Summary
Pioneering human induced pluripotent stem cell (iPSC)-based pre-clinical studies have raised safety concerns and pinpointed the need for safer and more efficient approaches to generate and maintain patient-specific iPSCs. One approach is searching for compounds that influence pluripotent stem cell reprogramming using functional screens of known drugs. Our high-throughput screening of drug-like hits showed that imidazopyridines—analogs of zolpidem, a sedative-hypnotic drug—are able to improve reprogramming efficiency and facilitate reprogramming of resistant human primary fibroblasts. The lead compound (O4I3) showed a remarkable OCT4 induction, which at least in part is due to the inhibition of H3K4 demethylase (KDM5, also known as JARID1). Experiments demonstrated that KDM5A, but not its homolog KDM5B, serves as a reprogramming barrier by interfering with the enrichment of H3K4Me3 at the OCT4 promoter. Thus our results introduce a new class of KDM5 chemical inhibitors and provide further insight into the pluripotency-related properties of KDM5 family members.
Subject Areas: Biological Sciences, Biochemistry, Molecular Biology
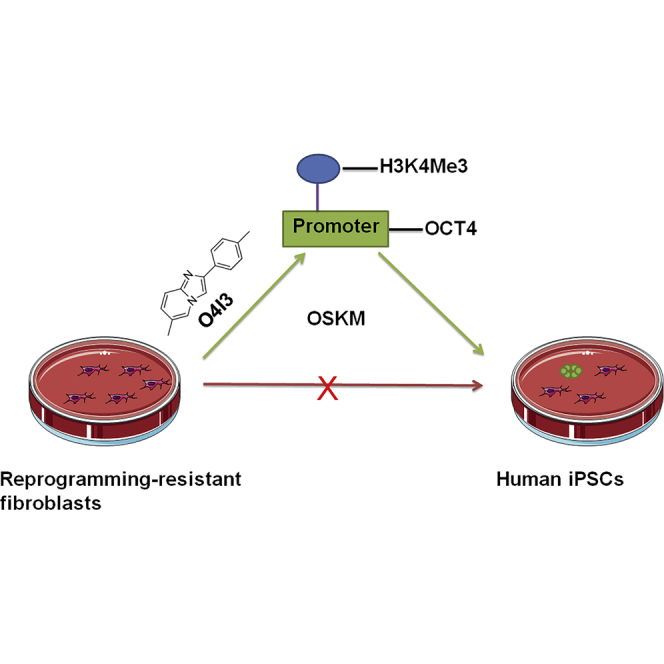
Graphical Abstract
Highlights
-
•
O4I3 supports the maintenance and generation of human iPSCs
-
•
O4I3 is a potent H3K4 demethylase KDM5 inhibitor in vitro and in cells
-
•
KDM5A, but not KDM5B, serves as an epigenetic barrier of reprogramming
-
•
Chemical or genetic inhibition of KDM5A tends to promote the reprogramming efficiency
Biological Sciences; Biochemistry; Molecular Biology
Introduction
Nuclear reprogramming from terminally differentiated somatic cells into induced pluripotent stem cells (iPSCs) is achieved by ectopic expression of several pluripotency-associated transcription factors, including OCT4, SOX2, KLF4, MYC, NANOG, or LIN28 (Takahashi et al., 2007, Takahashi and Yamanaka, 2006, Yu et al., 2007). This seminal technology provides a promising source for autologous organ transplantation, drug discovery, and disease modeling, opening a new era in the field of regenerative medicine (Smith et al., 2016, Takahashi and Yamanaka, 2016). When compared with somatic cell nuclear transfer, reprogramming of human fibroblasts (HFs) is a time-consuming (3–4 weeks) and inefficient (<0.01% of cells) process (Yamanaka and Blau, 2010). Somatic cell reprogramming can also be hampered by random mutations appearing in the course of reprogramming and during maintenance (Gore et al., 2011, Hussein et al., 2011, Ma et al., 2014). To reduce the risk of genetic and epigenetic variations in human iPSC (hiPSC), higher reprogramming efficiency and optimized cultivation conditions are of great importance. Furthermore, improved reprogramming efficiency for the generation of individual patient's own iPSCs will allow the development of personalized transplantation that avoids risks such as immune rejection caused by allogeneic transplantation (Morizane et al., 2013, Shiba et al., 2016). When compared with genetic manipulation, small molecules show advantages in controlling the reprogramming process and have been intensively investigated (Xie et al., 2017). Small molecules contribute to the establishment of more efficient and reliable protocols for the generation and maintenance of pluripotent stem cells (PSCs) (Watanabe et al., 2007). Although mouse chemically induced PSCs have been obtained by small-molecule cocktails (Hou et al., 2013), it seems that HFs are more resistant and chemical cocktails for generation of human iPSCs are not available yet.
A successful reprogramming process from fibroblasts to iPSCs requires proper histone modification, such as the repression of mesenchymal-associated markers and activation of pluripotency-associated genes (Mikkelsen et al., 2008). In general, hypermethylation of H3K4 is found close to transcription start sites (TSS) and is related to gene activation, whereas methylations of H3K27 and H3K9 represses gene expression (Papp and Plath, 2013). Very recently, analysis of the global landscape of histone markers confirmed the enrichment of H3K27Me3 and H3K9Me3, and the lack of H3K4Me3 at promoters of reprogramming-associated genes in the reprogramming-resistant naked mole rat fibroblasts (Tan et al., 2017). To overwhelm epigenetic barriers, a number of chemical inhibitors targeting histone methyltransferases (HMTs), histone demethylases (HDMs), and histone deacetylase (HDAC) have been introduced for reprogramming (Xie et al., 2017). KDM5, also known as JARID1, is a H3K4-specific HDM and is considered as a repressor of gene expression (Kooistra and Helin, 2012). Little is known about the function of KDM5 chemical inhibitors in somatic cell reprogramming.
We report that 2-arylpyrimidazoles, including zolpidem, (a US Food and Drug Administration-approved drug for insomnia treatment; Weitzel et al., 2000) act as pluripotency inducers to promote the generation of iPSCs from patient fibroblasts by targeting H3K4-specific demethylase, KDM5. We show that KDM5A, but not its family member KDM5B, is an epigenetic barrier in somatic cell reprogramming.
Results
Identification of Imidazopyridines as OCT4 Inducers by Cell-Based High-Throughput Screening
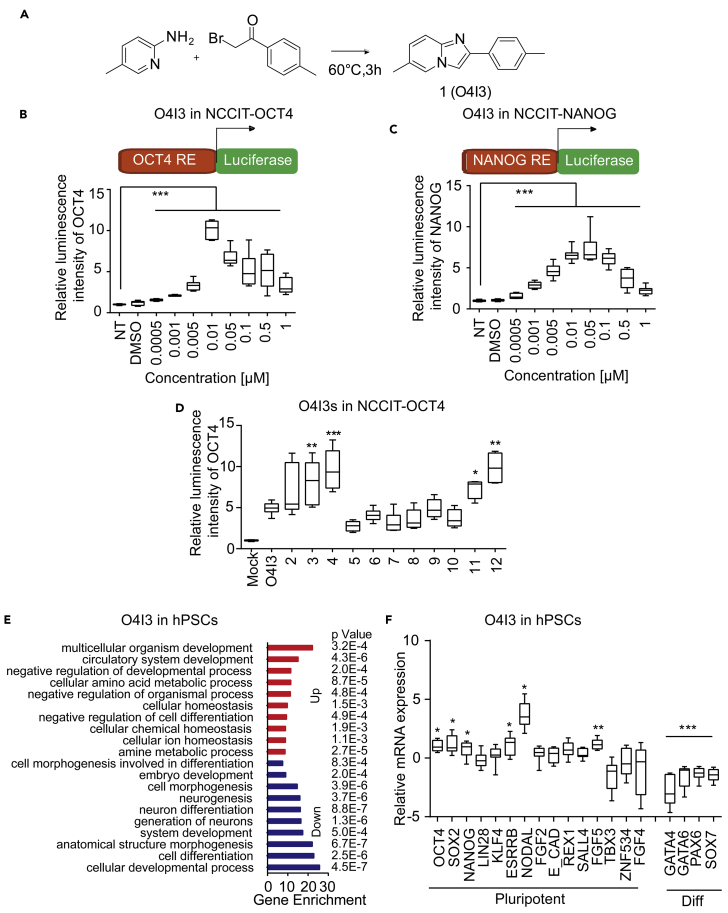
In an effort to find new chemical pluripotency inducers, we performed high-throughput screenings (HTSs) from a library containing ∼250,000 chemicals. The screen was based on cellular luciferase reporter assays, controlled by the promoter activity of pluripotency-associated transcription factors in HEK293 cells (Cheng et al., 2015a, Cheng et al., 2015b). Drug repurposing provides a successful way to develop new therapies with known, medically approved drugs within remarkably short periods (Cragg et al., 2014). With the expectation of having advantages in terms of pharmacodynamics and pharmacokinetics, we intentionally sought for chemicals sharing backbones with approved drugs. In the screen, we found nearly 4,000 imidazopyridine derivatives with significantly differential luciferase activities in the HEK-OCT4 reporter cell line (Figure S1A). To study this class of compounds in detail, we selected and synthesized the lead structure, 6-methyl-2-(p-tolyl)imidazo[1,2-a]pyridine (compound 1, Figure 1A), by heating ɷ-bromoacetophenone with an excess of 2-aminopyridine for 3 h to achieve a yield of >95% (Buu-HoÏ et al., 1954).
Figure 1.
Identification of Imidazopyridine Analogs as OCT4-Inducing Compounds
(A) Synthesis of the compound 1.
(B and C) (B) O4I3 activates OCT4 in NCCIT-OCT4 response element (RE)-driven luciferase reporter cells and (C) NANOG in NCCIT-NANOG RE-driven luciferase reporter cells after 48h treatment.
(D) Activity of O4I3 derivatives (50 nM) in NCCIT-OCT4 cells.
(E) Functional enrichment analysis shows statistically significant enrichment in Gene Ontology terms related to pluripotency, development, and homeostasis in O4I3-treated PSCs for 24 h. Red, upregulated genes; blue, downregulated genes. p values represent values after multitest adjustment (see details in Transparent Methods).
(F) qRT-PCR analysis of pluripotency- and differentiation (diff)-related gene expression in O4I3-treated hPSCs at a concentration of 10 nM for 48 h.
In (B), (C), and (F) statistical significance was compared with mock (01% DMSO) treatment, whereas in (D) it was compared with O4I3 treatment using two-way ANOVA and a post-hoc Tukey test. Data are represented as mean ± SD. ***p<0.001, **p<0.01, *p<0.05.
To examine the activity of endogenous OCT4 and its downstream gene NANOG, we generated reporter cell lines in human embryonic carcinoma cell lines (NCCIT), whose luciferase transcription is driven by response elements of either OCT4 (NCCIT-OCT4) or NANOG (NCCIT-NANOG). We found nearly 10-fold inductions of both OCT4 and NANOG by compound 1 at low nanomolar doses when compared with untreated cells (Figures 1B, 1C, and S1B). We then referred to it as OCT4-inducing compound 3 (O4I3). It has been shown that transient overexpression of OCT4 can lead to the differentiation of embryonic stem cells (ESCs) to extraembryonic endoderm and mesoderm (Niwa et al., 2000, Ovitt and Scholer, 1998). We found the induction of T and GATA4 in human PSCs (hPSCs) treated with 10 μM O4I3 (Figure S1C), suggesting that O4I3 might transiently hyperactivate OCT4 at high concentrations, which led to the differentiation and reduction of OCT4 activity (Figure 1B). We also tested the activity of zolpidem (containing the imidazopyridine backbone) in both reporter cell lines and detected an approximate 2-fold induction in NCCIT-OCT4 and NANOG reporter cells at a concentration of 10 μM (Figures S1D and S1E). Collectively, these results documented that O4I3 and zolpidem activated OCT4 at both the transcriptional and translational levels.
Pan-assay interference compounds are a class of compounds, referring to the hits from HTS, which give rise to an artificial signal in a drug-unlike manner (Baell and Walters, 2014). We chemically expanded O4I3 by replacing the CH3 group at the phenyl moiety with various substituents on the aryl moiety (Table S1) and found that compounds induced drug-like, structure-dependent activations in NCCIT-OCT4 cells (Figure 1D).
O4I3 Maintains Pluripotency of Human iPSCs
Functional annotation and enrichment analysis of DNA microarray in O4I3-treated hPSCs showed that O4I3 (10 nM) promoted hPSC homeostasis and repressed cellular differentiation (Figures 1E and S2A). qRT-PCR results confirmed that O4I3 stabilized or slightly increased the expression of pluripotency-associated genes (OCT4, SOX2, NANOG, and NODAL) and suppressed differentiation-related gene expression (GATA4, GATA6, PAX6, and SOX7) (Figure 1F). Cellular viability of hPSCs was increased up to 4-fold at low nanomolar concentrations of O4I3 when compared with cells treated with Rock inhibitor (Y-27632) (Figure S2B), a small molecule able to increase hPSC survival (Watanabe et al., 2007). Fluorescence-activated cell sorting (FACS) analysis confirmed that O4I3 (10 nM) supports pluripotency during the maintenance of hPSCs after at least 30 passages under feeder-free conditions as shown by the expression of TRA-1-81 and OCT4 (Figure S2C). In addition, zolpidem also promoted the maintenance of iPSCs at a concentration of 10 μM (Figure S2B).
O4I3 Promotes the Conversion of Human Resistant Fibroblasts into hiPSCs
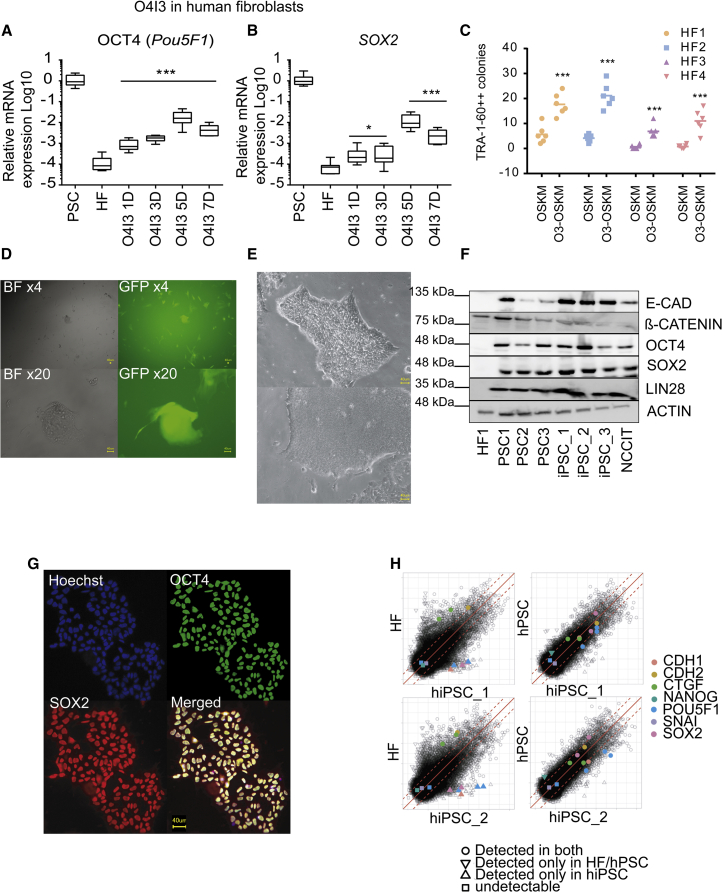
Analyzing the effects of O4I3 on HFs, we detected the activation of reprogramming-associated genes at the mRNA level (Figures 2A, 2B, and S3A), which included an approximately 10-fold induction of OCT4 (∼0.1% versus in hPSCs) and NANOG (∼1% versus hPSCs) and an about 100-fold increase of SOX2 (∼1% versus hPSCs) and CDH1 (∼10% versus hPSCs). We applied O4I3 in a commercially available episome-based reprogramming cocktail suitable for biosafety level 1 laboratory (Okita et al., 2011). These episomal vectors induce the expression of OCT4, SOX2, KLF4, LIN28, and L-MYC, and suppress p53 expression (referred to as OSKM). When compared with OSKM-only-treated fibroblasts, adding O4I3 (50 nM) to the cocktail increases the number of TRA-1-60-positive colonies (10–20 times) in both human primary fibroblasts (HF1 and HF2, Figure 2C).
Figure 2.
O4I3 Improves OSKM-Induced Reprogramming
(A and B) (A) O4I3 induces OCT4 and (B) SOX2 expression in human fibroblasts treated with O4I3 (50 nM) for the indicated time points (D, days).
(C) Efficiency of OSKM-induced reprogramming in the presence or absence of O4I3 in human fibroblasts 1–4 (HF1–HF4). ESC-like colonies were detected by TRA-1-60 staining.
(D) Reprogramming of resistant fibroblasts carrying LTR7-EGFP promoter. ESC-like GFP+ colonies were observed in bright field (BF) and GFP channel (GFP).
(E) ESC-like colonies detected from newly generated hiPSC lines.
(F) Expression of pluripotency-associated markers in the newly generated iPSC1, 2, and 3 when compared with hPSC1–3 as positive controls, determined by immunoblotting.
(G) Co-expression of OCT4 and SOX2 in the newly generated iPSCs.
(H) Scatterplots of gene expression data show high similarity between the newly generated iPSCs (hiPSC_1 and hiPSC_2) and hPSC (positive control), but not human fibroblasts (HF). All scatterplots are based on log2-transformed gene expression values. Red dashed lines depict the 2-fold change in expression values.
In (A) and (B) statistical significance was compared with mock (0.1% DMSO) treatment in fibroblasts, whereas in (C) it was compared with the efficiency obtained by ectopic expression of OSKM using two-way ANOVA followed by a post-hoc Tukey test. Data are represented as mean ± SD. ***p<0.001, **p<0.01, *p<0.05.
Studies have reported that senescence and other unknown cellular processes can confer donor fibroblasts resistance to reprogramming (Hayashi et al., 2016, Shore et al., 2006, Yamanaka, 2009). We tested if O4I3 enables reprogramming of refractory fibroblasts. For this, the following two HF cell lines resistant to OSKM-mediated reprogramming were selected: (1) the HFF-LTR7-EGFP cell line (at passage >40), which expresses EGFP under the control of the LTR7 promoter, known to be activated during reprogramming (Wang et al., 2014), and (2) HF4, isolated from a patient sample (Figure 2C). iPSC-like colonies appeared in both cases in the presence of O4I3 (Figures 2C and 2D) as well as in the presence of zolpidem at a concentration of 10 μM (Figure S3B).
Characterization of the Newly Generated hiPSCs
We expanded hiPSC-like colonies and cultivated them for at least 30 passages under feeder-free conditions. Established hiPSC lines showed typical human ESC morphology (Figure 2E) and expressed pluripotency markers such as OCT4, SOX2, E-Cadherin, and NANOG in a comparable manner to those in hPSCs (Figures 2F and 2G). More than 95% of new hiPSCs co-expressed TRA-1-60 and E-Cadherin, as well as TRA-1-81 and OCT4 analyzed by FACS (Figure S3C). Comparative DNA microarray data showed that pluripotency-associated genes (Pou5F1 encoding OCT4, CDH1 encoding E-Cadherin, EPCAM, and DNMT3B) were highly expressed in hiPSC_1 and hiPSC_2, whereas fibroblast-related genes (CDH2, TWIST1, TWIST2, and SNAI2) were suppressed (Figure S4A). Visualization of global gene expression profiles by both scatterplots and heatmap shows a higher similarity of gene expression patterns between the newly generated hiPSCs and hPSCs (as positive controls) when compared with that of HF (Figures 2H and S4B). Moreover, differentiated hiPSCs showed expression of markers of mesoderm (T, SLUG, and SNAI1), ectoderm (PAX6, Nestin, and Tuj1), and endoderm (AFP, FOXA2, and SOX17) (Figures S4C and S4D) using respective standard protocols, which further confirmed the pluripotency properties of the new hiPSCs.
O4I3 Is an Epigenetic Modulator
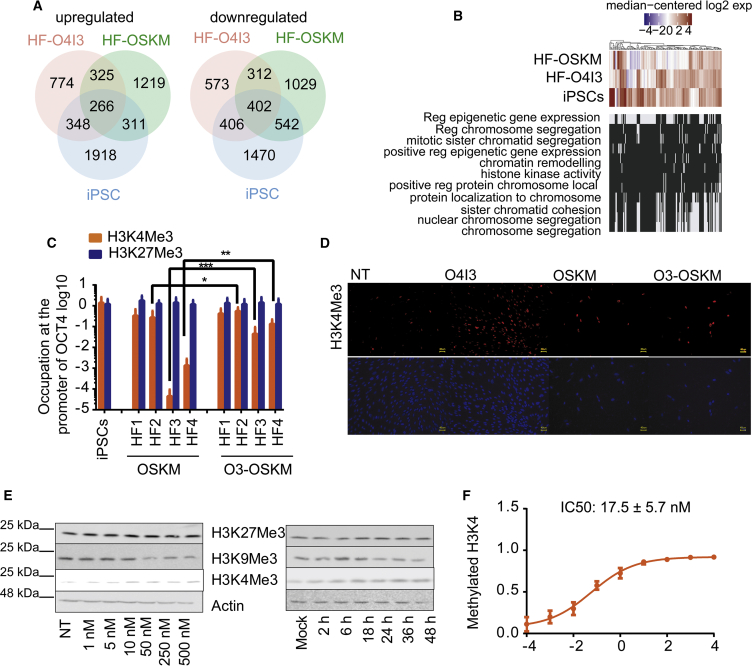
To obtain further insight into the mechanism of action of O4I3, we compared the global gene expression of HF, HF treated with O4I3 (HF-O4I3), HF transfected with episomal OSKM (HF-OSKM), and hiPSCs. Overlap in over 40% of all regulated genes was observed among HF-O4I3, HF-OSKM, and hiPSCs (Figure 3A), including genes involved in cell cycle progression and DNA replication (e.g., CDK1, MYC, and CDC25), and mesenchymal-epithelial-transition-associated genes (upregulation of CDH1 encoding E-Cadherin, EPCAM, CRB3, and OCLN, as well as downregulation of TWIST2). More than 30% of them were found to be involved in epigenetic modification and chromatin remodeling (Figures 3B and S5A), for example, OCT4 (POU5F1), LIN28, MYCN, KIFs, DNMT3B, HDAC2, TET1, EZH2, and JARID2, which are of importance in somatic reprogramming and maintenance of pluripotency (Apostolou and Hochedlinger, 2013, Mikkelsen et al., 2008). We performed Gene Ontology (GO)-based functional enrichment analysis of regulated genes in HF-O4I3 compared with HF and found that a number of specialized terms associated with epigenetic and chromatin regulation were on top of the regulated terms (Figure 3B), including sister chromatid segregation (GO:0000819) and sister chromatid cohesion (GO:0007062), ribonucleoprotein complex biogenesis (GO:0022613), organelle fission (GO:0048285), and DNA replication (GO:0006260). Gene enrichment analysis on RNA sequencing also showed that the top signaling pathways regulated by O4I3 in HFs included regulation of organismal process, system development and embryo implantation, as well as cell differentiation (Figure S5A)
Figure 3.
O4I3 Is an Epigenetic Modulator
(A) Venn diagram of up- and downregulated genes in O4I3 (50 nM)-treated human fibroblasts (HF) with ectopic expression of OCT4, SOX2, KLF4, and MYC (OSKM) as well as hiPSCs. HF-O4I3, HF cells treated with O4I3 (50 nM for 24 h); HF-OSKM, HF cells transfected with episomal OSKM; iPSCs, the newly generated iPSCs. Differential expression was calculated with respect to HF cells.
(B) Comparison of expression profiles of genes involved in epigenetic modification, chromatin remodeling, and related biological processes.
(C) Occupation of H3K4Me3 or H3K27Me3 at the promoter of OCT4 in HF cells transfected with OSKM alone or in the presence of 50 nM of O4I3 (O3-OSKM) for 3 days.
(D) O4I3 elevates the global levels of H3K4Me3 detected by immunocytochemistry. Cells were treated with 250 nM O4I3 for 3 days. Scale bar, 40 μm.
(E) Immunoblotting results show that O4I3 promotes global H3K4Me3 expression, whereas it represses H3K9Me3 protein levels in a concentration- and time-dependent manner. Cells were treated either with increasing concentrations of O4I3 for 24 h or with 250 nM of O4I3 for the indicated time points.
(F) O4I3 protects the methylation of H3K4, as observed by an in vitro methylation assay using total nuclear extraction.
In (C) statistical significance was compared with OSKM-treated fibroblasts using two-way ANOVA followed by a post-hoc Tukey test. Data are represented as mean ± SD. ***p<0.001, **p<0.01, *p<0.05.
Recently, Onder and co-workers performed a loss-of-function screen of 22 epigenetic regulators and found that the inhibition of DOT1L and eight other genes promoted iPSC generation (Onder et al., 2012). We found that O4I3 significantly repressed six of these nine genes, including DOT1L (Figure S5B).
O4I3 Promotes the Methylation of H3K4
hiPSC derivation is an epigenetic reprogramming process (Xie et al., 2017). Genome-wide analysis of histone modification and chromatin remodeling revealed the number of alternations occurring at the early stage of reprogramming, including the hypermethylation of H3K4 (Koche et al., 2011) and the demethylation of H3K27 and H3K9 (Chen et al., 2013, Tan et al., 2017). These loosen the compacted heterochromatin and promote transcription factors binding to the “open” chromatin to initiate the reprogramming (Koche et al., 2011, Soufi et al., 2012).
We investigated the transfection efficiency in HF1 and HF4 using the same episomal vector carrying cytomegalovirus (CMV)-driven GFP (Okita et al., 2011). We could not observe a significant difference between two cell lines, as determined by FACS analysis (Figure S5C). This result suggested that the resistance was unlikely associated with low transfection efficiency. To study the epigenetic effects of O4I3 and its relevance to reprogramming, we focused on two histone modifications at the promoter of OCT4, namely, H3K4Me3, known to be related to gene activation, and H3K27Me3, which indicates gene repression. Chromatin immunoprecipitation-qPCR results in two reprogrammable fibroblasts (HF1 and HF2) and in two reprogramming-resistant fibroblasts (HF3 and HF4) showed that OSKM was sufficient to induce abundant occupation of H3K4Me3 at the promoter of OCT4 in HF1 and HF2 in a comparable manner to those in iPSCs, while producing 1,000- to 10,000-fold less in reprogramming-resistant cells (Figures 3C and S5D). The level of H3K27Me3 at the OCT4 promoter was minimally affected in our experiments (Figure 3C). Analysis on the global level of H3K4Me3 by immunocytochemistry showed the increase of H3K4Me3 upon O4I3 treatment (Figures 3D and S5E). Immunoblotting confirmed a dose- and time-dependent increase of global H3K4Me3 expression in fibroblast, whereas H3K27Me3 remained mostly unaffected (Figure 3E). In an in vitro methylation assay, O4I3 protected methylated H3K4 with an IC50 value of 20 nM (Figure 3F). Trimethylation of H3K9 has been reported to block reprogramming by recruiting heterochromatin protein 1 to form heterochromatin at the core of pluripotency loci (Chen et al., 2013), which interferes with the hypermethylation of H3K4 (Binda et al., 2010). Accordingly, we found the reduction of global H3K9Me3 posterior to H3K4Me3 activation (Figures 3E and S5F).
O4I3 Is a Potent KDM5 Inhibitor
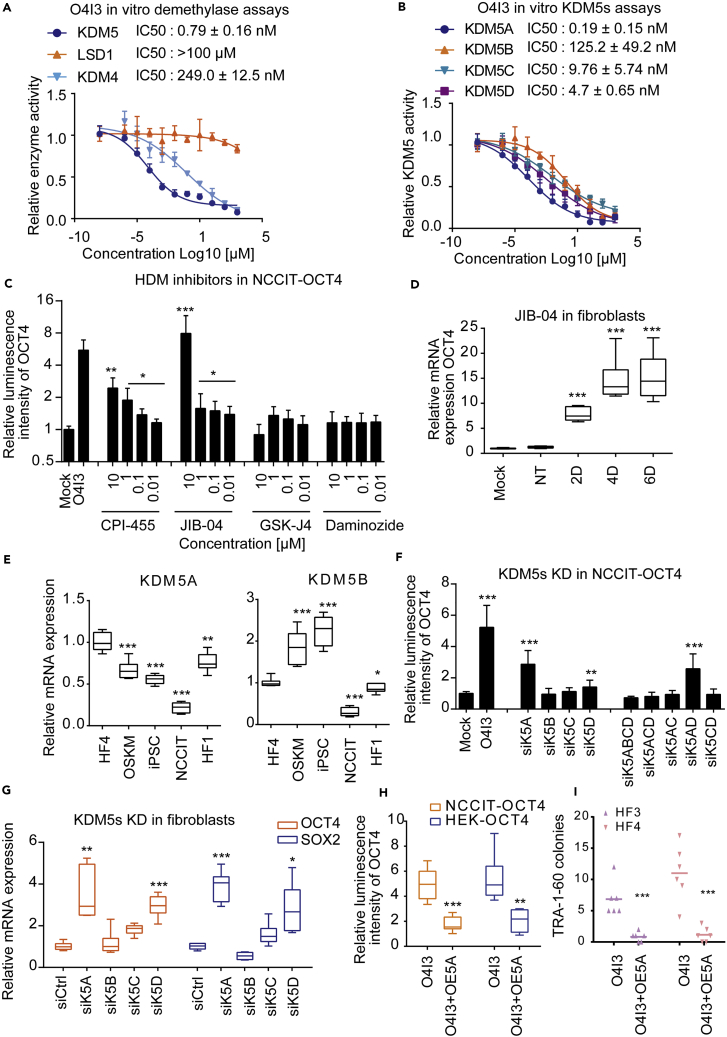
HMT and HDM are two major classes of enzymes, contributing to the regulation of histone methylation. Lysine-specific demethylase 1 (LSD1) and histone lysine demethylase 5 (KDM5, also known as JARID1) majorly catalyze demethylation of H3K4 (Kooistra and Helin, 2012). A few KDM5 chemical inhibitors have been reported to inhibit demethylation of H3K4, leading to an increase of global methylated H3K4 in various cell types (Johansson et al., 2016, Vinogradova et al., 2016, Wang et al., 2013). We tested the inhibitory effect of O4I3 on LSD1 and KDM5. KDM4 (also known as JMJD2), the HDM of H3K9 and H3K36, was also included. We found that O4I3 inhibited KDM5 with IC50 values of 0.79 nM, whereas it inhibited KDM4 with a 500-fold less potency (IC50: 249 nM). In the case of LSD1, we hardly detected the inhibitory effect of the molecule even at a concentration of 100 μM (Figure 4A).
Figure 4.
O4I3 Is a Selective KDM5A Inhibitor
(A) Comparison of O4I3 inhibitory effect on KDM5, LSD1, and KDM4 in vitro using the whole-cell nuclear extraction.
(B) The inhibitory effect of O4I3 on the members of KDM5 family of demethylases isolated from cells.
(C) A selective KDM5A inhibitor JIB-04 induces OCT4 expression in NCCIT-OCT4 cells. Four histone demethylase inhibitors (HDMs), namely, CPI-455, JIB-04, GSK-J4, and daminozide, were incubated with NCCIT-OCT4 reporter cells for 48 h.
(D) JIB-04 (5 μM) induces OCT4 expression in fibroblasts at the indicated time points (D, days).
(E) Comparison of KDM5A and KDM5B expression levels in fibroblast (HF1), resistant fibroblast (HF4), HF4 transfected with OSKM, iPSCs, and NCCIT.
(F) Knockdown of KDM5A (si5A) activates OCT4 in NCCIT-OCT4 reporter cells. Cells were transfected with various KDM5 siRNA concentrations for 48 h.
(G) Transient knockdown (48 h) of KDM5A induces OCT4 and SOX2 expression in fibroblasts.
(H) Overexpression of KDM5A (OE5A) compromises the effect of O4I3 (100 nM, 48 h) in NCCIT-OCT4 and HEK-OCT4 cells.
(I) The number of TRA-1-60-positive colonies was reduced in reprogramming-resistant fibroblasts with overexpression of KDM5A.
In (C), (D), and (F) statistical significance was compared with mock (0.1% DMSO) treatment, in (G) with non-targeting siRNA and in (E) with HF4, whereas in (H) and (I) it was compared with OSKM-treated fibroblasts using two-way ANOVA followed by a post-hoc Tukey test. Data are represented as mean ± SD. ***p<0.001, **p<0.01, *p<0.05.
In mammalian cells, the KDM5 family consists of four members, namely, KDM5A (known as JARID1A), KDM5B (known as JARID1B or PLU1), KDM5C (JARID1C), and KDM5D (JARID1D or SMCY) (Johansson et al., 2016). Selectivity was found for KDM5A with an IC50 value of 0.19 nM, whereas 20-, 40-, and 1,000-fold less potent IC50 values were obtained in the case of KDM5D, KDM5C, and KDM5B, respectively (Figure 4B). As expected, zolpidem protected H3K4 methylation and inhibited the expression of both KDM5 and KDM4 at micromolar concentrations (Figure S6A). Next, we tested four KDM chemical inhibitors. We found 3- and 5-fold induction of OCT4 expression in NCCIT-OCT4 reporter cell line in the presence of KDM5 inhibitors, CPI-455 (Vinogradova et al., 2016) and JIB-04 (Wang et al., 2013), respectively, but not in the presence of H3K27 demethylase inhibitor, GSK-J4 (Kruidenier et al., 2012) or the KDM2/7 inhibitor, daminozide (Rose et al., 2012), a plant growth regulator (Figure 4C). We detected the induction of OCT4, SOX2, and E-Cadherin expression in JIB-04-treated HFs (Figures 4D and S6B).
JIB-04 is a potent KDM5A inhibitor (IC50: 230 nM) and was found to produce a higher induction of OCT4 compared with the pan KDM5 inhibitor, CPI-445 (Figure 4C). We questioned if the members of the KDM5 family contribute differently to the induction of pluripotency. KDM5B has been shown to be crucial in the early embryonic development and plays an important role in ESC maintenance (Xie et al., 2011) and neural differentiation (Schmitz et al., 2011). However, little is known about the function of other members of the KDM5 family of genes in development. We thus re-analyzed the KDM5A-D expression during human preimplantation (Vassena et al., 2011) and compared their expression patterns with those of the putative pluripotency markers, OCT4 and NANOG. KDM5B showed a similar expression pattern to OCT4 and NANOG, whereas KDM5A behaved oppositely (Figure S6C). In the case of KDM5D, the difference was not significant and KDM5C remained unaffected between ESC and oocyte (Figure S6C). We further compared the expression of KDM5A-5D in fibroblasts, reprogrammed fibroblasts by OSKM, iPSCs, and NCCIT (Figures 4E and S7A). KDM5A was preferentially expressed in reprogramming-resistant fibroblasts, whereas KDM5B was more pluripotency related (Figure 4E), which was in agreement with previous gene expression profiling data (Figure S7B) (Takahashi et al., 2014), as well as immunoblotting results (Figure S7C). As JIB-04 also inhibits KDM4 and KDM6 demethylases, we repressed the expression of KDM5A-D using RNA interference (small interfering RNA [siRNA]) and found significantly higher OCT4 reporter activity in NCCIT-OCT4 cells (Figure 4F), as well as higher mRNA levels of OCT4 and SOX2 in HFs in the absence of KDM5A and KDM5D expression, but not in KDM5B and KDM5C knockdown cells (Figures 4G and S7D). Notably, the effects of KDM5D repression were found to be less significant when compared with those of KDM5A knockdown, most probably due to the various transfection efficiencies (Figure S7D). Moreover, knockdown of KDM5A also increased the global levels of H3K4Me3 with or without OSKM (Figure S7E). Overexpression of KDM5A compromised these effects caused by KDM5A inhibition (either O4I3 or JIB-04) in NCCIT-OCT4 reporter cells in a concentration-dependent manner, as well as in HEK-OCT4 reporter cells (Figures 4H, S7F, and S7G). Conversely, cells were resistant to KDM5A chemical inhibitors in the absence of KDM5A expression (Figure S7G). Of note, at concentrations of 10 and 100 nM, O413 treatment alone showed higher OCT4 levels when compared with that of O4I3 combined with siRNA-mediated KDM5A inhibition (Figure S7G), which may be due to the insufficient knockdown efficiency (Figure S7D). As expected, overexpression of KDM5A also affected the formation of TRA-1-60-positive colonies in OSKM-mediated reprogramming of HFs (Figure 4I). Taken together, our results confirm the importance of KDM5A in the induction of pluripotency markers and the process of reprogramming of HFs.
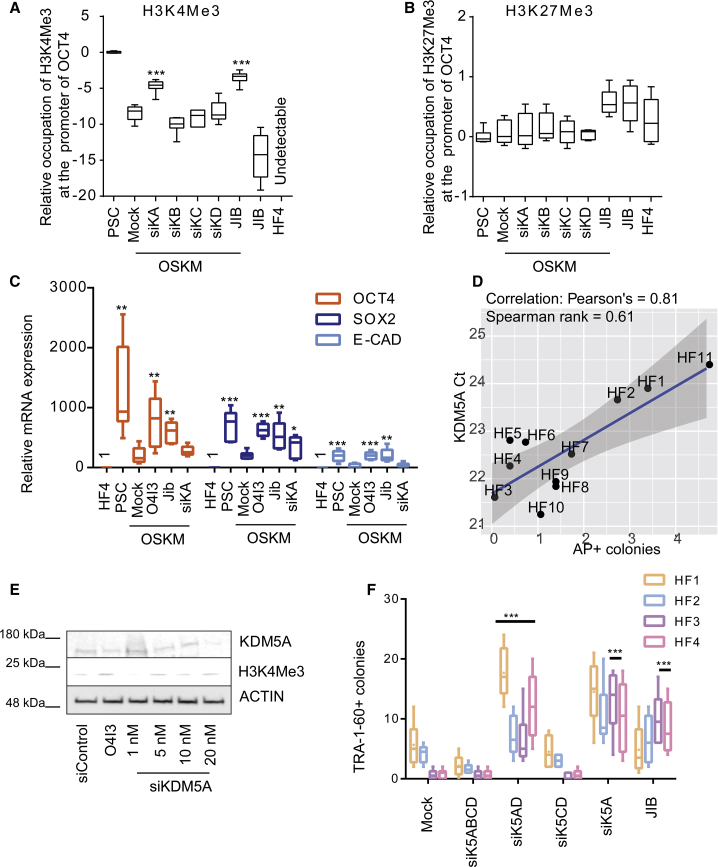
Inhibition of KDM5A Promotes Reprogramming of Resistant Fibroblasts
In reprogramming-resistant HF4, the level of H3K4Me3 at the promoter of OCT4 was increased by OSKM, but was still nearly 1,000-fold lower than that in PSCs (Figure 5A). In combination with OSKM overexpression, genetic repression of KDM5A expression using either KDM5A siRNA or chemical inhibition of KDM5A activity by JIB-04, significantly elevated the levels of H3K4Me3 (Figure 5A). However, a clear influence on H3K4 methylation was not observed in the presence of KDM5B-, KDM5C-, or KDM5D-siRNA (Figure 5A). Correspondingly, OSKM failed to force the expression of reprogramming-associated genes (POU5F1, SOX2, and CDH1) in the absence of KDM5A siRNA or JIB-04 in reprogramming-resistant fibroblasts (Figure 5C), implicating that OSKM was not sufficient to activate OCT4 to initiate reprogramming in those patient primary fibroblasts. Indeed, the reprogramming efficiency is negatively correlated with the expression of KDM5A in 11 HFs (Figure 5D). Moreover, KDM5A siRNA increased the global level of H3K4Me3 in a dose-dependent manner (Figures 5E and S7E). Of note, the alternation in the occupation of H3K27Me3 at the promoter of OCT4 was more refractory to chemical inhibition or genetic repression of KDM5s in our experiments (Figure 5B).
Figure 5.
KDM5A Is a Reprogramming Barrier
(A and B) (A) Suppression of KDM5A activity by siRNA or JIB-04 (5 μM, 48 h) promotes the enrichment of H3K4Me3 at the promoter of OCT4 in resistant fibroblasts, (B) but not that of H3K27Me3. Ct values were obtained from qRT-PCR.
(C) Suppression of KDM5A activity by JIB-04 or KDM5A siRNA (siKA) induces POU5F1, SOX2, and CDH1 mRNA levels during reprogramming, as determined by qRT-PCR.
(D) Correlation between reprogramming efficiency and KDM5A expression in HF1-HF11. The same amount of each cDNA was used as template for qRT-PCR where the Ct values indicate the expression of KDM5A.
(E) Knockdown effects of increasing concentrations of anti-KDM5A siRNA (siKDM5A, 48 h) on H3K4Me3 expression levels in HF.
(F) Reprogramming efficiency using various patient primary fibroblasts, as determined by the number of TRA-1-60-positive colonies. Cells were either transiently transfected with the indicated siRNA oligos in the first 5 days or treated with JIB-04 (5 μM, 48 h). siK5ABCD, anti-KDM5A/B/C/D siRNA; siK5AD, anti-KDM5A/D siRNA; siK5CD, anti-KDM5C/D siRNA; siK5A, anti-KDM5A siRNA; siK5B, anti-KDM5B siRNA; siK5C, anti-KDM5C siRNA; and siK5D, anti-KDM5D siRNA. In (A), (B), and (F) statistical significance was compared with OSKM-treated fibroblasts, whereas in (C) it was compared with DMSO (0.1%) treatment using two-way ANOVA and a post-hoc Tukey test. Data are represented as mean ± SD. ***p<0.001, **p<0.01, *p<0.05.
Transient suppression of KDM5A expression with either KDM5A siRNA or chemical inhibition mimicked the effect of O4I3 to either increase the episomal-based reprogramming efficiency in normal fibroblasts or to facilitate the formation of ESC-like TRA-1-60-positive colonies in reprogramming-resistant fibroblasts (Figures 5F and S8A). These ESC-like colonies could be isolated and expanded to establish stable hiPSC cell lines with the expression of pluripotency markers, like OCT4, SOX2, E-Cadherin, TRA-1-60, and TRA-1-81, and full differentiation potential (Figures S8B and S8C).
Discussion
In 2006, Yamanaka and his co-workers successfully converted mouse fibroblasts into iPSCs with viral overexpression of OSKM (Takahashi and Yamanaka, 2006). This technology provides a simple approach to manipulate cell fate and thereby greatly advances the research in the field of regenerative medicine (Takahashi et al., 2007, Takahashi and Yamanaka, 2016). After a decade of research, the molecular mechanisms by which OSKM mediated somatic cell reprogramming are still incompletely understood. Reprogramming is a stochastic and inefficient process (Yamanaka, 2009). Initially, Takahashi et al. reported a reprogramming efficiency of 0.01% in HFs using retroviral infection (Takahashi et al., 2007). Recently, non-integrating reprogramming methods have been established, including episomal transfection, one of a few approaches suitable for biosafety level 1 laboratory. However, there are several shortcomings being associated with this technique, including low reprogramming efficiency (0.01%) (Schlaeger et al., 2015). Seeking for small molecules able to promote somatic cell reprogramming, we screened ∼250,000 chemicals in cell-based assays and identified a series of O4Is (Cheng et al., 2015a, Cheng et al., 2015b). Here, we report that O4I3, a compound sharing an imidazopyridine backbone with the anti-insomnia drug zolpidem (Weitzel et al., 2000), not only promotes the viability of hPSC in single-cell expansion and elevates the episome-based reprogramming efficiency (from ∼0.01% to ∼0.1%) but also importantly facilitates the reprogramming of patient primary fibroblasts, which are resistant to reprogramming.
In general, the ability of transcription factors to bind their corresponding promoter elements is influenced by epigenetic properties of the chromatin structure, including DNA methylation, histone modifications, and ATP-dependent chromatin remodeling. A number of studies have shown that Yamanaka transcription factors recruit a number of epigenetic modifiers including histone post-translational-modifying enzymes, nucleosome remodeling factors, and DNA methylation enzymes at multiple stages of the reprogramming process (Smith et al., 2016, Soufi et al., 2012). In terminally differentiated somatic cells, genomic DNA related to pluripotency is tightly compacted into nucleosomes, the basic structure of chromatin consisting of the histone octamer (two copies, each consists of four histone molecules) wrapped with a length of 146-base pair DNA, to form higher-order chromatin structures, called heterochromatin, for gene silencing (Papp and Plath, 2013). At the initial stage of reprogramming, the pioneer factors, OCT4, SOX2, and KLF4 (OSK), bind to the nucleosomal pluripotency loci preferentially at regions far from the TSS (TSS-distal), and also at TSS-proximal, and induce H3K4 methylation first at the enhancer regions 50–500 kb from TSS (Soufi et al., 2012, Soufi et al., 2015) and later on at the promoters, resulting in gene expression changes (Atlasi and Stunnenberg, 2017, Soufi et al., 2015). Nevertheless, the influence of OSK on the pre-existing histone modifications, H3K27Me3, for instance, is very limited (Soufi et al., 2015). Furthermore, a genome-wide methylome analysis has previously revealed the gain of H3K4Me3 at promoters of pluripotency-associated genes during somatic cell reprogramming of mouse fibroblasts and B lymphocytes (Mikkelsen et al., 2008). Thus it is conceivable that applying chemicals that protect H3K4 methylation could overcome this epigenetic barrier during the reprogramming process. O4I3 not only enhanced the expression of global H3K4Me3 in cells and prevented the demethylation of H3K4 in vitro but also increased the occupancy of H3K4Me3 at the promoter of OCT4. Consequently, exogenous reprogramming factors bind to promoter regions marked by trimethylated H3K4, which makes the chromatin structure more accessible.
Histone methylation is a reversible process. In mammals, the COMPASS (complex proteins-associated with SET1)-like proteins belong to the “writers” of H3K4, whereas KDM5s specifically remove (“erase”) methyl groups from H3K4, unlike LSD1, which also demethylates H3K9Me1/2/3 (Kooistra and Helin, 2012). In this regard, the LSD1 inhibitor, parnate has been reported to promote reprogramming (Hou et al., 2013, Xie et al., 2017). Methylation of H3K9 represses gene expression and is considered as an epigenetic barrier for reprogramming (Chen et al., 2013). Thus parnate-induced pluripotency may be due to blocking the expression of differentiation-related genes via inhibition of H3K9 demethylation along with the activation of pluripotency markers through the enrichment of methylated H3K4 at their corresponding promoters or enhancers. We observed that O4I3 and zolpidem specifically inhibited KDM5 activity, an effect that could not be detected in the case of LSD1, suggesting that facilitation of H3K4 methylation at the promoter and enhancer regions of pluripotency genes might be the major reason for imidazopyridine-induced reprogramming in resistant fibroblasts.
Dynamic regulation of H3K4 methylation from oocyte stage to preimplantation suggests that KDM5s contribute to the embryonic development (Atlasi and Stunnenberg, 2017). Indeed, the essential role of KDM5B in ESC self-renewal and pluripotency has been reported (Schmitz et al., 2011, Xie et al., 2011). Re-analysis of gene expression data during human preimplantation (Vassena et al., 2011) interestingly showed a negatively correlated expression pattern of KDM5A when compared with that of KDM5B and to those of pluripotency markers, OCT4 and NANOG. We found that KDM5A was highly expressed in HFs and was repressed during cellular reprogramming. Thus the observation that O4I3 supported the maintenance and acquisition of pluripotency in HFs might be due to its selective inhibition on KDM5A. As a proof of concept, suppression of KDM5A expression either chemically, using the specific chemical inhibitor JIB-04 (Wang et al., 2013), or with siRNA mimicked the function of O4I3, in terms of induction of pluripotency markers, whereas ectopic expression of KDM5A compromised the effect of O4I3 on reprogramming. Recently, Pfaff et al. reported that de-repression of Kdm5A by miRNA-212/132 improved the reprogramming efficiency in murine embryonic fibroblasts (Pfaff et al., 2017). Mouse iPSCs are close to naive ESCs, whereas hiPSCs are rather primed pluripotent. Thus, it is interesting to investigate whether KDM5A plays opposing roles in naive and primed states of pluripotency.
The generation of patient-specific clinic-grade iPSCs is a prerequisite for applying transcription-factor-mediated reprogramming for personalized therapies. Compelling experimental results showed that the initiation of reprogramming is associated with hypermethylation of H3K4 at the promoters or enhancers of pluripotency genes. We identified a novel class of imidazopyridines as potent H3K4-specific KDM5 inhibitors through HTS and demonstrated that KDM5A, but not KDM5B, is an epigenetic barrier to reprogramming and that inhibition of KDM5A activity enables the induction of pluripotency in reprogramming-resistant patient-derived primary fibroblasts.
Limitations of the Study
There are several issues remaining to be addressed with regard to the inhibitory effect of O4I3 on KDM5 and its correlation with the induction of pluripotency, including (1) the influence of O413 on epigenetic modifiers other than those of the KDM5 family, such as other histone methyl transferases or demethylases; (2) the mode of interaction of O413 with KDM5 demethylase enzymes; and most importantly (3) the combinations of small molecules by which exogenous OCT4 can be replaced in the context of OSKM-mediated reprogramming.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We thank Prof. Stefan Wölfl for his kind support and valuable suggestions. We thank Luuk N. van Oosten for his critical comments to the manuscript. We thank Jie Cheng for designing chemical synthesis and Sawsan Saleh and Felix Braun for helping with chemical synthesis and characterization. We thank Mohamed A. Abu el Maaty for performing protein ELISA microarray analysis. We thank Viola Mayer for her technical support. We thank Maryam Dabiri for her support in the preparation of the cover image.
This work is supported by the Bundesministerium für Bildung und Forschung (BMBF) grant programs Drug-iPS (FKZ 0315398A and B) and SysToxChip (FKZ 031A303A and E). X.C. is a recipient of the Deutsche Forschungsgemeinschaft (DFG) grant program (CH 1690/2-1), and Y.D. is a recipient of the “Landesgraduiertenförderung (LGF) fellowship program for individual doctoral training” from Universität Heidelberg.
Author Contributions
Y.D. and R.A.G.-B. performed bioassessments. K.T. performed gene expression analyses as well as downstream data analyses. K.H., S.R., J.A., and R.M. established the reporter cell lines. J.U. isolated patient fibroblasts. J.W. performed RNA-seq experiment and data analysis. M.A.A.-N. was involved in the supervision of the bioinformatics analyses, manuscript correction, and discussion. X.C. designed and performed chemical syntheses as well as biological experiments, analyzed the data, and wrote the manuscript. All authors read and approved the manuscript before submission.
Declaration of Interests
J.A., R.M., and X.C. together with Heidelberg University hold a patent for O4I3 (EP: U21-16ERF).
Published: February 22, 2019
Footnotes
Supplemental Information includes Transparent Methods, eight figures, and three tables and can be found with this article online at https://doi.org/10.1016/j.isci.2019.01.012.
Data and Software Availibility
All DNA microarray data have been deposited in GEO under the accession number: GSE123668. RNA sequencing data have been deposited in the Bioproject database, NCBI. The accession number is SUB4899089.
Supplemental Information
References
- Apostolou E., Hochedlinger K. Chromatin dynamics during cellular reprogramming. Nature. 2013;502:462–471. doi: 10.1038/nature12749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atlasi Y., Stunnenberg H.G. The interplay of epigenetic marks during stem cell differentiation and development. Nat. Rev. Genet. 2017;18:643–658. doi: 10.1038/nrg.2017.57. [DOI] [PubMed] [Google Scholar]
- Baell J., Walters M.A. Chemical con artists foil drug discovery. Nature. 2014;513:481–483. doi: 10.1038/513481a. [DOI] [PubMed] [Google Scholar]
- Binda O., LeRoy G., Bua D.J., Garcia B.A., Gozani O., Richard S. Trimethylation of histone H3 lysine 4 impairs methylation of histone H3 lysine 9: regulation of lysine methyltransferases by physical interaction with their substrates. Epigenetics. 2010;5:767–775. doi: 10.4161/epi.5.8.13278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buu-HoÏ N.P., Jacquignon P., Xuong N.D., Lavit D. 2-ARYLPYRROCOLINES AND 2-ARYLPYRIMIDAZOLES. J. Org. Chem. 1954;19:1370–1375. [Google Scholar]
- Chen J., Liu H., Liu J., Qi J., Wei B., Yang J., Liang H., Chen Y., Chen J., Wu Y. H3K9 methylation is a barrier during somatic cell reprogramming into iPSCs. Nat. Genet. 2013;45:34–42. doi: 10.1038/ng.2491. [DOI] [PubMed] [Google Scholar]
- Cheng X., Dimou E., Alborzinia H., Wenke F., Gohring A., Reuter S., Mah N., Fuchs H., Andrade-Navarro M.A., Adjaye J. Identification of 2-[4-[(4-Methoxyphenyl)methoxy]-phenyl]acetonitrile and derivatives as potent Oct3/4 inducers. J. Med.Chem. 2015;58:4976–4983. doi: 10.1021/acs.jmedchem.5b00144. [DOI] [PubMed] [Google Scholar]
- Cheng X., Yoshida H., Raoofi D., Saleh S., Alborzinia H., Wenke F., Gohring A., Reuter S., Mah N., Fuchs H. Ethyl 2-((4-Chlorophenyl)amino)thiazole-4-carboxylate and derivatives are potent inducers of Oct3/4. J. Med.Chem. 2015;58:5742–5750. doi: 10.1021/acs.jmedchem.5b00226. [DOI] [PubMed] [Google Scholar]
- Cragg G.M., Grothaus P.G., Newman D.J. New horizons for old drugs and drug leads. J. Nat. Prod. 2014;77:703–723. doi: 10.1021/np5000796. [DOI] [PubMed] [Google Scholar]
- Gore A., Li Z., Fung H.L., Young J.E., Agarwal S., Antosiewicz-Bourget J., Canto I., Giorgetti A., Israel M.A., Kiskinis E. Somatic coding mutations in human induced pluripotent stem cells. Nature. 2011;471:63–67. doi: 10.1038/nature09805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y., Hsiao E.C., Sami S., Lancero M., Schlieve C.R., Nguyen T., Yano K., Nagahashi A., Ikeya M., Matsumoto Y. BMP-SMAD-ID promotes reprogramming to pluripotency by inhibiting p16/INK4A-dependent senescence. Proc.Natl.Acad.Sci. U S A. 2016;113:13057–13062. doi: 10.1073/pnas.1603668113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou P.P., Li Y.Q., Zhang X., Liu C., Guan J.Y., Li H.G., Zhao T., Ye J.Q., Yang W.F., Liu K. Pluripotent stem cells induced from mouse somatic cells by small-molecule compounds. Science. 2013;341:651–654. doi: 10.1126/science.1239278. [DOI] [PubMed] [Google Scholar]
- Hussein S.M., Batada N.N., Vuoristo S., Ching R.W., Autio R., Narva E., Ng S., Sourour M., Hamalainen R., Olsson C. Copy number variation and selection during reprogramming to pluripotency. Nature. 2011;471:58–U67. doi: 10.1038/nature09871. [DOI] [PubMed] [Google Scholar]
- Johansson C., Velupillai S., Tumber A., Szykowska A., Hookway E.S., Nowak R.P., Strain-Damerell C., Gileadi C., Philpott M., Burgess-Brown N. Structural analysis of human KDM5B guides histone demethylase inhibitor development. Nat. Chem. Biol. 2016;12:539–545. doi: 10.1038/nchembio.2087. [DOI] [PubMed] [Google Scholar]
- Koche R.P., Smith Z.D., Adli M., Gu H., Ku M., Gnirke A., Bernstein B.E., Meissner A. Reprogramming factor expression initiates widespread targeted chromatin remodeling. Cell Stem Cell. 2011;8:96–105. doi: 10.1016/j.stem.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooistra S.M., Helin K. Molecular mechanisms and potential functions of histone demethylases. Nature Rev. Mol. Cell Biol. 2012;13:297–311. doi: 10.1038/nrm3327. [DOI] [PubMed] [Google Scholar]
- Kruidenier L., Chung C.W., Cheng Z., Liddle J., Che K., Joberty G., Bantscheff M., Bountra C., Bridges A., Diallo H. A selective jumonji H3K27 demethylase inhibitor modulates the proinflammatory macrophage response. Nature. 2012;488:404–408. doi: 10.1038/nature11262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H., Morey R., O'Neil R.C., He Y., Daughtry B., Schultz M.D., Hariharan M., Nery J.R., Castanon R., Sabatini K. Abnormalities in human pluripotent cells due to reprogramming mechanisms. Nature. 2014;511:177–183. doi: 10.1038/nature13551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen T.S., Hanna J., Zhang X., Ku M., Wernig M., Schorderet P., Bernstein B.E., Jaenisch R., Lander E.S., Meissner A. Dissecting direct reprogramming through integrative genomic analysis. Nature. 2008;454:49–55. doi: 10.1038/nature07056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morizane A., Doi D., Kikuchi T., Okita K., Hotta A., Kawasaki T., Hayashi T., Onoe H., Shiina T., Yamanaka S. Direct comparison of autologous and allogeneic transplantation of iPSC-derived neural cells in the brain of a non-human primate. Stem Cell Rep. 2013;1:283–292. doi: 10.1016/j.stemcr.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa H., Miyazaki J., Smith A.G. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat. Genet. 2000;24:372–376. doi: 10.1038/74199. [DOI] [PubMed] [Google Scholar]
- Okita K., Matsumura Y., Sato Y., Okada A., Morizane A., Okamoto S., Hong H., Nakagawa M., Tanabe K., Tezuka K. A more efficient method to generate integration-free human iPS cells. Nat. Methods. 2011;8:409–412. doi: 10.1038/nmeth.1591. [DOI] [PubMed] [Google Scholar]
- Onder T.T., Kara N., Cherry A., Sinha A.U., Zhu N., Bernt K.M., Cahan P., Marcarci B.O., Unternaehrer J., Gupta P.B. Chromatin-modifying enzymes as modulators of reprogramming. Nature. 2012;483:598–602. doi: 10.1038/nature10953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovitt C.E., Scholer H.R. The molecular biology of Oct-4 in the early mouse embryo. Mol. Hum.Reprod. 1998;4:1021–1031. doi: 10.1093/molehr/4.11.1021. [DOI] [PubMed] [Google Scholar]
- Papp B., Plath K. Epigenetics of reprogramming to induced pluripotency. Cell. 2013;152:1324–1343. doi: 10.1016/j.cell.2013.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaff N., Liebhaber S., Mobus S., Beh-Pajooh A., Fiedler J., Pfanne A., Schambach A., Thum T., Cantz T., Moritz T. Inhibition of miRNA-212/132 improves the reprogramming of fibroblasts into induced pluripotent stem cells by de-repressing important epigenetic remodelling factors. Stem Cell Res. 2017;20:70–75. doi: 10.1016/j.scr.2017.03.003. [DOI] [PubMed] [Google Scholar]
- Rose N.R., Woon E.C., Tumber A., Walport L.J., Chowdhury R., Li X.S., King O.N., Lejeune C., Ng S.S., Krojer T. Plant growth regulator daminozide is a selective inhibitor of human KDM2/7 histone demethylases. J. Med.Chem. 2012;55:6639–6643. doi: 10.1021/jm300677j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaeger T.M., Daheron L., Brickler T.R., Entwisle S., Chan K., Cianci A., DeVine A., Ettenger A., Fitzgerald K., Godfrey M. A comparison of non-integrating reprogramming methods. Nat.Biotechnol. 2015;33:58–63. doi: 10.1038/nbt.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz S.U., Albert M., Malatesta M., Morey L., Johansen J.V., Bak M., Tommerup N., Abarrategui I., Helin K. Jarid1b targets genes regulating development and is involved in neural differentiation. EMBO J. 2011;30:4586–4600. doi: 10.1038/emboj.2011.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiba Y., Gomibuchi T., Seto T., Wada Y., Ichimura H., Tanaka Y., Ogasawara T., Okada K., Shiba N., Sakamoto K. Allogeneic transplantation of iPS cell-derived cardiomyocytes regenerates primate hearts. Nature. 2016;538:388–391. doi: 10.1038/nature19815. [DOI] [PubMed] [Google Scholar]
- Shore E.M., Xu M., Feldman G.J., Fenstermacher D.A., Cho T.J., Choi I.H., Connor J.M., Delai P., Glaser D.L., LeMerrer M. A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nat. Genet. 2006;38:525–527. doi: 10.1038/ng1783. [DOI] [PubMed] [Google Scholar]
- Smith Z.D., Sindhu C., Meissner A. Molecular features of cellular reprogramming and development. Nature reviews. Mol. Cell. Biol. 2016;17:139–154. doi: 10.1038/nrm.2016.6. [DOI] [PubMed] [Google Scholar]
- Soufi A., Donahue G., Zaret K.S. Facilitators and impediments of the pluripotency reprogramming factors' initial engagement with the genome. Cell. 2012;151:994–1004. doi: 10.1016/j.cell.2012.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soufi A., Garcia M.F., Jaroszewicz A., Osman N., Pellegrini M., Zaret K.S. Pioneer transcription factors target partial DNA motifs on nucleosomes to initiate reprogramming. Cell. 2015;161:555–568. doi: 10.1016/j.cell.2015.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Tanabe K., Ohnuki M., Narita M., Sasaki A., Yamamoto M., Nakamura M., Sutou K., Osafune K., Yamanaka S. Induction of pluripotency in human somatic cells via a transient state resembling primitive streak-like mesendoderm. Nat.Commun. 2014;5:3678. doi: 10.1038/ncomms4678. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Yamanaka S. A decade of transcription factor-mediated reprogramming to pluripotency. Nature reviews. Mol. Cell Biol. 2016;17:183–193. doi: 10.1038/nrm.2016.8. [DOI] [PubMed] [Google Scholar]
- Tan L., Ke Z., Tombline G., Macoretta N., Hayes K., Tian X., Lv R., Ablaeva J., Gilbert M., Bhanu N.V. Naked mole rat cells have a stable epigenome that resists iPSC reprogramming. Stem Cell Rep. 2017;9:1721–1734. doi: 10.1016/j.stemcr.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassena R., Boue S., Gonzalez-Roca E., Aran B., Auer H., Veiga A., Izpisua Belmonte J.C. Waves of early transcriptional activation and pluripotency program initiation during human preimplantation development. Development. 2011;138:3699–3709. doi: 10.1242/dev.064741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinogradova M., Gehling V.S., Gustafson A., Arora S., Tindell C.A., Wilson C., Williamson K.E., Guler G.D., Gangurde P., Manieri W. An inhibitor of KDM5 demethylases reduces survival of drug-tolerant cancer cells. Nat. Chem. Biol. 2016;12:531–538. doi: 10.1038/nchembio.2085. [DOI] [PubMed] [Google Scholar]
- Wang J., Xie G., Singh M., Ghanbarian A.T., Rasko T., Szvetnik A., Cai H., Besser D., Prigione A., Fuchs N.V. Primate-specific endogenous retrovirus-driven transcription defines naive-like stem cells. Nature. 2014;516:405–409. doi: 10.1038/nature13804. [DOI] [PubMed] [Google Scholar]
- Wang L., Chang J., Varghese D., Dellinger M., Kumar S., Best A.M., Ruiz J., Bruick R., Pena-Llopis S., Xu J. A small molecule modulates Jumonji histone demethylase activity and selectively inhibits cancer growth. Nat.Commun. 2013;4:2035. doi: 10.1038/ncomms3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K., Ueno M., Kamiya D., Nishiyama A., Matsumura M., Wataya T., Takahashi J.B., Nishikawa S., Nishikawa S., Muguruma K. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat.Biotechnol. 2007;25:681–686. doi: 10.1038/nbt1310. [DOI] [PubMed] [Google Scholar]
- Weitzel K.W., Wickman J.M., Augustin S.G., Strom J.G. Zaleplon: a pyrazolopyrimidine sedative-hypnotic agent for the treatment of insomnia. Clin.Ther. 2000;22:1254–1267. doi: 10.1016/s0149-2918(00)83024-6. [DOI] [PubMed] [Google Scholar]
- Xie L., Pelz C., Wang W., Bashar A., Varlamova O., Shadle S., Impey S. KDM5B regulates embryonic stem cell self-renewal and represses cryptic intragenic transcription. EMBO J. 2011;30:1473–1484. doi: 10.1038/emboj.2011.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie M., Tang S., Li K., Ding S. Pharmacological reprogramming of somatic cells for regenerative medicine. Acc. Chem. Res. 2017;50:1202–1211. doi: 10.1021/acs.accounts.7b00020. [DOI] [PubMed] [Google Scholar]
- Yamanaka S. Elite and stochastic models for induced pluripotent stem cell generation. Nature. 2009;460:49–52. doi: 10.1038/nature08180. [DOI] [PubMed] [Google Scholar]
- Yamanaka S., Blau H.M. Nuclear reprogramming to a pluripotent state by three approaches. Nature. 2010;465:704–712. doi: 10.1038/nature09229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J.Y., Vodyanik M.A., Smuga-Otto K., Antosiewicz-Bourget J., Frane J.L., Tian S., Nie J., Jonsdottir G.A., Ruotti V., Stewart R. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.