Abstract
Background
Acute myeloid leukemia (AML), an aggressive clonal disease, is genetically heterozygous. The prognostic role of expression of Breast Cancer Resistance Protein (BCRP) gene, which behaves as a multidrug transporter, in adult AML is ambiguous.
Objective
The objective is to assess the level of mRNA expression of BCRP gene in newly diagnosed cytogenetically normal adult Egyptian AML patients; and to clarify its potential influence and association between therapeutic responsiveness and disease free survival.
Methods
The BCRP gene expression was evaluated by quantifying its mRNA using real time RT-PCR in fifty newly diagnosed cytogenetically normal adult AML patients and 20 healthy normal controls. The expression was evaluated in relation to clinical and prognostic factors, response to treatment and the survival rate.
Results
BCRP mRNA was over expressed in adult AML patients compared to controls. This study showed a positive statistical correlation between BCRP gene expression and the percent of CD34 expression. Statistical analysis did not reveal any association between BCRP expression level and chemotherapeutic responsiveness or disease free survival rate.
Conclusion
The significance of BCRP gene expression and its function in AML is very complicated, therefore more standardized clinical studies are needed.
Keywords: BCRP, adult AML, gene expression, prognosis, Egypt
Introduction
Acute myeloid leukemia (AML), clonal malignant aggressive hematological disease, caused by accumulating acquired genetic abnormalities in hematopoietic stem cells1. It includes diverse categories according to molecular and other cytogenetic abnormalities2. Several prognostic markers were described in AML, including genetic mutations, polymorphisms or over expression of specific genes3.
One of these prognostic factors is the multidrug resistance (MDR) phenotype confirmed by the presence of transmembrane transporter proteins in leukemic cells4.
The Breast Cancer Resistance Protein (BCRP), synonymously known as ATP-binding-cassette protein, is coded by a gene located on chromosome 4q22, it encodes a protein composed of 655-amino acids, when over expressed it creates a drug resistance phenotype in tumor cell lines5. Such abnormal phenotype is due to the pump out of a large spectrum of chemicals, including chemotherapeutic drugs, outside the cells by the transport function of this protein. So it's expression has a particular influence on cancer treatment6.
The overexpression of BCRP gene enables cancer cell lines to self-renew and differentiate, which are their main characteristic feature5. These cells play a crucial role in MDR, where their BCRP overexpression enable them to resist chemotherapy and survive after being exposed to chemotherapeutics. It is possible that by inhibiting BCRP, the cancer cells could be targeted and eradicated5,7.
A limited number of clinical prognostic studies discussed BCRP mRNA expression in AML, with different selection criteria and interpretation methodology8–13. The aim of the current study is to assess the level of mRNA expression of BCRP gene by real time RT-PCR in 50 newly diagnosed cytogenetically normal adult Egyptian AML patients, trying to clarify its potential influence and its association between the therapeutic responsiveness and disease free survival.
Subjects and methods
Study population
The present study was applied on 50 newly diagnosed cytogenetically normal adult AML patients. They were 26(52%) males and 24(48 %). females The age of the included patients ranged from 18 to 71 years with a mean of 41.2±15.9 years. Patients were selected over six months from National Cancer Institute, Cairo, Egypt. The Ethical Committee of National Cancer Institute approved the current research protocol. All procedures performed in the current study were in accordance with the World Medical Association Declaration of Helsinki and its later amendments. Twenty age and sex-matched healthy unrelated blood donors participated in this study as a control group.
After collection of written informed consent from all participants, patients were subjected to full clinical assessment in addition to bone marrow examination. Immunohistochemistry staining and immuno-phenotyping was carried out to confirm the diagnosis and for classification of AML. Chromosomal karyotyping, molecular analysis with special emphasis on; t (8;21), inversion of chromosome 16, Nucleophosmin (NPM) gene mutations and FMS-like tyrosine kinase 3 -internal tandem duplication (FLT3-ITD) were done. Patients diagnosed as promyelocytic leukemia (M3), were not included in the study due to different therapeutic modalities and prognostic criteria. AML patients were followed up over the period of induction chemotherapy and for twelve months.
BCRP m-RNA expression:
From cases and control samples, cellular RNA was extracted, then the complementary DNA was synthesized using QIAamp RNA blood Mini Kit, supplied by Qiagen, and High capacity cDNA Archive kit, provided by Applied Bio systems, respectively, the manufacture instructions were followed for each kit. Every PCR amplification reaction had a total volume of 25 µL which included: 1 µL of the cDNA, 12.5 µLTaqMan universal PCR master mix (Applied Biosytems, Inc.), 1 µL of each forward and reverse primers of BCRP with the following sequences;5′-CAGTACTTCAGCATTCCACGAT-3′, and: 5′-GGCAGAAGTTTTGTCCCAAA-3′. In addition to 0.5µL of the probe of Taqman with the sequence of 5′-FAM-CATTATGCTGCAAAGCCGTAAATCCA-TAMRA-3′. The Glyceraldehyde 3-phosphate dehydrogenase (GADPH) gene was selected to be the housekeeping gene. The PCR reaction was programmed for; 10 min heating at 95°C, followed by 40 cycles of; 15 s heating at 95°C for denaturation and 1min at 60 °C for annealing and elongation. Each PCR run included negative control tube without addition of any cDNA template. The “comparative threshold method” (2-ΔΔCT) was used to calculate the relative expression level of BCRP gene13.
Treatment protocol and response to therapy
The AML patients were treated in accordance to the protocol of the Department of Medical Oncology, Kasr Al-Ainy Faculty of Medicine, CairoUniversity. Induction and consolidation were the two implemented phases of therapy. The remission was induced by 7-3 protocol, where fit patients (who were <60–65 years old, and selected fit patients up to age 75 years) received intensive therapy. Whereas less fit patients (who were70–75 years and older, or younger patients with significant co-morbidities) received lower intensive therapy. To achieve this inductionphase, a combination of cytarabine and anthracycline or anthracenedione was recommended (cytarabine 100–200 mg/m2 continuous IV infusion for 7 days, and for 3 days idarubicin 12 mg/m2/day, or daunorubicin 60–90 mg/m2/day were added). All patients were assessed for risk of relapse. Specific drug protocol, for consolidation therapy, were recommended based on the risk of relapse of the patient, high-dose cytarabine 3 g/m2 IV over 3 h every 12 h on days 1, 3 and 5 for 4 cycle.
After induction therapy, Complete remission(CR) was obtained when the patient had a cellular marrow with blast cells ≤5%, peripheral blood picture with a neutrophil count ≥1.5×103/ml, platelet count≥100×103/ml, and with no verification of leukemia in other sites. The patient was stated to be primary resistant if having cellular marrow with >5% blasts or verification of leukemia in other sites14.
Data analysis
Data arrangement and analysis were done using Statistical Analysis Systems, SAS vs8.02. The graphs were done using Harvard Graphics, vs4.
The data were summarized according to their type, where mean and standard deviations (SD) or median and range were used for numeric data, while percentages were used for categorical data. Mann-Witney test was performed to compare numeric variables. The Chi-square test was applied to compare groups regarding the categorical data, while for small sample size Fisher's exact test was used. The strength of association between two numeric variables was calculated using Spearman's correlation coefficient, where values close to 1 or -1 mean a perfect positive or negative correlation, respectively.
The disease free survival (DSF) was estimated starting from the time of remission to relapse or death or loss to follow and was evaluated by the Kaplan and Meier method, while the log rank test was used for comparison. Each P-value < 0.05 was considered to be significant.
Results
The main laboratory data of AML patients were summarized in table 1. AML patients were categorized into two groups according to the relative expression of BCRP gene: 34 AML patients (68%) were considered as a high expressors, with relative expression level higher than that of the controls. Sixteen AML patients (32%) were considered as a low expressors, with relative expression level within the range of the normal controls. There was no statistical significant difference between the two patients'category regarding their clinical and laboratory data.
Table (1).
Main clinical and Hematological findings in AML patients
| Parameter | Value | |
| Age (years) Median (range) |
39(18–71) | |
| Sex | Male | 26/50 (52%) |
| Female | 24/50(48%) | |
| Hepatomegaly | 12/50 (24%) | |
| Splenomegaly | 12/50(24%) | |
| Lymphadenopathy | 4/50(8%) | |
|
PB TLC (×109/L) Median (range) |
23.6 (0.9–273.1) | |
| Hb(gm/dl) | Median (range) | 7.4(3.4–12.4) |
| Mean±SD | 7.8gm/dL ± 1.9 | |
|
Platelets (×109/L) Median (range) |
30.5(6–241) | |
|
Peripheral blood Blast (%) Median (range) |
60.0(34–92) | |
|
Percent of BM blasts Median(range) |
67.0 (21–95) | |
| FAB Classification | M0 | 1/50(2%) |
| M1 | 13/50( 26%) | |
| M2 | 23/50(46%) | |
| M4 | 13/50( 26%) | |
| Molecular genetics | t (8; 21) | 4/50 (8%) |
| inv (16) | 1/50(2%) | |
A significant statistical difference was detected between the BCRP mRNA expression level and the percentage of blast cells expressing CD34 (p= 0.016, r= 0.330) in AML patients. Otherwise there was no statistical significant correlation could be found between BCRP gene expression and other clinical nor laboratory data of AML patients including the distribution among different FAB subtypes. After induction chemotherapy, Complete remission was achieved by 24 / 50 (48%) of AML patients. Among these cases, 15/24(62.5%) were high expressors and 9/24(37.5%) were low expressors. Twenty-six patients (52%) failed to achieve CR (12 patients showed resistance to induction treatment and 14 patients died during the course of induction). No statistical significant difference could be detected between high and low expressors groups according to the response of induction chemotherapy (Table 2).
Table (2).
Correlation of response rate with ABCG2 gene expression in 50 AML cases
| High BCRP m-RNA expression |
Low BCRP m-RNA expression |
P-value* | ||
| Response to induction chemotherapy |
CR** 24 AML Patients |
15/24(62.5%) | 9/24(37.5%) | 0.547 |
| Failed to achieve CR(resistance and death) 26 AML Patients |
19/26(73.1%) | 7/26(26.9%) |
P-values < 0.05 were considered significant.
CR; complete remission.
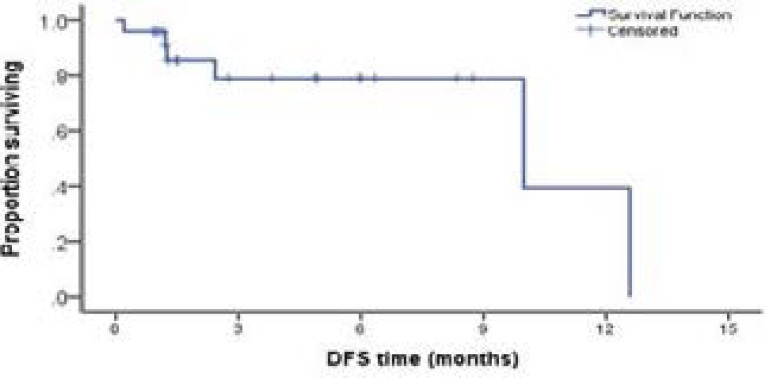
Follow up of AML patients for twelve months revealed that 18/24 (75%) patients who achieved CR after induction chemotherapy had a disease free survival(DFS)during the follow up period. Ten of them were high BCRP expressors, while eight were low BCRP expressors. Six patients failed to achieve 6 months DFS, five of them were high BCRP expressors and one patient was low BCRP m-RNA expressor. There was no statistically significant difference noticed between high and low BCRP expressors regarding their DFS rate (Figure 1).
Figure (1).
Kaplan Meier curve showing disease free survival in AML cases.
Discussion
Several factors affect the prognosis of adult AML, such as the age of the patient, the strength of post remission therapy, beside the biologic characteristics of the disease. Karyotyping at the time of diagnosis, the existence of transmembrane transporter proteins which grant multidrug resistance. The over-expression or the mutations affecting particular genes are also included in the factors affecting the disease outcome4.
The present work revealed that BCRP mRNA transcript was not overexpressed in the peripheral blood of normal healthy donors. Abbott et al.,8 found low levels of BCRP mRNA expression in normal samples, whether peripheral blood or bone marrow, which reflected the restricted BCRP expression to the rare repopulating hematopoietic stem cells15.
We chose the cutoff of expression level to be the highest reading of BCRP mRNA expression among the controls. Our results were in harmony with Abbott et al.,8 who classified 40 AML studies samples according the BCRP expression level into 3 levels: low expression level (lower than that of normal PB and BM), intermediate level of expression (greater than normal PB and BM but lower or equal to MDR cells clone 3.3), and high expression level (greater than MDR clone 3.3). Nasilowska-Adamska et al.,13 on the other hand, chose the median of BCRP mRNA expression level, in univariate analysis, as the cutoff for his results.
Of the 50 adult AML cases examined, 34 patients (68%) were high BCRP expressors. Similarly the majority of adult AML samples showed over expression of BCRP mRNA in comparison to normal controls in Abbott et al.,8 study, but this was lower than MDR cell lines. This finding can be explained by either the homogenous expression of BCRP by all leukemic cells, or that BCRP expression could be heterogeneous and restricted to a subpopulation of leukemic cells. Flow cytometry study of BCRP protein expression levels in adult AML found that the later scenario was more likely8,9.
In the current study, there was no statistically significant difference between AML patients with high BCRP expressors and low expressors regarding their clinical and laboratory data. Similarly, previous clinical studies on AML patients could not find an association between BCRP m-RNA expression and AML patient characteristics such as age , hemoglobin and total leucocytic count, nor among different FAB subtypes7,9,10,13.
During the study of the possible association between BCRP expression and other prognostic markers, a significant correlation between the BCRP mRNA expression level and the percentage of CD34 expression on leukemic blasts was found. Otherwise there was no statistically significant correlation between BCRP gene expression and other clinical nor laboratory data of AML patients. This was in agreement with the results reported in van den Heuvel-Eibrink et al., study12 who found a significant positive association between CD34 expression and BCRP gene expression. Abbott et al.,8 and Suvannasankha et al,9 could not find an association between and BCRP gene expression and other clinical nor other laboratory data, even the CD34 and or /CD33 expressing AML sub-population. In Nasilowska-Adamska et al., study13 the expression of BCRP mRNA in blast cells, which were positive for CD34, was of a marginal significance.
Controversy about the expression of BCRP mRNA in human pluripotent stem cells has been encountered, where Zeng et al.,16 reported that these cells were less resistant to certain cytotoxic chemicals due to BCRP expression. However, with more sensitive techniques Sarkadi et al.,17 suggested that BCRP expression was at a relatively high level in the human undifferentiated stem cells, which highlights its role in protecting this valuable stem cells from the damage caused by toxins, drugs or hypoxia.
The present work did not conceal a significant difference in BCRP mRNA expression as regard the early treatment response nor to the respect of DFS of the patients. Our results were consistent with Abbott et al.,8 and Suvannasankhaet al.,9.
Furthermore, Uggla et al.,11 could not find a difference between treatment AML responders and non-responders patients as regards BCRP expression, but in the group of responding patients, those with uppermost BCRP mRNA expression showed significantly shorter overall survival. On the contrary, the study of Nasilowska-Adamska et al.,13 found that, in a univariate analysis, the BCRP expression was associated with higher early relapse rate and significantly influenced the disease free survival in AML. Also van den Heuvel-Eibrink et al.,12 reported that BCRP expression was frequently up regulated in patients with relapsed AML. In addition, high BCRP expression level in AML patients has been associated with a relapsed or refractory state, lower complete response and shorter survival rates10,18. Damiani et al.,7,19 reported the same finding and stated that there was an up regulation in BCRP protein at relapsed AML samples when compared to newly diagnosed ones and it can identify AML patients with poor outcome even after stem cell transplantation.
As the biology of BCRP gene expression and its role in AML is much more complicated than in cell line models8, our study could not find a clear association between BCRP overexpression in adult AML cases and its possible contribution as a molecular predictor to the response of therapy. This could be attributed to the discordance between BCRP mRNA overexpression and subsequent translation into a functioning trans-membrane protein in a malignant and genetically complex disease such as AML20. Furthermore underlying genetic polymorphisms of the BCRP gene, as a part of the genetic heterogeneity in AML, could affect the expression levels with subsequent influence on the disease progression6 multiple genetic, together with heterogeneous functional proteins, interactions need to be clarified in AML.
In order to better understand the clinical role of BCRP in AML, we recommend the estimation of the BCRP expression in a bigger cohort of AML patients, study genetic polymorphisms that could affect the expression levels, standardize several factors including patient selection, used chemotherapeutic drugs and development of consensus recommendations for BCRP detection. Moreover, longer follow up period will clarify the possible influence of BCRP expression on DFS and overall survival of the disease.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Mrózek K, Marcucci G, Paschka P, Whitman SP, Bloomfield CD. Clinical relevance of mutations and geneexpression changes in adult acute myeloid leukemia with normal cytogenetics: are we ready for a prognostically prioritized molecular classification? Blood. 2007;109(2):431–448. doi: 10.1182/blood-2006-06-001149. 15. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, Bloomfield CD, Cazzola M, Vardiman JW. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–2405. doi: 10.1182/blood-2016-03-643544. 19. [DOI] [PubMed] [Google Scholar]
- 3.Harrison CJ, Hills RK, Moorman AV, Grimwade DJ, Hann I, Webb DK, Wheatley K, de Graaf SS, van den Berg E, Burnett AK, Gibson BE. Cytogenetics of childhood acute myeloid leukemia: United Kingdom Medical ResearchCouncil Treatment trials AML 10 and 12. J ClinOncol. 2010;28(16):2674–2681. doi: 10.1200/JCO.2009.24.8997. 1. [DOI] [PubMed] [Google Scholar]
- 4.Tallman MS. New strategies for the treatment of acute myeloid leukemia including antibodies and other novel agents, Hematology. Am Soc Hematol Educ Program. 2005:143–150. doi: 10.1182/asheducation-2005.1.143. [DOI] [PubMed] [Google Scholar]
- 5.Stacy AE, Jansson PJ, Richardson DR. Molecular pharmacology of ABCG2 and its role in chemoresistance. Mol Pharmacol. 2013;84(5):655–669. doi: 10.1124/mol.113.088609. PubMed. [DOI] [PubMed] [Google Scholar]
- 6.Sándor S, Jordanidisz T, Schamberger A, Várady G, Erdei Z, Apáti Á, Sarkadi B, Orbán TI. Functional characterization of the ABCG2 5′ non coding exon variants: Stem cell specificity, translationefficiency and the influence of drug selection. BiochimBiophys Acta. 2016;1859(7):943–951. doi: 10.1016/j.bbagrm.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 7.Damiani D, Tiribelli M, Calistri E, Geromin A, Chiarvesio A, Michelutti A, Cavallin M, Fanin R. The prognostic value of P-glycoprotein (ABCB) and breast cancer resistance protein (ABCG2) in adults with denovo acute myeloid leukemia with normal karyotype. Haematologica. 2006;91(6):825–828. [PubMed] [Google Scholar]
- 8.Abbott BL, Colapietro AM, Barnes Y, Marini F, Andreeff M, Sorrentino BP. Low levels of ABCG2 expression in adult AML blast samples. Blood. 2002;100(13):4594–4601. doi: 10.1182/blood-2002-01-0271. 15. [DOI] [PubMed] [Google Scholar]
- 9.Suvannasankha A, Minderman H, O'Loughlin KL, Nakanishi T, Ford LA, Greco WR, Wetzler M, Ross DD, Baer MR. Breast cancer resistance protein (BCRP/MXR/ABCG2) in adult acute lymphoblastic leukaemia: frequent expression and possible correlation with shorter disease-free survival. Br J Haematol. 2004;127(4):392–398. doi: 10.1111/j.1365-2141.2004.05211.x. [DOI] [PubMed] [Google Scholar]
- 10.Benderra Z, Faussat AM, Sayada L, Perrot JY, Chaoui D, Legrand O. Breast cancer resistance protein and pglycoprotein in 149 adult acute myeloid leukemias. Clin Cancer Res. 2004;10(23):7896–7902. doi: 10.1158/1078-0432.CCR-04-0795. PubMed. [DOI] [PubMed] [Google Scholar]
- 11.Uggla B, Ståhl E, Wågsäter D, Paul C, Karlsson MG, Sirsjö A, Tidefelt U. BCRP mRNA expression v. clinical outcome in 40 adult AML patients. Leuk Res. 2005;29(2):141–146. doi: 10.1016/j.leukres.2004.06.004. PubMed. [DOI] [PubMed] [Google Scholar]
- 12.van den Heuvel-Eibrink MM, van der Holt B, Burnett AK, Knauf WU, Fey MF, Verhoef GE, Vellenga E, Ossenkoppele GJ, Löwenberg B, Sonneveld P. C34-related coexpression of MDR1 and BCRP indicates a clinically resistant phenotype in patients with acutemyeloid leukemia (AML) of older age. Ann Hematol. 2007;86(5):329–337. doi: 10.1007/s00277-007-0269-7. PubMed. Stem Cells. 2010 Jan;28(1):174-176. DOI:10.1002/stem.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nasilowska-Adamska B, Solarska I, Paluszewska M, Malinowska I, Jedrzejczak WW, Warzocha KFLT3-ITD and MLL-PTD influence the expression of MDR-1, MRP-1, and BCRP mRNA but not LRP mRNA-assessed with RQ-PCR method in adult acute myeloid leukemia. Ann Hematol. 2014;93(4):577–593. doi: 10.1007/s00277-013-1898-7. [DOI] [PubMed] [Google Scholar]
- 14.Bacher U, Kern W, Schoch C. Evaluation of complete disease remission in acute myeloid leukemia: a prospective study based on cytomorphology, interphase fluorescence in situ hybridization, and immunophenotyping during follow-up in patients with acute myeloid leukemia. Cancer. 2006;106(4):839–847. doi: 10.1002/cncr.21665. PubMed. [DOI] [PubMed] [Google Scholar]
- 15.Zhou S, Schuetz JD, Bunting KD, Colapietro AM, Sampath J, Morris JJ, Lagutina I, Grosveld GC, Osawa M, Nakauchi H, Sorrentino BP. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med. 2001;7(9):1028–1034. doi: 10.1038/nm0901-1028. [DOI] [PubMed] [Google Scholar]
- 16.Zeng H, Park JW, Guo M, Lin G, Crandall L, Compton T, Wang X, Li XJ, Chen FP, Xu RH. Lack of ABCG2 expression and side population properties in human pluripotent stem cells. Stem Cells. 2009;27(10):2435–2445. doi: 10.1002/stem.192. [DOI] [PubMed] [Google Scholar]
- 17.Sarkadi B, Orbán TI, Szakács G, Várady G, Schamberger A, Erdei Z, Szebényi K, Homolya L, Apáti A. Evaluation of ABCG2 expression in human embryonic stem cells: crossing the same river twice? Stem Cells. 2010 Jan;28(1):174–176. doi: 10.1002/stem.262. [DOI] [PubMed] [Google Scholar]
- 18.Benderra Z, Faussat AM, Sayada L, Perrot JY, Tang R, Chaoui D, Marzac C, Marie JP, Legrand O. MRP3, BCRP, and P glycoprotein activities are prognostic factors in adult acute myeloid leukemia. Clin Cancer Res. 2005 Nov 1;11(21):7764–7772. doi: 10.1158/1078-0432.CCR-04-1895. [DOI] [PubMed] [Google Scholar]
- 19.Damiani D, Tiribelli M, Geromin A, Michelutti A, Cavallin M, Sperotto A, Fanin R. ABCG2 overexpression in patients with acute myeloid leukemia: Impact on stem cell transplantation outcome. Am J Hematol. 2015;90(9):784–789. doi: 10.1002/ajh.24084. PubMed. [DOI] [PubMed] [Google Scholar]
- 20.Suvannasankha A, Minderman H, O'Loughlin KL, Nakanishi T, Greco WR, Ross DD, Baer MR. Breast cancer resistance protein (BCRP/MXR/ABCG2) in acute myeloid leukemia: discordance between expression and function. Leukemia. 2004;18(7):1252–1257. doi: 10.1038/sj.leu.2403395. [DOI] [PubMed] [Google Scholar]