Summary
Aims
Diabetes screening strategies using glycated haemoglobin (HbA1c) as first‐instance diagnostic parameter may cause failure to detect individuals with abnormal glucose regulation and possible signs of microvascular complications despite “rule‐out” HbA1c levels. This cross‐sectional study examined the diagnostic performance of HbA1c in relation to fasting and two‐hour postload plasma glucose (FPG/2 h‐PG), and investigated whether individuals with normal HbA1c but abnormal FPG/2 h‐PG have a higher prevalence of moderately increased albuminuria as possible sign of early stage kidney damage.
Methods
A total of 2695 individuals (age 40‐79 years, 48% men) without prior diagnosis of diabetes and complete measurement of HbA1c, FPG, 2 h‐PG and urine albumin‐creatinine ratio (UACR) were taken from a large population‐based epidemiological study in the City of Leipzig, Germany.
Results
A total of 2439 individuals (90.5%, 95% CI: 89.4‐91.6) had normal HbA1c levels, <39 mmol/mol (<5.7%), while 234 (8.7%, 95% CI: 7.7‐9.8) had prediabetes, HbA1c ≥39 and <48 mmol/mol (≥5.7 and <6.5%), and 22 (0.8%, 95% CI: 0.5‐1.2) had diabetes, HbA1c ≥48 mmol/mol (≥6.5%), according to HbA1c. Among individuals with normal HbA1c, 35.6% (95% CI: 33.7‐37.5) had impaired fasting glucose or impaired glucose tolerance and 1.8% (95% CI: 1.4‐2.4) had diabetes according to FPG/2 h‐PG. Individuals with normal HbA1c but prediabetic or diabetic FPG/2 h‐PG had a significantly higher prevalence of moderately increased albuminuria (9.4%, 95% CI: 7.6‐11.5 and 13.3%, 95% CI: 5.8‐25.4, respectively) than individuals with normal HbA1c and normal FPG/2 h‐PG (3.9%, 95% CI: 3.0‐5.0).
Conclusions
The prevalence of prediabetes according to FPG/2 h‐PG among individuals with normal HbA1c is considerably high, and the prevalence of moderately increased albuminuria in this group is significantly elevated. Risk factors for diabetes such as age, gender and BMI may help to better identify this at‐risk group.
Keywords: albuminuria, diabetes, diagnostic performance, HbA1c
NOVELTY STATEMENT.
Diabetes screening strategies using glycated haemoglobin (HbA1c) as first‐instance diagnostic parameter may cause failure to detect individuals with abnormal glucose regulation and possible signs of microvascular complications despite “rule‐out” HbA1c levels.
Based on population‐based cross‐sectional data from 2695 individuals without prior diagnosis of diabetes, we found that individuals with normal HbA1c but prediabetic or diabetic levels of fasting and/or two‐hour postload plasma glucose had a significantly higher prevalence of moderately increased albuminuria than individuals with normal HbA1c and normal fasting and two‐hour postload plasma glucose levels.
Age, gender and BMI may help to better identify this at‐risk group, which otherwise would escape detection and early intervention.
1. INTRODUCTION
Over the past decades, the prevalence of type 2 diabetes has increased worldwide to a pandemic scale. It has been estimated that the number of individuals with yet undiagnosed diabetes was as high as 193 million in 2015 and is expected to increase further by 2040 to 642 million people with diabetes and 481 million people with prediabetes.1 Diabetes constitutes a major challenge for public healthcare systems as untreated hyperglycaemia can result in micro‐ and macrovascular complications such as diabetic retinopathy, chronic kidney failure, diabetic polyneuropathy or coronary artery disease and stroke.2 One early sign of diabetic complications is moderately increased albuminuria. It is an indicator of chronic kidney damage and it is correlated with the risk of cardiovascular events.3 To prevent the development or progression of such complications, early detection of individuals with yet unrecognized prediabetes and diabetes is of importance. The traditionally established measurements of fasting plasma glucose (FPG) and the oral glucose tolerance test (OGTT) have major drawbacks such as need for compliance to 8‐hour fasting and large intraindividual variability.4, 5 In the current position statement of the American Diabetes Association (ADA) and in the statement of the European Association for the Study of Diabetes (EASD), it is recommended to use glycated haemoglobin HbA1c as the preferred diagnostic tool for diabetes screening to overcome these limitations.6, 7 Some guidelines, including those of the German Diabetes Association, recommend a stepwise diagnostic approach using HbA1c as a first screening test with two different cut‐off values for the diagnostic decision, of which the lower cut‐off is used to rule out diabetes and the higher cut‐off is used to diagnose diabetes without measuring FPG and/or two‐hour postload glucose levels (2 h‐PG). In this setting, FPG and/or 2 h‐PG are only measured if the HbA1c concentration is between these two cut‐offs.8, 9, 10
Despite the advantages of HbA1c‐based diabetes screening, there is an ongoing debate about the usefulness of this marker to detect individuals with diabetes and prediabetes. A large number of studies have addressed this question in different populations, however resulting in ambiguous results and conclusions particularly with regard to optimal cut‐off values for diagnostic decisions.11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24 Importantly, stepwise diabetes screening strategies using only HbA1c as first‐instance diagnostic parameter may cause failure to detect individuals with abnormal glucose regulation and possible signs of microvascular complications despite HbA1c levels below the “rule‐out” cut‐off. In this study, we analysed the diagnostic performance of HbA1c compared to FPG and 2 h‐PG in a representative sample of adult individuals taken from an urban general population, who were not yet diagnosed with diabetes. We were particularly interested in assessing the prevalence of individuals with moderately increased albuminuria among individuals considered to have a normal glucose metabolism based on HbA1c testing.
2. MATERIALS AND METHODS
2.1. Study population
The present investigation was part of the “LIFE‐Adult‐Study”, a population‐based cohort study initiated in 2011. In 2014, this study has completed the cross‐sectional baseline examination of 10 000 randomly selected adults from Leipzig, a major city in Germany with approximately 580 000 inhabitants. The LIFE‐Adult‐Study is part of the “Leipzig Research Centre for Civilization Diseases” (LIFE), aiming to investigate disease prevalences, early onset markers, genetic predispositions, and the role of lifestyle factors for the development of major civilization diseases. Details of the objectives and the design of the LIFE‐Adult‐Study are described elsewhere.25 A total of 9479 subjects were aged 40‐79 years, which was the age range of interest for our specific research question. Of these, 8243 individuals had no personal history of diabetes. Of these, a total of 2695 individuals between 40 and 79 years of age without known diabetes had complete measurements of HbA1c and fasting plasma glucose (FPG), and had also performed a 75‐g oral glucose tolerance test (OGTT) with measurement of the two‐hour postload glucose level (2 h‐PG). Moreover, the urine albumin‐creatinine ratio (UACR) was determined in these individuals.
Not all 8243 individuals had complete measurements particularly because the OGTT was performed only in a subset of individuals for reasons of feasibility and costs. There were no large differences regarding age and sex distribution between the study sample and the individuals with incomplete measurements.
The LIFE‐Adult‐Study was approved by the responsible institutional ethics committee of the Medical Faculty of the University of Leipzig. Written informed consent was obtained from all participants prior to study enrolment.
2.2. Laboratory measurements
The OGTT was conducted after fasting for at least 8 hours. After initial peripheral blood drawing and intake of 75 g of glucose solution (Accu‐Chek® Dextrose O.G.T. Saft, Roche Diagnostics Deutschland GmbH, Mannheim, Germany), another peripheral blood sample was taken after 120 minutes for measurement of glucose concentration (2 h‐PG). All participants were also asked to provide a urine sample. The UACR was determined from morning spot urine samples. All samples were processed immediately and highly standardized in the LIFE preanalytical laboratory. Laboratory analyses were performed on the same day at the Institute of Laboratory Medicine of the University Hospital Leipzig (accredited by ISO 15189 and 17025) according to the “Quality Standards for Medical Laboratories of the German Chamber of Physicians (RiLiBÄK)”. HbA1c (turbidimetric assay), creatinine (enzymatic colorimetric assay) and albumin (turbidimetric assay) were analysed using a “Cobas 8000 Clinical Chemistry Analyzer” with test kits from Roche (Roche Diagnostics GmbH).
2.3. Statistical analysis
Based on their HbA1c measurements, study participants were divided into three groups: “normal” (HbA1c <5.7% [<39 mmol/mol]), “prediabetes” (HbA1c between ≥5.7% to <6.5% [≥39 to <48 mmol/mol]) and “diabetes” (HbA1c ≥6.5% [≥48 mmol/mol]). Likewise, all individuals were also classified into three groups based on their FPG and 2 h‐PG measurements: “normal” (FPG <5.6 mmol/L and 2 h‐PG <7.8 mmol/L), “prediabetes” (FPG between ≥5.6 to <7.0 mmol/L and/or 2 h‐PG between ≥7.8 and <11.0 mmol/L), and “diabetes” (FPG ≥7.0 mmol/L or 2 h‐PG ≥11.0 mmol/L). The cut‐offs chosen for these tripartitions are in accordance with the current clinical practice recommendations of the German Diabetes Association suggesting a stepwise diagnostic procedure with HbA1c as primary diagnostic parameter.10 This stepwise approach allows the diagnosis of diabetes to be made if HbA1c exceeds an upper cut‐off of ≥6.5% (≥48 mmol/mol), while diabetes (and prediabetes) is ruled out at a lower cut‐off of <5.7% (<39 mmol/mol). Only If HbA1c falls in between ≥5.7 and <6.5% (≥39 to <48 mmol/mol), additional measurement of FPG and/or 2 h‐PG is recommended. Participants with a urine albumin/creatinine ratio (UACR) of >20 mg/g (men) or >30 mg/g (women) were considered to have moderately increased albuminuria based on the clinical practice recommendations of the German Diabetes Association.26
Statistical comparisons between groups of individuals were performed using the t test (or the Mann‐Whitney U test where appropriate) for continuous variables, and the chi‐square test (or Fisher's exact test where required) for categorical variables. Receiver operating characteristic (ROC) analysis including calculation of the area under the ROC curve (ROC‐AUC) was used to quantify the performance of HbA1c to identify individuals with diabetes (or prediabetes plus diabetes) based on FPG and 2 h‐PG. Multivariate logistic regression analysis was employed to investigate whether gender, age, body mass index (BMI), and HbA1c are predictors for the presence of prediabetes (including diabetes) as judged by FPG and 2 h‐PG. Multivariate logistic regression analysis was also used to investigate the association between diabetes status according to FPG/2 h‐PG and the presence of moderately increased albuminuria adjusting for confounders such as age, gender, systolic blood pressure, high‐ and low‐density lipoprotein, and triglycerides. Two‐sided P‐values <0.05 were considered statistically significant. All analyses were conducted using IBM SPSS Statistics for Windows, Version 24.0 (IBM Corporation, Armonk, NY, USA).
3. RESULTS
Table 1 shows the characteristics of the study participants (n = 2695, 48% men) for those considered to have no diabetes, prediabetes, or diabetes according to the stepwise diagnostic procedure currently recommended by the German Diabetes Association. According to this algorithm, 70 (2.6%) individuals were considered to have a newly diagnosed diabetes, and 125 (4.6%) individuals were considered to have prediabetes. Individuals with diabetes were significantly more often male. On average, they were older, had a higher body mass index and waist‐to‐hip ratio, a higher systolic blood pressure, and higher levels of triglycerides and LDL cholesterol but lower levels of HDL cholesterol. There were also significant differences regarding serum urea and uric acid concentrations. The UACR was significantly increased in individuals with prediabetes and diabetes.
Table 1.
Characteristics of the study population (n = 2695)
| Diabetes status According to stepwise use of HbA1c (first step) and FPG/2 h‐PG (conditional second step) | Total n = 2695 (100.0%) | P a | |||
|---|---|---|---|---|---|
| Normal n = 2500 (92.8%) | Prediabetes n = 125 (4.6%) | Diabetes n = 70 (2.6%) | |||
| Age (y), no. (%) | |||||
| 40‐49 | 889 (35.6) | 15 (12.0) | 9 (12.9) | 913 (33.9) | <0.001 (a, b) |
| 50‐59 | 767 (30.7) | 41 (32.8) | 23 (32.9) | 831 (30.8) | |
| 60‐69 | 524 (21.0) | 35 (28.0) | 24 (34.3) | 583 (21.6) | |
| 70‐79 | 320 (12.8) | 34 (27.2) | 14 (20.0) | 368 (13.7) | |
| Sex, no. (%) | |||||
| Male | 1188 (47.5) | 72 (57.6) | 42 (60.0) | 1302 (48.3) | <0.001 (a) |
| Female | 1312 (52.5) | 53 (42.4) | 28 (40.0) | 1393 (51.7) | |
| BMI (kg/m2) | 26.7 ± 4.2 | 29.5 ± 4.5 | 31.5 ± 6.3 | 27.0 ± 4.4 | <0.001 (a, b, c) |
| WHR | 0.92 ± 0.09 | 0.97 ± 0.07 | 1.0 ± 0.08 | 0.92 ± 0.09 | <0.001 (a, b, c) |
| SBP (mm Hg) | 126.0 ± 15.7 | 128.4 ± 16.1 | 135.8 ± 20.6 | 126.4 ± 16.0 | <0.001 (b, c) |
| DBP (mm Hg) | 74.9 ± 9.6 | 74.8 ± 10.2 | 77.8 ± 11.2 | 75.0 ± 9.7 | 0.055 |
| Triglycerides (mmol/L) | 1.4 ± 1.1 | 1.6 ± 0.9 | 1.8 ± 0.9 | 1.4 ± 1.1 | <0.001 (a, b) |
| Total Cholesterol (mmol/L) | 5.6 ± 1.0 | 5.8 ± 1.2 | 5.6 ± 1.0 | 5.6 ± 1.0 | 0.264 |
| HDL (mmol/L) | 1.7 ± 0.5 | 1.5 ± 0.4 | 1.4 ± 0.4 | 1.6 ± 0.5 | <0.001 (a, b) |
| LDL (mmol/L) | 3.5 ± 0.9 | 3.8 ± 1.0 | 3.6 ± 1.0 | 3.5 ± 0.9 | 0.005 (a) |
| Creatinine (mmol/L) | 79.9 ± 23.0 | 82.1 ± 15.0 | 80.4 ± 18.5 | 80.0 ± 22.6 | 0.548 |
| Urea (mmol/L) | 5.0 ± 1.4 | 5.4 ± 1.6 | 5.1 ± 1.6 | 5.0 ± 1.4 | 0.003 (a) |
| Uric acid (mmol/L) | 313.5 ± 81.7 | 348.7 ± 78.2 | 375.3 ± 86.8 | 316.8 ± 82.5 | <0.001 (a, b, c) |
| eGFR (mL/min/1.73 m2) | 82.4 ± 14.4 | 80.2 ± 13.9 | 84.7 ± 18.4 | 82.4 ± 14.5 | 0.098 |
| uACR (mg/g) | 13.3 ± 80.5 | 26.3 ± 175.5 | 34.8 ± 82.8 | 14.5 ± 87.3 | 0.038 (b) |
| HbA1c (%) | 5.2 ± 0.3 | 5.9 ± 0.2 | 6.6 ± 1.6 | 5.3 ± 0.5 | |
| HbA1c (mmol/mol) | 33.5 ± 3.2 | 40.7 ± 2.7 | 48.2 ± 17.3 | 34.2 ± 5.0 | |
| FPG (mmol/L) | 5.4 ± 0.6 | 6.1 ± 0.4 | 7.7 ± 2.0 | 5.5 ± 0.8 | |
| 2 h‐PG (mmol/L) | 5.9 ± 1.7 | 7.0 ± 1.9 | 12.8 ± 4.6 | 2.1 ± 0.1 | |
BMI, body mass index; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; PG, postchallenge plasma glucose; SBP, systolic blood pressure; uACR, urine albumin‐creatinine ratio; WHR, waist‐to‐hip ratio.
Unless otherwise specified, data are shown as mean ± standard deviation.
P‐value represents the results of the overall comparison of the three groups (normal, prediabetes and diabetes). The letters a, b and c indicate that post hoc pairwise comparison was significant (P < 0.05): a, normal vs. prediabetes; b, normal vs. diabetes; c, prediabetes vs. diabetes.
Table 2 shows a comparison of the diabetes status (normal, prediabetes, diabetes) as determined by the HbA1c concentration vs. the diabetes status as determined by FPG/2 h‐PG levels. Based on HbA1c, 234 individuals (8.7%) had a prediabetic condition, and 22 individuals (0.8%) were considered to have diabetes. In contrast, the prevalence of prediabetes according to FPG/2 h‐PG was considerably higher (37%, 996 individuals), and also the prevalence of diabetes was higher (4.1%, 110 individuals). Among the 234 individuals with prediabetes according to HbA1c, 61 (26.1%) were normal according to FPG/2 h‐PG, and 48 (20.5%) were classified as having diabetes. Among 2439 individuals with normal HbA1c, 868 (35.6%) and 45 (1.8%) were considered to be prediabetic or diabetic, respectively, according to their FPG/2 h‐PG concentrations. The concordance rate between the classification based on HbA1c and FPG/2 h‐PG was 61.6%. The sensitivity and specificity of HbA1c to recognize diabetes (HbA1c ≥6.5% or ≥48 mmol/mol) according to the FPG/2 h‐PG definition was 15.5% and 99.8%, respectively. The sensitivity and specificity of HbA1c to recognize a normal status (HbA1c <5.7% or <39 mmol/mol) according to the FPG/2 h‐PG definition was 96.0% and 17.5%, respectively.
Table 2.
Comparison of the diabetes status (normal, prediabetes and diabetes) as determined by HbA1c vs. FPG/2 h‐PG
| Diabetes status according to FPG/2 h‐PG | Diabetes status according to HbA1c | |||
|---|---|---|---|---|
| Normal n = 2439 (90.5%) | Prediabetes n = 234 (8.7%) | Diabetes n = 22 (0.8%) | Total n = 2695 (100.0%) | |
| Normal | 1526 (62.6%) | 61 (26.1%) | 2 (9.1%) | 1589 (59.0%) |
| Prediabetes | 868 (35.6%) | 125 (53.4%) | 3 (13.6%) | 996 (37.0%) |
| Diabetes | 45 (1.8%) | 48 (20.5%) | 17 (77.3%) | 110 (4.1%) |
Figure 1 shows a ROC‐AUC analysis of the ability of the HbA1c levels in men and women to detect individuals with diabetes or prediabetes/diabetes according to FPG/2 h‐PG levels. For diabetes as the target category, ROC‐AUC was 0.81 (95% CI: 0.75‐0.88) in men and 0.87 (95% CI: 0.81‐0.93) in women. For prediabetes/diabetes, ROC‐AUC values were lower with 0.65 (95% CI: 0.62‐0.68) for men and 0.70 (95% CI: 0.67‐0.73) for women.
Figure 1.
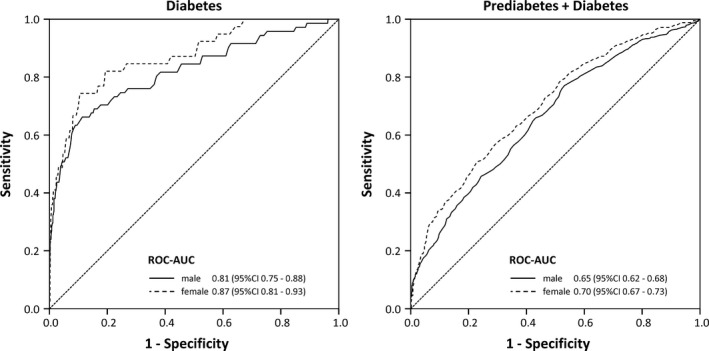
Receiver operating characteristic (ROC) curves for detecting diabetes or prediabetes/diabetes according to FPG/2 h‐PG based on HbA1c levels in men and women
Eight hundred and sixty‐eight (35.6%, 95% CI: 33.7‐37.5) of the individuals with normal HbA1c levels had prediabetes and 45 individuals (1.8%, 95% CI: 1.4‐2.4) had diabetes according to FPG/2 h‐PG (Table 2). Within the group of individuals with normal HbA1c, the overall prevalence of moderately increased albuminuria was 148/2439 (6.1%, 95% CI: 5.2‐7.1) (Table 3). While the prevalence was 3.9% in the group with normal FPG/2 h‐PG levels, it was significantly higher among individuals with prediabetes (9.4%) and diabetes (13.3%) according to FPG/2 h‐PG (P < 0.001). This difference remained significant after adjustment for confounders, such as gender, age, systolic blood pressure, LDL and HDL cholesterol, and triglycerides (P = 0.007). Individuals with prediabetes or diabetes according to FPG/2 h‐PG had a 1.80‐fold increased odds (95% CI: 1.25‐2.60) for moderately increased albuminuria compared to individuals with normal FPG/2 h‐PG levels in the adjusted analysis.
Table 3.
Prevalence of moderately increased albuminuria and UACR values by diabetes status according to HbA1c and FPG/2 h‐PG
| Diabetes status according to FPG/2 h‐PG | Diabetes status according to HbA1c | |||
|---|---|---|---|---|
| Normal | Prediabetes | Diabetes | Total | |
| Normal | 3.9% (60/1526) | 9.8% (6/61) | 0.0% (0/2) | 4.2% (66/1589) |
| 10.1 ± 42.3 | 21.6 ± 85.8 | 3.1 ± 0.8 | 10.5 ± 44.7 | |
| Prediabetes | 9.4% (82/868) | 10.4% (13/125) | 0.0% (0/3) | 9.5% (95/996) |
| 18.2 ± 122.1 | 26.3 ± 175.5 | 8.5 ± 5.6 | 19.1 ± 129.7 | |
| Diabetes | 13.3% (6/45) | 25.0% (12/48) | 35.3% (6/17) | 21.8% (24/110) |
| 17.6 ± 39.1 | 40.5 ± 95.4 | 27.1 ± 49.2 | 29.1 ± 70.8 | |
| Total | 6.1% (148/2439) | 13.2% (31/234) | 27.3% (6/22) | 6.9% (185/2695) |
| 13.1 ± 80.4 | 28.0 ± 142.0 | 22.4 ± 43.9 | 14.5 ± 87.3 | |
UACR values are shown as mean ± standard deviation.
Receiver operating characteristic analysis within the subgroup of individuals who are considered not to have diabetes or prediabetes according to the currently recommended stepwise screening algorithm (ie, individuals with HbA1c levels <5.7% [<39 mmol/L]) revealed that HbA1c was still significantly able to discriminate between individuals with normal FPG/2 h‐PG levels vs. those with prediabetic or diabetic FPG/2 h‐PG levels (Figure 2), however, with comparatively low AUC (0.63, 95% CI: 0.61‐0.66). Using multivariate logistic regression analysis, we examined whether additional and easily obtainable information such as gender, age, and BMI would improve the discrimination between the presence vs. absence of prediabetes/diabetes among individuals with normal HbA1c (Table 4). This analysis revealed that male sex, higher age, higher BMI, and higher HbA1c levels were independently predictive and were able to improve the AUC to 0.74 (95% CI: 0.72‐0.76, Figure 2).
Figure 2.
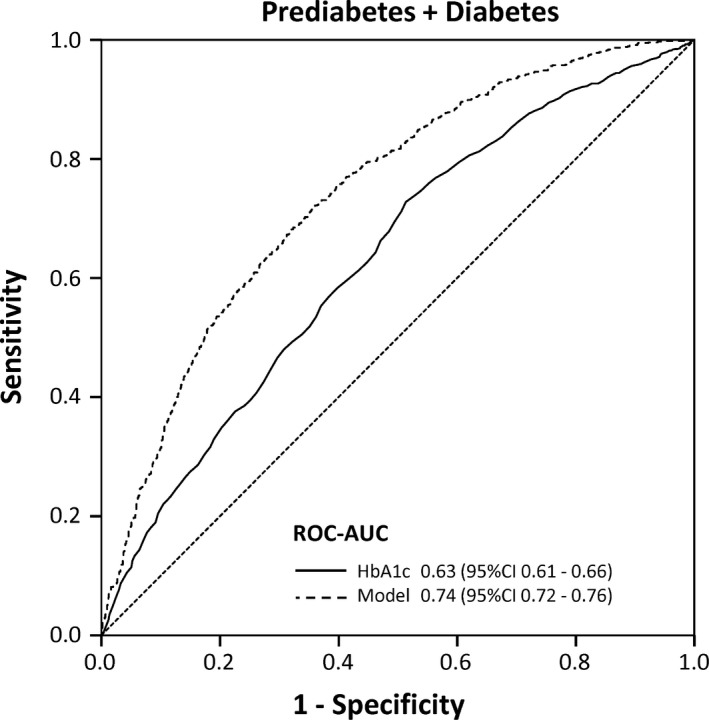
Receiver operating characteristic (ROC) curves for detecting prediabetes/diabetes (according to FPG/2 h‐PG definition) among individuals with normal HbA1c levels (<5.7% [<39 mmol/mol]). The solid curve shows the remaining discriminative performance of the HbA1c levels. The dashed line depicts the discriminative performance of the multivariate logistic regression model shown in Table 3 using information on gender, age and BMI in addition to the HbA1c level
Table 4.
Multivariate logistic regression analysis on predictors for the presence of prediabetes/diabetes among individuals with normal HbA1c
| Predictor | OR | 95% CI | P |
|---|---|---|---|
| Men (ref: Women) | 2.55 | 2.15‐3.03 | <0.001 |
| Age (per 10 y increase) | 1.51 | 1.38‐1.64 | <0.001 |
| BMI (per point increase) | 1.11 | 1.09‐1.13 | <0.001 |
| HbA1c (per 0.1% increase) | 1.07 | 1.05‐1.08 | <0.001 |
4. DISCUSSION
The present population‐based cross‐sectional study involving adults between 40 and 79 years without a prior diagnosis of diabetes demonstrates a large prevalence of prediabetes (35.6%) according to fasting and/or two‐hour postload plasma glucose levels among individuals with HbA1c levels which are considered to be normal (<5.7% [<39 mmol/L]). Our study also shows that the prevalence of moderately increased albuminuria among these prediabetic individuals is significantly increased compared to individuals who had normal levels of all three parameters HbA1c, FPG and 2 h‐PG, even after adjustment for different confounders known to be associated with albuminuria. Moreover, our study suggests that the discrimination between individuals with normal FPG/2 h‐PG levels and individuals with prediabetic or diabetic FPG/2 h‐PG levels among individuals with normal HbA1c might be improved using easily obtainable information such as age, gender and body mass index.
A number of mostly cross‐sectional and also some longitudinal studies have previously investigated the diagnostic performance of HbA1c measurements compared to FPG and/or postload glucose levels in different at‐risk populations and ethnicities, with different results and conclusions regarding the usefulness of HbA1c and optimal diagnostic cut‐off values.11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24 In line with our study, a number of these previous studies have also shown that a large proportion of individuals had a prediabetic condition according to their FPG/2 h‐PG levels despite normal HbA1c levels.11, 13, 14, 16, 22, 23 Our study results are also in agreement with previous studies that HbA1c has a considerably better performance to discriminate between individuals with diabetes and individuals not having diabetes compared to the performance to discriminate between normal individuals and individuals having prediabetes (or diabetes).17, 18, 20, 21
Some other studies have examined the association between glucose parameters and albuminuria. Bahar et al27 showed that the risk for prediabetic subjects with moderately increased albuminuria to develop diabetes mellitus was four times as high as for prediabetic subjects without moderately increased albuminuria. This finding underlines the need to detect individuals with moderately increased albuminuria, who are also at risk for diabetes. Suzuki et al28 showed that the prevalence of moderately increased albuminuria was significantly higher in subjects with combined impaired fasting glucose and impaired glucose tolerance than in subjects with only impaired glucose tolerance. In line with these results, our study revealed that individuals with normal HbA1c but elevated FPG and/or 2 h‐PG levels have a significantly higher prevalence of moderately increased albuminuria than individuals with normal FPG and 2 h‐PG levels. In contrast, Huang et al29 found that HbA1c, but not FPG or 2 h‐PG, was associated with risk of low‐grade albuminuria. The South London Diabetes Study found an increased prevalence of moderately increased albuminuria when comparing participants with T2DM according to FPG and/or 2 h‐PG and HbA1c to participants with T2DM with normal HbA1c.30
Our study shows that individuals with moderately increased albuminuria despite normal HbA1c escape early detection and treatment, if screening relies only on HbA1c. Our results also indicate that using clinical information such as age, gender, and BMI might be helpful to decide whom to recommend an additional measurement of FPG and/or 2 h‐PG despite normal HbA1c levels. The Canadian clinical practice guideline of the Canadian Diabetes Association, for example, did so by adding another HbA1c cut‐off and by including risk factors in the diagnostic algorithm.9 Moreover, a number of diabetes prediction models based on clinical data have been proposed in the past, for example the Leicester Diabetes Risk Score in primary care setting particularly with regard to detection of prediabetes.31 Further studies are needed to compare how these tools compare to the simple model based on age, gender and BMI proposed here.
Some limitations of the present study need to be addressed. First, the cross‐sectional approach does not allow proving a causal relationship between the increased FPG/2 h‐PG levels among individuals with normal HbA1c levels and the increased prevalence of moderately increased albuminuria, which would require long‐term longitudinal follow‐up studies. The first follow‐up of the LIFE‐Adult‐Study is currently under way. Second, the present analyses did not include other important early organ damages such as retinopathy. Third, there are several other causes of albuminuria apart from diabetic nephropathy, such as fever, infection, strenuous exercise within 24 hours, pregnancy and hypertension. Pregnancy was a general exclusion criterion in the LIFE‐Adult‐Study. Information on fever, infection and strenuous exercise was not collected. Therefore, an adjustment for these confounders was not possible. However, multivariate logistic regression analysis adjusting for systolic blood pressure revealed that among individuals with normal HbA1c levels the presence of moderately increased albuminuria was still significantly higher in individuals with prediabetic and diabetic levels of FPG and/or 2 h‐PG compared to individuals with normal FPG and 2 h‐PG levels. Furthermore, our study is limited to an urban and predominantly White Caucasian population in a specific eastern region of Germany. It has been shown that there are regional differences in diabetes prevalence within Germany, with higher prevalences in the eastern parts.32 Thus, our results might not be applicable to populations with other ethnicities, since it has been shown that results may vary depending on ethnicity and race.15 A large nationwide epidemiologic study based on a random sample of 200 000 individuals from the general population across Germany aiming to identify new and tailored strategies for early detection, prediction, and primary prevention of major diseases, including diabetes, is currently under way.33
In conclusion, the results of our study show an increased prevalence of moderately increased albuminuria among individuals with normal HbA1c level but impaired glucose regulation. These individuals escape detection and early treatment if only HbA1c is used for diabetes screening. Easily obtainable parameters such as age, gender and BMI might be used in addition to the HbA1c level to decide whether additional FPG and/or OGTT testing should be performed to detect individuals with impaired glucose regulation despite normal HbA1c levels.
ETHICAL APPROVAL
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
CONFLICT OF INTEREST
Nothing to declare.
AUTHOR CONTRIBUTIONS
Christoph Engel and Markus Loeffler were involved in the study concept and design. Kerstin Wirkner contributed to data acquisition. Ronny Baber contributed to the laboratory analyses. Mila Živković, Christoph Engel and Anke Toenjes analysed and interpreted the data. Mila Živković and Christoph Engel drafted the manuscript.
ACKNOWLEDGEMENTS
We wish to thank the citizens of the City of Leipzig for their willingness to participate in the study. We also thank the team of the LIFE‐Adult‐Study centre for their skilful technical assistance. We acknowledge support from the German Research Foundation (DFG) and Leipzig University within the program of Open Access Publishing.
Živković M, Tönjes A, Baber R, Wirkner K, Loeffler M, Engel C. Prevalence of moderately increased albuminuria among individuals with normal HbA1c level but impaired glucose tolerance: Results from the LIFE‐Adult‐Study. Endocrinol Diab Metab. 2018;1:e30 10.1002/edm2.30
Funding information
This publication is supported by LIFE—Leipzig Research Center for Civilization Diseases, an organizational unit affiliated to the Medical Faculty of the University Leipzig, Germany. LIFE is funded by means of the European Union, by the European Regional Development Fund (ERDF) and by funds of the Free State of Saxony within the framework of the excellence initiative.
REFERENCES
- 1. Ogurtsova K, da Rocha Fernandes JD, Huang Y, et al. IDF Diabetes Atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017;128:40‐50. [DOI] [PubMed] [Google Scholar]
- 2. Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405‐412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. de Zeeuw D, Parving HH, Henning RH. Microalbuminuria as an early marker for cardiovascular disease. J Am Soc Nephrol. 2006;17(8):2100‐2105. [DOI] [PubMed] [Google Scholar]
- 4. Troisi RJ, Cowie CC, Harris MI. Diurnal variation in fasting plasma glucose: implications for diagnosis of diabetes in patients examined in the afternoon. JAMA. 2000;284(24):3157‐3159. [DOI] [PubMed] [Google Scholar]
- 5. Ollerton RL, Playle R, Ahmed K, Dunstan FD, Luzio SD, Owens DR. Day‐to‐day variability of fasting plasma glucose in newly diagnosed type 2 diabetic subjects. Diabetes Care. 1999;22(3):394‐398. [DOI] [PubMed] [Google Scholar]
- 6. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2012;35(Suppl 1):S64‐S71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ryden L, Standl E, Bartnik M, et al. Guidelines on diabetes, pre‐diabetes, and cardiovascular diseases: executive summary. The Task Force on Diabetes and Cardiovascular Diseases of the European Society of Cardiology (ESC) and of the European Association for the Study of Diabetes (EASD). Eur Heart J. 2007;28(1):88‐136. [DOI] [PubMed] [Google Scholar]
- 8. Landgraf R. [National practice guideline therapy of type 2 diabetes]. MMW Fortschr Med. 2014;156 Spec No 1(1):76‐78. [PubMed] [Google Scholar]
- 9. Cheng AY. Canadian Diabetes Association 2013 clinical practice guidelines for the prevention and management of diabetes in Canada. Introduction. Can J Diabetes. 2013;37(Suppl 1):S1‐S3. [DOI] [PubMed] [Google Scholar]
- 10. Kerner W, Bruckel J. Definition, classification and diagnosis of diabetes mellitus. Exp Clin Endocrinol Diabetes. 2014;122(7):384‐386. [DOI] [PubMed] [Google Scholar]
- 11. Bhowmik B, Diep LM, Munir SB, et al. HbA(1c) as a diagnostic tool for diabetes and pre‐diabetes: the Bangladesh experience. Diabet Med. 2013;30(3):e70‐e77. [DOI] [PubMed] [Google Scholar]
- 12. Cavagnolli G, Comerlato J, Comerlato C, Renz PB, Gross JL, Camargo JL. HbA(1c) measurement for the diagnosis of diabetes: is it enough? Diabet Med. 2011;28(1):31‐35. [DOI] [PubMed] [Google Scholar]
- 13. Chilelli NC, Cosma C, Ragazzi E, et al. Screening with HbA1c identifies only one in two individuals with diagnosis of prediabetes at oral glucose tolerance test: findings in a real‐world Caucasian population. Acta Diabetol. 2014;51(5):875‐882. [DOI] [PubMed] [Google Scholar]
- 14. Kumaravel B, Bachmann MO, Murray N, et al. Use of haemoglobin A1c to detect impaired fasting glucose or Type 2 diabetes in a United Kingdom community based population. Diabetes Res Clin Pract. 2012;96(2):211‐216. [DOI] [PubMed] [Google Scholar]
- 15. Mostafa SA, Khunti K, Kilpatrick ES, et al. Diagnostic performance of using one‐ or two‐HbA1c cut‐point strategies to detect undiagnosed type 2 diabetes and impaired glucose regulation within a multi‐ethnic population. Diab Vasc Dis Res. 2013;10(1):84‐92. [DOI] [PubMed] [Google Scholar]
- 16. Mostafa SA, Khunti K, Srinivasan BT, Webb D, Gray LJ, Davies MJ. The potential impact and optimal cut‐points of using glycated haemoglobin, HbA1c, to detect people with impaired glucose regulation in a UK multi‐ethnic cohort. Diabetes Res Clin Pract. 2010;90(1):100‐108. [DOI] [PubMed] [Google Scholar]
- 17. Olson DE, Rhee MK, Herrick K, Ziemer DC, Twombly JG, Phillips LS. Screening for diabetes and pre‐diabetes with proposed A1C‐based diagnostic criteria. Diabetes Care. 2010;33(10):2184‐2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pinelli NR, Jantz AS, Martin ET, Jaber LA. Sensitivity and specificity of glycated hemoglobin as a diagnostic test for diabetes and prediabetes in Arabs. J Clin Endocrinol Metab. 2011;96(10):E1680‐E1683. [DOI] [PubMed] [Google Scholar]
- 19. Selvin E, Steffes MW, Gregg E, Brancati FL, Coresh J. Performance of A1C for the classification and prediction of diabetes. Diabetes Care. 2011;34(1):84‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shimodaira M, Okaniwa S, Hanyu N, Nakayama T. Optimal hemoglobin A1c levels for screening of diabetes and prediabetes in the Japanese population. J Diabetes Res. 2015;2015:932057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tankova T, Chakarova N, Dakovska L, Atanassova I. Assessment of HbA1c as a diagnostic tool in diabetes and prediabetes. Acta Diabetol. 2012;49(5):371‐378. [DOI] [PubMed] [Google Scholar]
- 22. Guo F, Moellering DR, Garvey WT. Use of HbA1c for diagnoses of diabetes and prediabetes: comparison with diagnoses based on fasting and 2‐hr glucose values and effects of gender, race, and age. Metab Syndr Relat Disord. 2014;12(5):258‐268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Heianza Y, Hara S, Arase Y, et al. HbA1c 5.7‐6.4% and impaired fasting plasma glucose for diagnosis of prediabetes and risk of progression to diabetes in Japan (TOPICS 3): a longitudinal cohort study. Lancet. 2011;378(9786):147‐155. [DOI] [PubMed] [Google Scholar]
- 24. Kato M, Noda M, Suga H, Nakamura T, Matsumoto M, Kanazawa Y. Haemoglobin A1c cut‐off point to identify a high risk group of future diabetes: results from the Omiya MA Cohort Study. Diabet Med. 2012;29(7):905‐910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Loeffler M, Engel C, Ahnert P, et al. The LIFE‐Adult‐Study: objectives and design of a population‐based cohort study with 10,000 deeply phenotyped adults in Germany. BMC Public Health. 2015;15:691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hasslacher C, Wolf G, Kempe P, Ritz E. Diabetic kidney disesase. Exp Clin Endocrinol Diabetes. 2014;122(7):391‐394. [DOI] [PubMed] [Google Scholar]
- 27. Bahar A, Makhlough A, Yousefi A, Kashi Z, Abediankenari S. Correlation between prediabetes conditions and microalbuminuria. Nephrourol Mon. 2013;5(2):741‐744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Suzuki H, Fukushima M, Usami M, et al. IGT with fasting hyperglycemia is more strongly associated with microalbuminuria than IGT without fasting hyperglycemia. Diabetes Res Clin Pract. 2004;64(3):213‐219. [DOI] [PubMed] [Google Scholar]
- 29. Huang X, Zhou Y, Xu B, et al. Glycated haemoglobin A1c is associated with low‐grade albuminuria in Chinese adults. BMJ Open. 2015;5(8):e007429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Azam M, Marwood L, Ismail K, et al. Diabetes complications at presentation and one year by glycated haemoglobin at diagnosis in a multiethnic and diverse socioeconomic population: results from the South London Diabetes Study. J Diabetes Res. 2015;2015:587673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gray LJ, Davies MJ, Hiles S, et al. Detection of impaired glucose regulation and/or type 2 diabetes mellitus, using primary care electronic data, in a multiethnic UK community setting. Diabetologia. 2012;55(4):959‐966. [DOI] [PubMed] [Google Scholar]
- 32. Tamayo T, Schipf S, Meisinger C, et al. Regional differences of undiagnosed type 2 diabetes and prediabetes prevalence are not explained by known risk factors. PLoS One. 2014;9(11):e113154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. The German National Cohort: aims, study design and organization. Eur J Epidemiol. 2014;29(5):371‐382. [DOI] [PMC free article] [PubMed] [Google Scholar]


