Summary
Aims
Little is known of the effect of sodium‐glucose cotransporter 2 (SGLT2) inhibitors on the renal tubules. We investigated the effect of the SGLT2 inhibitor, tofogliflozin (TOFO) on renal tubular indices, according to the degree of albuminuria, in type 2 diabetes mellitus (T2DM) patients with preserved renal function.
Materials and methods
A total of 988 patients, receiving TOFO, were enroled and divided into 3 groups, based on the urine albumin‐to‐creatinine ratio (UACR). The tubular indices (urinary N‐acetyl‐beta‐d‐glucosaminidase [NAG]‐to‐creatinine and urinary beta‐2 microglobulin [beta2MG]‐to‐creatinine ratios) and UACR were log‐transformed in the correlation analysis.
Results
Treatment with TOFO led to similar reductions in glycated haemoglobin (HbA1c) levels, from baseline to week 24, across all groups. The NAG level increased in the normoalbuminuria group and decreased in the macroalbuminuria group significantly (P < .001, both), but did not change in the microalbuminuria group. Significant reductions in the UACR were observed in both microalbuminuria and macroalbuminuria groups (P < .001, both). Significant negative correlations between changes in the NAG and beta2MG levels and their corresponding baseline values were observed in all participants. The reduction in the UACR was negatively correlated with baseline levels. The changes in the tubular indices were positively correlated with reductions in the UACR across groups.
Conclusions
Logarithmic reductions in the renal tubular indices, via SGLT2 inhibition, were observed in patients with T2DM. TOFO may not only improve the degree of albuminuria but may also have protective effects on the tubules.
Keywords: albuminuria, beta‐2 microglobulin, diabetic nephropathy, estimated glomerular filtration rate, N‐acetyl‐beta‐d‐glucosaminidase, sodium‐glucose cotransporter 2, tofogliflozin, tubular glomerular feedback, type 2 diabetes mellitus, urine albumin‐to‐creatinine ratio
1. INTRODUCTION
Diabetic nephropathy (DN) is characterised by a logarithmic increase in the level of urinary albumin, followed by a progressive decline in renal function. It is further characterised by functional changes in the glomerulus, such as glomerular hyperfiltration and expansion of the extracellular matrix in the mesangial areas, as well as by tubular injuries,1, 2 which ultimately result in progressive kidney dysfunction with an increase in the urine albumin‐to‐creatinine ratio (UACR) and reduction in the glomerular filtration rate (GFR). The key modifiable risk factors for DN include hypertension, hyperglycaemia, dyslipidemia and lifestyle factors.3 Albuminuria is typically caused by a defect in the glomerular filtration barrier; however, abnormalities in tubular albumin reabsorption may also contribute to the development of albuminuria.4
The overexpression of sodium‐glucose cotransporter 2 (SGLT2), which has been observed in human proximal tubular epithelial cells in patients with type 2 diabetes mellitus (T2DM),5 may exacerbate hyperglycaemia. Thus, its inhibition, by SGLT2 inhibitors, improves the degree of hyperglycaemia via an increase in the urinary glucose excretion.6, 7 The overexpression of SGLT2 is suggested to cause damage to the kidney, based on the results of preclinical experiments.8 SGLT2‐mediated glucose entry into tubular cells caused the cell more suscepitible to pro‐apoptotic effects.8 Thus the genetic deletion and pharmacological inhibition of SGLT2, in preclinical diabetes models,9, 10 could attenuate not only the associated glomerular hyperfiltration but also tubular apoptosis and atrophy. Therefore, SGLT2 inhibition is expected to provide beneficial effects in the case of DN.
Recently, 2 large studies showed that SGLT2 inhibitors improve renal outcomes, including the progression of albuminuria.11, 12 Although the specific overexpression of SGLT2 in human proximal tubular epithelial cells has been observed in T2DM patients, the renal tubular effects, via SGLT2 inhibition, have rarely been evaluated. The aim of this study was to investigate the effect of SGLT2 inhibitor, tofogliflozin (TOFO) on the renal tubular indices, according to the degree of albuminuria, in patients with T2DM from phase 2 and phase 3 TOFO studies.
2. METHODS
An integrated analysis was performed on 4 phase 2 and phase 3 TOFO studies (Table S1), with a duration of at least 24 weeks, that enroled patients with T2DM, and compared the use of the medication to that of a placebo, or different doses of TOFO. The CSG003JP study (TOFO 10, 20 and 40 mg monotherapy) was a 24‐week randomised, double‐blind, placebo‐controlled, combined phase 2 and 3 study.13 The CSG004JP study (TOFO 20 and 40 mg monotherapy) and the CSG005JP study (TOFO 20 and 40 mg as an add‐on to other oral anti‐diabetic agents) were both 52‐week randomised, controlled, open‐label, phase 3 studies.14 Details of the above study's design and results, including patient inclusion and exclusion criteria, have been previously reported.13, 14 The CSG006JP study (TOFO 40 mg as monotherapy or add‐on to sulfonylureas or dipeptidyl peptidase‐4 [DPP‐4] inhibitors) was a 24‐week, multicentre, open‐label study. Data from the 24‐week or 52‐week core treatment periods of each study were included in this integrated analysis.
All studies were conducted in accordance with the Declaration of Helsinki and Good Clinical Practice. The protocols were reviewed and approved by the institutional review board of each participating centre. All patients provided written informed consent before being enroled. This integrated analysis was also reviewed and approved by the institutional review board of St. Mary's Hospital.
This was an integrated analysis to evaluate the impact of treatment with TOFO on the renal tubular indices, such as N‐acetyl‐beta‐d‐glucosaminidase (NAG), and beta‐2 microglobulin (beta2MG), according to the degree of albuminuria, using data from subsets of patients with T2DM from 4 prospective phase 2 and phase 3 studies. Participants receiving TOFO were divided into 3 groups, based on their UACR levels at baseline. If the tubular indices and UACR at week 24 were not recorded in a patient, the last post‐baseline value measured before the scheduled visit at week 24, was used as the value at week 24 (using the last‐observation‐carried‐forward procedure). Laboratory variables including glycated haemoglobin (HbA1c), fasting plasma glucose (FPG), serum creatinine and serum cystatin C levels were also evaluated from baseline to week 24. The glomerular indices were calculated from serum creatinine and cystatin C levels. We estimated the eGFRMDRD using the Modification of the Diet in Renal Disease (MDRD) formula for the Japanese population15, 16and eGFRCys from the Japanese equation17 were estimated from the serum cystatin C values. The assessments also included effects on body weight, and systolic and diastolic blood pressure (BP) from the baseline to week 24. All measurements made within the groups were assessed using the last‐observation‐carried‐forward procedure.
2.1. Statistical analysis
Subjects receiving TOFO were divided into 3 groups, based on their baseline UACR levels: normoalbuminuria (UACR <30 mg/gCr), microalbuminuria (UACR 30‐<300 mg/gCr) and macroalbuminuria (UACR ≥300 mg/gCr) groups. Two participants were excluded from the analysis because their baseline eGFRMDRD values were <30 mL/min/1.73 m2. The changes in the tubular indices, UACR, laboratory variables, body weight and BP were compared from baseline to week 24. The differences across groups were also evaluated to investigate the impact of SGLT2 inhibition on the variables. The difference, according to the degree of albuminuria, in the TOFO 10 mg group was not investigated because the number of the participants was too low. The patient demographics of each group are summarised with appropriate descriptive statistics (means and standard deviation [SD] or medians for continuous variables and counts and percentages for categorical variables).
The assessments for HbA1c, FPG and body weight (change from the baseline in the variables, at week 24) were analysed using an analysis of covariance (ANCOVA) model, with the groups as a fixed effect and their baseline values as covariates, to examine the differences across groups and to verify their superiority. The other assessments, excluding the tubular indices and UACR, were analysed using a 1‐sample t test from the baseline to week 24. In addition, the differences in the assessments, across the groups, were analysed using ANOVA or a Fisher's exact test. The tubular indices and UACR were analysed using the Wilcoxon signed rank test from the baseline to week 24. In addition, the differences in the assessments, across the groups, were analysed with the Kruskal‐Wallis test. Finally, a correlation analysis between the tubular indices and the changes in variables was performed using Pearson product‐moment correlation coefficients. The tubular indices and UACR were log‐transformed with application to the univariate analysis. The (2‐sided) significance level for each test was .05.
3. RESULTS
The 988 participants with eGFRMDRD values >30 mL/min/1.73 m2 were divided into 3 groups—normoalbuminuria (n = 661), microalbuminuria (n = 278), and macroalbuminuria (n = 49) groups—according to their baseline UACR levels. The baseline demographic characteristics that might influence the UACR were significantly different between the groups (Table 1). In particular, the tubular indices—NAG and beta2MG—were significantly higher, whereas the UACR exponentially increased across the groups, according to the severity of DN; both the NAG and beta2MG levels were within their lower ranges in the normoalbuminuria group (Table 1). Renal function, assessed using glomerular indices, decreased with increasing UACR. Similarly, age, glycemic status, systolic BP, and the proportion of concomitant antihypertensive drugs used increased significantly with increasing levels of UACR (across the groups). In contrast, there was no significant difference across the groups, with regard to sex, TOFO dosage, diastolic BP or duration of DM (Table 1). Similar baseline characteristics according to the degree of albuminuria were observed in each dosage of tofogliflozin group (Table S3).
Table 1.
Baseline characteristics of the participants
| UACR (mg/gCr) | P across groups | |||
|---|---|---|---|---|
| <30 | 30‐<300 | ≥300 | ||
| N | 661 | 278 | 49 | |
| Age (y) | 57.7 (10.2) | 59.7 (10.5) | 60.1 (9.9) | .01 |
| Sex, n male/female | 435 / 226 | 193 / 85 | 33 / 16 | n.s. |
| TOFO 10 mg / 20 mg / 40 mg | 40 / 195 / 426 | 15 / 82 / 181 | 2 / 16 / 31 | n.s. |
| Body weight (kg) | 67.7 (13.7) | 69.3 (14.0) | 72.6 (15.8) | .03 |
| BMI (kg/m2) | 25.3 (4.2) | 26.0 (4.3) | 26.5 (4.2) | .01 |
| HbA1c (%) | 8.0 (0.9) | 8.2 (0.9) | 8.3 (1.0) | .001 |
| HbA1c (mmol/mol) | 64.2 (9.5) | 66.5 (10.2) | 67.7 (10.9) | |
| Fasting plasma glucose (mg/dL) | 159.5 (35.4) | 166.9 (35.0) | 171.2 (47.0) | .003 |
| Fasting plasma glucose (mmol/L) | 8.9 (2.0) | 9.3 (1.9) | 9.5 (2.6) | |
| Systolic BP (mm Hg) | 128.4 (13.9) | 133.0 (13.8) | 136.9 (13.3) | <.0001 |
| Diastolic BP (mm Hg) | 77.1 (9.8) | 78.7 (10.9) | 78.7 (12.6) | n.s. |
| Concomitant antihyperglycemic drugs (%) | 60.1 | 62.9 | 61.2 | n.s. |
| alpha‐GI / Biguanide / DPP‐4I / Glinide / Sulfonylurea / Thiazolidine | 64 / 62 / 68 / 16 / 113 / 74 | 26 / 36 / 35 / 4 / 50 / 24 | 6 / 1 / 5 / 2 / 15 / 1 | – |
| Concomitant antihypertensive drugs (%) | 39.6 | 55.4 | 67.4 | <.0001 |
| ARB / ACEI / CCB / beta‐blockers / diuretics | 195 / 10 / 139 / 19 / 51 | 118 / 9 / 105 / 13 / 17 | 23 / 3 / 29 / 2 / 5 | – |
| Duration of diabetes mellitus (y) | 6.8 (5.8) | 7.0 (6.0) | 7.5 (5.8) | n.s. |
| Creatinine (mg/dL) | 0.70 (0.15) | 0.73 (0.21) | 0.87 (0.29) | <.0001 |
| eGFRMDRD (mL/min/1.73 m2) | 88.8 (18.7) | 87.6 (23.6) | 74.5 (25.0) | <.0001 |
| eGFRcys (mL/min/1.73 m2) | 110.5 (22.5) | 104.4 (26.1) | 86.5 (27.3) | <.0001 |
| UACR (mg/gCr) | 11.2 | 68.3 | 775.4 | <.0001 |
| UACR (mg/mmolCr) | 1.3 | 7.7 | 87.7 | |
| Urinary NAG (U/gCr) | 6.4 | 8.8 | 14.5 | <.0001 |
| Urinary NAG (U/mmolCr) | 0.7 | 1.0 | 1.6 | |
| Urinary beta‐2MG (mg/gCr) | 115.2 | 161.3 | 347.4 | <.0001 |
| Urinary beta‐2MG (μg/mmolCr) | 13.0 | 18.2 | 39.3 | |
ACEI, ACE inhibitor; alpha‐GI, alpha‐glucosidase inhibitor; beta‐2MG, β2 microglobulin; BMI, body mass index; BP, blood pressure; CCB, calcium channel blocker; DPP‐4I, dipeptidyl peptidase‐4 inhibitor; eGFRMDRD, estimated glomerular filtration rate; eGFRcys, estimated glomerular filtration rate calculated from cystatin C; HbA1c, glycated haemoglobin; NAG, N‐acetyl‐β‐d‐glucosaminidase; TOFO, tofogliflozin; UACR, urine albumin‐to‐creatinine ratio.
Mean (standard deviation).
Median for UACR and tubular indices.
ANOVA across groups for continuous variables. Kruskal‐Wallis test across groups for urinary indices. Fisher's exact test across groups for categorical variables.
The NAG (median, +19%) level increased significantly from the baseline to week 24, in the normoalbuminuria group, whereas, in the macroalbuminuria group, a significant reduction in the NAG levels (median, −27%) was observed (Table 2). Additionally, the UACR was significantly reduced in all the groups, and significant differences were observed across the groups (Table 2). A median reduction of approximately 50% in the UACR was observed in the micro‐ and macroalbuminuria groups, while the degree of reduction in the UACR was significant but obscure in the normoalbuminuria group. With regard to glomerular indices, the eGFRMDRD was significantly decreased in the macro‐ and microalbuminuria groups, while significant reduction in the eGFRCys was found in all 3 groups (Table 2). Significant differences were observed in all the glomerular indices across the groups (Table 2).
Table 2.
Changes in the urine albumin‐to‐creatinine ratio, tubular indices, and glomerular indices, from baseline to week 24
| UACR (mg/gCr) | P across groups | |||
|---|---|---|---|---|
| <30 | 30‐<300 | ≥300 | ||
| UACR | ||||
| N | 658 | 271 | 49 | <.0001 |
| Median (mg/gCr) | −0.6* | −26.1** | −323.3** | |
| Median (mg/mmolCr) | −0.07* | −3.0** | −36.6** | |
| % | −6.5 | −48.5 | −49.7 | |
| Urinary NAG | ||||
| N | 658 | 271 | 49 | <.0001 |
| Median (U/gCr) | 1.2** | −0.07 | −3.9** | |
| Median (U/mmolCr) | 0.1** | −0.008 | −0.4** | |
| % | 18.5 | −1.2 | −26.9 | |
| Urinary beta2MG (mg/gCr) | ||||
| N | 658 | 271 | 49 | n.s. |
| Median (mg/gCr) | 4.6 | −1.4 | −39.2 | |
| Median (μg/mmolCr) | 0.5 | −0.2 | −4.4 | |
| % | 5.2 | −2.1 | −13.4 | |
| Creatinine (mg/dL) | ||||
| N | 661 | 278 | 49 | .0001 |
| Mean | 0.003 | 0.01* | 0.05** | |
| SD | 0.07 | 0.10 | 0.07 | |
| % | 0.80 | 2.47 | 5.73 | |
| eGFRMDRD (mL/min/1.73 m2) | ||||
| N | 661 | 278 | 49 | .01 |
| Mean | −0.3 | −1.6* | −4.2** | |
| SD | 9.6 | 11.8 | 7.4 | |
| % | 0.1 | −1.1 | −5.5 | |
| eGFRcys (mL/min/1.73 m2) | ||||
| N | 658 | 271 | 49 | .03 |
| Mean | −6.6** | −8.6** | −9.0** | |
| SD | 11.4 | 12.2 | 11.6 | |
| % | −5.7 | −7.8 | −10.1 | |
beta2MG, β2 microglobulin; eGFRMDRD, estimated glomerular filtration rate; eGFRcys, estimated glomerular filtration rate calculated from cystatin C; NAG, N‐acetyl‐β‐d‐glucosaminidase; n.s., not significant (across groups); UACR, urine albumin‐to‐creatinine ratio.
Median for UACR and tubular indices.
Mean (standard deviation).
One‐sample t test (vs baseline). Wilcoxon signed rank test (vs baseline) for urinary indices. ANOVA across groups for continuous variables. Kruskal‐Wallis test across groups for urinary indices.
*P < .05, **P < .001 vs baseline.
Significant improvements in the glycemic status (HbA1c and FPG), body weight and systolic BP were confirmed, on comparing the values at baseline and at week 24, with SGLT2 inhibition (Table S2). The magnitude of the changes in glycemic status, body weight and BP was similar across the groups (Table S2). While the diastolic BP was not reduced significantly in the macroalbuminuria group, significant decreases in the diastolic BP, from the baseline to week 24, were observed in the other groups (Table S2). The correlation analysis suggested that the changes in the tubular indices and UACR were associated with their baseline values (Figure 1). Log‐transformed NAG and beta2MG levels were significantly correlated with their corresponding baseline values (NAG, r = −.60 and beta2MG, r = −.49) (Figure 1). Similarly, the log‐transformed reduction in the UACR was significantly correlated with the baseline UACR (Pearson's coefficient, r = −.45) (Figure 1). Finally, the drug related adverse events of special interest in each group were evaluated (Table S5).
Figure 1.
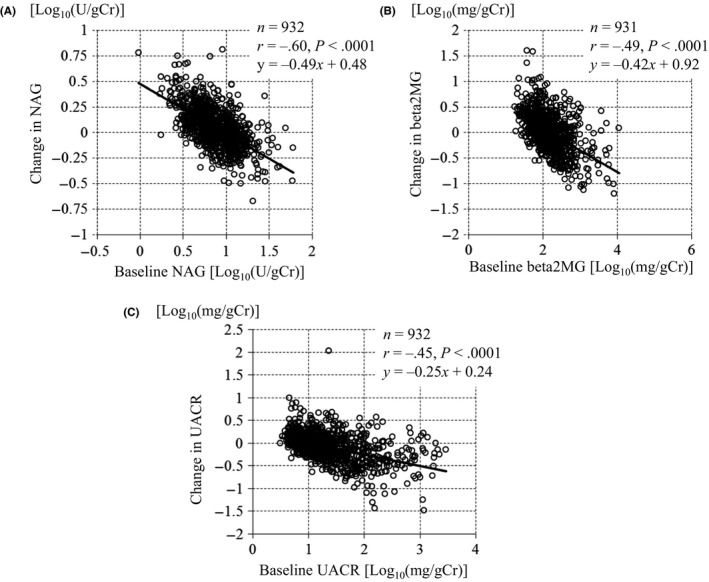
Correlations between the changes in tubular indices and the urine albumin‐to‐creatinine ratio (UACR), and their baseline levels. A, N‐acetyl‐beta‐d‐glucosaminidase (NAG), (B) Beta‐2 microglobulin (beta2MG), (C) UACR
4. DISCUSSION
This is the first study to evaluate the impact of TOFO on the renal tubules, in patients with T2DM, according to the degree of albuminuria. The results clearly showed that logarithmic reductions in the renal tubular indices, via SGLT2 inhibition, were observed in patients with T2DM. Thus, SGLT2 inhibition itself may produce logarithmic improvement not only in the case of albuminuria but also renal tubular injuries.
In recent times, the striking reductions in the relative risk of renal outcomes, including the progression of macroalbuminuria, were observed in the EMPA‐REG OUTCOME trial and CANVAS Program.11, 12 Cherney et al18 speculated that the direct effects of SGLT2 inhibition on albuminuria are not related to the improvements in the HbA1c levels, systolic BP, or body weight. In our study, SGLT2 inhibition resulted in an obscure reduction in the UACR, in the normoalbuminuria group. Prominent reductions in the UACR (approximately 50%) in the micro‐ and macroalbuminuria groups were observed; the degrees of improvement in the HbA1c level, BP, and body weight, in all groups, were similar (Table S2). Prominent reductions in the UACR, in the micro‐ and macroalbuminuria groups, were observed as early as week 12 (Figure 2). In addition, a logarithmic reduction in the UACR was significantly correlated with the baseline UACR, in all participants (Figure 1). Furthermore, we showed that significant correlations, similar to those of the UACR, were observed between the changes in the tubular indices (NAG and beta2MG) and their baseline values (Figure 1). Therefore, the effect of these characteristics of SGLT2 inhibition on albuminuria and tubular indices may suggest that SGLT2 inhibition itself is beneficial for the prevention of DN progression.
Figure 2.
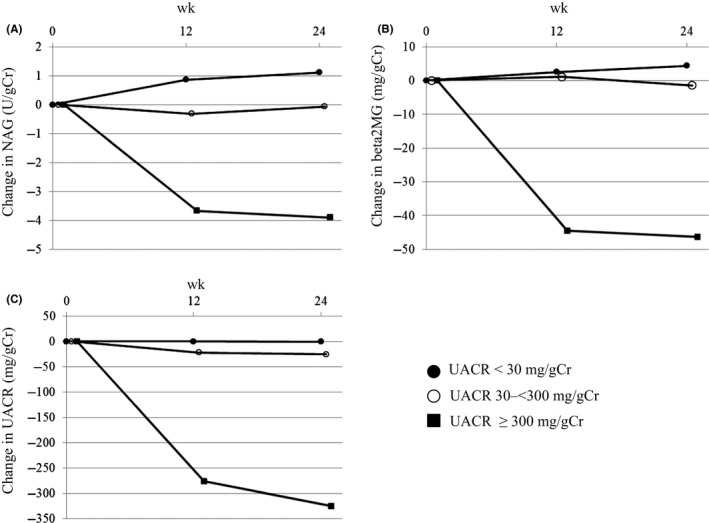
The process of the change in tubular indices and the urine albumin‐to‐creatinine ratio (UACR) in each group. A, N‐acetyl‐beta‐d‐glucosaminidase (NAG), (B) Beta‐2 microglobulin (beta2MG), (C) UACR. ●: UACR <30 mg/gCr, ○: UACR 30‐<300 mg/gCr, ■: UACR ≥300 mg/gCr, Median
We investigated the effects of TOFO on the tubular indices, by DN stages too. NAG levels were significantly reduced in the macroalbuminuria group; however, unexpectedly, they were significantly increased in the normoalbuminuria group (Table 2). Furthermore, we observed significant correlations between the changes in NAG levels and their baseline values (Figure 1). The elevation in the NAG levels, as observed in the normoalbuminuria group, might have been caused by the tubular cells’ functional response, as a larger amount of glucose in the urine might have flown into the backward tubule after SGLT2 inhibition. Differences in the response of NAG in each group could be attributed to the balance between the tubular cells’ functional response and the improvement in tubular damage. In the macroalbuminuria group, the improvements in the tubular damage were greater than the tubular cells’ functional response, suggesting that SGLT2 inhibition might have protective effects on the tubules. Following an increase in the DN stage, the baseline renal tubular indices increased (Table 1); afterwards, SGLT2 inhibition attenuated the resulting abnormalities, by DN stage (Table 2). In all the stages, significant positive correlations were found between changes in the UACR and tubular indices (Figures S1 and S2). This might suggest that there is a common improvement mechanism, through SGLT2 inhibition, underlying the UACR and tubular damage. When the individual DN stages were focused on, the UACR was found to be significantly reduced in the normoalbuminuria group. In the micro‐ and macroalbuminuria groups, in particular, the percentage changes in the UACR were not consistent with those in the tubular indices (Table 2). This finding was supported by the correlation analysis, which suggested that the regression line shifted downward in the micro‐ and macroalbuminuria groups (Figures S1 and S2). In the micro‐ and macroalbuminuria groups, the reductions in the UACR could be attributed to not only the tubular effects but also other glomerular effects including the improved function in mesangial cell and podocyte. Further clinical and preclinical studies need to be conducted for further elucidation.
In our study, greater reductions in the levels of estimated glomerular filtration rate (eGFR) at week 24 were observed, with increasing DN stage (Table 2). Cherney et al19 demonstrated the normalisation of tubuloglomerular feedback (TGF) via empagliflozin, in patients with type 1 DM with absolute hyperfiltration. They also suggested that empagliflozin caused afferent vasodilation and significantly improved intraglomerular hypertension.20 The reduction in the eGFR, in our study, could be attributed to the reset of glomerular hyperfiltration through SGLT2 inhibition, suggesting that anti‐hyperfiltration effects might contribute to reductions in the UACR. Improvements in the relative‐hyperfiltration, induced by SGLT2 inhibition, may impact the UACR through the TGF mechanism. The recovery of the eGFR might be attributed not only to the normalisation of the TGF but also other glomerular effects. In particular, preclinical experiments showed that SGLT2 is expressed in mesangial cells, and that the high uptake of glucose and Na+ through SGLT2, in conditions of hyperglycaemia, caused cellular dysfunction with associated hyperfiltration, while the inhibition of SGLT2 maintained cellular function.21, 22 Furthermore, the pharmacological inhibition of SGLT2 reduced the expansion of the mesangial areas in other experiments.23 Therefore, SGLT2 inhibition might produce an improvement in hemodynamic dysregulation, through the normalisation of TGF and other glomerular effects.
SGLT2 inhibitors are thought to be effective in lowering not only extracellular but also intracellular glucose levels. In the proximal tubular cells, intracellular hyperglycaemia, through SGLT2 overexpression, may increase the activity of polyol and hexosamine pathways, and induce the activation of protein kinase C and accumulation of advanced glycation products.24 The hyperglycaemia‐induced cellular abnormalities might produce oxidative stress, and stimulate the inflammation and fibrosis pathways, leading to tubulointerstitial damage, which might then cause the progression of diabetic complications including glomerular damages. In fact, in the proximal tubular cells, excessive glucose reabsorption causes cellular dysfunction.25 Experimental results show that SGLT2 inhibition weakens the cellular oxidative stress26 and knockdown of SGLT2 by small interfering RNAs (siRNAs) significantly inhibits high glucose‐induced reactive oxygen species generation and advanced glycation end‐products expression in proximal tubular cells.27 The current study showed that logarithmic improvements in the tubular indices were observed according to their baseline values (Figure 1). Therefore, SGLT2 might be the gatekeeper of glucose entry, and its inhibition might prevent the vicious cycle of intracellular hyperglycaemia in tubular cells. From the results of the EMPA‐REG OUTCOME, the effects of a lower dose and a higher dose of the SGLT2 inhibitor on slowing kidney disease progression were similar,11 which suggests that even a lower dose of SGLT2 inhibitor might be able to reduce the progression of diabetic complications through the improvement of intracellular hyperglycaemia. In the current study, reductions in the logarithmic tubular indices and UACR were observed irrespective of the dosage of TOFO (Table S4). Therefore, SGLT2 inhibitors are expected to cause marked reductions in tubular indices and the UACR early after the initiation of SGLT2 inhibition, by preventing the vicious cycle caused by intracellular hyperglycaemia.
This study has several limitations. Our study was performed in pooled analyses from 4 tofogliflozin studies, and we did not perform the comparison between placebo and tofogliflozin. Moreover, the number of participants in the current study was smaller, and the assigned dosage of tofogliflozin, in particular, in the macroalbuminuria group was 1‐sided. Further, we did not evaluate the effects of the concomitant medications including antihypertensive and antihyperglycaemic drugs. Moreover, the other parameters to evaluate the tubular and glomerular damages need to be measured and compared with those in healthy participants. Patients with renal insufficiency were excluded; thus, the evaluation in participants with lower eGFR levels and in the subgroups following both the degree of albuminuria and eGFR levels would be needed. Although histological confirmation was not performed, the patients’ backgrounds were consistent with those observed in previously conducted studies.28 A further prospective long‐term placebo‐controlled study of larger cohorts, in vitro molecular action, and pharmacological study are required to confirm our presumed explanation.
In conclusion, the use of an sodium‐glucose cotransporter 2 inhibitor logarithmically improved the renal tubular indices like urine albumin‐to‐creatinine ratio. Sodium‐glucose cotransporter 2 inhibitors may represent a promising treatment option for diabetic nephropathy through tubular effects.
CONFLICT OF INTEREST
KK has been an advisor to, and received honoraria for lectures from Astelas, Novo Nordisk Pharma, Sanwa Kagaku Kenkyusho, Takeda, Taisho Pharmaceutical, MSD, Kowa, Kissei, Sumitomo Dainippon Pharma, Novartis, Mitsubishi Tanabe Pharma, Nippon Boehringer Ingelheim, Daiichi Sankyo and Sanofi. AY is an employee of Kowa Pharmaceutical. HS is an employee of KOWA.
AUTHOR CONTRIBUTION
KN and YS contributed to the interpretation of data, writing of the first draft and revising the manuscript for important intellectual content. KK revised the manuscript for important intellectual content. AY researched data and reviewed the manuscript. HS created the database, performed statistical analyses and contributed to the interpretation of data. All authors have read and approved the final manuscript for submission.
Supporting information
ACKNOWLEDGEMENTS
The original studies on which the present article is based were funded by Chugai Pharmaceutical Co., Ltd.
Nunoi K, Sato Y, Kaku K, Yoshida A, Suganami H. Effects of sodium‐glucose cotransporter 2 inhibitor, tofogliflozin, on the indices of renal tubular function in patients with type 2 diabetes. Endocrinol Diab Metab. 2018;1:e15 10.1002/edm2.15
Contributor Information
Kiyohide Nunoi, Email: nu@st-mary-med.or.jp.
Kohei Kaku, Email: kka@med.kawasaki-m.ac.jp.
REFERENCES
- 1. Yamagishi S, Fukami K, Ueda S, Okuda S. Molecular mechanisms of diabetic nephropathy and its therapeutic intervention. Curr Drug Targets. 2007;8:952‐959. [DOI] [PubMed] [Google Scholar]
- 2. Vallon V, Thomson SC. Renal function in diabetic disease models: the tubular system in the pathophysiology of the diabetic kidney. Annu Rev Physiol. 2012;74:351‐375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dronavalli S, Duka I, Bakris GL. The pathogenesis of diabetic nephropathy. Nat Clin Pract Endocrinol Metab. 2008;4:444‐452. [DOI] [PubMed] [Google Scholar]
- 4. Dickson LE, Wagner MC, Sandoval RM, Molitoris BA. The proximal tubule and albuminuria: really!. J Am Soc Nephrol. 2014;25:443‐453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rahmoune H, Thompson PW, Ward JM, Smith CD, Hong G, Brown J. Glucose transporters in human renal proximal tubular cells isolated from the urine of patients with non‐insulin‐dependent diabetes. Diabetes. 2005;54:3427‐3434. [DOI] [PubMed] [Google Scholar]
- 6. Ikeda S, Takano Y, Cynshi O, et al. A novel and selective sodium‐glucose cotransporter‐2 inhibitor, tofogliflozin, improves glycaemic control and lowers body weight in patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2015;17:984‐993. [DOI] [PubMed] [Google Scholar]
- 7. Terauchi Y, Tamura M, Senda M, Gunji R, Kaku K. Efficacy and safety of tofogliflozin in Japanese patients with type 2 diabetes mellitus with inadequate glycaemic control on insulin therapy (J‐STEP/INS): results of a 16‐week randomized, double‐blind, placebo‐controlled multicentre trial. Diabetes Obes Metab. 2017;19:1397‐1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yamagishi S, Matsui T. Protective role of sodium‐glucose cotransporter 2 (SGLT2) inhibition against vascular complications in diabetes. Rejuvenation Res. 2015;19:107‐114. [DOI] [PubMed] [Google Scholar]
- 9. Vallon V, Rose M, Gerasimova M, et al. Knockout of Na‐glucose transporter SGLT2 attenuates hyperglycemia and glomerular hyperfiltration but not kidney growth or injury in diabetes mellitus. Am J Physiol Renal Physiol. 2013;304:F156‐F167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thomson SC, Rieg T, Miracle C, et al. Acute and chronic effects of SGLT2 blockade on glomerular and tubular function in the early diabetic rat. Am J Physiol Regul Integr Comp Physiol. 2012;302:R75‐R83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323‐334. [DOI] [PubMed] [Google Scholar]
- 12. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in t2 diabetes. N Engl J Med. 2017;377:644‐657. [DOI] [PubMed] [Google Scholar]
- 13. Kaku K, Watada H, Iwamoto Y, et al. Efficacy and safety of monotherapy with the novel sodium/glucose cotransporter‐2 inhibitor tofogliflozin in Japanese patients with type 2 diabetes mellitus: a combined Phase 2 and 3 randomized, placebo‐controlled, double‐blind, parallel‐group comparative study. Cardiovasc Diabetol. 2014;13:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tanizawa Y, Kaku K, Araki E, et al. Long‐term safety and efficacy of tofogliflozin, a selective inhibitor of sodium‐glucose cotransporter 2, as monotherapy or in combination with other oral antidiabetic agents in Japanese patients with type 2 diabetes mellitus: multicenter, open‐label, randomized controlled trials. Expert Opin Pharmacother. 2014;15:749‐766. [DOI] [PubMed] [Google Scholar]
- 15. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130:461‐470. [DOI] [PubMed] [Google Scholar]
- 16. Matsuo S, Imai E, Horio M, et al. Revised equations for estimating glomerular filtration rate (GFR) from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982‐992. [DOI] [PubMed] [Google Scholar]
- 17. Horio M, Imai E, Yasuda Y, et al. GFR estimation using standardized serum cystatin C in Japan. Am J Kidney Dis. 2013;61:197‐203. [DOI] [PubMed] [Google Scholar]
- 18. Cherney D, Lund SS, Perkins BA, et al. The effect of sodium glucose cotransporter 2 inhibition with empagliflozin on microalbuminuria and macroalbuminuria in patients with type 2 diabetes. Diabetologia. 2016;59:1860‐1870. [DOI] [PubMed] [Google Scholar]
- 19. Cherney DZ, Perkins BA, Soleymanlou N, et al. Renal hemodynamic effect of sodium‐glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation. 2014;129:587‐597. [DOI] [PubMed] [Google Scholar]
- 20. Skrtić M, Yang GK, Perkins BA, et al. Characterisation of glomerular haemodynamic responses to SGLT2 inhibition in patients with type 1 diabetes and renal hyperfiltration. Diabetologia. 2014;57:2599‐2602. [DOI] [PubMed] [Google Scholar]
- 21. Wakisaka M, Nagao T, Yoshinari M. Sodium glucose cotransporter 2 (SGLT2) plays as a physiological glucose sensor and regulates cellular contractility in rat mesangial cells. PLoS One. 2016;11:e0151585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wakisaka M, Nagao T. Sodium glucose cotransporter 2 in mesangial cells and retinal pericytes and its implications for diabetic nephropathy and retinopathy. Glycobiology. 2017;27:691‐695. 10.1093/glycob/cwx047. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Terami N, Ogawa D, Tachibana H, et al. Long‐term treatment with the sodium glucose cotransporter 2 inhibitor, dapagliflozin, ameliorates glucose homeostasis and diabetic nephropathy in db/db mice. PLoS One. 2014;9:e100777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615‐1625. [DOI] [PubMed] [Google Scholar]
- 25. Morrisey K, Steadman R, Williams JD, Phillips AO. Renal proximal tubular cell fibronectin accumulation in response to glucose is polyol pathway dependent. Kidney Int. 1999;55:160‐167. [DOI] [PubMed] [Google Scholar]
- 26. Langham RG, Kelly DJ, Gow RM, et al. Increased renal gene transcription of protein kinase C‐beta in human diabetic nephropathy: relationship to long‐term glycaemic control. Diabetologia. 2008;51:668‐674. [DOI] [PubMed] [Google Scholar]
- 27. Maeda S, Matsui T, Takeuchi M, Yamagishi S. Sodium‐glucose cotransporter 2‐mediated oxidative stress augments advanced glycation end products‐induced tubular cell apoptosis. Diabetes Metab Res Rev. 2013;29:406‐412. [DOI] [PubMed] [Google Scholar]
- 28. Kim SS, Song SH, Kim IJ, et al. Clinical implication of urinary tubular markers in the early stage of nephropathy with type 2 diabetic patients. Diabetes Res Clin Pract. 2012;97:251‐257. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


