Summary
Aims
Using the novel FreeStyle Libre (FSL), glucose monitoring (FGM) system becomes increasingly popular among people with type 1 diabetes (T1D) and is associated with less and shorter hypoglycaemic events without deterioration of HbA1c. There are not yet data reporting the impact of FGM in people with T1D in real‐life conditions. We sought of evaluating the tolerance, the acceptance and the efficacy of the FGM system in routine medical practice.
Methods
This 12‐month observational study included 120 individuals with T1D evaluated every 3 months. After having been instructed about FGM utilization, participants were trained to optimize the glycaemic control.
Results
Participants stopped immediately of measuring capillary blood glucose (2.88 ± 0.12 per day) (mean ± SEM) after having received the first FSL device and the number of scans per day increased up to 8.87 ± 0.58 per day. HbA1c levels decreased from 8.51% ± 0.14% at baseline to 7.77% ± 0.09% after 3 months to slightly increase to 7.92% ± 0.09% at 12 months, in correlation with the number of scans per day. The number (but not the duration) of hypoglycaemic events slightly increased from 16.9 ± 1.44 per month at baseline to 24.0 ± 2.91 per month at 12 months, after reaching a peak of 26.4 ± 2.31 per month at 6 months. They were correlated with improved HbA1c.
Conclusion
Our study shows that using the FGM system improves HbA1c levels in people with T1D along with a moderate increase in the number of mild hypoglycaemic events. The new FGM system facilitates the therapeutic empowerment of people with T1D, but in a context of structured education.
Keywords: diabetes management, FreeStyle libre, glucose monitoring, real‐life conditions
1. INTRODUCTION
Nowadays, real‐time continuous glucose monitoring (CGM) is increasingly used for diabetes management.1, 2 Ongoing improvements, since early 2000, have been brought in terms of safety, user acceptance and accuracy. The recent availability of devices with MARD (mean absolute relative difference) below 10% compared with capillary blood glucose monitoring (BGM) allows CGM‐derived glucose values of being used for self‐adjustment of insulin dosages without adjuvant use of BGM.3
Few major problems though still hamper the use of CGM for glucose management on a large scale, that is, the need of daily finger‐stick BGM for device calibration, the short sensor lifetime and the price.4 An alternative technology, the FreeStyle Libre (FSL) glucose monitoring (FGM), recently made available by Abbott Diabetes Care, overcome these pitfalls.4 FSL continuously measures interstitial fluid glucose levels in real time. In contrast to CGM systems, FGM is based on the use of low cost, factory‐calibrated 14‐day small size patch glucose sensors with MARD close to 10%.5, 6, 7 Interstitial fluid glucose levels are measured every minute, recorded every 15 minutes and stored for a period of 90 days. Real‐time glucose values are visualized on demand by transfer from the sensor, as soon as it is brought in close vicinity of the sensor. The reader displays the actual glucose value, a trend arrow (based on the last 15‐minute glucose levels) which gives information on rate and direction of glucose changes, and retrospective glucose readings over the last 8 hours. Data can be uploaded from the reader through a software (Ambulatory Glucose profile, [AGP]) that generates standardized glucose summaries stored as PDF files.8, 9, 10 As they provide reliable information about glucose control and variability, incidence, depth and duration of hypoglycaemic events, AGP‐generated graphs can be easily used by the patient, either alone or with health care professionals (HCP) as an educational tool.
“Real‐world” data looking at more than 400 million individual glucose measurements recently reported that the best HbA1c values and time in range (between 70 mg/dL and 180 ml/dL) were observed among users performing the highest number of scans per day.11
These data are in accordance with those previously reported in the IMPACT trial which showed in 328 well‐controlled individuals with type 1 diabetes that the time spent in nocturnal hypoglycaemia and the number of serious hypoglycaemia (<55 mg/dL) were significantly reduced among individuals wearing the device. These results came along significant improvement in parameters of quality of live.12 Similar results were found in individuals with type 2 diabetes in the REPLACE program which also showed that time spent in hypoglycaemia was significantly reduced after 6 and 12 months along with improvement in treatment satisfaction.13, 14
In this study, we sought of analysing FGM‐induced effects on the glycaemic control (HbA1c and hypoglycaemic events) in a cohort of individuals with type 1 diabetes followed in real‐live conditions as those occurring in the daily clinical practice in an outpatient diabetes clinic.
2. STUDY DESIGN AND PARTICIPANTS
This study was started as soon as the FGM technology became available in Belgium (January 2016 onwards). A total of 135 individuals with type 1 diabetes were prospectively recruited from consecutive visits in our outpatient clinic (CHR Mons‐Hainaut, Belgium). Study eligibility criteria were type 1 diabetes diagnosed for more than 1 year in male and female adults, aged 18 years or older and having provided informed consent. Because of the real‐life design of this clinical trial, there were no specific exclusion criteria, except the current use of another CGM system, the nonacceptance of this new method of glucose monitoring, a greatly altered medical condition, the nonadherence to the clinic visit every 3 months, the inability of using the FGM system, the known allergy to medical‐grade adhesives, and pregnancy. The study was performed according a protocol approved by the local Ethic Review Committee.
Baseline diabetes and anthropometric characteristics were recorded from each participant using standardized records. Each of them was then individually trained on how to set the device up on the back of the upper arm every 2 weeks. The device was immediately unblinded allowing patients to readily visualize glucose values. During the next 2 weeks, participants did not receive specific instructions for insulin dose self‐management. They were though asked to keep recording glucose levels in a diary and to take appropriate measures as they were previously trained to do when using finger sticks. After 2 weeks, they were seen again to check for technical pitfalls and to make sure they correctly understood how to use the device and to set a new sensor up. Their glucose data were then uploaded from the reader and AGP‐derived reports were generated. The data were thoroughly reviewed together with HCP who provided insulin titration algorithms to tightly adapt insulin dosages regarding glucose levels. Instructions were given to anticipate as much as possible hypoglycaemic episodes, to optimize fasting glucose levels and to reduce postprandial glucose excursions. Participants were invited to scan the sensor as many times as they believe to be necessary for glucose self‐management and insulin dose adaptations. Each of them was then seen every 3 months over a period of 1 year for weight measurement, insulin dosage recordings, sensor data uploading and diabetes education.
For the statistical analysis, 3 groups of individuals were considered; the entire cohort, the subgroup of them with HbA1c ≥ 7.5% at baseline (more than 2‐thirds of the cohort) and the subgroup of them with HbA1c < 7.5% at baseline. This splitting was performed to identify which individual with type 1 diabetes is taking the most benefit from the FGM system.
HbA1c was measured in all participants at 0, 3, 6, 9 and 12 months (T0, T3, T6, T9, T12) using a Bio‐Rad 10 Hemoglobin Testing System (Bio‐Rad laboratories, Inc, Temse, Belgium).
Linear mixed models15 were used for longitudinal analysis according to the recommendation of Molenberghs and Kenward16 using the R software (R Core Team, 2016), version 3.2.2. The statistical significance among groups was estimated using a paired T test and the correlation analysis was performed using Pearson or Spearman tests (GraphPad Prism, San Diego, CA, USA). P < .05 was considered as statistically significant.
3. RESULTS
3.1. Patient characteristics at baseline
Out of the initial cohort of 135 individuals, 3 stopped for local allergy reason. Six others for various reasons (visibility of the device, too many detachments and the wish of using alternative CGM methods). There were lost of view. Three female participants who became pregnant during the trial were withdrawn from the final analysis. A total of 120 individuals with type 1 diabetes were finally recruited for the study. They were 40.1 ± 1.2 year old (mean±SEM) with duration of diabetes of 16.8 ± 1 years, BMI of 25.9 ± 0.5 kg/m2 and baseline HbA1c levels of 8.5% ± 0.1%. At the entry of the study, they were receiving 0.71 ± 0.02 U/kg/d as total daily dose (TDD) of insulin. Of the 120 participants, 90 were treated with daily multiple doses of insulin (MDI) and the 30 remaining with continuous subcutaneous insulin infusion (CSII) (Table 1A).
Table 1.
(A) Characteristics of participants at baseline. A total of 120 individuals with type 1 diabetes were included in this observational prospective study. (B) Number of daily scans. Results are expressed as means ± SEM
| (A) | |||
|---|---|---|---|
| Mean ± SEM | Minimum | Maximum | |
| Age (years) | 40.11 ± 1.278 | 18 | 76 |
| Diabetes duration (years) | 16.84 ± 1.033 | 1 | 47 |
| HbAlc(%) | 8.5 ± 0.1385 | 5.6 | 16 |
| BMI (kg/m2) | 25.93 ± 0.51 | 17.72 | 47.27 |
| Insulin (U/Kg.d) | 0.7151 ± 0.026 | 0.3333 | 1.809 |
| (B) | ||||||
|---|---|---|---|---|---|---|
| T0‐15d | T0 | T3 | T6 | T9 | T12 | |
| Number of scans (mean ± SEM) | 2.885 ± 0.12 n = 110 | 8.871 ± 0.58 n = 101 |
8.23 ± 0.45 n = 103 NS |
8.01 ± 0.5 n = 91 P < .05 vs T0 |
8.49 ± 0.58 n = 89 NS |
8.8 ± 0.69 n = 73 NS |
BMI, Body mass index.
At all time, P < .00001 as compared to T0‐15d.
The average number of glucose controls per day by BGM before receiving the first sensor (T0‐15d) was 2.88 ± 0.12/d. As soon as participants had access to FGM and received the green light for insulin dose adjustment (T0: 2 weeks after having received the first sensor), they stopped nearly immediately of checking capillary blood glucose levels by BGM. By contrast, the number of sensor scans per day significantly raised up to 8.87 ± 0.58/d and remained stable throughout the study period (8.85 ± 0.69/d at T12) (Table 1B).
There was a slight increase in BMI values which reached the statistical significance only at T6 (26.69 ± 0.62 kg/m2) as compared to T0 (25.93 ± 0.51 kg/m2) (P < .05). This moderate weight gain was observed only in individuals with baseline HbA1c levels ≥7.5% (Table 2).
Table 2.
BMI (body mass index) and insulin dosages
| T0 | T3 | T6 | T9 | T12 | |
|---|---|---|---|---|---|
| BMI (kg/m2) (mean ± SEM) |
25.93 ± 0.51 n = 114 |
26.28 ± 0.58 n = 100 NS |
26.69 ± 0.62 n = 91 P < .05 vs T0 |
26.39 ± 0.59 n = 80 NS |
26.5 ± 0.63 n = 64 NS |
| BMI (kg/m2), HBAlc >7.5% at T0 (mean ± SEM) |
26.45 ± 0.59 n = 91 |
26.92 ± 0.67 n = 80 NS |
27.57 ± 0.72 n = 71 P < .05 vs T0 |
27.35 ± 0.70 n = 59 P < .05 vs T0 |
27.32 ± 0.76 n = 46 NS |
| BMI (kg/m2), HBA1c < 7.5% at T0 (mean ± SEM) |
23.89 ± 0.81 n = 23 |
23.75 ± 0.87 n = 20 NS |
23.57 ± 0.9 n = 20 NS |
23.69 ± 0.85 n = 21 NS |
24.37 ± 0.91 n = 18 NS |
| Long‐acting insulin (U/d) (mean ± SEM) |
28.28 ± 1.653 n = 117 |
25.81 ± 1.489 n = 107 P < .05 vs T0 |
26.49 ± 1.694 n = 96 P < .05 vs T0 |
24.54 ± 1.397 n = 82 P < .05 vs T0 |
24.16 ± 1.456 n = 67 P < .05 vs T0 |
| Long‐acting insulin (U/d), HBAlc > 7.5% at T0 (mean ± SEM) |
29.91 ± 1.99 n = 93 |
27.30 ± 1.800 n = 83 P < .05 vs T0 |
28.38 ± 2.059 n = 74 P < .05 vs T0 T0 |
26.45 ± 1.695 n = 60 P < .05 vs T0 |
25.83 ± 1.713 n = 49 NS |
| Long‐acting insulin (U/d), HBAlc < 7.5% at T0 (mean ± SEM) |
21.98 ± 1.892 n = 24 |
20.68 ± 2.039 n = 24 NS |
20.12 ± 2.144 n = 22 NS |
19.33 ± 2.071 n = 22 P < .05 vs T0 |
19.61 ± 2.536 n = 18 NS |
| Rapid‐acting insulin (U/d) (mean ± SEM) |
28.37 ± 1.556 n = 114 |
29.10 ± 1.924 n = 102 NS |
29.97 ± 1.982 n = 93 P < .05 vs T0 |
30.03 ± 2.023 n = 80 P < .001 vs T0 P < .05 vs T3 |
29.08 ± 2.09 n = 63 NS |
| Rapid‐acting insulin (U/d), HBAlc > 7.5% at T0 (mean ± SEM) |
29.87 ± 1.827 n = 91 |
30.65 ± 2.334 n = 80 NS |
31.65 ± 2.427 n = 72 NS |
32.24 ± 2.56 n = 59 P < .001 vs T0 |
31.03 ± 2.758 n = 44 NS |
| Rapid‐acting insulin (U/d), HBAlc < 7.5% at T0 (mean ± SEM) |
22.43 ± 2.37 n = 23 |
23.45 ± 2.476 n = 22 NS |
24.19 ± 2.48 n = 21 NS |
23.81 ± 2.355 n = 21 NS |
24.58 ± 2.488 n = 19 NS |
| Total insulin/weight (U/kg/d) (mean ± SEM) |
0.71 ± 0.026 n = 114 |
0.69 ± 0.027 n = 92 P < .05 vs T0 |
0.70 ± 0.031 n = 85 NS |
0.69 ± 0.029 n = 73 NS |
0.69 ± 0.035 n = 54 NS |
| Total insulin/weight (U/kg/d), HBAlc > 7.5% at T0 (mean ± SEM) |
0.74 ± 0.031 n = 91 |
0.7 ± 0.034 n = 72 P < .05 vs T0 |
0.72 ± 0.038 n = 67 NS |
0.71 ± 0.036 n = 53 NS |
0.73 ± 0.044 n = 38 NS |
| Total insulin/weight, (U/kg/d), HBAlc < 7.5% at T0 (mean ± SEM) |
0.62 ± 0.034 n = 23 |
0.63 ± 0.034 n = 20 NS |
0.64 ± 0.046 n = 18 NS |
0.62 ± 0.041 n = 20 NS |
0.60 ± 0.051 n = 16 NS |
3.2. The HbA1c levels significantly decrease in individuals with type 1 diabetes using FGM
When the whole cohort of participants was taken into consideration, there was a significant reduction in HbA1c levels with a decrease from 8.51% ± 0.14% to 7.77% ± 0.95% already at visit T3 compared to T0 (P < .0001). This value flattened at 6 months to slightly (but significantly) increase up to 7.91% ± 1.11% and 7.92% ± 0.09% at T9 and T12, respectively (Figure 1A). The decrease in HbA1c levels was driven by the subgroup of individuals with baseline HbA1c ≥ 7.5%. Among those participants, HbA1c levels dropped from 8.95% ± 0.14% to 8.03% ± 0.09%, 7.99% ± 0.1%, 8.2% ± 0.13% and 8.16% ± 0.09% at T3, T6, T9 and T12 (P < .0001), respectively (Figure 1B). By contrast, there was no change in HbA1c levels in the subgroup of participants with baseline HbA1c < 7.5%. There was even a progressive increase in HbA1c levels which reached the significance at T12 (7.14% ± 0.16%) as compared to T0 (6.8% ± 0.11%; P < .05; Figure 1C).
Figure 1.
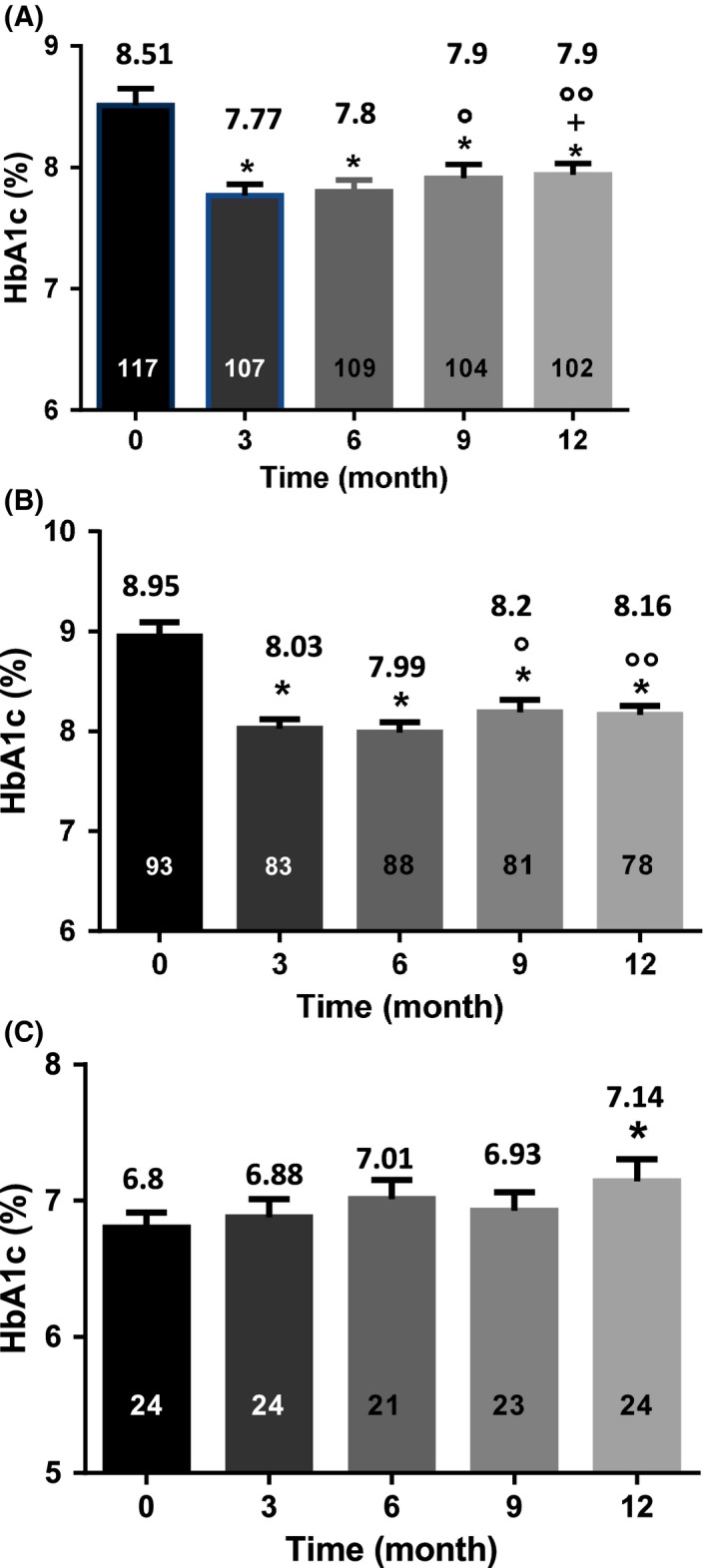
HbA1c values over time. A, HbA1c values (indicated above the columns) in the entire cohort. HbA1c values significantly decreased at all time‐points even though they were slightly increasing after 9 months. B, HbA1c values (indicated above the columns) in the subgroup of individuals with HbA1c ≥ 7.5% at T0. HbA1c values significantly decreased at all time‐points. C, HbA1c values (indicated above the columns) in the subgroup of individuals with HbA1c < 7.5% at T0. No change in HbA1c levels was observed except at T12 where there were slightly increased. The numbers in the columns represent the number of individuals. *, P < .0001 as compared to T0; +, P < .01 vs T3; °, P < .05; °°, P < .001 vs T6 (A and B). *, P < .05 as compared to T0 (C). T0 = 15 days after setting of the first sensor
As shown in Figure 2, there was a negative correlation between the number of scans per day and the decrease in HbA1c levels. This negative correlation was observed at all time‐points and in the 2 subgroups of patients regardless baseline HbA1c levels.
Figure 2.
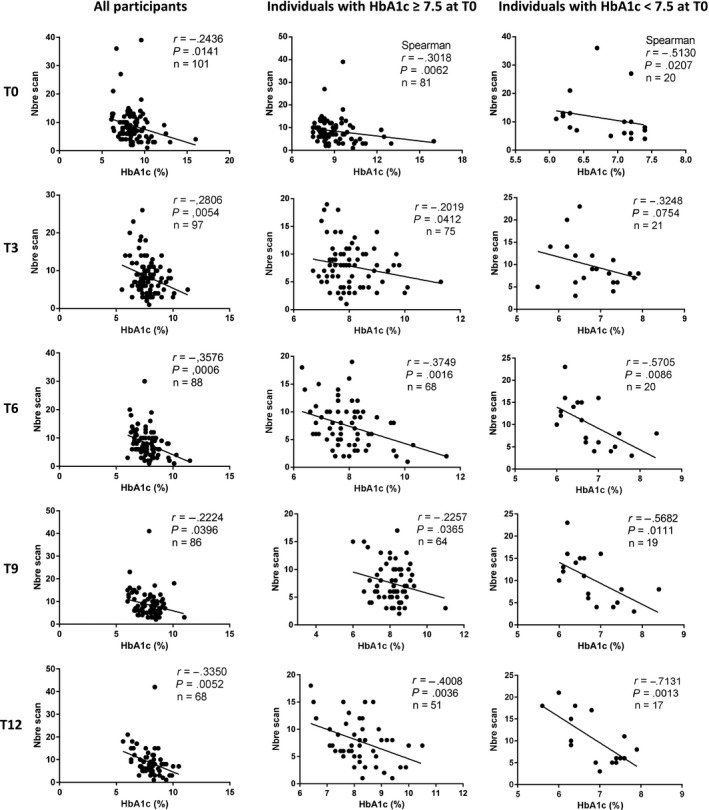
Correlation between HbA1c values and the number of daily scans. The correlation was calculated using Pearson correlation coefficient (r), excepted if indicated. A negative correlation was observed between the number of scans and the HbA1c values at all time‐points
3.3. The number, but not the duration, of hypoglycaemic events increases in individuals with type 1 diabetes using FGM
There has been no episode of severe hypoglycaemia (requiring third‐party assistance and/or injection of glucagon) during the entire period of observation.
When considering the whole cohort of participants, the number of hypoglycaemic events (defined as glucose levels < 70 mg/dL [3.9 mmol/L]) significantly increased from 16.9 ± 1.44 events/month to 22.9 ± 2.03 events/month at T3 (P < .001). The number of hypoglycaemic episodes at T6, T9 and T12 was alike to T3, but again significantly increased compared to T0 (P < .05) (Figure 3A). The increased number of mild hypoglycaemic events was mostly observed in the subgroup of participants with baseline HbA1c levels ≥7.5% (Figure 3B). This increase was transient with a peak at T6 (26.2 ± 2.7 events/month) compared to T0 (17 ± 1.6 events/month) which then decreased at T12 (20.38 ± 2.06 events/month). A positive trend was also observed in the subgroup of participants with baseline HbA1c levels <7.5%, but, compared to T0 (16.4 ± 3.7 events/month), the statistical significance was observed only at T3 (28.5 ± 5.6 events/month) and not at the other time‐points, likely because of the lower number of individuals in this subgroup (Figure 3C).
Figure 3.
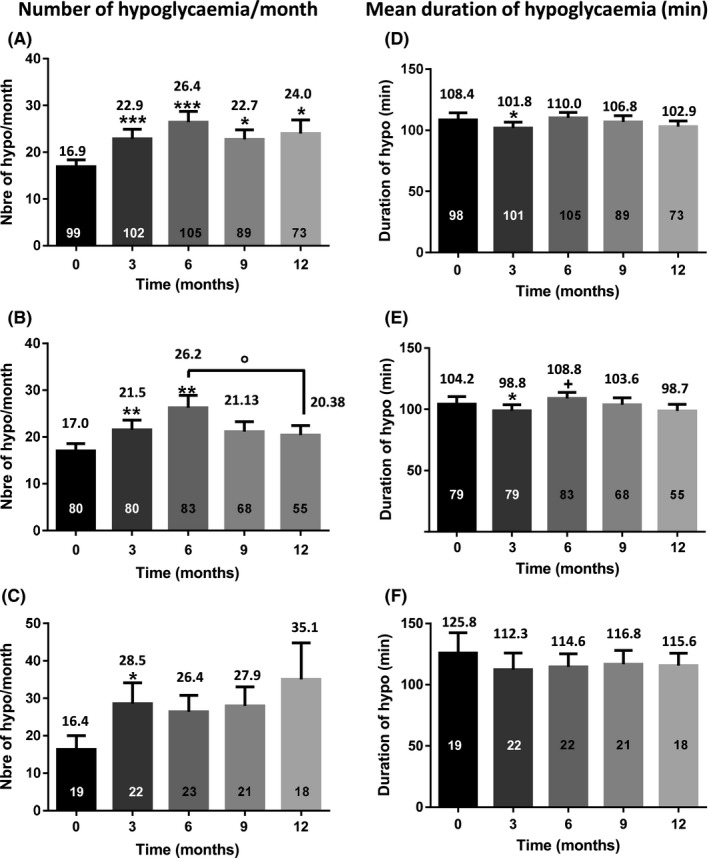
Number of hypoglycaemic events/month (A, B and C) and mean duration of a hypoglycaemic event (D, E, and F). A,D, The entire cohort. B,E, Participants with HbA1c ≥ 7.5 at T0. C,F, Participants with HbA1c < 7.5 at T0. The results are indicated above each column. The numbers in the columns represent the number of individuals. *, P < .05; **, P < .01; ***, P < .001 as compared to T0; +, P < .05 vs T3; °, P < .05 vs T6
When the whole cohort was taken into consideration, there were no frank changes in the duration of hypoglycaemic events. It slightly decreased at T3 as compared to T0, but only in the subgroup of participants with baseline HbA1c levels ≥7.5% (Figure 3D‐F).
As expected and reported in Figure S1, there was a negative correlation between the number of hypoglycaemic events and HbA1c levels.
3.4. The total daily insulin dose (TDD) remained stable over time with changes in the long‐acting/rapid‐acting insulin ratio
Insulin TDD was higher in participants with baseline HbA1c levels ≥7.5% (0.74 ± 0.03 U/kg/d) compared to those with baseline HbA1c levels <7.5% (0.62 ± 0.03 U/kg/d). TDD remained stable over time, with a transient decrease that was significant at T3 in the entire cohort and in the subgroup of participants with baseline HbA1c levels ≥7.5%. As shown in Table 2, the doses of long‐acting insulin significantly decreased over time, especially in participants with baseline HbA1c levels ≥7.5%. By contrast, the doses of rapid‐acting insulin increased over time to reach the statistical significance at T6 (29.97 ± 1.98 U/d) and T9 (30.03 ± 2.02 U/d) compared to T0 (28.37 ± 1.55 U/d). This increase was mostly observed in the subgroup of individuals with baseline HbA1c levels ≥7.5%.
4. DISCUSSION
In accordance with previous studies,4, 5, 6, 7, 11, 12, 13, 14 our data confirm that the FGM system is easy to use and quite convenient for people with type 1 diabetes. The confidence of the participants in this new technology was confirmed by the fast‐increased rate of sensor scans per day and the associated drop in the rate of conventional BGM as soon as they had access to FSL‐associated at‐demand‐readings for insulin dose adjustment. The high degree of satisfaction was confirmed by the high level of utilization still observed by the end of the study period. Some finger strips were still used though, mainly during the first weeks of the study (between T0‐15d and T0), to cross‐check results provided by FSL. BGM was then rapidly abandoned when people acquired confidence in FGM because of the verified concordance between BGM and FGM values. Nevertheless, because technical failures can always occur, participants were still encouraged to keep measuring capillary blood glucose in case of doubt about glucose data provided by FSL or discordance between their feelings and data displayed by the reader.
The main finding of the study was the significant decrease in HbA1c levels already observed at T3 which lasted up to the end of the observation period. The improvement in HbA1c values was mainly observed in the subgroup of individuals with baseline HbA1c levels ≥7.5%. When considering well‐controlled individuals for whom there was no HbA1c improvement (HbA1c even slightly increased at 12 months), it therefore comes out that less‐controlled individuals seem to be those who take the most advantage of this new technology. These people are usually less motivated or more reluctant of using the often‐considered hassle and inconvenient conventional BGM. A population‐based study even lately indicated that up to 1‐third of individuals with type 1 diabetes do perform no BGM at all.17 Our study conducted in real‐life conditions therefore suggests that the access to the FGM technology tends to improve the willingness of individuals with poor glycaemic control to better take care of the disease.
Regarding the subgroup of well‐controlled participants with baseline HbA1c levels <7.5%, our results are partly in accordance with those reported by Bolinder et al.12 In this randomized controlled study, therefore different from the observational design of our study, there was a 38% reduction in the time spent in hypoglycaemia at night and a 50% reduction in serious hypoglycaemia in the intervention group. HbA1c levels were unchanged after 6 months. In contrast with our study, the intervention group in the Bolinder study was exclusively composed of highly motivated and well‐empowered individuals who were deeply involved in daily diabetes self‐management. Noteworthy, although participants in the intervention group had direct access to the glucose data, and again in contrast with our study, no specific instruction was given for them to interpret these data. In our study, the education strategy was different as participants, as soon as 2 weeks after having received the first sensor, were strongly encouraged of using glucose data displayed by the reader to modify insulin dosages according to treatment algorithms provided by HCPs. Compared to less‐controlled participants, the glycaemic control did not further improve among those well‐controlled at baseline. HbA1c levels even moderately increased by the end of the observation period, but at a level (7.14%) that can be still considered as acceptable regarding the international recommendations. In the meantime, there was a positive trend in the number of mild hypoglycaemia which can be explained by the fact that although the amount of long‐acting insulin was reduced, the amount of rapid‐acting insulin increased overtime when measured in the cohort taken as a whole. After questioning participants, it appeared that, because of their increased confidence in the FGM technology, they were inclined of taking more audacious therapeutic decisions to challenge for instance postprandial glucose excursions. There was in general less fear of hypoglycaemic events because of the participant's ability to quickly recognize and correct them and to better anticipate events thanks to the glucose trends arrows that were considered, beside displayed actual glucose levels, as the most helpful tool provided by FGM. This advantage largely overcame the absence of real‐time alerts for high or low glucose levels conventional CGM systems usually offer.
Same observations were made among less‐controlled individuals who, because of higher HbA1c levels at baseline, saw their glycaemic control significantly and rapidly improved. This shift in using more prandial insulin occurred despite no relevant changes in insulin TDD of likely because day‐to‐day changes in insulin profiles were made gradually. It is worth noting that the increased rate of hypoglycaemic events (at least in the subgroup of less‐controlled individuals) was transient and reached a maximum at 6 months to decrease significantly at 12 months. Again, after questioning participants, it clearly came out that, because of the increased level of acceptance of the new glucose monitoring system in deep contrast with conventional BGM, less‐controlled participants were also more actively committed in controlling their disease, while fearing less hypoglycaemia. The participant “enthusiasm” was sometimes cooled down by HCPs who endlessly insisted on the importance of hypoglycaemia avoidance, again emphasizing the relevance of an ongoing personalized education oriented towards the management of potential drawbacks.
The improvement in HbA1c values observed in our study is also in agreement with recent papers that reported improved glycaemic control in people with type 1 and type 2 diabetes who are using CGM systems on a regular basis.18, 19, 20
There was a clear and significant correlation between the number of scans per day and improved HbA1c levels, again in accordance with previous studies, for instance the real‐world data which showed a significant decrease in HbA1c levels among individuals who checked daily glucose levels at the highest rate.11 The performance of the FGM system therefore depends on the frequency of sensor utilization. Any advantage taken from the system is rapidly lost as soon as the rate of scans per day decreases. It is therefore of crucial importance to keep constant the motivation of people with diabetes for them keeping the scanning rate at the highest level. The intervention of the medical/paramedical team as support of ongoing education is therefore mandatory. Beyond the psychological support, it helps people with diabetes to keep taking overtime the best therapeutic decisions in terms of insulin self‐adjustment on a daily basis.
In summary, our data indicate that the FGM system used in day‐to‐day clinical practice is a well‐tolerated technology that is readily adopted by the vast majority of people with type 1 diabetes. Beyond the unquestionable comfort this new technology offers, it improves per se the glycaemic control in people with type 1 diabetes followed in real‐life conditions. This new technology provides real‐time reliable glucose information and glucose trends that are proactively used by people with type 1 diabetes for fine‐tuning therapeutic decision and adaptation of insulin dosages. This beneficial effect is obtained in a context of structured therapeutic education and is correlated with the number of scans per day. As long as the motivation is kept intact, the FGM technology contributes to empower people with type 1 diabetes. It reinforces their confidence in the ability to better improve the glycaemic profile, while foreseeing possible hypoglycaemic events that can be then anticipated or at least better controlled.
CONFLICT OF INTERESTS
This study was not supported by external financial support. IMC is a member of the Abbott Diabetes Care European advisory board and received honoraria for consulting or lecture fees from Abbott Diabetes Care.
AUTHOR CONTRIBUTION
IP conceived and designed the study, participated to data collection, results interpretation and critical revision of the manuscript. FP and CH participated to data collection. ACG participated to data collection, performed the data analysis and figures design, contributed to the manuscript. IMC participated to data collection and results interpretation, and wrote manuscript. All authors approved the final version to be published.
Supporting information
ACKNOWLEDGEMENTS
Authors want to thank the individuals attending the outpatient clinic who gave their consent to collect the data presented in this study. They would also like to thank Mr. Jean‐François Fils for having thoroughly reviewed the statistical analysis of the study.
Paris I, Henry C, Pirard F, Gérard A‐C, Colin IM. The new FreeStyle libre flash glucose monitoring system improves the glycaemic control in a cohort of people with type 1 diabetes followed in real‐life conditions over a period of one year. Endocrinol Diab Metab. 2018;1:e23 10.1002/edm2.23
REFERENCES
- 1. McGill JB, Ahmann A. Continuous glucose monitoring with multiple daily insulin treatment: outcome studies. Diabetes Technol Ther. 2017;19:S3‐S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rodbard D. Continuous glucose monitoring: a review of recent studies demonstrating improved glycemic outcomes. Diabetes Technol Ther. 2017;19:S25‐S37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kovatchev BP, Patek SD, Ortiz EA, Breton MD. Assessing sensor accuracy for non‐adjunct use of continuous glucose monitoring. Diabetes Technol Ther. 2015;17:177‐186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Garg SK, Akturk HK. Flash glucose monitoring: the future is here. Diabetes Technol Ther. 2017;19:S1‐S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bailey T, Bode BW, Christiansen MP, Klaff LJ, Alva S. The performance and usability of a factory‐calibrated flash glucose monitoring system. Diabetes Technol Ther. 2015;17:787‐794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Distiller LA, Cranston I, Mazze R. First clinical experience with retrospective Flash Glucose Monitoring (FGM) analysis in South Africa: characterizing glycemic control with ambulatory glucose profile. J Diabetes Sci Technol. 2016;10:1294‐1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Olafsdottir AF, Attvall S, Sandgren U, et al. A clinical trial of the accuracy and treatment experience of the flash glucose monitor freestyle libre in adults with Type 1 diabetes. Diabetes Technol Ther. 2017;19:164‐172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bergenstal RM, Ahmann AJ, Bailey T, et al. Recommendations for standardizing glucose reporting and analysis to optimize clinical decision making in diabetes: the ambulatory glucose profile. J Diabetes Sci Technol. 2013;7:562‐578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mazze RS, Lucido D, Langer O, Hartmann K, Rodbard D. Ambulatory glucose profile: representation of verified self‐monitored blood glucose data. Diabetes Care. 1987;10:111‐117. [DOI] [PubMed] [Google Scholar]
- 10. Mazze RS, Strock E, Wesley D, et al. Characterizing glucose exposure for individuals with normal glucose tolerance using continuous glucose monitoring and ambulatory glucose profile analysis. Diabetes Technol Ther. 2008;10:149‐159. [DOI] [PubMed] [Google Scholar]
- 11. Dunn TC, Xu Y, Hayter G, Ajjan RA. Real‐world flash glucose monitoring patterns and associations between self‐monitoring frequency and glycaemic measures: a European analysis of over 60 million glucose tests. Diabetes Res Clin Pract. 2017;137:37‐46. [DOI] [PubMed] [Google Scholar]
- 12. Bolinder J, Antuna R, Geelhoed‐Duijvestijn P, Kroger J, Weitgasser R. Novel glucose‐sensing technology and hypoglycaemia in type 1 diabetes: a multicentre, non‐masked, randomised controlled trial. Lancet. 2016;388:2254‐2263. [DOI] [PubMed] [Google Scholar]
- 13. Haak T, Hanaire H, Ajjan R, Hermanns N, Riveline JP, Rayman G. Flash glucose‐sensing technology as a replacement for blood glucose monitoring for the management of insulin‐treated type 2 diabetes: a multicenter. Open‐Label Randomized Controlled Trial. Diabetes Ther. 2017;8:55‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Haak T, Hanaire H, Ajjan R, Hermanns N, Riveline JP, Rayman G. Use of flash glucose‐sensing technology for 12 months as a replacement for blood glucose monitoring in insulin‐treated type 2 diabetes. Diabetes Ther. 2017;8:573‐586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Verbeke G, Molenberghs G. Linear Mixed Models for Longitudnal Data. Springers series in statistics. Berlin, Germany: Springer‐Verlag; 2000. Ref Type: Serial (Book, Monograph) [Google Scholar]
- 16. Molenberghs G, Kenward MG. Missing Data in Clinical Studies. Chichester, UK: G. John Wiley and Sons Lds.; 2007. Ref Type: Edited Book. [Google Scholar]
- 17. Cameron D, Harris FM, Evans JM. Patterns of self‐monitoring of blood glucose in insulin‐treated diabetes: analysis of a Scottish population over time. Diabetes Obes Metab. 2016;18:729‐731. [DOI] [PubMed] [Google Scholar]
- 18. Beck RW, Riddlesworth TD, Ruedy K, et al. Continuous glucose monitoring versus usual care in patients with type 2 diabetes receiving multiple daily insulin injections: a randomized trial. Ann Intern Med. 2017;167:365‐374. [DOI] [PubMed] [Google Scholar]
- 19. Beck RW, Riddlesworth T, Ruedy K, et al. Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: the DIAMOND Randomized Clinical Trial. JAMA. 2017;317:371‐378. [DOI] [PubMed] [Google Scholar]
- 20. Lind M, Polonsky W, Hirsch IB, et al. Continuous glucose monitoring vs conventional therapy for glycemic control in adults with Type 1 diabetes treated with multiple daily insulin injections: the gold randomized clinical trial. JAMA. 2017;317:379‐387. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


