Abstract
Purpose
Fluorouracil plus cisplatin and radiation twice a day (FCT) is an established chemoradiation (CRT) regimen for selective bladder-sparing treatment of muscle-invasive bladder cancer. Gemcitabine and once daily radiation (GD) is a well-supported alternative. The current trial evaluates these regimens.
Methods
Patients with cT2-4a muscle-invasive bladder cancer were randomly assigned to FCT or GD. Patients underwent transurethral resection and induction CRT to 40 Gy. Patients who achieved a complete response (CR) received consolidation CRT to 64 Gy and others underwent cystectomy. We administered adjuvant gemcitabine/cisplatin chemotherapy. The primary end point was the rate of freedom from distant metastasis at 3 years (DMF3). The trial was not statistically powered to compare regimens, but to assess whether either regimen exceeded a DMF3 benchmark of 75%. Toxicity and efficacy end points, including CR and bladder-intact distant metastasis free survival at 3 years (BI-DMFS3), were assessed.
Results
From December 2008 to April 2014, 70 patients were enrolled, of which 66 were eligible for analysis, 33 per arm. Median follow-up was 5.1 years (range, 0.4 to 7.8 years) for eligible living patients. DMF3 was 78% and 84% for FCT and GD, respectively. BI-DMFS3 was 67% and 72%, respectively. Postinduction CR rates were 88% and 78%, respectively. Of 33 patients in the FCT arm, 21 (64%) experienced treatment-related grade 3 and 4 toxicities during protocol treatment, with 18 (55%), two (6%), and two patients (6%) experiencing grade 3 and 4 hematologic, GI, and genitourinary toxicity, respectively. For the 33 patients in the GD arm, these figures were 18 (55%) overall and 14 (42%), three (9%) and two patients (6%), respectively.
Conclusion
Both regimens demonstrated DMF3 greater than 75%. There were fewer toxicities observed in the GD arm. Either gemcitabine and once daily radiation or a cisplatin-based regimen could serve as a base for future trials of systemic therapy.
INTRODUCTION
Selective bladder preservation using trimodality therapy is an established treatment of muscle-invasive bladder cancer (MIBC) with outcomes that are comparable to those of radical cystectomy.1-4 This treatment paradigm has been studied and refined in a succession of Radiation Therapy Oncology Group (RTOG) clinical trials, which demonstrated high rates of complete response (CR), bladder preservation in the majority of patients, and survival rates comparable to those of radical cystectomy.1 This approach is now recognized in the American Urological Association, European Association of Urology, and National Comprehensive Cancer Network guidelines.5-7
Contemporary RTOG trials have incorporated cisplatin-based concurrent chemotherapy with twice-a-day radiation and adjuvant chemotherapy. A regimen that incorporated fluorouracil plus cisplatin as radiosensitizing chemotherapy demonstrated a high response rate and acceptable toxicity in the phase I and II trial RTOG 95-06 (ClinicalTrials.gov identifier: NCT00002791).8 A subsequent randomized phase II trial, RTOG 0233 (ClinicalTrials.gov identifier: NCT00055601) demonstrated the response rate and rate of distant metastasis that were similar to those of a regimen that combined cisplatin and paclitaxel, with lower rates of toxicity.9 This regimen has come to represent the RTOG standard.
Radiosensitization with cisplatin is not ideal for all patients, particularly for those with impaired renal function or hearing deficits. Clinical trials investigating cisplatin-free regimens have been completed.10-14 A phase I trial at the University of Michigan established the safety of a regimen that incorporated concurrent twice-a-week low-dose gemcitabine and once daily radiation.11 The maximum tolerated dose of gemcitabine was 27 mg/m2. Response rate was high, and bladder preservation and survival rates were comparable to those observed in existing series. This regimen seemed to be an attractive alternative.
RTOG 0712 (ClinicalTrials.gov identifier: NCT00777491) was designed as a randomized phase II trial in which patients received fluorouracil plus cisplatin with radiation twice a day (FCT) or gemcitabine plus once daily radiation (GD), and the primary end point was freedom from distant metastasis at 3 years (DMF3).
METHODS
Patients
Patients were at least 18 years of age with pathologically confirmed American Joint Committee on Cancer clinical stage T2-4a, Nx or N0, or M0 bladder cancer. All had Zubrod performance status scores of 0 or 1, were candidates for surgery and systemic therapy combined with pelvic radiation, and were free of tumor-related hydronephrosis. Patients with involvement of the prostatic urethra that was completely resected with no stromal invasion were eligible. Radiographically suggestive lymph nodes required pathologic evaluation; patients with confirmed lymph node metastasis were not eligible. Eligible patients had adequate bladder function. Patients had a serum creatinine of 1.5 mg/dL or less or otherwise demonstrated adequate renal function. Additional exclusion criteria were prior chemotherapy, pelvic radiation, concurrent use of nephrotoxic or ototoxic drugs, prior allergic reaction to a study drug, or a severe active comorbidity. The protocol was approved by the institutional review board at each site, and patients signed a study-specific informed consent form.
Study Design
This randomized phase II trial was conducted at 10 centers in the United States and Canada. Patients were stratified by clinical T stage (T2 v T3-4a); using the treatment allocation scheme described by Zelen,15 patients were randomly assigned in a 1:1 ratio to FCT or GD as the chemoradiation component of a selective bladder preservation regimen. Treatment was not blinded.
Treatment
Patients were treated with selective bladder preservation using trimodality therapy. A maximally safe transurethral resection of bladder tumor (TURBT) was performed. After TURBT, patients were stratified and randomly assigned. Induction chemoradiation began within 8 weeks of TURBT and consisted of a radiation dose of 40 Gy with concurrent chemotherapy. After induction, patients underwent a cystoscopic assessment with tumor site biopsy. Those who achieved CR continued on to consolidation chemoradiation, receiving an additional 24 Gy. Others were referred for prompt cystectomy. Adjuvant chemotherapy that consisted of gemcitabine plus cisplatin was offered to all patients after completion of local therapy.
Chemoradiation
FCT.
Induction therapy consisted of 13 days of concomitant boost radiation with 1.6 Gy delivered to a pelvic volume every morning. In the afternoon, 1.5 Gy was delivered to the bladder for the first 5 days and 1.5 Gy to the bladder tumor for the final 8 days. The minimal interval between treatments was 4 hours. Total induction doses were 20.8 Gy to the pelvis, 28.3 Gy to the bladder, and 40.3 Gy to the bladder tumor. Chemotherapy doses were fluorouracil 400 mg/m2 on days 1 to 3 and 15 to 17 and cisplatin 15 mg/m2 on days 1 to 3, 8 to 10, and 15 to 17 during induction therapy.
Consolidation therapy consisted of 1.5 Gy delivered to a pelvic volume twice a day for 8 days for a total of 24 Gy. The resulting total radiation doses were 44.8 Gy to the pelvis, 52.3 Gy to the bladder, and 64.3 Gy to the bladder tumor. Chemotherapy doses were fluorouracil 400 mg/m2 on days 1 to 3 and 8 to 10 and cisplatin 15 mg/m2 on days 1, 2, 8, and 9 during consolidation therapy.
GD.
Induction therapy consisted of once daily radiation for 20 days with 2 Gy per day delivered to a pelvic volume for the first 10 days, followed by 2 Gy delivered to the bladder for 4 days and 2 Gy to the bladder tumor for the final 6 days. Total induction doses were 20 Gy to the pelvis, 28 Gy to the bladder, and 40 Gy to the bladder tumor. Chemotherapy consisted of gemcitabine 27 mg/m2 delivered twice a week on days 1, 4, 8, 11, 15, 18, 22, and 25 during induction therapy.
Consolidation therapy consisted of 2 Gy per day delivered to a pelvic volume for 12 days for a total of 24 Gy. The resulting total radiation doses were 44 Gy to the pelvis, 52 Gy to the bladder, and 64 Gy to the bladder tumor. Chemotherapy consisted of gemcitabine 27 mg/m2 delivered twice a week on days 1, 4, 8, 11, and 15 during consolidation therapy.
Adjuvant chemotherapy.
Adjuvant chemotherapy began 12 weeks after consolidation chemoradiation or 8 weeks after radical cystectomy. It consisted of gemcitabine 1,000 mg/m2 on days 1 and 8 and cisplatin 70 mg/m2 on day 8, repeated every 21 days for four cycles. If cisplatin was not tolerated, paclitaxel 150 mg/m2 was allowed as a substitution.
Assessments
At baseline, all patients underwent physical examination; hematologic and biochemical analyses; assessment of bladder function; computed tomography of the chest, abdomen, and pelvis; bone scan; and examination under anesthesia.
Tumor Control
Patients with conserved bladders were observed with cystoscopy; tumor site rebiopsy; examination under anesthesia; urine cytology; and chest, abdomen, and pelvis computed tomography scans every 3 months in the first year, every 4 months the second year, every 6 months for the next 3 years, and annually thereafter.
Adverse Events
Adverse events were graded using the Common Terminology Criteria for Adverse Events, version 3.0.
Statistical Methodology and Analysis
The primary end point was the rate of DMF3 after random assignment. Sample size was calculated using the Clopper-Pearson method with a one-sided significance level of .1.16 Assuming the lower boundary of the 90% CI of the DMF3 was 0.75, with a sample size of 32 patients per arm, there would be a 10% chance of observing a DMF3 of less than 75% if the true rate was 86%. Patients who did not receive any protocol treatment or who had fewer than 3 years of follow-up without an event were not evaluable. If either arm had a rate of DMF3 of at least 75%, it would be considered promising for inclusion in a phase III trial. The study was not designed to compare treatment arms.
Secondary end points included efficacy measures, such as CR rate and bladder-intact distant metastasis free survival rate at 3 years (BI-DMFS3). BI-DMFS was defined as the time since random assignment to the development of distant metastasis, undergoing cystectomy, or death from any cause, whichever came first. Patients who were free of any of these events with fewer than 3 years of follow-up were not evaluable. We also used the Kaplan-Meier method to estimate the BI-DMFS rate over time for each arm. Protocol treatment completion and toxicities were assessed. Completion rate for a specified phase of treatment was defined as the proportion of eligible patients who completed that phase per protocol. Toxicities that occurred during treatment and those that occurred after treatment but within 180 days were summarized separately. Logistic regression was used to model the occurrence of treatment-related grade 3 or greater events during treatment. Time to the first treatment-related grade 3 or greater event after treatment was analyzed using the competing risk approach,17 with death as a competing risk.
RESULTS
Patients
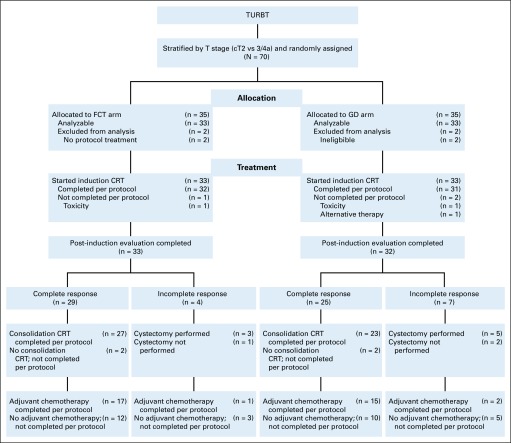
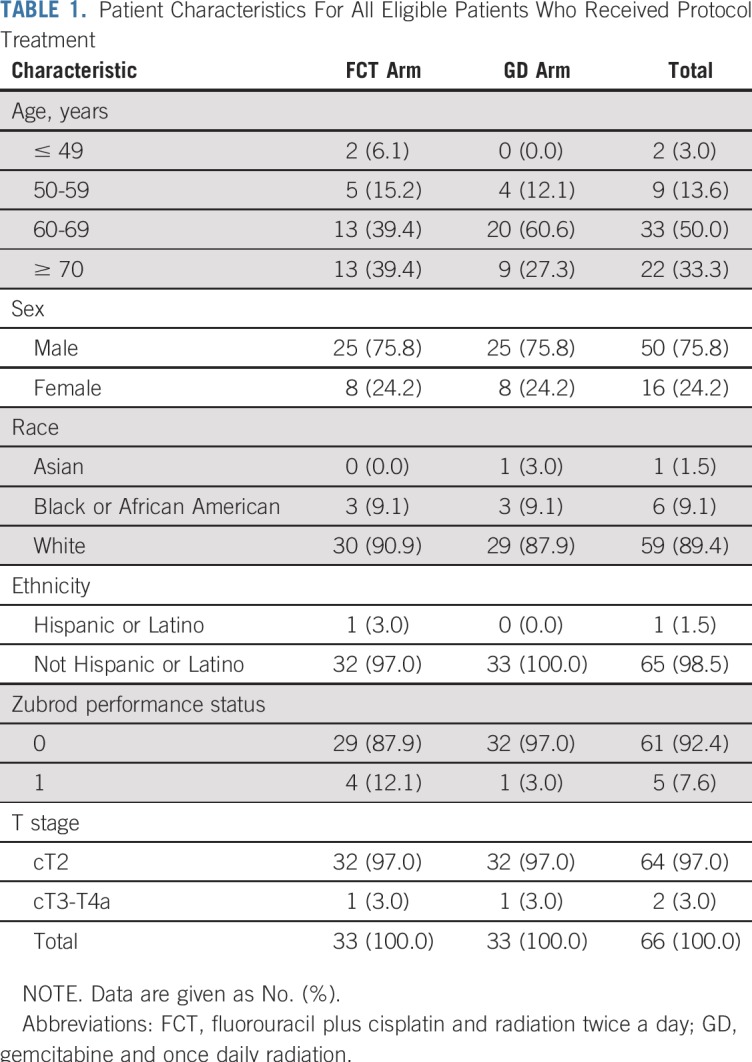
From December 2008 through February 2014, 70 patients were registered and randomly assigned, 35 to each treatment arm (Fig 1). Two patients in each arm were either ineligible or did not receive any protocol treatment and were excluded from subsequent analyses, which left 66 eligible patients, 33 per treatment arm. Baseline characteristics were balanced between the two arms (Table 1). Analyses were based on all data reported through May 2017. Median follow-up was 4.3 years (range, 0.4 to 7.8 years) for all 66 eligible patients and 5.1 years (range, 0.4 to 7.8 years) for the 51 patients alive at the time of reporting.
FIG 1.
CONSORT 2010 diagram. CRT, chemoradiation; FCT, fluorouracil plus cisplatin and radiation twice a day; GD, gemcitabine and once daily radiation; TURBT, transurethral resection of bladder tumor.
TABLE 1.
Patient Characteristics For All Eligible Patients Who Received Protocol Treatment
Primary Outcome
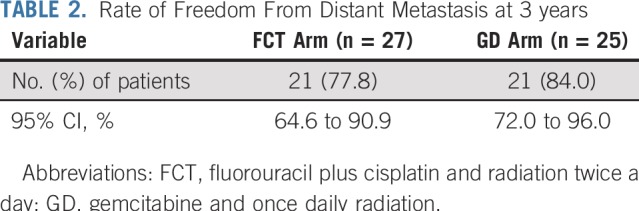
Twenty-seven (82%) and 25 (76%) patients in the FCT and GD arms, respectively, were evaluable for the primary end point. Reasons for exclusion included inadequate follow-up (n = 2), consent withdrawal within 3 years (n = 7), and death (n = 5) before 3 years without distant metastasis. With the observed numbers of evaluable patients, there was a 13% to 14% chance of observing a DMF3 rate of less than 75% if the true rate was 86%. In the FCT arm, the DMF3 rate was 78% (21 of 27 evaluable patients), with a 95% CI of 65% to 91%. In the GD arm, the rate was 84% (21 of 25 evaluable patients), with a 95% CI of 72% to 96% (Table 2). The difference was not significant on post hoc analysis (P = .73, Fisher’s exact test). Both arms met the 75% benchmark defined in the trial design.
TABLE 2.
Rate of Freedom From Distant Metastasis at 3 years
Treatment Completion
In the FCT arm, 32 (97%) of 33 eligible patients completed induction therapy, 27 (93%) of 29 complete responders completed consolidation therapy, and 18 (56%) of 32 completed adjuvant chemotherapy. In the GD arm, 31 (94%) of 33 eligible patients completed induction therapy, 23 (92%) of 25 complete responders completed consolidation therapy, and 17 (55%) of 31 completed adjuvant chemotherapy. Prompt cystectomy was performed in three of four non–complete responders in the FCT arm and five of seven in the GD arm.
Adverse Events
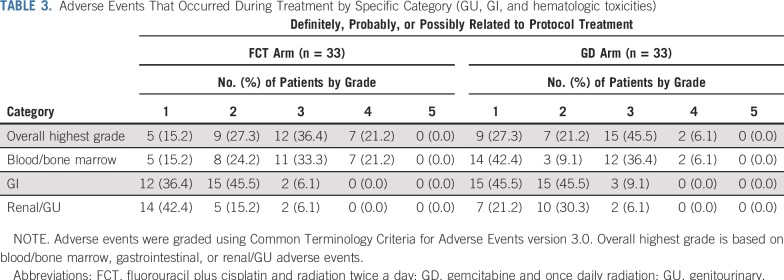
Of 33 patients in the FCT arm, 21 (64%) experienced treatment-related grade 3 and 4 toxicities during protocol treatment, and 18 (55%), two (6%), and two patients (6%) experienced hematologic, GI, and genitourinary toxicities, respectively (Table 3). Treatment-related grade 3 and 4 toxicities occurred during induction chemoradiation, consolidation chemoradiation, and adjuvant chemotherapy in six (18%) of 33 evaluable patients, six (21%) of 29, and 18 (67%) of 27, respectively. There was one death likely related to protocol treatment in the FCT arm after adjuvant chemotherapy as a result of an intracranial hemorrhage. For 33 patients in the GD arm, 18 (55%) experienced treatment-related grade 3 and 4 toxicities during protocol treatment, and 14 (42%), three (9%), and two patients (6%) experienced hematologic, GI, and genitourinary toxicities, respectively (Table 3). Treatment-related grade 3 and 4 toxicities occurred during induction chemoradiation, consolidation chemoradiation, and adjuvant chemotherapy in 11 (33%) of 33 evaluable patients, seven (29%) of 24, and 13 (59%) of 22, respectively. In the immediate interval after the end of protocol treatment, eight (25%) of 32 patients in the FCT arm and five (16%) of 31 in the GD arm experienced treatment-related grade 3 and 4 hematologic, GI, or genitourinary toxicities. There was no significant difference in the occurrence of treatment-related grade 3 or greater toxicity during protocol treatment between arms, with an odds ratio of 0.68 in favor of the GD arm (P = .45). There was also no significant difference in the first occurrence of treatment-related grade 3 or greater toxicity after the end of treatment, with a hazard ratio of 0.81 in favor of the GD arm (P = .64).
TABLE 3.
Adverse Events That Occurred During Treatment by Specific Category (GU, GI, and hematologic toxicities)
Secondary Outcomes
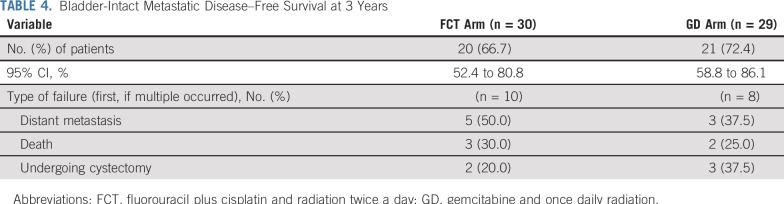
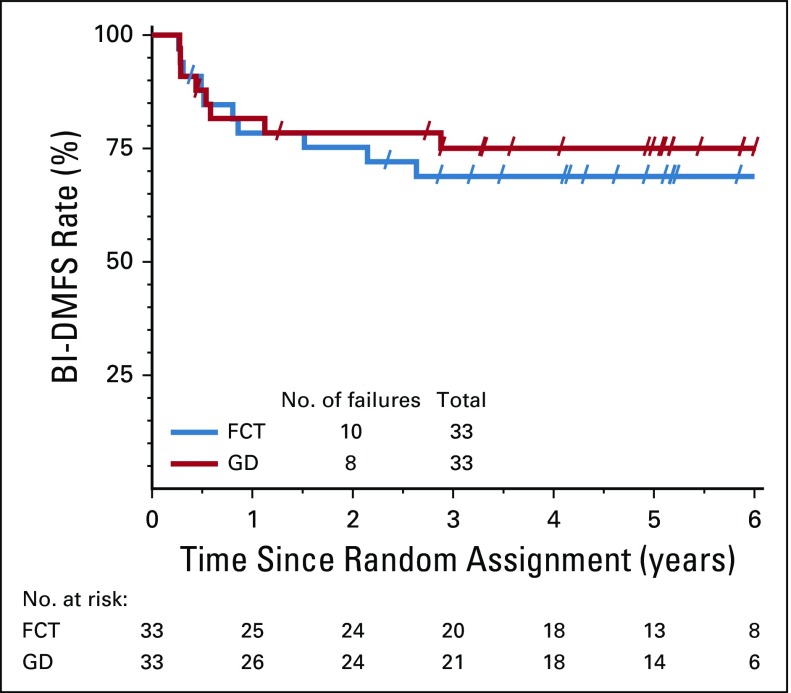
The CR rate to induction chemoradiation was 88% (29 of 33 evaluable patients) for the FCT arm and 78% (25 of 32 evaluable patients) for the GD arm. The BI-DMFS3 rate was 67% (20 of 30 evaluable patients; 95% CI, 52% to 81%) for the FCT arm and 72% (21 of 29 evaluable patients; 95% CI, 59% to 86%) for the GD arm (Table 4). Of the 10 patients with a documented experience of failure in the FCT arm, five developed distant metastases, three died, and two underwent cystectomy as the first type of failure. Of the eight patients who experienced failure in the GD arm, the results were three, two, and three patients, respectively. The Kaplan-Meier curves for BI-DMFS are shown in Figure 2. The hazard ratio of 0.79 (95% CI, 0.31 to 2.00) favored the GD arm, but was not significant (P = .62, log-rank test).
TABLE 4.
Bladder-Intact Metastatic Disease–Free Survival at 3 Years
FIG 2.
Bladder-intact distant metastasis–free survival (BI-DMFS). FCT, fluorouracil plus cisplatin and radiation twice a day; GD, gemcitabine and once daily radiation.
Overall, by 3 years 11 patients died (six in the FCT arm and five in the GD arm). There were eight cystectomies performed (three in the FCT arm and five in the GD arm) all performed for less than a CR to induction therapy.
DISCUSSION
The primary end point in this randomized phase II trial was freedom from distant metastasis at 3 years. This end point was chosen because distant metastasis is the primary mode of failure for patients with bladder cancer and generally precedes a bladder cancer–related death. Both treatment arms met the 75% benchmark.
Both regimens were well tolerated and the majority of grade 3 and 4 events were hematologic, with only a handful of GI or genitourinary events, which were equally distributed between the arms. We observed no excess GI toxicity with the gemcitabine regimen, even with the inclusion of pelvic fields that were not included in the University of Michigan predecessor trial11,12.
CR rates to induction chemoradiation were 88 and 78% for the FCT and GD arms, respectively. These rates compare favorably with prior RTOG trials and long-term single-institutional series for which rates ranging from 69% to 72% were reported.1-3 The University of Michigan achieved a CR rate of 91% for patients who received a similar regimen using gemcitabine and once daily radiation for T2 bladder cancers without a break such that response was assessed after the completion of the entire course of chemoradiation.11,12 Regardless, the rates of CR in RTOG 0712 seem to be favorable.
Rates of BI-DMFS3 were 67% and 72% for the FCT and GD arms, respectively. These results compare favorably with similar composite end points in pooled analysis of RTOG trials and single-institution series.1,3 Still, continued follow-up will be required as these series reported 10-year outcomes with distant metastasis rates and disease-specific survival of 32% to 35% and 42% to 65%, respectively.1-3 Among long-term survivors, 80% retained their native bladder.
Many patients with bladder cancer are elderly or have comorbidities, which limits their ability to receive aggressive treatment. Analysis of treatment patterns in the National Cancer Database demonstrated that 52.5% of patients received aggressive therapy and only 7.6% received definitive chemoradiation or radiation alone.18 Aggressive therapy was less common with advancing age. Turgeon et al19 reported high rates of CR and bladder preservation with low toxicity rates in a series of elderly patients who were treated with hypofractionated intensity-modulated radiation therapy and once weekly chemotherapy. Continued efforts to develop better-tolerated and more convenient therapy are warranted.
Many patients with bladder cancer are not ideal candidates for cisplatin, which prompted investigation of alternate regimens. The Institute of Cancer Research UK Bladder Cancer 2001 (BC2001) trial was a phase III trial that evaluated the efficacy of chemoradiation with fluorouracil and mitomycin compared with radiation alone for patients with MIBC.10 There was a significant improvement in locoregional control with no increase in adverse events for patients who received chemoradiation. These results established fluorouracil and mitomycin as a viable alternative to cisplatin-based regimens. Other groups, including the University of Michigan, investigated the use of gemcitabine as a radiosensitizer. Caffo et al13 and Sangar et al14 combined once weekly gemcitabine with radiation and reported CR rates of 100% and 80%, respectively, with bladder-intact survival rates of 75% and 88%, respectively, almost 2 years since treatment. Choudhury et al20 reported high response rates and durable local control in a phase II trial. The University of Michigan regimen demonstrated a CR rate of 91%. At 5 years, bladder-intact survival, overall survival, and disease-specific survival rates were 62%, 76%, and 82%, respectively.11 This regimen formed the basis of our GD arm.
RTOG protocols have typically incorporated twice-a-day radiation with a mid-treatment break with cystoscopic response assessment. Whether acceleration influences efficacy or acute toxicity is uncertain. One randomly assigned trial that compared once-per-day and twice-per-day radiation for patients who received radiation alone for MIBC revealed no improvement in efficacy for the accelerated fractionation arm, but did demonstrate an increase in acute bowel reactions.21 Unlike the current study, no concurrent chemotherapy or mid-treatment break was included. In our study, as the two arms had differing chemotherapy regimens, no definitive comment can be made on the toxicity of once-per-day versus twice-a-day radiation; however, efficacy and toxicity observed in the GD arm were favorable. The daily radiation schedule is more convenient for patients and better suited to routine clinical practice.
Successor multigroup bladder preservation trials have turned their focus toward incorporating adjuvant systemic agents into the treatment regimen instead of refining the chemoradiation component. SWOG/NRG 1806 is a phase III trial in development which will study whether the addition of atezolizumab, an immune checkpoint inhibitor, will improve outcomes (J. Efstathiou, personal communication, August 2018). This trial will incorporate once daily radiation without a mid-treatment assessment and allow several concurrent chemotherapy regimens, including once weekly low-dose gemcitabine as per our trial, once weekly cisplatin, or fluorouracil and mitomycin. NRG/RTOG 0712 provides reassurance that these two regimens can create a comparable bladder-sparing foundation upon which to add and test novel systemic therapies.
In conclusion, our study demonstrates that both FCT and GD represent favorable regimens with DMF3 rates of 78% and 84%, respectively. These regimens were well tolerated and had high CR and bladder preservation rates. Both could be considered for additional study. Concurrent low-dose gemcitabine is a reasonable alternative to a cisplatin-based regimen. Once-per-day-radiation is a reasonable alternative to accelerated twice-a-day radiation, which may allow for the wider adoption of bladder preservation.
Footnotes
Presented at the 2018 Genitourinary Cancers Symposium, San Francisco, CA, February 8-10, 2018, and the 2017 American Society for Radiation Oncology Annual Meeting, San Diego, CA, September 24-27, 2017.
Supported by Grants No. U10-CA180868 (NRG Oncology Operations) and U10-CA180822 (NRG Oncology Statistics and Data Management Center) from the National Cancer Institute.
AUTHOR CONTRIBUTIONS
Conception and design: John J. Coen, Cheryl T. Lee, Chin-Lee Wu, William Parker, Anthony L. Zietman, Howard M. Sandler, William U. Shipley
Provision of study materials or patients: John J. Coen, Chin-Lee Wu, Jason A. Efstathiou, Omer Kucuk, William U. Shipley
Collection and assembly of data: John J. Coen, Peixin Zhang, Philip J. Saylor, Chin-Lee Wu, Anthony L. Zietman, Ashesh B. Jani
Data analysis and interpretation: John J. Coen, Peixin Zhang, Philip J. Saylor, Cheryl T. Lee, Chin-Lee Wu, Timothy Lautenschlaeger, Anthony L. Zietman, Jason A. Efstathiou, Ashesh B. Jani, Omer Kucuk, Luis Souhami, Joseph P. Rodgers, Howard M. Sandler, William U. Shipley
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Bladder Preservation With Twice-a-Day Radiation Plus Fluorouracil/Cisplatin or Once Daily Radiation Plus Gemcitabine for Muscle-Invasive Bladder Cancer: NRG/RTOG 0712—A Randomized Phase II Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Cheryl T. Lee
Other Relationship: Merck
Anthony T. Zietman
Leadership: Elsevier
Jason A. Efstathiou
Consulting or Advisory Role: Bayer, Blue Earth Diagnostics, Taris Biomedical, EMD Serono
Ashesh B. Jani
Consulting or Advisory Role: Blue Earth Diagnostics
Luis Souhami
Honoraria: Varian Medical Systems
Consulting or Advisory Role: Janssen Oncology, Bayer
Travel, Accommodations, Expenses: Varian Medical Systems
Howard M. Sandler
Stock and Other Ownership Interests: Advanced Bioinformatics
Consulting or Advisory Role: Janssen Pharmaceuticals, Blue Earth Diagnostics, Ferring, Dendreon
Other Relationship: Caribou Publishing
No other potential conflicts of interest were reported.
REFERENCES
- 1.Mak RH, Hunt D, Shipley WU, et al. : Long-term outcomes in patients with muscle-invasive bladder cancer after selective bladder-preserving combined-modality therapy: A pooled analysis of Radiation Therapy Oncology Group protocols 8802, 8903, 9506, 9706, 9906, and 0233. J Clin Oncol 32:3801-3809, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Efstathiou JA, Spiegel DY, Shipley WU, et al. : Long-term outcomes of selective bladder preservation by combined-modality therapy for invasive bladder cancer: The MGH experience. Eur Urol 61:705-711, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Rödel C, Grabenbauer GG, Kühn R, et al. : Combined-modality treatment and selective organ preservation in invasive bladder cancer: Long-term results. J Clin Oncol 20:3061-3071, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Stein JP, Lieskovsky G, Cote R, et al. : Radical cystectomy in the treatment of invasive bladder cancer: Long-term results in 1,054 patients. J Clin Oncol 19:666-675, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Chang SS, Bochner BH, Chou R, et al. : Treatment of nonmetastatic muscle-invasive bladder cancer: American Urological Association/American Society of Clinical Oncology/American Society for Radiation Oncology/Society of Urologic Oncology clinical practice guideline summary. J Oncol Pract 13:621-625, 2017 [DOI] [PubMed] [Google Scholar]
- 6.Alfred Witjes J, Lebret T, Compérat EM, et al. : Updated 2016 EAU guidelines on muscle-invasive and metastatic bladder cancer. Eur Urol 71:462-475, 2017 [DOI] [PubMed] [Google Scholar]
- 7. National Comprehensive Cancer Network. NCCN Guidelines - Bladder Cancer (Version 2.2018) [Internet]. 2018 [cited 2018 Mar 6];Available from: https://www.nccn.org/professionals/physician_gls/pdf/bladder.pdf.
- 8.Kaufman DS, Winter KA, Shipley WU, et al. : The initial results in muscle-invading bladder cancer of RTOG 95-06: Phase I/II trial of transurethral surgeryplus radiation therapy with concurrent cisplatin and 5-fluorouracil followed by selective bladder preservationor cystectomy depending on the initial response. Oncologist 5:471-476, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Mitin T, Hunt D, Shipley WU, et al. : Transurethral surgery and twice-daily radiation plus paclitaxel-cisplatin or fluorouracil-cisplatin with selective bladder preservation and adjuvant chemotherapy for patients with muscle invasive bladder cancer (RTOG 0233): A randomised multicentre phase 2 trial. Lancet Oncol 14:863-872, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.James ND, Hussain SA, Hall E, et al. : Radiotherapy with or without chemotherapy in muscle-invasive bladder cancer. N Engl J Med 366:1477-1488, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Oh KS, Soto DE, Smith DC, et al. : Combined-modality therapy with gemcitabine and radiation therapy as a bladder preservation strategy: Long-term results of a phase I trial. Int J Radiat Oncol Biol Phys 74:511-517, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Kent E, Sandler H, Montie J, et al. : Combined-modality therapy with gemcitabine and radiotherapy as a bladder preservation strategy: Results of a phase I trial. J Clin Oncol 22:2540-2545, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Caffo O, Fellin G, Graffer U, et al. : Phase I study of gemcitabine and radiotherapy plus cisplatin after transurethral resection as conservative treatment for infiltrating bladder cancer. Int J Radiat Oncol Biol Phys 57:1310-1316, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Sangar VK, McBain CA, Lyons J, et al. : Phase I study of conformal radiotherapy with concurrent gemcitabine in locally advanced bladder cancer. Int J Radiat Oncol Biol Phys 61:420-425, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Zelen M: The randomization and stratification of patients to clinical trials. J Chronic Dis 27:365-375, 1974 [DOI] [PubMed] [Google Scholar]
- 16.Clopper CJ, Pearson ES: The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika 26:404-413, 1934 [Google Scholar]
- 17.Fine JP, Gray RJ: A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94:496, 1999 [Google Scholar]
- 18.Gray PJ, Fedewa SA, Shipley WU, et al. : Use of potentially curative therapies for muscle-invasive bladder cancer in the United States: Results from the National Cancer Data Base. Eur Urol 63:823-829, 2013 [DOI] [PubMed] [Google Scholar]
- 19.Turgeon G-A, Souhami L, Cury FL, et al. : Hypofractionated intensity modulated radiation therapy in combined modality treatment for bladder preservation in elderly patients with invasive bladder cancer. Int J Radiat Oncol Biol Phys 88:326-331, 2014 [DOI] [PubMed] [Google Scholar]
- 20.Choudhury A, Swindell R, Logue JP, et al. : Phase II study of conformal hypofractionated radiotherapy with concurrent gemcitabine in muscle-invasive bladder cancer. J Clin Oncol 29:733-738, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Horwich A, Dearnaley D, Huddart R, et al. : A randomised trial of accelerated radiotherapy for localised invasive bladder cancer. Radiother Oncol 75:34-43, 2005 [DOI] [PubMed] [Google Scholar]