Summary
Objective
Anti‐Müllerian Hormone (AMH) concentration is high at birth in males, demonstrating the presence of functional testicular tissue in the prepubertal period, and acting as a useful marker in the investigation of paediatric reproductive disorders. AMH also provides a tool in the investigation of female virilization, premature ovarian failure and polycystic ovarian syndrome in childhood. Robust, assay‐specific paediatric AMH reference intervals are therefore required for clinical interpretation of results. The aim of this study was to derive age‐specific AMH reference intervals for males and females aged 0‐18 years.
Design and Patients
Plasma samples were obtained from patients at Royal Manchester Children's Hospital and analysed for AMH using the automated Beckman Coulter Access AMH Assay. Patients under investigation for paediatric reproductive or endocrine disorders were excluded from the study.
Measurements
Seven hundred and 2 patient plasma samples (465 male, 237 female) were subject to AMH measurement, and results were analysed in order to derive continuous and discrete reference intervals for the paediatric age range.
Results
Clear discrimination between male and female AMH results was evident in the prepubertal age range, with some overlap between the genders following pubertal onset.
Conclusions
We have derived age‐related reference intervals for plasma AMH in the paediatric age range (0‐18 years) using the automated Beckman Coulter Access AMH assay which will aid in the investigation of paediatric endocrine disorders such as disorders of sexual development.
Keywords: Anti‐Müllerian Hormone, disorders of sex development, immunoassay, ovarian reserve, paediatrics, reference values, testicular hormones
1. INTRODUCTION
Anti‐Müllerian Hormone (AMH), a dimeric glycoprotein, is a member of the transforming growth factor‐β (TGF‐β) family of cytokines which plays an essential role in the normal differentiation of reproductive structures. In males, AMH is produced by Sertoli cells from the 7th week of gestation and plays an important role in the foetus triggering the involution of Müllerian ducts, allowing for testicular development.1 In females, by contrast, AMH is produced by ovarian granulosa cells of antral and pre‐antral follicles from the 36th week of gestation and plays a role in control of ovarian follicle growth.2
Male AMH is high at birth persisting at an elevated level postnatally until puberty. Although onset and continued basal expression of foetal male AMH is gonadotropin independent, Follicle Stimulating Hormone (FSH) stimulates testicular Sertoli cell proliferation up regulating AMH transcription.3 However, with the onset of puberty, the increased intratesticular production of androgens, along with expression of the Sertoli cell androgen receptor, overcomes the stimulatory effect of FSH on AMH production, leading to the downregulation of AMH.4 In females, AMH levels rise through infancy and increase at puberty, before remaining relatively stable until the third decade of life.5, 6
The differing expression of AMH between male and females during childhood makes AMH a valuable marker of gonadal function in paediatric reproductive disorders. One of the main clinical uses of AMH in paediatrics is as a marker for the presence of testicular tissue. When AMH has been used to investigate cases of bilateral anorchia and complete gonadal dysgenesis, an undetectable AMH result has provided sufficient diagnostic certainty to avoid the need for invasive surgical exploration.3 The primary method of gonadal evaluation in prepubertal children has been to determine the response of serum testosterone to the administration of hCG (human chorionic gonadotropin), although a failure of testosterone to increase does not necessarily imply a lack of testicular tissue as the same reduced response would be evident in the presence of a testosterone biosynthetic defect.7 AMH will only be produced if testicular Sertoli cells are present and can therefore aid in the diagnostic pathway for disorders of sexual development (DSD).
In cases of hypogonadotropic hypogonadism, both prepubertal and pubertal males are likely to have low AMH levels due to decreased FSH stimulation, although AMH increases in response to FSH treatment.8 In patients with untreated hypogonadotropic hypogonadism, AMH concentration will be elevated for age postpuberty as there will be insufficient testosterone production for downregulation. AMH will, however, be lower than expected for the patient's Tanner stage, reflecting the lack of FSH stimulus.8 Patients with primary hypogonadism such as Klinefelter's syndrome (47XXY) have a normal AMH during infancy but this decreases with age, as Sertoli cell function reduces from mid‐puberty, resulting in high FSH and small testis volume. Patients with constitutional delay of puberty, or with defective androgen production or sensitivity, maintain high prepubertal AMH levels.3
In prepubertal females, an AMH plasma level can differentiate between female virilization caused by the presence of testicular tissue or a granulosa cell tumour, and virilization caused by increased adrenal androgen expression. AMH plasma concentration has also been shown to correlate with, and therefore provide a marker of, ovarian reserve.9 Although low at birth in females, AMH increases slightly through childhood and decreases to undetectable levels shortly before clinical menopause. Whilst blood gonadotropin levels vary across the menstrual cycle, AMH has the advantage of a relatively stable blood concentration, therefore providing an attractive marker of premature ovarian failure,10 with the potential to predict onset in Turner's syndrome patients.11 AMH can provide a particularly useful marker of ovarian reserve in the prepubertal population, where FSH is not a reliable measurement; and where assessment may be required for patients who may be about to undergo cranial irradiation or treatment with gonadotoxic agents as part of cancer therapy enabling decisions around egg harvesting.10, 12, 13 Furthermore, AMH has also shown potential as a marker for polycystic ovarian syndrome (PCOS) in adolescents, showing a raised concentration compared to controls14, 15 which correlates with ovarian volume16 and androgen level.17
Commercial assays for the measurement of circulating anti‐Müllerian hormone (AMH) have been available for many years. The 2 most commonly used first‐generation enzyme‐linked immunosorbent assays (ELISAs) for AMH were the Diagnostic Systems Laboratories (DSL) AMH ELISA and the Immunotech (IOT) AMH ELISA, with the IOT kit consistently reporting statistically significant higher values than the DSL kit.18 In 2010, Beckman Coulter combined these technologies to produce a second‐generation (Gen II) AMH ELISA.19 This assay was subsequently modified to minimize interference from complement20, 21, 22 and has recently been adapted for the Beckman Access automated platform.
Whilst AMH measurement may be useful in the initial evaluation of an infant with a suspected DSD, age‐specific reference intervals are required order to interpret results.7 Previous studies have investigated AMH concentration in the male and female paediatric population from 0 to 18 years.7, 23, 24 However, these have all been carried out using the now redundant first‐generation AMH protocols, bringing the results into question due to the reported issues with standardization of these assays.25 Further studies using the second generation of AMH assays have considered only the male4 or female5, 6, 26 population; therefore, limiting the use of the reference intervals in diagnosis of DSD as there is limited information concerning the discrimination between the different genders.
The aim of our study was to improve the clinical utility of plasma AMH measurement by determining robust reference intervals for male and females across the paediatric age range using the Beckman Coulter Access AMH assay.
2. MATERIALS AND METHODS
2.1. Sample collection, storage and analysis
Unselected sequential plasma samples were collected over a 6‐month period from surplus routine samples sent to the laboratory at Royal Manchester Children's Hospital, Manchester University NHS Foundation Trust, Manchester, UK. Surplus specimens of acellular plasma marked for disposal were used following completion of all diagnostic investigations. Specimens were fully anonymized and were used solely for the purpose of improving care for patients. It is understood that studies for patient benefit such as the present study can be conducted without explicit consent (further information is available at https://www.hta.gov.uk/). Specimens were collected in blood tubes preloaded with Lithium Heparin and centrifuged within 30 minutes of receipt in the laboratory to separate plasma from cellular components. After analysis, samples were stored at +4°C for no more than 12 hours before the plasma supernatant was removed from cells into a secondary container in order to maintain AMH stability27 and stored frozen at −80°C until analysis.
Specimens were collected from 469 phenotypically male and 235 phenotypically female patients aged between 1 day and 18 years. All samples were allocated a study number, labelled with the sex and age in days, and fully anonymized from any other patient data. Patients undergoing investigation for any suspected disorder of sexual development or other endocrinopathy were excluded from the study.
Samples were analysed for AMH using the Access AMH assay (Beckman Coulter, High Wycombe, U.K.) according to manufacturer's instructions using a volume of 180 μL of plasma for females and 20 μL of plasma for males, made up to 200 μL using manufacturer's diluent. The assay showed intermediate precision marked by a CV of <5% across 3 different levels of quality control material (7, 34 and 103 pmol/L). The manufacturer's data on limit of quantification for this assay are 0.036 pmol/L, with a linear range up to 181 pmol/L. Due to anticipated high AMH concentrations, all male samples were analysed following an offline 1:10 dilution using the manufacturer's diluent.
Samples were analysed for testosterone using a liquid chromatography high‐resolution accurate mass‐mass spectrometry (LC‐HRAM/MS) assay. Extraction of testosterone from plasma samples was performed using HLB Prime 96‐well elution plates (Waters, Manchester, UK), and compounds were separated using an Aquity HSS T3 column, 2.1 mm × 5.0 mm, 1.8 μm (Waters) using a method developed for the Exactive Plus with an HESI source (ThermoScientific, Waltham, USA).
2.2. Results analysis
Results obtained were analysed in several ways, following division into 11 different categories according to age. Due to the relatively small numbers in some of these age groupings, data were firstly analysed using smoothed splines with quantile regression with models built using the R package quantreg,28 with taus of 0.05, 0.5 and 0.95. Nonlinear cubic splines were calculated with the degrees of freedom for spline fitting defined by minimizing the Akaike's Information Criterion (AIC) for the 3 quantiles under consideration; the value of 7 was used for the male model, and 5 for the female model. The AMH value predicted by the model, at the 3 levels, was calculated for every day between the ages of 0 and 18.
In order to generate discrete reference intervals, results were further analysed using robust statistics in order to limit the influence of outliers. Due to the limited number of samples in the neonatal female age groups, results between groups were compared using Tukey's studentized range (Honest Significant Difference, HSD) test in ANOVA analysis, in order to determine if there were significant differences, and then combined. The 95% quantiles were generated using a robust fit to a gamma distribution using the Robust R package 0.4.16. About 95% confidence intervals for the robust procedure were derived using a bootstrap sampling method with 1000 iterations29 using the Bootstrap R (S‐Plus) function version 1.3‐19.
3. RESULTS
The Beckman Coulter Access AMH assay was used to measure AMH in 704 plasma samples from children aged between 1 day and 18 years. Whilst female samples were analysed neat, male samples were subject to a 1 in 10 dilution prior to analysis in order to ensure results fell within the linear range of the assay. The requirement for a larger volume for female samples than the diluted male samples resulted in a smaller cohort of females (235) to males (469).
Statistically significant differences were observed between AMH concentration of male and female plasma samples across the age range (Figure 1). AMH showed a wide spread for the male cohort at birth, with a range from 76 pmol/L to 1156 pmol/L in the neonatal period. In males, AMH was high at birth and remained elevated throughout infancy finally decreasing around 12 years of age (Figure 1). Conversely, AMH was relatively low in females following birth (with a range from 0.07 pmol/L to 7.94 pmol/L) and gradually increased during infancy and childhood (Figure 1). Clear discrimination was evident between AMH concentrations of both genders during infancy, with results converging around 13 years of age, although AMH concentration in males continued to be higher (Figure 1).
Figure 1.
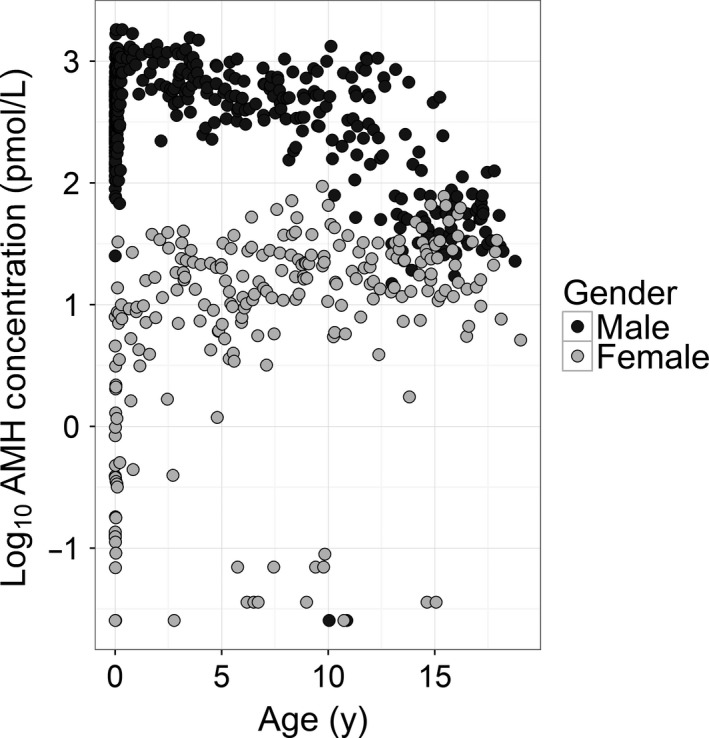
Concentration of AMH by gender and age. AMH concentration was measured in plasma samples from 704 children (235 female, 469 male) using the Beckman Access analyser and assay. Results were then segregated according to age and gender. AMH concentration was considered on a log scale to compare the results obtained from both genders simultaneously
Stratification of samples into different age categories allowed variation in AMH concentration through the paediatric age range to be considered further (Figure 2). In females, AMH was found to be low or undetectable in all samples in groups 1 to 5 representing the neonatal period (Figure 2). AMH rose gradually during infancy, reaching a plateau during, and following, the pubertal years in groups 9‐11. Male samples showed much greater variability of AMH concentration in each of the different age categories (Figure 2). In the neonatal period (age groups 1 ‐5), median AMH was found to be much higher in the male cohort (436 pmol/L) than in the equivalent female age range (1.24 pmol/L), reflecting the presence of functioning Sertoli tissue in males. Despite the wide range of results which were observed for males (76 to 1156 pmol/L), there was clear discrimination between the 2 genders with the highest observed female result (7.94 pmol/L) much lower than the lowest male value. Although AMH was high at birth in the male cohort, concentration continued to rise during the neonatal period peaking in the first year of life (Figure 2, age group 6) and showing a decline during the pubertal years in age groups 9‐11 (Figure 2).
Figure 2.
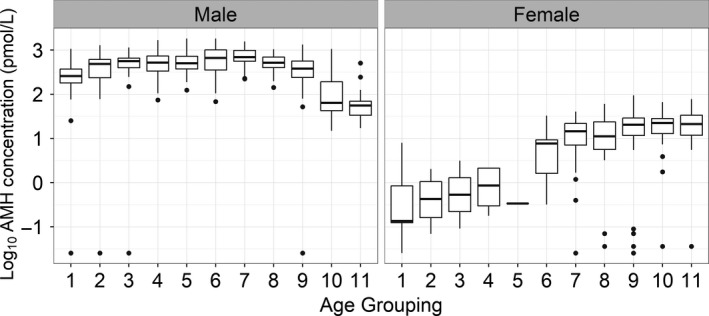
Box plot of AMH results for each of 11 age categories. The median of the group is indicated by the horizontal bar within the box which represents the interquartile range of the data. The vertical line represents the maximum and minimum of the data range. Outliers are plotted outside of these lines. AMH concentration is plotted on a log scale to allow easy comparison between genders. The sample size for each group is indicated in Table 1
We hypothesized that differing concentrations of testosterone due to entering puberty at different ages may account for some of the observed variation in the AMH concentration of different age groups. As such, all male samples were analysed for testosterone using LC‐HRAM/MS and the correlation of testosterone and AMH concentration was considered (Figure 3). In general, high levels of testosterone, found predominantly in age groups 10 and 11 (12‐18.9 yrs), were associated with low concentrations of AMH. Conversely high concentrations of AMH were associated with low concentrations of testosterone during infancy in age groups 7 to 9 (1 to 11.9 yrs). AMH and testosterone were both raised in a number of neonatal samples in age groups 1 to 6, a likely reflection of the lack of expression of the androgen receptor in Sertoli cells in the neonatal age range.
Figure 3.
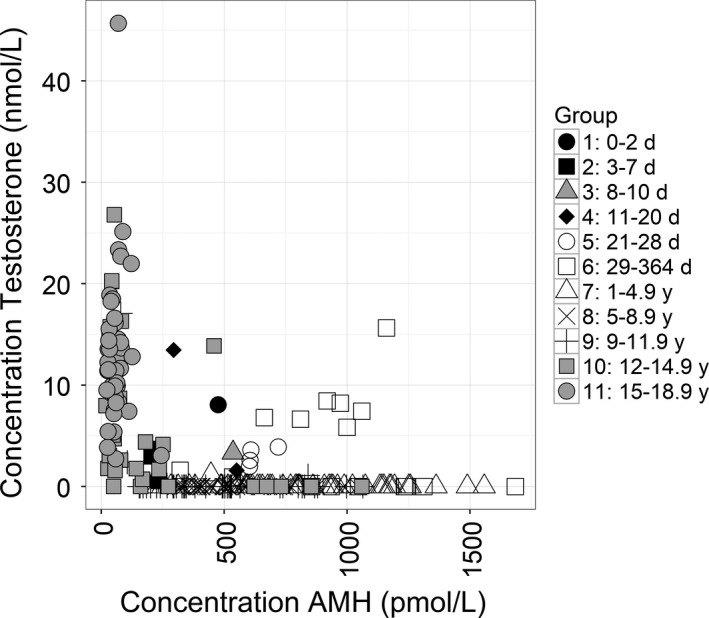
Correlation of Testosterone and AMH concentration in males. Testosterone and AMH concentration were measured in all male samples and the results segregated according to age range as indicated. AMH concentration was measured using the Beckman Coulter Access AMH assay with Testosterone measured on the same sample using LC‐HRAM/MS analysis. The sample size for each group is indicated in Table 1
In order to account for the spread of data across the age ranges and to consider the data as a continuous data set, 2 different quantile regression models were built, 1 for each gender, using the R package Quantreg28 (Figure 4). To make the best use of nonlinear relationships, nonlinear cubic splines were calculated. As expected the male trend demonstrated a high concentration of AMH at birth, increasing to a peak within the first year of life, before decreasing and plateauing during infancy, and decreasing further at puberty (Figure 4). AMH in females started low at birth, increasing to a peak in infancy, with a small decrease before again increasing as participants entered puberty shortly before 10 years of age.
Figure 4.
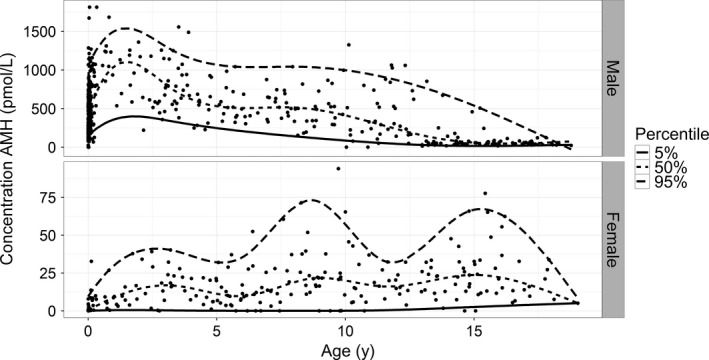
Quantile regression with smoothed splines. AMH results from both male (469 samples) and females (235 samples) were analysed using quantile regression with smoothing splines using the R package Quantreg with taus of 0.05, 0.5 and 0.95. The sample size for each group is indicated in Table 1
Whilst quantile regression analysis allowed the consideration of the data set as continuous, allowing daily variation in age to alter the expected AMH concentration, AMH results were further analysed to produce discrete reference intervals which are more practically useful. Due to the limited sample number, some of the age groups were combined for the female cohort, after analysis of the results using Tukey's HSD test in order to define whether there was any significant difference between age groups. Where they were deemed to be comparable, the age groups in the female neonatal period were combined together (Table 1). In order to limit the influence of outliers, the robust method of statistical analysis was then used to calculate 95% reference interval with 95% confidence interval (Table 1). The results clearly show discrimination between the 2 genders across the paediatric age range. The median AMH value increased with age for the male cohort up to 9 years old before decreasing through puberty. The female cohort showed an increasing median AMH with age apart from a minor decrease in median concentration for the 5‐7.9 year age category which is of uncertain significance, especially as the 2.5th and 97.5th centiles fit with the trend towards an increase in concentration.
Table 1.
Male and Female paediatric AMH reference ranges for different age ranges. The 95% confidence interval at 2.5th and 97.5th centiles were generated using a robust fit to a gamma distribution. In the female cohort, all samples from the neonatal period were grouped together due to the limited number of participants
| Age group | N | Female AMH (pmol/L) | ||
|---|---|---|---|---|
| 2.5th percentile (90% CI) | Median | 97.5th percentile (90% CI) | ||
| Female | ||||
| 0‐28 d | 24 | 0.002 (0‐0.0037) | 0.37 | 4.08 (0‐7.57) |
| 29‐364 d | 17 | 0.05 (0‐0.10) | 7.36 | 38.58 (19.97‐64.21) |
| 1‐4.9 y | 42 | 1.28 (0‐2.17) | 14.58 | 50.77 (38.91‐64.13) |
| 5‐7.9 y | 42 | 0.80 (0‐1.45) | 11.44 | 51.35 (31.59‐68.74) |
| 8‐11.9 y | 47 | 2.29 (0‐4.11) | 20.42 | 68.16 (41.20‐90.45) |
| 12‐14.9 y | 33 | 3.18 (0‐5.82) | 17.87 | 55.38 (34.18‐69.25) |
| 15‐18.9 y | 30 | 2.44 (0‐3.76) | 21.14 | 74.21 (44.72‐102.90) |
| Age group | N | Male AMH (pmol/L) | ||
|---|---|---|---|---|
| 2.5th percentile (90% CI) | Median | 97.5th percentile (90% CI) | ||
| Male | ||||
| 0‐2 d | 51 | 72.73 (39.9‐96.67) | 258.1 | 628.54 (485.27‐752.02) |
| 3‐7 d | 45 | 119.30 (14.43‐138.18) | 486.1 | 1112.11 (1061.98‐1402.15) |
| 8‐10 d | 14 | 193.19 (0‐309.23) | 563.4 | 1074.24 (723.40‐1551.83) |
| 11‐20 d | 37 | 211.15 (166.49‐300.56) | 522.3 | 987.52 (618.99‐1085.19) |
| 21‐28 d | 26 | 201.06 (109.81‐285.01) | 504.7 | 1055.39 (757.45‐1236.30) |
| 29‐364 d | 66 | 287.97 (245.28‐400.50) | 662.9 | 1242.42 (775.52‐1462.13) |
| 1‐4.9 y | 58 | 282.08 (181.84‐337.86) | 690.91 | 1525.92 (1379.12‐1810.30) |
| 5‐8.9 y | 39 | 221.18 (150.05‐257.38) | 517.29 | 1062.66 (879.79‐1268.55) |
| 9‐11.9 y | 49 | 84.25 (0‐87.47) | 380.89 | 1109.54 (1050.65‐1475.83) |
| 12‐14.9 y | 36 | 3.57 (0‐5.17) | 64.66 | 444.49 (343.15‐704.72) |
| 15‐18.9 y | 48 | 16.13 (8.25‐20.12) | 55.61 | 120.35 (86.72‐142.49) |
4. DISCUSSION
Reference intervals are 1 of the most powerful tools provided by a clinical laboratory allowing for easy and correct interpretation of patient results by clinicians. Recent guidelines advocate the use of AMH in the paediatric endocrinology clinic, but state that up to date age‐, sex‐ and method‐related reference intervals are necessary for interpretation.3 During this study, we have derived reference intervals for AMH across the paediatric age range which should enable increased use of this biomarker within this population.
Results from our cohort agree with previous studies showing that AMH is high at birth in males, reflecting functioning Sertoli cells, and remains relatively high for the whole prepubertal period.8 AMH downregulation at puberty was found to be accompanied by an increase in testosterone, due to the increase in Leydig cell function. Apart from during the neonatal period, when the androgen receptor is not expressed in Sertoli cells, AMH concentration was found to be low or undetectable in samples with a high testosterone, whilst AMH was high in prepubertal samples with a low testosterone concentration. AMH concentration was found to be much lower in the female cohort at birth, rising during infancy and reaching a plateau in puberty in agreement with published data.6 In the neonatal and infancy period, there was clear discrimination between AMH concentration for each gender; therefore, demonstrating that AMH is a powerful tool for the assessment of Sertoli cell activity, and the presence of functioning testicular tissue, in the paediatric population.
The higher concentration of AMH in the male cohort resulted in all these samples being subject to a 1 in 10 dilution prior to analysis. The larger volume of sample required by the female cohort reduced the number of participants. The reference interval was therefore based on a larger cohort of males (469) than females (235). The use of anonymized surplus samples from a clinical laboratory limits our knowledge of the patient population, although the samples are likely to reflect the multi‐ethnic population which our laboratory serves as a tertiary referral centre. Patients under investigation for any endocrinopathy were excluded from the study prior to sample collection and anonymization; however, it was assumed that the included samples were from healthy participants. Tanner stage information allowing for specific partitioning of patients according to pubertal status was also unavailable, although testosterone acted as a surrogate marker in the male cohort.
Anti‐Müllerian Hormone concentration in this cohort was measured using the automated Beckman Coulter Access AMH assay. This has been shown to have very good correlation with AMH measured using the modified Gen II ELISA.30 Indeed, it has been proposed that correlation is good enough for adult reference intervals to be interchangeable between assays,30 although not all authors have reported a direct equivalence in values.31
Anti‐Müllerian Hormone reference interval studies have been previously described for the male and females covering the paediatric age range;7, 23, 24 however, these have been based upon AMH assays which are now redundant. Further studies have focused on only 1 gender4, 5, 6, 26 therefore limiting the clinical utility of the reference intervals. A recent study has followed a cohort of both genders over a 10‐year period from 5 to 14 years,32 with serial AMH measurements; however, this again has been unable to provide working reference intervals due to the lack of samples pre‐5 years of age reflecting the age range when the vast majority of queries about ambiguous genitalia and DSD are likely.
The measured AMH concentrations across the paediatric age range were used to consider a reference interval in 2 ways; firstly a continuous model of AMH concentration across the whole age range, and secondly discrete ranges defined by distinct age ranges. Continuous age‐dependent reference intervals are more reliable than discrete ranges as they do not make any assumptions about the patient but allow for consideration of their exact age and as such better reflect biological normality.33 The data in this study were therefore subject to smoothing, as a method of reducing interference from outliers, and a continuous data range was generated which can be used following the chart. The 5th, 50th and 95th centiles were determined for each data set (Figure 4). The 95th centile for the female cohort was influenced by outliers due to the smaller sample size available (Figure 4), accentuating the shape of the curve. However, the procedure used optimized the number of spine inflexions for all 3 curves and had minimal effect on the median curve.
Although potentially optimal in terms of clinical utility, a continuous data range is less practical than discrete age ranges as it is incompatible with laboratory information systems. The results were therefore also analysed to provide discrete reference intervals for different age groups. Current guidelines define a reference interval as 2 limiting values which 95% of the population fall within34 ideally determined using 120 samples in order to have statistical significance.35 However, CLSI guidelines recognize that this number of samples may be difficult to achieve in the paediatric population where age‐ and sex‐specific ranges are the most clinically useful reference intervals.36 Indeed, these guidelines go onto suggest that the robust method of statistical analysis can allow for the generation of reference intervals from a smaller number of samples.36 As such, although we were unable to stratify samples into age groups with 120 participants, reference intervals were determined using the robust method of analysis. The male cohort was divided into 5 age groups prior to 28 days of age in order to highlight the significant change in AMH level during this period (Table 1). The median AMH values for the 8‐10 and 21‐28 day age groups are slightly out of sequence with the other age groups; however, the reference interval for these age categories is in keeping with the other data points. A larger sample size would have been preferred and likely have reduced the confidence interval for each grouping.
The reference intervals determined by this study show there is no overlap between the male and female cohort in the prepubertal age range. This pattern is in agreement with previously published studies which have used redundant AMH assays.24 An AMH concentration falling within the male paediatric reference interval is therefore highly indicative of the presence of functioning testicular tissue.1 A female presenting with an AMH within the male reference interval should be examined both for the presence of testes or the presence of a granulosa cell tumor.3 The reference, and continuous age dependent, intervals determined during this study will allow for effective use of AMH as a marker of functioning testicular tissue and ovarian reserve in the paediatric population.
CONFLICT OF INTEREST
The work was supported by Beckman Coulter through provision of reagent kits and some technical and statistical assistance free of charge and Waters through provision of LC‐MS extraction plates. Beyond this, neither company had any part in the preparation of this manuscript. The authors have no conflicts of interest.
AUTHOR CONTRIBUTION
All authors contributed to study design. HJ and AY carried out sample collection and analysis and prepared the manuscript, NB provided statistical analysis, LT, CC and KH were responsible for the conception of the study.
ACKNOWLEDGMENTS
We thank Francis Fung for assistance at the inception of this study and commencement of sample collection. We are also grateful to Edward Hinchliffe for assistance with testosterone measurement.
Jopling H, Yates A, Burgoyne N, Hayden K, Chaloner C, Tetlow L. Paediatric Anti‐Müllerian Hormone measurement: Male and female reference intervals established using the automated Beckman Coulter Access AMH assay. Endocrinol Diab Metab. 2018;1:e21 10.1002/edm2.21
REFERENCES
- 1. Lindhardt JM, Hagen CP, Johannsen TH, et al. Anti‐müllerian hormone and its clinical use in paediatrics with special emphasis on disorders of sex development. Int J Endocrinol. 2013;2013:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anckaert E, Öktem M, Thies A, et al. Mulitcenter analytical performance evaluation of a fully automated anti‐Müllerian hormone assay and reference interval determination. Clin Biochem. 2016;49:260‐267. [DOI] [PubMed] [Google Scholar]
- 3. Ahmed SF, Achermann JC, Arlt W, et al. Society for Endocrinology UK guidance on the initial evaluation of an infant or an adolescent with a suspected disorder of sex development (Revised 2015). Clin Endocrinol. 2016;84:771‐788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aksglaede L, Sørensen K, Boas M, et al. Changes in anti‐Müllerian hormone (AMH) throughout the life span: a population‐based study of 1027 healthy males from birth (cord blood) to the age of 69 years. J Clin Endocrinol Metab. 2010;95:5357‐5364. [DOI] [PubMed] [Google Scholar]
- 5. Hagen CP, Aksglaede L, Sørensen K, et al. Serum levels of anti‐Müllerian hormone as a marker of ovarian function in 926 healthy females from birth to adulthood and in 172 Turner syndrome patients. J Clin Endocrinol Metab. 2010;95:5003‐5010. [DOI] [PubMed] [Google Scholar]
- 6. Lie Fong S, Visser JA, Welt CK, et al. Serum anti‐müllerian hormone levels in healthy females: a nomogram ranging from infancy to adulthood. J Clin Endocrinol Metab. 2012;97:4650‐4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ahmed SF, Keir L, McNeilly J, Galloway P, O'Toole S, Wallace AM. The concordance between serum anti‐Müllerian hormone and testosterone concentrations depends on durationof hCG stimulation in boys undergoing investigation of gonadal function. Clin Endocrinol (Oxf). 2010;72:814‐819. [DOI] [PubMed] [Google Scholar]
- 8. Grinspon RP, Rey RA. Anti‐müllerian hormone and sertoli cell function in paediatric male hypogonadism. Horm Res Paediatr. 2010;73:81‐92. [DOI] [PubMed] [Google Scholar]
- 9. Dewailly D, Andersen CY, Balen A, et al. The physiology and clinical utility of anti‐Mullerian hormone in women. Hum Reprod Update. 2014;20:370‐385. [DOI] [PubMed] [Google Scholar]
- 10. Lunsford AJ, Whelan K, McCormick K, McLaren JF. Anti‐Müllerian hormone as a measure of reproductive function in female childhood cancer survivors. Fertil Steril. 2014;101:227‐231. [DOI] [PubMed] [Google Scholar]
- 11. Lunding SA, Aksglaede L, Anderson RA, et al. AMH as predictor of premature ovarian insufficiency: a longitudinal study of 120 turner syndrome patients. J Clin Endocrinol Metab. 2015;100:E1030‐E1038. [DOI] [PubMed] [Google Scholar]
- 12. Miyoshi Y, Ohta H, Namba N, et al. Low serum concentrations of anti‐Müllerian hormone are common in 53 female childhood cancer survivors. Horm Res Paediatr. 2013;79:17‐21. [DOI] [PubMed] [Google Scholar]
- 13. Gupta AA, Chong AL, Deveault C, et al. Anti‐Mullerian Hormone (AMH) in female adolescent cancer patients prior to, during and after completion of therapy: a pilot feasibility study. J Pediatr Adolesc Gynecol. 2016;29:599‐603. [DOI] [PubMed] [Google Scholar]
- 14. Parco S, Novelli C, Vascotto F, Princi T. Serum anti‐Müllerian hormone as a predictive marker of polycystic ovarian syndrome. Int J Gen Med. 2011;4:759‐763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mumford SL, Legro RS, Diamond MP, et al. Baseline AMH level associated with ovulation following ovulation induction in women with Polycystic Ovary Syndrome. JCEM. 2016;101:3288‐3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pawelczak M, Kenigsberg L, Milla S, Liu YH, Shah B. Elevated serum anti‐Müllerian hormone in adolescents with polycystic ovary syndrome: relationship to ultrasound features. J Pediatr Endocrinol Metab. 2012;25:983‐989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sopher AB, Grigoriey G, Laura D, et al. Anti‐Müllerian hormone may be a useful adjunct in the diagnosis of polycystic ovary syndrome in nonobese adolescents. J Pediatr Endocrinol Metab. 2014;27:1175‐1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rustamov O, Smith A, Roberts SA, et al. The measurement of Anti‐Müllerian Hormone: a critical appraisal. J Clin Endocrinol Metab. 2014;99:723‐732. [DOI] [PubMed] [Google Scholar]
- 19. Kumar A, Kalra B, Patel A, McDavid L, Roudebush WE. Development of a second generation anti‐Müllerian hormone (AMH) ELISA. J Immunol Methods. 2010;362:51‐59. [DOI] [PubMed] [Google Scholar]
- 20. Rustamov O, Smith A, Roberts SA, et al. Anti‐Müllerian Hormone: poor assay reproducibility in a large cohort of subjects suggests sample instability. Hum Reprod. 2012;27:3085‐3091. [DOI] [PubMed] [Google Scholar]
- 21. Bonifacio M, Bradley CK, Karia S, Livingstone M, Bowman MC, McArthur SJ. The original Beckman Coulter Generation II assay significantly underestimates AMH levels compared with the revised protocol. J Assist Reprod Genet. 2015;32:1691‐1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Han X, McShane M, Sahertian R, Whiye C, Ledger W. Pre‐mixing serum samples with assay buffer is a prerequisite for reproducible anti‐ Müllerian hormone measurement using the BeckmanCoulter Gen II assay. Hum Reprod. 2014;29:1042‐1045. [DOI] [PubMed] [Google Scholar]
- 23. Lee MM, Donahoe PK, Hasegawa T, et al. Mullerian inhibiting substance in humans: normal levels from infancy to adulthood. J Clin Endocrinol Metab. 1996;81:571‐576. [DOI] [PubMed] [Google Scholar]
- 24. Guibourdenche J, Lucidarme N, Chevenne D, et al. Anti‐Müllerian hormone levels in serum from human foetuses and children: pattern and clinical interest. Mol Cell Endocrinol. 2003;211:55‐63. [DOI] [PubMed] [Google Scholar]
- 25. Craciunas L, Roberts SA, Yates AP, Smith A, Fitzgerald C, Pemberton PW. Modification of the Beckman‐Coulter second‐generation enzyme linked immunosorbent assay protocol improves the reliability of serum antimüllerian hormone measurement. Fertil Steril. 2015;103:554‐559. [DOI] [PubMed] [Google Scholar]
- 26. Kelsey TW, Wright P, Nelson SM, Anderson RA, Wallace WH. A validated model of serum anti‐müllerian hormone from conception to menopause. PLoS ONE. 2011;6:e22024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Demirdjian G, Bord S, Lejeune C, et al. Performance characteristics of the Access AMH assay for the quantitative determination of anti‐Mὒllerian hormone (AMH) levels on the Access* family of automated immunoassay systems. Clin Biochem. 2016;49:1267‐1273. [DOI] [PubMed] [Google Scholar]
- 28. Koenker R (2016). quantreg: Quantile Regression. R package version 5.29. https://cran.r-project.org/web/packages/quantreg/index.html Accessed October 16, 2017.
- 29. Davison AC, Hinkley DV. Bootstrap Methods and Their Applications. Cambridge: Cambridge University Press; 1997. [Google Scholar]
- 30. Pearson K, Long M, Prasad J, Wu YY, Bonifacio M. Assessment of the Access AMH assay as an automated, high‐performance replacement for the AMH Generation II manual ELISA. Reprod Biol Endocrinol. 2016;16:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nelson S, Pastuszek E, Kloss G, et al. Two new automated, compared with two enzyme‐linked immunosorbent, antimüllerian hormone assays. Fertil Steril. 2015;104:1016‐1021. [DOI] [PubMed] [Google Scholar]
- 32. Jeffrey A, Streeter AJ, Hosking J, Wilkin TJ, Nelson SM. Anti‐Müllerian hormone in children: a ten‐year prospective longitudinal study (EarlyBird 39). J Paediatr Endocrinol Metab. 2015;28:1153‐1162. [DOI] [PubMed] [Google Scholar]
- 33. Hübner U, Englisch C, Werkmann H, et al. Continuous age‐dependent reference ranges for thyroid hormones in neonates, infants, children and adolescents established using the ADVIA Centaur Analyzer. Clin Chem Lab Med. 2002;40:1040‐1047. [DOI] [PubMed] [Google Scholar]
- 34. Colantonio DA, Kyriakopoulou L, Khun Chan M, et al. Closing the gaps in paediatric laboratory reference intervals: a CALIPER database of 40 biochemical markers in a health and multi‐ethnic population of children. Clin Chem. 2012;58:854‐868. [DOI] [PubMed] [Google Scholar]
- 35. Horn PS, Pesce AJ. Reference intervals: an update. Clin Chim Acta. 2003;334:5‐23. [DOI] [PubMed] [Google Scholar]
- 36. Clinical and Laboratory Standards Institute (CLSI) . Defining, Establishing and Verifiying Reference Intervals in the Clinical Laboratory: Approved Guidelines, 3rd edn CLSI document EP28‐A3c. Wayne, PA: Clinical and laboratory Standards institute; 2008. [Google Scholar]


