Summary
The subsurface represents a largely unexplored frontier in microbiology. Here, coal seams present something of an oasis for microbial life, providing moisture, warmth, and abundant fossilized organic material. Microbes in coal seams are thought to syntrophically mobilize fossilized carbon from the geosphere to the biosphere. Despite the environmental and economic importance of this process, little is known about the microbial ecology of coal seams. In the current study, ecological succession and spatial niche partitioning are explored in three coal seam microbial communities. Scanning electron microscopic visualization and 16S rRNA sequencing track changes in microbial communities over time, revealing distinct attached and planktonic communities displaying patterns of ecological succession. Attachment to the coal surface is biofilm mediated on Surat coal, whereas microbes on Sydney and Gunnedah coal show different attachment processes. This study demonstrates that coal seam microbial communities undergo spatial niche partitioning during periods of succession as microbes colonize coal environments.
Subject Areas: Coal Geochemistry, Biogeoscience, Microbiology, Microbiome
Graphical Abstract

Highlights
-
•
Coal surfaces and waters have distinctly different microbial communities
-
•
Microbes attach to coal surfaces via multiple adhesion strategies
-
•
Adhesion strategies include biofilm formation and direct cell attachment
-
•
Coal microbe succession patterns provide insights into possible community roles
Coal Geochemistry; Biogeoscience; Microbiology; Microbiome
Introduction
Despite global efforts to reduce the dependence on fossil fuels, coal remains a key fuel for energy generation. Microbially produced coal seam gas (CSG) has been suggested as a way to aid transition from traditional fossil fuels to renewables. Improving our understanding of microbial communities responsible for the production of CSG, particularly with respect to how these communities function and the roles played by the individual taxa in this process, is critical for understanding this important geochemical process, as well as for developing methods for stimulating CSG production (Rice and Claypool, 1981, Ritter et al., 2015).
Coal seam microbial communities exist in the deep subsurface within water-filled cleats of coal seams. They have often been spatially isolated and adapted to survive on recalcitrant carbon sources under chemically reduced conditions with limited terminal electron acceptors (Strąpoć et al., 2011). Studies of microbial communities inhabiting these environments have largely focused on surveying communities associated with coal seam formation waters (Ritter et al., 2015). These studies have mostly aimed to increase methane production rates in situ. These studies typically use either biostimulation, introducing nutrients (Ritter et al., 2015, Colosimo et al., 2016), or bioaugmentation, introducing exogenous microbes, to enhance gas yield (Jones et al., 2010, Wang et al., 2016, Fuertez et al., 2017).
An important gap in our understanding of coal seam microbiology is the metabolic and ecological roles played by the majority of the bacterial taxa observed in coal seams. The archaea largely have well-defined roles in hydrogenotrophic, acetoclastic, and methylotrophic methanogenesis owing to their taxonomically constrained metabolisms, whereas the metabolic roles played by the majority of bacterial taxa remain poorly understood (Garcia et al., 2000, Colosimo et al., 2016). One way to investigate the metabolic contributions of specific microbes is through examining their patterns of succession when the microbial community is introduced to an environment. This approach has been used in fields of macroecology, where functional roles or niches are often inferred from an organism's traits. For example, the competitive, stress-tolerant, and ruderal (CSR) classification is used in plant ecology to explain how plant traits will influence their patterns of succession (Grime, 2006). In microbial ecology, an organism's traits are often difficult to elucidate if axenic cultures are not readily obtainable. In this case the succession patterns of organisms can be used to hypothesize on the traits possessed by the organisms.
One example of a system in which the succession patterns of individual microorganisms has been used to infer functional traits of microorganisms is leaf colonization and decomposition. This system provides a well-studied, analogous system to coal seams. In these systems when a limited heterogeneous resource (the fallen leaf) is introduced, a succession of fungal and bacterial species are observed, rising and falling in abundance as they compete, sequentially utilize, and exhaust the resource, starting with the most labile components and proceeding through increasingly recalcitrant ones (Frankland, 1992, Remacle, 1971). An examination of succession in coal seams, a similar endogenously heterotrophic environment, where the abundances of individual taxa are tracked over time may similarly provide insights into the ecological roles of individual bacterial taxa (Fierer et al., 2010).
Another ecological concept that warrants particular attention in the coal seam context is spatial niche partitioning. Microbes in coal seams are known to exist in water-filled cleats, and studies have shown that different microbial communities are observed on solid coal samples compared with associated formation waters (Klein et al., 2008, Guo et al., 2012, Wei et al., 2013, Lawson et al., 2015, Barnhart et al., 2013). The observation of a biofilm formed by a coal seam microbial community on a coal surface suggests that spatial partitioning between the coal surface and associated waters is an important split in coal seam microbial communities (Vick et al., 2016). Cell attachment and spatial partitioning may be driven by a requirement to access insoluble carbon sources in the coal. Spatial partitions may thus mirror a functional split wherein primary fermenters of the coal organic matter attach to the substrate to access insoluble compounds (Furmann et al., 2013). This phenomenon has been documented on aerobically incubated oil sand's bitumen (Wyndham and Costerton, 1981) and in the animal gut where anaerobic primary degraders attach to plant biomass, increasing the degradation rates of insoluble compounds such as cellulose (Costerton, 1992, Minato et al., 1966).
The current study examines succession and spatial partitioning to address previously unexplored questions about microbial ecology in coal seam environments. These include the following questions: What are the successional patterns of coal seam microbial taxa and can they be used to assign functional roles? How does studying the planktonic and attached communities separately inform us about the roles and niches of microbial taxa in coal seams? How universal are these patterns between different coal seam environments? Addressing these questions may provide important insights into the microbes inhabiting deep subsurface environments where microbial communities utilize complex fossilized resources for energy and biomass.
The patterns of succession in microbial communities growing on coal from three different coal basins, under physicochemical conditions mimicking each coal seam environment, are explored in the current study. Coal-degrading microcosms were used to study this succession with inoculation of crushed coal supplemented with nitrogen and phosphorus sources mimicking the introduction of meteoric water into a newly exposed coal seam or the liberation of nitrogen- or phosphorus-rich compounds from an exposed mineral source in the coal seam. Community profiles for the attached and planktonic communities were tracked over time from inoculation through to a more mature state with methane production, as a terminal metabolite, measured at each time point. Physical interactions of the attached communities and the coal surface were examined by scanning electron microscopy to investigate modes of attachment and biofilm formation.
Results
Coal Seam Microbial Communities Differ in Structure between Attached and Planktonic Samples for All Coal Types
16S rRNA gene amplicon sequencing performed on the attached and planktonic cells of the 189 microcosms, as well as inoculum, resulted in 379 community profiles after two samples failed PCR amplification. Operational taxonomic unit (OTU) tables with coal seam microBiome (CSMB, see Vick et al (2018)) assignments are available in Table S1.
At the first time point the communities were dominated by Proteobacteria and Firmicutes. Proteobacteria formed a larger proportion of attached communities (p < 0.0005), whereas Firmicutes made up a larger proportion of planktonic communities (p < 0.0001), a finding shared by other studies of coal seam microbial communities (Barnhart et al., 2013, Susilawati et al., 2015). The Bacteroidetes, Actinobacteria, and Euryarchaeota had significantly higher relative abundances in the planktonic fractions (p < 0.183, p < 0.011, and p < 0.0002, respectively), whereas the Deferribacteres were limited to Surat communities. The Gunnedah and Sydney communities showed more divergence at the phylum level between attached and planktonic communities than the Surat communities at this time point (Figure 1).
Figure 1.
Community Composition and Diversity Indices for Coal Seam Microcosms.
(A) Relative abundances at the phylum level for the attached and planktonic communities at the initial sampling time point (day 3) and at final time point (day 113, Gunnedah; day 99, Sydney; and day 111, Surat).
(B) Shannon's and Simpson's diversity indices for the described communities at all nine time points. Sorensen's index is shown for adjacent time points for all communities. The mean is shown as a dashed line with standard error of the mean indicated by outer edges of the colored regions.
Linear discriminant analysis Effect Size (LEfSe) analysis was performed to determine the OTUs that drove the differences in planktonic and attached communities for each coal type and at each time point. Overall, 523 OTUs were identified as driving differences between planktonic and attached communities across all three coal basins. Of these, 182 drove differences in the Gunnedah microcosms, 270 drove differences in the Sydney microcosms, and 333 drove differences in the Surat microcosms (Table S2).
By the final time point Euryarchaeota were at higher relative abundance overall and showed higher relative abundances in the planktonic than attached communities (p < 0.0015). Firmicutes had become more abundant than Proteobacteria, and whereas the planktonic and attached split was still significant for the relative abundances of Proteobacteria (p < 0.0010), it was not for Firmicutes (p < 0.069). Bacteroidetes remained abundant members of the community, although no longer significantly different between the planktonic and attached fractions (p < 0.397). Actinobacteria in the Sydney communities had also changed, from a higher relative abundance in the attached to a higher relative abundance in the planktonic fractions (p < 0.0276) (Figure 1).
OTUs from the phylum Firmicutes, enriched in planktonic communities of all three coals, were largely from the Clostridiales, an order commonly known to form syntrophies with methanogenic archaea (Sobieraj and Boone, 2006, Bizukojc et al., 2010, Eichler and Schink, 1984). OTUs from the Proteobacteria observed in higher abundances in the attached communities were diverse in their phylogenetic distribution and included OTUs in high relative abundance from the alpha-, beta-, and gammaproteobacterial groups. The Gammaproteobacteria included Acinetobacter species, which have been associated with hydrocarbon degradation, often through induced adhesion to hydrocarbons via cell surface proteins (Hanson et al., 1994, Baldi et al., 1999). The Betaproteobacteria include Aquabacterium species, involved in alkane degradation and commonly found in association with freshwater biofilms (Kalmbach et al., 1999, Masuda et al., 2014). Finally, the alphaproteobacterial Sphingomonas genus known to be important degraders of insoluble polyaromatic hydrocarbons in various environments was also observed (Bastiaens et al., 2000, Uyttebroek et al., 2006).
Microcosms also showed spatiotemporal variation in biodiversity. For Gunnedah microcosms, the least biodiverse of the three, the planktonic community was more biodiverse than the attached community (Figure 1B). Sydney and Surat microcosms had greater biodiversity than Gunnedah microcosms but did not show the same spatial variations, whereas Surat communities showed temporal changes, becoming more diverse as time increased (Figure 1). Beta diversity measures (Sorensen's index) were highly similar for planktonic and attached communities from the same coal basin at all time points. Overall, Surat and Sydney coal basin communities increased in similarity between adjacent time points over time, whereas the Gunnedah coal basin communities had similar Sorensen's index values over time until a decrease in similarity between days 59 and 113 (Figure 1).
Microbial Communities Display Distinctive Patterns of Ecological Successional
At the OTU level all three communities were significantly different from one another (p < 0.001) and had distinct attached and planktonic communities at almost all time points (p < 0.05). Planktonic communities showed larger basin-level divergence than attached (Figure 2). Surat showed a clearer temporal change in structure than Gunnedah and Sydney in both attached and planktonic communities, with replicates at early time points being more variable than at later time points. The attached and planktonic communities for all coals showed temporal shifts in community structure with these being greatest early and reducing over time, although Surat communities showed shifts until a later time point than Sydney and Gunnedah (Figure 2).
Figure 2.
Principle-Component Analysis (PCA) of Microbial Communities From the Surat, Sydney and Gunnedah Microcosms.
Colored stars on combined community PCAs indicate the inoculum community, Surat indicated by green circles, Sydney by purple and Gunnedah by orange). Blue and red circles indicate the planktonic and attached communities respectively. Colored circles show the individual microcosm community replicates with shading from dark to light indicating the age of the microcosms since inoculation.
Examination of the most abundant OTUs showed that individual taxa responded both spatially and temporally. Of the three communities, OTUs in Surat showed the most obvious temporal changes, although trends could be observed in both the attached and planktonic communities on all coals. OTUs appeared to increase and decrease in both a linear, e.g., OTU_17 in the Surat attached community, and polynomial manner, e.g., OTU_8 in the Surat planktonic community. Other relative abundance changes were not continuous but appeared to reverse at intermediate time points, e.g., OTU_3 and OTU_13 in the Sydney and Surat planktonic communities, respectively. Many of these trends were spatially specific with trends in the planktonic communities not always mirrored in the attached communities, e.g., OTU_13 in the Surat communities. Also, where an OTU showed a distinct temporal trend on one coal, rarely was the same trend observed on other coals for the same OTU (Figures 3 and 4). LEfSe analysis of the OTU abundance data between communities at adjacent time points illustrated which OTUs where driving the changes in communities between these time points (Table S3). Of these OTUs identified by LEfSe, 425 drove differences between microbial communities from adjacent time points in the planktonic communities and 290 in the attached in one or more of the adjacent time point comparisons.
Figure 3.
Relative Abundances of the Top 30 Most Abundant OTUs in the 16S rRNA Gene Amplicon Surveys of the Attached Communities
Average abundances are indicated by the size of the black circle with the standard error of the mean indicated by the inner and outer edges of the colored rings. Phylum-level assignment for OTUs is shown on the right. Fine-scale taxonomic assignment of OTUs is provided in Table S1.
Figure 4.
Relative Abundances of the Top 30 Most Abundant OTUs in the 16S rRNA Gene Amplicon Surveys of the Planktonic Communities
Average abundances are indicated by a black circle with the standard error of the mean indicated by the inner and outer edges of the colored rings. Phylum-level assignment for OTUs is shown on the right. Fine-scale taxonomic assignment of OTUs is provided in Table S1.
Visualization of Succession in Attached Communities
Visualization of coal surfaces after incubation with coal-associated microbial communities was performed to observe attachment strategies driving the split between attached and planktonic microbial communities in coal seams. Broad observations of the morphotypes, methods of attachment, and notable interactions between different microbes and particular features of the coal surface are outlined below at each time point.
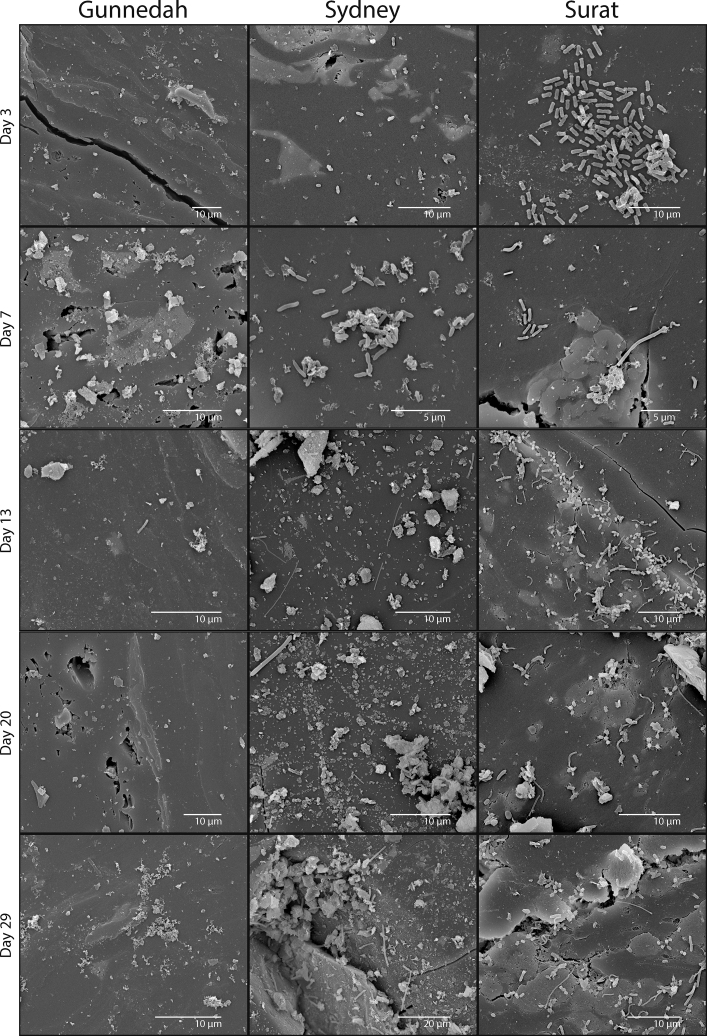
By day 3, cells were observed on the coal surface in Sydney and Surat, but not in the Gunnedah microcosms. The cells on the Sydney coal were of a single morphotype: short (∼2 μm) rods enlarged at either end, appearing singly on the coal surface with no obvious attachment. Cells on the Surat coal were also composed of a single morphology but were longer (∼3–4 μm) rods of even width appearing in micro-colonies (Figure 5).
Figure 5.
Representative Scanning Electron Micrographs of the Surface of Coal Disks Incubated with Coal Seam Microbial Communities during Early Stages of Incubation.
Coal disks are visualized for microcosms at days 3, 7, 13, 20, and 29 after inoculation.
By day 7, Sydney coal showed an increase in the same morphotype observed at day 3. Cells were still observed singly on the coal surface without obvious signs of attachment but in greater numbers and occasionally undergoing cell division. The Surat coal showed new morphotypes appearing in addition to those seen at day 3. Vibrioid cells ∼3 μm long, occasionally with numerous polar flagella and long (≥5 μm) rod-shaped morphotypes, were occasionally observed (Figure 5). At this time point, adherent cells were infrequently observed for the Gunnedah coal.
At day 13, rare, single (∼3 μm) rod-shaped cells were observed on Gunnedah coal. On Sydney coal, a further new morphotype was observed that was long (>10 μm) and filamentous. Cell density continued to increase for the Surat community with the first observations of a new (∼0.65 μm) coccoid morphotype showing distinct short, string-like structures radially around the cell anchoring to the coal. These new attaching morphotypes were observed in high numbers and appeared to strongly localize around cracks in the coal. Another long, string-like morphotype similar to that seen on the Sydney coal was observed on the Surat coal, occasionally with a polar bulbous section (Figure 5).
Between days 20 and 59, cells were infrequently observed on Gunnedah coal surfaces with short rod morphotypes increasingly observed. Cells on Sydney coal increased in density, and the fine, string-like morphotypes increased in abundance. A new large, long, rod-shaped morphotype (∼10 μm) was also observed occasionally on the coal surface during this period. The Surat community continued to increase in cell number, type, and extracellular material, with this biofilm nucleating from cracks in the coal and spreading outward. By day 59 a new square-ended rod-shaped morphotype with a wrinkled appearance also appeared on Surat coal disks (Figures 5 and 6).
Figure 6.
Representative Scanning Electron Micrographs of the Surface of Coal Disks Incubated with Coal Seam Microbial Communities during Later Stages of Incubation.
Coal disks are visualized for microcosms at days 38, 48, and 59 and at a final time point 99, 111, and 113 days after inoculation for the Sydney, Surat, and Gunnedah Basins, respectively.
By the final time point, string-like morphotypes were also present on Gunnedah coal, although the overall cell density remained low and no signs of biofilm or attachment were evident. Sydney coal showed the same range of morphotypes, although cell density had increased since day 59. Similar to Gunnedah coal, no evidence of biofilm or attachment was seen on Sydney coal. For the Surat community, coal showed further biofilm coverage. Various rod-shaped cells increased in abundance compared with the cocci and formed a mesh covering almost the complete surface of the coal (Figure 6).
Methane Generation in Coal-Degrading Microcosms
Methane production in Surat and Gunnedah microcosms was evident by day 7. By day 13, methane production was evident in all three communities plateauing at ∼1.4% in Gunnedah microcosms. Methane production plateaued next in Sydney microcosms at day 20 at ∼1.2%, whereas it continued till day 29 in Surat microcosms before it too plateaued at ∼7.0%. In all microcosms a second “bump” of methane production was observed before ceasing. This second increase occurred between days 29 and 38 for Sydney and Gunnedah microcosms and between days 48 and 59 for Surat microcosms (Figure 7).
Figure 7.
Methane Production by Coal Seam Microcosms.
Methane concentrations in the headspace of coal seam microcosms. Error bars indicate standard error of the mean. All measurements shown had seven replicates with the exception of indicated Sydney microcosms, which had replicates of *n = 1, **n = 4, ***n = 6, and ****n = 3.
Physicochemical Characteristics of Formation Waters and Petrological Characteristics of Coals
All formation waters were alkaline (bicarbonate buffered) and moderately brackish with electrical conductivities ∼2, 9, and 4 times less than seawater for Gunnedah, Surat, and Sydney, respectively. All waters were also lacking in meaningful concentrations of nitrogen and phosphorus (Table S4).
Sydney coal was the highest rank (1.41% mean, “maximum” vitrinite reflectance), whereas Surat (0.50%) and Gunnedah (0.64%) coals were of a similar lower rank. The major maceral in Sydney and Surat coal was vitrinite at 55.4% and 69.6% by volume, respectively. The vitrinite was composed mostly of telovitrinite in Sydney (44% of the sample) and Surat (44.3%) coals and detrovitrinite in Gunnedah coal (22.7%). Inertinite was the dominant maceral in Gunnedah coal where it made up 48.3% of the sample. Inertinite was also observed in high abundance in Sydney coal at 32.6% but was much less in Surat coal. The inertinite in all three coals was mostly composed of semifusinite. Liptinite was also variable between coals, making up 21.3% and 12% of Surat and Gunnedah coals, respectively, but was absent in Sydney coal (Table S5).
Proximate analysis of coals showed that, with the higher rank of Sydney coal, volatile matter was less (20.6 wt.%) and fixed carbon (69.6 wt.%) was more than for Surat and Gunnedah coals, which had 44.1 wt % and 36.2 wt % volatile matter and 44.7 wt % and 55.7 wt % fixed carbon, respectively. Elemental analyses show that Sydney Basin coal had larger amounts of carbon and less oxygen and hydrogen than the other coals (Table S5).
Discussion
Despite its importance in global geochemical processes the microbial ecology of the terrestrial subsurface remains poorly understood because of physical isolation and extreme physicochemical characteristics. Within the terrestrial subsurface, coal seams present an interesting environment where fossilized carbon, containing organic molecules not typically encountered by most organisms, is degraded to methane. The current study, looking at three different microbial communities on their native coals, represents a comprehensive examination of the distinct differences in attached and planktonic communities living on coal and the successional patterns of these communities over time. This study also reports on the observation of community-specific attachment strategies, including biofilm formation and direct cell attachment to coal.
Attached and Planktonic Communities Are Distinct in Coal Seams
Previous studies of coal seams have shown that microbial communities associated with coal solids often differ from those in associated waters. Although these studies show differences in microbial communities associated with mine waters and coal blocks (Wei et al., 2013, Wei et al., 2014), coal cuttings and production waters (Guo et al., 2012, Lawson et al., 2015, Klein et al., 2008), and coal solids and culture media (Zhang et al., 2015), it is not always clear how much of this variation arises from true spatial partitioning. To explicitly examine spatial partitioning, three different coal seam communities were examined on their native coals and formation waters over a ∼110-day incubation to determine how universal attachment was in different coal environments and whether it is a phenomenon seen in both colonizing and mature stages. Significantly different attached and planktonic communities were observed at both the OTU and phylum levels for all three coal communities and at all time points of incubation. Differences in these attached and planktonic communities at the phylum level included distinct differences in the relative abundances of the Euryarchaeota, Firmicutes, Bacteroidetes, Proteobacteria, and Actinobacteria. Some of the differences in the attached and planktonic communities were universal to all three coals, such as the association of Firmicutes and euryarchaeotal species with the planktonic communities and the proteobacterial species with the attached communities, a finding supported by other studies (Guo et al., 2012, Barnhart et al., 2013). Other differences were coal type (e.g., Actinobacteria, Cloacimonetes) or temporally (e.g., Firmicutes, Bacteroidetes) influenced.
The statistically significant differences observed between the planktonic and attached communities in all three coal seam microcosms at all time points suggest that this spatial separation is important in these coal seam communities. This spatial separation mirrors a major functional division observed in communities from other anaerobic microbial environments, including the rumen and animal gut where attached microbes are commonly involved in the initial degradation of cellulose and other insoluble substrates (Minato et al., 1966, Costerton, 1992). As the organic matter in coal is primarily composed of insoluble components, the study of the attached and planktonic communities as discreet entities should be considered in future studies and may provide insights into the organisms responsible for the initial degradation of coal compounds, the identification of which remains a fundamental question in coal seam microbial ecology (Ritter et al., 2015).
Patterns of Succession Observed in Coal Seam Microbial Communities
Coal seam microbial communities have typically been studied at either a single time point or before and after the addition of a nutrient or additive (Ritter et al., 2015). One notable exception is a study by Susilawati and colleagues (2015), which observed temporal changes in one Indonesian microbial community grown on three different coals over a period of 20 days (Susilawati et al., 2015). Jones and colleagues (2010) also tracked the abundances of a select few organisms within a microbial community growing on coal over a period of 102 days; however, these microbial communities were derived from a wetland sediment rather than a native coal community (Jones et al., 2010). The current study has expanded upon this examination of temporal changes in coal seam microbial communities and examined three different Australian coal seam communities grown with their native coals and matched formation waters.
Generally these changes, across all coal communities, include a decrease in the relative abundance of the Proteobacteria over time with a concomitant increase in the relative abundances of the Euryarchaeota and Firmicutes, a finding supported by observations in Indonesian coals (Susilawati et al., 2015). This, along with the observation that proportions of Proteobacteria are higher in the attached communities, whereas the Euryarchaeota and Firmicutes appear in higher abundances planktonically, suggests that the Proteobacteria play a role in the breakdown of insoluble coal material for use by secondary fermenting Firmicutes taxa and methanogenic archaea.
Examining the communities at the OTU level reveals temporally driven changes to individual members of the community, most obvious in Surat Basin microcosms. Replicate microcosms were initially quite divergent but became much more tightly clustered at the final time points according to principal-component analysis. This suggests that the community composition during early colonization processes is variable, perhaps due to stochastic factors associated with inoculation rates of rapidly growing taxa. Communities become increasingly similar as time goes on, suggesting that as the microbial communities modify their environment through decomposition of the coal substrate and production of downstream metabolites, a subset of specialized taxa, including the methanogens, which are not as influenced by initial inoculation and growth rates, take over. This effect appears to be characteristic of succession in diverse microbial communities and suggests that the Surat communities may be reaching a point of climax or stability (Dini-Andreote et al., 2015, Kielak et al., 2016). However, it should be noted that these coal seam microcosms represent endogenous heterotrophic environments and as such this stability may change if the current pools of compounds supplying carbon and energy are depleted and metabolism turns to a different pool of resources (Fierer et al., 2010).
At the individual OTU level there were a number of successional patterns observed for the various taxa in the coal seams. Some, such as OTU_8, a Citrobacter sp., and OTU_5, an Acetobacterium sp., spiked in abundance at very early time points before quickly declining, particularly in the planktonic communities (Figure 4). This observation was also identified in the LEfSe analysis where these OTUs are involved in driving the changes between the day 3 and day 7 communities for the Sydney and Gunnedah communities, respectively (Table S3). These taxa may be analogous to ruderal species in plant communities, which are able to utilize a readily available niche and grow rapidly, but are then replaced by competitors or stress-tolerant species with somewhat slower growth rates. Ruderal life strategies have previously been associated with genomic features such as 16S rRNA gene copy number of diverse bacterial species, which may help explain these observed succession patterns in the coal seam (Klappenbach et al., 2000). Conversely, several taxa, such as OTU_31, a Coriobacteriaceae sp. and OTU_7, a Thermoanaerobacteraceae sp., gradually increase in abundance over time in the planktonic community of the Sydney microcosms and attached community of the Gunnedah microcosms, respectively, only reaching high abundances at later time points. These taxa are likely more specialized in their metabolisms and are probably utilizing compounds slowly liberated from the coal. Another interesting successional pattern observed for OTU_3 (Methanocalculus sp.) in the planktonic community of the Sydney microcosms and OTU_13 (Firmicutes) in the planktonic community of the Surat microcosms was a rapid increase in abundance until an intermediate time point followed by an abrupt drop and continued low abundance. These taxa could be presumed to be utilizing a limited resource in the coal or formation water, or a downstream metabolite of it based upon the observation of these trends in the planktonic and not attached communities, which becomes depleted or for which they are outcompeted. Future examinations of succession in coal seam communities may benefit from tracking organic intermediates in the culture media to correlate changes in metabolite concentrations with organism abundances with the aim of elucidating the metabolisms of these particular taxa (Orem et al., 2010). Interestingly, for all these taxa showing distinct successional patterns, in none of the observed instances were the successional patterns the same across the multiple coal types examined despite the OTUs presence in all three communities. It is speculated that these coals may either have very distinctly different biodegradable components or that the communities have a high degree of functional redundancy, allowing for different taxa to fill metabolic niches depending on their competitive advantage at the various physicochemical parameters present in the coal environments. This hypothesis, however, would have to be tested in further studies and would require a greater understanding of the functional clades that bacteria form in coal seam environments. One question of interest would be whether coal seam communities converge in terms of the functional clades present and their relative abundances in the attached and planktonic coal seam communities as succession on a coal resource progresses.
Attached Communities from Different Coal Seams Differ in Their Attachment Strategies
Previously coal attachment via biofilms (Raudsepp et al., 2015, Hazrin-Chong and Manefield, 2012) and direct cell interactions (Hazrin-Chong et al., 2014, Bagdigian and Myerson, 1986, Chen and Skidmore, 1988) has been observed for microbes grown on coal aerobically, whereas little examination of this phenomena has been made for native anaerobic microbial consortia (Vick et al., 2016). Here, distinct attached communities of microbes were seen in all three coal communities, whereas scanning electron microscopic visualization of the coal surface identified biofilm formation in only Surat Basin microcosms. This suggests an alternative attachment strategy in the other communities, similar to that observed for Pseudomonas protegens on coal (Hazrin-Chong et al., 2014), which may involve adhesin, other protein-mediated attachment, or more general hydrophobic-cell-to-substrate interactions (Marshall and Cruickshank, 1973). Surat Basin microcosms showed the development of a biofilm that recruited successive morphotypes and increased in density over time. Surat coal was initially colonized by a rod-shaped, micro-colony-forming morphotype. This morphotype may be represented by OTU_8, a Citrobacter sp. based on the predominance of this OTU at early time points in attached Surat communities and morphological features of Citrobacter sp. isolated from related formation waters (unpublished data). Another morphotype that appeared in abundance at an early time point on Surat coals was a vibrioid cell with multiple polar flagella, which may be represented by OTU_26, a Geovibrio sp., or OTU_15, a Desulfovibrio sp., both of which appeared to show similar abundances to the morphotype in Surat attached communities.
Surat Basin biofilms appeared to nucleate in patches starting with a cocci morphotype, and once a certain density was reached a number of rod-shaped morphotypes colonized the upper layer of the biofilm. This appears similar to observations made by Costerton (1992) who noted that in rumen particles a primary degrader of an insoluble substrate would form a biofilm plaque to concentrate enzymes or other compounds for substrate solubilization and degradation. This would be followed by attachment of a secondary fermenter to the upper layers of the biofilm removing inhibitory metabolic by-products, resulting in an increased substrate degradation rate (Costerton, 1992). This suggests that the coccoid morphotype may be of particular interest to studies of primary fermenters as it appeared to be the first to form attachment to the coal through a biofilm-mediated process after which different morphotypes, presumably representing secondary fermenters, were recruited to the biofilm.
Interestingly, previous work has shown that a microbial community from the Sydney coal basin is capable of appreciable biofilm formation when grown on a Bowen Basin coal (Vick et al., 2016), indicating that coal seam communities hold the potential to form biofilm attachments but fail to do so when presented with unsuitable coals. This hypothesis is supported by observations of significantly higher methane production in Surat microcosms, suggesting that this coal is more amenable to microbial degradation. It remains to be shown, however, what physicochemical factors of this coal make it more amenable to biodegradation as the Surat microcosms differ to the other microcosms in multiple physicochemical parameters including decreased salinity of the formation water and higher volatile organic matter in the coal. It was observed, from LEfSe analysis, that more OTUs drove differences between the attached and planktonic communities in the Surat microcosms than the Sydney and Gunnedah microcosms; it may be some of these OTUs, observed as driving attachment in the Surat but not the other microcosms, which selectively form biofilms when presented with a suitable coal. Exploration of the apparent directed nucleation of biofilms at certain regions and types of coal could provide important insights into understanding the components of coal targeted by microbes for biodegradation. Future exploration using techniques including Nanoscale Secondary Ion MassSpectrometry (nanoSIMS), energy-dispersive X-ray spectroscopy, or Attenuated total reflectance Fourier-Transform infrared spectroscopy (ATR-FTIR) may help to identify the chemical characteristics of the regions of coal that elicit attachment. Combining these chemical characterizations with classical petrological and molecular ecological techniques may provide insights into whether particular maceral groups or layers of bedding represent hotspots for microbial attachment and catabolism.
Conclusions
The current study demonstrates the presence of two distinct spatially separated microbial communities, attached to the coal or living planktonically, across three different coal environments. Although attachment of microbes to the coal surface was common to all the coals, the mode of attachment was variable with Sydney and Gunnedah Basin communities showing direct cell attachment and the Surat Basin community developing a biofilm. The current study also shows that distinct patterns of succession are observed for these attached and planktonic communities during a period of over a hundred days. Taken together, these results highlight the importance of spatial and temporal partitioning in driving microbial community structures in coal seam environments and suggest that future studies of coal should involve the design of experiments with spatial and temporal variations in mind. Future work examining the functional characteristics of microbes associated with attachment phenotypes at certain time points in succession also has the potential to address fundamental questions about the primary fermenting bacteria in the coal seam, a key area of investigation in coal seam microbial ecology (Ritter et al., 2015).
Limitations of the Study
Coal seam microbial communities in eastern Australia have been shown to differ in some aspects from other seams in North America, particularly in the dominant classes of methanogens observed (Vick et al., 2018). These differences may indicate microbial communities with different functional components, and as such these communities may undergo different attachment and succession processes.
One question raised by the current study is whether different coal seam microbial communities undergo convergent or divergent patterns of succession. Our current understanding of which taxa constitute the various functional clades within coal seam communities was insufficient to address this question in the current study. Developing an understanding of the functional roles of bacterial taxa in coal seam microbial communities should be the priority of research in the field of coal seam microbiology and will be necessary to address questions of convergence or divergence between coal seam microbial communities as they colonize a coal environment.
In the current study we examine coal disks that were analyzed in a way that facilitates descriptive, but not quantitative analysis. Images used in the current study come from duplicated coal disks, and images were taken in a manner demonstrative of the processes observed across the disk surfaces. These images were not taken at random positions across the disk surface and not in quantities sufficient for statistically sound quantitative measurement. Future imaging studies would benefit from an approach that allows for statistically sound quantitative measurements of biofilm coverage and cell density, among other measures.
In addition, although many attempts to mimic the subsurface coal environment were made, including using matched coals and formation waters, there are factors such as hydrostatic pressure that could not be mimicked in a laboratory setting and may have effects of microbial communities and their succession patterns and niche partitioning.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
The authors would like to thank Mr. Stephane Armand for assistance with the use of gas chromatography equipment, Mr. Paul Marvig for the construction of the polished coal disks, and the Macquarie University Microscopy department, in particular Dr. Sue Lindsay, Dr. Nadia Suarez-Bosche, and Ms, Nicole Vella, for their assistance and guidance in generating scanning electron micrographs. Mr. Silas Vick was supported by a Macquarie University-funded postgraduate scholarship. This work was supported by a CSIRO Energy strategic research initiative.
Author Contributions
Conceptualization, S.H.W.V., D.J.M., and I.T.P.; Methodology, S.H.W.V. and D.J.M.; Investigation, S.H.W.V.; Writing – Original Draft, S.H.W.V.; Writing – review and editing, D.J.M., S.G.T., and I.T.P.; Data Curation, S.H.W.V. and P.G.; Formal analysis, S.H.W.V., K.L.P., N.S., and S.G.; Supervision D.J.M., S.G.T., and I.T.P.
Declaration of Interests
The authors declare no conflict of interests relating to the work presented in the current manuscript.
Published: February 22, 2019
Supplemental Information
Positive values (red) indicate OTUs that are significantly more attached, and negative values (blue) indicate those that are significantly more planktonic.
Positive values (red) indicate OTUs that are increasing significantly between the time points indicated, and negative values (blue) indicate OTUs showing a significant decrease.
References
- Bagdigian R.M., Myerson A.S. The adsorption of Thiobacillus ferrooxidans on coal surfaces. Biotechnol. Bioeng. 1986;28:467–479. doi: 10.1002/bit.260280402. [DOI] [PubMed] [Google Scholar]
- Baldi F., Ivos̆Ević N., Minacci A., Pepi M., Fani R., Svetlic̆Ić V., Žutić V. Adhesion of Acinetobacter venetianus to diesel fuel droplets studied with in situ electrochemical and molecular probes. Appl. Environ. Microbiol. 1999;65:2041–2048. doi: 10.1128/aem.65.5.2041-2048.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnhart E.P., De León K.B., Ramsay B.D., Cunningham A.B., Fields M.W. Investigation of coal-associated bacterial and archaeal populations from a diffusive microbial sampler (DMS) Int. J. Coal Geol. 2013;115:64–70. [Google Scholar]
- Bastiaens L., Springael D., Wattiau P., Harms H., Verachtert H., Diels L. Isolation of adherent polycyclic aromatic hydrocarbon (PAH)-degrading bacteria using PAH-sorbing carriers. Appl. Environ. Microbiol. 2000;66:1834–1843. doi: 10.1128/aem.66.5.1834-1843.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizukojc M., Dietz D., Sun J., Zeng A.-P. Metabolic modelling of syntrophic-like growth of a 1, 3-propanediol producer, Clostridium butyricum, and a methanogenic archeon, Methanosarcina mazei, under anaerobic conditions. Bioprocess Biosyst. Eng. 2010;33:507–523. doi: 10.1007/s00449-009-0359-0. [DOI] [PubMed] [Google Scholar]
- Chen C.Y., Skidmore D.R. Attachment of Sulfolobus acidocaldarius cells on coal particles. Biotechnol. Prog. 1988;4:25–30. [Google Scholar]
- Colosimo F., Thomas R., Lloyd J.R., Taylor K.G., Boothman C., Smith A.D., Lord R., Kalin R.M. Biogenic methane in shale gas and coal bed methane: a review of current knowledge and gaps. Int. J. Coal Geol. 2016;165:106–120. [Google Scholar]
- Costerton J.W. Pivotal role of biofilms in the focused attack of bacteria on insoluble substrates. Int. Biodeterior. Biodegradation. 1992;30:123–133. [Google Scholar]
- Dini-Andreote F., Stegen J.C., Van Elsas J.D., Salles J.F. Disentangling mechanisms that mediate the balance between stochastic and deterministic processes in microbial succession. Proc. Natl. Acad. Sci. U S A. 2015;112:E1326–E1332. doi: 10.1073/pnas.1414261112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichler B., Schink B. Oxidation of primary aliphatic alcohols by Acetobacterium carbinolicum sp. nov., a homoacetogenic anaerobe. Arch. Microbiol. 1984;140:147–152. [Google Scholar]
- Fierer N., Nemergut D., Knight R., Craine J.M. Changes through time: integrating microorganisms into the study of succession. Res. Microbiol. 2010;161:635–642. doi: 10.1016/j.resmic.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Frankland J.C. Mechanisms in fungal succession. In: Carroll G.C., Wicklow D.T., editors. The Fungal Community: its organization and role in the ecosystem. Second Edition. Marcel Dekker; 1992. pp. 383–401. [Google Scholar]
- Fuertez J., Boakye R., Mclennan J., Adams D.J., Sparks T., Gottschalk A. Developing methanogenic microbial consortia from diverse coal sources and environments. J. Nat. Gas Sci. Eng. 2017;46:637–650. [Google Scholar]
- Furmann A., Schimmelmann A., Brassell S.C., Mastalerz M., Picardal F. Chemical compound classes supporting microbial methanogenesis in coal. Chem. Geol. 2013;339:226–241. [Google Scholar]
- Garcia J.-L., Patel B.K., Ollivier B. Taxonomic, phylogenetic, and ecological diversity of methanogenic archaea. Anaerobe. 2000;6:205–226. doi: 10.1006/anae.2000.0345. [DOI] [PubMed] [Google Scholar]
- Grime J.P. John Wiley & Sons; 2006. Plant Strategies, Vegetation Processes, and Ecosystem Properties. [Google Scholar]
- Guo H., Liu R., Yu Z., Zhang H., Yun J., Li Y., Liu X., Pan J. Pyrosequencing reveals the dominance of methylotrophic methanogenesis in a coal bed methane reservoir associated with eastern Ordos Basin in China. Int. J. Coal Geol. 2012;93:56–61. [Google Scholar]
- Hanson K., Kale V.C., Desai A.J. The possible involvement of cell surface and outer membrane proteins of Acinetobacter sp. A3 in crude oil degradation. FEMS Microbiol. Lett. 1994;122:275–279. [Google Scholar]
- Hazrin-Chong N.H., Manefield M. An alternative SEM drying method using hexamethyldisilazane (HMDS) for microbial cell attachment studies on sub-bituminous coal. J. Microbiol. Methods. 2012;90:96–99. doi: 10.1016/j.mimet.2012.04.014. [DOI] [PubMed] [Google Scholar]
- Hazrin-Chong N.H., Marjo C.E., Das T., Rich A.M., Manefield M. Surface analysis reveals biogenic oxidation of sub-bituminous coal by Pseudomonas fluorescens. Appl. Microbiol. Biotechnol. 2014;98:6443–6452. doi: 10.1007/s00253-014-5832-2. [DOI] [PubMed] [Google Scholar]
- Jones E.J., Voytek M.A., Corum M.D., Orem W.H. Stimulation of methane generation from nonproductive coal by addition of nutrients or a microbial consortium. Appl. Environ. Microbiol. 2010;76:7013–7022. doi: 10.1128/AEM.00728-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmbach S., Manz W., Wecke J., Szewzyk U. Aquabacterium gen. nov., with description of Aquabacterium citratiphilum sp. nov., Aquabacterium parvum sp. nov. and Aquabacterium commune sp. nov., three in situ dominant bacterial species from the Berlin drinking water system. Int. J. Syst. Evol. Microbiol. 1999;49:769–777. doi: 10.1099/00207713-49-2-769. [DOI] [PubMed] [Google Scholar]
- Kielak A.M., Scheublin T.R., Mendes L.W., Van Veen J.A., Kuramae E.E. Bacterial community succession in pine-wood decomposition. Front. Microbiol. 2016;7:231. doi: 10.3389/fmicb.2016.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klappenbach J.A., Dunbar J.M., Schmidt T.M. rRNA operon copy number reflects ecological strategies of bacteria. Appl. Environ. Microbiol. 2000;66:1328–1333. doi: 10.1128/aem.66.4.1328-1333.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein D.A., Flores R.M., Venot C., Gabbert K., Schmidt R., Stricker G.D., Pruden A., Mandernack K. Molecular sequences derived from Paleocene Fort union formation coals vs. associated produced waters: implications for CBM regeneration. Int. J. Coal Geol. 2008;76:3–13. [Google Scholar]
- Lawson C.E., Strachan C.R., Williams D.D., Koziel S., Hallam S.J., Budwill K. Patterns of endemism and habitat selection in coalbed microbial communities. Appl. Environ. Microbiol. 2015;81:7924–7937. doi: 10.1128/AEM.01737-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall K., Cruickshank R. Cell surface hydrophobicity and the orientation of certain bacteria at interfaces. Arch. Microbiol. 1973;91:29–40. doi: 10.1007/BF00409536. [DOI] [PubMed] [Google Scholar]
- Masuda H., Shiwa Y., Yoshikawa H., Zylstra G.J. Draft genome sequence of the versatile alkane-degrading bacterium Aquabacterium sp. strain NJ1. Genome Announc. 2014;2 doi: 10.1128/genomeA.01271-14. e01271–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minato H., Endo A., Ootomo Y., Uemura T. Ecological treatise on the rumen fermentation. J. Gen. Appl. Microbiol. 1966;12:53–69. [Google Scholar]
- Orem W.H., Voytek M.A., Jones E.J., Lerch H.E., Bates A.L., Corum M.D., Warwick P.D., Clark A.C. Organic intermediates in the anaerobic biodegradation of coal to methane under laboratory conditions. Org. Geochem. 2010;41:997–1000. [Google Scholar]
- Raudsepp M., Gagen E., Evans P., Tyson G., Golding S., Southam G. The influence of hydrogeological disturbance and mining on coal seam microbial communities. Geobiology. 2015;14:163–175. doi: 10.1111/gbi.12166. [DOI] [PubMed] [Google Scholar]
- Remacle J. Succession in the oak litter microflora in forests at Mesnil-Eglise (Ferage), Belgium. Oikos. 1971;22:411–413. [Google Scholar]
- Rice D.D., Claypool G.E. Generation, accumulation, and resource potential of biogenic gas. Am. Assoc. Pet. Geol. Bull. 1981;65:5–25. [Google Scholar]
- Ritter D., Vinson D., Barnhart E., Akob D.M., Fields M.W., Cunningham A.B., Orem W., Mcintosh J.C. Enhanced microbial coalbed methane generation: a review of research, commercial activity, and remaining challenges. Inter. J. Coal Geol. 2015;146:28–41. [Google Scholar]
- Sobieraj M., Boone D.R. Springer; 2006. Syntrophomonadaceae. The Prokaryotes. [Google Scholar]
- Strąpoć D., Mastalerz M., Dawson K., Macalady J., Callaghan A.V., Wawrik B., Turich C., Ashby M. Biogeochemistry of microbial coal-bed methane. Annu. Rev. Earth Planet. Sci. 2011;39:617–656. [Google Scholar]
- Susilawati R., Evans P.N., Esterle J.S., Robbins S.J., Tyson G.W., Golding S.D., Mares T.E. Temporal changes in microbial community composition during culture enrichment experiments with Indonesian coals. Int. J. Coal Geol. 2015;137:66–76. [Google Scholar]
- Uyttebroek M., Ortega-Calvo J.-J., Breugelmans P., Springael D. Comparison of mineralization of solid-sorbed phenanthrene by polycyclic aromatic hydrocarbon (PAH)-degrading Mycobacterium spp. and Sphingomonas spp. Appl. Microbiol. Biotechnol. 2006;72:829–836. doi: 10.1007/s00253-006-0337-2. [DOI] [PubMed] [Google Scholar]
- Vick S.H., Tetu S.G., Sherwood N., Pinetown K., Sestak S., Vallotton P., Elbourne L.D., Greenfield P., Johnson E., Barton D. Revealing colonisation and biofilm formation of an adherent coal seam associated microbial community on a coal surface. Int. J. Coal Geol. 2016;160:42–50. [Google Scholar]
- Vick S.H., Greenfield P., Tran-Dinh N., Tetu S.G., Midgley D.J., Paulsen I.T. The Coal Seam Microbiome (CSMB) reference set, a lingua franca for the microbial coal-to-methane community. Int. J. Coal Geol. 2018;186:41–50. [Google Scholar]
- Wang H., Lin H., Rosewarne C.P., Li D., Gong S., Hendry P., Greenfield P., Sherwood N., Midgley D.J. Enhancing biogenic methane generation from a brown coal by combining different microbial communities. Int. J. Coal Geol. 2016;154:107–110. [Google Scholar]
- Wei M., Yu Z., Zhang H. Microbial diversity and abundance in a representative small-production coal mine of central China. Energy Fuels. 2013;27:3821–3829. [Google Scholar]
- Wei M., Yu Z., Jiang Z., Zhang H. Microbial diversity and biogenic methane potential of a thermogenic-gas coal mine. Int. J. Coal Geol. 2014;134:96–107. [Google Scholar]
- Wyndham R., Costerton J. In vitro microbial degradation of bituminous hydrocarbons and in situ colonization of bitumen surfaces within the Athabasca oil sands deposit. Appl. Environ. Microbiol. 1981;41:791–800. doi: 10.1128/aem.41.3.791-800.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Liang Y., Pandey R., Harpalani S. Characterizing microbial communities dedicated for conversion of coal to methane in situ and ex situ. Int. J. Coal Geol. 2015;146:145–154. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Positive values (red) indicate OTUs that are significantly more attached, and negative values (blue) indicate those that are significantly more planktonic.
Positive values (red) indicate OTUs that are increasing significantly between the time points indicated, and negative values (blue) indicate OTUs showing a significant decrease.