Summary
Background
Melatonin is a hormone synthesized mainly by the pineal gland, and secreted only at night. Melatonin has been proposed as a modulator of glucose metabolism.
Methods
Here we studied the metabolic effects of melatonin administration alone (s.c. 10 mg/kg) or in combination with metformin (p.o. 300 mg/kg), a widely used anti‐diabetic drug. These treatments were tested on glucose tolerance, insulin sensitivity and food intake in Zucker fatty rats (i.e., bearing a missense mutation in the leptin receptor gene) and high‐fat fed Sprague‐Dawley rats.
Results
Melatonin alone or in combination did not significantly modify glucose tolerance in either model. Melatonin alone in high‐fat fed Sprague‐Dawley improved insulin sensitivity to the level of metformin. In addition, combined treatment further ameliorated insulin sensitivity (+13%), especially during the late phase of rising glycemia. The lack of similar effects in Zucker rats suggests an involvement of leptin signaling in mediating the positive effects of melatonin. Body mass gain in Sprague‐Dawley rats was decreased by both metformin, and combined metformin and melatonin. While melatonin alone did not markedly affect food intake, its combination with metformin led to a more pronounced anorexia (‐17% food intake during the last week), as compared to metformin alone.
Conclusions
Melatonin improves the beneficial effects of metformin on insulin sensitivity and body mass gain in high‐fat fed Sprague‐Dawley rats. Therefore, the combination of melatonin and metformin could be beneficial to develop dual therapies to treat or delay type 2 diabetes associated with obesity.
Keywords: diet‐induced obesity, glucose tolerance, insulin sensitivity, metabolic syndrome
1. INTRODUCTION
Diabetes and obesity are global epidemics affecting more and more people worldwide. In addition to genetic susceptibility, overeating and/or sedentary lifestyle, other factors have been recently identified. In particular, disruptions in circadian rhythms due to recurrent exposition to bright light at night, shift work and chronic jetlag lead to increased risks of developing several metabolic problems like cardiovascular diseases,1 overweight and type 2 diabetes (T2D).2, 3, 4 Thus, it is important to develop new therapeutic strategies that prevent or limit the adverse effects of circadian disruption on metabolic homeostasis.
Melatonin is a hormone synthesised mainly by the pineal gland and secreted only at night. This specific timing is tightly controlled by the master clock located in the suprachiasmatic nuclei of the hypothalamus that are reset by the ambient light/dark cycle. The daily rhythm of circulating melatonin is a powerful chronobiotic signal because it modulates the phase of circadian oscillations in peripheral and central structures, including the master clock.5 Exposure to light at night not only resets the master clock, but immediately inhibits melatonin as long as lights are switched on,6 thus perturbing internal coupling within the circadian network and external synchronisation to local time.
In addition to its time‐giving properties,5, 6, 7 melatonin has been proposed as a modulator of glucose metabolism and feeding behaviour, although the underlying mechanisms are not fully characterised yet.3 Pinealectomy which abolishes circulating melatonin leads to diabetogenic symptoms in rodents,8 such as glucose intolerance, drastic reduction in GLUT4 expression in white adipose tissue and muscle, reduced fat cell responses to insulin altered rate of insulin secretion,9, 10 and hyperglycaemia at night.11 Melatonin receptors (MT1 and MT2) knock‐out (KO) in mice abolishes the daily rhythm in blood glucose,12 while mice KO for MT1 display increased insulin resistance.13 Furthermore, single nucleotide polymorphisms in the MT2 receptor (also called MTNR1B) are associated with higher incidence of T2D in humans.14, 15, 16 Lower secretion of melatonin in nurses has been identified as a risk factor for developing T2D.17 Besides glucose metabolism, growing evidence indicates that melatonin can modulate feeding behaviour, at least in rodents. Oral supplementation of melatonin in drinking water causes a drop in total food intake and a decrease in meal size,18 as well as a reduction in body mass gain and in adiposity, combined with improved sensitivity to insulin.19
Together, these findings suggest that melatonin is a factor possibly involved in metabolic health and the pathogenic effects of altered timing. As such, exogenous melatonin alone or in combination with other treatments may have anti‐diabetic properties. Metformin is widely used for treating patients with T2D because it inhibits gluconeogenesis, lowers fasting blood glucose and reduces food intake.20 We hypothesise that the combination of melatonin with metformin would improve the effects of either melatonin or metformin alone to alleviate the diabetic symptoms. For that purpose, we investigated the effects of melatonin and/or metformin supplementation on glucose tolerance, insulin sensitivity and food intake in two rat models of T2D and obesity: missense mutation (fatty, fa) in the leptin receptor gene (ie, Zucker fatty rats) and high‐fat diet‐induced obesity in Sprague Dawley rats.
2. MATERIAL AND METHODS
2.1. Animals and housing conditions
A total of 32 male Zucker rats (fa/fa, strain code: 85; Charles River Laboratories, Saint Germain Nuelles, France), 8‐week old upon arrival, and 32 male Sprague Dawley rats (strain code: 400; Charles River Laboratories), 4‐week old upon arrival, were housed in individual cages at 22 ± 1°C under a 12 hours light/dark cycle (lights on at 07.00 am) and had ad libitum access to food (standard low‐fat diet; ref. 105, Scientific Animal Food and Engineering, Augy, France) and UV‐treated tap water during 2 weeks of habituation. A piece of wood for gnawing was added in each cage.
All experiments were approved by the Regional Ethical Committee of Strasbourg for Animal experimentation (CREMEAS) and French Ministry of Higher Education and Research (APAFIS #00426.02) in accordance with the U.S. National Institutes of Health Guide for the Care and Use of Laboratory Animals (1996), the French National Law implementing the European Union Directive 2010/63/EU.
2.2. Experimental design
2.2.1. Experiment 1
Zucker rats fed with chow diet were divided into four groups (n = 8) that received a treatment every evening (1‐2 hours before lights off) during 2 weeks. The 1st group (control) received an injection of hydroxyl‐ethyl‐cellulose 0.5% (HEC; Sigma‐Aldrich, Saint‐Quentin Fallavier, France) by oral gavage, and s.c. injection of ringer solution containing 5% alcohol. The 2nd group received an injection of metformin (300 mg/kg in HEC; Sigma‐Aldrich) by oral gavage, and s.c. injection of ringer with 5% alcohol. The 3rd group received an injection of HEC by oral gavage and s.c. injection of melatonin (10 mg/kg in a ringer solution with 5% alcohol; Sigma‐Aldrich). The 4th group was treated with injection of metformin (300 mg/kg in HEC) by oral gavage and s.c. injection of melatonin (10 mg/kg in 5% alcohol ringer). The different routes of administration and dosages for metformin and melatonin were selected based on previous studies in rats.21, 22
After 2 weeks of treatment, Zucker rats were fasted overnight and submitted to an oral glucose tolerance test (oral gavage with d‐glucose 2 g/kg; Sigma‐Aldrich) 2 hours after the lights were turned on. Microsamples (<0.5 μL) of blood were collected from tail veins, and blood glucose was determined immediately with an Accu‐Check glucometer (Roche Diagnostics, Meylan, France).
One week later under daily treatment, Zucker rats were fasted overnight and tested for insulin sensitivity (ip, insulin 1 U/kg, Umuline, Lilly, France) 2 hours after the lights were turned on. Microsamples of tail blood were taken as above.
One further week later under daily treatment, food was removed at lights on. Two hours after lights on, all Zucker rats were deeply euthanised with a high dose of sodium pentobarbital (ip, 150 mg/kg) and their blood was sampled by intracardiac puncture, collected in tubes with 4% EDTA (10 mL for 1 mL of blood) and centrifuged for 10 minutes at 4600 g at 4°C. Body mass was determined every week and food intake was evaluated by weighing the cumulative food intake over a week during the last 2 weeks of treatment.
2.2.2. Experiment 2
During 3 months of baseline, all Sprague Dawley rats were fed with a pelleted very‐high‐fat and carbohydrate‐free diet (5.9 kcal/g, Scientific Animal Food and Engineering, Augy, France; 75% fat content, for details on diet composition, see HDF3 in23). The diet was vacuum‐packed and stored at 8°C before use. Rats were given fresh food once a week. Body mass and food intake were determined every week.
The protocol for Sprague Dawley rats under high‐fat diet (including 4 treatments, oral glucose tolerance test and insulin sensitivity test) was the same as above, except that epididymal fat tissue was dissected and weighed on the day of sacrifice. One ill animal from the fourth group had to be removed from the experiment, leaving n = 7 in the metformin+melatonin group.
2.3. Assays of plasma metabolites
Plasma glucose was determined with GOD‐PAP Kit (LP80009, Biolabo, Maizy, France). Concentrations of nonesterified fatty acids (NEFA) were measured by NEFA‐HR(2) kit (Wako Chemicals GmbH, Neuss, Germany). Plasma triglycerides (TG) and glycerol levels were measured with serum triglyceride determination kit (TR‐0100, Sigma‐Aldrich). Plasma levels of total cholesterol, low‐density lipoprotein (LDL)‐cholesterol and high‐density lipoprotein (HDL)‐cholesterol were evaluated by a colorimetric direct method (LP80106, 90206, 90416, respectively; Biolabo).
2.4. Hormonal assays
Leptin and insulin concentrations were determined by a rat leptin ELISA kit (EZRL‐83K, Millipore‐Merck, Molsheim, France) and an ultra‐sensitive rat insulin ELISA kit (#90060, Crystal Chem Inc., Downers Grove, IL, USA), respectively.
2.5. Statistical analysis
Data are presented as mean ± SEM. Area under the curve (AUC) was determined relative to baseline. One‐ or two‐way analyses of variance (ANOVA) with repeated measures were performed to assess the effects of treatment and/or time, followed by post hoc Fisher LSD test (SigmaPlot version 12, SPSS Inc, Chicago, IL, USA). P values >0.05 were considered nonsignificant (NS).
3. RESULTS
3.1. Zucker rats
3.1.1. Body mass and food intake
Body mass determined before and after the 4‐week treatment was significantly increased by time (F 1,28 = 149.7, P < 0.001), but unaffected by the treatment (F 3,28 = 0.6, P > 0.1; Table 1A). In parallel, food intake measured during the 3rd and 4th week of treatment did not differ significantly according to time (F 1,28 = 0.6, P > 0.1) or treatment (F 1,28 = 1.2, P > 0.1).
Table 1.
Bodyweight (BW) and food intake (FI) before and after the treatment
| (A) Zucker rats | ||||
|---|---|---|---|---|
| BW before (g) | BW after (g) | FI Week 3 (g) | FI week 4 (g) | |
| Control | 366.6 ± 15.7* | 384.2 ± 16.5& | 26.7 ± 1.0 | 27.7 ± 1.2 |
| Metformine | 365.3 ± 11.2* | 392.3 ± 10.2& | 27.9 ± 0.7 | 29.8 ± 0.5 |
| Melatonin | 379.7 ± 13.1* | 410.4 ± 15.7& | 29.3 ± 1.3 | 28.9 ± 1.5 |
| Met + MEL | 377.5 ± 9.0* | 407.2 ± 10.2& | 30.5 ± 1.6 | 29.8 ± 1.9 |
| (B) Sprague Dawley rats | |||||
|---|---|---|---|---|---|
| BW before (g) | BW after (g) | FI before (g) | FI Week 3 (g) | FI week 4 (g) | |
| Control | 528.6 ± 18.5 | 528.6 ± 19.1a | 14.0 ± 0.5* | 11.4 ± 0.4& d | 11.3 ± 0.2& a |
| Metformin | 531.2 ± 14.2* | 477.5 ± 16.7& ac | 14.0 ± 0.3* | 8.6 ± 0.7& bc | 10.4 ± 0.6£ a |
| Melatonin | 528.1 ± 20.1 | 534.33 ± 25.4a | 14.1 ± 0.8* | 12.5 ± 1.0& a | 11.8 ± 0.8*a |
| Met + MEL | 523.6 ± 17.1* | 463.8 ± 10.5& bc | 13.4 ± 0.3* | 6.6 ± 0.4& b | 8.6 ± 0.2& b |
For a given treatment group (horizonal lines), means with different symbols (*, &, or £) are significantly different for a given parameter (ie, bodyweight or food intake) between steps (P < 0.001). For a given parameter at a given step (columns), means with different letters (a, b, c, or d) are significantly different between treatment groups (P < 0.001). Data are means ± SEM.
3.1.2. Glucose tolerance
An oral bolus of glucose given in the early morning triggered a significant increase in plasma glucose levels, which declined during the 3 following hours. Two‐way ANOVA detected a significant effect of time (F 7,196 = 84.7, P < 0.001), a significant [time × treatment] interaction (F 21,196 = 3.4, P < 0.001), and only a trend for a main effect of treatment (F 3,196 = 2.7, P = 0.06). There was a global improvement of glucose tolerance in Zucker rats by metformin treatment compared to control procedure and melatonin. The combination of metformin and melatonin did not modify markedly the beneficial effect of metformin alone. Fasting blood glucose corresponding to T0 did not differ significantly between treatments. Metformin alone and metformin+melatonin led to lower levels of plasma glucose during the first 90 minutes after glucose load, as compared to the control group. Melatonin treatment increased plasma glucose only during the acute response (ie, 15 minutes after glucose load), as compared to control rats. One‐way ANOVA on area under the curve (0 to 2 hours after glucose load) highlighted an effect of treatment (F 3,28 = 5.2, P = 0.006), metformin+melatonin group showing reduced values (P < 0.05) and metformin group being very close to significant threshold (P = 0.051), as compared to the control group, while melatonin alone was without effect on area under the curve reflecting glucose tolerance (Figure 1).
Figure 1.
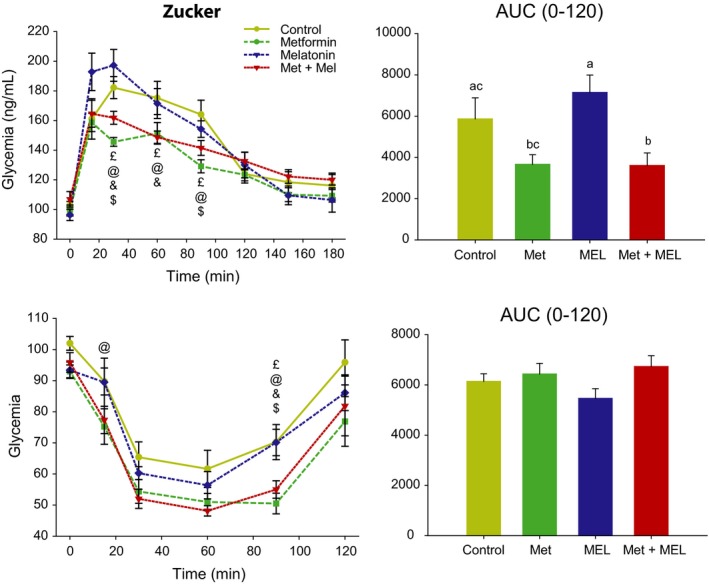
Upper left panel: oral glucose tolerance test (OGTT) in Zucker rats fasted overnight. Symbols were used to highlight differences between treatment groups for a given time point: £, control group different from Metformin+Melatonin group; @, control group different from Metformin group; &, Metformin group different from Metformin+Melatonin group; $, Metformin group different from Melatonin group. Upper right panel: corresponding area under the curve of OGTT (AUC). Groups with different letters are significantly different (P < 0.05). Lower left panel: ip, insulin sensitivity test (ITT) in Zucker rats fasted overnight. Symbols for statistical differences, as above. Lower right panel: corresponding area under the curve of ITT (AUC). AUC, area under the curve. Met, metformin, MEL, Melatonin
3.1.3. Insulin sensitivity
Administration of insulin acutely reduced plasma glucose levels, which returned to basal values 2 hours after the treatment. Two‐way ANOVA indicates significant effects of time (F 7,196 = 87.2, P < 0.001) and treatment (F 3,196 = 4.1, P = 0.016), without significant interaction. Daily treatments with metformin and metformin+melatonin led to improved insulin sensitivity, while injections of melatonin alone were without significant effect, as compared to rats treated with vehicle. One‐way ANOVA on area under the curve (0‐2 hours after insulin injection) did not detect any effect of the treatment (F 3,28 = 1.2, P = 0.3; Figure 1).
3.1.4. Plasma parameters
None of the plasma metabolites or hormones was significantly modified by the 4 treatments (P > 0.05; Figures 2 and 3).
Figure 2.
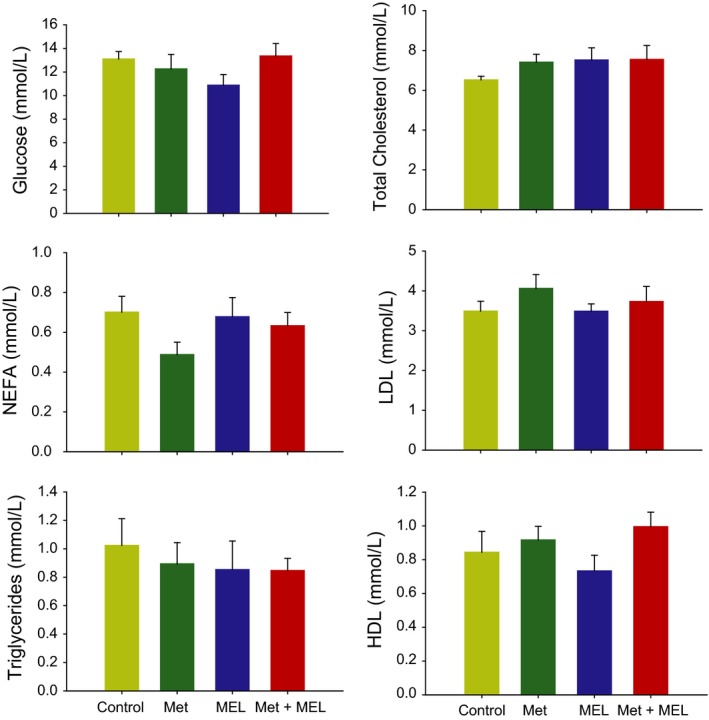
Plasma metabolites in the 4 groups of Zucker rats. Low‐density lipoproteins, LDL; high‐density lipoproteins, HDL; NEFA, nonesterified fatty acids; Met, metformin; MEL, Melatonin
Figure 3.
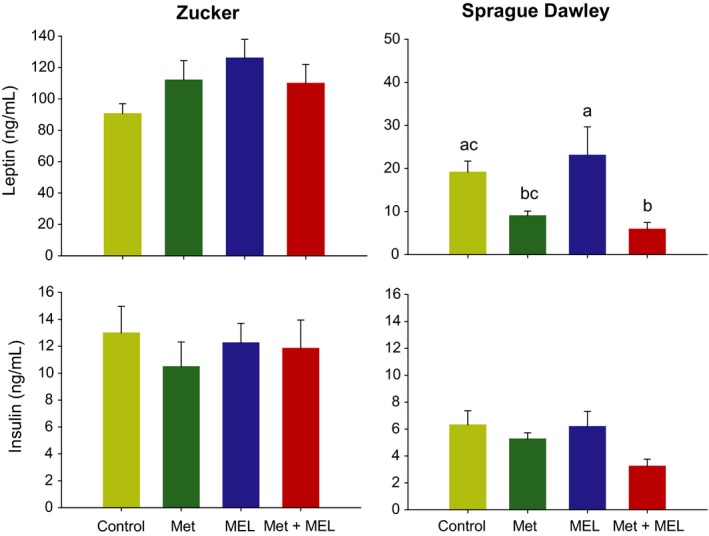
Plasma levels of leptin (upper panels) and insulin (lower panels) in Zucker (right panels) an Sprague Dawley rats (left panels). Groups with different letters are significantly different (P < 0.05). Met, metformin; MEL, Melatonin
3.2. High‐fat‐fed Sprague Dawley rats
3.2.1. Body mass, adipose tissue and food intake
Body mass in high‐fat‐fed Sprague Dawley rats changed according to time (F 1,27 = 108.5, P < 0.001). There was also a significant [time × treatment] interaction (F 3,27 = 45.2, P < 0.001); Table 1B). Before starting the treatment, the 4 groups of rats had a similar body mass around 530 g. While daily treatments with either vehicle melatonin were without significant effect, treatments with metformin alone or in combination with melatonin produced a significant reduction in body mass (P < 0.001; Table 1B). The mass of epididymal white adipose tissue was not significantly modified by the 4 treatments (Control: 14.09 ± 2.17 g; Metformin: 10.61 ± 1.22 g; Melatonin: 15.35 ± 2.54 g; Metformin+Melatonin: 9.35 ± 1.35 g; F 3,27 = 2.1, P > 0.1). The body mass loss can, in part, be explained by a reduction in food intake. Mean food intake before and during the 3rd and 4th week of treatment was significantly affected by time (F 2,27 = 126.7, P < 0.001) and treatment (F 3,27 = 9.8, P < 0.001). Compared to rats treated with vehicle, melatonin led to a reduction in food consumption during the 3rd week, while metformin was associated with a deeper reduction of food intake. These differences were no longer significant during the last week of treatment. By contrast, the combination of metformin and melatonin led to a long‐lasting anorexia until the end of the experiment. Notably, rats receiving metformin+melatonin ate −17% less compared to those treated with metformin alone during the 4th week (Table 1B).
3.2.2. Glucose tolerance
Glucose load in high‐fat‐fed rats led to increased levels of plasma glucose which remain elevated during the next 3 hours. Tolerance to glucose was affected by both time (F 7,189 = 58.1, P < 0.001) and treatment (F 3,189 = 5.9, P < 0.01). Overall, vehicle and melatonin treatments did not differ from each other, while treatments with metformin alone or in combination to melatonin significantly improved glucose tolerance by maintaining lower glycaemia compared to the control group during the last 2 hours of the test (ie, 5 time‐points). One‐way ANOVA on area under the curve (0–3 hours after glucose load) also detected a significant effect of treatment (F 3,27 = 3.8, P < 0.05), highlighting the improvement of glucose tolerance in both groups with metformin or metformin+melatonin, compared to the control group. One‐way ANOVA on area under the curve confirmed the beneficial effect of metformin and metformin+melatonin, and also revealed an effect of melatonin. Of note, the combination of metformin plus melatonin tended to have a potentiating effect on glucose tolerance (Figure 4).
Figure 4.
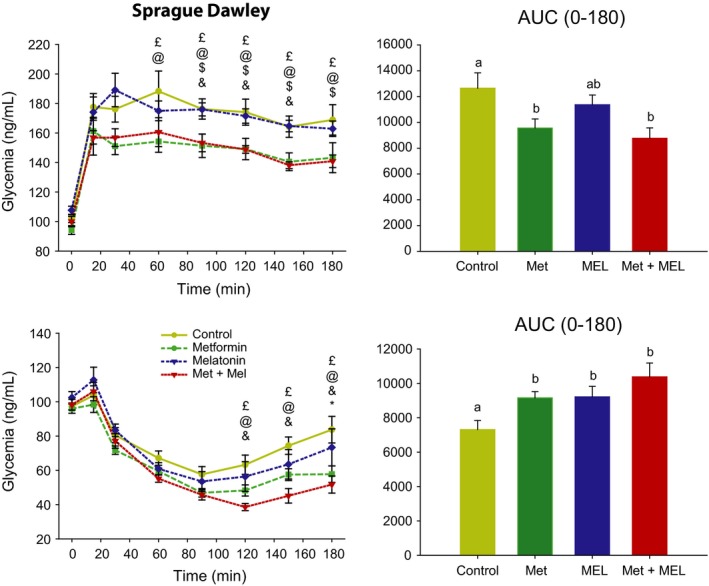
Upper left panel: oral glucose tolerance test (OGTT) in Sprague Dawley rats fasted overnight. Symbols were used to highlight differences between treatment groups for a given time point: £, control group different from Metformin+Melatonin group; @, control group different from Metformin group; &, Metformin group different from Metformin+Melatonin group; $, Metformin group different from Melatonin group. Upper right panel: corresponding area under the curve of OGTT (AUC). Groups with different letters are significantly different (P < 0.05). Lower left panel: ip, insulin sensitivity test (ITT) in Sprague Dawley rats fasted overnight. Symbols for statistical differences, as above. Lower right panel: corresponding area under the curve of ITT (AUC). Groups with different letters are significantly different (P < 0.05). AUC, area under the curve. Met, metformin, MEL, Melatonin
3.2.3. Insulin sensitivity
While basal levels of plasma insulin were not significantly affected by treatment (Figure 3, see below), insulin injections induced hypoglycaemia that was modified both by time (F 7,189 = 113.8, P < 0.001) and treatment (F 3,189 = 3.4, P < 0.05). Overall, as compared to vehicle group, insulin sensitivity was improved by metformin and melatonin plus metformin (especially during the last 2 hours of the test), while melatonin alone was without significant effect. Comparable findings are obtained when comparing the area under the curve (F 3,27 = 4.6, P < 0.01; Figure 4).
3.2.4. Plasma parameters
Most analysed plasma metabolites (ie, glucose, NEFA, HDL, cholesterol and triglycerides) were unaffected by the treatment, except LDL that was significantly modified (F 3,27 = 7.026, P < 0.001). LDL levels were higher after melatonin administration, with or without metformin (Figure 5). Regarding insulin, there was also a trend for an effect of treatment (F 3,27 = 2.5, P = 0.08), with the metformin+melatonin association leading only to an apparent reduction in insulinaemia. By contrast, leptin levels were strongly changed according to the treatment (F 3,27 = 4.6, P < 0.01; Figure 3).
Figure 5.
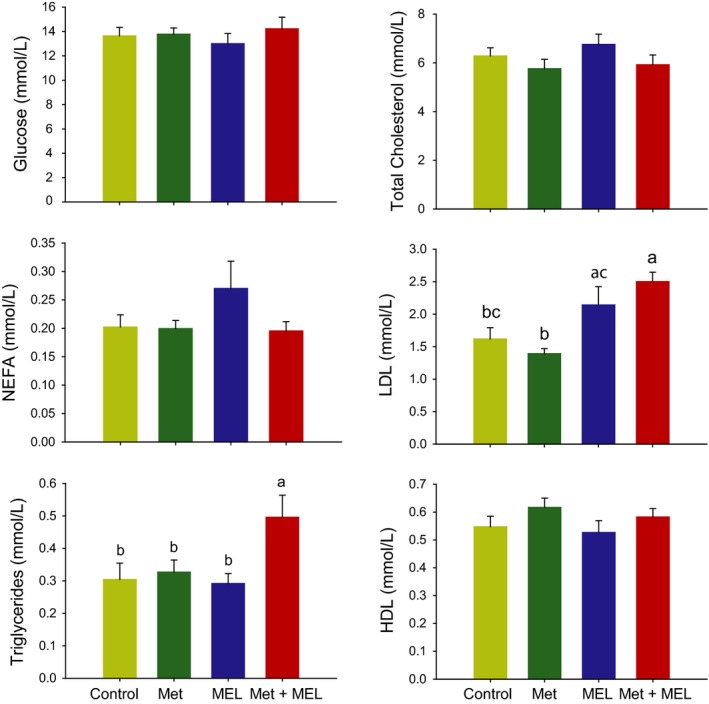
Plasma metabolites in the 4 groups of Sprague Dawley rats. Low‐density lipoproteins, LDL; high‐density lipoproteins, HDL; NEFA, nonesterified fatty acids; Met, metformin; MEL, Melatonin. Groups with different letters are significantly different (P < 0.05)
4. DISCUSSION
A number of dual therapies are developed to improve the treatment of obese subjects with T2D. Metformin, a widely used anti‐diabetic drug, has been for instance associated with insulin sensitiser.24 Here, using two animal models of T2D and obesity: Zucker fatty rats and high‐fat‐fed Sprague Dawley rats, we tested the effectiveness of combining metformin with melatonin, a time‐giving hormone also able to modulate glucose metabolism. The combined treatment tends to further improve insulin sensitivity (+13%), as compared to metformin or melatonin alone. In addition, the combined treatment led to a more pronounced anorexia (−17% food intake during the last week of treatment), as compared to metformin alone. Such differences were not detected in Zucker rats, thus suggesting that impaired leptin signalling prevents the beneficial effects of melatonin.
4.1. Anti‐diabetic and anorectic effects of metformin
Metformin was initially recognised as an anti‐diabetic drug preventing hyperglycaemia, via AMPK activation in the liver. Further investigations, however, highlighted wider roles of metfomin, eventually mediated by AMPK‐independent mechanisms.20 Among others, metformin has anorectic effects in rats fed with chow or high‐fat diet, thus favouring loss of body fat and normalisation of plasma glucose and glucose tolerance.21 The present results in high‐fat‐fed Sprague Dawley rats clearly confirm these findings. To assess whether metformin‐induced body mass loss is exclusively due to its anorectic effects will require additional experiments of pair‐feeding. In obese subjects also, a 2‐week treatment with metformin leads to body mass loss and associated decline in plasma leptin, in correlation with a reduction in food intake.25
By contrast, while a previous report also found anorectic effect of metformin in Zucker rats,26 we did not detect any change in food intake, nor in body mass loss after repeated administration of the same dose of metformin in these rats. Another study in Zucker rats also did not find a significant effect of metformin alone.27 Differences in routes of administration (ie, subcutaneous versus oral gavage) can partly explain such discrepancies. Alternatively, the lack of effect of metformin in our Zucker rats may result from their altered leptin signalling. Of note, metformin treatment induces not only a reduction in plasma glucagon, but also an increased release of plasma leptin,28 both effects likely contributing to diminish food intake. Furthermore, metformin‐induced reduction in appetite has been shown to involve activation of leptin signalling pathway in the hypothalamus, including increased expression of leptin receptor and increased in P‐STAT3.29, 30
4.2. Effects of melatonin on glucose metabolism, body mass and food intake
Besides its action as a chronobiotic agent (ie, an agent affecting function of circadian clocks), exogenous melatonin has been proposed as a beneficial regulator of glucose metabolism, mostly using pinealectomy or KO mice for melatonin receptors.8, 9, 10, 11, 12 Melatonin has been proposed to inhibit insulin secretion.31 The present experiments using repeated administration of melatonin in both high‐fat‐fed Sprague Dawley rats and chow‐fed Zucker rats do not readily confirm this observation because plasma levels of insulin remain unaffected by melatonin administration. Another work in mice has shown that mice lacking MT1 receptor have down‐regulated insulin signalling pathway while melatonin administration in wild‐type mice exposed to constant light restores insulin sensitivity.32 In addition, the present data indicate that treatment with melatonin improves insulin sensitivity of high‐fat‐fed Sprague Dawley, to a level similar to that of metformin. A previous work has already reported that melatonin provided in drinking water reduces insulin resistance in high‐fat‐fed Wistar rats.33 Based on the mouse study cited above, this suggests that the improvement of insulin sensitivity is mediated via MT1 receptors.
A series of studies also established that oral supplementation of melatonin to middle‐aged and older (10‐ to 22‐month‐old) Sprague Dawley rats not only restores the plasma profiles of melatonin and leptin at young rats levels (4‐month‐old), but also reduces body mass and visceral fat, reversing in part the metabolic syndrome established with the progression of age.34, 35 In addition, other works showed that the treatment of elderly animals with melatonin reduces body mass, as well as re‐establishing full insulin signalling.19 In these studies as well as in high‐fat‐fed younger rats,36 body mass loss after melatonin treatment is not related to changes in feeding behaviour. Our results are in full accordance with this interpretation because melatonin‐treated Sprague Dawley rats also show similar changes. Such body mass loss with anorexia could be due to decreased ability to store energy in the white adipose tissue. The lack of difference in epididymal fat mass or in plasma leptin after repeated treatment with melatonin does not valid this hypothesis. Alternatively, melatonin may increase energy expenditure, this hypothesis being supported by the observation of an increased nocturnal activity after melatonin treatment.37 Further investigation using indirect calorimetry will help to solve this issue.
Other studies, however, have found that melatonin can fine‐tune the pattern of feeding. In particular, mice KO for MT1 spend more time feeding.38 Moreover, exogenous melatonin in rats leads to overall reduced food intake, due to smaller and shorter meals.18 Such anorectic effect, however, was found only in nondiabetic Wistar, but not in diabetic rats. This might explain why we do not observe a similar modulator role of melatonin on the amount of food consumed in high‐fat‐fed Sprague Dawley rats, nor in Zucker rats.
The metabolic action of melatonin could be mediated either centrally39 and/or peripherally. Among peripheral tissues, gut is a candidate to be affected by melatonin treatment. In support to this view, a previous work has demonstrated that melatonin prevents diet‐induced obesity in mice via counteracted changes in the composition of the microbiome (ie, dysbiosis), decrease in low‐grade inflammation and oxidative stress, dyslipidaemia, as well as stimulation of browning in inguinal subcutaneous adipose tissue and improvement of insulin resistance.40
4.3. Effects of melatonin in combination with metformin on glucose metabolism, body mass and food intake
The combination of metformin and melatonin does not improve beneficial effects of metformin alone on glucose tolerance in high‐fat‐fed Sprague Dawley rats. By contrast, the combined treatment tends to ameliorate insulin sensitivity compared to metformin alone, especially during the late phase of rising glycaemia. This improvement is not detectable in Zucker rats, suggesting that functional leptin receptors may to some extent play a role. Further investigation will be necessary to unravel the mechanistic interactions between melatonin and leptin signalling. Importantly, in a model of diet‐induced obesity coupled with circadian desynchronisation in Sprague Dawley rats, the combined treatment with melatonin and metformin has also been shown to improve insulin sensitivity, partly via anti‐apoptotic effects on pancreatic β cells.41 Antioxidants properties of the combination of metformin and melatonin have been established in rats with induced polycystic ovary syndrome. Thus, it is possible that in high‐fat‐fed rats, the combined administration leads to a decrease in peripheral oxidative stress.
Of note, the combination is effective to further reduce feeding consumption and for a longer time, as compared to the anorectic effects of metformin alone in Sprague Dawley. Therefore, the observed reduction in body mass gain may, in part, result from a reduced food intake.
4.4. Biomedical perspectives
T2D and obesity are increasing worldwide. These metabolic disorders are frequently associated with circadian disturbances.3 Conversely, circadian desynchronisation due to bright light at night, shift work and chronic jetlag leads to increased occurrence of metabolic risks.42 Here we show that melatonin administration, especially in combination with metformin, diminishes metabolic disturbances. In humans, melatonin treatment has been shown to reduce insulin secretion, especially in subjects carrying a genetic variant (ie, rs10830963) in the MT2 receptor.43
A part of the beneficial effects of melatonin may be mediated by its chronobiotic effects (ie, its effects of the circadian clocks) that would, for instance, restore a phase‐alignment between the central clock and many secondary clocks in peripheral tissues. Here s.c. 10 mg/kg dosage of melatonin has been injected in late afternoon, a timing associated with a shifting effect on the central clock.5 In accordance with the importance of taking into account the timing of injection, a previous study has demonstrated that oral administration of 30 mg/kg melatonin in rats is effective to reduce body mass gain when it is given in late afternoon, but not in the morning.36 Another, nonexclusive, hypothesis is that melatonin treatment actually decreases oxidative stress in metabolic tissues such as liver and pancreas. Further experiments are warranted to investigate this possibility and what could be the synergistic effects of melatonin and metformin at the intra‐cellular level.
Considered together, the present findings indicate that the combined treatment with melatonin and metformin may be beneficial to develop novel bitherapies to treat or delay T2D with obesity.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
AUTHORS CONTRIBUTIONS
RFDF, CSK, BGL, PP and EC designed the experiments, HR and SD performed the experiments, HR, SD and RFDF analysed the data, RFDF made the figures and wrote the first version of the manuscript that was corrected by all co‐authors.
ACKNOWLEDGEMENTS
We thank Sylviane Gourmelen (INCI, CNRS UPR3212), as well as Dominique Ciocca and Nicolas Lethenet (Chronobiotron platform, UMS3415, CNRS, University of Strasbourg) for help with experiments. This work was supported by the Centre National de la Recherche Scientifique, University of Strasbourg (EC and PP) and the Institut de Recherches Internationales Servier (CSK and BGL).
Dantas‐Ferreira RF, Raingard H, Dumont S, et al. Melatonin potentiates the effects of metformin on glucose metabolism and food intake in high‐fat‐fed rats. Endocrinol Diab Metab. 2018;1:e39 10.1002/edm2.39
REFERENCES
- 1. Woon PY, Kaisaki PJ, Braganca J, et al. Aryl hydrocarbon receptor nuclear translocator‐like (BMAL1) is associated with susceptibility to hypertension and type 2 diabetes. Proc Natl Acad Sci USA. 2007;104(36):14412‐14417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Delezie J, Challet E. Interactions between metabolism and circadian clocks: reciprocal disturbances. Ann N Y Acad Sci. 2011;1243:30‐46. [DOI] [PubMed] [Google Scholar]
- 3. Forrestel AC, Miedlich SU, Yurcheshen M, Wittlin SD, Sellix MT. Chronomedicine and type 2 diabetes: shining some light on melatonin. Diabetologia. 2017;60(5):808‐822. [DOI] [PubMed] [Google Scholar]
- 4. Qian J, Scheer FA. Circadian system and glucose metabolism: implications for physiology and disease. Trends Endocrinol Metab. 2016;27(5):282‐293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pevet P, Challet E. Melatonin: both master clock output and internal time‐giver in the circadian clocks network. J Physiol Paris. 2011;105(4–6):170‐182. [DOI] [PubMed] [Google Scholar]
- 6. Redlin U. Neural basis and biological function of masking by light in mammals: suppression of melatonin and locomotor activity. Chronobiol Int. 2001;18(5):737‐758. [DOI] [PubMed] [Google Scholar]
- 7. Houdek P, Novakova M, Polidarova L, Sladek M, Sumova A. Melatonin is a redundant entraining signal in the rat circadian system. Horm Behav. 2016;83:1‐5. [DOI] [PubMed] [Google Scholar]
- 8. Cipolla‐Neto J, Amaral FG, Afeche SC, Tan DX, Reiter RJ. Melatonin, energy metabolism, and obesity: a review. J Pineal Res. 2014;56(4):371‐381. [DOI] [PubMed] [Google Scholar]
- 9. Zanquetta MM, Seraphim PM, Sumida DH, Cipolla‐Neto J, Machado UF. Calorie restriction reduces pinealectomy‐induced insulin resistance by improving GLUT4 gene expression and its translocation to the plasma membrane. J Pineal Res. 2003;35(3):141‐148. [DOI] [PubMed] [Google Scholar]
- 10. Lima FB, Matsushita DH, Hell NS, Dolnikoff MS, Okamoto MM, Cipolla Neto J. The regulation of insulin action in isolated adipocytes. Role of the periodicity of food intake, time of day and melatonin. Braz J Med Biol Res. 1994;27(4):995‐1000. [PubMed] [Google Scholar]
- 11. la Fleur SE, Kalsbeek A, Wortel J, van der Vliet J, Buijs RM. Role for the pineal and melatonin in glucose homeostasis: pinealectomy increases night‐time glucose concentrations. J Neuroendocrinol. 2001;13(12):1025‐1032. [DOI] [PubMed] [Google Scholar]
- 12. Owino S, Contreras‐Alcantara S, Baba K, Tosini G. Melatonin signaling controls the daily rhythm in blood glucose levels independent of peripheral clocks. PLoS One. 2016;11(1):e0148214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Contreras‐Alcantara S, Baba K, Tosini G. Removal of melatonin receptor type 1 induces insulin resistance in the mouse. Obesity. 2010;18(9):1861‐1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bouatia‐Naji N, Bonnefond A, Cavalcanti‐Proenca C, Sparso T, Holmkvist J, Marchand M. A variant near MTNR1B is associated with increased fasting plasma glucose levels and type 2 diabetes risk. Nat Genet. 2009;41(1):89‐94. [DOI] [PubMed] [Google Scholar]
- 15. Sparso T, Bonnefond A, Andersson E, Bouatia‐Naji N, Holmkvist J, Wegner L. G‐allele of intronic rs10830963 in MTNR1B confers increased risk of impaired fasting glycemia and type 2 diabetes through an impaired glucose‐stimulated insulin release: studies involving 19,605 Europeans. Diabetes. 2009;58(6):1450‐1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dupuis J, Langenberg C, Prokopenko I, Saxena R, Soranzo N, Jackson AU. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet. 2010;42(2):105‐116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McMullan CJ, Schernhammer ES, Rimm EB, Hu FB, Forman JP. Melatonin secretion and the incidence of type 2 diabetes. JAMA. 2013;309(13):1388‐1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Montano ME, Molpeceres V, Mauriz JL, et al. Effect of melatonin supplementation on food and water intake in streptozotocin‐diabetic and non‐diabetic male Wistar rats. Nutr Hosp. 2010;25(6):931‐938. [PubMed] [Google Scholar]
- 19. Zanuto R, Siqueira‐Filho MA, Caperuto LC, Bacurau RF, Hirata E, Peliciari‐Garcia RA. Melatonin improves insulin sensitivity independently of weight loss in old obese rats. J Pineal Res. 2013;55(2):156‐165. [DOI] [PubMed] [Google Scholar]
- 20. Rena G, Hardie DG, Pearson ER. The mechanisms of action of metformin. Diabetologia. 2017;60(9):1577‐1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lv WS, Wen JP, Li L, et al. The effect of metformin on food intake and its potential role in hypothalamic regulation in obese diabetic rats. Brain Res. 2012;1444:11‐19. [DOI] [PubMed] [Google Scholar]
- 22. Bothorel B, Barassin S, Saboureau M, et al. In the rat, exogenous melatonin increases the amplitude of pineal melatonin secretion by a direct action on the circadian clock. Eur J Neurosci. 2002;16(6):1090‐1098. [DOI] [PubMed] [Google Scholar]
- 23. Sinitskaya N, Gourmelen S, Schuster‐Klein C, Guardiola‐Lemaitre B, Pevet P, Challet E. Increasing the fat‐to‐carbohydrate ratio in a high‐fat diet prevents the development of obesity but not a prediabetic state in rats. Clin Sci (Lond). 2007;113(10):417‐425. [DOI] [PubMed] [Google Scholar]
- 24. Suzuki M, Odaka H, Suzuki N, Sugiyama Y, Ikeda H. Effects of combined pioglitazone and metformin on diabetes and obesity in Wistar fatty rats. Clin Exp Pharmacol Physiol. 2002;29(4):269‐274. [DOI] [PubMed] [Google Scholar]
- 25. Paolisso G, Amato L, Eccellente R, et al. Effect of metformin on food intake in obese subjects. Eur J Clin Invest. 1998;28(6):441‐446. [DOI] [PubMed] [Google Scholar]
- 26. Rouru J, Pesonen U, Koulu M, et al. Anorectic effect of metformin in obese Zucker rats: lack of evidence for the involvement of neuropeptide Y. Eur J Pharmacol. 1995;273(1–2):99‐106. [DOI] [PubMed] [Google Scholar]
- 27. Yasuda N, Inoue T, Nagakura T, et al. Metformin causes reduction of food intake and body weight gain and improvement of glucose intolerance in combination with dipeptidyl peptidase IV inhibitor in Zucker fa/fa rats. J Pharmacol Exp Ther. 2004;310(2):614‐619. [DOI] [PubMed] [Google Scholar]
- 28. Barnea M, Haviv L, Gutman R, Chapnik N, Madar Z, Froy O. Metformin affects the circadian clock and metabolic rhythms in a tissue‐specific manner. Biochim Biophys Acta. 2012;1822(11):1796‐1806. [DOI] [PubMed] [Google Scholar]
- 29. Aubert G, Mansuy V, Voirol MJ, Pellerin L, Pralong FP. The anorexigenic effects of metformin involve increases in hypothalamic leptin receptor expression. Metabolism. 2011;60(3):327‐334. [DOI] [PubMed] [Google Scholar]
- 30. Lee CK, Choi YJ, Park SY, Kim JY, Won KC, Kim YW. Intracerebroventricular injection of metformin induces anorexia in rats. Diabetes Metab J. 2012;36(4):293‐299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Picinato MC, Haber EP, Cipolla‐Neto J, Curi R, de Oliveira Carvalho CR, Carpinelli AR. Melatonin inhibits insulin secretion and decreases PKA levels without interfering with glucose metabolism in rat pancreatic islets. J Pineal Res. 2002;33(3):156‐160. [DOI] [PubMed] [Google Scholar]
- 32. Owino S, Sanchez‐Bretano A, Tchio C, et al. Nocturnal activation of melatonin receptor type 1 signaling modulates diurnal insulin sensitivity via regulation of PI3K activity. J Pineal Res. 2018;64(3):e12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cano Barquilla P, Pagano ES, Jimenez‐Ortega V, Fernandez‐Mateos P, Esquifino AI, Cardinali DP. Melatonin normalizes clinical and biochemical parameters of mild inflammation in diet‐induced metabolic syndrome in rats. J Pineal Res. 2014;57(3):280‐290. [DOI] [PubMed] [Google Scholar]
- 34. Puchalski SS, Green JN, Rasmussen DD. Melatonin effect on rat body weight regulation in response to high‐fat diet at middle age. Endocrine. 2003;21(2):163‐167. [DOI] [PubMed] [Google Scholar]
- 35. Wolden‐Hanson T, Mitton DR, McCants RL, et al. Daily melatonin administration to middle‐aged male rats suppresses body weight, intraabdominal adiposity, and plasma leptin and insulin independent of food intake and total body fat. Endocrinology. 2000;141(2):487‐497. [DOI] [PubMed] [Google Scholar]
- 36. Prunet‐Marcassus B, Desbazeille M, Bros A, et al. Melatonin reduces body weight gain in Sprague Dawley rats with diet‐induced obesity. Endocrinology. 2003;144(12):5347‐5352. [DOI] [PubMed] [Google Scholar]
- 37. Terron MP, Delgado‐Adamez J, Pariente JA, Barriga C, Paredes SD, Rodriguez AB. Melatonin reduces body weight gain and increases nocturnal activity in male Wistar rats. Physiol Behav. 2013;118:8‐13. [DOI] [PubMed] [Google Scholar]
- 38. Fischer C, Mueller T, Pfeffer M, Wicht H, von Gall C, Korf HW. Melatonin receptor 1 deficiency affects feeding dynamics and pro‐opiomelanocortin expression in the arcuate nucleus and pituitary of mice. Neuroendocrinology. 2017;105(1):35‐43. [DOI] [PubMed] [Google Scholar]
- 39. Shima T, Chun SJ, Niijima A, et al. Melatonin suppresses hyperglycemia caused by intracerebroventricular injection of 2‐deoxy‐D‐glucose in rats. Neurosci Lett. 1997;226(2):119‐122. [DOI] [PubMed] [Google Scholar]
- 40. Xu P, Wang J, Hong F, et al. Melatonin prevents obesity through modulation of gut microbiota in mice. J Pineal Res. 2017;62(4):e12399. [DOI] [PubMed] [Google Scholar]
- 41. Thomas AP, Hoang J, Vongbunyong K, Nguyen A, Rakshit K, Matveyenko AV. Administration of melatonin and metformin prevents deleterious effects of circadian disruption and obesity in male rats. Endocrinology. 2016;157(12):4720‐4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Arble DM, Bass J, Behn CD, Butler MP, Challet E, Czeisler C. Impact of sleep and circadian disruption on energy balance and diabetes: a summary of workshop discussions. Sleep. 2015;38(12):1849‐1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tuomi T, Nagorny CLF, Singh P, Bennet H, Yu Q, Alenkvist I. Increased melatonin signaling is a risk factor for type 2 diabetes. Cell Metab. 2016;23(6):1067‐1077. [DOI] [PubMed] [Google Scholar]


