Summary
Aims
TECOS was a randomized, double‐blind, placebo‐controlled trial assessing the impact of sitagliptin vs. placebo on cardiovascular outcomes when added to usual care in patients with type 2 diabetes. We report the use of concomitant diabetes medications and the risk for progression to insulin during follow‐up.
Materials and Methods
TECOS enrolled 14 671 participants with HbA1c 6.5%‐8.0% on monotherapy with metformin, pioglitazone, sulfonylurea (SU), or dual therapy with two oral agents or insulin with or without metformin. Subsequent diabetes management was by the participant's usual care physician. Time to initiation of insulin and risk of hypoglycaemia were estimated using Cox proportional hazards models.
Results
The most common glucose‐lowering regimens at baseline were metformin monotherapy (30.2%), SU monotherapy (8.5%), metformin/SU therapy (35.1%), and insulin with or without metformin (13.9% and 8.6%, respectively). Over a median 3.0 years’ follow‐up, diabetes therapy was intensified in 25.2% of participants (sitagliptin 22.0%, placebo 28.3%). Medications most commonly added were SU (8.3%) or insulin (8.8%). Insulin initiation in the usual care setting occurred at mean (standard deviation) HbA1c of 8.5 (1.5)%. Sitagliptin did not impact rates of severe hypoglycaemia, but delayed progression to insulin when added to metformin or metformin/SU regimens.
Conclusion
Consistent with the trial's pragmatic design, TECOS participants underwent typical progression of diabetes medications. Sitagliptin was associated with lower HbA1c, without increased risk for severe hypoglycaemia and was associated with delayed progression to insulin when added to metformin with or without SU.
Keywords: DPP‐4 inhibitor, hypoglycaemia, insulin, sitagliptin, type 2 diabetes
1. INTRODUCTION
Type 2 diabetes mellitus (T2DM) is a progressive disease usually requiring the addition of multiple antihyperglycaemic agents to achieve and maintain adequate glycaemic control over time. The typical pattern of diabetes treatment begins with lifestyle adjustment and monotherapy with metformin, progresses to dual therapy with oral agents and/or injectable treatments, and most patients with T2DM will eventually require therapy with insulin. In the United Kingdom Prospective Diabetes Study, 53% of newly diagnosed T2DM patients treated with sulfonylurea (SU) monotherapy required the addition of insulin over 6 years, albeit at a time when availability of non‐insulin treatments was limited.1 Similarly, among 368 382 patients with T2DM registered in the Swedish National Diabetes Registry in 2017, 57 805 patients (15.7%) used insulin in combination with other glucose‐lowering drugs, and 32 342 patients (8.8%) used insulin alone.2 A US‐based cross‐sectional, age‐adjusted assessment from the Centers for Disease Control and Prevention reports that 18% of American adults with diabetes use insulin alone and another 13% use insulin in combination with oral agents.3
Although an effective and safe treatment at any stage of diabetes, transition to insulin for many patients is considered a marker of “end‐stage disease” or of a failure of diabetes care. Barriers to insulin use include patients’ fear of injections or side effects of insulin (most commonly hypoglycaemia) or of stigma and discrimination. Clinicians’ lack of time, knowledge or experience in providing appropriate patient education and training in insulin use poses additional obstacles.4, 5
The Trial Evaluating Cardiovascular Outcomes with Sitagliptin (TECOS), a randomized, placebo‐controlled cardiovascular outcomes trial comparing sitagliptin with placebo when added to usual care, provides an opportunity to examine international patterns of pharmacological diabetes treatment, thresholds for the addition of insulin and the degree to which sitagliptin therapy, compared with placebo, delays the onset of insulin use in a large, international cohort.
2. RESEARCH DESIGN AND METHODS
2.1. Study design and participants
The design, protocol and primary results of TECOS (NCT00790205) have been published previously.6, 7 The study was designed and run independently by the Duke Clinical Research Institute (DCRI) and the University of Oxford Diabetes Trials Unit (DTU) in an academic collaboration with the sponsor, Merck Sharp & Dohme (Kenilworth, NJ, USA). The protocol was approved by the ethics committees associated with all participating trial sites, and all participants provided written informed consent for trial participation.
Briefly, 14 671 participants from 38 countries were enrolled between December 2008 and July 2012. Eligible participants were ≥50 years old (with no upper age limit) with T2DM, atherosclerotic cardiovascular disease, and HbA1c values of 6.5%‐8.0% (48‐64 mmol/mol) on stable dose mono‐ or dual‐combination therapy with metformin, pioglitazone, or SU, or insulin with or without metformin. Study patients were randomized in a double‐blind fashion to the addition of sitagliptin or placebo to usual care at doses appropriate for their estimated glomerular filtration rate (eGFR). Patients with an eGFR <30 mL/min per 1.73 m2 were not eligible. During follow‐up, treatment for T2DM and its comorbidities was provided by usual care providers based on local guidelines, with an intent to achieve comparable glycaemic control in the two treatment groups. The addition of any antihyperglycaemic agent was permitted with the exception of glucagon‐like peptide‐1 (GLP‐1) receptor agonists or open‐label dipeptidyl peptidase‐4 (DPP‐4) inhibitors. Use of rosiglitazone was discouraged. Data regarding use of concomitant medications by drug class, occurrence of severe hypoglycaemia (hypoglycaemia requiring the assistance of another individual), serious adverse events (SAEs) and adverse events (AEs) resulting in study drug discontinuation were recorded at all visits. Drug classes for glucose‐lowering medications were as follows: metformin, SU, thiazolidinedione, insulin (included short‐ and long‐acting preparations), pramlintide, non‐SU secretagogues, alpha‐glucosidase inhibitors, GLP‐1 receptor agonists and open‐label DPP‐4 inhibitors.
2.2. Statistical analysis
The intention‐to‐treat population was used for all analyses. Baseline characteristics are summarized using mean (standard deviation) or median (25th, 75th percentile) for continuous variables, and number (percentage) for categorical variables. Outcomes include change in HbA1c, proportion experiencing a severe hypoglycaemic event, proportion with a severe or treatment‐emergent AE and time to the addition of insulin (for those not using insulin at baseline).
Medication initiation dates were used where available (primarily for insulin), but if missing, study visit dates approximated medication initiation. Comparisons between randomized treatment allocation with regard to the initiation of a new glucose‐lowering medication were tested using Cox proportional hazards regression models, stratified by region, with reporting of hazard ratios (HRs), 95% confidence intervals (CIs) and P‐values. The mean HbA1c value at the time of insulin initiation was calculated for those patients with at least one HbA1c measurement within the preceding 6 months. HbA1c values during follow‐up were compared for treatment groups using repeated‐measures analysis of variance models that adjusted for time, region and baseline HbA1c. Cox proportional hazards regression models used to test for possible associations between randomized treatment allocation and severe hypoglycaemic events included randomized treatment, baseline medication group and treatment‐by‐medication group interactions. The proportional hazards assumption in the Cox regression was checked, but violations occurred only after 3 years of follow‐up, at a time when 92% of hypoglycaemia events and 88% of insulin initiation events had already occurred.
AEs collected within TECOS included those that met SAE reporting criteria, and those that resulted in cessation of study medication; however, study end‐points and expected diabetes complications (as listed on the TECOS clinical event list7) were not included in the AE analyses. Other non‐serious AEs were not collected. AEs were analysed in the all‐patients‐as‐treated population, comprising all randomized patients who received at least one dose of study medication. AEs were analysed as binary variables. Counts and proportions of patients with events were reported, along with Miettinen‐Nurminen 95% CIs for the risk difference between sitagliptin and placebo.
3. RESULTS
Baseline characteristics by treatment group have been published,8 with characteristics by medication class shown in Table 1. The most common glucose‐lowering regimens were metformin monotherapy (30.2%, N = 4435), SU monotherapy (8.5%, N = 1246), metformin/SU therapy (35.1%, 5152) and insulin (including short‐ and long‐acting preparations) with or without metformin (13.9% [N = 2032], 8.6% [N = 1255], respectively). A more detailed breakdown of baseline medication use is provided in Table S1. Further results listed here are only for those medication classes used at baseline in >5% of the population, which included metformin, SU, metformin/SU combination, insulin and insulin/metformin combination.
Table 1.
Baseline patient characteristics according to baseline diabetes medication class
| Metformin only (N = 4435) | Sulfonylurea only (N = 1246) | Sulfonylurea/metformin (N = 5152) | Insulin (N = 1255) | Insulin/metformin (N = 2032) | |
|---|---|---|---|---|---|
| Age at randomization (y)a | 65 (59, 70) | 68 (61, 74) | 65 (59, 71) | 68 (62, 73) | 65 (60, 71) |
| Female sex | 1316 (29.7%) | 422 (33.9%) | 1408 (27.3%) | 398 (31.7%) | 621 (30.6%) |
| Race (%) | |||||
| White | 3189 (71.9) | 867 (69.6) | 2870 (55.7) | 1016 (81.0) | 1601 (78.8) |
| Black | 123 (2.8) | 29 (2.3) | 154 (3.0) | 61 (4.9) | 58 (2.9) |
| Asian | 815 (18.4) | 271 (21.7) | 1797 (34.9) | 70 (5.6) | 222 (10.9) |
| Other | 308 (6.9) | 79 (6.3) | 331 (6.4) | 108 (8.6) | 151 (7.4) |
| Hispanic or Latino (%) | 554 (12.5) | 141 (11.3) | 571 (11.1) | 202 (16.1) | 284 (14.0) |
| Durationb of type 2 diabetes (y) | 6 (3, 10) | 8 (5, 13) | 11 (6, 16) | 17 (11, 24) | 16 (11, 22) |
| Qualifying HbA1c (%) | 7.0 (6.7, 7.4) | 7.1 (6.8, 7.6) | 7.3 (6.9, 7.7) | 7.4 (7.0, 7.8) | 7.4 (7.0, 7.8) |
| Body mass index (kg/m2) | 29.7 (26.5, 33.3) | 28.7 (25.5, 32.2) | 28.4 (25.5, 32.1) | 31.0 (27.4, 34.7) | 31.2 (27.8, 35.2) |
| Systolic blood pressure (mm Hg) | 133 (123, 143) | 134 (125, 145) | 134 (124, 145) | 132 (122, 145) | 135 (125, 147) |
| Diastolic blood pressure (mm Hg) | 80 (70, 85) | 80 (70, 84) | 80 (70, 84) | 75 (68, 82) | 78 (70, 84) |
| eGFR (mL/min/1.73 m2)c | 75 (62, 90) | 69 (56, 84) | 74 (61, 90) | 61 (49, 78) | 72 (60, 87) |
| eGFR <60 mL/min/1.73 m2 | 746 (17.0%) | 402 (32.6%) | 1018 (19.9%) | 536 (43.5%) | 473 (23.5%) |
| Urine albumin creatinine ratio (g/mol creatinine) | 0.9 (0.4, 2.7) | 0.9 (0.1, 3.2) | 1.3 (0.5, 4.0) | 1.9 (0.6, 8.2) | 1.6 (0.6, 5.8) |
| Total cholesterol (mg/dL) | 160.0 (135.1, 193.4) | 168.0 (144.0, 201.0) | 158.0 (134.0, 189.0) | 158.0 (132.9, 191.0) | 153.0 (130.0, 181.5) |
| LDL cholesterol (mg/dL) | 84.5 (65.6, 110.4) | 94.6 (72.0, 122.0) | 83.6 (65.3, 108.0) | 83.0 (65.6, 109.0) | 79.0 (61.0, 102.0) |
| HDL cholesterol (mg/dL) | 42.5 (36.0, 50.2) | 42.5 (35.0, 51.0) | 41.0 (35.0, 49.0) | 42.5 (34.7, 51.0) | 40.2 (34.4, 48.3) |
| Triglycerides (mg/dL) | 144.2 (105.0, 199.0) | 143.0 (101.0, 200.9) | 142.5 (105.0, 200.0) | 135.0 (95.0, 194.0) | 141.6 (100.9, 203.5) |
| Prior coronary heart disease (%) | 3240 (73.1) | 915 (73.4) | 3768 (73.1) | 958 (76.3) | 1545 (76.0) |
| Myocardial infarction (%) | 1934 (43.6) | 582 (46.7) | 2025 (39.3) | 607 (48.4) | 897 (44.1) |
| ≥50% coronary stenosis (%) | 2284 (51.5) | 621 (49.8) | 2751 (53.4) | 649 (51.7) | 1051 (51.7) |
| Prior PCI (%) | 1789 (40.9) | 433 (35.1) | 1903 (37.5) | 490 (39.7) | 839 (41.8) |
| Prior CABG (%) | 1001 (22.6) | 287 (23.0) | 1214 (23.6) | 410 (32.7) | 612 (30.1) |
| Cerebrovascular disease (%) | 1059 (23.9) | 354 (28.4) | 1218 (23.6) | 368 (29.3) | 467 (23.0) |
| Peripheral arterial disease (%) | 708 (16.0) | 184 (14.8) | 824 (16.0) | 270 (21.5) | 375 (18.5) |
| Prior congestive heart failure (%) | 729 (16.4) | 347 (27.8) | 754 (14.6) | 348 (27.7) | 404 (19.9) |
| Cigarette smoking status (%) | |||||
| Current | 556 (12.5) | 146 (11.7) | 568 (11.0) | 134 (10.7) | 217 (10.7) |
| Former | 1799 (40.6) | 429 (34.4) | 1907 (37.0) | 548 (43.7) | 903 (44.4) |
| Never | 2080 (46.9) | 671 (53.9) | 2677 (52.0) | 573 (45.7) | 912 (44.9) |
| Medications taken at time of randomization (%) | |||||
| Statins | 3584 (80.8) | 923 (74.1) | 4121 (80.0) | 966 (77.0) | 1673 (82.3) |
| Ezetimibe | 240 (5.4) | 45 (3.6) | 208 (4.0) | 82 (6.5) | 141 (6.9) |
| ACE inhibitor or ARB | 3439 (77.5) | 939 (75.4) | 4029 (78.2) | 1028 (81.9) | 1677 (82.5) |
| Diuretics | 1646 (37.1) | 528 (42.4) | 1926 (37.4) | 698 (55.6) | 998 (49.1) |
| Calcium channel blockers | 1438 (32.4) | 415 (33.3) | 1732 (33.6) | 445 (35.5) | 753 (37.1) |
| Beta blockers | 2827 (63.7) | 815 (65.4) | 3197 (62.1) | 844 (67.3) | 1305 (64.2) |
| Aspirin | 3521 (79.4) | 929 (74.6) | 4087 (79.3) | 924 (73.6) | 1604 (78.9) |
| Other platelet antagonists | 886 (20.0) | 264 (21.2) | 1264 (24.5) | 249 (19.8) | 391 (19.2) |
ACE, angiotensin‐converting enzyme; ARB, angiotensin‐receptor blocker; CABG, coronary artery bypass graft; PCI, percutaneous coronary intervention.
Data are median (standard deviation) or n (%).
Age is missing among patients enrolled in Lithuania because the entire birth date including year was not available.
Duration = (year of randomization − year of diagnosis) + 1.
MDRD formula was used to calculate the eGFR. Site‐reported values are presented in the table.
Metformin and SU monotherapy users had shorter median (IQR) duration of diabetes (6 [3, 10] and 8 [5, 13] years, respectively), users of metformin/SU combination therapy had an intermediate median diabetes duration (11 [6, 16] years), while insulin (17 [11, 24]) and insulin/metformin (16 [11, 22]) users had the longest duration (Table 1 and Figure 1). Asian participants were numerically less likely to be using insulin. Renal function parameters were consistent with clinical guidelines for medication use (highest eGFR for metformin, lower in insulin and SU users) as were proportions of patients with heart failure (lowest in metformin‐containing regimens).
Figure 1.
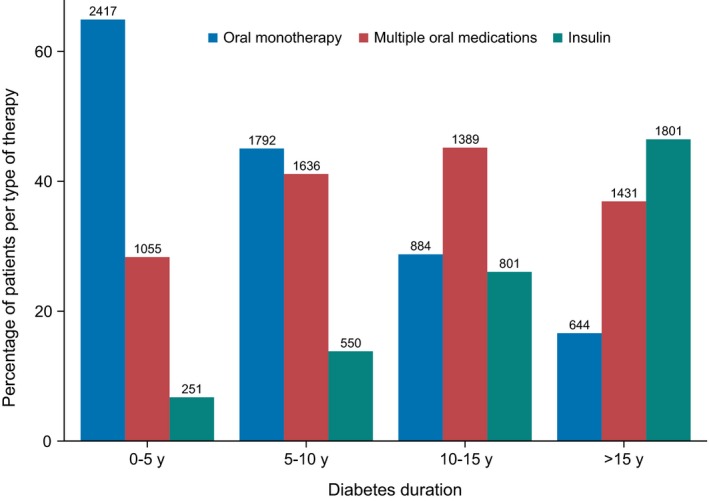
Composition of diabetes medication regimen by duration of diabetes. Insulin indicates both short‐ and long‐acting preparations used alone or in combination with other agents
Calculated over the duration of follow‐up, estimated overall mean HbA1c (95% CI) was 0.28% (−0.30 to −0.26) lower in the sitagliptin group compared with the placebo group and was similarly reduced in all groups by baseline diabetes medication class (Table S2). Diabetes medications were added during follow‐up in 25.2% of participants, but in fewer participants in the sitagliptin group (22.0%, N = 1616) compared with the placebo group (28.3%, 2080) (Figure 2). The most commonly added medications were SU (8.3%, N = 1215) and insulin (8.8%, N = 1286). Figure 2 also shows the addition of concomitant diabetes medication according to baseline medication use. Patients receiving baseline metformin monotherapy were most likely to add SU (21%), whereas those receiving baseline SU monotherapy were most likely to receive metformin (32%). Those receiving metformin/SU combination therapy were most likely to have insulin added to their regimen (17%), although alpha‐glucosidase inhibitors (6%), thiazolidinediones (4%) or open‐label DPP‐4 inhibitors (3%) were also used.
Figure 2.
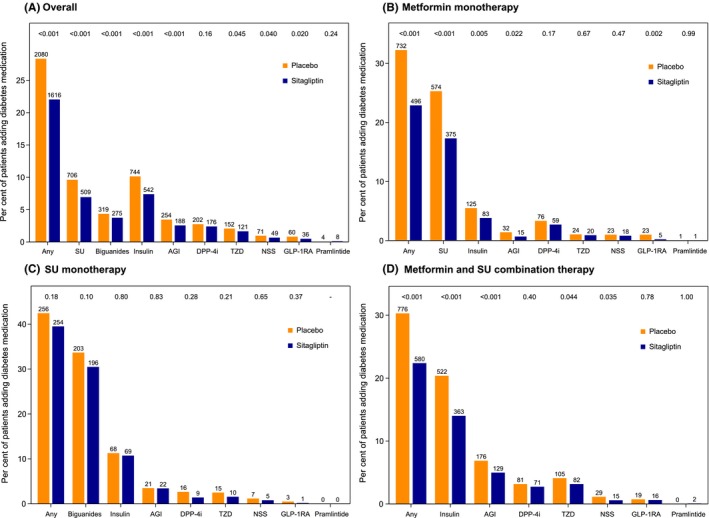
Concomitant diabetes medication added during follow‐up by randomized treatment group (A) overall and (B‐D) according to baseline diabetes medication use. The addition of any antihyperglycaemic agent was permitted by the TECOS protocol, with the exception of GLP‐1 receptor agonists or open‐label DPP‐4 inhibitors. Use of rosiglitazone was discouraged. Hazard ratios, confidence intervals and P‐values are from Cox proportional hazards regression models for the association between randomized treatment and time to addition of the given medication, stratified by region. AGI, alpha‐glucosidase inhibitor; DPP‐4i, dipeptidyl peptidase‐4 inhibitor; GLP‐1 RA, glucagon‐like peptide‐1 receptor agonist; NSS, non‐sulfonylurea secretagogue; SU, sulfonylurea; TZD, thiazolidinedione
In those patients not using insulin at baseline, insulin was initiated in 4.7% of metformin monotherapy users over a median follow‐up of 3.0 years, 11.0% of SU monotherapy users over a median follow‐up of 3.1 years and 17.2% of metformin/SU combination therapy users over a median 3.2 years’ follow‐up. Overall, the mean (SD) HbA1c value at the time of insulin initiation was 8.5% (1.5%) and was lower for those on metformin monotherapy (8.3% [1.6%]) or SU monotherapy (8.4% [1.4%]), but higher for those receiving dual oral agent treatment (8.6% [1.5%]). The likelihood that insulin would be added was significantly lower with sitagliptin overall (HR 0.70; 95% CI 0.63‐0.79; P < 0.001), in patients using metformin alone (HR 0.67; 95% CI 0.51‐0.89; P = 0.005) or using metformin/SU combination therapy (HR 0.64; 95% CI 0.56‐0.73; P < 0.0001) at baseline, but when added in those using SU alone at baseline, sitagliptin had no significant impact on the time to insulin use (HR 0.96; 95% CI 0.68‐1.34; P = 0.80) (Figure 3).
Figure 3.
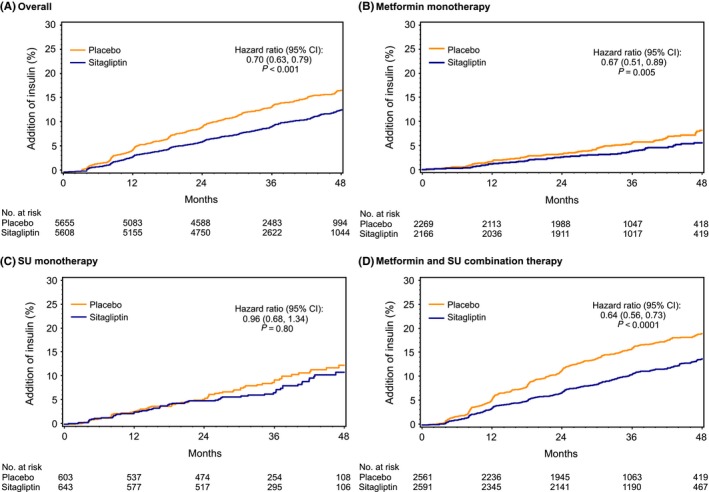
Initiation of chronic insulin therapy by randomized treatment group (A) overall and (B‐D) according to baseline diabetes medication use
Event rates for incident severe hypoglycaemia were lowest in patients using metformin monotherapy at baseline, somewhat higher in those using SU or metformin/SU combination therapy and highest in those using insulin alone or in combination (Table 2). The likelihood of severe hypoglycaemia was not significantly different for sitagliptin compared with placebo, either overall or according to baseline glucose‐lowering medication use. Similar patterns were observed when baseline non‐insulin users were censored after the initiation of SU or insulin (data not shown). There were no differences between treatment groups in the frequency of AEs or SAEs (Table S3) according to baseline medication use.
Table 2.
Incident severe hypoglycaemic events according to baseline therapy
| Sitagliptin | Placebo | HR (95% CI) | P‐value | |||
|---|---|---|---|---|---|---|
| Events/total (%) | Events per 100 pt‐y | Events/total (%) | Events per 100 pt‐y | |||
| Overall | 160/7332 (2.2) | 0.78 | 143/7339 (1.9) | 0.70 | 1.12 (0.89, 1.40) | — |
| Baseline insulin | ||||||
| No | 99/5608 (1.8) | 0.60 | 75/5655 (1.3) | 0.45 | 1.32 (0.98, 1.78) | 0.092 |
| Yes | 61/1724 (3.5) | 1.47 | 68/1684 (4.0) | 1.70 | 0.89 (0.63, 1.26) | |
| Baseline diabetes medication | ||||||
| Metformin only | 19/2166 (0.9) | 0.30 | 18/2269 (0.8) | 0.27 | 1.09 (0.57, 2.08) | 0.61 |
| Sulfonylurea only | 9/643 (1.4) | 0.49 | 7/603 (1.2) | 0.41 | 1.25 (0.47, 3.36) | |
| Metformin + sulfonylurea | 66/2591 (2.5) | 0.86 | 47/2561 (1.8) | 0.63 | 1.38 (0.95, 2.01) | |
| Insulin only | 27/657 (4.1) | 1.79 | 25/598 (4.2) | 1.82 | 1.03 (0.60, 1.77) | |
| Insulin + metformin | 32/1008 (3.2) | 1.31 | 38/1024 (3.7) | 1.55 | 0.85 (0.53, 1.36) | |
HR (95% CI) is for comparison of sitagliptin to placebo for the time to first severe hypoglycaemic event overall and by baseline medication class used.
4. CONCLUSIONS
The TECOS study was conducted in conjunction with usual community‐based diabetes care according to local treatment guidelines. Our analysis shows that TECOS participants, a cohort with reasonably well‐controlled diabetes at baseline, broadly followed expected patterns of diabetes medication use. The majority of patients were using one or two oral agents at baseline, and the choice of baseline medication paralleled median diabetes duration, ranging from the shortest duration in metformin monotherapy users (6 years) to the longest (17 years) in those receiving insulin monotherapy. Intensification of therapy also showed progression from mono‐ to dual‐oral agent therapy and then to insulin alone or in combination. Severe hypoglycaemia was more common in patients using regimens containing SU or insulin, but rates were not impacted by randomization to sitagliptin or placebo.
This paper examines the real‐world progression of glucose‐lowering diabetes therapy (per the trial protocol)7 “as deemed necessary by the usual care physician … to achieve an appropriate, individualized glycaemic goal in line with national guidelines,” rather than protocol‐imposed targets. TECOS provides a structured opportunity to make these observations globally on the treatment standards in effect at the time the trial was conducted. Insulin was added in 8.8% of TECOS participants during a median 3.0 years of follow‐up, with the median HbA1c of 8.5% at the time of initiation higher than the 6.5%‐7% target suggested in contemporaneous international guidelines.9, 10 These findings echo those from other cohorts demonstrating delayed insulin initiation with respect to glycaemic control. The Insulin Titration—Gaining an Understanding of the Burden of Type 2 Diabetes in Europe (INSTIGATE) study showed a mean HbA1c of 9.15% at the time of insulin initiation in a Spanish cohort, 38% of whom were treated with dual oral agent therapy.11 A multinational observational study including 17,374 participants documented pre‐insulin HbA1c values of 8.9 ± 1.6%.12 The reasons for delayed insulin initiation are multifactorial, including patient‐centred and physician‐related factors.13, 14 Although TECOS participants appear to have fared slightly better, with lower HbA1c values at insulin initiation, this may reflect the selection of better controlled patients for the trial, more attentive care for trial participants than is seen in general practice, or contemporaneous guidelines recommending less stringent HbA1c targets for patients with diabetes and established cardiovascular complications.16 Delays in treatment intensification have been called a “dysglycaemic legacy,” associated with increased development of cardiovascular and renal complications.17 Of note, the rate of insulin initiation was higher in PROspective pioglitAzone Clinical Trial In macroVascular Events (PROactive), but this is not surprising, given that insulin was really the only additional glucose‐lowering agent available at that time. The impact of other agents on insulin initiation rates would be of interest, but the TECOS protocol did not permit the use of GLP‐1 receptor agonists, the use of thiazolidinediones was strictly limited, and the infrequent use of SGLT‐2 inhibitors in the 2008‐14 TECOS time period meant there were too few participants to make comparisons between these groups for time to insulin initiation.
Within TECOS, treatment with sitagliptin was associated with improved glycaemic control and a delayed use of insulin in patients receiving metformin monotherapy or combination therapy with metformin and SU. The finding that sitagliptin had no impact on time to insulin when added to SU monotherapy may be related to patient selection for SU therapy, resulting in a cohort with minimal capacity for additional beta‐cell stimulation by a DPP‐4 inhibitor. It is also possible that a delay does exist, supported by the apparent separation of the time to insulin curves at 2 years, but that the sample size in this subgroup was too small to detect a difference between treatment groups statistically. More rapid progression to insulin in patients using SU was also shown in ADOPT (A Diabetes Outcome Progression Trial)18 and may be an intrinsic property of SU therapy due to chronic insulin stimulation.19
In clinical practice, intensification of diabetes regimens may confer increased risk of hypoglycaemia. In TECOS, patients randomized to sitagliptin achieved lower HbA1c values throughout follow‐up without experiencing an increased risk for severe hypoglycaemia. The same was true regardless of baseline therapy, although it should be noted that the TECOS protocol encouraged down‐titration of concomitant medications if severe hypoglycaemia developed, rather than study drug discontinuation. This is in contrast to earlier studies suggesting that rates of hypoglycaemia were higher when sitagliptin was added to medications with intrinsic increased risk for hypoglycaemia (eg, SU or insulin), but not when added to medications without significant increased hypoglycaemia risk (eg, metformin).20, 21
Interpretation of these findings is limited by several features of TECOS. Medications available for intensification were limited both by the protocol, which prohibited use of both GLP‐1 receptor agonists and open‐label DPP‐4 inhibitors, and by temporal factors. The trial ran between December 2008 and March 2015, predating common clinical use of SGLT‐2 inhibitors. The trial entry criteria resulted in enrolment of patients with relatively well‐controlled diabetes (HbA1c 6.5%‐8.0%), which may not be typical of patients generally considered for intensification of their diabetes treatments. Furthermore, although TECOS enrolled a secondary cardiovascular prevention population, baseline cardiovascular risk factors were relatively well controlled, which again may not be typical of the general T2DM population in many areas. Finally, our observations are limited by the median 3.1‐year follow‐up time for the study. This analysis provides insights into the natural history of diabetes progression for those with later stage, well‐controlled disease. Even over a relatively short 3‐year follow‐up, one‐quarter of participants had their diabetes regimen intensified. TECOS participants underwent a pattern of diabetes medication intensification consistent with guidelines relevant during the period of the trial, given the restrictions on medication choices imposed by the study protocol. Progression to insulin was late according to the level of glucose control, again consistent with similar cohorts, but use of sitagliptin did delay insulin progression in most combination therapy regimens, and combination therapies with insulin did not increase the risk of severe hypoglycaemia. These data underscore the importance of clinical vigilance in diabetes care since progression of the disease continues even in the absence of substantial perturbations of glycaemic control. Future work to understand the predictors of progression in those with advanced, well‐controlled diabetes could inform personalized diabetes management efforts.
DISCLOSURES
Bethel: grants, personal fees, and other support from Merck, Sharp & Dohme, other support from Boehringer‐Ingelheim, Novo Nordisk, Theracos, AstraZeneca, and GlaxoSmithKline, and non‐financial support from Bayer. Engel: employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc. Stevens and Alvarsson: none. Lokhnygina: grants from Merck, Janssen Research & Development, AstraZeneca, GlaxoSmithKline, and Bayer HealthCare AG. Ding: employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc Josse: grants or personal fees from Amgen, AstraZeneca, Boehringer‐Ingelheim, Eli Lilly, Janssen, and Merck. Hramiak: personal fees from Amgen, AstraZeneca/Bristol Myers Squibb, Boehringer Ingelheim, Eli Lilly, GlaxoSmithKline, Hoffmann‐La Roche, Insulet, Janssen‐Ortho (JNJ), Merck Frosst, Novo Nordisk, Sanofi, and Takeda, and research support from AstraZeneca/Bristol Myers Squibb, Eli Lilly, GlaxoSmithKline, Janssen‐Ortho (JNJ), Lexicon, Medtronic, Merck Frosst, and Novo Nordisk. Green: grants from Merck Sharp & Dohme, AstraZeneca, Intarcia and GlaxoSmithKline; Consultant for Boehringer‐Ingelheim, NovoNordisk, and Daiichi. Peterson: grants and personal fees from Janssen, grants from Eli Lilly, and personal fees from AstraZeneca, Bayer, and Sanofi. Holman: grants and personal fees from Merck, grants from Bayer, AstraZeneca, and Bristol‐Myers Squib, personal fees from AMGEN, Bayer, Intarcia, Novartis, Novo Nordisk, and other support from GlaxoSmithKline, Janssen, and Takeda.
AUTHOR CONTRIBUTIONS
MAB was a Clinical Lead for TECOS and designed this analysis, produced the first manuscript draft, interpreted the results, reviewed and edited the manuscript, and provided final approval for the version to be published. SSE contributed to the analysis design and interpretation of the results, and reviewed/edited the manuscript. SRS, YL and JD performed statistical analysis and reviewed/edited the manuscript. RGJ, MA, IH and JBG reviewed/edited the manuscript. JBG was a Clinical Lead for TECOS and Chair of the TECOS clinical event adjudication committee (CEC), and reviewed/edited the manuscript. EDP was Joint Chair of the TECOS Executive Committee and reviewed/edited the manuscript. RRH was Joint Chair of the TECOS Executive Committee and reviewed/edited the manuscript.
Supporting information
ACKNOWLEDGEMENTS
The authors acknowledge editorial assistance from Peter Hoffmann of the Duke Clinical Research Institute and input from Ioan Andrei Vereşiu, MD, PhD, of Iuliu Hatieganu, University of Medicine and Pharmacy, Cluj‐Napoca, Romania. RRH is an NIHR Senior Investigator.
Bethel MA, Engel SS, Stevens SR, et al; on behalf of the TECOS Study Group . Progression of glucose‐lowering diabetes therapy in TECOS. Endocrinol Diab Metab. 2019;2:e53 10.1002/edm2.53
Funding information
The TECOS study was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
DATA ACCESSIBILITY
Requests to access the data for this study from qualified researchers trained in human subject confidentiality protocols may be submitted at dcri.org/data‐sharing.
REFERENCES
- 1. Wright A, Burden A, Paisey RB, Cull CA, Holman RR, UK Prospective Diabetes Study Group . Sulfonylurea inadequacy: efficacy of addition of insulin over 6 years in patients with type 2 diabetes in the U.K. Prospective Diabetes Study (UKPDS 57). Diabetes Care. 2002;25:330–336. [DOI] [PubMed] [Google Scholar]
- 2. Swedish National Diabetes Register. https://www.ndr.nu/#/knappen. Accessed March 16, 2018.
- 3. Centers for Disease Control and Prevention . Age‐adjusted percentage of adults with diabetes using diabetes medication, by type of medication, United States, 1997–2011. https://www.cdc.gov/diabetes/statistics/meduse/fig2.htm. Accessed March 16, 2018.
- 4. Ng CJ, Lai PS, Lee YK, Azmi SA, Teo CH. Barriers and facilitators to starting insulin in patients with type 2 diabetes: a systematic review. Int J Clin Pract. 2015;69:1050–1070. [DOI] [PubMed] [Google Scholar]
- 5. Abu Hassan H, Tohid H, Mohd Amin R, Long Bidin MB, Muthupalaniappen L, Omar K. Factors influencing insulin acceptance among type 2 diabetes mellitus patients in a primary care clinic: a qualitative exploration. BMC Fam Pract. 2013;14:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Green JB, Bethel MA, Paul SK, et al. Rationale, design, and organization of a randomized, controlled Trial Evaluating Cardiovascular Outcomes with Sitagliptin (TECOS) in patients with type 2 diabetes and established cardiovascular disease. Am Heart J. 2013;166(6):983–989.e7. [DOI] [PubMed] [Google Scholar]
- 7. Green JB, Bethel MA, Armstrong PW, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373:232–242. [DOI] [PubMed] [Google Scholar]
- 8. Bethel MA, Green JB, Milton J, et al. Regional, age and sex differences in baseline characteristics of patients enrolled in the Trial Evaluating Cardiovascular Outcomes with Sitagliptin (TECOS). Diabetes Obes Metab. 2015;17:395–402. [DOI] [PubMed] [Google Scholar]
- 9. American Diabetes Association . Standards of medical care in diabetes—2008. Diabetes Care. 2008;31(Suppl. 1):S12–S54. [DOI] [PubMed] [Google Scholar]
- 10. Nathan DM, Buse JB, Davidson MB, et al. American Diabetes Association; European Association for Study of Diabetes. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy. Diabetes Care. 2009;32:193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Costi M, Dilla T, Reviriego J, Castell C, Goday A. Clinical characteristics of patients with type 2 diabetes mellitus at the time of insulin initiation: INSTIGATE observational study in Spain. Acta Diabetol. 2010;47(Suppl 1):169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Khunti K, Damci T, Meneghini L, Pan CY, Yale JF, SOLVE Study Group . Study of Once Daily Levemir (SOLVE™): insights into the timing of insulin initiation in people with poorly controlled type 2 diabetes in routine clinical practice. Diabetes Obes Metab. 2012;14:654–661. [DOI] [PubMed] [Google Scholar]
- 13. Russell‐Jones D, Pouwer F, Khunti K. Identification of barriers to insulin therapy and approaches to overcoming them. Diabetes Obes Metab. 2018;20:488–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mata‐Cases M, Franch‐Nadal J, Real J, et al. Therapeutic inertia in patients treated with two or more antidiabetics in primary care: factors predicting intensification of treatment. Diabetes Obes Metab. 2018;20:103–112. [DOI] [PubMed] [Google Scholar]
- 15. Khunti K, Millar‐Jones D. Clinical inertia to insulin initiation and intensification in the UK: a focused literature review. Prim Care Diabetes. 2017;11:3–12. [DOI] [PubMed] [Google Scholar]
- 16. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient‐centered approach: position Statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35:1364–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Paul SK, Klein K, Thorsted BL, Wolden ML, Khunti K. Delay in treatment intensification increases the risks of cardiovascular events in patients with type 2 diabetes. Cardiovasc Diabetol. 2015;14:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kahn SE, Haffner SM, Heise MA, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Eng J Med. 2006;355:2427–2443. [DOI] [PubMed] [Google Scholar]
- 19. Maedler K, Carr RD, Bosco D, Zuellig RA, Berney T, Donath MY. Sulfonylurea induced beta‐cell apoptosis in cultured human islets. J Clin Endocrinol Metab. 2005;90:501–506. [DOI] [PubMed] [Google Scholar]
- 20. Visbøll T, Rosenstock J, Yki‐Järvinen H, et al. Efficacy and safety of sitagliptin when added to insulin therapy in patients with type 2 diabetes. Diabetes Obes Metab. 2010;12:167–177. [DOI] [PubMed] [Google Scholar]
- 21. Hermansen K, Kipnes M, Luo E, et al. Efficacy and safety of the dipeptidyl peptidase‐4 inhibitor, sitagliptin, in patients with type 2 diabetes mellitus inadequately controlled on glimepiride alone or on glimepiride and metformin. Diabetes Obes Metab. 2007;9:733–745. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Requests to access the data for this study from qualified researchers trained in human subject confidentiality protocols may be submitted at dcri.org/data‐sharing.


