Summary
Objective
To determine the glucose‐lowering mechanism of action and the effects of a quick‐release bromocriptine‐QR, a D2‐dopamine agonist (Cycloset) on vascular function in patients with type 2 diabetes (T2D).
Study design and methods
Fifteen poorly controlled T2D treated with metformin plus glucagon‐like peptide‐1 receptor agonists (GLP‐1RA) were studied after 4 months of Cycloset, 3.2 mg/d. Subjects received a 5‐hour double‐tracer (iv 3‐3H‐glucose and oral 14C‐glucose) mixed meal test (MMT) to quantitate rates of endogenous glucose production (EGP), oral glucose appearance (RaO) and disappearance (Rd) pre‐ and post‐Cycloset. Vascular assessments included 2‐day continuous BP monitoring, reactive hyperaemia index (RHI) and arterial stiffness (AS).
Results
HbA1c decreased from 8.3 ± 0.3% to 7.7 ± 0.2% (P < 0.05), fasting plasma glucose did not change (143 ± 4 vs 147 ± 5) and mean plasma glucose during MTT decreased from 223 ± 3 to 210 ± 4 mg/dL (P < 0.05) after Cycloset. Basal EGP (2.2 ± 0.2 vs 2.1 ± 0.2 mg/kg min) was unchanged, but there was greater MMT suppression (1.1 ± 0.1 vs 0.7 ± 0.1, P < 0.05). After Cycloset, RaO declined from 2.0 ± 0.1 to 1.7 ± 0.2 mg/kg min and peripheral oral glucose appearance from 53.1 ± 3.2 to 44.4 ± 3.1 g (P < 0.01). There were no changes in plasma insulin or glucagon concentration. Systolic (134 ± 4 vs 126 ± 6), diastolic (78 ± 3 vs 73 ± 4), mean BP (97 ± 5 vs 90 ± 4) and pulse pressure (54 ± 2 vs 51 ± 2 mm Hg) were reduced; RHI increased from 1.4 ± 0.1 to 1.9 ± 0.3 au and AS decreased modestly (19.8 ± 4.1 to 16.2 ± 3.7 au, P = NS).
Conclusions
Addition of Cycloset to GLP‐1 RA improved vascular indices and postprandial hyperglycaemia in T2DM primarily by lowering oral glucose appearance, suggesting that hepatic glucose uptake was enhanced. Improved vascular indices may explain the reduction in cardiovascular events observed with Cycloset therapy in patients with T2DM.
Keywords: bromocriptine, glucose efficacy, vascular protection
1. INTRODUCTION
Cycloset (quick‐release bromocriptine) is a sympatholytic D2‐dopamine agonist approved for the treatment of type 2 diabetes (T2D). Based on animal and human studies, timed bromocriptine administration within 2 hours of awakening has been shown to increase hypothalamic dopamine levels and inhibit excessive sympathetic tone within the central nervous system (CNS), resulting in blood glucose lowering.1, 2, 3 There is little to no evidence to suggest that Cycloset augments insulin secretion and whether tissue insulin sensitivity improves remains questionable. Addition of Cycloset to poorly controlled patients with T2D treated with diet alone, metformin, sulfonylureas or a thiazolidinedione produces a 0.5%‐0.7% decrement in HbA1c.4 Published results suggest that Cycloset acts primarily to reduce elevated postprandial glucose levels,4 but the mechanism(s) underlying this action have yet to be established. In a double‐blind, placebo‐controlled study of patients with T2D treated with metformin, Cycloset was shown to reduce the composite cardiovascular (CV) end‐point of myocardial infarction, stroke and CV mortality by 55%.5 The reasons behind this drug's beneficial effect on CV disease also remain unexplained.
As patients with T2D are at high risk for atherosclerotic cardiovascular complications, antidiabetic agents that improve glycaemic control and also reduce CV risk are desirable. Hyperglycaemia is a minor risk factor for coronary artery disease and stroke compared with other more established risk factors such as dyslipidaemia, hypertension, obesity and the insulin resistance (metabolic) syndrome.6, 7 However, even after the treatment of dyslipidaemia and hypertension, weight loss and improved glycaemic control, patients with T2D still remain at high risk for atherosclerotic cardiovascular complications.7 Among preatherosclerotic conditions, vascular endothelial dysfunction is a common abnormality.8, 9
To better understand the glucose‐lowering mechanisms and CV protective effects of Cycloset, we investigated glucose kinetics using a dual‐isotope technique and simultaneously assessed vascular function and CV risk factors. We chose to study poorly controlled patients with T2D treated with a GLP‐1 RA as unpublished data (VeroScience, Tiverton, RI, USA) suggested increase glycaemic efficacy of Cycloset in this group. If these two agents were to act via different mechanisms.10, 11 Cycloset and liraglutide combination therapy might have an additive or even synergistic effect to reduce postprandial plasma glucose levels in patients with T2D. Further, as liraglutide, similar to Cycloset, has been reported to reduce CV events in high‐risk patients with T2D,12 we sought to determine whether the addition of Cycloset would positively affect vascular parameters that could result in enhanced CV outcomes.
2. STUDY DESIGN AND METHODS
2.1. Subjects
Fifteen Hispanic (11 females; 4 males) poorly controlled (HbA1c >7.5%) type 2 diabetic patients treated with a stable dose of liraglutide (1.2‐1.8 mg/d; n = 15) plus metformin (n = 12) and low‐dose glargine insulin (n = 3) participated in the study. Subjects had mean (±SEM) age = 57 ± 9 years, BMI = 33.4 ± 4.4 kg/m2, A1c = 8.3 ± 0.5% and diabetes duration = 10.2 ± 5.6 years. Body weight (88 ± 13 kg) was stable (±3 lbs) over the 3 months prior to the study. Only subjects with a daytime feeding/night‐time sleeping schedule were included. All subjects followed a standard ADA‐recommended diet for diabetes, did not participate in any excessively heavy exercise programmes and were not taking any medications known to alter glucose metabolism (with the exception of metformin and insulin) or the neurosynaptic function. Individuals with evidence of major organ system disease, diabetic proliferative retinopathy and symptomatic neuropathy, as determined by physical examination, history and screening laboratory tests, were excluded. The study was approved by the University of Texas Health Science Center at San Antonio (UTHSCSA) IRB and informed written consent was obtained from all participants prior to the study. All study procedures were conducted at the Clinical Research Center at the Texas Diabetes Institute (TDI), University Hospital System, affiliated with the UTHSCSA.
2.2. Study design
On a separate day, subjects who met the entry criteria had a DEXA (Hologic Dexa Scanner, Bedford, MA, USA) in the morning following a 10‐ to 12‐hour overnight fast. Within 3‐5 days, subjects returned to the CRC at 0700 hours following a 10‐ to 12‐hour overnight fast for noninvasive hemodynamic testing and a 5‐hour meal tolerance test (MMT) with dual‐isotope technique (see below). Subjects refrained from use of caffeine, tobacco, vitamins or medications that might affect vascular tone for 24 hours prior to the study. Upon arrival at the CRC subjects rested for 15 minutes and a cuff was placed around the dominant arm for the measurement of blood pressure. Next, noninvasive assessment of endothelial function was conducted using the Endo‐PAT 2000 device (see below). After ~180 minutes, subjects received a 5‐hour dual‐isotope MMT. Subjects injected their normal dose of liraglutide and ingested the usual dose of metformin 30 minutes prior to MMT, but the insulin dose (n = 3) was withheld. Upon completion of the MTT, subjects were dismissed wearing a continuous ambulatory blood pressure (BP) monitor (Spacelabs Healthcare, Inc., Issaquah, WA, USA). Two days later, subjects returned and the BP data (mean systolic and diastolic BP, pulse rate, pulse pressure) were downloaded. On the following day, subjects were instructed to start Cycloset at the dose of 0.8 mg/d in the morning. After 1 week and weekly thereafter, Cycloset was increased to 1.6 mg, then to 2.4 mg until the maximum dose of 3.2 mg once daily was reached. During this period, subjects were instructed to self‐monitor capillary blood glucose and report any hypoglycaemia or other adverse effects. Subjects received frequent telephone calls, text messaging and monthly visits to the TDI Research Center to ensure compliance with the regimen. In two patients, the Cycloset dose was decreased to 2.4 mg/d for symptoms of orthostatic hypotension, lightheadedness, nausea and vomiting. All baseline studies including laboratory tests, DEXA, Endo‐PAT, double‐isotope MMT and continuous ambulatory BP monitoring were repeated 4 months after Cycloset was added to the therapeutic regimen.
2.3. Study procedures
2.3.1. Dual‐energy X‐ray absorptiometry (DEXA)
After an overnight fast, a DEXA scan to determine body composition was performed by a licensed radiology technician using a Discovery 010‐1596 Hologic DEXA scanner (Hologic Inc., Bedford, MA, USA). Subjects rested comfortably in supine position in a quiet room and the entire body was scanned. The acquired images were integrated and analysed by a software ibm computer program (Hologic Inc., Bedford, MA, USA) and the total amount of fat, fat‐free mass and total body water were estimated.
2.3.2. Endo‐PAT 2000
It was used to evaluate endothelial vasodilator function. Two plethysmography probes were placed on the index finger of each hand to record endothelium‐mediated changes in the digital pulse waveform, known as the peripheral arterial tone (PAT) signal. A blood pressure cuff at the level of the biceps was rapidly inflated to 60 mm Hg above the patient's systolic pressure or 200 mm Hg, whichever was higher. The complete cessation of blood flow to the hand was verified by the absence of a PAT signal from the occluded arm. After 5 minutes of occlusion, the cuff pressure was quickly released and 5‐minute postocclusion measurements were recorded. Readings in the contralateral arm were used as the control value. Following release of the arm cuff, a downstream hyperaemic response and endothelium‐mediated changes in the PAT signal are elicited. Endo‐PAT measurements were performed in the fasting state in a quiet, dimly lighted, temperature‐controlled examination room. Subjects remained supine and comfortable for 15 minutes with both arms supported at the patient's side. Blood pressure was measured in the arm that was not occluded during the Endo‐PAT study with a second blood pressure cuff. The Endo‐PAT system calculates the response to reactive hyperaemia and creates a PAT ratio using the post‐ and preocclusion values. These values are normalized against measurements from the contralateral arm, which serve as control for nonendothelial dependent systemic effects. The results are expressed as the reactive hyperaemia index (RHI), which gives an indication of the postischaemic endothelial vasodilator function. The Endo‐PAT system also measures the Augmentation Index (AI), which provides an index of arterial stiffness. The (AI) results are automatically corrected for the prevailing pulse rate and stored in the system.
2.3.3. Continuous ambulatory blood pressure monitor
Two‐day continuous ambulatory BP monitoring (Spacelabs Healthcare, Inc.) was obtained in all subjects after completion of the double‐isotope MMT. The BP monitoring device was placed and removed at the TDI Research Center, where the data were downloaded and recorded for analyses. Systolic and diastolic BP were continuously measured and mean BP was automatically calculated, along with average pulse rate and pulse pressure values.
2.3.4. Double‐isotope mixed meal test
Subjects reported to the TDI Research Center at 0700 hours following a 10‐ to 12‐hour overnight fast. A catheter was placed in an antecubital vein for the infusion of all test substances. A second catheter was inserted retrogradely into a hand vein on the dorsum of the hand which was placed in a heated box for withdrawal of arterialized blood samples. At −180 minutes, baseline blood samples were obtained and a prime (40 μCi × FPG/100) − continuous (0.40 μCi/min) infusion of 3‐3H‐glucose was started. Blood samples for determination of plasma glucose, insulin, C‐peptide, glucagon and FFA concentrations and tritiated glucose and 14C‐glucose radioactivity were obtained at −180, −30, −20, −10 and 0 minutes. At time zero, subjects ingested a mixed meal (75 g of glucose, 25 g of fat, 20 g of protein, 600 kcal) over 15‐20 minutes. The glucose in the meal was labelled with 100 μCi of 1‐14C‐glucose. Plasma samples for substrates, hormones and radioactivity were obtained every 15 minutes for 5 hours.
2.4. Calculations
During the fasting postabsorptive state, the rate of EGP equals the rate of glucose uptake by all tissues in the body and is calculated as the tritiated glucose infusion rate (DPM/min) divided by the plasma tritiated glucose specific activity (DPM/mg). Following meal ingestion, non‐steady‐state conditions prevail and the rates of total (RaT) and oral (RaO) glucose appearance in the systemic circulation are calculated using Steele's equation from the plasma 14C‐glucose and 3H‐glucose specific activities, respectively, as previously described.13 The difference between RaT and RaO yields the rate of EGP. Subtraction of RaO (calculated over the entire 300‐minute period postmeal) from 75 g (the ingested glucose load) gives the splanchnic (primarily reflects hepatic) glucose uptake. The incremental glucose area above baseline (ΔAUC) during the MMT was calculated using the trapezoidal rule. Insulin secretion was calculated for the entire 300 minutes of the post‐MMT period as: (I 0‐300/G 0‐300) and (CP0‐300/G 0‐300) and for the first 120 minutes post‐MMT as: (I 0‐120/G 0‐120); where I is plasma insulin ΔAUC, G is the plasma glucose ΔAUC, and CP is plasma C‐peptide ΔAUC. The Matsuda Index (MI) and the Insulin Secretion/Insulin Resistance (Disposition Index, DI) were calculated as previously published.14
2.5. Statistical analyses
The change in HbA1c, body weight and composition, fasting and post‐MMT plasma glucose and FFA concentrations and rates of endogenous (EGP) and exogenous glucose appearance (RaO) in the peripheral circulation between the baseline study and the study performed 4 months after Cycloset treatment were analysed using a paired t test. Similar comparisons were made for the changes in plasma insulin, C‐peptide, glucagon and insulin secretion indices, as well as for all measured vascular parameters using the paired t test. Post hoc testing was carried out with the Bonferroni correction. Values are presented as mean ± SEM. A P value <0.05 was considered statistically significant.
2.6. Sample size calculations
Assuming that Cycloset would cause a 20% decrease in the rate of total glucose appearance in the systemic circulation (RaT) with a SD of 15%, we computed that 15 subjects would provide 90% power to detect a significant change at an alpha of 0.05.
3. RESULTS
After 4 months of Cycloset, the HbA1c declined from 8.3 ± 0.3% to 7.7 ± 0.2% (P < 0.05). FPG did not change (145 ± 3 vs 147 ± 4 mg/dL), whereas the mean post‐meal plasma glucose (PG) concentration (Figure 1) decreased from 223 ± 3 to 210 ± 4 mg/dL (P < 0.01). The incremental area above the fasting plasma glucose concentration decreased from 23 469 ± 2346 to 18 482 ± 1848 mg/dL*300 min (P < 0.01). The fasting plasma FFA concentration (530 ± 12 vs 510 ± 20 μmol/L) and the per cent suppression (51% vs 50%) in plasma FFA concentration during the MMT were not significantly different before and after Cycloset.
Figure 1.
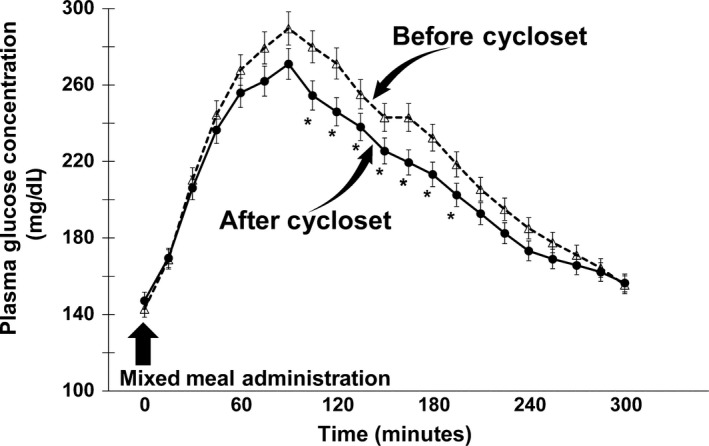
Plasma glucose concentration following the ingestion of a mixed meal before and 4 mo after Cycloset therapy. The fasting plasma glucose concentration did not change, whereas the mean post‐mixed meal plasma glucose concentration decreased after Cycloset therapy. There was a ~22% decline in the incremental area above the fasting plasma glucose, calculated during the 300 min following the mixed meal administration, after Cycloset therapy. *P < 0.01 Before vs After Cycloset
The basal rate of EGP was 2.2 ± 0.2 mg/kg min (191 ± 12 mg/min) and did not change significantly (2.1 ± 0.2 mg/kg min [184 ± 9 mg/min]) after 4 months of Cycloset. The per cent suppression of basal EGP during the MMT was comparable pre‐ and post‐therapy (~38% vs ~40%), respectively, although between 240 and 300 minutes EGP suppression was greater after Cycloset (~63%) than pretherapy (~52%). The mean pre‐ and post‐therapy EGP between 240 and 300 minutes decreased to 1.1 ± 0.1 (92 ± 8 mg/min) and to 0.7 ± 0.1 mg/kg min (67 ± 7 mg/min), respectively (P < 0.05) (Figure 2). The rate of oral glucose appearance (RaO) during the 0‐ to 300‐minute MMT was significantly decreased 4 months after the addition of Cycloset, from a mean 2.0 ± 0.1 to 1.7 ± 0.2 mg/kg min (177 ± 11 to 149 ± 10 mg/min) and total oral glucose load appearance in peripheral blood declined from 53.1 ± 3.2 to 44.4 ± 3.1 g (P < 0.01). The significant decrement in peripheral appearance of the oral glucose load was documented between 90 and 210 minutes during the MMT (Figure 3). There was no significant change in the total rate of glucose disposal during the MMT, 3.3 ± 0.3 vs 3.0 ± 0.3 mg/kg min (301 ± 27 vs 275 ± 31 mg/min), respectively, prior to and after 4 months of Cycloset therapy (Figure 4).
Figure 2.
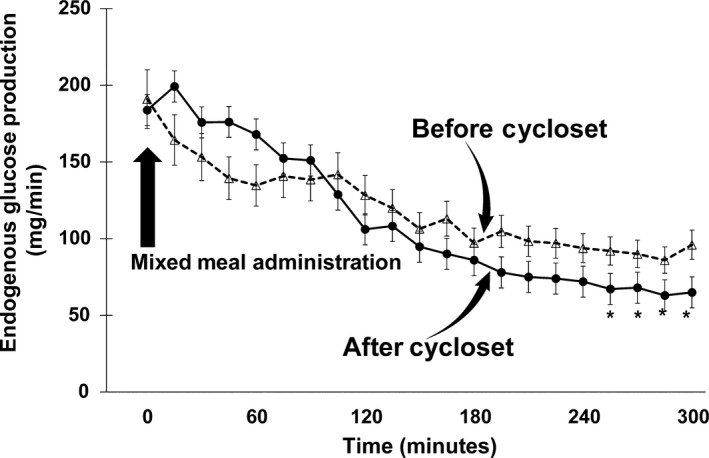
Rate of endogenous glucose production following the ingestion of a mixed meal before and after 4 mo of Cycloset therapy. The basal rate of endogenous glucose production did not change, and the per cent suppression over the 300 min post‐mixed meal was comparable before and after Cycloset therapy. However, between 240 and 300 min post‐mixed meal, the decrease in endogenous glucose production was slightly greater after Cycloset than before Cycloset. *P < 0.05 Before vs After Cycloset
Figure 3.
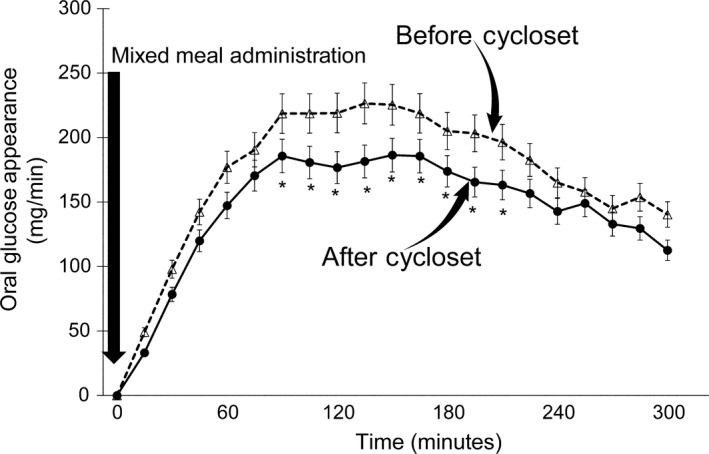
Rate of oral glucose appearance (RaO) following the ingestion of a mixed meal before and after 4 mo of Cycloset therapy. The rate of oral glucose appearance (RaO) during the 0‐300 min of the mixed meal test was significantly decreased, and the total oral glucose load appearance in peripheral blood declined by nearly 20% after Cycloset therapy. The decrement in peripheral appearance of the oral glucose load was documented between 90 and 210 min during the mixed meal test. *P < 0.01 Before vs After Cycloset
Figure 4.
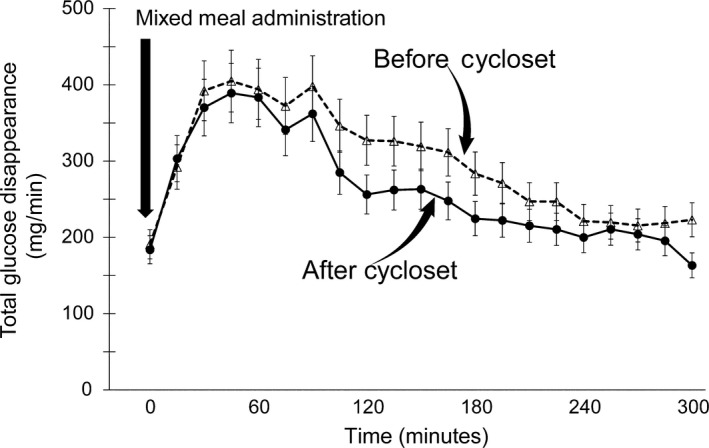
Rate of total glucose disappearance (Rd) following the ingestion of a mixed meal before and after 4 mo of Cycloset therapy. Basal and post‐mixed meal total glucose disappearance rates over the 300‐min period of observation were comparable before and after Cycloset therapy
The fasting plasma insulin concentration (20.0 ± 2.1 vs 22.2 ± 2.3 μU/mL) and the mean plasma insulin concentration during the MMT (43.3 ± 6.1 vs 51.1 ± 8.0 μU/mL) did not change significantly after Cycloset therapy. The fasting plasma C‐peptide concentration (4.8 ± 0.3 vs 4.6 ± 0.3 ng/mL) and the mean plasma C‐peptide during the MMT (10.1 ± 1.3 vs 10.0 ± 1.3 ng/mL) also did not change. Similarly, the fasting plasma glucagon concentration (61.2 ± 4.1 vs 55.4 ± 3.1 pg/mL) and the mean plasma glucagon during the MMT (60.0 ± 7.2 vs 56.7 ± 6.2 pg/mL) did not change. Cycloset did not change the insulin secretion indices calculated either as the change in plasma insulin divided by the change in plasma glucose over 300 minutes (0.32 ± 0.07 vs 0.42 ± 0.05 [μU/mL]/[mg/dL]), by the change in plasma C‐peptide over the change in plasma glucose over 300 minutes (0.07 ± 0.02 vs 0.08 ± 0.03 [ng/mL]/[mg/dL]), by the change in plasma insulin over the change in plasma glucose during the initial 120 minutes (0.22 ± 0.03 vs 0.24 ± 0.05 [μU/mL]/[mg/dL]), by the change in plasma C‐peptide over the change in plasma glucose during the initial 120 minutes (0.04 + 0.01 vs 0.05 + 0.01 [ng/mL]/[mg/dL]) or by the Insulin Secretion/Insulin Resistance (Disposition Index) (0.41 ± 0.40 vs 0.38 ± 0.34) of the MMT. The addition of Cycloset also had no effect on the Matsuda Index (1.8 ± 0.2 vs 1.6 ± 0.2) assessed over the initial 120 minutes.
Four months of Cycloset therapy had no effect on the pulse rate (74 ± 8 vs 73 ± 10 bpm), but the systolic (134 ± 4 vs 126 ± 6 mm Hg), diastolic (78 ± 3 vs 73 ± 4 mm Hg) and mean arterial blood pressure (97 ± 5 vs 90 ± 4 mm Hg) all decreased significantly (P < 0.05). The pulse pressure also decreased, but nonsignificantly from 54 ± 2 to 51 ± 2 mm Hg. The reactive hyperaemia index (RHI) obtained in each subject with the Endo‐PAT and expressed in arbitrary units (au) was compared to standardized normal values derived from age‐ and gender‐matched individuals, provided by the manufacturer. RHI values below 1.61 were considered abnormal. In 10 subjects with abnormally low baseline RHI values, there was an increase from 1.4 ± 0.1 to 1.9 ± 0.3 au after Cycloset treatment (P < 0.05). No significant change in RHI was noted in the five subjects who had a normal baseline RHI (2.5 ± 0.2 vs 2.1 ± 0.2 au). The individual Augmentation Index (AI) retrieved from the same Endo‐PAT measurements and corrected for the pulse rate represents an estimate of arterial stiffness. AI showed an improvement in 9 out of the 15 subjects, although the modest decline in AI did not reach statistical significance (19.8 ± 4.1 vs 16.2 ± 3.7 au).
There were no significant changes in body weight (88.1 ± 13.8 vs 87.1 ± 13.7 kg), BMI (33.4 ± 4.4 vs 33.1 ± 4.7 kg/m2) or per cent body fat (39.1 ± 6.7% vs 39.3 ± 7.4%) following the addition of Cycloset. In general, a stepwise gradual increase in Cycloset dose was well tolerated and only three patients had the final dose reduced to 2.4 mg/d because of nausea and lightheadedness.
4. DISCUSSION
Consistent with previous publication,4 the present results demonstrate that addition of the quick‐release sympatholytic D2‐dopamine agonist, Cycloset, to a GLP‐1 receptor agonist in patients with poorly controlled type 2 diabetes reduces the HbA1c by ~0.6%, (from 8.3 ± 0.3% to 7.7 ± 0.2%) during a 4‐month period. This decrease is explained entirely by a significant ~22% reduction in the post‐meal glycaemic excursion, as determined by the incremental area under the plasma glucose concentration above the baseline fasting glucose. There was no change in the fasting plasma glucose concentration after 4 months of Cycloset therapy. Using the double‐tracer technique, we demonstrated that the rate of systemic appearance of the ingested glucose load was significantly diminished after the mixed meal was consumed, which contributed the most to the postmeal decline in plasma glucose. A mild, but significant suppression of EGP noted during the last hour of the 300‐minute post‐MMT observation period also contributed to lower post‐meal hyperglycaemia. The rate of glucose disposal following ingestion of the mixed meal was unchanged by Cycloset therapy and did not interfere with the attenuation of the post‐meal glycaemic excursion. The curve depicting the changes in plasma glucose concentration over time following the mixed meal ingestion coincide very closely with the pattern of the changes in the rates of oral glucose appearance in the systemic circulation earlier (90‐210 minutes) and with the late EGP suppression between 240‐300 minutes (Figures 1, 2, 3). The reduced rate of oral glucose appearance is consistent with previous publications.15, 16 Assuming that there is no delay in gastrointestinal absorption (not measured in the present study), these observations suggest that Cycloset increases hepatic glucose uptake. Although dopamine binding predominantly to D3 receptors within the intestine may slow gut motility, activation of central dopamine D2 receptors (as with bromocriptine) acts as a primary regulator to increase gut motility, via activation of vagal and reduction in sympathetic efferent activities to the gut.17, 18 Further, this effect cannot be explained by changes in plasma insulin, glucagon or free fatty acid concentrations. This is the first demonstration in humans that a drug that dampens central sympathetic activity can augment hepatic glucose uptake and is consistent with studies in animals, which have documented an important role of sympathetic overactivity in the development of impaired glucose homeostasis.19, 20 In addition, our observation that Cycloset therapy was accompanied by a late post‐meal EGP suppression is consistent with a sympatholytic effect, brought about by the use of Cycloset on reducing hepatic glucose production, as shown in animal models.21, 22 Binding of bromocriptine to D2‐dopamine receptors in hypothalamic centres is known to restrain sympathetic nervous system activity and, act as a sympatholytic agent.3, 23 As a result, stimulation of hepatic glucose uptake and suppression of glucose production are not surprising. As similar findings have been reported in type 2 diabetic patients not treated with liraglutide,24 the enhanced splanchnic (hepatic) uptake of glucose following meal ingestion cannot be explained by background therapy with a GLP‐1 receptor agonist.
Cycloset therapy was associated with significant decreases in blood pressure and in pulse pressure. Vascular endothelial dysfunction improved in all 10 individuals with an abnormally low RHI and there was a trend for arterial stiffness, assessed by the Endo‐PAT to decline. Vascular endothelial dysfunction measured by the Endo‐PAT reflects the degree of vasodilation in the arterial microcirculation following a brief period of ischaemia. Under normal circumstances, postischaemic vasodilator agents, predominantly nitric oxide, are released into the regional circulation to enhance tissue reperfusion and oxygenation and to promote clearance of toxic waste products from the ischaemic area (also known as reactive hyperaemia).25 In patients with diabetes, subnormal postischaemic reactive hyperaemia has been ascribed to reduced availability of nitric oxide.25, 26 Our data indicate that the majority of patients with abnormal reactive hyperaemia at baseline showed significant improvements following treatment with Cycloset. Whether this represents a direct effect of the drug on the vasculature or an indirect effect mediated by the sympatholytic activity and/or improved glycaemic control (ΔA1c = 0.6%) associated with Cycloset therapy cannot be determined from this study. These results, however, may explain, in part, the cardiovascular protection that has been reported with this antidiabetic agent.27, 28, 29, 30 In this study, we did not observe any change in the heart rate following exposure to Cycloset, which is in agreement with earlier reports showing either minimal29 or no change30 in heart rate. The fact that heart rate did not increase despite a significant fall in arterial blood pressure, however, may be of clinical importance and is expected to occur with central inhibition of sympathetic tone.
The present study has several limitations and the most notable are: first, the relatively small number of subjects who were studied with the purpose of analysing several variables, while statistical power was calculated based solely on anticipated differences in the rate of systemic glucose appearance. The absence of a control group may be viewed as a shortcoming of the study design, although the main objective of the study was to elucidate the glucose‐lowering mechanisms with documentation of changes in glucose kinetics, which makes comparisons with a control group less relevant. Thirdly, 14 of the 15 subjects were Hispanic. Whether our results can be extended to T2D individuals of other ethnic backgrounds needs confirmation.
In summary, addition of Cycloset to poorly controlled type 2 diabetes individuals treated with liraglutide resulted in a significant decrease in HbA1c, which was explained by a reduction in postprandial hyperglycaemia following meal ingestion. These results suggest that Cycloset therapy increases hepatic glucose uptake following glucose ingestion, and it may even help to suppress endogenous glucose output. The decline in arterial blood pressure and pulse pressure, the improvement in endothelial dysfunction and the tendency for arterial stiffness to decline could provide cardiovascular protection and explain, at least in part, the cardiovascular benefits reported with the use of Cycloset.
CONFLICT OF INTERESTS
Ralph A. DeFronzo is a member of the advisory boards of Astra Zeneca, Janssen, Lexicon, Boehringer‐Ingelheim Lilly Alliance and Novo Nordisk. Ralph A. DeFronzo is a member of the speakers’ bureau of Novo Nordisk, Merck and AstraZeneca. Ralph DeFronzo has grant support from AstraZeneca and Janssen. Eugenio Cersosimo is a member of the advisory boards of Boehringer‐Ingelheim Lilly Alliance and Sanofi. Eugenio Cersosimo is a member of the speaker bureau of AstraZeneca, Janssen and Boehringer‐Ingelheim Lilly Alliance. Eugenio Cersosimo has grant support from AstraZeneca and Janssen. Mariam Alatrach, Christina Agyin, John Adams, Robert Chilton and Curtis Triplitt have no conflict of interests to declare.
AUTHOR CONTRIBUTION
MA, CA, JA, CT and EC conducted the studies and analysed and interpreted the data. RD, RC and EC designed the study, reviewed, analysed and interpreted the data and wrote the manuscript. RD represents that these data are original and not yet made public and is responsible for the content of this publication.
ACKNOWLEDGEMENTS
This study was supported by VeroScience, LLC (Investigator‐Initiated Grant to RAD), the Texas Diabetes Institute of the University Health System (technicians and laboratory equipment and supplies) and the Division of Diabetes, University of Texas Health Science Center at San Antonio (nursing staff, faculty time & effort, additional laboratory technicians, equipment, materials and supplies).
Alatrach M, Agyin C, Adams J, et al. Glucose lowering and vascular protective effects of cycloset added to GLP‐1 receptor agonists in patients with type 2 diabetes. Endocrinol Diab Metab. 2018;1:e34 10.1002/edm2.34
REFERENCES
- 1. Kamath V, Jones CN, Yip JC, et al. Effects of quick‐release form of bromocriptine (Ergoset) on fasting and postprandial plasma glucose, insulin, lipid, and lipoprotein concentrations in obese nondiabetic hyperinsulinemic women. Diabetes Care. 1997;20:1697‐1701. [DOI] [PubMed] [Google Scholar]
- 2. Pijl H, Ohashi S, Matsuda M, et al. Bromocriptine: a novel approach to the treatment of type 2 diabetes. Diabetes Care. 2000;23:1154‐1161. [DOI] [PubMed] [Google Scholar]
- 3. Cincotta AH. Hypothalamic role in the insulin resistance syndrome In: Hansen B, Shaffrir E, eds. Insulin Resistance Syndrome. London: Taylor and Francis; 2002:271‐312. [Google Scholar]
- 4. DeFronzo RA. Bromocriptine: a sympatholytic, D2‐dopamine agonist for the treatment of type 2 diabetes. Diabetes Care. 2011;34:789‐794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chamarthi B, Ezrokhi M, Rutty D, Cincotta AH. Impact of bromocriptine‐QR therapy on cardiovascular outcomes in type 2 diabetes mellitus subjects on metformin. Postgrad Med. 2016;128(8):761‐769. [DOI] [PubMed] [Google Scholar]
- 6. DeFronzo RA, Ferrannini E. Insulin resistance: a multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care. 1991;14:173‐194. [DOI] [PubMed] [Google Scholar]
- 7. DeFronzo RA. Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: the missing links. The Claude Bernard Lecture 2009. Diabetologia. 2010;53:1270‐1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ludmer PL, Selwyn AP, Shook TL, et al. Paradoxical vasoconstriction induced by acetylcholine in vessel atherosclerotic coronary arteries. N Engl J Med. 1986;315:1046‐1051. [DOI] [PubMed] [Google Scholar]
- 9. DeVriese AS, Verbeuren TJ, deVoorde JV, Lameire NH, Vanhoutte PM. Endothelial dysfunction in diabetes. Br J Pharmacol. 2000;130(5):963‐974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cervera A, Wajcberg E, Sriwijitkamol A, et al. Mechanism of action of exenatide to reduce postprandial hyperglycemia in type 2 diabetes. Am J Physiol Endocrinol Metab. 2008;294:E846‐E852. [DOI] [PubMed] [Google Scholar]
- 11. Cersosimo E, Gastaldelli A, Cervera A, et al. Effect of exenatide on splanchnic and peripheral glucose metabolism in type 2 diabetic subjects. J Clin Endocrinol Metab. 2011;96:1763‐1770. [DOI] [PubMed] [Google Scholar]
- 12. Blonde L, Russell‐Jones D. The safety and efficacy of liraglutide with or without oral antidiabetic drug therapy in type 2 diabetes: an overview of the LEAD 1‐5 studies. Diabetes Obes Metab. 2009;11:26‐34. [DOI] [PubMed] [Google Scholar]
- 13. Ferrannini E, Simonson DC, Katz LD, et al. The disposal of an oral glucose load in patients with non‐insulin dependent diabetes. Metabolism. 1988;37:79‐85. [DOI] [PubMed] [Google Scholar]
- 14. Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462‐1470. [DOI] [PubMed] [Google Scholar]
- 15. Scislowski PWD, Tozzo E, Zhang Y, Phaneuf S, Prevelige R, Cincotta AH. Biochemical mechanisms responsible for the attenuation of diabetic and obese conditions in ob/ob mice treated with dopaminergic agonists. Int J Obes Relat Metab Disord. 1999;23:425‐431. [DOI] [PubMed] [Google Scholar]
- 16. Cincotta AH, Meier AH. Bromocriptine inhibits in vivo free fatty acid oxidation and hepatic glucose output in seasonally obese hamsters (Mesocricetus auratus). Metabolism. 1995;44(10):1348‐1355. [DOI] [PubMed] [Google Scholar]
- 17. Toti L, Travagli RA. Gastric dysregulation induced by micro‐injection of 6_OHDA in the substantia nigra pars compacta of rats is determined by alterations in the brain‐gut axis. Am J Physiol Gastrointest Liver Physiol. 2014;307:G1013‐G1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lomax AE, Sharkey KA, Furness JB. The participation of the sympathetic innervation of the gastrointestinal tract in disease states. Neurogastroenterol Motil. 2010;22:7‐18. [DOI] [PubMed] [Google Scholar]
- 19. Shimazu T. Neuronal regulation of hepatic glucose metabolism in mammals. Diabetes Metab Rev. 1987;3:185‐206. [DOI] [PubMed] [Google Scholar]
- 20. Nonogaki K. New insights into sympathetic regulation of glucose and fat metabolism. Diabetologia. 2000;43(5):533‐549. [DOI] [PubMed] [Google Scholar]
- 21. van den Hoek AM, van Heijningen M, van der Elst JPS, et al. Intra‐cerebro‐ventricular administration of neuropeptide Y induces hepatic insulin resistance via sympathetic innervation. Diabetes. 2008;57:2304‐2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yi CX, Serlie MJ, Ackermans MT, et al. A major role for perifornical orexin neurons in the control of glucose metabolism in rats. Diabetes. 2009;58:1998‐2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Luo S, Zhang Y, Ezrokhi M, Li Y, Tsai T‐H, Cincotta AH. Circadian peak dopaminergic activity response at the biological clock pacemaker (suprachiasmatic nucleus) area mediates the metabolic responsiveness to a high fat diet. J Neuroendocrinol. 2018;30:e12563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bajaj M, Suraamornkul S, Pratipanawatr T, et al. Pioglitazone reduces hepatic fat content and augments splanchnic glucose uptake in patients with type 2 diabetes. Diabetes. 2003;52:1364‐1370. [DOI] [PubMed] [Google Scholar]
- 25. Cersosimo E, DeFronzo RA. Insulin resistance and endothelial dysfunction: the road map for cardiovascular diseases. Diabetes Metab Res Rev. 2006;22:423‐436. [DOI] [PubMed] [Google Scholar]
- 26. Wajcberg E, Thappil N, Fernandez M, et al. Comprehensive assessment of vascular reactivity in Hispanic children and adults with and without diabetes mellitus. Pediatr Diabetes. 2006;7:329‐335. [DOI] [PubMed] [Google Scholar]
- 27. Parkes D. Bromocriptine. N Engl J Med. 1979;301:873‐878. [DOI] [PubMed] [Google Scholar]
- 28. Chamarthi B, Gaziano M, Blonde L, et al. Timed bromocriptine‐QR therapy reduces progression of cardiovascular disease and dysglycemia in subjects with well‐controlled type 2 diabetes mellitus. J Diabetes Res. 2015;2015:157698 10.1155/2015/157698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gaziano JM, Cincotta AH, O'Connor CM, et al. Randomized clinical trial of quick‐release bromocriptine among patients with type 2 diabetes on overall safety and cardiovascular outcomes. Diabetes Care. 2010;33(7):1503‐1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gaziano JM, Cincotta AH, Vinik A, Blonde L, Bohannon N, Scranton R. Effect of bromocriptine‐QR (a quick‐release formulation of bromocriptine mesylate) on major adverse cardiovascular events in type 2 diabetes. J Am Heart Assoc. 2012;1(5):e002279. [DOI] [PMC free article] [PubMed] [Google Scholar]


