Summary
Aims
The understanding of second‐line use of glucose‐lowering drugs (GLDs) in the general population with type 2 diabetes (T2D) treatment is important as recent results have shown cardiovascular benefits with sodium‐glucose cotransporter‐2 inhibitors (SGLT‐2i) and glucagon‐like peptide‐1 receptor agonists (GLP‐1RA). Our aim was to describe second‐line GLD treatment patterns in four Nordic countries.
Methods
All T2D patients treated with GLD between 2006 and 2015 were identified in prescribed drug registries in Denmark, Finland, Norway and Sweden, and linked with National Patient and Cause of Death Registries. Second‐line treatment was defined as a prescription of a second GLD class following ≥6 months of metformin monotherapy. Index was the date of first dispense of the second‐line drug.
Results
A rapid uptake of newer GLDs (GLP‐1RA, DPP‐4i and SGLT‐2i) over the 10‐year observation period was seen in Denmark, Finland and Norway, while slower in Sweden. In 2015, 33,880 (3.1%) of 1,078,692 T2D patients initiated second‐line treatment, and newer GLDs were more commonly used in Finland (92%), Norway (71%) and Denmark (70%) vs Sweden (44%). In 2015, the use of older GLDs (insulin and sulphonylureas) was 7‐fold greater in Sweden compared to Finland (49% vs 7%), and 1.6‐fold greater compared with Denmark and Norway (49% vs 30% and 29%, respectively).
Conclusions
Despite comparable demography and healthcare systems in four neighbouring countries, surprisingly large differences in second‐line use of newer GLDs were found. With recent evidence of potential cardiovascular benefits with newer GLDs, such differences may have an important impact on cardiovascular outcomes.
Keywords: observational study, second‐line, SGLT2, type 2 diabetes
1. INTRODUCTION
For several years, global guidelines have advocated metformin as first‐line pharmacological treatment of type 2 diabetes (T2D), but second‐line treatment choices are considered equal and open for individualization based on choices and considerations among patients and healthcare professionals.1 However, due to variances in reimbursement and national guidelines, there may well be differences between prescription patterns between countries.
Knowledge of second‐line treatment patterns has become even more important as recent studies have reported secondary preventive cardiovascular (CV) benefits of several of the new glucose‐lowering drugs (GLDs).2, 3, 4, 5, 6, 7, 8 Moreover, large observational studies have recently shown associations with increased risk of severe hypoglycaemia, CV and all‐cause mortality with older GLDs (sulphonylureas and insulins) in second‐line treatment.9, 10, 11, 12, 13, 14, 15, 16 Consequently, there are large differences with regard to potential effects and side effects, drug administration, costs and evidence grades for the six different GLD classes currently recommended as second‐line options, that is dipeptidyl peptidase‐4 inhibitors (DPP‐4is), sodium‐glucose cotransporter‐2 inhibitors (SGLT‐2is), glucagon‐like peptide‐1 receptor agonists (GLP‐1RA), sulphonylureas, thiazolidinediones and insulins.1 An important first step is to understand to which extent different second‐line GLDs are used in the broad T2D population, to determine inertia to follow new guidelines and willingness taking newer drugs into use.
The aim of this study was to describe second‐line treatment after metformin monotherapy in four Nordic countries during the last decade using nationwide registers (covering a total population of >25 million inhabitants), and to examine potential treatment differences between the neighbouring countries.
2. MATERIAL AND METHODS
2.1. Data sources
The present work is part of the D360 Nordic programme, a large‐scale diabetes investigation program which utilizes the unique features of full coverage nationwide healthcare registries and public healthcare systems covering more than 25 million inhabitants in all the Nordic countries, to include all T2D patients with filled GLD prescriptions.17 Detailed data on the data sources, see Supporting Information Appendix S1—section 1.
The four Nordic countries Denmark, Finland, Norway and Sweden have comprehensive, nationwide public healthcare systems (Supporting Information Appendix S1—section 1).2, 7 All citizens have a unique personal identification number (person‐ID), which is mandatory for all administrative purposes (including any contact with the healthcare system and drug purchases), thus providing a full population medical history. Individual patient‐level data from the Prescribed Drug Registers, the Cause of Death Registers, and the National Patient Registers covering all hospitalizations with discharge diagnoses and all outpatient hospital visits were linked using the person‐ID. The linked databases were separately managed by Steno Diabetes Center Copenhagen, Gentofte, Denmark (Danish data), StatFinn & EPID Research, Espoo, Finland (Finnish data) and Statisticon AB, Uppsala, Sweden (Swedish and Norwegian data).
In Denmark (the DAFFODIL study database), data were made available following an application to The Danish Data Protection Agency18 and to Statistics Denmark19 with final approval by the Danish Health Data Agency. In Finland (the DAHLIA study database), the study protocol was approved by the ethical review board of the Hjelt Institute, University of Helsinki Medical Faculty (Dnro 96/13/03/00/15). In Norway (the DAPHNE study database), the study protocol was approved by the Regional Ethics Committee, Helse Sør‐Øst (ref.nr. 2015/1337/REK sør‐øst A) and authorization by the Norwegian Data Inspectorate (Datatilsynet). In Sweden (the DAISY study database), the protocol was approved by the Stockholm Regional Ethics Committee (reference number 2013/2206‐31) with data linkage performed by the Swedish National Board of Health and Welfare.
2.2. Study population
All T2D patients aged 18 years and above who filled a GLD prescription from the beginning of year 2006 to the end of year 2015 were included. Patients with a diagnosis of type 1 diabetes, gestational diabetes or polycystic ovarian syndrome were excluded (Supporting Information Appendix S1—section 2).
Second‐line treatment was defined as ≥6 months (two reiteration prescription cycles of 3 months) of metformin monotherapy (at any dose), followed by a filled prescription of a second GLD class such as DPP‐4i, SGLT‐2i, GLP1‐RA, sulphonylurea, insulin or other GLD (glitazones, acarbose and glinides). The index date was defined as the date of first filled prescription of the second‐line drug.
2.3. Baseline data
Patient characteristics included age at index date, sex, index date, date of first‐line metformin GLD dispense and information on patient frailty (defined as at least one hospitalization of three or more consecutive days during the year prior to index date), detailed in Supporting Information Table S1b.10, 13, 20 Comorbidities were searched for in all available data prior to and including the index date, with an exception for severe hypoglycaemia (within 12 months prior to index date) and cancer (within 5 years prior to index date), detailed Supporting Information Table S1c. Prior medications were defined as any dispense 12 months prior to and including index date, detailed Supporting Information Table S1d.
2.4. Statistical analysis
Demographic data are presented as mean (SD) or n (%). Annual proportions of GLD class used for second‐line treatment were calculated by dividing the number of patients filling a second GLD class prescription by the total number of second‐line patients at the year of interest. No statistical comparisons were between country prescription patterns. In Sweden, we utilized the possibility to compare second‐line treatment between individual counties (the 21 healthcare regions) for the years 2006‐2015. All analyses were conducted using sas, version 9.3 (SAS Institute Inc., Cary, NC, USA) or r statistical software (R version 3.1.1 or 3.2.3).21
3. RESULTS
3.1. Baseline
In 2015, there was a total of 1 078 692 GLD‐treated T2D patients in the four countries (Denmark, 180 742; Finland, 367 356; Norway, 177 171; and Sweden, 353 423), Table 1. A total of 33 880 (3.1%) patients initiated second‐line treatment, and this proportion was very similar throughout the countries. In 2015, Swedish and Finnish patients were older (65.0 years and 65.3 years), vs Norwegian and Danish patients (61.7 years and 62.0 years). The proportion of female patients receiving second‐line treatment was approximately 40% in all countries. Prevalent CV disease was most common in Finland (36%) and least prevalent in Denmark (26%). Treatments reducing cardiovascular disease risk, such as angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers, statins, low‐dose aspirin and beta blockers, were more extensively used in Denmark (90%), Sweden (89%) and Finland (90%) compared to Norway (80%) (Table 1).
Table 1.
Baseline description of the prevalent populations, year 2015, for Norway, Sweden, Denmark and Finland
| Denmark | Finland | Norway | Sweden | |
|---|---|---|---|---|
| No. of second‐line patients | 6343 | 9123 | 5019 | 13 395 |
| % of T2D full population | 3.5% | 2.5% | 2.8% | 3.8% |
| Time on metformin, mean years (SD) | 4.4 (2.9) | 5.0 (3.1) | 4.7 (3.2) | 4.8 (2.9) |
| Age, mean (SD) | 62.0 (12.7) | 65.3 (12.3) | 61.7 (12.8) | 65.0 (12.1) |
| Female, n | 2518 (39.7) | 4083 (44.8) | 1952 (38.9) | 5318 (39.7) |
| Comorbidities | ||||
| CVD | 1661 (26.2) | 3234 (35.5) | 1347 (26.8) | 4189 (31.3) |
| Myocardial infarction | 333 (5.2) | 667 (7.3) | 318 (6.3) | 1294 (9.7) |
| Unstable angina | 148 (2.3) | 487 (5.3) | 135 (2.7) | 605 (4.5) |
| Angina pectoris | 561 (8.8) | 923 (10.1) | 441 (8.8) | 1028 (7.7) |
| Heart failure | 360 (5.7) | 827 (9.1) | 306 (6.1) | 1000 (7.5) |
| Atrial fibrillation | 583 (9.2) | 1328 (14.6) | 445 (8.9) | 1424 (10.6) |
| Stroke | 332 (5.2) | 968 (10.6) | 240 (4.8) | 1254 (9.4) |
| Peripheral artery disease | 330 (5.2) | 499 (5.5) | 317 (6.3) | 643 (4.8) |
| Microvascular disease | 944 (14.9) | 1625 (17.8) | 950 (18.9) | 2202 (16.4) |
| Chronic kidney disease | 121 (1.9) | 105 (1.2) | 160 (3.2) | 170 (1.3) |
| Lower limb amputations | 25 (0.4) | 29 (0.3) | 13 (0.3) | 35 (0.3) |
| Cancera | 649 (10.2) | 1214 (13.3) | 502 (10.0) | 1271 (9.5) |
| Drug treatments | ||||
| CVD risk treatment | 5734 (90.4) | 8185 (89.7) | 4037 (80.4) | 11 929 (89.1) |
| Antihypertensives ACEi or ARBs | 4703 (74.1) | 7434 (81.5) | 3412 (68.0) | 10392 (77.6) |
| Statins | 4615 (72.8) | 5421 (59.4) | 2865 (57.1) | 8758 (65.4) |
| Low‐dose aspirin | 1789 (28.2) | — | 1689 (33.7) | 3931 (29.3) |
| Beta blockers | 1762 (27.8) | 4549 (49.9) | 1693 (33.7) | 5639 (42.1) |
| Second‐line treatment | ||||
| Time on metformin, mean years (SD) | 4.4 (2.9) | 5.0 (3.1) | 4.7 (3.2) | 4.8 (2.9) |
| DPP‐4i | 3555 (56.0) | 8165 (89.5) | 2763 (55.1) | 4551 (34.0) |
| SGLT‐2i | 360 (5.7) | 193 (2.1) | 536 (10.7) | 579 (4.3) |
| GLP‐1RA | 510 (8.0) | 46 (0.5) | 247 (4.9) | 769 (5.7) |
| Sulphonylurea | 1317 (20.8) | 120 (1.3) | 1121 (22.3) | 4068 (30.4) |
| Insulin | 597 (9.4) | 510 (5.6) | 328 (6.5) | 2451 (18.3) |
| Other | 4 (0.1) | 89 (1.0) | 24 (0.5) | 977 (7.3) |
ACE, angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; CVD, cardiovascular disease.
Cancer diagnose within 5 years prior to index.
3.2. Second‐line treatment from year 2006 to 2015
The second‐line treatment patterns of filled GLD prescriptions showed rapid changes during the observation period years 2006‐2015 in Finland, Denmark and Norway, whereas the uptake of the newer GLDs (DPP‐4i, SGLT‐2i and GLP1‐RA) was slower in Sweden (Figure 1). Conversely, the use of sulphonylurea decreased substantially and insulin use remained low over the last decade in Finland, Denmark and Norway. This is in contrast to Sweden, where the use of both sulphonylurea and insulin remained at a higher level and started to decrease much later compared to the other Nordic countries.
Figure 1.
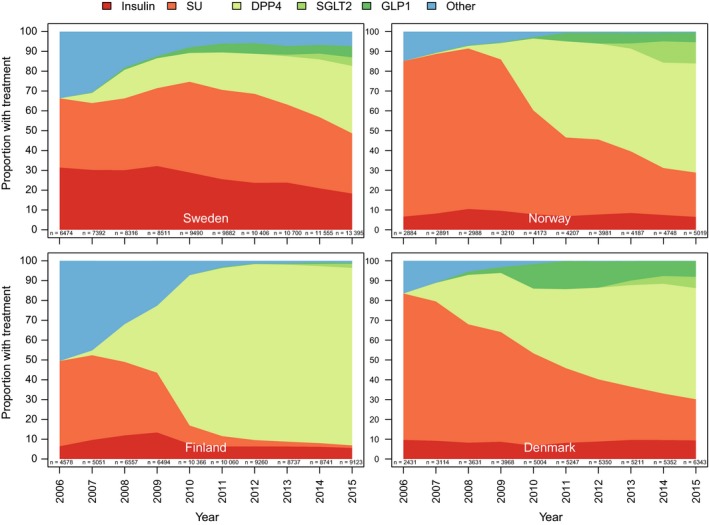
Second‐line line initiation after mono‐metformin in the four Nordic countries (Denmark, Finland, Norway and Sweden) during years 2006‐2015
3.3. Second‐line treatment year 2015
In 2015, second‐line treatment is initiated after about 5 years (4.7‐5.0 years) in Norway, Finland and Sweden but slightly shorter in Denmark (4.4 years). Newer GLDs was extensively used as second‐line agents in three of the Nordic countries (Finland 92%, Norway 71% and Denmark 70%), but was lower in Sweden (44%), Figure 2. DDP‐4i was the most commonly used second‐line therapy in all countries. Conversely, the use of older GLDs (such as insulin and sulphonylureas) as second‐line agents in Sweden was 7‐fold greater compared to Finland (49% vs 7%) and 1.6‐fold greater compared to Denmark and Norway (49% vs 30% and 29%, respectively). Compared to the other Nordic countries, Finland differed by a substantially lower use (7%) of older GLDs during year 2015.
Figure 2.
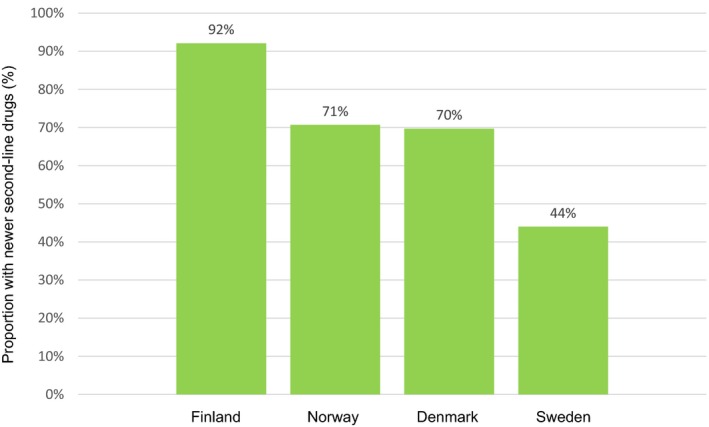
Proportion of second‐line initiation of newer glucose‐lowering drugs (DPP‐4i, SGLT‐2i and GLP1‐RA) in the four Nordic countries (Denmark, Finland, Norway and Sweden) during the year 2015
3.4. Regional differences in second‐line treatment within Sweden
In 2015, there was a large difference in use of newer GLDs as second‐line treatment between “high and low user” counties in Sweden. The highest use was found in Värmland (80%), Halland (67%) and Örebro (64%), whereas Gotland (25%), Norrbotten (25%) and Västra Götaland (29%) were at the lower end. Overall, the regional use of older GLDs displayed opposite differences to the above as expected. (Supporting Information Figures S1 and S2) In general, the use of newer GLDs has increased in all counties from 2006 to 2015 (Supporting Information Figure S2a–u) but with large variations in the time course. There were also marked differences in the use of individual drug classes, both among new GLDs and sulphonylurea and insulin.
4. DISCUSSION
In national T2D populations, covering more than one million pharmacologically treated patients, we found that approximately 3% were annually initiated on second‐line treatment after metformin monotherapy, an observation that was consistent across the four Nordic countries. In general, the use of sulphonylureas is decreasing, however, at a slower pace in Sweden compared to the other three countries. Insulin use was stable and at a low level in all countries except Sweden, where the use of insulin was slightly decreasing but more than twice as high compared to the other Nordic countries during the last decade. Surprisingly, we found very large differences in second‐line treatment initiation between countries, despite similar demography, healthcare education levels and nationwide public healthcare systems. During the observation period, use of newer GLDs was markedly higher in Finland, intermediate in Denmark and Norway, while it remained low in Sweden. Interestingly, we also noted large local variations of the use of newer GLDs within one country, Sweden, between healthcare regions. Curtis et al22 have recently reported extensive geographical differences across England to a similar degree that we have seen in Sweden. These observations are of interest as there was no definitive evidence regarding CV safety and secondary CV preventive effects of the newer GLDs during that observation period. Whether and how the treatment patterns change after the published CV outcome trials for newer GLDs6, 8 and after recent and ongoing revisions of guidelines will be of interest to follow in future studies.
Interestingly, the time on metformin monotherapy was slightly shorter in Denmark (~4 years), compared to the other countries (~5 years). The shorter time on metformin monotreatment might indicate a more proactive disease management in Denmark, consistent with findings in other studies.23, 24
All four Nordic countries have public healthcare systems that guarantee all citizen access to relevant care, treatment and reimbursed drugs. The health care is typically divided into a large primary sector, and a more specialized secondary sector including outpatient clinics and hospitals. For a chronic condition as T2D, initial treatment and prescriptions will be made by the primary care physician, and later it may be relevant to refer the patient to a specialized outpatient clinic. It can therefore be assumed the majority of decisions regarding second‐line treatment are made in the primary care, and comparisons in our analysis are in fact mostly dependent on differences in organization and reimbursement of GLDs in the four countries. Interestingly, a similar fraction of the T2D population in each country was initiated on second‐line therapy (approximately 3.2%) in 2015, perhaps indicating similarities in patient and physicians’ treatment habits across the countries. In a recent comparison between a primary care database in the UK and a primary care and internal medicine database in Germany, distinctly different patterns of second‐line treatment prescriptions were found in 10 000 patients.25 In the German population, metformin was most frequently combined with DPP‐4i, whereas 57% of the included UK population was prescribed SU and metformin as second‐line choice. Although these data did not include SGLT‐2is or GLP‐1RAs, the analysis clearly demonstrates differences between two countries where treatment is mainly driven by primary care.
National reimbursement strategies may play a role in explaining the differences in our findings. Between the four countries, there are different and changing co‐payer levels, which all are likely to influence the mutual doctor–patient decision on which second‐line treatment to initiate, when also taking the price the patients have to pay, into consideration. For instance, in many counties in Sweden there are both a guideline and a reimbursement system that mandate basal insulin or SU to be used as second‐line treatment in general practice, while, for example in Denmark, there is a free choice between all drug classes, where primary care clinics are semi‐private self‐owned businesses with less stringent monitoring of prescription patterns.
Differences in treatment guidelines can also partly explain the findings. Apart from international guidelines, there are both national and also regional within‐country differences regarding guidelines as well as recommendations from regional healthcare authorities and primary healthcare associations. Järvinen et al26, 27 have compared the national guidelines for the treatment of T2D in the Nordic countries and show differences, which seemingly is only partly reflected in our data. It is clear from that paper that, until recently, Swedish guidelines advocated NPH (neutral protamine Hagedorn, isophane) insulin and SU as second‐line therapy, also shown in other observational studies.13, 27, 28 Interestingly, new guidelines were introduced in Sweden during 2017 advocating individualized treatment choice as well as use of SGLT2i or GLP1‐RA in T2D patients with established cardiovascular disease. The consequences in terms of treatment pattern change are presently evaluated.
Organization of T2D care, traditions and referral patterns most probably also influence the observed differences between the Nordic countries. In many parts of Denmark, the primary care physicians are encouraged to refer the patients to a secondary sector outpatient clinic for diabetes courses and education together with optimization of pharmacological treatment. This is in contrast to the organization in many parts of Sweden where primary care centres commonly have trained staff available to handle initiation of insulin therapy.13, 28 The higher use of statin in Denmark despite the least prevalent CVD population might also reflect the differences in organization and attitudes on CV prevention in T2D across countries.
Finally, it is possible that there are country‐specific differences to the various well‐known barriers to treatment intensification, that is barriers to insulin initiation or addition of more drugs, equally frequent in patients and healthcare professionals, as reviewed by Khunti et al29
Apart from the prescription patterns of GLDs, we found an interesting difference in use of cardiovascular preventive drugs (renin‐angiotensin system blocking treatment, statins, low‐dose aspirin and beta blockers) across the Nordic countries, which were lower in Norway (80%) as compared with the other three countries (89%‐90%). Differences in implementation of multifactorial risk factor management or attitudes towards polypharmacy could be an explanation, but our data limit the conclusions that can be made.
The differences seen in our analyses may have a potential impact on both clinical outcomes and overall healthcare costs in the four countries, now and in the future. Adherence to guidelines using a multifactorial intervention strategy, based on the findings in the Steno 2 Study, has the potential to prolong survival and reduce the extent and cost of complications.30 In addition, following the improvements in clinically relevant outcome demonstrated in recent years with SGLT‐2i2, 3, 6, 7, 8, 31 and GLP‐1RA,4, 5 it is tempting to speculate that populations with a high use of these GLDs will have a better overall prognosis with respect to survival and cardiovascular and other diabetes complications than populations with high use of other GLDs. There may also be beneficial long‐term effects on health economy. When the use of newer GLDs with CV protective effect is sufficiently extensive in clinical practice, future studies should address whether trends in CV morbidity and mortality risks are affected and how this translates into healthcare utilization costs.32
4.1. Strengths
Strengths of the present work are the population‐based, nationwide and unselected real‐world design, which provides a high external validity and large population. In addition, the utilized registers have full coverage for hospitalizations, filled drug prescriptions and cause of death with established and entirely public healthcare systems and few patients lost to follow‐up. CV diagnoses in the registries have been reported to have high validity.33, 34, 35, 36, 37
4.2. Limitations
This analysis is based on registries and therefore carries some limitations relating to the completeness and quality of the registries. Also, there may be some differences between registers from the four countries, although we have done our best to equalize any differences known. Particularly differences in classification of diabetes type may influence observed differences in, for example, insulin use. Since there are no ICD‐10 codes for Latent autoimmune diabetes of adults (LADA) diagnosis, it is difficult to determine the proportion of patients in any of the countries. With relatively low proportion of LADA patients and probably similar prevalence in the countries, we suggest that this has little impact when comparing treatment patterns. However, we cannot rule out that patients with early failure on metformin and/or second‐line insulin treatment could harbour a higher proportion of LADA patients.
From our analysis, we can only determine which prescriptions were filled at the pharmacy, which does not equal actual ingestion of the drug. As such, we have no information on medication adherence once picked up from the pharmacy. In order to reliably define an established metformin monotherapy, we required at least two dispenses over 6 months since the reiteration cycle is 3 months. This means that second‐line index earlier than 6 months will not be reflected in the results. It is not possible in this descriptive analysis to analyse the actual cause for the differences seen, as many different factors seem to interact. The present work has no information on laboratory measurements, lifestyle parameters, primary healthcare data, or socioeconomic data, and consequently, there may be remaining explanatory factors for choosing GLDs. However, in a representative subsample in Denmark, Norway and Sweden from the D360 program, we found similar relevant laboratory measurements when comparing the three countries which could support similar blood glucose targets of the T2D patients.38
5. CONCLUSION
Approximately 3% of the total Nordic T2D population is annually initiated on second‐line glucose‐lowering treatment following metformin monotherapy. Although the rapid uptake of newer GLDs was observed in the majority of the included countries, there were surprisingly large differences in second‐line use of newer GLDs, 2.1 times between countries in 2015 despite similar healthcare education, populations and nationwide public healthcare systems. Also, even larger within‐country variations were observed, up to 3.2 times in Sweden. Since newer GLDs have shown beneficial effects on cardiovascular outcomes and total mortality, information from studies like ours is important when planning new treatment strategy recommendations both from nationwide and local perspectives.
DISCLOSURES
FP reports having received research grants from AstraZeneca and Novartis and lecture fees from Novartis, Eli Lilly, MSD, AstraZeneca and Boehringer Ingelheim and having served as a consultant for Astra Zeneca, Amgen, Novo Nordisk and MSD. TN has received unrestricted grants from AstraZeneca and NovoNordisk and is on the national board of NovoNordisk, Sanofi‐Aventis, Amgen, Eli Lilly and Boehringer Ingelheim. MEJ holds shares in Novo Nordisk and has received grants and lecture fees from Astra Zeneca. JWE has received honoraria or research grants from AstraZeneca, NovoNordisk, Bristol‐Myers‐Squibb, Sanofi and MSD. PF holds a full‐time position at AstraZeneca. DN has received consultancy fees from Novo Nordisk, Astra Zeneca and Eli Lilly. MT is employed by an independent statistical consultant company, Statisticon AB, Uppsala, Sweden, for which AstraZeneca Nordic‐Baltic is a client. HLG reports honoraria from Sanofi, Novo Nordisk, Lilly, Boehringer Ingelheim. AN has honoraria from MSD, Astra Zeneca, Eli Lilly, Boehringer Ingelheim, Novo Nordisk. JB holds a full‐time position at AstraZeneca as epidemiologist. KIB, grants to his institution from AstraZeneca for this study and for lectures and consulting from Novo Nordisk, Sanofi, Lilly, Boehringer Ingelheim and Merck Sharp & Dohme. JGE has received honoraria or research grants from AstraZeneca, NovoNordisk and Boehringer Ingelheim. JTL has received lecture and consultancy fees from Sanofi, Merck, NovoNordisk and Astrazeneca. MLJ holds shares in Novo Nordisk. FH is employed by Statfinn & EPID Research which performs commissioned pharmacoepidemiological studies, and thus, its employees have been and currently are working in collaboration with several pharmaceutical companies (including AstraZeneca).
AUTHOR CONTRIBUTIONS
All authors participated in the research design. MT, FH and MLJ performed the data management and statistical analyses after discussion with all authors. All authors participated in data interpretation and in writing the manuscript. All authors took final responsibility in the decision to submit for publication.
Supporting information
ACKNOWLEDGEMENTS
We are grateful to Susanna Jerström and Helena Goike at AstraZeneca for logistic support and valuable comments on the manuscript. Urban Olsson, Statisticon AB, is acknowledged for database management. Solomon Christopher, Minna Vehkala (StatFinn & EPID Research, Espoo, Finland) are all acknowledged for database management and statistical work. Data from the Norwegian Patient Register have been used in this publication. The interpretation and reporting of these data are the sole responsibility of the authors, and no endorsement by the Norwegian patient register is intended nor should be inferred. All authors are guarantors of the manuscript.
Persson F, Bodegard J, Lahtela JT, et al. Different patterns of second‐line treatment in type 2 diabetes after metformin monotherapy in Denmark, Finland, Norway and Sweden (D360 Nordic): A multinational observational study. Endocrinol Diab Metab. 2018;1:e36 10.1002/edm2.36
Funding information
This work was sponsored by AstraZeneca AB.
REFERENCES
- 1. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient‐centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38(1):140‐149. [DOI] [PubMed] [Google Scholar]
- 2. Birkeland KI, Jorgensen ME, Carstensen B, et al. Cardiovascular mortality and morbidity in patients with type 2 diabetes following initiation of sodium‐glucose co‐transporter‐2 inhibitors versus other glucose‐lowering drugs (CVD‐REAL Nordic): a multinational observational analysis. Lancet Diabetes Endocrinol. 2017;5(9):709‐717. [DOI] [PubMed] [Google Scholar]
- 3. Kosiborod M, Cavender MA, Fu AZ, et al. Lower risk of heart failure and death in patients initiated on sodium‐glucose cotransporter‐2 inhibitors versus other glucose‐lowering drugs: the CVD‐REAL Study (comparative effectiveness of cardiovascular outcomes in new users of sodium‐glucose cotransporter‐2 inhibitors). Circulation. 2017;136(3):249‐259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with Type 2 diabetes. N Engl J Med. 2016;375(19):1834‐1844. [DOI] [PubMed] [Google Scholar]
- 5. Marso SP, Daniels GH, Brown‐Frandsen K, et al. Liraglutide and cardiovascular outcomes in Type 2 Diabetes. N Engl J Med. 2016;375(4):311‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in Type 2 diabetes. N Engl J Med. 2017;377(7):644‐657. [DOI] [PubMed] [Google Scholar]
- 7. Persson F, Nystrom T, Jorgensen ME, et al. Dapagliflozin is associated with lower risk of cardiovascular events and all‐cause mortality in people with type 2 diabetes (CVD‐REAL Nordic) when compared with dipeptidyl peptidase‐4 inhibitor therapy: a multinational observational study. Diabetes Obes Metab. 2018;20(2):344‐351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in Type 2 diabetes. N Engl J Med. 2015;373(22):2117‐2128. [DOI] [PubMed] [Google Scholar]
- 9. Ekstrom N, Svensson AM, Miftaraj M, et al. Cardiovascular safety of glucose‐lowering agents as add‐on medication to metformin treatment in type 2 diabetes: report from the Swedish National Diabetes Register. Diabetes Obes Metab. 2016;18(10):990‐998. [DOI] [PubMed] [Google Scholar]
- 10. Eriksson JW, Bodegard J, Nathanson D, Thuresson M, Nystrom T, Norhammar A. Sulphonylurea compared to DPP‐4 inhibitors in combination with metformin carries increased risk of severe hypoglycemia, cardiovascular events, and all‐cause mortality. Diabetes Res Clin Pract. 2016;117:39‐47. [DOI] [PubMed] [Google Scholar]
- 11. Mogensen UM, Andersson C, Fosbol EL, et al. Cardiovascular safety of combination therapies with incretin‐based drugs and metformin compared with a combination of metformin and sulphonylurea in type 2 diabetes mellitus–a retrospective nationwide study. Diabetes Obes Metab. 2014;16(10):1001‐1008. [DOI] [PubMed] [Google Scholar]
- 12. Morgan CL, Mukherjee J, Jenkins‐Jones S, Holden SE, Currie CJ. Combination therapy with metformin plus sulphonylureas versus metformin plus DPP‐4 inhibitors: association with major adverse cardiovascular events and all‐cause mortality. Diabetes Obes Metab. 2014;16(10):977‐983. [DOI] [PubMed] [Google Scholar]
- 13. Nyström T, Bodegard J, Nathanson D, Thuresson M, Norhammar A, Eriksson JW. Second line initiation of insulin compared with DPP‐4 inhibitors after metformin monotherapy is associated with increased risk of all‐cause mortality, cardiovascular events, and severe hypoglycemia. Diabetes Res Clin Pract. 2017;123:199‐208. [DOI] [PubMed] [Google Scholar]
- 14. Ou SM, Shih CJ, Chao PW, et al. Effects on clinical outcomes of adding dipeptidyl peptidase‐4 inhibitors versus sulfonylureas to metformin therapy in patients with Type 2 diabetes mellitus. Ann Intern Med. 2015;163(9):663‐672. [DOI] [PubMed] [Google Scholar]
- 15. Roumie CL, Greevy RA, Grijalva CG, et al. Association between intensification of metformin treatment with insulin vs sulfonylureas and cardiovascular events and all‐cause mortality among patients with diabetes. JAMA. 2014;311(22):2288‐2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Seong JM, Choi NK, Shin JY, et al. Differential cardiovascular outcomes after dipeptidyl peptidase‐4 inhibitor, sulfonylurea, and pioglitazone therapy, all in combination with metformin, for type 2 diabetes: a population‐based cohort study. PLoS ONE. 2015;10(5):e0124287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lindh A, Persson F, Sobocki P, Bodegard J, Lindarck N. Nordic longitudinal data from electronic medical records and full population national registers: unique opportunities for new Insights in benefit of diabetes patients. Value Health. 2015;18(7):A726. [Google Scholar]
- 18. Agency TDDP . Introduction to the Danish Data Protection Agency Copenhagen, Denmark: Datatilsynet. 2011. https://www.datatilsynet.dk/english/the-danish-data-protection-agency/introduction-to-the-danish-data-protection-agency/. Accessed May 14, 2018.
- 19. Denmark S . Data for research Copenhagen. Denmark: Statistics Denmark. 2018. https://www.dst.dk/en/TilSalg/Forskningsservice. Accessed May 14, 2018.
- 20. Norhammar A, Bodegård J, Nyström T, Thuresson M, Eriksson JW, Nathanson D. Incidence, prevalence and mortality of type 2 diabetes requiring glucose‐lowering treatment, and associated risks of cardiovascular complications: a nationwide study in Sweden, 2006‐2013. Diabetologia. 2016;59(8):1692‐1701. [DOI] [PubMed] [Google Scholar]
- 21. 2015 CT . R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2015. [Google Scholar]
- 22. Curtis HJ, Dennis JM, Shields BM, et al. Time trends and geographical variation in prescribing of drugs for diabetes in England 1998‐2017. Diabetes Obes Metab. 2018;20(9):2159‐2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Birkeland KI, Bodegard J, Persson F, et al. Primary care management of type 2 diabetes mellitus in Denmark, Norway and Sweden: a long term observational study, Munich, Germany. 2016. https://www.easd.org/virtualmeeting/home.html#!resources/primary-care-management-of-type-2-diabetes-mellitus-in-denmark-norway-and-sweden-a-long-term-observational-study. Accessed April 10, 2018.
- 24. Persson F, Bodegard J, Birkeland KI, et al. HbA1c and second line glucose lowering drug initiation in Denmark, Norway and Sweden: an observational study comparing T2DM management in primary care. Munich, Germany. 2016. https://www.easd.org/virtualmeeting/home.html#!resources/hba1c-and-second-line-glucose-lowering-drug-initiation-in-denmark-norway-and-sweden-an-observational-study-comparing-type-2-diabetes-mellitus-management-in-primary-care. Accessed April 10, 2018.
- 25. Khunti K, Godec TR, Medina J, et al. Patterns of glycaemic control in patients with type 2 diabetes mellitus initiating second‐line therapy after metformin monotherapy: retrospective data for 10 256 individuals from the United Kingdom and Germany. Diabetes Obes Metab. 2018;20(2):389‐399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jarvinen S, Laine MK, Eriksson JG. Comparison of use of diabetic medication and clinical guidelines in four Nordic countries. Ann Med. 2016;48(3):162‐168. [DOI] [PubMed] [Google Scholar]
- 27. Swedish National Guidelines for Diabetes Care. 2015. [120]. http://www.socialstyrelsen.se/Lists/Artikelkatalog/Attachments/19803/2015-4-12.pdf. Accessed May 1, 2018.
- 28. Nystrom T, Bodegard J, Nathanson D, Thuresson M, Norhammar A, Eriksson JW. Novel oral glucose‐lowering drugs are associated with lower risk of all‐cause mortality, cardiovascular events and severe hypoglycaemia compared with insulin in patients with type 2 diabetes. Diabetes Obes Metab. 2017;19(6):831‐841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Khunti K, Millar‐Jones D. Clinical inertia to insulin initiation and intensification in the UK: a focused literature review. Prim Care Diabetes. 2017;11(1):3‐12. [DOI] [PubMed] [Google Scholar]
- 30. Gaede P, Oellgaard J, Carstensen B, et al. Years of life gained by multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: 21 years follow‐up on the Steno‐2 randomised trial. Diabetologia. 2016;59(11):2298‐2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Patorno E, Goldfine AB, Schneeweiss S, et al. Cardiovascular outcomes associated with canagliflozin versus other non‐gliflozin antidiabetic drugs: population based cohort study. BMJ. 2018;360:k119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nathanson D, Sabale U, Eriksson JW, et al. Health care cost development in a type 2 diabetes patient population on glucose lowering drug treatment: a nation‐wide observational study 2006‐2014. Pharmacoeconomics. 2017. 10.1007/s41669-017-0063-y [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Brynildsen J, Hoiseth AD, Nygard S, et al. Diagnostic accuracy for heart failure ‐ data from the Akershus Cardiac Examination 2 Study. Tidsskr Nor Laegeforen. 2015;135(19):1738‐1744. [DOI] [PubMed] [Google Scholar]
- 34. Ingelsson E, Arnlov J, Sundstrom J, Lind L. The validity of a diagnosis of heart failure in a hospital discharge register. Eur J Heart Fail. 2005;7(5):787‐791. [DOI] [PubMed] [Google Scholar]
- 35. Kumler T, Gislason GH, Kirk V, et al. Accuracy of a heart failure diagnosis in administrative registers. Eur J Heart Fail. 2008;10(7):658‐660. [DOI] [PubMed] [Google Scholar]
- 36. Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sundboll J, Adelborg K, Munch T, et al. Positive predictive value of cardiovascular diagnoses in the Danish National Patient Registry: a validation study. BMJ Open. 2016;6(11):e012832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Persson F, Bodegard J, Birkeland K, et al. HbA1c and second line glucose lowering drug initiation in Denmark, Norway and Sweden: an observational study comparing T2DM management in primary care. 52nd EASD Annual Meeting, Munich, Germany. 2016.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


