Summary
Background
Left ventricular (LV) diastolic dysfunction commonly is observed in individuals with type 2 diabetes mellitus (T2DM). We employed transthoracic echocardiography (TTE) and cardiac magnetic resonance imaging (CMRI) to investigate the hypothesis that LV diastolic dysfunction in T2DM is associated with poor glycemic control.
Methods
Forty subjects, 21 with normal glucose tolerance (NGT) and 19 with T2DM, were studied with CMRI and TTE to assess LV function. Early‐to‐late transmitral flow ratio (E/A) and deceleration time (DecT) were assessed with both modalities. Normalized (to body surface area) end‐diastolic volume (EDV/BSA) and normalized peak LV filling rate (pLVFR/BSA) were assessed with CMRI. Early transmitral flow velocity to septal velocity (E/e’) and isovolumetric relaxation time (IVRT) were measured using TTE. Dimensional parameters were normalized to body surface area (BSA).
Results
CMRI measurements demonstrated impaired E/A (1.13 ± 0.34 vs 1.62 ± 0.42, P < .001), increased DecT (174 ± 46 ms vs 146 ± 15, P = .005), as well as lower EDV/BSA (63 ± 10 vs 72 ± 9 mL/m2, P < .01) and pLVFR/BSA (189 ± 46 vs 221 ± 48 mL s−1 m−2, P < .05) in T2DM subjects. TTE measurements revealed lower E/A (1.1 ± 0.4 vs 1.4 ± 0.2, P < .001) and E/e’ (6.8 ± 1.5 vs 8.7 ± 2.0, P < .0001) with higher DecT (203 ± 22 ms vs 179 ± 18, P < .001) and IVRT (106 ± 14 ms vs 92 ± 10, P < .001) in T2DM. Multiple parameters of LV function: E/ACMRI (r = −.50, P = .001), E/ATTE (r = −.46, P < .005), pLVFR/BSA (r = −.35, P < .05), E/e’ (r = −.46, P < .005), EDV/BSACMRI (r = −.51, P < .0001), EDV/BSATTE (r = −.42, P < .01) were negatively correlated with HbA1c. All but E/e’ also were inversely correlated with fasting plasma glucose (FPG).
Conclusions
Impaired LV diastolic function (DF) was found in T2DM subjects with both CMRI and TTE, and multiple LVDF parameters correlated negatively with HbA1c and FPG. These results indicate that impaired LVDF is inversely linked to glycemic control in T2DM patients.
Keywords: clinical studies, echocardiography, magnetic resonance imaging, type 2 diabetes
1. INTRODUCTION
Impaired left ventricular diastolic function (LVDF) is a common finding in patients with type 2 diabetes mellitus (T2DM). Approximately 30% of individuals with T2DM manifest impaired LVDF without demonstrated coronary artery disease (CAD) or hypertension.1, 2, 3, 4 The pressure difference between left atrium (LA) and LV during diastole is the result of LV relaxation and transmitral flow, followed by LA contraction at the end of diastole. Prominent features of diabetic myocardial dysfunction5, 6, 7 include impaired LVDF with altered ventricular compliance and altered transmitral flow patterns during LV filling.8, 9, 10
Abnormal LVDF develops in stages, starting with delayed relaxation, to pseudonormal filling and finally to restrictive filling.11, 12, 13, 14, 15 Analysis of the transmitral velocity curve provides information about filling pressures and patient prognosis.16 Transmitral flow is dependent on multiple interrelated factors including the rate and extent of ventricular relaxation, atrial and ventricular compliance, mitral valve displacement, suction factor and left atrial pressure.17, 18
Transthoracic echocardiography (TTE) is routinely used to evaluate LVDF.8, 9, 10 Parameters measured include pulse‐wave Doppler transmitral blood flow and tissue Doppler imaging (TDI) of myocardial wall velocities, changes in LV volumes and pulmonary venous flow.17, 18 The ratio of early mitral valve flow velocity (E) to the tissue Doppler early diastolic lengthening velocities (e’) correlates closely with LV filling pressures.19 Cardiac magnetic resonance imaging (CMRI) also can be used to measure blood flow alterations, including reduction in early diastolic filling rate (E velocity) and deceleration time (DecT), which is the gradual deceleration of the early LV filling time.20, 21, 22, 23 Other parameters important to LVDF are the A wave and isovolumic relaxation time (IVRT), which are related to the higher effort expressed in late ventricular filling.1, 4, 17
The aim of this study was to examine the relationship between parameters of diastolic function, measured with both CMRI and TTE, and glycemic control in T2DM patients.
2. METHODS
2.1. Study population
Nineteen T2DM subjects and 21 age/gender/ethnicity‐matched normal glucose‐tolerant (NGT) control subjects participated in the study (Table 1). None of the control subjects had any history of cardiovascular disease and all had a normal echocardiogram. All T2DM subjects had undergone a diagnostic cardiac catheterization within the previous 6 months. Fifteen of the 19 T2DM subjects were confirmed to have coronary artery disease (CAD), but none had evidence of abnormal wall movement, abnormal systolic ejection fraction, valvular heart disease, or untreated coronary artery blockage. None of the T2DM subjects had evidence of proliferative retinopathy, peripheral neuropathy, or microalbuminuria. Exclusion criteria included clinical symptoms of heart failure or ischaemic coronary artery disease, hypertension (≥ 140/90 mm Hg) and standard exclusion criteria for MRI studies (aneurism clips, pacemakers, etc.). Symptoms related to ischaemic heart disease and New York Heart Association (NYHA) functional class, time of T2DM diagnosis and medication regimen were assessed (Table 1). The study was approved by the Institutional Review Board (IRB) of the UTHSCSA, and all subjects gave informed written consent.
Table 1.
Characteristics of study population
| Patient parameter | NGT (N = 21) | T2DM (N = 19) | P‐value |
|---|---|---|---|
| Age (y) | 45 ± 9 | 51 ± 8 | NS |
| Sex (M/F) | 9/12 | 11/8 | NS |
| Ethnicity (H/B/C) | 17/1/3 | 14/1/5 | NS |
| Diabetes duration (y) | NA | 3.4 ± 2.5 | |
| Weight (kg) | 73.4 ± 14.3 | 87.6 ± 12.8 | .0006 |
| Body mass index (kg·m−2) | 26.9 ± 3.9 | 31.7 ± 4.7 | .0004 |
| Body surface area (m2) | 1.82 ± 0.2 | 2.00 ± 0.2 | .001 |
| Body Fat (%) | 31.8 ± 8.5 | 34.7 ± 7.3 | NS |
| Waist circumference (cm) | 93.2 ± 11 | 105.2 ± 10.7 | .001 |
| Total cholesterol (mg·dL−1) | 177 ± 26 | 177 ± 51.4 | NS |
| HbA1c (%) | 5.4 ± 0.4 | 7.1 ± 1.4 | .0001 |
| Fasting plasma glucose (mg·dL−1) | 92 ± 6 | 151 ± 44 | .0001 |
| Triglycerides (mg·dL−1) | 110 ± 82 | 217 ± 146 | .002 |
| HDL (mg·dL−1) | 55 ± 11 | 42 ± 13 | .001 |
| LDL (mg·dL−1) | 102 ± 20 | 88 ± 25 | .03 |
| Systolic blood pressure (mm Hg) | 115 ± 10 | 123 ± 10 | .01 |
| Diastolic blood pressure (mm Hg) | 75 ± 8 | 79 ± 9 | .04 |
| Heart rate (bpm) | 62 ± 9 | 69 ± 11 | NS |
| Smoking (N;%) | 2 (10%) | 5 (26%) | NS |
| Medications (N;%) | |||
| Antidiabetic Medications | |||
| Metformin | — | 14 (74%) | — |
| Sitagliptin | — | 1 (5%) | — |
| Glipizide | — | 9 (47%) | — |
| Statins | — | 16 (84%) | — |
| Fibrates | — | 1 (5%) | — |
| Niacin | — | 2 (11%) | — |
| Antiplatelet drugs | — | 15 (79%) | — |
| ACE Inhibitors/ARBs | — | 14 (74%) | — |
| Ca antagonists | — | 2 (11%) | — |
| Beta‐blockers | — | 12 (63%) | — |
| Diuretics | — | 1 (5%) | — |
Data are presented as mean ± SD or n (%).
B, Blacks; C, Caucasian; H, Hispanics.
On the day of screening, HbA1c was measured by affinity chromatography (Biochemical Methodology, Drower 4,350; Isolab, Akron, OH). Plasma total cholesterol, HDL cholesterol and triglyceride levels were measured enzymatically on a Hitachi 704 autoanalyzer. LDL cholesterol was calculated from the Friedwald equation. On the day of enrolment, weight, height, waist circumference, blood pressure and heart rate were recorded after 5 minutes of reclining. Per cent body fat was determined by dual‐energy X‐ray absorptiometry (DEXA), as previously described.24
2.2. Transthoracic echocardiography
TTE was performed in the standard parasternal long‐ and short‐axis views and from apical orientations using a clinical system with a duplex 2.5‐4.0 MHz transducer (Logiq 9, GE Healthcare, Waukesha, WI). During 2D imaging, 3‐5 cardiac cycles were captured, and 10 cardiac cycles were recorded during Doppler imaging. The clinical function protocol included standard, comprehensive TTE systolic and diastolic assessments.19 Two‐dimensional (2D) TTE ventricular volumes, LV mass and LV ejection fraction, were calculated from the 4‐chamber and 2‐chamber areas using the modified Simpson's rule. Mitral inflow images were acquired in apical 4‐chamber and 5‐chamber views via pulsed‐wave Doppler sampling performed at the mitral valve leaflet tips perpendicular to the valve annulus. Tissue Doppler profiles were acquired in apical 4‐chamber view to determine mitral septal peak velocity (e’) and its relation to early diastolic velocity (E/e′) and left atrial diameter to help assess LV filling pressures.
The systolic and diastolic evaluations by echocardiography were performed by a single experienced echo‐cardiographer (MMW) who was blinded to CMR results. The diagnosis of impaired LVDF was graded based on the ratio of the diastolic transmitral flow velocity (E) to the peak diastolic transmitral flow velocity (A) and deceleration time (DecT) as the main parameters.6, 9, 10, 19 In patients with equivocal tissue Doppler results, pulmonary venous flow profiles also were measured to evaluate impaired LVDF.13
Two investigators (MMW and RJC) independently and blindly interpreted the measurements using standard classifications: normal, delayed relaxation, pseudonormalization, restrictive physiology or indeterminate by TTE. The results were based on criteria published in the ASE guidelines using adult cut‐off values.19, 25 The diagnosis of impaired LVDF was graded as described in previous studies.6, 9, 10, 20, 26, 27, 28 Adjustments were made for heart rate, age, body mass index (BMI), weight and body surface area (BSA).
2.3. Cardiac MRI
CMR was performed on 3.0 T MRI system (TIM Trio, Siemens Medical Solutions, Malvern, PA) with a six‐channel phased‐array torso coil and corresponding six posterior spine coil elements. Standard cardiac two‐, three‐ and four‐chamber localizer views were obtained using a gradient‐echo sequence (7 mm thick, 2.2 × 1.3 mm2 pixel). Cine imaging with retrospective gating was performed using a balanced steady‐state free precession sequence with iPAT=2, TR/TE = 2.44/1.22 ms, 25‐30 cardiac phases, matrix 224 × 288, FOV 336 × 430 mm2, 1.5 × 1.5 mm2 pixel. Contiguous short‐axis slices were acquired during repetitive breath‐holds at end‐expiration. Mitral inflow images were obtained with a phase‐contrast gradient‐echo sequence with through‐plane velocity encoding (Venc=100 cm/s) at the mitral valve. Slice thickness was 8 mm, FOV=228 × 430 mm, matrix=192 × 102, with 2.89 × 2.89 × 8.0 mm pixels, flip angle=10o, TR/TE=5.8/3.6 ms, acquiring 25‐30 cardiac phases.
CMR data were analysed using dedicated software (CMR42, Circle Cardiovascular Imaging Inc., Calgary AB) to perform global and regional LV function analyses from short‐axis images to determine LV volumes (trabeculae and papillary muscles included) and myocardial mass and cardiac output. Phase‐contrast CMR images were processed to produce transmitral flow profiles. Body surface area (BSA) was calculated using the Mosteller formula.29
2.4. Statistical analysis
Data are expressed as mean ± SD or percentages. Statistical analyses were performed using the R 3.4.2 statistical software (RStudio IDE, Version 1.0.153). Normality of data was assessed using the Shapiro‐Wilk test. Student's t test was used to evaluate the null hypothesis between the NGT and T2DM groups for continuous variables, with P < .05 deemed significant. The chi‐squared test of independence was used to evaluate the null hypothesis between the NGT and T2DM groups for categorical variables. Pearson's correlation was used to evaluate associations amongst imaging parameters. Spearman's correlation was used to assess associations between LV diastolic function and metabolic parameters, which failed the normality test. The bias and limits of agreement (LoA) between imaging methods were obtained by the Bland‐Altman analysis.30 Multivariate linear regression analysis was conducted by stepwise multiple linear regression with E/A, as the dependent variable and HbA1c, fasting glucose, BMI, per cent body fat, plasma triglyceride, HDL and systolic blood pressure as the independent variables. 31 Logarithmic transformation was used on parameters whose distributions were deemed non‐normal. The Tukey's ladder transformation process was used for variables that did not fit a non‐normal distribution following logarithmic transformation.
3. RESULTS
The clinical and metabolic characteristics of the study population are presented in Table 1. All subjects had either normal LVDF values (E/A ≥ 0.8, septal e′ ≥8, lateral e′ ≥10, deceleration time 140 to 240 ms) or Grade 1, mildly impaired LVDF (E/A < 0.8, septal e′ <8, lateral e′ <10, deceleration time >240 ms). T2DM and NGT subjects were well matched for age and gender. BMI, per cent body fat and waist circumference were higher in T2DM vs NGT subjects. T2DM subjects had reduced plasma HDL cholesterol and higher plasma triglyceride levels compared to NGT subjects. Systolic and diastolic blood pressure was slightly higher in T2DM subjects compared to NGT individuals. On mean, T2DM subjects were in reasonably good glycemic control as documented by HbA1c = 7.1 ± 1.4% (range = 5.3%‐10.6%).
3.1. LV systolic function
LV systolic functional measurements by TTE (Table 2) and CMRI (Table 3) were not significantly different between T2DM and NGT groups and were within the normal range. Ejection fraction was normal in both T2DM and NGT groups. Neither T2DM nor NGT groups showed evidence of changes in LVMI or left ventricular hypertrophy.32, 33 There was good agreement by Bland‐Altman analysis for the measurements of cardiac index by TTE and CMRI (bias = 0.66 L·min−1·m−2, LoA: −1.84 L·min−1 m−2 to 0.53 L·min−1·m−2) (Figure S1A) and of ejection fraction by TTE and CMR (bias = 1.47%, LoA: −11.4%‐14.4%; Figure S1B). Measurement of BSA‐normalized LV diastolic volumes by TTE and CMRI demonstrated a larger average difference between modalities (bias = −15.6 mL·m−2, LoA: −33‐0.53 mL·m−2; Figure S2A). However, LV myocardial mass measurements were comparable by TTE and CMRI (bias = 0.66 g·m−2, LoA: −1.84‐0.53 g·m−2; Figure S2B).
Table 2.
Measured transthoracic echocardiographic (TTE) parameters
| Parameter | NGT (N = 21) | T2DM (N = 19) | P‐value |
|---|---|---|---|
| Left atrial diameter (cm) | 3.4 ± 0.4 | 3.9 ± 0.5 | .001 |
| Ejection fraction (%) | 64.6 ± 2.3 | 64.3 ± 2.7 | NS |
| Fractional shortening (%) | 35.2 ± 1.8 | 34.5 ± 2.2 | NS |
| Cardiac index (L·min−1·m−2) | 2.3 ± 0.5 | 2.2 ± 0.4 | NS |
| Left ventricular mass index (g·m−²) | 81.7 ± 14.0 | 86.2 ± 13.2 | NS |
| Stroke volume (mL) | 67.2 ± 12 | 68 ± 13 | NS |
| Stroke volume/BSA (mL·m−²) | 37.4 ± 7.8 | 34.1 ± 7.2 | NS |
| End‐diastolic volume (mL) | 95.8 ± 10.1 | 100.2 ± 12.9 | NS |
| End‐diastolic volume/BSA (mL·m−²) | 53 ± 6 | 50 ± 5 | .05 |
| End‐systolic volume (mL) | 34.2 ± 4.3 | 35.6 ± 4.9 | NS |
| End‐systolic volume/BSA (mL·m−²) | 19.0 ± 2.9 | 17.8 ± 2.2 | NS |
Data are presented as mean ±SD.
Table 3.
CMRI measures of systolic and diastolic function
| NGT (N = 21) | T2DM (N = 19) | P‐value | |
|---|---|---|---|
| Systolic parameters | |||
| Ejection fraction (%) | 64 ± 0.1 | 62 ± 0.1 | NS |
| End‐systolic volume (mL) | 81 ± 14 | 83 ± 11 | NS |
| Stroke volume (mL) | 50 ± 15 | 47 ± 16 | NS |
| Cardiac output (L·min−1) | 5.4 ± 0.9 | 5.9 ± 1.5 | NS |
| Myocardial mass (g) | 107 ± 28 | 122 ± 33 | NS |
| LV peak ejection rate (mL·s−1) | 427 ± 67 | 477 ± 170 | NS |
| Diastolic parameters | |||
| End‐diastolic volume (mL) | 131 ± 23 | 126 ± 26 | NS |
| E/A (flow) | 1.62 ± 0.42 | 1.13 ± 0.34 | <.001 |
| E/A (max velocity) | 1.4 ± 0.4 | 1.1 ± 0.3 | <.001 |
| Deceleration time (ms) | 145.9 ± 14.6 | 174.4 ± 46.0 | .005 |
| LV peak filling rate (mL·s−1) | 405 ± 105 | 379 ± 96 | NS |
| Normalized to BSA | |||
| End‐diastolic volume/BSA (mL·m−2) | 71.8 ± 9.4 | 62.5 ± 10.2 | <.01 |
| End‐systolic volume/BSA (cm) | 27.3 ± 8.17 | 23.4 ± 4.9 | NS |
| Stroke volume/BSA (mL·m−2) | 42.9 ± 5.3 | 37.7 ± 7.7 | <.05 |
| Cardiac index (L·min−1·m−2) | 3.00 ± 0.46 | 2.91 ± 0.62 | NS |
| Left ventricular mass index (g·m−²) | 58.1 ± 10.1 | 60.6 ± 14.8 | NS |
| LV peak ejection rate/BSA (mL·s−1·m−2) | 235 ± 34 | 235 ± 68 | NS |
| LV peak filling rate/BSA (mL·s−1·m−2) | 221 ± 48 | 189 ± 46 | <.05 |
Data are presented as mean ±SD or %.
BSA, body surface area; LV, left ventricle.
3.2. LV diastolic function
Doppler measurements by echocardiography and flow assessment by CMRI showed that the E/A ratio measured by both methods was significantly decreased in T2DM vs NGT subjects (Tables 3 and 4). E/A values obtained by Doppler flow (NGT=1.4 ± 0.2, T2DM=1.1 ± 0.4, P < .001) and by phase‐contrast CMRI (NGT=1.62 ± 0.42, T2DM=1.13 ± 0.34, P < .001) both were significantly reduced in T2DM vs NGT. Bland‐Altman analysis between the pulsed‐wave Doppler E/A values and phase‐contrast CMRI E/A values showed good correlation (r = .73, P < .02) and agreement (bias = 0.02, LoA: −0.48 to +0.55; Figure S3A,B). The CMRI measurement of BSA‐normalized peak LV filling rate (189 ± 46 mL·s−1·m−2) was significantly lower in T2DM subjects compared to NGT subjects (221 ± 48 mL·s−1·m−2, P < .05; Figure S4A,B).
Table 4.
Echocardiographic Doppler flow and Doppler tissue parameters
| Patient parameter | NGT (N = 21) | T2DM (N = 19) | P‐value |
|---|---|---|---|
| E wave (cm·s−1) | 83.9 ± 15.7 | 77.8 ± 14.6 | NS |
| A wave (cm·s−1) | 62.5 ± 14.2 | 74.1 ± 18.3 | .01 |
| E/A ratio | 1.4 ± 0.2 | 1.1 ± 0.4 | .0005 |
| Deceleration Time (ms) | 179.1 ± 17.6 | 203.3 ± 21.7 | .0003 |
| e’ septal wave peak velocity (cm·s−1) | 12.6 ± 2.5 | 9.2 ± 1.3 | .006 |
| E/e’ ratio | 6.8 ± 1.5 | 8.7 ± 2.0 | <.0001 |
| Velocity propagation (cm·s−1) | 53.9 ± 5.8 | 42 ± 5.6 | <.0001 |
| Isovolumetric relaxation time (ms) | 91.6 ± 10.3 | 106.1 ± 14.1 | <.0001 |
| S/D ratio | 1.3 ± 0.3 | 1.1 ± 0.3 | .04 |
| PV Ar (cm·s−1) | 22.2 ± 3.9 | 26.1 ± 4.6 | .005 |
| E wave/Propagation velocity ratio | 1.6 ± 0.3 | 1.9 ± 0.3 | .003 |
Data are presented as mean ± SD.
TTE measurements of DecT were significantly higher in T2DM (203.3 ± 21.7 ms) vs NGT (179.1 ± 17.6 ms, P < .005). This also was true for DecT obtained with phase‐contrast CMRI (T2DM:174.4 ± 46 ms; NGT: 145.9 ± 14.6 ms, P < .01). However, the CMRI DecT values were generally lower than those obtained by TTE, the correlation between TTE and CMR DecT was not significant and Bland‐Altman analysis showed poor agreement.
TTE provided additional parameters for evaluation of LV diastolic function in T2DM. Left atrial diameter was within the normal range in all T2DM subjects but was significantly greater in T2DM vs NGT (3.9 ± 0.5 vs 3.4 ± 0.4 cm, P < .001). Isovolumetric relaxation time and pulmonary venous (PV) velocities in T2DM were within the range expected for mildly impaired LV diastolic function (DecT > 200 ms; IVRT ≥100 ms; pulmonary venous flow (S > D); annular e < 8 cm/s). E/A measured in T2DM (1.1 ± 0.4) did not reach the criterion (<0.8) for diastolic dysfunction but was significantly lower in T2DM vs NGT (Table 4). Doppler tissue velocity measurements showed that a septal peak e′ in T2DM (9.2 ± 1.3 cm/s) was lower than in NGT (12.6 ± 2.5 cm/s, P < .01) and that the E/e’ ratio (8.7 ± 2) in T2DM was higher than in NGT (6.8 ± 1.5, P < .0001). Peak atrial velocity (PVAr) (reflects retrograde pressure over the pulmonic veins secondary to increased pressure in left atrium) was significantly greater in T2DM (26.1 ± 4.6 cm/s) vs NGT (22.2 ± 3.9 cm/s, P < .01).
3.3. Correlations with diastolic function
Data from the NGT and T2DM groups were combined to determine correlations of diastolic function and metabolic parameters. Combining these data produced inherently bimodal data sets. Therefore, HbA1c, FPG, DecT by CMR and E/A by TTE data did not have normal distributions across both groups. E/A by CMRI was significantly and negatively correlated with HbA1c (ρ = −.61, P = .00003) and with FPG (r = −.60, P = .00004; Figure 1A,B). E/A by TTE also was significantly and negatively correlated with HbA1c (ρ = −.49, P < .001) and FPG (ρ = −.51, P = .0008; Figure 2A,B). DecT by CMRI was significantly correlated with FPG (ρ = .38, P = .02) but not HbA1c (ρ = .24, P = .15), while DecT obtained by TTE was significantly correlated with both HbA1c (ρ = .38, P = .015) and FPG (ρ = .46, P = .002; Figure 3A,B). EDV/BSA by CMRI was significantly and negatively correlated with HbA1c (ρ = −.59, P = .00007) and FPG (ρ = −.42, P < .01; Figure 4A,B). EDV/BSA by TTE was significantly and negatively correlated with HbA1c (ρ = −.56, P = .00016) and with FPG (ρ = −.37, P = .019). The mitral septal peak velocity (e’) was significantly and negatively correlated with HbA1c (ρ = −.58, P < .001) and FPG (ρ = −.74, P = .00004; Figure 5A,B). E/A by CMRI was significantly and negatively correlated with FFA (ρ = −.47, P = .02) and DecT obtained by TTE was significantly correlated with FFA (ρ = .44, P = .005). Because hypertension has been shown to be related to the development of diastolic dysfunction,34 we looked for correlations between both systolic and diastolic blood pressure and E/A, DecT, and EDV/VSA but none were found (P > .50). This is not surprising as blood pressure, although high in T2DM vs NGT, was only minimally increased (123/79 vs 115/75; Table 1). Because T2DM subjects were more obese than NGT individuals, we also looked for correlations between measures of obesity (BMI, % body fat, and waist circumference) and indices of diastolic function, but failed to observe any significant relationships (P > .30).
Figure 1.
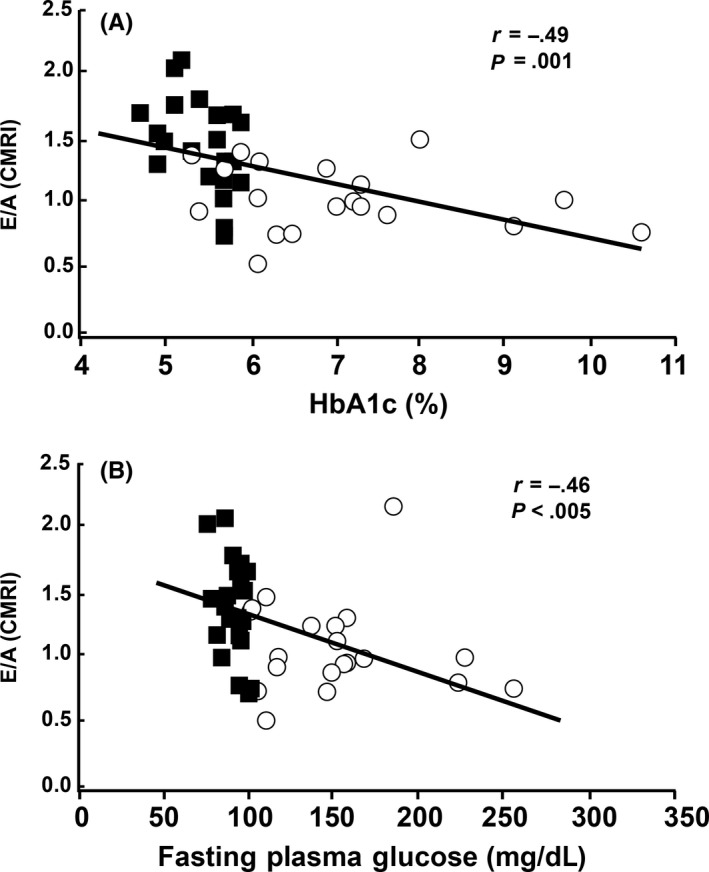
A, E/A values obtained by phase‐contrast CMRI were significantly and negatively correlated with both HbA1c (ρ =−.61, P < .0001) and B, fasting plasma glucose. (ρ = −. 60, P < .0001) Blue squares indicate NGT subjects and red circles indicate T2DM subjects
Figure 2.
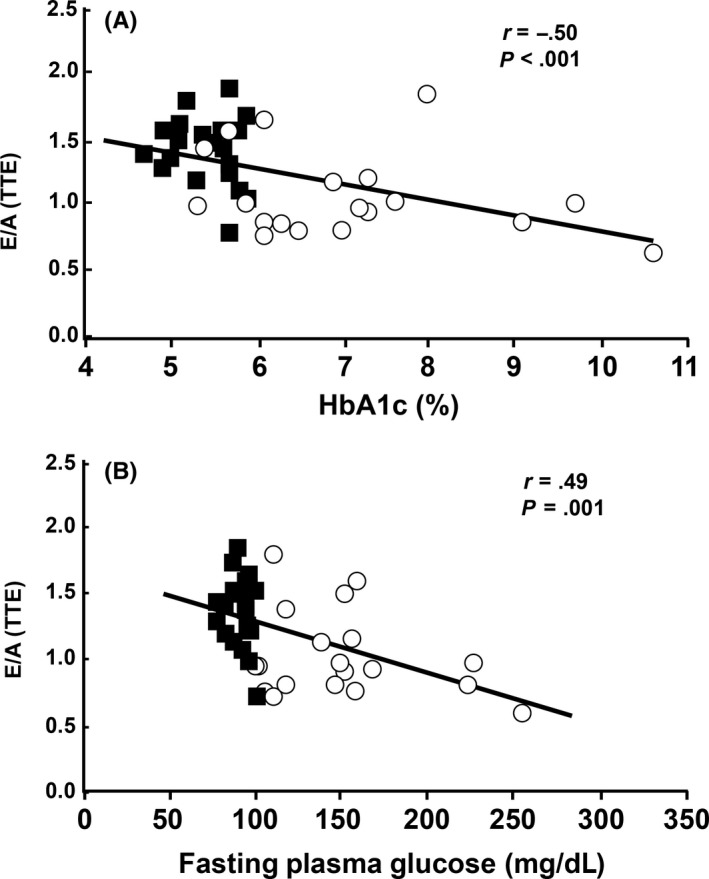
A, The E/A values obtained by transthoracic Doppler echocardiography were significantly and negatively correlated with HbA1c (ρ = −.49, P = .001) and B, with fasting plasma glucose (ρ = −.5, P<=.001). Blue squares indicate NGT subjects and red circles indicate T2DM subjects
Figure 3.
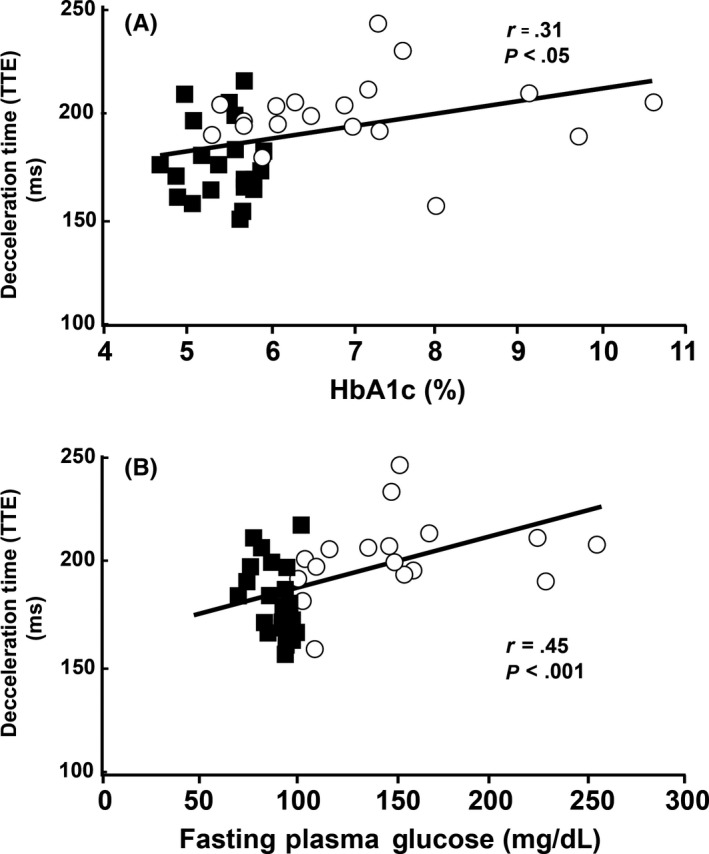
A, Deceleration times obtained from transthoracic Doppler echo were significantly correlated with HbA1c (ρ = .38, P = .015) and B, with fasting plasma glucose (ρ = .46, P < .005). Blue squares indicate NGT subjects and red circles indicate T2DM subjects
Figure 4.
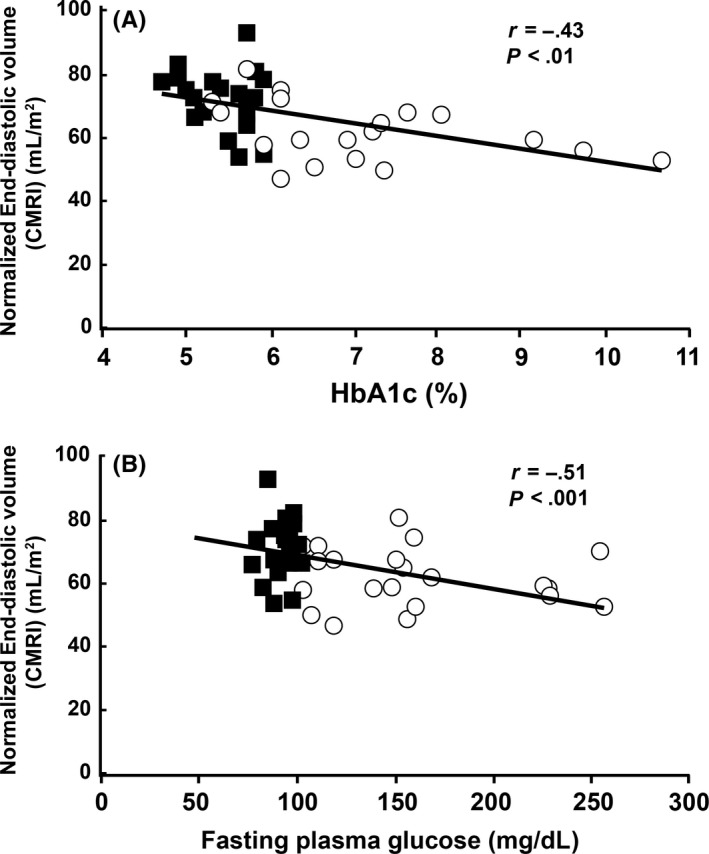
A, The normalized end‐diastolic volumes measured with CMRI were significantly and negatively correlated with HbA1c (ρ = −.59, P = 7 × 10−5) and B, with fasting plasma glucose (ρ = −.42, P < .01). Blue squares indicate NGT subjects and red circles indicate T2DM subjects
Figure 5.
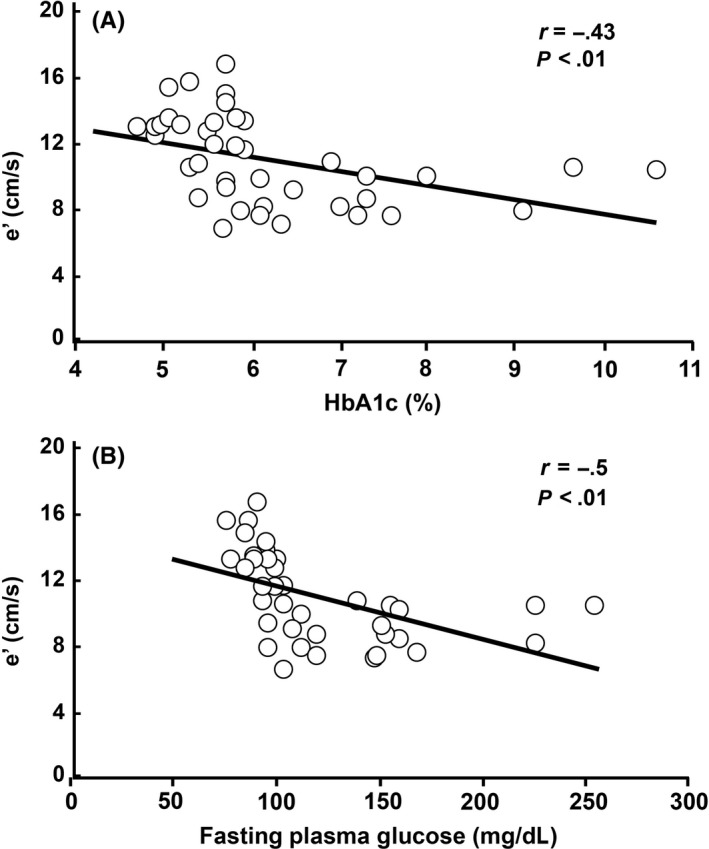
A, Tissue Doppler measurements of mitral septal peak velocity (e’) were significantly and negatively correlated with HbA1c (ρ = −.58, P = 8 × 105) and B, with FPG (ρ = −.74, P = 4 × 10−8). Blue squares indicate NGT subjects and red circles indicate T2DM subjects
3.4. Multivariate linear regression analysis
In a linear multivariate regression model with E/A as the dependent variable and HbA1c, fasting plasma glucose, BMI, per cent body fat, plasma triglyceride, HDL, and systolic blood pressure as the independent variables, only glucose control was significantly predictive of E/A. When HbA1c was included as a measure of glucose control, it was a significant predictor of E/A (P = .04); HDL and FFA were close to significance (P = .08). (Table 5) The coefficient of determination (r 2) for the regression model including the three parameters (HbA1c, FFA and HDL) was .35 (P = .0003). Replacing HbA1c with FPG as a measure of glycemic control did not affect the predictive power of glycemic control for E/A.
Table 5.
Results of multivariate analysis with E/A as the dependent variable resulted in r 2 = .35 (P = .0003)
| Dependent variables | β estimate | P‐value |
|---|---|---|
| Intercept | 1.82 | .01 |
| log (HbA1c) | −0.63 | .04 |
| log (FFA) | −0.23 | .08 |
| HDL | 0.01 | .08 |
4. DISCUSSION
The present study demonstrates that: (i) even reasonably well‐controlled T2DM patients (mean A1c = 7.1%) manifest evidence of impaired LV diastolic function despite completely normal LV systolic function. All subjects had normal systolic function, with EF > 50% by CMRI and EF > 60% by TTE; (ii) in the NGT group, most diastolic parameters fell within normal range; (iii) impaired diastolic function was associated with the level of glycemic control, as determined by the HbA1c and fasting plasma glucose concentration. The E/A ratio, by both imaging modalities, was significantly lower in T2DM vs NGT, while the DecT values, measured with both modalities, were significantly increased in T2DM vs NGT. The PLVFR/BSA also was lower in the T2DM group, while the E/e’ ratio was significantly higher in T2DM; the PLVR/BSA correlated inversely with HbA1c (r = −.31, P < .05). Both the end‐diastolic volume (Figure 4) and e’ (Figure 5) also correlated inversely with both the HbA1c and FPG glucose concentration.
Diastolic dysfunction is a complex condition, characterized by abnormal LV relaxation, filling diastolic distensibility and diastolic stiffness. Multiple TTE parameters are suggestive of diastolic dysfunction including E/A < 0.5, DecT > 280 ms, E/e’ >8 and LV mass index >122 g m−2.19, 35 In the current study, four NGT subjects and 17 T2DM subjects met the E/e′ threshold for diastolic dysfunction. As multiple parameters documented that diastolic function in the T2DM group was compromised compared to the control NGT group, we characterize this condition as “impaired diastolic dysfunction” to mimic the prediabetic state “impaired glucose tolerance.” This observation has important potential clinical observations as it may allow the clinician to identify those T2DM individuals with normal LV systolic function who are at risk to develop clinically significant diastolic dysfunction and diastolic heart failure.
As discussed previously, several imaging parameters were significantly and negatively correlated with HbA1c and FPG, including E/A, EDV/BSA and e’, while DecT was significantly and positively correlated with HbA1c and FPG levels. Thus, glycemic control was associated with impaired diastolic function in T2DM individuals even though the level of glycemic control (mean HbA1c = 7.1%) was reasonably good according to goals established by the American Diabetes Association.36 Although the mean HbA1c was 7.1%, the HbA1c range extended from 5.3% to 10.6%. Whether one uses the E/A ratio or the deceleration time (Figures 1 and 3), it is clear that even individuals with an HbA1c ≤ 7.0% manifest a decline in diastolic function. Previous studies have demonstrated that diastolic function is abnormal in T2DM subjects, but these studies primarily included T2DM patients with more severe heart disease and poor glycemic control.37, 38 The present study demonstrates that impaired LV diastolic function is evident even in well‐controlled T2DM patients. The triglyceride/HDL ratio, an index of insulin resistance, has been shown to be weakly correlated with diastolic dysfunction in insulin‐treated T2DM patients.39 However, in the present study, neither the triglyceride/HDL ratio nor the triglyceride or HDL concentrations individually correlated with any parameter of diastolic function. Hypertension also has been shown to be associated with diastolic dysfunction.33 In the present study, we failed to observe any correlation between systolic or diastolic blood pressure and any index of diastolic function. However, it should be noted that the blood pressure was very well controlled in the diabetic group. We also failed to find a correlation between any measure of obesity (BMI, per cent body fat, and waist circumference) and any parameter of diastolic function.
Lastly, in the multivariate linear regression analysis, only glycemic control parameters (ie, HbA1c and FPG) were found to be significant predictors of E/A, suggesting that glycemic control is related to diastolic dysfunction independent of other factors that may affect cardiac function (ie, blood pressure, BMI). Thus, the level of glycemic control (HbA1c) was the best correlate of underlying diastolic dysfunction in the present study. To the best of our knowledge, the current study is the first to demonstrate a significant association between multiple imaging parameters of diastolic function and the HbA1c and fasting plasma glucose in normotensive subjects with T2DM.
Systolic functional parameters measured by CMRI and TTE were in good general agreement, especially cardiac index, ejection fraction and myocardial mass. Amongst diastolic parameters, E/A and DecT, determined by both modalities, were in good agreement and differentiated T2DM from NGT, albeit with some overlap between groups.19, 35 Even though the DecT has been reported to correlate poorly with LV filling pressures,36 we observed a significant difference between T2DM and NGT groups by both TEE and CMRI. The lack of correlation between DecT values measured by TTE and by CMRI is attributed to the poor temporal resolution of CMRI compared to TTE. The isovolumic relaxation time was not found to be useful for identifying impaired diastolic function in the T2DM group.
The present study was limited in that the cine CMRI studies required multiple breath‐holds (up to 5) for full coverage of the left ventricle, which could degrade volume measurements in subjects who did not hold their breath consistently. In an attempt to decrease the impact of this problem, subjects were asked to hold their breath at end‐expiration. Furthermore, only two‐dimensional phase‐contrast CMRI was used, which is more dependent on positioning of image slices perpendicular to the direction of flow than 3D phase‐contrast methods. Likewise, the accuracy of the TTE Doppler measurements is dependent on accurate positioning of the ultrasound probe head with respect to acoustic windows. Despite these procedural limitations, we nonetheless were able to detect the subclinical presence of diastolic dysfunction in T2DM patients. Lastly, all 19 T2DM patients previously had undergone cardiac catheterization and 15 received treatment to reverse coronary arterial blockages. The increase in coronary perfusion from PTCA could have resulted in an improvement in diastolic dysfunction, explaining why E/A and DecT were only modestly reduced. Nonetheless, this did not obscure the inverse correlation between diastolic dysfunction and HbA1c and fasting plasma glucose concentration.
It is possible that the glycemic parameters (HbA1c and FPG) measured in the present study are the result of an underlying pathophysiologic disturbance, that is insulin resistance, which is more directly responsible for the diastolic dysfunction. Thus, further studies are required to determine what other metabolic/biochemical markers may be useful in identifying diabetic patients who have early diastolic dysfunction so that therapies can be instituted to reverse/delay the development of heart failure. With respect to this, one previous study37 demonstrated a weak correlation between HOMA‐IR and Ele’. A more precise measurement of insulin resistance with the euglycemic insulin clamp is indicated to examine the role of impaired insulin action in the development of diastolic dysfunction in patients with T2DM.
CONFLICT OF INTERESTS
Ralph DeFronzo is on the advisory boards for Astra Zeneca, Novo Nordisk, Janssen, Lexicon and Boehringer‐Ingelheim. He has research support from Bristol Myers Squibb, Boehringer‐Ingelheim, Takeda and Astra Zeneca. He is also on the speaker's bureau of Novo‐Nordisk and Astra Zeneca. None of the other authors have any conflicts of interests to report. The research reported here was funded, in part, by a grant from Takeda, Inc. All data collection, analysis and interpretation were carried out independently by the authors.
Supporting information
ACKNOWLEDGEMENT
All authors contributed to the performance of the study. RAD, MAG and GDC designed the study and wrote the original draft of the manuscript which subsequently was reviewed by all authors.
Clarke GD, Molina‐Wilkins M, Solis‐Herrera C, et al. Impaired left ventricular diastolic function in T2DM patients is closely related to glycemic control. Endocrinol Diab Metab. 2018;1:e14 10.1002/edm2.14
Geoffrey D. Clarke and Marjorie Molina‐Wilkins contributed equally to the performance of the study.
REFERENCES
- 1. Hirota Y. A clinical study of left ventricular relaxation. Circulation. 1980;62:756‐763. [DOI] [PubMed] [Google Scholar]
- 2. Devereux RB, Roman MJ, Liu JE, et al. Congestive heart failure despite normal left ventricular systolic function in a population‐based sample: the Strong Heart Study. Am J Cardiol. 2000;86:1090‐1096. [DOI] [PubMed] [Google Scholar]
- 3. Bursi F, Weston SA, Redfield MM, et al. Systolic and diastolic heart failure in the community. JAMA. 2006;296:2209‐2216. [DOI] [PubMed] [Google Scholar]
- 4. Zabalgoitia M, Ismaeil MF, Anderson L, Maklady FA. Prevalence of diastolic dysfunction in normotensive, asymptomatic patients with well‐controlled type 2 diabetes mellitus. Am J Cardiol. 2001;87:320‐323. [DOI] [PubMed] [Google Scholar]
- 5. Khan JN, Wilmot EG, Leggate M, et al. Subclinical diastolic dysfunction in young adults with type 2 diabetes mellitus: a multiparametric contrast‐enhanced cardiovascular magnetic resonance pilot study assessing potential mechanisms. Eur Heart J Cardiovasc Imaging. 2014;15:1263‐1269. [DOI] [PubMed] [Google Scholar]
- 6. Von Bibra H, Sutton MSJ. Diastolic dysfunction in diabetes and the metabolic syndrome: promising potential for diagnosis and prognosis. Diabetologia. 2010;53:1033‐1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Patil VC, Patil HV, Shah KB, Vasani JD, Shetty P. Diastolic dysfunction in asymptomatic type 2 diabetes mellitus with normal systolic function. J Cardiovasc Dis Res. 2011;2:213‐222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Appleton CP, Hatie LK, Popp RL. Relation of transmitral flow velocity patterns to left ventricular diastolic function: new insights from a combined hemodynamic and Doppler echocardiographic study. J Am Coll Cardiol. 1988;12:426‐440. [DOI] [PubMed] [Google Scholar]
- 9. Meissner JS, Pajaro OE, Yellin IL. Investigation of left ventricular filling dynamics. Einstein Quartely J Biol Med. 1986;4:47‐57. [Google Scholar]
- 10. Firstenberg MS, Greenberg NL, Main ML, et al. Determinants of diastolic myocardial tissue Doppler velocities: influences of relaxation and preload. J Appl Physiol. 2001;90:299‐307. [DOI] [PubMed] [Google Scholar]
- 11. Stoddard MF, Pearson AC, Kern MJ, Ratcliff J, Mrosek DG, Labovitz AJ. Left ventricular diastolic function: comparison of pulsed Doppler echocardiographic and hemodynamic indexes in subjects with and without coronary artery disease. J Am Coll Cardiol. 1989;13:327‐336. [DOI] [PubMed] [Google Scholar]
- 12. Hammermeister KE, Warbasse JR. The rate of change of left ventricular volume in man. II. Diastolic events in health and disease. Circulation. 1974;49:739‐747. [DOI] [PubMed] [Google Scholar]
- 13. Klein AL, Burstow DJ, Tajik AJ, Zachariah PK, Bailey KR, Seward JB. Effects of age on left ventricular dimensions and filling dynamics in 117 normal persons. Mayo Clin Proc. 1994;69:212‐224. [DOI] [PubMed] [Google Scholar]
- 14. Yellin EL, Meisner JS. Physiology of diastolic function and transmitral pressure‐flow relations. Cardiol Clin. 2000;18:411‐433, vii. [DOI] [PubMed] [Google Scholar]
- 15. Thomas JD, Weyman AE. Echocardiographic Doppler evaluation of left ventricular diastolic function: physics and physiology. Circulation. 1991;84:977‐990. [DOI] [PubMed] [Google Scholar]
- 16. Nishimura RA, Tajik AJ. Evaluation of diastolic filling of left ventricle in health and disease: Doppler echocardiography is the clinician's Rosetta Stone. J Am Coll Cardiol. 1997;30:8‐18. [DOI] [PubMed] [Google Scholar]
- 17. Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251‐259. [DOI] [PubMed] [Google Scholar]
- 18. Yu CM, Lin H, Yang H, Kong SL, Zhang Q, Lee SW. Progression of systolic abnormalities in patients with “isolated” diastolic heart failure and diastolic dysfunction. Circulation. 2002;105:1195‐1201. [DOI] [PubMed] [Google Scholar]
- 19. Paulus WJ, Tschöpe C, Sanderson JE, et al. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Euro Heart J. 2007;28:2539‐2550. [DOI] [PubMed] [Google Scholar]
- 20. Rathi VK, Doyle M, Yamrozik J, et al. Routine evaluation of left ventricular diastolic function by cardiovascular magnetic resonance: a practical approach. J Cardiovasc Magn Reson. 2008;10:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gatehouse PD, Keegan J, Crowe LA, et al. Applications of phase contrast flow and velocity imaging in cardiovascular MRI. Eur Radiol. 2005;15:2172‐2184. [DOI] [PubMed] [Google Scholar]
- 22. Krishnamurthy R, Pednekar A, Cheong B, Muthupillai R. High temporal resolution SSFP cine MRI for estimation of left ventricular diastolic parameters. J Magn Reson Imag. 2010;31:872‐880. [DOI] [PubMed] [Google Scholar]
- 23. Caudron J, Fares J, Bauer F, Dacher JN. Evaluation of left ventricular diastolic function with cardiac MR imaging. Radiographics. 2010;31:239‐259. [DOI] [PubMed] [Google Scholar]
- 24. DeFronzo RA, Tripathy D, Schwenke DC, et al. Pioglitazone for diabetes prevention in impaired glucose tolerance. New Engl J Med. 2011;364:1104‐1115. [DOI] [PubMed] [Google Scholar]
- 25. Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocard. 2009;22:107‐133. [DOI] [PubMed] [Google Scholar]
- 26. Oh JK, Appleton CP, Hatle LK, Nishimura RA, Seward JB, Tajik AJ. The noninvasive assessment of left ventricular diastolic function with two‐dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 1997;10:246‐270. [DOI] [PubMed] [Google Scholar]
- 27. Lester SJ, Tajik AJ, Nishimura RA, Oh JK, Khandheria BK, Seward JB. Unlocking the mysteries of diastolic function: deciphering the Rosetta Stone 10 years later. J Am Coll Cardiol. 2008;51:679‐689. [DOI] [PubMed] [Google Scholar]
- 28. Kitzman DW, Little WC. Left ventricle diastolic dysfunction and prognosis. Circulation. 2012;125:743‐745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Verbraecken J, Van de Heyning P, De Backer W, Van Gaal L. Body surface area in normal‐weight, overweight, and obese adults. A comparison study. Metabolism. 2006;55:515‐524. [DOI] [PubMed] [Google Scholar]
- 30. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;327:307‐310. [PubMed] [Google Scholar]
- 31. Dalgaard P. Introductory Statistics With R, 2nd edn New York, NY: Springer Science & Business Media; 2008:190‐193. [Google Scholar]
- 32. Devereux RB, Reichek N. Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation. 1977;55:613‐618. [DOI] [PubMed] [Google Scholar]
- 33. Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2015;18:1440‐1463. [DOI] [PubMed] [Google Scholar]
- 34. Beevers G, Lip GYH, O'Brien E. ABC of hypertension: the pathophysiology of hypertension. BMJ. 2001;322:912‐916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Al Jaroudi WA, Thomas JD, Rodriguez LL, Jaber WA. Prognostic value of diastolic dysfunction: state of the art review. Cardiology in Review. 2014;22:79‐90. [DOI] [PubMed] [Google Scholar]
- 36. American Diabetes Association . Standards of medical care in diabetes‐2014. Diabetes Care. 2104;37:S14. [DOI] [PubMed] [Google Scholar]
- 37. Shah AM, Shin SH, Takeuchi M, et al. Left ventricular systolic and diastolic function, remodeling, and clinical outcomes among patients with diabetes following myocardial infarction and the influence of direct renin inhibition with aliskiren. Eur J Heart Failure. 2012;14:185‐192. [DOI] [PubMed] [Google Scholar]
- 38. Fontes‐Carvalho R, Ladeiras‐Lopes R, Bettencourt P, Leite‐Moreira A, Azevedo A. Diastolic dysfunction in the diabetic continuum: association with insulin resistance, metabolic syndrome and type 2 diabetes. Cardiovasc Diabetol. 2015;14:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Von Bibra H, Paulus WJ, Sutton MSJ, Leclerque C, Schuster T, Schumm‐Draeger PM. Quantification of diastolic dysfunction via the age dependence of diastolic function—Impact of insulin resistance with and without type 2 diabetes. Intl J Cardiol. 2015;182:368‐374. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


