Fifty years ago, Armitage and Doll1 in the United Kingdom and Nordling2 in the United States used epidemiologic data to show that the age-specific incidence of a variety of cancers followed a remarkably similar pattern, with rates increasing to the sixth power of age. They suggested that this relationship could be explained if a cancer cell was the result of six or seven successive mutations in a specified order. Remarkably, recent studies of cancer genomes have confirmed this observation, and most cancers have between three and seven mutations in genes causally implicated in cancer pathogenesis (so-called driver genes).3 Early precancerous states can be identified for several tumor types and often contain single cancer-initiating mutations.4-7 The acquisition of somatic mutations detected in the blood leading to the clonal expansion of mutated hematopoietic cells is referred to as clonal hematopoiesis (CH). CH is commonly detected in healthy individuals, but confers an increased risk of hematologic disease.4,7 CH mutations generally occur at low frequencies in genes implicated in myeloid neoplasms such as DNMT3A, TET2, ASXL1, and TP53.8 Aging is the strongest known risk factor for CH, with the prevalence increasing greatly with each decade of life.9-11 CH is associated with an increased risk of hematologic malignancies (especially myeloid neoplasms), shorter overall survival, and increased risk of cardiovascular disease (CVD).9-11
Recent studies suggest CH is more prevalent in patients with solid cancer, with approximately 30% harboring CH mutations in their blood.12 With the increased use of genetic sequencing to inform clinical decision-making in oncology, the possibility of unintentional discovery of CH in the setting of genomic analysis is increasingly likely. First, CH can be discovered incidentally when sequencing blood for the purpose of germline testing.13,14 Second, tumor sequencing is often accompanied by blood sequencing as a matched control, and from analysis of the blood sequencing data, CH can be uncovered as an incidental finding.12 Third, CH can be discovered in tests of cell-free DNA, much of which comes from WBCs and can confound these results.15,16 Fourth, CH can be detected on solid tumor sequencing due to blood contamination of tumor samples, leading to false-positive tumor somatic mutation calls.17-20 Finally, CH can be identified during the evaluation of unexplained cytopenias, through the National Comprehensive Cancer Network recommended sequencing of myelodysplastic syndrome (MDS)–associated genes in patients with cytopenias when there is suspicion for MDS.21 Thus, the identification of CH in patients with solid tumors is a reality that oncologists must be prepared to encounter. Here, we discuss the implications of CH in populations of patients with cancer, the existing knowledge gaps in this area, and our recommendations for how to approach CH when it is encountered clinically.
The clinical implications of CH detection raise the need to define standardized clinical criteria for its definition and clinical management, given that CH detection is influenced by a variety of factors. These factors include sequencing depth, the set of genes sequenced, the minimum percentage of blood cells with the mutation (ie, variant allele fraction [VAF]) used for CH calling, as well as inclusion of appropriate controls that account for background mutation rate, germline variation, and sequencing artifacts. The frequency of CH increases greatly when considering mutations involving a small number of total leukocytes (ie, at VAF < 1% to 2%).22 Thus, the depth of sequencing and the variant calling strategy may influence the prevalence of detected CH and, therefore, its clinical relevance in any given study. Because a VAF of 2% is used in many current clinical sequencing assays as the cutoff for variant calling, a cutoff for CH as a somatic mutation in the peripheral blood at a VAF 2% or greater has been suggested23 and will be used for the purpose of this commentary. However, the actual cutoff with clinical and biologic significance remains to be delineated, as CH at a VAF less than 2% may expand after exposure to oncologic therapy24 and could contribute to sequelae, including development of therapy-related myeloid neoplasms (t-MNs).25,26 Discrepancies also exist in the definition of the genes involved in CH, with some studies restricting CH to that occurring in leukemia-driver genes.9 This generally results in a lower reported prevalence of CH compared with studies that include somatic mutations in any gene.12,22 Because of the low VAFs observed in most patients with CH, an additional challenge exists in discriminating sequencing artifact from true, low VAF CH mutations, particularly when sequencing peripheral blood samples in isolation. This can be addressed by comparison of a suspected peripheral blood variant with a matched reference sample (eg, a tumor sample)12 or through comparison with a reference unmatched control.27
Despite issues in direct comparison between studies, due to differences in sequencing and analytic methods, there is evidence that patients with solid tumors have a higher prevalence of CH compared with the general adult population. The factors that contribute to the observed higher frequency of CH in patients with solid tumors are not completely defined but may include exposure to oncologic therapy. CH with TP53 and PPM1D is associated with exposure to radiation and chemotherapy,12 and TP53 mutations are known to precede development of t-MNs.28 In addition, shared risk factors between cancer and CH, such as smoking, may account for the increased prevalence of CH.12 CH is clinically relevant in patients with solid tumors because it is associated with a variety of adverse outcomes.12 CH in patients with solid tumor is associated with an increased risk for t-MNs, including MDS and acute myeloid leukemia. Therapy-related myeloid neoplasms represent lethal secondary malignancies that portend very poor prognosis and are generally refractory to therapy (overall survival, 6 to 12 months). In independent studies, we observed a higher prevalence of CH at the time of primary (solid) cancer diagnosis in individuals who developed t-MN compared with matched patients who did not develop t-MNs (62% v 27%, P = .02; and 71% v 31%, P = .008, respectively).25,26 These findings were replicated in a cohort of patients with lymphoma treated with standard chemotherapy25 and autologous stem cell transplantation.29
In the general population, patients with CH in the setting of cytopenias are at high risk of occult myeloid neoplasms and ultimate development of overt disease.30,31 It has been demonstrated that cases of CH with a VAF greater than or equal to 10%, multiple mutations, and spliceosome gene mutations have high positive predictive values (ranging from 0.86 to 1.0) for the presence of occult myeloid neoplasm among patients being evaluated for cytopenias.31 Whether these high predictive rates translate to solid-tumor populations has not been established and requires a more comprehensive understanding of the relationships among CH, cytopenias, and exposure to oncologic therapy. Nevertheless, this suggests that subsets of patients with CH and cytopenias are at risk for occult myeloid malignancy.
There is some evidence that CH may have an impact on cancer-related survival. CH in patients with solid tumors and lymphoma has been associated with a reduced overall survival, independent of t-MN risk, that worsens with increasing VAF.12,29 Validation studies are warranted to test these early observations and elucidate the mechanism underpinning these associations.
CH is also strongly associated with an increased risk of CVD, including coronary heart disease (hazard ratio [HR], 2.0; P = .02), ischemic stroke (HR, 2.6; P = .003), and early-onset myocardial infarction (odds ratio, 4.0; P < .001).9,10 In these studies, the strength of the association between CH and CVD was comparable to well-validated traditional cardiovascular risk factors such as high cholesterol, smoking, and hypertension. Individuals harboring the JAK2 V617F mutation or CH with VAF greater than 10% had a particularly increased risk for CVD (HR, 12.1 and 2.2, respectively).10 Mechanistic studies demonstrated that genetically altered mice with reduced TET2 expression in hematopoietic cells, including monocytes/macrophages, developed accelerated atherosclerosis10,32 and heart failure33 associated with increased macrophage activation and expression of the proinflammatory cytokine, interleukin-1β (IL-1β). Evidence of increased IL-1β activity in mouse models of CH suggests the intriguing concept that novel anti-inflammatory therapies targeting IL-1β34 might be particularly effective, but at present, there are no evidence-based approaches to reducing CH-associated CVD risk. Furthermore, the generalizability of this observation to the spectrum of acquired mutations observed in CH is unknown. These strong associations are critically relevant to cancer survivors and may contribute to the accelerated rates of CVD observed in this context compared with the general population.35
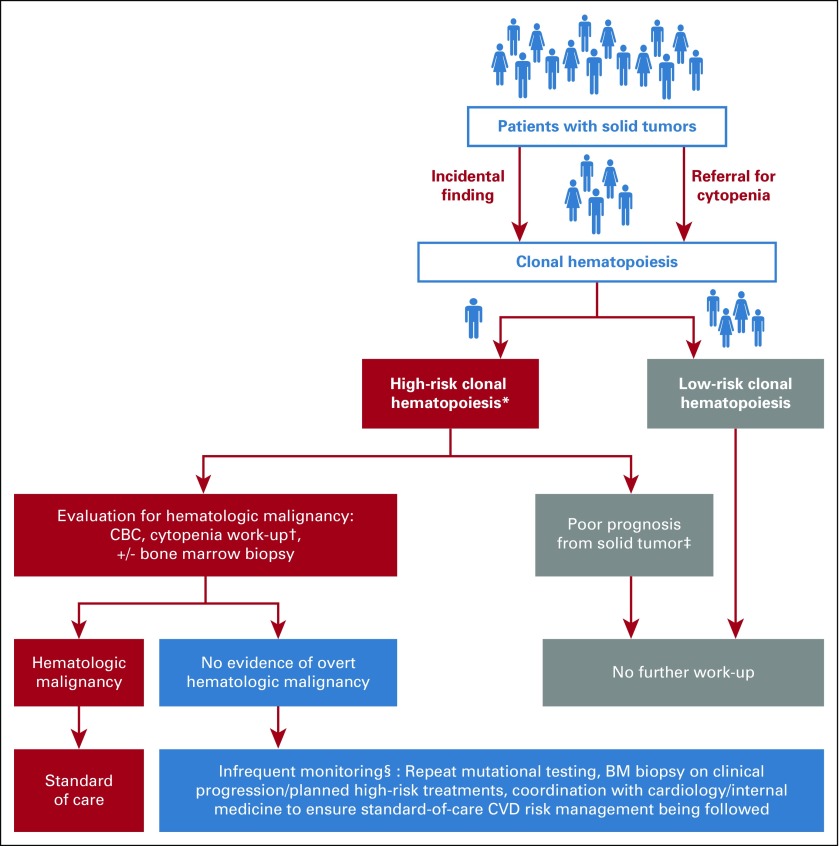
Currently, upon detection of CH, there is no standard of care for the reporting and monitoring of these patients. In the absence of evidence-based guidelines, we discuss our general management strategies for patients with solid tumors who have CH. First, because of the lack of established interventional strategies, we do not inform all patients of CH when discovered as an incidental finding. However, when patients with CH have clinical signs of bone marrow dysfunction (most commonly evidenced by abnormal indices on a CBC count) and/or high-risk mutational characteristics, such as a VAF greater than or equal to 10% or more than one mutation, notification of CH should be considered to allow evaluation for an occult hematologic disorder and to follow for subsequent risk of t-MN. In patients with significant cytopenias, it is critical to evaluate for alternative causes on history, physical, and laboratory results, such as iron deficiency anemia and anemia of chronic kidney disease (Fig 1). If no alternative etiology for cytopenias is identified on initial work-up, collecting a bone marrow biopsy specimen may be warranted to evaluate for an underlying hematologic neoplasm. For patients who have no overt evidence of hematologic disease, infrequent follow-up, including periodic monitoring of CBC counts, is recommended. As the risk and time-course for transformation from CH to overt hematologic disease is better characterized, molecular and clinical features may be used to inform follow-up periods. We recommend repeated CBC counts every 3 to 12 months depending on the progression rate of patients with and without blood count abnormalities. In patients who have progressive blood count abnormalities or other symptoms concerning for evolving hematologic disease, repeated mutational testing combined with repeated bone marrow biopsy specimen assessment should be considered. Importantly, because of the increased risk of CVD, we recommend consultation with cardiologists or primary care physicians to ensure that modifiable risk factors for CVD are adequately managed. Because CH is not included in CVD risk calculators or clinical guidelines, we recommend that standard guidelines for CVD risk reduction be followed.36-39
FIG 1.
Flow diagram of our general management strategies for patients with solid tumors with findings of clonal hematopoiesis (CH). (*) We currently consider high-risk CH that, when detected as an incidental finding, would warrant work-up for an underlying hematologic disorder as the following: CH in the presence of significant blood count abnormalities, the presence of a single CH mutation at a high variant allele fraction (> 10%) or multiple CH mutations. Ongoing research efforts will likely further refine this and/or identify additional high-risk features, such as hotspot TP53 mutations, DNMT3A R882 variants, and so forth. (†) Cytopenia work-up including history and physical, comprehensive metabolic panel; peripheral smear; thyroid-stimulating hormone, ferritin, folate, haptoglobin, lactate dehydrogenase, vitamin B1, vitamin B6, vitamin B12, methylmalonic acid, and copper levels; prothrombin time or partial thromboplastin time; and international normalized ratio. Specific for anemia, we suggest measuring the following: reticulocyte count, flow cytometry for paroxysmal nocturnal hemoglobinuria (if low haptoglobin and/or high lactate dehydrogenase in appropriate clinical context), serum protein electrophoresis, and serum immunofixation. Specific for thrombocytopenia we suggest measuring immature platelet fraction. Specific for neutropenia, we suggest measuring antineutrophil antibody level. (‡) For patients anticipated to have a poor prognosis (< 6 to 12 months) from their primary solid tumor, we would not suggest further work-up for CH. (§) Monitoring interval can be determined by the treating clinician, though we would suggest 3- to 12-month evaluations (more frequently for patients receiving ongoing cytotoxic therapy, and less frequently for patients in long-term follow-up). Current guidelines recommend targeting a blood pressure less than 130/80 mm Hg,36 lifestyle modifications, and pharmacotherapy with a thiazide diuretic, calcium channel blocker, or angiotensin-converting enzyme inhibitor/angiotensin receptor blocker. Preclinical and clinical studies suggest statins have potent anti-inflammatory effects, including suppression of interleukin-1β release,36 and should be prescribed to all patients with an estimated 10-year atherosclerotic CVD risk greater than 7.5%.38,39 BM, bone marrow; CVD, cardiovascular disease.
Although the capacity to use CH as an independent clinical decision-making tool is tempting, the evidence is not currently sufficient to recommend such a management approach. Here, we identify some of the key knowledge gaps that need to be answered by future studies. First, a consensus on the definition of clinically meaningful CH should be established. For example, what is the minimum VAF threshold that should be considered clinically meaningful CH? Should only mutations in leukemia-driver genes be considered when identifying CH or should all genes with low VAF mutations be included? Second, the gene-specific risk associated with CH needs to be established, because not all CH gene mutations may carry the same leukemogenic potential or risk of CH-driven sequelae. Identifying a risk-adapted classification of CH is especially crucial to inform future therapeutic studies aimed at reducing CH-associated risks. Third, a context-dependent interaction between CH and various types of cellular stressors needs to be established. For example, if a subgroup of patients with solid tumors and CH is at a highest risk of progression to t-MN and if specific therapies are shown to promote the continued expansion of specific CH clones, an opportunity for risk-directed therapy modification could be envisioned, particularly in the setting of cancers with only a modest survival benefit from adjuvant therapy. Fourth, because exposure to oncologic therapy is related to an increased risk of both CVD and CH in patients with solid tumors, mitigating the risks of CVD will be an important survivorship issue. Whether predictive models for CVD should be modified to include CH and whether CH should factor into guidelines for primary and secondary CVD require further evidence before changes are recommended. Establishment of a publicly available database of CH variants would aid in standardizing criteria and enabling population-based meta-analysis to deliver meaningful associations.40,41 Ultimately, collaborative, multi-institution prospective studies with many patients are needed to validate existing observations, establish longitudinal kinetics of CH, and identify therapeutic strategies to mitigate CH risk for those patients with and those without cancer alike.
ACKNOWLEDGMENT
K.L.B is funded by the American Society of Hematology. N.K.G. is funded by the National Institutes of Health/National Cancer Institute Moffitt Cancer Center Postdoctoral Training Program in Molecular Epidemiology of Cancer (Grant No. T32-CA147832). C.C.C is funded by the Conquer Cancer Foundation and the University of North Carolina Oncology Clinical Translational Research Training Program (Grant No. K12-CA120780). K.T receives support from the Khalifa Scholar Physician Scientist Award, Physician Scientist Program at MD Anderson Cancer Center and the Leukemia Specialized Programs of Research Excellence Career Enhancement Program. K.L.B., N.K.G., C.C.C., K.T., R.L., and E.P. contributed equally to this work.
AUTHOR CONTRIBUTIONS
Conception and design: Kelly L. Bolton, Nancy K. Gillis, Catherine C. Coombs, Koichi Takahashi, Ahmet Zehir, Brian C. Jensen, Ross Levine, Elli Papaemmanuil, Eric Padron
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Managing Clonal Hematopoiesis in Patients With Solid Tumors
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Catherine C. Coombs
Honoraria: H3 Biomedicine; Pharmacyclics
Consulting or Advisory Role: AbbVie
Travel, Accommodations, Expenses: Incyte; Arog Pharmaceuticals
Koichi Takahashi
Honoraria: Kyowa Hakko Kirin; Celgene; Pharmacyclics
Consulting or Advisory Role: SymBio Pharmaceuticals
Research Funding: Onconova Therapeutics (Inst); MEI Pharma (Inst)
Travel, Accommodations, Expenses: SymBio Pharmaceuticals; Helsinn Therapeutics
Rafael Bejar
Honoraria: Celgene; Genoptix; Foundation Medicine; Alexion Pharmaceuticals; AbbVie/Genentech; Astex Pharmaceuticals
Consulting or Advisory Role: Celgene; Genoptix; Foundation Medicine
Research Funding: Celgene
Patents, Royalties, Other Intellectual Property: Licensed a prognostic gene mutation signature to Genoptix from 2011 to 2016
Travel, Accommodations, Expenses: Celgene; Genoptix
Luis A. Diaz Jr
Leadership: Personal Genome Diagnostics
Stock and Other Ownership Interests: PapGene; Personal Genome Diagnostics; Jounce Therapeutics
Consulting or Advisory Role: Merck; Personal Genome Diagnostics; Genentech; Cell Design Labs
Research Funding: Merck (Inst)
Patents, Royalties, Other Intellectual Property: PapGene, PGDx, and other entities have licensed several patent applications from Johns Hopkins, where L.A.D. is an inventor. These relationships are subject to certain restrictions under Johns Hopkins University policy, and the terms of these arrangements are managed by the university in accordance with its conflict-of-interest policies.
Travel, Accommodations, Expenses: Merck
Simon Mantha
Research Funding: Janssen
Other Relationship: Daboia Consulting
Virginia Klimek
Research Funding: H3 Biomedicine (Inst); Karyopharm Therapeutics (Inst)
Elli Papaemmanuil
Stock and Other Ownership Interests: Tesaro (I)
Honoraria: Novartis; Celgene
Research Funding: Celgene
Patents, Royalties, Other Intellectual Property: Royalties for an AKT inhibitor through the Cancer Research UK (I)
Travel, Accommodations, Expenses: Celgene; Novartis; Tesaro (I)
Ross Levine
Leadership: Qiagen
Stock and Other Ownership Interests: Loxo
Honoraria: Incyte
Consulting or Advisory Role: Novartis
Research Funding: Roche; Celgene
Eric Padron
Honoraria: Incyte; Karyopharm Therapeutics; Incyte (Inst); Cell Therapeutics (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Armitage P, Doll R. The age distribution of cancer and a multi-stage theory of carcinogenesis. Br J Cancer. 1954;8:1–12. doi: 10.1038/bjc.1954.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nordling CO. A new theory on cancer-inducing mechanism. Br J Cancer. 1953;7:68–72. doi: 10.1038/bjc.1953.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martincorena I, Raine KM, Gerstung M, et al. Universal patterns of selection in cancer and somatic tissues Cell 1711029–1041 e21, 2017. [Erratum: Cell 173:1823, 2018] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Busque L, Mio R, Mattioli J, et al. Nonrandom X-inactivation patterns in normal females: Lyonization ratios vary with age. Blood. 1996;88:59–65. [PubMed] [Google Scholar]
- 5.Martincorena I, Roshan A, Gerstung M, et al. High burden and pervasive positive selection of somatic mutations in normal human skin. Science. 2015;348:880–886. doi: 10.1126/science.aaa6806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 7.Busque L, Patel JP, Figueroa ME, et al. Recurrent somatic TET2 mutations in normal elderly individuals with clonal hematopoiesis. Nat Genet. 2012;44:1179–1181. doi: 10.1038/ng.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Papaemmanuil E, Gerstung M, Bullinger L, et al. Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med. 2016;374:2209–2221. doi: 10.1056/NEJMoa1516192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jaiswal S, Fontanillas P, Flannick J, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371:2488–2498. doi: 10.1056/NEJMoa1408617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jaiswal S, Natarajan P, Silver AJ, et al. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N Engl J Med. 2017;377:111–121. doi: 10.1056/NEJMoa1701719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Genovese G, Kähler AK, Handsaker RE, et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N Engl J Med. 2014;371:2477–2487. doi: 10.1056/NEJMoa1409405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coombs CC, Zehir A, Devlin SM, et al. Therapy-related clonal hematopoiesis in patients with non-hematologic cancers is common and associated with adverse clinical outcomes. Cell Stem Cell. 21:374–382 e4, 2017. doi: 10.1016/j.stem.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coffee B, Cox HC, Kidd J, et al. Detection of somatic variants in peripheral blood lymphocytes using a next generation sequencing multigene pan cancer panel. Cancer Genet. 2017;211:5–8. doi: 10.1016/j.cancergen.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Weitzel JN, Chao EC, Nehoray B, et al. Somatic TP53 variants frequently confound germ-line testing results. Genet Med. doi: 10.1038/gim.2017.196. [epub ahead of print on November 30, 2017] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu Y, Ulrich BC, Supplee J, et al. False positive plasma genotyping due to clonal hematopoiesis. Clin Cancer Res. doi: 10.1158/1078-0432.CCR-18-0143. [epub ahead of print on March 22, 2018] [DOI] [PubMed] [Google Scholar]
- 16.Strickler JH, Loree JM, Ahronian LG, et al. Genomic landscape of cell-free DNA in patients with colorectal cancer. Cancer Discov. 2018;8:164–173. doi: 10.1158/2159-8290.CD-17-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Severson EA, Riedlinger GM, Connelly CF, et al. Detection of clonal hematopoiesis of indeterminate potential in clinical sequencing of solid tumor specimens. Blood. 2018;131:2501–2505. doi: 10.1182/blood-2018-03-840629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ptashkin RN, Mandelker DL, Coombs CC, et al. Clonal Hematopoiesis Confounds Clinical Genomic Profiling of Solid Tumors. JAMA Oncol. doi: 10.1001/jamaoncol.2018.2297. [epub ahead of print on June 5, 2018] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coombs CC, Gillis NK, Tan X, et al. Identification of clonal hematopoiesis mutations in solid tumor patients undergoing unpaired next-generation sequencing assays. Clin Cancer Res. doi: 10.1158/1078-0432.CCR-18-1201. [epub ahead of print on June 4, 2018] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones S, Anagnostou V, Lytle K, et al. Personalized genomic analyses for cancer mutation discovery and interpretation. Sci Transl Med. 2015;7:283ra53. doi: 10.1126/scitranslmed.aaa7161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Comprehensive Cancer Network Myelodysplastic syndromes (version 2) 2018 doi: 10.6004/jnccn.2015.0038. https://www.nccn.org/professionals/physician_gls/pdf/mds.pdf [DOI] [PMC free article] [PubMed]
- 22.Zink F, Stacey SN, Norddahl GL, et al. Clonal hematopoiesis, with and without candidate driver mutations, is common in the elderly. Blood. 2017;130:742–752. doi: 10.1182/blood-2017-02-769869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steensma DP, Bejar R, Jaiswal S, et al. Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood. 2015;126:9–16. doi: 10.1182/blood-2015-03-631747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wong TN, Miller CA, Jotte MRM, et al. Cellular stressors contribute to the expansion of hematopoietic clones of varying leukemic potential. Nat Commun. 2018;9:455. doi: 10.1038/s41467-018-02858-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takahashi K, Wang F, Kantarjian H, et al. Preleukaemic clonal haemopoiesis and risk of therapy-related myeloid neoplasms: A case-control study. Lancet Oncol. 2017;18:100–111. doi: 10.1016/S1470-2045(16)30626-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gillis NK, Ball M, Zhang Q, et al. Clonal haemopoiesis and therapy-related myeloid malignancies in elderly patients: A proof-of-concept, case-control study. Lancet Oncol. 2017;18:112–121. doi: 10.1016/S1470-2045(16)30627-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. NHLBI Exome Sequencing Project (ESP). Exome variant server. http://evs.gs.washington.edu/EVS/
- 28.Wong TN, Ramsingh G, Young AL, et al. Role of TP53 mutations in the origin and evolution of therapy-related acute myeloid leukaemia. Nature. 2015;518:552–555. doi: 10.1038/nature13968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gibson CJ, Lindsley RC, Tchekmedyian V, et al. Clonal hematopoiesis associated with adverse outcomes after autologous stem-cell transplantation for lymphoma. J Clin Oncol. 2017;35:1598–1605. doi: 10.1200/JCO.2016.71.6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kwok B, Hall JM, Witte JS, et al. MDS-associated somatic mutations and clonal hematopoiesis are common in idiopathic cytopenias of undetermined significance. Blood. 2015;126:2355–2361. doi: 10.1182/blood-2015-08-667063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malcovati L, Gallì A, Travaglino E, et al. Clinical significance of somatic mutation in unexplained blood cytopenia. Blood. 2017;129:3371–3378. doi: 10.1182/blood-2017-01-763425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fuster JJ, MacLauchlan S, Zuriaga MA, et al. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science. 2017;355:842–847. doi: 10.1126/science.aag1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sano S, Oshima K, Wang Y, et al. Tet2-mediated clonal hematopoiesis accelerates heart failure through a mechanism involving the IL-1β/NLRP3 inflammasome. J Am Coll Cardiol. 2018;71:875–886. doi: 10.1016/j.jacc.2017.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 35.Carver JR, Shapiro CL, Ng A, et al. American Society of Clinical Oncology clinical evidence review on the ongoing care of adult cancer survivors: Cardiac and pulmonary late effects. J Clin Oncol. 2007;25:3991–4008. doi: 10.1200/JCO.2007.10.9777. [DOI] [PubMed] [Google Scholar]
- 36.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:2199–2269. [Erratum: J Am Coll Cardiol 71:2273-2275, 2018] [Google Scholar]
- 37.Waehre T, Yndestad A, Smith C, et al. Increased expression of interleukin-1 in coronary artery disease with downregulatory effects of HMG-CoA reductase inhibitors. Circulation. 2004;109:1966–1972. doi: 10.1161/01.CIR.0000125700.33637.B1. [DOI] [PubMed] [Google Scholar]
- 38.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25) Suppl 2:S1–S45. doi: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 39.Stone NJ, Robinson JG, Lichtenstein AH, et al. ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;2014;63:2889–2934. doi: 10.1016/j.jacc.2013.11.002. (25, 25 Pt B) [Erratum: J Am Coll Cardiol 63:3024-302, 2014 (25 Pt B); and 66(24):2812, 2015] [DOI] [PubMed] [Google Scholar]
- 40.Cerami E, Gao J, Dogrusoz U, et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gao J, Aksoy BA, Dogrusoz U, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]