Summary
Aims
Knowledge about adult‐onset (AO) type 1 diabetes remains insufficient. We sought to characterize the initial 5 years of AO type 1 diabetes and hypothesized that initial factors predictive of subsequent glycaemic control might exist.
Materials and methods
A retrospective cohort study based on electronic medical records of 280 subjects with newly diagnosed AO type 1 diabetes (>18 years of age, excluding secondary and latent autoimmune diabetes) with available data for the initial 5‐year treatment.
Results
Characteristics at diagnosis: 61% men, mean age 37 ± 12 years, BMI 23 ± 3.3 (kg/m2), systolic/diastolic blood pressure: 123 ± 15/76 ± 9 mm Hg and LDL cholesterol: 2.9 ± 0.9 mmol/L. HbA1c decreased from 106 mmol/mol (11.8%) at diagnosis to 52 mmol/mol (6.9%) at 6 months and then increased gradually to 67 mmol/mol (8.3%) after 5 years. Strict glycaemic control (<53 mmol/mol (7%)) was achieved by 66% within 6‐9 months and 30% after 5 years. Comparing patients with and without strict glycaemic control after 5 years revealed no differences in HbA1c, C‐peptide or any other diabetes‐related parameter at the time of diagnosis. However, reaching strict control within 6‐9 months after diagnosis was strongly associated with remaining in strict control after 5 years (OR: 9.2 (CI‐95% 4.0‐20.9; P < 0.0001)). Conversely, patients who did not achieve early strict control were very unlikely to be well controlled after 5 years.
Conclusions
Long‐term tight glycaemic control in subjects with AO type 1 diabetes is both achievable and to some extent predictable. Whether alternative strategies shortly after diagnosis would benefit patients with insufficient glycaemic control should be investigated.
Keywords: adult‐onset type 1 diabetes, cohort study, glycaemic control, type 1 diabetes
1. INTRODUCTION
Adult‐onset (AO) type 1 diabetes represents half of the patients who are diagnosed with type 1 diabetes. Nonetheless, this group of patients is considerably less well described in the literature, compared to patients with onset during childhood or adolescence. Since adult patients often represent a more heterogeneous group with an admixture of type 2 diabetes compared to a younger age group, disease classification and diagnosis is an added challenge.1 An intriguing example is latent autoimmune diabetes of the adult (LADA), the diagnosis of which may depend upon the physician's choice of initial treatment.2
Many aspects of type 1 diabetes differ between patients with onset in childhood and adolescence compared to adult onset. For example, age‐dependent differences have been described concerning pathogenesis,3 pattern of autoantibodies and interaction with genetic predisposition,4 disease progression in terms of decline in β‐cell function and response to immuno‐modulatory treatment.5 Timely diagnosis is important since it will have a direct impact on the choice and modality of treatment. Diagnostic criteria and challenges aside, we hypothesized that there might be factors, either at presentation or during the first years of treatment, that may serve as discriminators between patients who achieve robust glycaemic control and those who do not. Such factors may be informative of unmet needs in terms of treatment modality or intensity at an early time point.
2. MATERIALS AND METHODS
2.1. Study design and population
We conducted a retrospective cohort study based on electronic medical records from the period 2001‐2012. We included all patients above 18 years of age with a diagnosis of newly onset type 1 diabetes, who had been followed for at least 5 years. “Newly onset” was defined as having the initial visit at Steno Diabetes Center (SDC; Gentofte, Denmark) within 4 weeks of diagnosis. Diagnosis was based on classical clinical type 1 diabetes criteria, immediate need for insulin treatment and clinical presentation (history of weight loss or diabetic ketoacidosis), whereas the presence of autoantibodies was not mandatory. Hence, patients with latent autoimmune diabetes of the adult (LADA), defined as GAD antibody‐positive patients with lack of immediate insulin treatment (within 6 months after diagnosis), were not included in the current analysis. A total of 280 patients matched these selection criteria. Baseline (time of diagnosis) parameters included in the analyses were as follows: age, gender, baseline C‐peptide, GAD antibody level, yearly measurement of albuminuria; albumin‐to‐creatinine ratio (ACR), lipid profile, along with quarterly data on HbA1c, total daily insulin dose, weight and blood pressure. Furthermore, information regarding smoking, exercise and Problem Areas In Diabetes 1 (PAID‐1) score6 was also included.
Patients are referred to SDC from a well‐defined geographical area of Copenhagen (DK). All patients with newly diagnosed type 1 diabetes at the centre follow a standardized patient educational programme; they are seen by physicians, nurses and dietitians for multiple scheduled visits the first year, followed by 3‐4 yearly visits predominantly by physicians. The educational programme and standards for visits have essentially remained the same during the observational period.
2.2. Measures
All laboratory and anthropometric measurements were recorded using standardized procedures at the SDC accredited laboratory. HbA1c was measured by ion exchange high‐performance liquid chromatography (TOSOH Automated Glycohemoglobin Analyzer, Minato‐ku, Tokyo, Japan). Total‐cholesterol, HDL‐cholesterol and fasting plasma triglycerides (TG) were measured enzymatically after hydrolysation and oxidation using dry chemistry reagent slides (Ortho‐Clinical Diagnostics, Illkirch Cedex, France). LDL cholesterol was calculated using the Friedewald equation (Friedewald WT 1972). Urine albumin concentration was measured by quantitative immunological turbidimetry (Hitachi 717 analyser, Boeringer, Mannheim, Germany) on a single urine specimen. Automated oscillometric blood pressure recorders were used (AND UA‐787plus, A&D medical, San Jose, California, USA). GAD antibodies were measured with an immunoassay (Sunrise Touchscreen, Tucan Austria).
Lifestyle factors (regular exercise and smoking) and diabetes‐related distress were assessed during follow‐up. Regular exercise (Yes/No) was defined as a minimum of 30 minutes of exercise 7 times per week. Smoking was defined as either yes or no. PAID‐1 scores were dichotomized in a “Low” group (scores 0 and 1) and a “High” group (scores 2 to 4). Information regarding exercise and smoking was considered valid when recorded in the fourth or fifth year of study as these habits might have changed after getting a diabetes diagnosis, whereas the last recorded PAID‐1 score was accepted without considering which year it was recorded.
2.3. Outcomes
Glycaemic control was categorized both early after diagnosis and at the 5‐year follow‐up based on HbA1c level. “Early strict control” was defined as an HbA1c <53 mmol/mol (<7%) anytime during the 2nd or 3rd quarter after diagnosis, and “Late strict control” was defined as an HbA1c <53 mmol/mol (<7%) anytime during the fifth year after diagnosis.
2.4. Statistical analysis
Subject characteristics were summarized using means and standard deviations for continuous variables and frequencies for categorical variables. To account for the naturally skewed distribution of ACR, the data were log10 transformed before determining the mean.
The association between the dependent variable “Late strict glycaemic control” and the categorical variable (gender) and the continuous variables (age, baseline weight, weight 5 years after diagnosis, baseline HbA1c and C‐peptide levels, and insulin dose 5 years after diagnosis) was examined by univariate analyses; chi‐square test and Kruskal‐Wallis test were used, respectively.
To assess the importance of diabetes‐related measurements and year of onset for “Late strict control,” a multivariate, logistic regression analysis was performed including the independent variables HbA1c, C‐peptide level, BMI and GAD antibody status at diagnosis and first‐year LDL level. Additionally, year of diagnosis and “Early strict glycaemic control,” as defined above, was included in the model. The analyses were carried out using PROC GENMOD. The initial models included main effects but no interaction terms. The model was then reduced using backward elimination.
Odds ratios with 95% confidence limits were estimated to quantify the association between the dependent variable “Late strict control” and the independent variables “Early strict control” and lifestyle factors (smoking, exercise and PAID‐1 score) by univariate Fisher's exact probability statistics. Additionally, risk ratios were calculated for statistically significant associations to estimate the causal inference. SAS Enterprise Guide 7.12 (SAS Institute Inc., Cary, NC, USA) was used for all the statistical assessments. P < 0.05 was considered statistically significant.
2.5. Ethics approval
Permission to use data from the electronic medical records was obtained from the Danish Data Protection Agency (ref. number: SDC‐2014‐009).
3. RESULTS
Clinical characteristics of patients at the time of diagnosis are shown in Table 1. Of the 280 patients included in the study, 171 men and 109 women, 84% were GAD‐positive at presentation (data available for N = 159, as GAD was not implemented as onset standard test until August 2005). For all patients, HbA1c decreased after diagnosis. By the second quarter after diagnosis, the mean HbA1c had decreased from 106 ± 25 to 52 ± 13 mmol/mol (11.8 ± 2.29% to 6.9 ± 1.19%; P < 0.0001). Thereafter, a gradual increase during the following 5 years was observed, reaching a mean of 67 ± 20 mmol/mol (8.3 ± 1.83%; Figure 1).
Table 1.
Clinical characteristics of the patients at the time of diagnosis; age, body mass index (BMI), glycated haemoglobin (HbA1c), systolic and diastolic blood pressure, low‐density lipoprotein (LDL cholesterol), proinsulin c‐peptide (C‐peptide) and urine albumin‐to‐creatinine ratio
| Characteristics | N | Mean (SD) |
|---|---|---|
| Age at onset (y) | 280 | 36.9 (12.4) |
| Body mass index (kg/m2) | 194 | 23.0 (3.3) |
| HbA1c (mmol/mol (%)) | 280 | 106 (25) (11.8 (2.29)) |
| Blood pressure (Systolic, mm Hg) | 242 | 123 (15) |
| Blood pressure (Diastolic, mm Hg) | 242 | 76 (9) |
| LDL cholesterol (mmol/L) | 258 | 2.9 (0.9) |
| C‐peptide (pmol/L) | 265 | 365 (241) |
| Albumin‐to‐creatinine ratioa | 270 | 8.9 (2.5) |
To account for the naturally skewed distribution of albumin‐to‐creatinine ratio (ACR), the data were log10 transformed before determining the mean.
Figure 1.
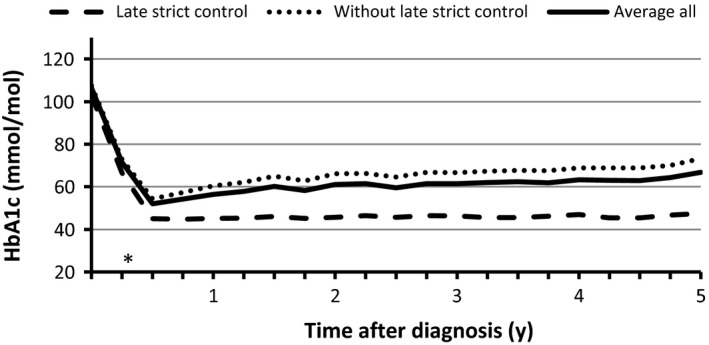
HbA1c during 5 years’ follow‐up from time of diagnosis, measured quarterly. Mean HbA1c for all patients and comparison of patients with and without Late strict control (HbA1c <53 mmol/mol (<7%) anytime during the fifth year after diagnosis). *P < 0.001
Over the 5 years, there were small increases in systolic and diastolic blood pressure, HDL‐cholesterol and BMI (P < 0.0001), a marked increase in total daily insulin dose (P < 0.0001) and small decreases in TG and LDL cholesterol (P = 0.001).
When defining strict glycaemic control as an HbA1c <53 mmol/mol (<7%), we found that 66% of patients achieved Early strict control (within 6‐9 months). At the 5‐year follow‐up, the corresponding proportion had decreased to 30% of patients (Late strict control). In all 77 out of the 179 patients (43%) who achieved Early strict control achieved Late strict control as well. By contrast, only 7 out of 92 patients (8%) without Early strict control achieved Late strict control (missing data about early control: n = 9).
Patients with Late strict control did not differ in baseline HbA1c compared to patients without Late strict control. However, the regular measurements of HbA1c during the 5 years of follow‐up clearly demonstrated that the HbA1c of the two groups began to separate already 3‐6 months after diagnosis (P < 0.001; Figure 1). The risk ratio for patients who obtained Early strict control was 5.7 for maintaining strict control after 5 years, compared to patients that did not obtain Early strict control.
The comparison of other parameters and 5‐year follow‐up data between patients obtaining or not obtaining Late strict control revealed no differences between groups, apart from total daily insulin dose. Mean total daily insulin dose was higher for patients not obtaining Late strict glycaemic control. This difference occurred early after diagnosis and reached significance after 9 months (P = 0.02) and even stronger after 5 years (P < 0.0001; Figure 2A). In contrast, neither gender (P = 0.7), age (P = 0.3), baseline HbA1c (P = 0.3), baseline C‐peptide (P = 0.4), baseline weight (P = 0.5), nor weight after 5 years (P = 0.6; Figure 2B) differed between the groups. Additionally, women who had been more closely monitored due to pregnancy during the study period (n = 13) did not differ from other women in the study (P = 0.5). Mean values of the quantitative variables are presented in Table 2.
Figure 2.
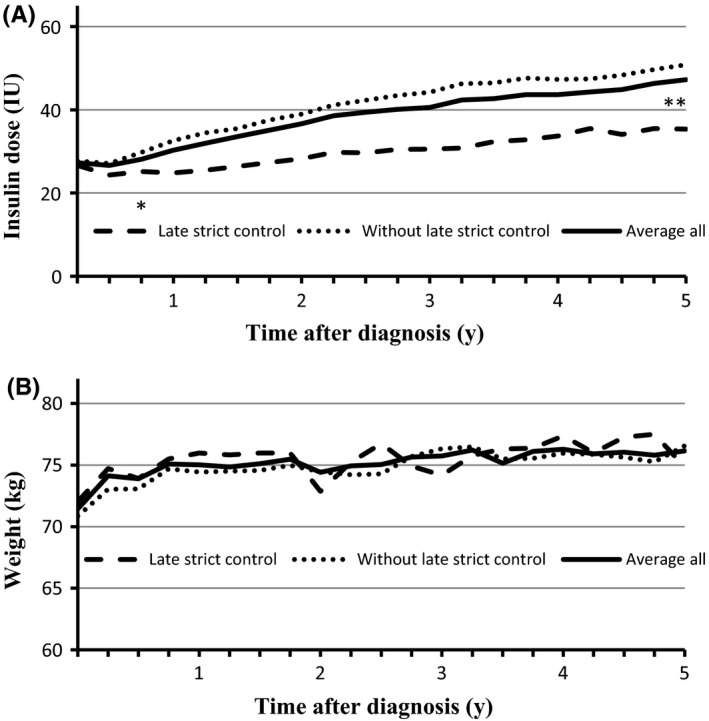
A, Insulin dose; B, Body weight—both measured quarterly during five years’ follow‐up. Mean values for all patients and comparison of patients with and without Late strict control (HbA1c <53 mmol/mol (<7%) anytime during the fifth year after diagnosis). *P = 0.0222; **P < 0.0001
Table 2.
Comparison of patients with and without strict glycaemic control after 5 years; age at diagnosis, weight at time of diagnosis (baseline), weight after 5 years, glycated haemoglobin (HbA1c) at time of diagnosis (baseline), low‐density lipoprotein (LDL cholesterol), proinsulin c‐peptide (C‐peptide), total daily insulin dose after 5 years. The P‐value indicates the significance of the association between late strict control and the given parameter
| Late strict controla | Yes (N = 84) | No (N = 196) | Number of observations | P‐value |
|---|---|---|---|---|
| Age at onset (y) | 37.8 | 36.6 | 280 | 0.3 |
| Weight, baseline (kg) | 71.6 | 70.8 | 194 | 0.5 |
| Weight, after five years (kg) | 76.6 | 75.5 | 278 | 0.6 |
| HbA1c, baseline (mmol/mol (%)) | 103 (11.6) | 107 (11.9) | 280 | 0.3 |
| LDL cholesterol (mmol/L) | 3.0 | 2.8 | 258 | 0.7 |
| C‐peptide, baseline (pmol/L) | 369.2 | 355.7 | 265 | 0.4 |
| Total daily insulin dose after 5 years (IU) | 34.8 | 50.6 | 279 | <0.0001 |
Late strict control: HbA1c <53 mmol/mol (<7%) anytime during the fifth year after diagnosis.
A multivariate model confirmed the impact of Early strict control on long‐term glycaemic outcome; the strongest predictor for patients to obtain Late strict control was achieving Early strict control (P < 0.0001), with an odds ratio for the association of 9.2 (CI‐95% 4.0‐20.9; P < 0.0001). The remaining parameters tested in the model were all nonsignificant [baseline HbA1c (P = 1.0), C‐peptide (P = 0.09), BMI (P = 0.4), first‐year LDL level (P = 0.4), GAD antibody status (P = 0.3) and year of diagnosis (P = 0.377)].
Concerning lifestyle factors at follow‐up, the majority of patients were nonsmokers although there was significant underreporting of smoking status (112 of 158, 122 observations missing) and most patients reported to be exercising regularly (182 of 241; 39 observations missing). Not smoking was positively associated with obtaining Late strict control with an odds ratio of 3.4 (CI‐95% 1.3‐8.8; P = 0.01), while stating to exercise regularly was not statistically significant (P = 0.1; OR = 1.8; CI‐95% 0.9‐3.6). Data on diabetes‐related stress, as assessed by PAID‐1 score, were available in a subgroup of patients (99/280 patients); 58% had a low PAID‐1 score (<2) and 42% had a high score (≥2). A low PAID‐1 score was positively associated with obtaining Late strict control (OR = 2.9; CI‐95% 1.2‐6.9; P = 0.021). Hence, patients without strict glycaemic control were more likely to experience diabetes‐related stress.
4. DISCUSSION
In this retrospective cohort study of a comparatively large group of patients with AO type 1 diabetes, we found that adequate glycaemic control, as suggested by international treatment guidelines, was achieved by two‐thirds of the patients within the first half year following diagnosis, and that less than a third of patients remained at this level of glycaemic control after 5 years. Whereas neither baseline HbA1c nor C‐peptide was predictive of subsequent glycaemic control, the HbA1c level reached early after diagnosis clearly was. It was almost 6 times as likely that patients, who achieved strict control within the first 6‐9 months of treatment, maintained glycaemic control long term, compared to those who did not. This may have clinical implications in how resources are prioritized and how care is personalized for this group of patients.
Clinical characteristics of our cohort appeared classical in relation to a diagnosis of type 1 diabetes. HbA1c at diagnosis was high, and BMI, blood pressure, urine albumin‐to‐creatinine ratio and lipid levels were within normal ranges. A minority of the patients were GAD‐negative (16%), in which case, the diagnosis was based on clinical appearance. Others have reported similar levels of autoantibody‐negative patients; the presence of autoantibodies being the highest among paediatric patients, to then decrease with age at diagnosis of type 1 diabetes among adults.7 As expected, we also observed a higher proportion of males. The preponderance for males is more pronounced in adults compared with children.7, 8
Previous studies in paediatric cohorts have also shown that the Hba1c level reached early after diagnosis predicts future glycaemic control.9, 10, 11 One study even found that poor glycaemic control early after diagnosis was associated with the presence of microvascular complications years later.11 To the best of our knowledge, this is the first time that a similar observation of glycaemic prediction is described in a cohort of patients with AO type 1 diabetes. In the paediatric setting, psychosocial along with biological factors have been associated with long‐term glycaemic control.10, 12 In our investigation, these observations should be interpreted in the adult setting. In terms of lifestyle and disease‐related distress, both smoking and PAID‐1 score turned out to be associated with not achieving Late strict control. These findings are in line with other investigations in adults.13 Given the significant association between smoking, PAID‐1 score and poor glycaemic control, and the trend of an association between regular exercise and good glycaemic control, it is possible that also psychosocial factors may contribute to long‐term glycaemic outcome—nevertheless, the study does not allow a clear conclusion in terms of causality.
In patients with LADA, markers of autoimmunity invariably relate to a more rapid progress of beta cell failure.14 Interestingly, in this investigation where we carefully tried to exclude patient with LADA, we did not observe any association between either GAD status, or of C‐peptide at time of diagnosis and subsequent glycaemic control. We did, however, observe lower insulin requirements in well‐regulated patients at the 5‐year follow‐up. The difference in insulin dose between groups was evident already after 9 months of treatment, suggesting a difference in rate and degree of remission and residual beta‐cell function. This is supported by the fact that the gradual increase in weight during follow‐up was not associated with achieving strict glycaemic control (Figure 2B). Hence, despite the lack of predictive value of autoimmune markers at time of diagnosis, underlying differences in biology seem to affect glycaemic outcome. This interpretation is obviously limited by the lack of repeated C‐peptide measurements during follow‐up in the current investigation. But it remains an intriguing opportunity that patients characterized by early glycaemic control may have a greater chance at prolonging their independence from external insulin administration as a result of early intervention.
The classification of adult‐onset autoimmune diabetes is not always evident.15 In our study, we carefully excluded LADA patients, the aim being to describe as accurately as possible a cohort of pure AO type 1 diabetes. The distinction between AO type 1 diabetes and LADA is predominantly based on the timing of insulin treatment, and a distinct diagnosis of LADA based on a pathophysiologic marker does not exist.16 Nonetheless, studies have shown genetic group‐wise differences between LADA patients and AO type 1 diabetes.17 One might speculate whether the subgroup of patients with well‐controlled diabetes after 5 years in our cohort to some extent still represent a LADA‐like end of the spectrum of AO type 1 diabetes with a less aggressive underlying autoimmune process, as illustrated by the lower insulin requirement. By definition, it is likely that some overlap exists between these patients and LADA patients with a delayed diagnosis. Awaiting the possible finding of a distinct diagnostic marker, an empirical approach with focus on treatment targets rather than disease classification might be more useful. Outcome‐based criteria may be the pragmatic solution, and repeated measurements of C‐peptide along with HbA1c may serve as inspiration for discussing treatment and prognosis with the individual patient.18
The referral of patients from a selected area may be a weakness of this study. By contrast, the rather uniform structure around patient education, planning of visits and follow‐up may strengthen the data. Patients are referred to the centre from a well‐defined but rather large geographical area of Copenhagen. Although we do not have information regarding ethnicity, the population of Copenhagen is rather homogenous, and the vast majority of our patients are Caucasians. According to national guidelines, all Danish patients with type 1 diabetes are referred to specialized centres. All patients with newly diagnosed type 1 diabetes at our centre follow a designated patient educational programme; they are seen by physicians, nurses and dietitians for multiple scheduled visits the first year, followed by 3‐4 yearly visits predominantly by physicians. The educational programme for newly diagnosed patients and the standards for visits have essentially remained the same during the observational period. In the multivariate model, year of onset was not independently associated with long‐term glycaemic control. Despite possible differences in socioeconomic status due to the geographical area, we assume that our observations of glycaemic prediction are representative of the type 1 diabetes population in general.
In conclusion, strict glycaemic control in subjects with AO type 1 diabetes can be maintained over time. The level of HbA1c reached early after diagnosis seems to be a good predictor for long‐term glycaemic control and, clearly, patients who fail to obtain adequate control shortly after onset warrants increased attention. There is an apparent lack of other predictive variables for long‐term glycaemic control as well as prognosis in general, as measured at the time of diagnosis. Gaining more knowledge about this group of patients will help us individualize therapy from early after diagnosis and hopefully lead to the improved glycaemic outcome for patients with AO type 1 diabetes in general.
CONFLICT OF INTEREST
Louise Boysen and Dan Hesse have no conflict of interests. Martin Ridderstråle is currently an employee of Novo Nordisk A/S but was employed at SDC at the time of the study and declares no conflict of interest.
AUTHOR CONTRIBUTION
All three authors have contributed substantially to the design of the study and the acquisition, analysis and interpretation of data. All three authors have participated in drafting the article and revising it critically and have approved the final version to be published.
Hesse D, Boysen L, Ridderstråle M. Adult‐onset type 1 diabetes: Predictors of glycaemic control. Endocrinol Diab Metab. 2018;1:e38 10.1002/edm2.38
REFERENCES
- 1. Gale EA. Latent autoimmune diabetes in adults: a guide for the perplexed. Diabetologia. 2005;48(11):2195‐2199. [DOI] [PubMed] [Google Scholar]
- 2. Laugesen E, Ostergaard JA, Leslie RD, Danish Diabetes Academy W, Workshop S. Latent autoimmune diabetes of the adult: current knowledge and uncertainty. Diabet Med. 2015;32(7):843‐852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Poudel A, Savari O, Striegel DA, et al. Beta‐cell destruction and preservation in childhood and adult onset type 1 diabetes. Endocrine. 2015;49(3):693‐702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Graham J, Hagopian WA, Kockum I, et al. Genetic effects on age‐dependent onset and islet cell autoantibody markers in type 1 diabetes. Diabetes. 2002;51(5):1346‐1355. [DOI] [PubMed] [Google Scholar]
- 5. Hao W, Gitelman S, DiMeglio LA, Boulware D, Greenbaum CJ. Type 1 diabetes TrialNet study G. Fall in C‐peptide during first 4 years from diagnosis of type 1 diabetes: variable relation to age, HbA1c, and insulin dose. Diabetes Care. 2016;39(10):1664‐1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McGuire BE, Morrison TG, Hermanns N, et al. Short‐form measures of diabetes‐related emotional distress: the Problem Areas In Diabetes scale (PAID)‐5 and PAID‐1. Diabetologia. 2010;53(1):66‐69. [DOI] [PubMed] [Google Scholar]
- 7. Sabbah E, Savola K, Ebeling T, et al. Genetic, autoimmune, and clinical characteristics of childhood‐ and adult‐onset type 1 diabetes. Diabetes Care. 2000;23(9):1326‐1332. [DOI] [PubMed] [Google Scholar]
- 8. Kyvik KO, Nystrom L, Gorus F, et al. The epidemiology of type 1 diabetes mellitus is not the same in young adults as in children. Diabetologia. 2004;47(3):377‐384. [DOI] [PubMed] [Google Scholar]
- 9. Shalitin S, Phillip M. Which factors predict glycemic control in children diagnosed with type 1 diabetes before 6.5 years of age? Acta Diabetol. 2012;49(5):355‐362. [DOI] [PubMed] [Google Scholar]
- 10. Lawes T, Franklin V, Farmer G. HbA1c tracking and bio‐psychosocial determinants of glycaemic control in children and adolescents with type 1 diabetes: retrospective cohort study and multilevel analysis. Pediatr Diabetes. 2014;15(5):372‐383. [DOI] [PubMed] [Google Scholar]
- 11. Samuelsson U, Steineck I, Gubbjornsdottir S. A high mean‐HbA1c value 3‐15 months after diagnosis of type 1 diabetes in childhood is related to metabolic control, macroalbuminuria, and retinopathy in early adulthood–a pilot study using two nation‐wide population based quality registries. Pediatr Diabetes. 2014;15(3):229‐235. [DOI] [PubMed] [Google Scholar]
- 12. Thompson SJ, Auslander WF, White NH. Comparison of single‐mother and two‐parent families on metabolic control of children with diabetes. Diabetes Care. 2001;24(2):234‐238. [DOI] [PubMed] [Google Scholar]
- 13. Sturt J, Dennick K, Due‐Christensen M, McCarthy K. The detection and management of diabetes distress in people with type 1 diabetes. Curr Diab Rep. 2015;15(11):101. [DOI] [PubMed] [Google Scholar]
- 14. Turner R, Stratton I, Horton V, et al. UKPDS 25: autoantibodies to islet‐cell cytoplasm and glutamic acid decarboxylase for prediction of insulin requirement in type 2 diabetes. UK prospective diabetes study group. Lancet. 1997;350(9087):1288‐1293. [DOI] [PubMed] [Google Scholar]
- 15. Ostergaard JA, Laugesen E, Leslie RD. Should there be concern about autoimmune diabetes in adults? Current evidence and controversies. Curr Diab Rep. 2016;16(9):82. [DOI] [PubMed] [Google Scholar]
- 16. Leslie RD, Palmer J, Schloot NC, Lernmark A. Diabetes at the crossroads: relevance of disease classification to pathophysiology and treatment. Diabetologia. 2016;59(1):13‐20. [DOI] [PubMed] [Google Scholar]
- 17. Andersen MK, Lundgren V, Turunen JA, et al. Latent autoimmune diabetes in adults differs genetically from classical type 1 diabetes diagnosed after the age of 35 years. Diabetes Care. 2010;33(9):2062‐2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huda MS, Hitman GA. C‐peptide: should we be testing this routinely in the type 1 diabetes clinic? Diabet Med. 2015;32(10):1259‐1260. [DOI] [PubMed] [Google Scholar]


