Abstract
Background
Metastasis is the leading cause of cancer deaths. Migration of tumor cells is an important stage in metastasis. Therefore, recent studies have focused on clarifying migration and migration-dependent cell functions such as angiogenesis, wound healing, and invasion.
Objectives
In the present study, we aimed to investigate the effect of acetazolamide, which is a classical carbonic anhydrase inhibitor, on the cell viability, migration, and colony forming capacity of human LS174T colorectal cancer cells.
Methods
Three different cell culture techniques (MTT test, wound healing and clonogenic assay) were performed in this in vitro study on colorectal cancer cells.
Results
Acetazolamide reduced the cell viability, migration and colony formation ability of cells depending on dose. There was no significant difference between the cells treated with acetazolamide with 1 µM dose and the control. However, it can be concluded that acetazolamide exerts its effect on human colorectal cancer cells at 10-1000 µM concentrations.
Conclusion
Acetazolamide was observed to significantly inhibit the cell viability, colony forming capacity, and migration ability in the culture medium of LS174T cells. This inhibitor effect of acetazolamide was observed to be dependent on the concentration in medium.
Keywords: LS174T, carbonic anhydrase inhibitor, colorectal carcinoma
Introduction
Among all cancer types, colorectal cancer is the third most common cancer type in males (0.7 million, 10.1% of all cancer types) and the second most common cancer type in females by following the breast cancer (0.61 million, 9.2% of all cancer types). Colorectal cancer mortality rate ranks the fourth in males and third in females.1
The most common cause of death is metastasis in cancer patients. Therefore, metastasis has become a focus both in scientific and clinical researches in cancer patients. Though great progresses have been made in clarifying the molecular and cellular basis of cancer-related deaths in recent years. The underlying mechanism of metastatic disease has still not been fully clarified, and methods for preventing metastasis have remained limited.2 Cancer cells acquire the ability to move through their own motility in tissues during invasion and metastasis. The control of cancer cell migration is a major problem in tumor treatment. Cancer cell migration has also a critical importance in several physiological and pathological processes including tumor growth, wound healing, immunity, angiogenesis and metastasis.3 Due to their rapid metabolism and split rate, cancer cells need more oxygen and nutrient than other cells. New blood vessels (angiogenesis) are formed to meet this need. Tumor blood vessels are enlarged and pores with wide gap junctions are formed in endothelial cells depending on the imbalance in the levels of angiogenic regulators.1
Aquaporins are small transmembrane water channel proteins found in different human tissues.4 Most of the AQPs are distributed in epithelium and endothelium as well as in other cells and tissues such as erythrocytes, astrocytes, adipocytes and skeletal muscle.5 A significant correlation was found between the severity and prognosis of histological tumors of cancer patients and AQP expression in several cancer types. Therefore, AQPs have been suggested to be used as therapeutic biological markers in cancer patients, and AQP inhibitors may be a novel therapeutic target in tumor cells and microvessels.4
Aquaporin-1 (AQP1) has been shown to be over-expressed in brain, lung, breast, colorectal, and ovarian cancers.6,7 As well as AQP1, certain types of AQPs such as AQP3, AQP5 and AQP9 are expressed in colorectal cancer.7 There was a correlation between the over-expression of AQP3 and tumor differentiation and metastasis in some patients with colon cancer.8 Furthermore, AQP5 and AQP9 have been associated with drug resistance in colon cancer.9
There are also several AQP inhibitor compounds, which are used to inhibit biological functions of the tumor.10–12 Acetazolamide is one of them and is found to inhibit the AQP1 expression protecting the tumor from cytotoxic edema through the preservation of extracellular acidification, and to support tumor metastasis in the glioma.12 Acetazolamide is a potent CA inhibitor which catalyzes the stabilization of carbondioxide and carbonic acid, and has important effects on the urine formation process, reabsorption of sodium bicarbonate and acid release.13 It reduces the tumor growth in vivo, when used as monotherapy and has also additive effects by delaying tumor growth, when used in combination with other chemotherapeutic compounds.14
In the present study, we aimed to investigate the effect of acetazolamide on the cell viability, migration, and colony forming capacity of human LS174T colorectal cancer cells. We chose the LS174T colorectal cancer model in which the expressions IX and XII have previously been observed.15
Methods
Preparation of cell culture and passage
Human colon cancer cells were used in our study. LS174T cell line was obtained from German Cancer Research Center (Heidelberg, Germany). Cells were cultured in an incubator with 5% CO2 and 37°C temperature. RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS) and L-glutamine (2 mM) was used as a cell medium. The cells were passaged two or three times a week to keep the cells in logarithmic growth phase, and were washed with phosphate-buffered saline (PBS) and trypsinized (0.25% trypsin/EDTA). Pelleted for 5 min at 1,500 rpm and re-suspended at the desired concentration in RPMI-1640 medium.
Cell proliferation
Viability of the cells was evaluated through the method of staining of living cells with MTT (3- [4,5-dimethylthiazol-2-yl] −2,5-diphenyltetrazolium bromide). LS174T cells were cultured in 96-well culture plates as being 4x103 cells in each well (Sarstedt, Germany). Acetazolamide was added at 1 µM, 10 µM, 100 µM, 1000 µM concentrations after 24 h of incubation. 10 µL MTT (Applichem, Germany) solution, which was prepared in 5 mg/mL PBS, was added for per well 24 h after the addition of acetazolamide. 100 µL of isopropanol/HCl solution was added to the plates, which were kept in the incubator for 3–4 h, after the removal of medium. Formazan crystals were made to be dissolved. Optical densities were measured at 570 nm wavelength by using ELISA plate reader (Bio-Tech, USA).16,17
In vitro wound healing assay
LS174T cells were cultured in plates as being 6x104 cells in each well. They were subjected to a 24 h standard incubation (5% CO2, 37°C). A straight line was drawn by using a 200 µL sterile plastic pipette tip. Then, cell residues were removed by using RPMI-1640 medium. 0.5 mL of acetazolamide was added at concentrations of 1 µM, 10 µM, 100 µM, and 1000 µM. The wound healing effect was evaluated at 0, 24, 48, and 72 h through photographs.18
Clonogenic assay
A colony formation assay was performed to evaluate cell proliferation. Culture was made to be 500 cells for each of the six well plates. Acetazolamide was added at increasing concentrations (1–1000 µM) after 24 h of incubation. This was carried out every 5 days. Cells were washed with PBS and stained with crystal violet after two weeks. Colonies with more than 50 cells were counted and analyzed.19
Statistical analysis
The ImageJ 1.48 software was used to evaluate wound healing photos. Statistical analysis was performed using the SPSS version 15.0 software (SPSS Inc., Chicago, IL, USA), and descriptive statistics were expressed in mean ± standard deviation (SD). The Shapiro-Wilk normality test was used to evaluate whether the variables showed a normal distribution. One-way analysis of variance (ANOVA) was used for the independent groups. A p value of <0.05 was considered statistically significant.
Results
Cell proliferation assay
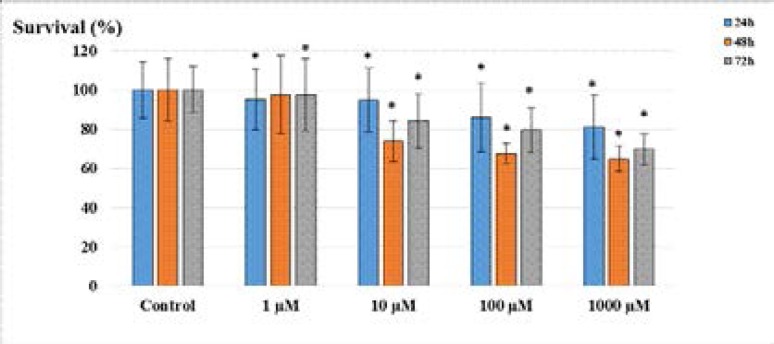
Acetazolamide administration in LS174T cell line was observed to inhibit the cell proliferation at all concentrations. According to the MTT test results, the results obtained at 24, 48, and 72 h were found to be statistically significant different (p<0.05). The inhibitory effect of acetazolamide in LS174T cell line was significant within the first 48 h, while the inhibition decreased at 72 h (Figure 1).
Figure 1.
Survival rates of LS174T cells after the administration of acetazolamide. (*p<0.05; compared to the control).
A statistically significant decrease (p<0.05) in cell viability was observed as the dose increased in the first 24 hours (1 µM; 95.1%, 10 µM; 94.8%, 100 µM; 85.7%, 1000 µM; 81.1%). Inhibition of cell viability was observed (p<0.05) at increasing concentrations after 48 hours incubation, 10 µM; 73.8%, 100 µM; 67.5%, 1000 µM; 64.9%. When cell viability is assessed after 48 hours of incubation, 1 µM concentration of acetazolamide inhibited cell viability ratio of 97.4% (p>0.05). After 72 hours of incubation, the results were 1 µM; 97.5%, 10 µM; 84.1%, 100 µM; 79.4%, 1000 µM; 69.8% (p<0.05).
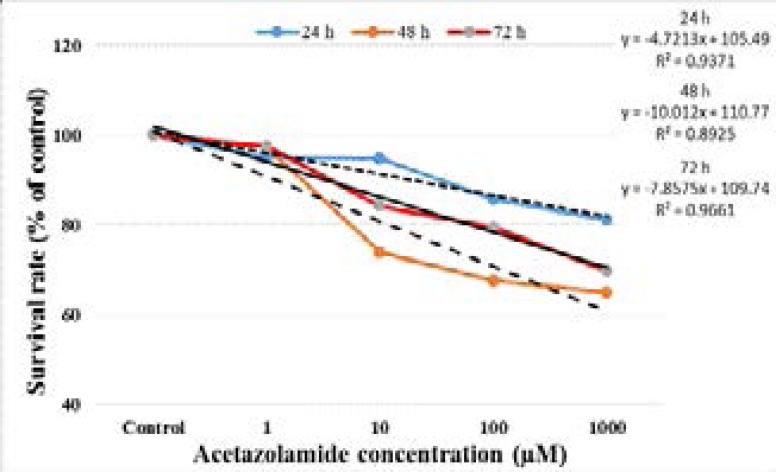
The survival curves of LS174T cells after acetazolamide administration at different doses are shown in Figure 2. Acetazolamide showed an antiproliferative effect on LS174T cells due to concentration and time.
Figure 2.
Survival curves of LS174T cells following exposure to acetazolamide. The values represent the means ± SD.
In vitro wound healing (scratch) assay
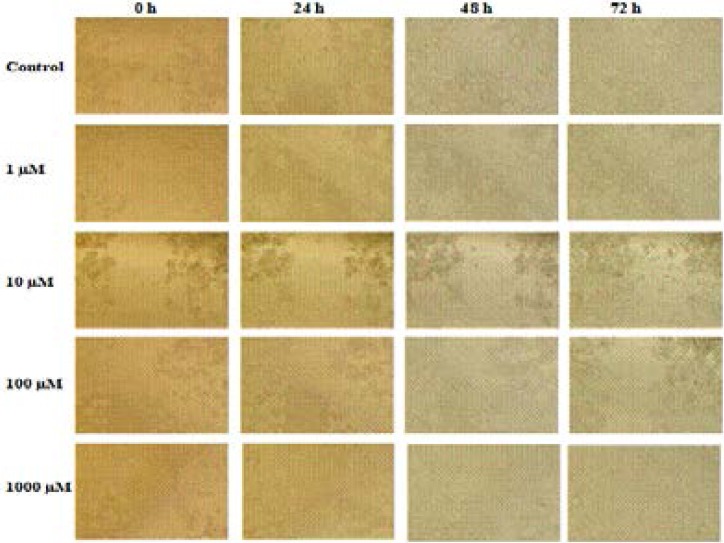
LS174T cells (4x103 cells in 0.5 mL) were cultured to 24 well plates, and a straight line was drawn with a 200 µl pipette tip to open a cleft. After 24 h, acetazolamide was added. The images of the cells were taken at 24, 48 and 72 h (Figure 3).
Figure 3.
Wound healing responses of LS174T cell line following exposure to acetazolamide. ImageJ 1.48 software was used for the measurements.
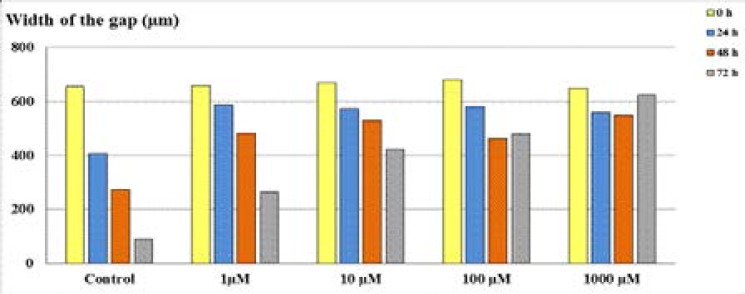
In wound healing results, the cleft, which was opened at the first 24 and 48 h, was observed to be healed at the lowest level, and the ability of cells to migrate was restrained. The results of wound closure area showed that acetazolamide treatment improved cellular migration which enables significantly accelerated wound closure (Figure 4).
Figure 4.
The width of the gaps of LS174T cells after the administration of acetazolamide.
Clonogenic assay
LS174T cells were cultured as being 500 cells in 2 mL for each of the six well plates. After 24 h, it was treated with acetazolamide at increasing concentrations. The treatment was repeated after five days, and the test was ended at day 10. As a result of the test, colonies with more than 50 cells were counted for the LS174T cell line (Table 1).
Table 1.
The treatment acetazolamide was reduced clonogenic survival on LS174T cells. Cells were treated for 24 h with increasing doses (1–1000 µM) of acetazolamide
| Acetazolamide treatment | Number of colonies counted |
Reduction of colony count compared to control (%) |
| Control | 130 | - |
| 1 µM | 100 | 23.1 |
| 10 µM | 75 | 42.8 |
| 100 µM | 59 | 54.7 |
| 1000 µM | 38 | 70.8 |
Discussion
Aquaporins are a family of proteins consisting of 13 small (∼30-kDa monomers) hydrophobic integral membrane water channel proteins which are involved in the transcellular and transepithelial water movement, fluid transport, and cell migration.20,21 They play a central role in brain water homeostasis, secretion of exocrine glands, urine concentration, skin hydration, fat metabolism, and neural signal transduction. In addition to these, AQPs are also engaged in the pathogenesis of tumor-related edema, tumor cell proliferation, cell migration and tumor angiogenesis, and cancer formation.4 In recent studies, the relation between AQP and cancer has been addressed.7,22–28 AQP1 expression in plasma membrane of HT20 colon cancer cells and adenovirus-mediated increased expression of AQP1 were found to increase the plasma membrane water permeability and migration rate in both wound healing and invasive transwell migration assays. Water permeability of AQP1 mediated plasma membrane was also suggested to be of utmost importance for colon cancer cell migration, and it was related to tumor invasion and metastasis.23 The tumor microenvironment includes the complex physiological networks contributing to the plasticity of tumor cells and the heterogeneity of the tumor tissue.
Hypoxia is an important component which produces intratumoral oxygen gradients. One of the adaptive responses of tumor cells to hypoxia is the increased expression and functional activation of carbonic anhydrase IX (CA IX).4 CA IX, which is metalloprotein containing zinc, catalyzes the conversion of carbon dioxide to bicarbonate ion and proton reversibly.4,29,30 Through this catalytic activity, CA IX contributes to the regulation of intracellular and extracellular pH impairments caused by hypoxia-induced changes in cellular metabolism producing excess acid.4 Overexpression of CA IX is generally associated with poor prognosis and decreased disease-free survival after successful treatment due to its important role in tumor progression, acidification and metastasis in different types of cancer. Therefore, it is considered as a prognostic indicator in oncology.29 In a previous study, Tülüce and colleagues found that the novel sulfonamide derivative CA IX inhibitor (H-4i) has more cytotoxic and apoptotic activity through the production of ROS on colorectal cancer cells than in normal cells.30 Acetazolamide is one of the sulfonamide-derived CA inhibitors. There are several studies suggesting that acetazolamide, which is used clinically in glaucoma treatment, can be also useful in cancer chemotherapy. It was shown to inhibit some carbonic anhydrase enzymes and AQPs in certain types of cancer. According to some authors, acetazolamide can reduce the number of invasive cells by inhibiting the cell proliferation.31,32 In our study, acetazolamide in concentrations of 10 µM and higher was found to reduce the number of cells significantly, which are grown in cell culture plates. After acetazolamide administration, both the rate of colony formation and the ability of cells to migrate were observed to be reduced.
In several studies, there are some evidences with regard to the inhibition effect of acetazolamide alone on invasive potential of cancer cells in vitro. Based on literature information it can be suggested that the inhibition of CA enzymes by acetazolamide can reduce the invasive potential of cancer cells by inhibiting the activation of these matrix degrading enzymes.
Acetazolamide which was used is an inhibitor of colorectal cancer-associated carbonic anhydrases (CAIX and CAXII) and water channel proteins (AQP1 and AQP5) in this study. We attempted to make the microenvironment of tumor cells alkaline by suppressing the expression of these proteins. Migration and invasion of the cells were thought to be suppressed in this way. As dose-dependent, acetazolamide inhibited the cell viability, migration ability, and ability to colonize of cells.
Acknowledgment
The study was supported by Inonu University Scientific Research Projects Coordination Unit with project number 2014/29. The authors would like to thank Prof. Dr. Martin R Berger (The German Cancer Research Center, Heidelberg, Germany).
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Severino P, De Hollanda LM, Santini A, Reis LV, Souto SB, Souto EB, et al. Nanobiomaterials in Cancer Therapy, Advances in nanobiomaterials for oncology nanomedicine. Elsevier; 2016. pp. 91–115. [Google Scholar]
- 2.Martin TA, Ye L, Sanders AJ, Lane J, Jiang WG. Madame Curie Bioscience Database [Internet] Austin (TX): Landes Bioscience; 2000–2013. Cancer Invasion and Metastasis: Molecular and Cellular Perspective. [Google Scholar]
- 3.Yamazaki D, Kurisu S, Takenawa T. Regulation of cancer cell motility through actin reorganization. Cancer Science. 2005;96(7):379–386. doi: 10.1111/j.1349-7006.2005.00062.x. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang J, Feng L, Zhu Z, Zheng M, Wang D, Chen Z, et al. Aquaporins as diagnostic and therapeutic targets in cancer: How far we are? Journal of Translational Medicine. 2015;13:96. doi: 10.1186/s12967-015-0439-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verkman AS. Aquaporins in clinical medicine. Annual Review of Medicine. 2012;63:303–316. doi: 10.1146/annurev-med-043010-193843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mobasheri A, Airley R, Hewitt SM, Marples D. Heterogeneous expression of the aquaporin 1 (AQP1) water channel in tumors of the prostate, breast, ovary, colon and lung: a study using high density multiple human tumor tissue microarrays. International Journal of Oncology. 2005;26(5):1149–1158. [PubMed] [Google Scholar]
- 7.Moon C, Soria JC, Jang SJ, Lee J, Obaidul Hoque M, Sibony M, et al. Involvement of aquaporins in colorectal carcinogenesis. Oncogene. 2003;22(43):6699–6703. doi: 10.1038/sj.onc.1206762. PubMed. [DOI] [PubMed] [Google Scholar]
- 8.Li A, Lu D, Zhang Y, Li J, Fang Y, Li F, et al. Critical role of aquaporin-3 in epidermal growth factor-induced migration of colorectal carcinoma cells and its clinical significance. Oncology Reports. 2013;29(2):535–540. doi: 10.3892/or.2012.2144. PubMed. [DOI] [PubMed] [Google Scholar]
- 9.Shi X, Wu S, Yang Y, Tang L, Wang Y, Dong J, et al. AQP5 silencing suppresses p38 MAPK signaling and improves drug resistance in colon cancer cells. Tumor Biology. 2014;35(7):7035–7045. doi: 10.1007/s13277-014-1956-3. [DOI] [PubMed] [Google Scholar]
- 10.Bergamo A, Sava G. Ruthenium anticancer compounds: myths and realities of the emerging metal-based drugs. Dalton Transactions. 2011;40(31):7817–7823. doi: 10.1039/c0dt01816c. [DOI] [PubMed] [Google Scholar]
- 11.Niemietz CM, Tyerman SD. New potent inhibitors of aquaporins: silver and gold compounds inhibit aquaporins of plant and human origin. FEBS Letters. 2002;531(3):443–447. doi: 10.1016/s0014-5793(02)03581-0. [DOI] [PubMed] [Google Scholar]
- 12.Pedersen SF, Hoffmann EK, Mills JW. The cytoskeleton and cell volume regulation. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 2001;130(3):385–399. doi: 10.1016/s1095-6433(01)00429-9. [DOI] [PubMed] [Google Scholar]
- 13.Zhang J, An Y, Gao J, Han J, Pan X, Pan Y, et al. Aquaporin-1 translocation and degradation mediates the water transportation mechanism of acetazolamide. Plos One. 2012;7(9):e45976. doi: 10.1371/journal.pone.0045976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teicher BA, Liu SD, Liu JT, Holden SA, Herman TS. A carbonic anhydrase inhibitor as a potential modulator of cancer therapies. Anticancer Research. 1993;13(5A):1549–1556. [PubMed] [Google Scholar]
- 15.Chiche J, Ilc K, Laferriere J, Trottier E, Dayan F, Mazure NM, Brahimi-Horn MC, Pouyssegur J. Hypoxia-inducible carbonic anhydrase IX and XII promote tumor cell growth by counteracting acidosis through the regulation of the intracellular pH. Cancer Research. 69:358–368. doi: 10.1158/0008-5472.CAN-08-2470. [DOI] [PubMed] [Google Scholar]
- 16.Uhlman ME, Georges RB, Boleij A, Eyol E, Kubarenko A, Adwan H, et al. Influence of osteopontin expression on the metastatic growth of CC531 rat colorectal carcinoma cells in rat liver. Cancer Gene Therapy. 2011;18(11):795–805. doi: 10.1038/cgt.2011.48. [DOI] [PubMed] [Google Scholar]
- 17.Eyol E, Murtaga A, Zhivkova-Galunska M, Georges R, Zepp M, Djandji D, et al. Few genes are associated with the capability of pancreatic ductal adenocarcinoma cells to grow in the liver of nude rats. Oncology Reports. 2012;28(6):2177–2187. doi: 10.3892/or.2012.2049. [DOI] [PubMed] [Google Scholar]
- 18.Kaleağasıoğlu F, Berger MR. Differential effects of erufosine on proliferation, wound healing and apoptosis in colorectal cancer cell lines. Oncology Reports. 2014;31(3):1407–1416. doi: 10.3892/or.2013.2942. [DOI] [PubMed] [Google Scholar]
- 19.Tekedereli I, Alpay SN, Akar U, Yuca E, Ayugo-Rodriguez C, Han HD, et al. Therapeutic silencing of Bcl-2 by systemically administered siRNA nanotherapeutics inhibits tumor growth by autophagy and apoptosis and enhances the efficacy of chemotherapy in orthotopic xenograft models of ER (-) and ER (+) breast cancer. Molecular Therapy-Nucleic Acids. 2013;10(2):e121. doi: 10.1038/mtna.2013.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ribatti D, Ranieri G, Annese T, Nico B. Aquaporins in cancer. Biochimica et Biophysica Acta. 2014;1840(5):1550–1553. doi: 10.1016/j.bbagen.2013.09.025. [DOI] [PubMed] [Google Scholar]
- 21.Verkman AS, Mitra AK. Structure and function of aquaporin water channels. American Journal of Physiology-Renal Physiology. 2000;278(1):F13–F28. doi: 10.1152/ajprenal.2000.278.1.F13. [DOI] [PubMed] [Google Scholar]
- 22.Yang JH, Shi YF, Chen XD, Qi WJ. The influence of aquaporin-1 and microvessel density on ovarian carcinogenesis and ascites formation. International Journal of Gynecological Cancer. 2006;16(1):400–405. doi: 10.1111/j.1525-1438.2006.00476.x. [DOI] [PubMed] [Google Scholar]
- 23.Jiang Y. Aquaporin-1 activity of plasma membrane affects HT20 colon cancer migration. IUBMB Life. 2009;61(10):1001–1009. doi: 10.1002/iub.243. PubMed. [DOI] [PubMed] [Google Scholar]
- 24.Hu J, Verkman AS. Increased migration and metastatic potential of tumor cells expressing aquaporin water channels. FASEB Journal. 2006;20(11):1892–1894. doi: 10.1096/fj.06-5930fje. PubMed. [DOI] [PubMed] [Google Scholar]
- 25.Otterbach F, Callies R, Adamzik M, Kimmiq R, Siffert W, Schmid KW, et al. Aquaporin (AQP1) expression is a novel characteristic feature of a particularly aggresive subgroup of basal-like breast carcinomas. Breast Cancer Research and Treatment. 2010;120(1):67–76. doi: 10.1007/s10549-009-0370-9. [DOI] [PubMed] [Google Scholar]
- 26.Huang Y, Murakami T, Sano F, Kondo K, Nakaigawa N, Kishida T, et al. Expression of aquaporin 1 in primary renal tumors: a prognostic indicator for clear-cell renal cell carcinoma. European Urology. 2009;56(4):690–698. doi: 10.1016/j.eururo.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 27.Machida Y, Ueda Y, Shimasaki M, Sato K, Sagawa M, Katsuda S, et al. Relationship of aquaporin 1, 3, and 5 expression in lung cancer cells to cellular differentiation, invasive growth, and metastasis potential. Human Pathology. 2011;42(5):669–678. doi: 10.1016/j.humpath.2010.07.022. [DOI] [PubMed] [Google Scholar]
- 28.Bin K, Shi-Peng Z. Acetazolamide inhibits aquaporin-1 expression and colon cancer xenograft tumor growth. Hepatogastroenterology. 2011;58(110–111):1502–1506. doi: 10.5754/hge11154. [DOI] [PubMed] [Google Scholar]
- 29.Tafreshi NK, Lloyd MC, Bui MM, Gillies RJ, Morse DL. Carbonic anhydrase IX as an imaging and therapeutic target for tumors and metastases. Subcellular Biochemistry. 2014;75:221–254. doi: 10.1007/978-94-007-7359-2_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tülüce Y, Ahmed BA, Koyuncu İ, Durgun M. The cytotoxic, apoptotic and oxidative effects of carbonic anhydrase IX inhibitor on colorectal cancer cells. The Journal of Bioenergetics and Biomembranes. 2018;50(2):107–116. doi: 10.1007/s10863-018-9749-9. [DOI] [PubMed] [Google Scholar]
- 31.Parkkila S, Rajaniemi H, Parkkila AK, Kivela J, Waheed A, Pastorekova S, et al. Carbonic anhydrase inhibitor suppresses invasion of renal cancer cells in vitro. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(5):2220–2224. doi: 10.1073/pnas.040554897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiang Y, Ma B, Li T, Gao JW, Yu HM, Li XJ. Acetazolamide inhibits aquaporin-1 protein expression and angiogenesis. Acta Pharmacologica Sinica. 2004;25(6):812–816. [PubMed] [Google Scholar]