Abstract
The purpose of this study was to investigate if the enhanced bioaccumulation of lutein in retina and brain of breastfed, compared to formula-fed, infant monkeys was associated with higher levels of serum total and HDL cholesterol, apolipoproteins, or mRNA/protein expression of carotenoid-related genes. Newborn rhesus macaques were either breastfed, fed a carotenoid-supplemented formula, or fed an unsupplemented formula for 6 months (n = 8, 8, 7). Real-time qPCR and western blotting were performed in two brain regions [occipital cortex (OC); cerebellum (CB)] and two retina regions [macular retina (MR); peripheral retina (PR)]. Breastfed infants had higher serum total cholesterol, HDL cholesterol, apoA-I, and apoB-100 levels than the combined formula-fed groups (P<0.05). Diet type did not alter expression of the nine genes (CD36, SCARB1, SCARB2, LDLR, STARD3, GSTP1, BCO1, BCO2, RPE65) examined except for SCARB2 in the retina and brain regions. In conclusion, dietary regimen did not impact the expression of carotenoid-related genes except for SCARB2. However, carotenoid-related genes were differentially expressed across brain and retina regions. Breastfed infants had higher serum total and HDL cholesterol, and apolipoproteins, suggesting that lipoprotein levels might be important for delivering lutein to tissues, especially the macular retina, during infancy.
Keywords: lutein, brain, retina, lipoproteins, carotenoid-related genes, rhesus macaques
1. Introduction
Humans are not able to synthesize lutein, a yellow xanthophyll, de novo. Therefore, tissue deposition of lutein is dependent upon dietary sources, such as green leafy vegetables, fruits, and egg yolks. Lutein is distributed ubiquitously in tissues; most notably, lutein selectively accumulates in the retina and brain. Lutein and its isomers zeaxanthin and meso-zeaxanthin constitute macular pigment in the primate fovea, where they have an essential role in eye health and visual performance [1]. Dietary consumption of lutein and zeaxanthin is associated with a decreased risk of age-related macular degeneration (AMD), a leading cause of visual loss in the elderly [2, 3]. In addition, lutein preferentially accumulates in the neural tissues of infants and older adults [4–6] and it has been suggested that lutein may play a role in cognitive health [7].
Various factors involved in lutein’s selective accumulation in the retina have been widely studied. In circulation, polar xanthophylls are transported mainly by HDL particles compared to the hydrophobic carotenes [8]. Lutein is taken up into the retina from circulation via several receptors including CD36 and class B scavenger receptors (SR-B1 and SR-B2) [9]. In retina tissue, steroidogenic acute regulatory domain protein 3 (STARD3) selectively binds and protects lutein [10] while glutathione S-transferase PI (GSTP1) binds zeaxanthin [11]. β-Carotene 9,10’ oxygenase-2 (BCO2), along with β-carotene-15,15’-oxygenase (BCO1), are known mammalian carotenoid cleavage enzymes. Human BCO1 appears to be the primary enzyme to cleave carotenes [12]. BCO2 isolated from ferret and chicken cleaves a variety of carotenoids including xanthophylls [13, 14]. Interestingly, Li et al. [15] suggested that the inactivity of primate BCO2 due to the loss of an alternative splicing site contributes to selective accumulation of lutein and zeaxanthin in primate retina. In contrast, Babino et al. [16] proposed that the enzymatic function of human BCO2 is conserved and BCO2 expression is regulated by oxidative stress. Recently it was discovered that RPE65, known as retinoid isomerase, can convert lutein to meso-zeaxanthin in vertebrate eyes [17]. Furthermore, it has been shown that multiple single nucleotide polymorphisms (SNPs) in genes related to lutein absorption, transport, and metabolism are associated with macular pigment optical density (MPOD), and also may be related to the risk of AMD [18]. In the brain, the mechanism for lutein accumulation is not well understood, but it is assumed to be similar to retina, as the blood-brain barrier and blood-retina barrier are structurally and functionally similar. In addition, brain lutein concentrations and MPOD are highly correlated in primates [19, 20].
In early life, infants are dependent upon lutein intake from breast milk and/or infant formula to achieve optimal lutein status. Recently, we compared the influence of breast milk, a carotenoid-supplemented formula or an unsupplemented formula on lutein biodistribution patterns in tissues of infant rhesus macaques after 6 months of life [21]. Breastfed infants accumulated significantly more lutein in all tissues examined than those fed the lutein supplemented formula despite similar lutein levels in each. It is likely that the macronutrient matrix of breast milk enhances carotenoid bioavailability compared to commercial formulas [21, 22]. However, we speculate that in addition to the differences in lutein bioavailability between breast and formula milk, differential expression of carotenoid-related genes might explain a portion of the differences in lutein accumulation in brain and retina between the diet groups. We probed whether breast milk or formulas high and low in carotenoids, fed to infant monkeys from birth to six months, differentially impacted 1)serum levels of total or HDL cholesterol or apolipoprotein and 2) the expression of transporters, binding proteins, and carotenoid cleavage enzymes in retina and selected brain regions.
2. Methods
2.1. Animals and Diets
The procedures in this study were approved by the Institutional Animal Care and Use Committee of Oregon Health and Science University and were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. From the day after birth until 6 months, newborn rhesus macaques (Macaca mulatta) were either breastfed (n=8), fed a carotenoid-supplemented formula (n=8) (Similac Advance with OptiGRO), or fed an unsupplemented formula (n=7) based on the Similac Advance base formulation, with only the native carotenoids present in the lipid components. Based on our analysis by reverse-phase HPLC, lutein, zeaxanthin, β-carotene and lycopene, respectively, were found at the following levels in breast milk: 251, 105, 0, and 0 nmol/kg; supplemented formula: 237, 19.0, 74.2, and 338 nmol/kg; and unsupplemented formula: 38.6, 2.3, 21.5, and 0 nmol/kg [21]. The breastfeeding dams were fed Monkey Diet Jumbo 5037 (Lab Diet) and fresh fruits and vegetables.
After 6 months of feeding, infant monkeys were humanely euthanized by a veterinary pathologist under deep pentobarbital anesthesia. At the time of death, fasting blood samples were collected and centrifuged at 800 × g for 15 min to obtain serum. From the brain, samples were dissected from occipital cortex and cerebellum and preserved in RNAlater™ solution. From each retina, 4 mm diameter biopsy punches were used to obtain samples of the macular and peripheral retina. Once the vitreous was removed, the neural retina was dissected from the underlying retinal pigment epithelium and choroid. All collected samples were frozen in liquid nitrogen and then stored at −80° C until analysis.
2.2. Carotenoid analysis in serum HDL and non-HDL particles
All extractions and analyses were performed under yellow light to minimize light-induced damage of carotenoids. Carotenoids in serum HDL and non-HDL particles were analyzed by using a protocol provided by Emily Mohn at Tufts University. Briefly, 200 μL of serum was mixed with 500 μL of precipitation solution (0.44 mM phosphotungstic acid, 20 mM MgCl2) and 2 μL of 0.52 M pyrogallol. After vortexing, the mixture was incubated at room temperature for 15 minutes and then centrifuged at 2300 x g for 15 min at 4°C. After centrifugation, the supernantant was removed to obtain HDL-containing serum. For analyzing carotenoids in non-HDL particles, the pellet was mixed with 200 μL of 0.85% saline, vortexed, and sonicated. Saline (0.85%, 2mL), echinenone as an internal standard, chloroform/ methanol (2:1, v/v) and 2 μL 0.52 M pyrogallol were added and vortexed. For analyzing carotenoids in HDL particles,1.5 mL 0.85% saline and 100 μL internal standard were added to the isolated HDL-containing supernantant (300 μL), and then 9mL of chloroform/methanol(9 mL, 2:1 v/v) and 2 μL 0.52 M pyrogallol were added and vortexed. For both extractions, mixtures were centrifuged at 1000 g for 10 min at 4°C. The upper aqueous layer was transferred and the hexane extraction process was repeated two more times.
Carotenoid analyses were carried out on an Alliance HPLC system (e2695 Separation Module) equipped with 2998 photodiode array detector (Waters). The extracts were separated on a reverse-phase C30 column (4.6 × 150 mm, 3 μm; YMC) maintained at 18°C. A phase gradient method was used for carotenoid separation, based on the method of Yeum et al. [23]. The lower limit of detection for carotenoids was 0.2 pmol. The average recovery of the internal standard was 92.4 ± 1.0 %. Carotenoids were identified via absorption spectra, retention times, and comparison to standards and quantified by an internal standard curve method.
2.3. Total cholesterol, HDL cholesterol, and apolipoprotein analyses
Serum total cholesterol levels were determined with a commercial kit (Wako Diagnostics) according to the manufacturer’s protocol.HDL cholesterol levels were analyzed in the isolated HDL-containing serum using the same kit. Serum apolipoprotein (apo) A-I, B-48, and B-100 levels were assessed with commercial ELISA kits (MyBioSource) according to the manufacturer’s instructions.
2.4. Total RNA extraction and quantitative real-time PCR analysis
Total RNA for nine carotenoid-related genes was isolated from macular and peripheral regions of RPE-choroid and retina and from brain regions (occipital cortex and cerebellum) using RNeasy® Lipid Tissue Mini Kit (Qiagen), according to the manufacturer’s instructions. cDNA was synthesized from 400ng of total RNA using RT2 First Strand Kit (Qiagen). RNA purity and concentrations were determined using a μCuvette G1.0 (Eppendorf) in a BioPhotometer (Eppendorf). Amplification reactions were performed on the QuantStudio™ 7 Flex Real-Time PCR System (Life Technologies) following the manufacturer’s protocol for the SYBR® Green ROX qPCR Mastermix (Qiagen). After testing three different reference genes [actin gamma 1 (ACTG1), ribosomal protein L13a (RPL13a), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH)], ACTG1 was chosen as a reference gene because it had the lowest variability among different regions of brain, RPE-choroid, and retina samples. Relative gene expression levels were analyzed using the 2−ΔΔCt method. Primer sequences are described in Table 1.
Table 1.
Primer sequences utilized in RT-PCR analysis.
| Gene | Forward | Reverse |
|---|---|---|
| CD36 | TGGTCAAGCCATCAAACAAA | GCAACAAACATCACCACACC |
| SCARB1 | AGATCATGTGGGGCTACCAG | GTTCCACTTGTCCACGAGGT |
| SCARB2 | GCTCTGGGGCTACAAAGATG | TTCCCATTCCATTCCACAAT |
| LDLR | TACAGCACCCAGCTTGACAG | TTTCCTCTTCACACCCTTGG |
| STARD3 | GCAAGAACTTGGAGCAGGAG | GCAGCTCAGAGAGGATGACC |
| GSTP1 | CCGCACTCTTGGGCTCTATGG | AGTCATCCTTGCCTGCCTCGT |
| RPE65 | GATGCTTACGTACGGGCAAT | TTGTCGGTAACCTCCACTCC |
| BCO1 | CATTGTGGAAAAGGGGAAGA | GGCTGCTCAAGGAAGATGA |
| BCO2 | TGATGTGGTTTGGAGAGAAGATGGT | TGCTTTCATTCTGGTTGGGAGTG |
2.5. Western Blotting
Total proteins from frozen brain samples were extracted with RIPA buffer (Thermo Scientific) containing 1% protease inhibitor cocktail (Sigma) at 4°C. Protein concentrations were determined by the Pierce BCA protein assay kit (Thermo Fisher Scientific). 20μg of proteins were separated on SDS-PAGE and transferred onto an Immobilon-P PVDF membrane (Millipore). Following blocking with 5% non-fat milk, membranes were probed with specific primary antibodies and subsequently incubated with HRP-linked secondary antibodies for chemiluminescent detection. The primary antibodies used and their dilutions are listed as follows: STARD3 at 1:3000, GSTP1 at 1:3000, GAPDH at 1:3000 (all from Cell Signaling). The band intensities were visualized by ImageQuant LAS 4000 (GE Healthcare) and quantified using Quantity One software (Bio-Rad).
2.6. Statistical analysis
Data analyses were performed using SPSS version 24 (IBM SPSS Statistics). Unequal variances were evaluated prior to ANOVA analysis using Levene’s test. Two-way ANOVA was performed to assess effects on gene expression of diet and brain region, or diet and retina region. Gene expression in brain, retina, or RPE-choroid regions was analyzed by one-way ANOVA, with Bonferroni’s multiple comparison test. In case of unequal variances, the Welch’s ANOVA was used to compare group means, followed by Dunnett’s T3 test for posthoc comparisons. Two-tailed Student’s t-test was performed to assess differences in serum cholesterol and apolipoproteins between breastfed and all formula-fed monkeys (with the two formula groups combined). The correlations between pairs of variables were determined by Pearson’s linear correlation coefficient. All data are presented as mean ± SEM. All differences or relationships were considered significant when P<0.05.
3. Results
Lutein and zeaxanthin levels in serum, brain and retina dependent upon dietary sources after 6 months of feeding
As previously reported [21], after 6 months of feeding, lutein and zeaxanthin differentially accumulated in numerous tissues of infant monkeys in the different feeding groups (Table 2). Breastfed infant monkeys had several-fold higher lutein levels than either formula group in serum, both retina regions (macular retina, MR; peripheral retina, PR), and both brain regions (occipital cortex, OC; cerebellum, CB). Compared to unsupplemented formula, supplemented formula significantly enhanced lutein levels in the serum, both brain regions, and PR, but not in the MR. Serum and brain zeaxanthin were only detectable in the breastfed monkeys. Breastfed monkeys had higher zeaxanthin concentrations in both retina regions compared to the formula-fed monkeys. Supplemented formula increased zeaxanthin levels in the PR, but not in the MR compared to unsupplemented formula. As expected, lutein and zeaxanthin concentrations were higher in the MR than in the PR in all dietary groups. In brain, lutein concentrations were higher in the OC than in CB in all feeding groups.
Table 2.
Lutein and zeaxanthin concentrations in serum, retina (macular retina, MR; peripheral retina, PR), and brain (occipital cortex, OC; cerebellum, CB) of infant rhesus macaques after 6 months of breastfeeding or formula feeding1.
| BF | SF | UF | ||
|---|---|---|---|---|
| Lutein | ||||
| Serum [nmol/L] | 499 ± 78.0a | 66.9 ± 4.97b | 10.6 ± 3.78c | |
| Retina [pmol/g]† | ||||
| PR | 877 ± 194a | 156 ± 34.8b | 34.9 ± 12.6C | |
| Brain [pmol/g]† | ||||
| CB | 80.4 ± 15.4a | 16.9 ± 1.26b | 3.28 ±1.65C | |
| Zeaxanthin | ||||
| Serum [nmol/L] | 124 ± 20.1 | nd2 | nd | |
| Retina [pmol/g]† | ||||
| PR | 258 ± 58.2a | 15.3 ± 5.98b | 2.67 ± 2.67b | |
| Brain [pmol/g]† | ||||
| CB | 37.8 ± 21.2 | nd | nd | |
BF, breastfed (n=8); SF, supplemented formula-fed (n=8); UF, unsupplemented formula-fed (n=7).
Values are means ± SEM.
nd = not detected. Values in a row with different superscript letters are significantly different, P<0.05 by one-way ANOVA or Welch’s test followed by Bonferroni or Dunnett’s T3 pairwise posthoc tests.
Adapted from Reference [21].
Serum total cholesterol, HDL cholesterol, and apolipoprotein levels
No differences were found between the two formula-fed groups in any lipoprotein measure, and therefore the two groups were combined for subsequent analyses. Serum total and HDL cholesterol levels were higher in the breastfed infants than in the combined formula-fed groups (1.2-fold and 1.7-fold higher, respectively) (Table 3). Serum lutein concentrations were positively and significantly correlated with HDL cholesterol (R2=0.197, P=0.034) but the positive correlation with total cholesterol did not reach statistical significance (R2=0.163, P=0.056). The levels of apoA-I, the major protein marker of HDL, were significantly higher in the breastfed infants compared to the combined formula-fed infants. ApoB-100 and apoB-48 are protein constituents of LDL. Levels of ApoB-100, but not ApoB-48, were significantly higher in the breastfed infants than in the combined formula-fed infants.
Table 3.
Total cholesterol, HDL cholesterol, and apolipoprotein levels in serum of infant rhesus macaques after 6 months of breastfeeding or formula feeding1.
| BF | SF | UF | Breastfed vs. Combined formula-fed (P-value) |
|
|---|---|---|---|---|
| Total cholesterol [mmol/L] | 7.23 ± 0.54 | 5.94 ± 0.21 | 5.78 ± 0.58 | 0.021* |
| HDL cholesterol [mmol/L] | 0.75 ± 0.15 | 0.45 ± 0.13 | 0.42 ± 0.08 | 0.048* |
| ApoA-I [μg/mL] | 188 ± 39.2a | 106± 18.2b | 80.5 ± 12.6b | 0.050* |
| ApoB-48 [μg/mL] | 16.6 ± 56.0 | 22.2 ± 6.22 | 21.3 ± 1.41 | 0.273 |
| ApoB-100 [μg/mL] | 993 ± 160a | 641 ± 104ab | 504 ± 73.8b | 0.010* |
BF, breastfed (n=8); SF, supplemented formula-fed (n=8); UF, unsupplemented formula-fed (n=7).
Values are means ± SEM. Values in a row with different superscript letters are significantly different, P<0.05 by one-way ANOVA by Bonferroni posthoc test.
P<0.05, Significant different between the breastfed and combined formula-fed groups by Student’s t-test.
Carotenoid levels in HDL and non-HDL fractions
To better understand the differential lutein accumulation in brain and macular tissues between breastfed and formula-fed infants, we investigated the amounts and distribution of carotenoids in the serum HDL and non-HDL fractions. Lutein concentrations in serum HDL and non-HDL fractions were significantly higher in breastfed infants compared to both formula-fed groups (Table 4). Supplemented formula significantly increased lutein concentrations in HDL and non-HDL fractions compared to unsupplemented formula. Breastfeeding also significantly increased β-carotene concentrations in HDL and non-HDL fractions compared to both formula groups. Zeaxanthin was only detectable in the serum of the breastfed group. Lycopene was detectable in the serum of the supplemented formula group and one breastfed infant.
Table 4.
Carotenoid levels in serum HDL and non-HDL fractions of infant rhesus monkeys either breastfed, fed a carotenoid-supplemented formula or fed an unsupplemented formula for six months.
| BF | SF | UF | |
|---|---|---|---|
| Lutein | |||
| Serum HDL [nmol/L] | 369 ± 58.6a | 50.4 ± 4.14b | 8.62 ± 3.15C |
| Serum HDL [nmol/L] | 130 ± 20.8a | 16.6 ± 1.45b | 1.96 ± 0.69c |
| Zeaxanthin | |||
| Serum HDL [nmol/L] | 88.2 ± 14.7 | nd2 | nd |
| Serum non-HDL [nmol/L] | 35.6 ± 5.81 | nd | nd |
| β-carotene | |||
| Serum HDL [nmol/L] | 66.7 ± 11.4a | 30.2 ± 2.39b | 17.9 ± 2.31b |
| Serum HDL [nmol/L] | 68.4 ± 12.5a | 25.3 ± 1.59b | 8.93 ± 1.22C |
| Lycopene | |||
| Serum HDL [nmol/L] | nd | 319 ±7.36 | nd |
| Serum non-HDL [nmol/L] | 15.0 ± 15.0 | 177 ±5.04 | nd |
BF, breastfed (n=8); SF, supplemented formula-fed (n=8); UF, unsupplemented formula-fed (n=7).
Values are means ± SEM. 2nd = not detected. Values in a row with different superscript letters are significantly different, P<0.05 by one-way ANOVA or Welch’s test followed by Bonferroni or Dunnett’s T3 pairwise posthoc tests.
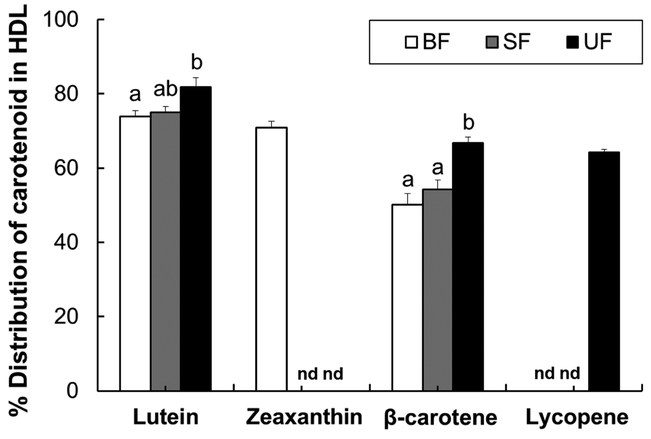
The majority of lutein was found in the HDL fraction (range: 66–89%) in all dietary groups (Figure 1).β-carotene was equally distributed between HDL and non-HDL particles both in the breastfed and the supplemented formula group, while the percentage of β-carotene in the HDL fraction was significantly higher in the unsupplemented formula group compared to both the breastfed and supplemented formula groups. Zeaxanthin concentrations were significantly greater in the serum HDL fraction than in the non-HDL fraction of the breastfed group. Lycopene was also mainly transported by HDL in the supplemented formula group.
Figure 1.
Percent distribution of carotenoid in the serum HDL fraction of infant rhesus monkeys either breastfed (BF, n=8), fed a carotenoid-supplemented formula (SF, n=8) or fed an unsupplemented formula (UF, n=7) for six months. Data are expressed as means ± SEM. Labeled means for each carotenoid without a common letter differ, P<0.05, by one-way ANOVA followed by Bonferroni posthoc tests. nd = not detected.
The correlation between the serum lutein: β-carotene ratio and lutein concentrations in retina and brain tissues
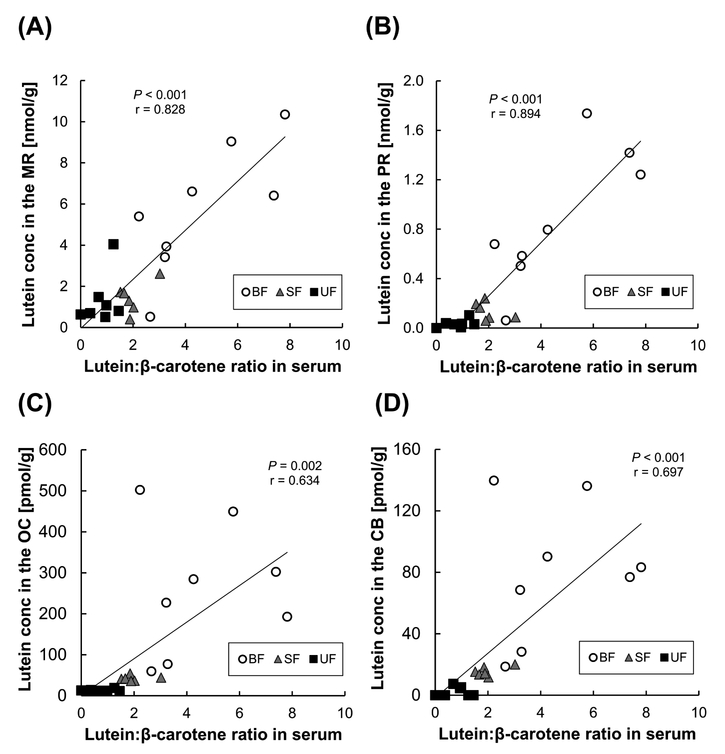
Additionally, we looked at the correlation between the lutein: β-carotene ratio in serum and lutein concentrations in two retina regions and two brain regions (Figure 2). The lutein: β-carotene ratio in serum was highest in the breastfed group (4.6 ± 0.8), followed by the supplemented formula group (2.0 ± 0.2) and the unsupplemented formula group (0.9 ± 0.1) excluding one sample with undetected β-carotene. The lutein: β-carotene ratio in serum was strongly correlated with lutein levels in MR (r =0.828, P<0.001), PR (r =0.847, P<0.001), OC (r =0.634, P=0.002) and CB (r =0.697,P<0.001). Furthermore, in the supplemented formula-fedinfants, the lutein: lycopene ratio in serum was strongly correlated with lutein levels in OC (r =0.817, P=0.013), but not in MR, PR, and CB.
Figure 2.
Relation between the lutein: β-carotene ratio in serum and lutein concentrations in two retina regions [macular retina, MR (A); peripheral retina, PR (B)] and between lutein: β-carotene ratio in serum and lutein concentrations in two brain regions [occipital cortex, OC (C); cerebellum, CB (D)]. BF, breastfed (n=8); SF, supplemented formula-fed (n=6); UF, unsupplemented formula-fed (n=7). Pearson correlation coefficient (r) and P-value are indicated.
Gene expression of receptors, binding proteim, and cleavage enzymes in macular and peripheral areas of RPE-choroid or retina
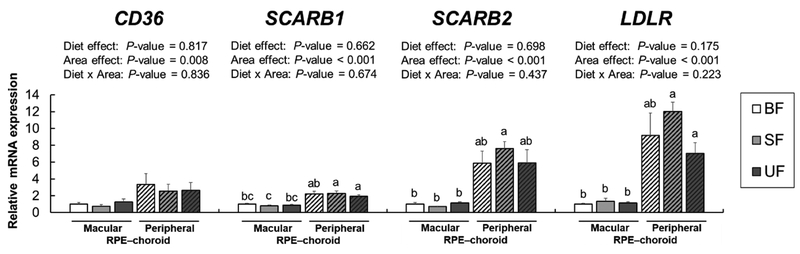
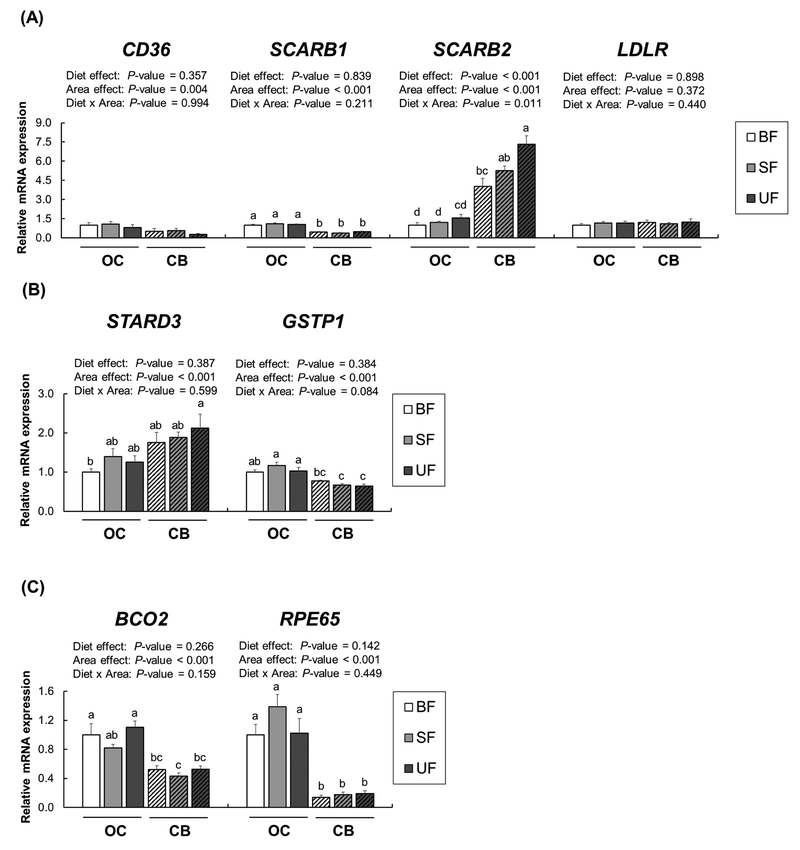
The mRNA expression of carotenoid-related receptors (CD36, SCARBl, SCARB2, and LDLR) in the macular and peripheral RPE-choroid was determined by RT-PCR (Figure 3). Class B scavenger receptors are known to mediate HDL uptake (CD36, SR-B1, and SR-B2), and LDLR are known to be localized in the RPE [24–26]. Expression of all receptor genes examined were significantly down-regulated in the macular RPE-choroid compared to the peripheral RPE-choroid (CD36, P= 0.008; SCARBl, SCARB2, and LDLR, P<0.001). No significant effects of diet or diet × region were seen in the mRNA levels of these receptors.
Figure 3.
Relative receptor mRNA expression in macular (solid bars) and peripheral (striped bars) RPE–choroid in infant rhesus monkeys either breastfed (BF, n=8), fed a carotenoid supplemented formula (SF, n=8) or fed an unsupplemented formula (UF, n=7) for six months. Relative mRNA levels were determined by real-time RT-PCR and normalized by ACTG1. The mean values for the macular RPE–choroid of the breastfed infant monkeys was set at 1.0. Data are expressed as means ± SEM. Diet and area effects were determined by two-way ANOVA. Labeled means for each gene without a common letter differ, P<0.05, by Welch’s test followed by Dunnett’s T3 pairwise posthoc test.
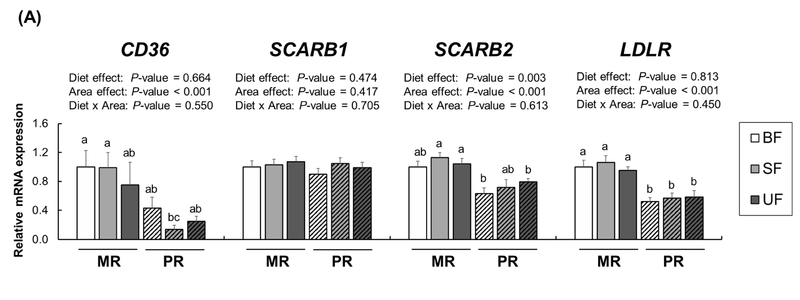
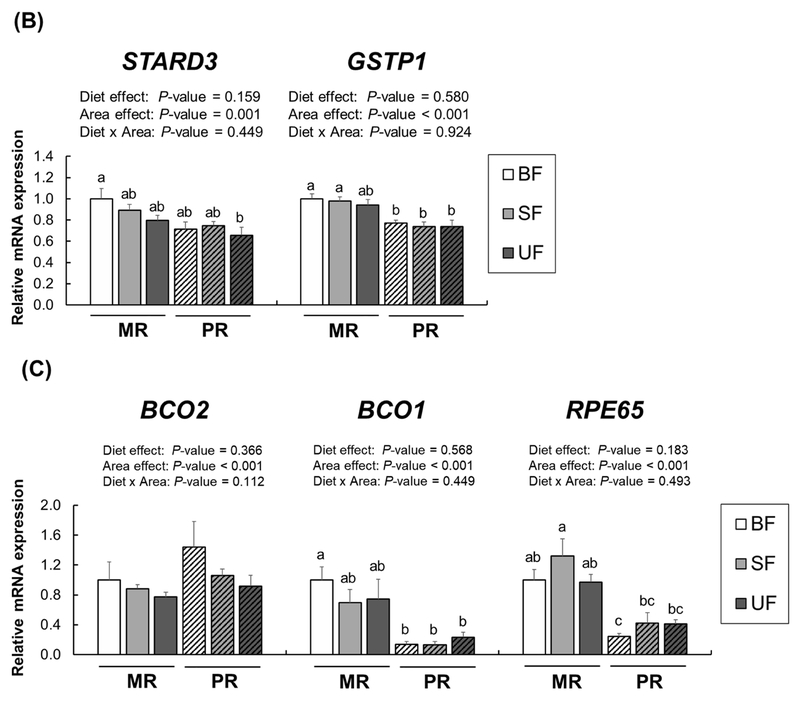
We also analyzed expression of these receptors in the macular and peripheral retina (Figure 4A). Several lipid receptors, including CD36, SR-B1, and LDLR, are also known to be localized in the retina as internal lipid transport mechanisms [25, 26]. While no significant diet × region interactions were found in mRNA expression of these receptors, expression of CD36, SCARB2, and LDLR, but not SCARBl, was higher in the MR than in the PR (CD36, SCARB2,and LDLR, P<0.001). There was a significant diet effect of SCARB2 expression in both retina regions (P=0.003). We also measured xanthophyll binding proteins and carotenoid metabolic proteins (Figure 4B). A two-way ANOVA revealed no significant effects of diet or diet × region interactions in mRNA levels of STARD3 and GSTPl; however, expression of both STARD3 and GSTPl was upregulated in the MR compared to the PR (P<0.001 for both). With no effects of diet or diet × region interactions in BCO2, BCOl, and RPE65, we again found a significant region effect (Figure 4C); BCO2 expression was higher in the PR compared to the MR whereas expression of BCOl and RPE65 showed the opposite pattern (P<0.001 for both).
Figure 4.
Relative mRNA expression for receptors (A), xanthophyll binding proteins (B), and cleavage enzymes (C) in the macular retina (MR, solid bars) and the peripheral retina (PR, striped bars) of infant rhesus monkeys either breastfed (BF, n=8), fed a carotenoid-supplemented formula (SF, n=8) or fed an unsupplemented formula (UF, n=7) for six months. Relative mRNA levels were determined by real-time RT-PCR and normalized by ACTG1. The mean value for the MR of the breastfed infant monkeys was set at 1.0. Data are expressed as means ± SEM. Diet and area effects were determined by two-way ANOVA. Labeled means for each gene without a common letter differ, P<0.05, by one-way ANOVA or Welch’s test followed by Bonferroni or Dunnett’s T3 pairwise posthoc tests.
Gene expression of receptors, binding proteins, and cleavage enzymes in two brain regions
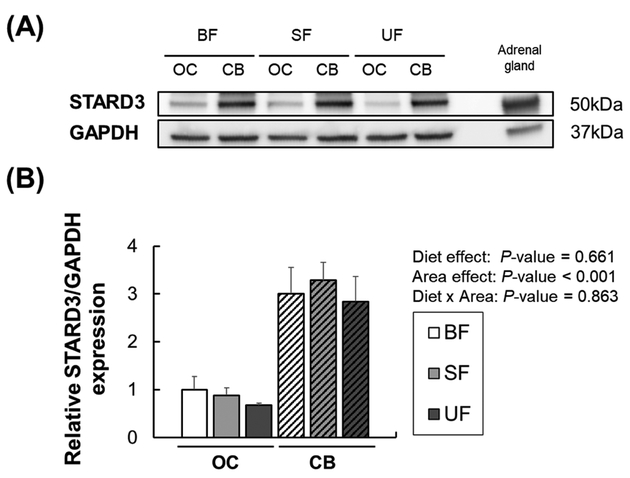
The expression of carotenoid-related genes was evaluated by RT-PCR in the OC and CB. We decided to analyze OC and CB due to their differences in lutein accumulation (OC > CB), as reported previously [21]. No significant effects of diet or diet × region interactions were found for CD36, SCARB1, and LDLR expression. However, the expression of CD36 and SCARB1 was significantly higher in the OC compared to the CB (P=0.004 and P< 0.001, respectively), whereas there was no difference in LDLR expression (Figure 5A). There was a significant diet effect of SCARB2 expression in brain regions (P<0.001); post-hoc pairwise comparisons showed that the breastfed group had significantly lower SCARB2 expression compared to the unsupplemented formula group in the CB. In addition, SCARB2 expression was significantly higher in the CB compared to the OC (P=0.004). While there were no significant effects of diet or diet × region interactions in the expression of xanthophyll binding proteins (STARD3, GSTP1), expression of both genes was significantly different between the two regions (Figure 5B). STARD3 mRNA expression was higher in the CB than in the OC (P<0.001), and this pattern was confirmed by Western blotting (P<0.001) (Figure 6). GSTP1 mRNA expression was significantly higher in the OC than in the CB (P<0.001); however, no significant differences were found in GSTP1 protein levels between the two regions (data not shown). Expression of genes related to carotenoid metabolism (BCO2, RPE65) showed no significant effects of diet or diet × region interactions (Figure 5C), but BCO2 and RPE65 expression levels were higher in the OC than in the CB (P<0.001 for both). The limited amount of retina and RPE-choroid tissue made it impossible to investigate protein expression in these tissues.
Figure 5.
Receptors (A), xanthophyll binding proteins (B), and cleavage enzymes (C) mRNA expression in the occipital cortex (OC, solid bars) and the cerebellum (CB, striped bars) of infant rhesus monkeys either breastfed (BF, n=8), fed a carotenoid-supplemented formula (SF, n=8) or fed an unsupplemented formula (UF, n=7) for six months. Relative mRNA levels were determined by real-time RT-PCR and normalized by ACTG1. The mean value for the OC of the breastfed infant monkeys was set at 1.0. Data are expressed as means ± SEM. Diet and area effects were determined by two-way ANOVA. Labeled means for each gene without a common letter differ, P<0.05, by one-way ANOVA followed by Bonferroni posthoc tests.
Figure 6.
Representative Western blot photographs (A) and relative protein levels (B) of STARD3 in occipital cortex (OC, solid bars) and cerebellum (CB, striped bars) of infant rhesus monkeys either breastfed (BF), fed a carotenoid-supplemented formula (SF) or fed an unsupplemented formula (UF) for six months. GAPDH was used as a loading control. The mean value for the OC of the breastfed infant monkeys was set at 1.0. Mouse adrenal tissue was used as a positive control. Data are expressed as means ± SEM (n=3 per dietary group in each brain region). Diet and area effects were determined by two-way ANOVA. No significant group differences were found.
4. Discussion
We previously determined that breastfeeding substantially increased lutein accumulation in various tissues of infant rhesus macaques compared to infant formula-feeding, despite closely matched concentrations of lutein in breast milk and the carotenoid-supplemented infant formula [21]. Given that serum lutein concentrations were strongly correlated with lutein concentrations in multiple tissues of infant monkeys, irrespective of diet, we assumed that intestinal absorption processes might be an important determinant of lutein uptake and tissue deposition. In a Commentary [22] that accompanied our previous paper, Dallas and Traber proposed that the higher bioavailability of lutein in breast milk might mainly result from the complex structure of the milk fat globule and milk lipases which might optimize lutein absorption compared to commercial infant formulas [27]. In addition, lipid composition differs between breast milk and infant formula, especially with respect to cholesterol and various fatty acids [28], which might contribute to differential absorption and tissue uptake of lutein [29]. With the acknowledgement that several factors intrinsic to breast milk enhance the bioavailability of lutein, the current work explored whether breast milk or infant formulas differentially impacted the mRNA levels of carotenoid-related genes and their protein products, and whether changes in expression were in part responsible for the differential lutein deposition in brain and retina. In contrast to our hypothesis, the expression of carotenoid-related genes in brain and retina, except for SCARB2 in brain regions, were not associated with different types of feeding in infant monkeys over the first 6 postnatal months.
Notably, it was demonstrated that serum total and HDL cholesterol, apoA-I, and apoB-100 levels were higher in the breastfed infants than in formula-fed infants, suggesting that more HDL and LDL particles were present in the breastfed infants compared to the formula-fed infants. The increased number of lipoproteins via breastfeeding may contribute to the delivery of more lutein into the macular retina and brain of the breastfed infants compared to formula-fed infants since: 1) HDL is the primary vehicle for lutein delivery, 2) lutein is also efficiently delivered by LDL according to in vitro studies [9, 30], and 3) both HDL and LDL levels are higher in breastfed infants compared to formula-fed infants. Enhanced HDL and LDL cholesterol levels have been observed in breastfed human infants compared to those fed formula [31–33], due to the higher presence of cholesterol in breast milk than in infant formula, with particularly high levels in the early lactation period.
Furthermore, Thomas et al. [30] showed in ARPE-19 cells that there was an inhibitory interaction between uptake of lutein and β-carotene from LDL. We found that the lutein: β-carotene ratio in serum was strongly correlated with lutein levels in macular retina as well as occipital cortex. Our findings imply that competition between circulating lutein and β-carotene may be one factor for determining lutein uptake into the macular retina and occipital cortex. In addition, in human adults, intestinal absorption of lutein and β-carotene was affected by their interaction [34].
A novel finding from this work was that various carotenoid-related genes were differentially expressed among brain and retinal regions, independent of diet. The differential gene expression between the OC and CB and between the macular and peripheral retina appears to help explain the differential lutein deposition in these areas. The relationship between the expression of several genes and xanthophyll content in brain regions was previously demonstrated by Mohn et al. [35]; that study showed that genetic origin of rhesus monkeys (Indian vs. Chinese) was related to the expression of genes involved in carotenoid and fatty acid metabolism and immune response in prefrontal cortex, cerebellum, and striatum. However, that study did not compare gene expression among the brain regions.
Notably, STARD3 mRNA expression was upregulated in the macular retina compared to the peripheral retina, which may explain the higher lutein accumulation in the macula (range: 0.405 ~ 10.4 nmol/g) compared to the periphery (range: 0 ~ 1.74 nmol/g). Unlike the retina, in the two brain regions STARD3 expression patterns (CB > OC) were opposite to lutein accumulation patterns (OC > CB). Higher STARD3 expression in the CB compared to the OC may be due to STARD3’s involvement not only in binding to lutein but in cholesterol transport [36]. In older human adults, cholesterol content is higher in the CB than in the OC [37], and in the OC cholesterol concentrations (10.7 mg/g [37]) are substantially higher than lutein concentrations (53.8 μg/g [6]). Previously, Tanprasertsuk et al. [38] showed a strong correlation between STARD3 protein expression and brain lutein concentrations in human infants. However, we did not find a significant correlation between the two variables in brain or retina in this intervention study.
In conclusion, contrary to our hypothesis, infant monkeys fed breast milk or infant formulas with low or high levels of carotenoids did not show differential expression of genes involved in carotenoid binding, transport and metabolism, in the retina or brain, with the exception of SCARB2. Given that breastfed infant monkeys had higher total and HDL cholesterol, apoA-I and apoB-100 levels in serum than formula-fed infant monkeys, combined with higher lutein concentrations in HDL and non-HDL fractions, suggests that lipoprotein levels may be one determinant of the higher lutein accumulation in the retina and other tissues during infancy. Differential bioavailability of carotenoids between breast milk and formula is apparently the primary driver for enhanced lutein tissue accumulation of breast-fed compared to formula-fed monkeys. However, the differential expression of carotenoid-related genes among retina and brain regions may explain some of the differences in lutein accumulation in those areas.
Highlights.
Lipoprotein levels might be critical for delivering lutein to retina and other tissues during infancy.
Dietary regimen does not impact the expression of genes involved in carotenoid transport, binding, and metabolism, except for SCARB2, in the brain and retina of infant monkeys.
Carotenoid-related genes are differentially expressed across brain and retina regions of infant monkeys.
5. Acknowledgement
We appreciate Emily Mohn’s generosity in providing the protocol for HDL isolation and carotenoid extraction. This work was supported by Abbott Nutrition through the Center for Nutrition, Learning, and Memory (CNLM) at the University of Illinois at Urbana-Champaign, and by NIH grant P51OD011092.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: This work was funded by Abbott Nutrition and MJK is employed by Abbott Nutrition. SJ, MN, and JWE have no COI.
References
- [1].Mares J, Lutein and zeaxanthin isomers in eye health and disease, Annual Review of Nutrition 36 (2016) 571–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Seddon JM, Ajani UA, Sperduto RD, Hiller R, Blair N, Burton TC, Farber MD, Gragoudas ES, Haller J, Miller DT, et al. , Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration. Eye Disease Case-Control Study Group, JAMA 272(18) (1994) 1413–20. [PubMed] [Google Scholar]
- [3].G. Age-Related Eye Disease Study Research, SanGiovanni JP, Chew EY, Clemons TE, Ferris FL 3rd, Gensler G, Lindblad AS, Milton RC, Seddon JM, Sperduto RD, The relationship of dietary carotenoid and vitamin A, E, and C intake with age-related macular degeneration in a case-control study: AREDS Report No. 22, Arch Ophthalmol 125(9) (2007) 1225–32. [DOI] [PubMed] [Google Scholar]
- [4].Craft NE, Haitema TB, Garnett KM, Fitch KA, Dorey CK, Carotenoid, tocopherol, and retinol concentrations in elderly human brain, J Nutr Health Aging 8(3) (2004) 156–62. [PubMed] [Google Scholar]
- [5].Vishwanathan R, Kuchan MJ, Sen S, Johnson EJ, Lutein and preterm infants with decreased concentrations of brain carotenoids, J Pediatr Gastroenterol Nutr 59(5) (2014) 659–65. [DOI] [PubMed] [Google Scholar]
- [6].Johnson EJ, Vishwanathan R, Johnson MA, Hausman DB, Davey A, Scott TM, Green RC, Miller LS, Gearing M, Woodard J, Nelson PT, Chung HY, Schalch W, Wittwer J, Poon LW, Relationship between serum and brain carotenoids, alpha-tocopherol, and retinol concentrations and cognitive performance in the oldest old from the Georgia Centenarian Study, J Aging Res 2013 (2013) 951786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Mohn ES, Johnson EJ, Lutein and cognition across the lifespan, Nutrition Today 52(4) (2017) 183–189. [Google Scholar]
- [8].Wang W, Connor SL, Johnson EJ, Klein ML, Hughes S, Connor WE, Effect of dietary lutein and zeaxanthin on plasma carotenoids and their transport in lipoproteins in age-related macular degeneration, Am J Clin Nutr 85(3) (2007) 762–9. [DOI] [PubMed] [Google Scholar]
- [9].Shyam R, Vachali P, Gorusupudi A, Nelson K, Bernstein PS, All three human scavenger receptor class B proteins can bind and transport all three macular xanthophyll carotenoids, Archives of Biochemistry and Biophysics 634 (2017) 21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Li B, Vachali P, Frederick JM, Bernstein PS, Identification of StARD3 as a lutein-binding protein in the macula of the primate retina, Biochemistry 50(13) (2011) 2541–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bhosale P, Larson AJ, Frederick JM, Southwick K, Thulin CD, Bernstein PS, Identification and characterization of a Pi isoform of glutathione S-transferase (GSTP1) as a zeaxanthin-binding protein in the macula of the human eye, J Biol Chem 279(47) (2004) 4944754. [DOI] [PubMed] [Google Scholar]
- [12].dela Seña C, Narayanasamy S, Riedl KM, Curley RW, Schwartz SJ, Harrison EH, Substrate specificity of purified recombinant human β-carotene 15,15’-oxygenase (BCO1), Journal of Biological Chemistry 288(52) (2013) 37094–37103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hu K-Q, Liu C, Ernst H, Krinsky NI, Russell RM, Wang X-D, The Biochemical Characterization of ferret carotene-9’,10’-monooxygenase catalyzing cleavage of carotenoids in vitro and in vivo, Journal of Biological Chemistry 281(28) (2006) 19327–19338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].dela Seña C, Sun J, Narayanasamy S, Riedl KM, Yuan Y, Curley RW, Schwartz SJ, Harrison EH, Substrate specificity of purified recombinant chicken β-carotene 9’,10’-oxygenase (BCO2), Journal of Biological Chemistry 291(28) (2016) 14609–14619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Li B, Vachali PP, Gorusupudi A, Shen Z, Sharifzadeh H, Besch BM, Nelson K, Horvath MM, Frederick JM, Baehr W, Bernstein PS, Inactivity of human β,β-carotene-9’,10’-dioxygenase (BCO2) underlies retinal accumulation of the human macular carotenoid pigment, Proceedings of the National Academy of Sciences 111(28) (2014) 10173–10178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Babino D, Palczewski G, Widjaja-Adhi MAK, Kiser PD, Golczak M, von Lintig J, Characterization of the role of β-carotene 9,10-dioxygenase in macular pigment metabolism, Journal of Biological Chemistry 290(41) (2015) 24844–24857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Shyam R, Gorusupudi A, Nelson K, Horvath MP, Bernstein PS, RPE65 has an additional function as the lutein to meso-zeaxanthin isomerase in the vertebrate eye, Proceedings of the National Academy of Sciences 114(41) (2017) 10882–10887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bohn T, Desmarchelier C, Dragsted LO, Nielsen CS, Stahl W,Ruhl R, Keijer J, Borel P, Host-related factors explaining interindividual variability of carotenoid bioavailability and tissue concentrations in humans, Mol Nutr Food Res 61(6) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Vishwanathan R, Neuringer M, Snodderly DM, Schalch W, Johnson EJ, Macular lutein and zeaxanthin are related to brain lutein and zeaxanthin in primates, Nutr Neurosci 16(1) (2013) 21–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Vishwanathan R, Schalch W, Johnson EJ, Macular pigment carotenoids in the retina and occipital cortex are related in humans, Nutritional Neuroscience 19(3) (2016) 95–101. [DOI] [PubMed] [Google Scholar]
- [21].Jeon S, Ranard KM, Neuringer M, Johnson EE, Renner L, Kuchan MJ, Pereira SL, Johnson EJ, Erdman JW Jr., Lutein is differentially deposited across brain regions following formula or breast feeding of infant rhesus macaques, The Journal of Nutrition 148(1) (2018) 3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Dallas DC, Traber MG, How does breast milk enhance lutein absorption?, The Journal of Nutrition 148(1) (2018) 1–2. [DOI] [PubMed] [Google Scholar]
- [23].Yeum KJ, Booth SL, Sadowski JA, Liu C, Tang G, Krinsky NI, Russell RM, Human plasma carotenoid response to the ingestion of controlled diets high in fruits and vegetables, Am J Clin Nutr 64(4) (1996) 594–602. [DOI] [PubMed] [Google Scholar]
- [24].Duncan KG, Bailey KR, Kane JP, Schwartz DM, Human retinal pigment epithelial cells express scavenger receptors BI and BII, Biochem Biophys Res Commun 292(4) (2002) 1017–22. [DOI] [PubMed] [Google Scholar]
- [25].Tserentsoodol N, Gordiyenko NV, Pascual I, Lee JW, Fliesler SJ, Rodriguez IR, Intraretinal lipid transport is dependent on high density lipoprotein-like particles and class B scavenger receptors, Mol Vis 12 (2006) 1319–33. [PubMed] [Google Scholar]
- [26].Tserentsoodol N, Sztein J, Campos M, Gordiyenko NV, Fariss RN, Lee JW, Fliesler SJ, Rodriguez IR, Uptake of cholesterol by the retina occurs primarily via a low density lipoprotein receptor-mediated process, Mol Vis 12 (2006) 1306–18. [PubMed] [Google Scholar]
- [27].Singh H, Gallier S, Nature’s complex emulsion: The fat globules of milk, Food Hydrocolloids 68 (2017) 81–89. [Google Scholar]
- [28].Katoku Y, Yamada M, Yonekubo A, Kuwata T, Kobayashi A, Sawa A, Effect of the cholesterol content of a formula on the lipid compositions of plasma lipoproteins and red blood cell membranes in early infancy, Am J Clin Nutr 64(6) (1996) 871–7. [DOI] [PubMed] [Google Scholar]
- [29].Marriage BJ, Williams JA, Choe YS, Maki KC, Vurma M, DeMichele SJ, Mono and diglycerides improve lutein absorption in healthy adults: a randomised, double-blind, cross over, single-dose study, Br J Nutr 118(10) (2017) 813–821. [DOI] [PubMed] [Google Scholar]
- [30].Thomas SE, Harrison EH, Mechanisms of selective delivery of xanthophylls to retinal pigment epithelial cells by human lipoproteins, Journal of Lipid Research 57(10) (2016) 18651878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Wong WW, Hachey DL, Insull W, Opekun AR, Klein PD, Effect of dietary cholesterol on cholesterol synthesis in breast-fed and formula-fed infants, J Lipid Res 34(8) (1993) 1403–11. [PubMed] [Google Scholar]
- [32].Bianchi C, Brambilla P, Cella D, Ragogna F, Tettamanti C, Del Puppo M, Kienle MG, Chiumello G, Ruotolo G, Influence of breast- and formula-feeding on plasma cholesterol precursor sterols throughout the first year of life, J Pediatr 131(6) (1997) 928–31. [DOI] [PubMed] [Google Scholar]
- [33].Isomura H, Takimoto H, Miura F, Kitazawa S, Takeuchi T, Itabashi K, Kato N, Type of milk feeding affects hematological parameters and serum lipid profile in Japanese infants, Pediatr Int 53(6) (2011) 807–13. [DOI] [PubMed] [Google Scholar]
- [34].Kostic D, White WS, Olson JA, Intestinal absorption, serum clearance, and interactions between lutein and beta-carotene when administered to human adults in separate or combined oral doses, Am J Clin Nutr 62(3) (1995) 604–10. [DOI] [PubMed] [Google Scholar]
- [35].Mohn ES, Erdman JW Jr., Neuringer M, Kuchan MJ, Johnson EJ, Brain xanthophyll content and exploratory gene expression analysis: subspecies differences in rhesus macaque, Genes Nutr 12 (2017) 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Rigotti A, Cohen DE, Zanlungo S, STARTing to understand MLN64 function in cholesterol transport, Journal of Lipid Research 51(8) (2010) 2015–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].SoOderberg M, Edlund C, Alafuzoff I, Kristensson K, Dallner G, Lipid composition in different regions of the brain in Alzheimer’s disease/senile dementia of Alzheimer’s type, Journal of Neurochemistry 59(5) (1992) 1646–1653. [DOI] [PubMed] [Google Scholar]
- [38].Tanprasertsuk J, Li B, Bernstein PS, Vishwanathan R, Johnson MA, Poon L, Johnson EJ, Relationship between concentrations of lutein and StARD3 among pediatric and geriatric human brain tissue, PLoS One 11(5) (2016) e0155488. [DOI] [PMC free article] [PubMed] [Google Scholar]