankyrin or other parameters alone. Thus, gankyrin could serve as a meaningful indicator for predicating outcome of RCC patients, especially when combined with the current clinical parameters or indicators. The combined parameters help distinguish different subgroups of RCC patients for particular therapeutic modalities. It should be pointed out that although this type of studies by us and others provide novel biomarkers with superiority in predicting disease progression and prognosis of RCC patients, further clinical studies are needed to confirm the applicability of these clinical tests for RCC patient stratification and for accurately predicting outcomes and treatment response of patients.
Gankyrin plays an oncogenic role in cell proliferation and apoptosis, is overexpressed in many tumors, including most hepatocellular carcinomas (HCCs) and confers tumorigenicity to non-malignant cells. Transgenic mice that overexpress gankyrin specifically in the hepatocytes surprisingly developed vascular tumors (hemangioma/hemangiosarcomas) in the liver. Liu et al. showed that gankyrin binds to and sequester factor inhibiting hypoxia-inducible factor-1 (FIH-1), which results in decreased interaction between FIH-1 and hypoxia-inducible factor-1α (HIF-1α) and increased activity of HIF-1 to promote VEGF production and vascular tumorigenesis [28]. The study suggests that gankyrin might play a physiological role in hypoxic responses besides its roles as an oncoprotein. Gankyrin overexpression also results in decreased cellular levels of p53 and pRb, two key tumor suppressors, leading ultimately to the onset of oncogenic cell functions and fate [29].
Last but not least, many studies have demonstrated gankyrin may be a promising target for future therapies in a broad range of cancers. The oncogenic role of gankyrin stems from its inhibition of two important tumor suppressor proteins, retinoblastoma protein (pRb) [30] and p53 [31], and also its modulation of several vital cellular signaling pathways including Wnt/β-Catenin [32], NF-κB [33], STAT3/Akt [34], IL-1β/IRAK-1 [35], RhoA/ROCK [36] and hypoxia-inducible factor-1 (FIH-1) [28]. Actually, the pathways have been also implicated in RCC progression [[37], [38], [39]], which may explain why gankyrin over-expression might lead to more aggressive RCC. These observations provide strong rationale to consider the therapeutic potential of gankyrin inhibition for cancer treatment. Li et al. used adenovirus-delivered siRNA to target gankyrin in hepatocellular carcinoma [40]; Panobinostat (LBH589), a hydroxamic acid-derived histone deacetylase inhibitor, has shown promising anticancer effects through down-regulating gankyrin/STAT3/Akt pathway [34]. Perhaps more directly relevant is the discovery of a small molecule named cjoc42 that is capable of binding to gankyrin. Cjoc42 can inhibit gankyrin activity and prevents the decrease in p53 protein levels normally associated with high expression of gankyrin, and cjoc42 restores p53-dependent transcription and sensitivity to DNA damage. [41]. Nevertheless, the mechanisms of gankyrin's tumorigenic activities are far from being completely understood. Therefore, more efforts are still needed to characterize the comprehensive molecular traits of gankyrin before we can target this protein safely and efficaciously in patients.
Author contributions
C.W. and X.C designed the study, analyzed the data and wrote the paper. C.W., Y. Li., C.C. X.Z., J.M. performed the experiments, analyzed the data, performed the animal studies and statistical analysis. H.H., Y.W., T.H., J.Z., X.P., C.C. performed the animal studies and provided the RCC patient samples. J.Z., N.J., C.H., X.M., Y.S. and X.C. supervised the study. C.H, X.M., Y.S. and X.C. edited and reviewed the manuscript.
Funding and acknowledgement
This work was supported by National Natural Science Foundation of China (Nos. 81773154, 81772747 and 81301861), Medical Discipline Construction Project of Pudong New Area Commission of Health and Family Planning (PWYgf2018-03), the Shanghai Medical Guidance (Chinese and Western Medicine) Science and Technology Support Project (No. 17411960200) and Outstanding Leaders Training Program of Pudong Health Bureau of Shanghai (No. PWR12016-05).
Declaration of interests
The authors declare no potential conflicts of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2018.12.011.
Contributor Information
Chuan-yi Hu, Email: huchuanyi2001@163.com.
Xiaojing Ma, Email: xim2002@med.cornell.edu.
Ying-hao Sun, Email: sunyhsmmu@126.com.
Xin-gang Cui, Email: cuixingang@smmu.edu.cn.
Appendix A. Supplementary data
Supplementary material 1
Supplementary table 1-7
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Posadas E.M., Limvorasak S., Figlin R.A. Targeted therapies for renal cell carcinoma. Nat Rev Nephrol. 2017;13(8):496–511. doi: 10.1038/nrneph.2017.82. [DOI] [PubMed] [Google Scholar]
- 3.Choueiri T.K., Motzer R.J. Systemic therapy for metastatic renal-cell carcinoma. N Engl J Med. 2017;376(4):354–366. doi: 10.1056/NEJMra1601333. [DOI] [PubMed] [Google Scholar]
- 4.Joosten S.C., Hamming L., Soetekouw P.M., Aarts M.J., Veeck J., van Engeland M. Resistance to sunitinib in renal cell carcinoma: from molecular mechanisms to predictive markers and future perspectives. Biochim Biophys Acta. 2015;1855(1):1–16. doi: 10.1016/j.bbcan.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Ljungberg B., Bensalah K., Canfield S., Dabestani S., Hofmann F., Hora M. EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol. 2015;67(5):913–924. doi: 10.1016/j.eururo.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 6.Frank I., Blute M.L., Cheville J.C., Lohse C.M., Weaver A.L., Zincke H. An outcome prediction model for patients with clear cell renal cell carcinoma treated with radical nephrectomy based on tumor stage, size, grade and necrosis: the SSIGN score. J Urol. 2002;168(6):2395–2400. doi: 10.1016/S0022-5347(05)64153-5. [DOI] [PubMed] [Google Scholar]
- 7.Ljungberg B., Hanbury D.C., Kuczyk M.A., Merseburger A.S., Mulders P.F., Patard J.J. Renal cell carcinoma guideline. Eur Urol. 2007;51(6):1502–1510. doi: 10.1016/j.eururo.2007.03.035. [DOI] [PubMed] [Google Scholar]
- 8.Zheng J., Wang L., Peng Z., Yang Y., Feng D., He J. Low level of PDZ domain containing 1 (PDZK1) predicts poor clinical outcome in patients with clear cell renal cell carcinoma. EBioMedicine. 2017;15:62–72. doi: 10.1016/j.ebiom.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhai W., Sun Y., Guo C., Hu G., Wang M., Zheng J. LncRNA-SARCC suppresses renal cell carcinoma (RCC) progression via altering the androgen receptor(AR)/miRNA-143-3p signals. Cell Death Differ. 2017;24(9):1502–1517. doi: 10.1038/cdd.2017.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Z., Zhang J., Zhang Z., Feng Z., Wei J., Lu J. The putative tumor suppressor microRNA-30a-5p modulates clear cell renal cell carcinoma aggressiveness through repression of ZEB2. Cell Death Dis. 2017;8(6):e2859. doi: 10.1038/cddis.2017.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen D., Chen W., Xu Y., Zhu M., Xiao Y., Shen Y. Upregulated immune checkpoint HHLA2 in clear cell renal cell carcinoma: a novel prognostic biomarker and potential therapeutic target. J Med Genet. 2018 doi: 10.1136/jmedgenet-2018-105454. [DOI] [PubMed] [Google Scholar]
- 12.Chen J., Bai M., Ning C., Xie B., Zhang J., Liao H. Gankyrin facilitates follicle-stimulating hormone-driven ovarian cancer cell proliferation through the PI3K/AKT/HIF-1alpha/cyclin D1 pathway. Oncogene. 2016;35(19):2506–2517. doi: 10.1038/onc.2015.316. [DOI] [PubMed] [Google Scholar]
- 13.Qin X., Wang X., Liu F., Morris L.E., Wang X., Jiang B. Gankyrin activates mTORC1 signaling by accelerating TSC2 degradation in colorectal cancer. Cancer Lett. 2016;376(1):83–94. doi: 10.1016/j.canlet.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 14.Zhen C., Chen L., Zhao Q., Liang B., Gu Y.X., Bai Z.F. Gankyrin promotes breast cancer cell metastasis by regulating Rac1 activity. Oncogene. 2013;32(29):3452–3460. doi: 10.1038/onc.2012.356. [DOI] [PubMed] [Google Scholar]
- 15.Ortiz C.M., Ito T., Tanaka E., Tsunoda S., Nagayama S., Sakai Y. Gankyrin oncoprotein overexpression as a critical factor for tumor growth in human esophageal squamous cell carcinoma and its clinical significance. Int J Cancer. 2008;122(2):325–332. doi: 10.1002/ijc.23106. [DOI] [PubMed] [Google Scholar]
- 16.Zheng T., Hong X., Wang J., Pei T., Liang Y., Yin D. Gankyrin promotes tumor growth and metastasis through activation of IL-6/STAT3 signaling in human cholangiocarcinoma. Hepatology. 2014;59(3):935–946. doi: 10.1002/hep.26705. [DOI] [PubMed] [Google Scholar]
- 17.McShane L.M., Altman D.G., Sauerbrei W., Taube S.E., Gion M., Clark G.M. REporting recommendations for tumor MARKer prognostic studies (REMARK) Nat Clin Pract Urol. 2005;2(8):416–422. [PubMed] [Google Scholar]
- 18.Zigeuner R., Hutterer G., Chromecki T., Imamovic A., Kampel-Kettner K., Rehak P. External validation of the Mayo Clinic stage, size, grade, and necrosis (SSIGN) score for clear-cell renal cell carcinoma in a single European centre applying routine pathology. Eur Urol. 2010;57(1):102–109. doi: 10.1016/j.eururo.2008.11.033. [DOI] [PubMed] [Google Scholar]
- 19.Wang C., Peng G., Huang H., Liu F., Kong D.P., Dong K.Q. Blocking the feedback loop between neuroendocrine differentiation and macrophages improves the therapeutic effects of enzalutamide (MDV3100) on prostate cancer. Clin Cancer Res. 2018;24(3):708–723. doi: 10.1158/1078-0432.CCR-17-2446. [DOI] [PubMed] [Google Scholar]
- 20.Viola P., Maurya M., Croud J., Gazdova J., Suleman N., Lim E. A Validation study for the use of ROS1 immunohistochemical staining in screening for ROS1 translocations in lung cancer. J Thorac Oncol. 2016;11(7):1029–1039. doi: 10.1016/j.jtho.2016.03.019. [DOI] [PubMed] [Google Scholar]
- 21.Wang C., Cheng L. Gankyrin as a potential therapeutic target for cancer. Invest New Drugs. 2017;35(5):655–661. doi: 10.1007/s10637-017-0474-8. [DOI] [PubMed] [Google Scholar]
- 22.Barata P.C., Rini B.I. Treatment of renal cell carcinoma: current status and future directions. CA Cancer J Clin. 2017;67(6):507–524. doi: 10.3322/caac.21411. [DOI] [PubMed] [Google Scholar]
- 23.Fu Q., Xu L., Wang Y., Jiang Q., Liu Z., Zhang J. Tumor-associated macrophage-derived interleukin-23 interlinks kidney cancer glutamine addiction with immune evasion. Eur Urol. 2018 doi: 10.1016/j.eururo.2018.09.030. [DOI] [PubMed] [Google Scholar]
- 24.Giraldo N.A., Becht E., Vano Y., Petitprez F., Lacroix L., Validire P. Tumor-infiltrating and peripheral blood T-cell immunophenotypes predict early relapse in localized clear cell renal cell carcinoma. Clin Cancer Res. 2017;23(15):4416–4428. doi: 10.1158/1078-0432.CCR-16-2848. [DOI] [PubMed] [Google Scholar]
- 25.Mikami S., Mizuno R., Kosaka T., Saya H., Oya M., Okada Y. Expression of TNF-alpha and CD44 is implicated in poor prognosis, cancer cell invasion, metastasis and resistance to the sunitinib treatment in clear cell renal cell carcinomas. Int J Cancer. 2015;136(7):1504–1514. doi: 10.1002/ijc.29137. [DOI] [PubMed] [Google Scholar]
- 26.Sheng Y., Ng C.P., Lourie R., Shah E.T., He Y., Wong K.Y. MUC13 overexpression in renal cell carcinoma plays a central role in tumor progression and drug resistance. Int J Cancer. 2017;140(10):2351–2363. doi: 10.1002/ijc.30651. [DOI] [PubMed] [Google Scholar]
- 27.Hsieh J.J., Purdue M.P., Signoretti S., Swanton C., Albiges L., Schmidinger M. Renal cell carcinoma. Nat Rev Dis Primers. 2017;3 doi: 10.1038/nrdp.2017.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Y., Higashitsuji H., Higashitsuji H., Itoh K., Sakurai T., Koike K. Overexpression of gankyrin in mouse hepatocytes induces hemangioma by suppressing factor inhibiting hypoxia-inducible factor-1 (FIH-1) and activating hypoxia-inducible factor-1. Biochem Biophys Res Commun. 2013;432(1):22–27. doi: 10.1016/j.bbrc.2013.01.093. [DOI] [PubMed] [Google Scholar]
- 29.Chapman A.M., McNaughton B.R. Synthetic proteins potently and selectively bind the oncoprotein gankyrin, modulate its interaction with S6 ATPase, and suppress Gankyrin/MDM2-dependent ubiquitination of p53. ACS Chem Biol. 2015;10(8):1880–1886. doi: 10.1021/acschembio.5b00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sdek P., Ying H., Chang D.L., Qiu W., Zheng H., Touitou R. MDM2 promotes proteasome-dependent ubiquitin-independent degradation of retinoblastoma protein. Mol Cell. 2005;20(5):699–708. doi: 10.1016/j.molcel.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 31.Higashitsuji H., Liu Y., Mayer R.J., Fujita J. The oncoprotein gankyrin negatively regulates both p53 and RB by enhancing proteasomal degradation. Cell Cycle. 2005;4(10):1335–1337. doi: 10.4161/cc.4.10.2107. [DOI] [PubMed] [Google Scholar]
- 32.Dong L.W., Yang G.Z., Pan Y.F., Chen Y., Tan Y.X., Dai R.Y. The oncoprotein p28GANK establishes a positive feedback loop in beta-catenin signaling. Cell Res. 2011;21(8):1248–1261. doi: 10.1038/cr.2011.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen Y., Li H.H., Fu J., Wang X.F., Ren Y.B., Dong L.W. Oncoprotein p28 GANK binds to RelA and retains NF-kappaB in the cytoplasm through nuclear export. Cell Res. 2007;17(12):1020–1029. doi: 10.1038/cr.2007.99. [DOI] [PubMed] [Google Scholar]
- 34.Song X., Wang J., Zheng T., Song R., Liang Y., Bhatta N. LBH589 Inhibits proliferation and metastasis of hepatocellular carcinoma via inhibition of gankyrin/STAT3/Akt pathway. Mol Cancer. 2013;12(1):114. doi: 10.1186/1476-4598-12-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Su B., Luo T., Zhu J., Fu J., Zhao X., Chen L. Interleukin-1beta/Iinterleukin-1 receptor-associated kinase 1 inflammatory signaling contributes to persistent Gankyrin activation during hepatocarcinogenesis. Hepatology. 2015;61(2):585–597. doi: 10.1002/hep.27551. [DOI] [PubMed] [Google Scholar]
- 36.Man J.H., Liang B., Gu Y.X., Zhou T., Li A.L., Li T. Gankyrin plays an essential role in Ras-induced tumorigenesis through regulation of the RhoA/ROCK pathway in mammalian cells. J Clin Invest. 2010;120(8):2829–2841. doi: 10.1172/JCI42542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khan M.N., Bhattacharyya T., Andrikopoulos P., Esteban M.A., Barod R., Connor T. Factor inhibiting HIF (FIH-1) promotes renal cancer cell survival by protecting cells from HIF-1alpha-mediated apoptosis. Br J Cancer. 2011;104(7):1151–1159. doi: 10.1038/bjc.2011.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang X., Cheng Y., Zhu Y., Li H., Ge W., Wu X. Epigenetic silencing of ASPP1 confers 5-FU resistance in clear cell renal cell carcinoma by preventing p53 activation. Int J Cancer. 2017;141(7):1422–1433. doi: 10.1002/ijc.30852. [DOI] [PubMed] [Google Scholar]
- 39.Ueno K., Hirata H., Majid S., Tabatabai Z.L., Hinoda Y., Dahiya R. IGFBP-4 activates the Wnt/beta-catenin signaling pathway and induces M-CAM expression in human renal cell carcinoma. Int J Cancer. 2011;129(10):2360–2369. doi: 10.1002/ijc.25899. [DOI] [PubMed] [Google Scholar]
- 40.Li H., Fu X., Chen Y., Hong Y., Tan Y., Cao H. Use of adenovirus-delivered siRNA to target oncoprotein p28GANK in hepatocellular carcinoma. Gastroentrology. 2005;128(7):2029–2041. doi: 10.1053/j.gastro.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 41.Chattopadhyay A., O'Connor C.J., Zhang F., Galvagnion C., Galloway W.R., Tan Y.S. Discovery of a small-molecule binder of the oncoprotein gankyrin that modulates gankyrin activity in the cell. Sci Rep. 2016;6 doi: 10.1038/srep23732. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1
Supplementary table 1-7