Abstract
Isoprene and other plastidial isoprenoids are produced primarily from recently-assimilated photosynthates via the 2-C-methyl-D-erythritol 4-phosphate pathway. However, when environmental conditions limit photosynthesis, a fraction of carbon for MEP pathway can come from extrachloroplastic sources. The flow of extrachloroplastic carbon depends on species, leaf developmental and environmental conditions. The exchange of common phosphorylated intermediates between the MEP pathway and other metabolic pathways can occur via plastidic phosphate translocator. The C1 and C2 carbon intermediates can contribute to chloroplastic metabolism, including photosynthesis and isoprenoid synthesis. Integration of these metabolic processes provide an example of metabolic flexibility, and results in the synthesis of primary metabolites for plant growth and secondary metabolites for plant defense, allowing effective use of environmental resources under multiple stresses.
Keywords: DMADP, energy control, MEP pathway, plant stress, VOCs
The availability of carbon sources used for plastidic MEP pathway flux with emphasis on isoprene
Among the more than 1700 volatile organic compounds (VOC) already identified, isoprene is the most abundant species emitted by plants [1–3]. It is estimated that the annual global emissions of isoprene from land vegetation ranges from 440 to 660 Tg C year-1 [3,4]. Due to its high reactivity to major tropospheric oxidants, isoprene can impact air chemistry and climate by affecting the concentrations of carbon monoxide and ozone, among other oxidants, and by prolonging the lifespan of methane. Isoprene reactions also contribute strongly to the formation of secondary organic aerosols, thereby further altering the climate [5,6]. From ecological and evolutionary perspectives, isoprene and other volatile plastidial isoprenoids play important biological functions, including biotic and abiotic stress protection [2,7,8].
Plant isoprene emission was discovered six decades ago [9], and since then, significant advances have been made concerning the biosynthetic processes and the emission rate of isoprene [10]. Despite progress in understanding the regulatory mechanisms of the MEP pathway [11], current knowledge of the interplay between the synthesis and emission of isoprene and other cellular processes remains rather limited. Moreover, the integrated coordination and metabolite trafficking between cell compartments, and consequently, the use of different metabolite sources and photosynthetic energy for plastidial volatile isoprenoids synthesis is controversial. While the regulatory controls driving these processes are not yet fully elucidated, these processes provide an elegant example of flexible metabolic processes which respond to the carbon and energy needs of the plant through short- and long-term variations in environmental conditions. Understanding of the different regulatory mechanisms involved in controlling MEP pathway carbon contributions is critical to place biological and environmental observations in a proper context [12–14].
Isoprene is formed in the chloroplasts by isoprene synthase from its immediate precursor, dimethylallyl diphosphate (DMADP, see Glossary). In the chloroplasts, both DMADP and isopentenyl diphosphate (IDP) are generated by the methylerythritol phosphate (MEP) pathway from pyruvate and glyceraldehyde 3-phosphate (G3-P) [15,16] (Figure 1). The pathway is also responsible for the synthesis of a range of key plastidic isoprenoids including phytohormones (gibberellin, cytokinin, abscisic acid, strigolactones); chloroplastic pigments (side chains of chlorophylls and carotenoids); electron carriers (side chain of plastoquinone); tocopherols and phylloquinone [17–19]. In addition, the MEP pathway supports the synthesis of important secondary metabolites, including the C5 hemiterpenes (isoprene and methylbutenol); many C10 monoterpenes (e.g. limonene, terpinolene, myrcene, α- and β-pinene, ocimene); and some C20 diterpenes [16,20–22]. DMADP and IDP are also produced by the cytosolic mevalonate (MVA) pathway, where they are used for synthesis of brassinosteroids, sterols, dolichols, ubiquinone, triterpenes (squalene) and sesquiterpenes (farnesene, caryophyllene, sesquiphellandrene, nerolidol) [15,19,21,22]. Considering the huge diversity, and a large carbon flux going into isoprenoid synthesis, isoprenoid production consumes a significant fraction of photosynthetic carbon and energy, and thus, could potentially compete with the primary metabolism [23,24].
Glossary.
Dimethylallyl diphosphate (DMADP): immediate precursor of isoprene biosynthesis.
Formaldehyde dehydrogenase: an enzyme that catalyses the oxidation of formaldehyde to formate.
Formate dehydrogenase: an enzyme that catalyses the oxidation of formate to CO2.
Isopentenyl diphosphate (IDP): allylic isomer of dimethylallyl diphosphate (DMADP). There are two biosynthetic routes to IDP, the classical mevalonic acid (MVA) pathway in the cytosol and the methylerythritol phosphate (MEP) pathway in the plastid.
Jmax: maximum linear electron transport rate.
Malate valve: a pathway for exporting reducing equivalents among different cellular compartments via a specific malate-oxaloacetate translocator and activation of malate dehydrogenase (MDH) enzymes. Isoenzymes of MDH are found in each cellular compartment. NAD-dependent isoforms of MDH are present in mitochondria, peroxisomes, cytosol, and plastids, while the chloroplasts contain the redox-controlled NADP-MDH. The MDH are responsible by reversible conversion of oxaloacetate to malate using the oxidation of NAD(P)H to NAD(P).
Mehler reaction: in chloroplasts, when ferredoxin becomes highly reduced, e.g. under environmental conditions when the photosynthetic photon flux is in excess of that used for carbon assimilation, the electrons can be transferred from photosystem I to oxygen to form superoxide radicals (O2–•).
Oxylipin pathways: plant oxylipins constitute a multiplicity of lipophilic signalling molecules derived from the oxygenation of polyunsaturated fatty acids. These molecules are involved in responses to biotic and abiotic stresses as well as in plant development.
Pentose and triose phosphate intermediates: The set of pentose intermediates, together with the triose phosphate pool, constitute a large group of interconvertible metabolites often called the triose phosphate/pentose phosphate pool.
Plastidic phosphate translocator (PT) family: the plastidic phosphate translocator mediates a counter-exchange of phosphorylated C3, C5, C6 metabolites in exchange of inorganic phosphate. The PT family comprises four members; triose phosphate/phosphate, phosphoenolpyruvate/phosphate, glucose 6-phosphate and xylulose 5-phosphate/phosphate translocator.
Prenyltransferase: constitutes a large family of enzymes which catalyse the chain elongation of C5 building blocks via sequential condensation reactions with one or more allylic diphosphates groups in order to generate the isoprenoid carbon skeletons with different chain lengths, from GDP (C10) to natural rubber (C>10,000).
Vcmax: maximum carboxylation rate by Rubisco.
Xanthophyll cycle: the xanthophylls are a group of oxygenated pigment carotenoids found in all photosynthetic organisms, which comprise three carotenoids violaxanthin, antheraxanthin, and zeaxanthin. The xanthophyll cycle involves the conversion of violaxanthin to antheraxanthin and then to zeaxanthin. This cycle participates in the thermal dissipation of excess absorbed light energy.
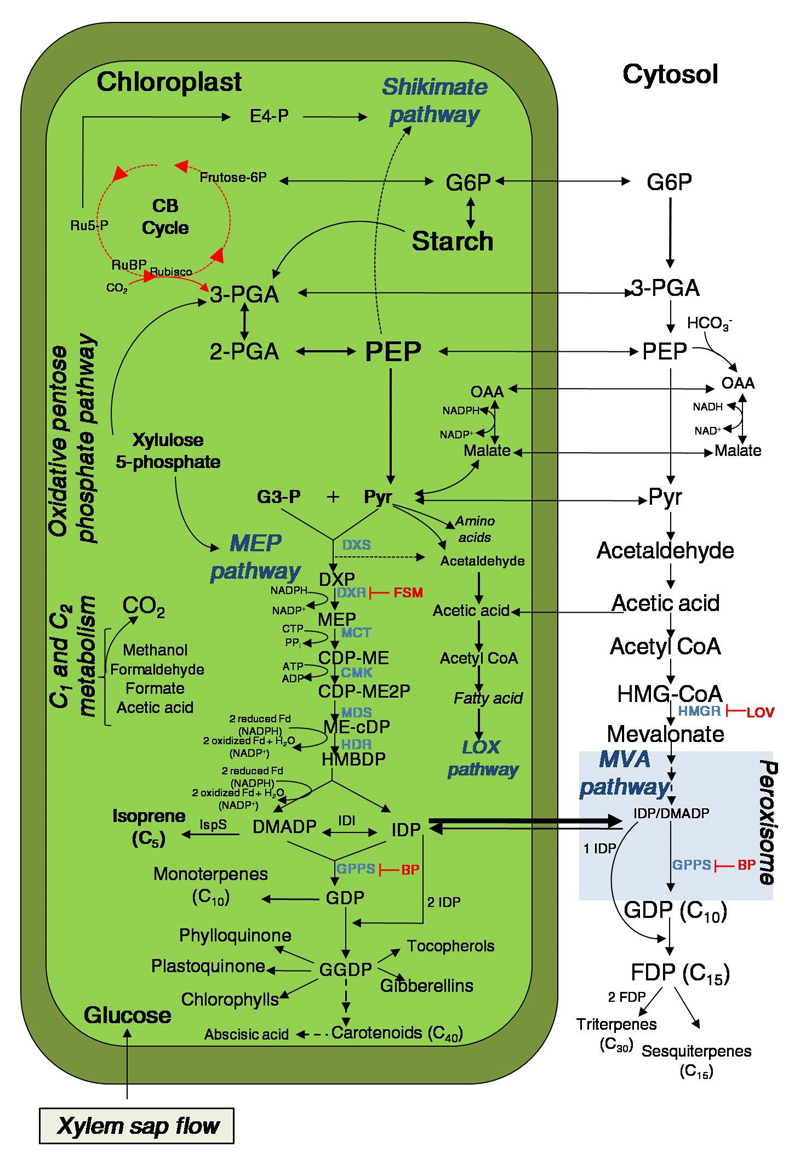
Figure 1.
Synthesis of major plant volatile isoprenoids, isoprene and monoterpenes, occurs via the 2-C-methyl-D-erythritol 4-phosphate (MEP) pathway in chloroplast, however other multiple biosynthetic pathways can contribute carbon intermediates to MEP pathway. Due to the compartmental nature of the chloroplast metabolism and cytosol, the intermediate exchange among different pathways is hampered. The key pathways interacting with the MEP pathway are Carbon 1 (C1) metabolic pathway, oxidative pentose pathway, Calvin-Benson (CB) cycle, shikimate pathway, cytosolic glycolysis and mevalonate (MVA) pathway. Potential exchange of intermediates through the plastid membrane is also shown, as well as intermediate carbon partitioning. In addition, biosynthesis of different classes of isoprenoids formed from the same five carbon (C5) precursors in both compartments is shown. Solid-line arrows indicate carbon flow from extra-chloroplast intermediate molecules and exchange of carbon intermediates between the compartments. The inhibitors of the MVA and MEP pathway are shown in red. Fosmidomycin (FMS) inhibits DXR, the enzyme responsible for the formation of MEP from DXP in the MEP pathway [30,89]. Bisphosphonates (BP) inhibitors (e.g. alendronate and zoledronate) are used to inhibit prenyltransferases reactions, suppressing the formation of isoprenoids formed from GDP in chloroplast and cytosol [44,88]. The lovastatin (LOV) (also referred to as mevinolin), is a specific inhibitor of HMGR in the MVA pathway [75,79,87]. Abbreviations: Metabolites: CDP-ME, 4-(cytidine 5’-diphospho)-2-C-methyl-D-erythritol; CDP-ME2P, 2-Phospho-4-(cytidine 5’-diphospho)-2-C-methyl-D-erythritol; DMADP, dimethylallyl diphosphate; E4-P; erythrose 4-phosphate; FDP, farnesyl diphosphate (FDP; C15); GDP, geranyl diphosphate (GDP, C10); geranylgeranyl diphosphate (GGDP, C20); G6P, glucose 6-phosphate; G3-P, glyceraldehydes-3-phosphate; HCO3-, bicarbonate ion; HMBDP, 1-Hydroxy-2-methylbut-2-enyl-butenyl 4-diphosphate; HMG-CoA, 3-hydroxy-3-methyl-glutaryl-coenzyme A; IDP, isopentenyl diphosphate; MEP, 2-C-methyl-D-erythritol 4-phosphate; ME-cDP, 2-C-methyl-D-erythritol 2,4-cyclodiphosphate; OAA, oxaloacetate; PEP, phosphoenolpyruvate; 2-PGA, 2- phosphoglycerate; 3-PGA, 3-phosphoglycerate; RuBP, ribulose-1,5-bisphosphate; Ru5-P, Ribulose 5-phosphate. Enzymes: CMK, 4-(cytidine 5’-diphospho)-2-C-methyl-D-erythritol kinase; DXS, 1-Deoxy-D xylulose 5-phosphate synthase; DXR, 1-Deoxy-D-xylulose 5-phosphate reductoisomerase; HDR, 4-Hydroxy-3-methylbut-2-enyl-diphosphate reductase; HMGR, 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase; IDI, isopentenyl diphosphate isomerase; IspS, isoprene synthase; MCT, 2-C-methyl-D-erythritol 4-phosphate cytidylyltransferase; MDS, 2-C-methyl-D-erythritol 2,4-cyclodiphosphate synthase. Cofactors: ADP, adenosine diphosphate; ATP, adenosine triphosphate; CTP, cytidine triphosphate; NADH, nicotinamide adenine dinucleotide (form reduced); NAD+, nicotinamide adenine dinucleotide (form oxidized); NADPH, nicotinamide adenine dinucleotide phosphate (form reduced); NADP+, nicotinamide adenine dinucleotide phosphate (form oxidized). Additional abbreviations: Fd; ferredoxin-dependent reaction; PPi; pyrophosphate.
Photosynthesis is the principal carbon and energy source for isoprene synthesis [25]. Light-dependent isoprene emission is related to electron transport rate [26,27], triose phosphates, ATP and NADPH levels, and to the flux and proportion of intermediates passing through the MEP pathway [24,25,28]. Under non-stressed conditions, 13CO2-feeding experiments have demonstrated that approximately 90% of the carbon atoms emitted as isoprene will be rapidly labelled by 13C [29–32], most likely from the recently assimilated carbon incorporated in molecules of triose phosphates. However, under non-photorespiratory conditions, a persistent emission of [12C]isoprene is seen during the 13CO2-labelling experiment, indicating that even under non-stressed conditions, the carbon sources available for isoprene synthesis can be derived from different cellular precursors [33].This same report shows that, even after the transfer of leaves to 12CO2 in the light, the 13C-labelling of one carbon atom remains for several hours and this effect is more pronounced in fosmidomycin-fed leaves [33]. The persistence of [13C1]isoprene emissions upon return to normal 12CO2 suggests that extrachloroplastic carbon sources consist of carbon species, presumably stored carbohydrates, with a slower turnover and 13C-labelled species derived from recently assimilated photosynthate exported from chloroplasts. The fraction of unlabelled carbon in isoprene emission is typically greater when environmental conditions (e.g., high temperatures, drought stress [34,35] and low CO2 concentrations [36] are limiting photosynthesis [37], suggesting that non-recently-assimilated, so-called ‘alternative carbon sources’ can be mobilized for isoprene synthesis especially under such conditions [29,30,37]. Furthermore, the fraction of unlabelled carbon can vary with species [29], leaf-developmental stage (see Box 2 for further discussion) [30] and it can also vary among different classes of isoprenoids, e.g., among non-oxygenated monoterpenes β-pinene, myrcene and (E)-β-ocimene, and oxygenated compounds linalool and 3-methyl-3-buten-1-ol; and sesquiterpenes (E)-β-farnesene, α-farnesene, among others [38,39].The alternative carbon sources can arise from multiple origins, including chloroplastic ones such as starch breakdown [33], the chloroplastic pyruvate pool [29,36], and deoxyxylulose phosphate molecules derived from the pentose pathway [40]; and extra-chloroplastic ones such as xylem-transported carbohydrates [37,41,42] and possibly also from the cytosolic phosphoenolpyruvate (PEP) pool [43–46]. Additionally, it has been suggested that re-fixation of CO2 released by mitochondrial respiration and/or photo-respiratory processes can provide carbon substrates for MEP pathway [30,47–49] (Figure 1).
Box 2. Age-dependent isoprene emission in leaves.
In developing leaves, DMADP formation is limited and isoprene emission is constrained [26]. In addition, only about 50% of the small amounts of isoprene produced are labelled by 13C – a similar proportion to the labelled DMADP pool [30]. This suggests an alternative source of DMADP for isoprene production in early leaf development. In this stage of development, a relatively large amount of carbon is diverted to essential non-volatile chloroplastic isoprenoids, which are needed for the assembly of the photosynthetic apparatus [91]. Moreover, in the early stages of leaf development, photosynthetic structures are not fully formed, further compromising the availability of substrates and energy for isoprene synthesis [26,123].
Isoprene emission rates are also lower in older leaves, however, a larger DMADP pool size and reduced isoprene synthase activity have also been observed in these older leaves [26,91]. Accumulation of C5 building blocks is highest when the production of prenyl diphosphate precursors exceeds consumption by downstream reactions [24,124]. Accordingly, a rise in the DMADP pool in old leaves can be linked to a reduction in either prenyltransferase reactions or overall foliage physiological activity before senescence is triggered. Leaf aging may result in a lower demand for synthesis of essential isoprenoid compounds and a shift in substrate allocation towards volatile isoprenoids [91]. The reduction of foliage physiological activity with leaf aging can lead to enhanced formation of reactive oxygen species (ROS), and reduce carbon allocation towards primary metabolites [125]. It has been suggested that a higher DMADP pool size could be used to maintain a high isoprene emission rate and relieve oxidative damage [26]. Additionally, it has been suggested that the excess of prenyl diphosphate groups not used for the formation of essential isoprenoids may be reallocated for volatile isoprenoid production as an evolutionary strategy to avoid wasting of sequestered phosphate in the form of DMADP and IDP [19]. Therefore, it appears that differences in the isoprene emission rate at different developmental stages can result from differences in physiological maturation, availability of DMADP and enzymatic competition for substrate by downstream prenyltransferase reactions [26,91,126,127].
Species capable of emitting isoprene or monoterpenes tend to emit either one or the other; this pattern alters depending on leaf development [17,127] or when under attacks from pathogens, or via wounding and abiotic stress factors. Monoterpenes are formed from the prenyl diphosphate precursor GDP (Figure 1), which is itself made by condensation of one IDP and one DMADP molecule. It has been postulated that the shift from monoterpenes to isoprene during leaf development probably reflects a lower DMADP pool in young leaves, in combination with the very high Michaelis–Menten constant (Km) of isoprene synthase enzyme compared to other allylic diphosphate-utilizing enzymes [91,127–130] and limited isoprene synthase activity which results from low expression in young leaves [26]. Thus, the biosynthesis of monoterpenes may be favored when C5 prenyl phosphates are diminished by primary metabolic pathways during early leaf developmental stage [127]. Additionally, young leaves are more attractive for herbivores and pathogens, and the increase in monoterpene production could thus contribute in protection against attacks of herbivores and pathogens.
Interestingly, a lack of complete labelling of the Calvin-Benson (CB) cycle intermediates in 13CO2–feeding experiments has also been observed, with the non-labelled primary photosynthate fraction typically being ca. 10% in non-stressed leaves [50]. Recently, a new hypothesis was developed to explain this [51] which might, at least partly, explain the incomplete 13C labelling of isoprene. This assumption is based on the activation of carbon flux through the hypothesized glucose-6P (G6P) shunt around the CB cycle from pools of glucose phosphate (Figure 1). It has been proposed that this alternative carbon flow is responsible for the lack of complete labelling of the CB intermediates metabolite when the leaves are exposed to 13C-labelled CO2. Specifically, it was suggested that the presence of a high G6P pool can increase carbon fluxes through the G6P shunt. The G6P/Frutose-6P (F6P) ratio can be regulated by the activity of the phosphoglucoisomerase (PGI), which responds to the phosphoglycerate (PGA) concentration in chloroplasts. The PGA is a negative allosteric effector of PGI: high PGA levels limit the production of G6P from F6P, while simultaneously stimulating starch synthesis. In contrast, when PGA is low, PGI activity is high, increasing the pools of G6P in chloroplasts. Thus, it is possible that under conditions that restrict photosynthesis (e.g., low [CO2], water deficit and high temperature) - which are conditions that generally lead to the accumulation of G6P - the activation of the G6P shunt from the pools of glucose phosphate results in an increased pool of non-labelled carbon flowing into the MEP pathway and thus into isoprene molecules. This is further supported by the higher rate of starch degradation under photorespiratory conditions, which results in a glucose phosphate pool that can feed into the G6P shunt and subsequently could improve the precursor availability for MEP pathway [52,53].
Even after decades of intense research on the biochemical and physiological mechanisms of isoprene emission, several key issues have not been fully elucidated: (1) the evolutionary controls on isoprene emission across plant genera [27,54,55]; (2) the mechanistic processes by which isoprene exerts its protective effect [56] and (3) the control mechanisms associated with the availability of reducing power and carbon skeletons used for isoprene synthesis [27,28,30,48,57]. Here, we shall focus on the last question by highlighting recent evidence concerning control and regulation of metabolic exchange between the MEP and other biosynthetic pathways, as well as the relationship between isoprene synthesis and leaf energetic status.
The implications of carbohydrate metabolism on patterns of isoprene synthesis and emission
The close relationship between photosynthesis and carbon flux through the MEP pathway means that the mechanisms of photosynthetic regulation are key factors in controlling carbon and energy allocation for isoprene biosynthesis [27,41,58]. There is evidence that the isoprene emission rate correlates with both primary photosynthetic metabolite content as well as with photosynthetic energy supply, but it has been difficult to tease apart the proportional importance because energy supply and photosynthetic metabolite concentrations are also correlated. Under light-saturated conditions, higher isoprene emission rates are correlated with higher starch, triose phosphate and sucrose contents, in addition to the increased availability of both ATP and NADPH [41,58,59]. During the day, variations in VOC emissions and the synthesis of plastidic terpenoids have been explained by changes in the availability of precursors [41,58,60]. In particular, flux through the MEP pathway is accelerated in the light period, mostly due to the photosynthesis-dependent supply of sugars and energy [25,59]. Indeed, diurnal variations in triose phosphate pools are highly correlated with the levels of intermediates of the MEP pathway [25], but triose phosphate concentrations are also correlated with PEP and pyruvate concentrations [58].
Besides fluctuations in partitioning of photoassimilates and energy flow levels [60,61], there is evidence that some diurnal variability involves circadian clock dependent regulation [25]. The circadian regulation of isoprene synthesis is linked with rhythmic variations in gene expression, protein amount, and enzyme activity of the MEP pathway [59,62,63], but also with non-circadian-clock regulated fluctuations in partitioning of photoassimilates and energy flow levels [60,61,64].
The above observations suggest that isoprene synthesis can be responsive to changes in plastidic carbohydrate availability and that the DMADP pool size is limited by levels of energetic products generated in photosynthesis [27,57,65,66]. Indeed, the fraction of assimilated carbon lost as isoprene increases with leaf energetic status [67,68]. Changes in isoprene emission can also co-vary with other, more effective energy quenching pathways, including the Mehler reaction, xanthophyll cycle, and some energy sink processes such as photorespiration and nitrate reduction [65,69–71].
Apart from direct controls, experimental and theoretical analyses suggest a possible indirect integration of isoprene synthesis with carbohydrate metabolism [24,25,33,37,42,58]. The carbohydrate-mediated regulation of isoprenoid precursor production in plants seems to involve the differential regulation of gene expression and enzyme activity, as well as the production of precursors [72–75]. The integrated regulation between MEP-derived isoprenoid production and carbohydrate metabolism is supported by the identification and characterization of an Arabidopsis thaliana PRL1-deficient (Pleiotropic Regulatory Locus 1) mutant [73]. This gene encodes a conserved WD-protein that functions as a regulator of members of the SnRK1 (sucrose non-fermenting1-related protein kinase) family. These protein kinases are involved in the modulation of sugar and starch levels in leaves [76] and modulation of the activity of other important metabolic enzymes, including the central enzyme of the cytosolic MVA pathway, hydroxymethylglutaryl-CoA reductase (HMGR) [73,77]. The mutation of the prl1gene resulted in enhanced availability of MEP pathway substrates, with a concomitant increase in the levels of plastidic isoprenoid pigments. Similar results were also found in Arabidopsis wild-type plants growing in a medium supplemented with sucrose [72,73]. It can be deduced from such reports that the loss of function of prl1might result in increased sugar levels and redirection to the production of chloroplastic pigments via the MEP pathway. Additionally, reductions in carotenoids and pigments observed in sucrose-limited conditions have been associated with a decrease in the hexose pool and changes in gene expression and activity of the enzyme phytoene synthase [72].
Cross-talk between the MVA and MEP pathways: carbon supplementation for isoprene synthesis
The enzymes called prenyltransferases catalyse the sequential condensation of DMADP with IDP (Figure 1) and yield the polyprenyl diphosphate precursors geranyl diphosphate (GDP, C10), farnesyl diphosphate (FDP; C15), and geranylgeranyl diphosphate (GGDP, C20). Although the MVA and MEP pathways operate in independent sub-cellular compartments in plants, there is evidence that the some of these diphosphates can travel between the sub-cellular compartments including cytosol, peroxisomes, mitochondria and chloroplast [21,78–83], providing metabolic cross-talk and compensatory potential. Other studies have shown that enzymes and isoprenoid precursors that have been commonly thought to be confined to either MEP or MVA pathway and thus located either only in the chloroplasts or in the cytosol, can be targeted to other subcellular compartments [81,83,84]. For example, there is evidence that FDP (classically thought to be confined to cytosol) can be synthetized in the chloroplasts or transported to chloroplasts in some species [81]. Also, the cytosolic and mitochondrial localization of GGDP has been confirmed in A. thaliana and pea (Pisum sativum) chloroplasts [85]. Moreover, some results suggest that plastidic GDP can be exported to the cytosol and promote the synthesis of monoterpenes via the action of cytosolic bifunctional sesquiterpene/monoterpene synthases [82,86]. This evidence collectively indicates the possibility that chloroplastic isoprene synthesis may rely on substrates originating from multiple subcellular compartments.
Feeding experiments using labelled intermediates of MEP pathway or cytosolic MVA pathway have been useful in the quantitative assessment of metabolic flux through either of the pathways. Continuous feeding with isotope-labelled [2-13C] MVA to intact cotton seedlings resulted in approximately 50% of the labelled precursors of monoterpenes, 40% of the phytol chain of chlorophyll and 50% of β-carotene; all of which are compounds typically and exclusively associated with the MEP pathway [21]. With respect to cytosolic isoprenoid products generally related to the MVA pathway, plants fed with the isotope-labelled MEP precursor, [5,5-2H2] deoxy-D-xylulose (DX) resulted in approximately 33% of labelled precursors for the synthesis of sesquiterpenes, 50% of the C25 heliocidic terpenoid aldehydes and approximately 20% of sitosterol molecules [21]. Other representative examples of this cross-talk come through the incorporation of [2-13C] MVA into plastoquinone, and [5-2H] mevalonate or [5,5-2H2] DX labelling into dolichols [87]. These observations demonstrate the active participation of both pathways in biosynthesis of cytoplasmic and plastid isoprenoids compounds of plants.
Cross-talk between the MEP and cytosolic MVA pathways has been directly proven by experiments using pathway-selective inhibitors [75,87–89]. Application of fosmidomycin results in a strong reduction in isoprene emission rate, and this reduction is associated with an almost complete depletion of the DMADP pool (Figure 1). However, full inhibition of isoprene emission, as well as the decrease in pools of ME-cDP, has not been demonstrated even after continued fosmidomycin feeding for more than 1 h [88,90]. Furthermore, the residual isoprene emissions of leaves previously fed with fosmidomycin were not by 13C and the level of the incomplete labelling of isoprene was associated with the fraction of re-fixed respiratory CO2[30]. Together, these observations suggest the presence of substantial intermediate supply from the cytosol. The incorporation studies with [1,1,1,4-2H4]DX have showed that cytoplasmic isoprenoids, in the presence of lovastatin (a specific inhibitor of 3-hydroxy-3-methylglutaryl-CoA; see Figure 1) can be synthesized from MEP pathway[89]. In addition, the use of lovastatin or fosmidomycin[a specific inhibitor of 1-deoxy-D-xylulose 5-phosphate (DXP) reductoisomerase (DXR)] has resulted in a decrease in the formation of cytosol-synthesized dolichols [87]. This evidence further supports the hypothesis of a possible trafficking of intermediates between the MVA and MEP pathway, as well as concomitant regulation of both pathways in the biosynthesis of plastid and cytoplasmic isoprenoids in plants.
Bisphosphonates, which inhibit prenyltransferase reactions, have also been used to examine the MEP pathway flux and intermediate exchange [88,91]. Interestingly, bisphosphonate-inhibited leaves had increased levels of ME-cDP as well as the immediate isoprene substrate DMADP or IDP [88]. Accumulation of C5 prenyl phosphates can reduce the carbon input into the MEP pathway through negative feedback inhibition of the activity of 1-deoxy-D-xylulose 5-phosphate synthase (DXS) by IDP and DMADP [88,92]. Interestingly, simultaneous feeding of bisphosphonate inhibitors and fosmidomycin resulted in major differences in the isoprene emission rate and accumulation of isoprenoid precursors when compared to fosmidomycin-only-inhibited leaves [88]. In fosmidomycin-inhibited leaves, the depletion of DMADP pool is due to limited input and overall reduction of MEP-intermediates to DMADP. However, the application of bisphosphonate inhibitors to fosmidomycin-inhibited leaves resulted in a large increase in ME-cDP and a greater DMADP pool and isoprene emission rate, which suggests that the transport of intermediates from the cytosol occurs when C5 prenyl phosphates accumulate [88].The inhibition of the MVA pathway by lovastatin has been observed within 48 hours of application, but, surprisingly, inhibition of the cytosolic pathway led to an increase in production, or perhaps reduced catabolism, of chloroplast-synthesized photosynthetic pigments (carotenoids and chlorophyll) [79].This suggests a possible indirect effect on metabolite supply to the MEP pathway as a result of the blockage, which may be linked to its operating at the level of metabolites prior to HMGR. Additionally, in fosmidomycin-treated plants, a dramatic reduction in chlorophyll and carotenoids levels can be observed within 48 hours of application, along with an increase in the levels of sterols in the cytosol [79]. Also, 96 hours after the addition of lovastatin, the cytosolic and chloroplastic levels of metabolites were significantly lower in fosmidomycin-treated leaves than in the control samples. This might indicate that the compensation for metabolic imbalance favors cytosolic isoprenoid biosynthesis [78]. Regardless, these results provide additional evidence that a metabolic compensation can happen through the flux of metabolic intermediates between the pathways. Thus, the cross-talk between pathways might act as a major factor for fine-tuning the carbon flux to maintain the synthesis of vital isoprenoids under conditions of both end product accumulation and feedback inhibition. However, the extent and the exact identities of the transported phosphorylated intermediates have not yet been fully elucidated.
Exchange of common intermediates between the MEP pathway and other metabolic pathways
ME-cDP is an immediate precursor to the final two energy-dependent reactions of the MEP pathway (catalysed by ferredoxin-dependent (iron-sulphur cluster) reductases; (Figures 1 and 2). The use of DXS overexpression mutants or inhibitors of the MEP pathway has revealed an accumulation of ME-cDP when either the flux through the MEP pathway increases [92] or as a response to decreases in the activity of the enzymes downstream of DMADP [88]. ME-cDP can also accumulate under certain types of stress (for example; pathogen attack or oxidative stress) [90,93] which leads to the efflux of this metabolite to the cytosol [90]. In fact, previous studies have indicated that ME-cDP can act as a retrograde signal in order to initiate transcription of stress response genes in the nucleus [93,94], and this can only occur if ME-cDP is capable of travelling across the chloroplast envelope [90]. Indeed, a slow efflux of ME-cDP from plastids has been demonstrated as a plastid-to-nucleus retrograde signaling metabolite that induces expression of nuclear stress-response gene [90,92] (Figure 2). This statement is confirmed by strong correlation between accumulation of ME-cDP content and levels of salicylic acid and expression levels of hydroperoxide lyase (HPL; a stress-inducible nuclear gene encoding a plastid-localized HPL branch of the oxylipin pathway) [93], however, exactly how the pool of ME-cDP could be mobilized from the MEP pathway is currently unknown.
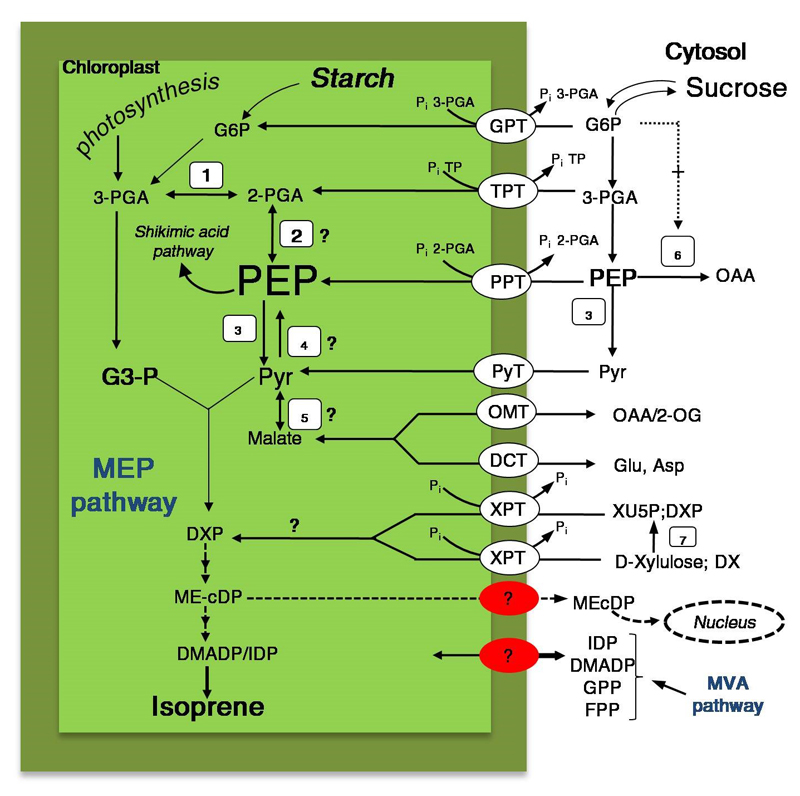
Figure 2.
A review of the current understanding of chloroplastic phosphoenolpyruvate (PEP) synthesis associated with pyruvate formation and specific transporters that mediate the transport of intermediates through the plastid membrane. The primary products of photosynthesis, triose phosphates, are exported via the triose phosphate/phosphate translocator (TPT), or metabolized in chloroplasts. The PEP, can be imported via PEP/phosphate translocator (PPT), or synthetized by plastidic enolase (2) or, to a lesser extent, by the pyruvate, orthophosphate dikinase (4) enzyme. PEP in the chloroplasts can enter the shikimic acid pathway or, after pyruvate (Pyr) formation, enter the MEP pathway. There are several possible sources for Pyr inside the chloroplasts: 1) export of cytosolic PEP through a PEP/phosphate translocator (PPT) [107] and its conversion to pyruvate via glycolysis involving phosphoglycerate mutase (PGL) and enolase (ENO) [148]; 2) synthesis from PEP by pyruvate kinase (PK) [149]; 3) via NADP-dependent malate dehydrogenase within chloroplast [150,151]; or 4) import from the cytosol via plastid Na+-coupled pyruvate transporter (PyT) [152]. The malate can be transported across the plastid inner envelope membrane through the so-called malate valve (or malate shunt) facilitated by 2-oxoglutarate (2-OG)-malate transporter (OMT) or via plastidial dicarboxylate transporter (DCT), which acts as an antiporter exchanging malate against glutamate (Glu) and aspartate (Asp) [119,151,153]. The product of starch and sucrose degradation, glucose 6-phosphate (G6P), is imported into the chloroplast by a hexose translocator (GPT). This specific metabolite is considered to be a positive allosteric effector of the PEP carboxylase enzyme (6). The figure shows a possible traffic of 2-C-methyl-D-erythritol 2,4-cyclodiphosphate (ME-cDP) from plastid into cytosol and its transfer to the nucleus. In addition, pentose intermediates, D-xylulose and 1-deoxy-D-xylulose (DX), can be phosphorylated to and/or subsequently translocated into the MEP pathway from xylulose 5-phosphate (XPT) carrier. Metabolites: DXP, 1-Deoxy-D xylulose 5-phosphate; XU5P, xylulose 5-phosphate. Enzymes numbering: phosphoglycerate mutase (1); enolase (2); pyruvate kinase (3); pyruvate orthophosphate dikinase (4); NADP-malate dehydrogenase (5); PEP carboxylase (6); xylulose kinase (7). The questions marks indicate steps for which the enzymes involved in isoprenoid metabolism or transport activities (red circles) remain to be elucidated.
In fosmidomycin-treated plants of A. thaliana, the reduction of hydroxymethylbutenyl diphosphate synthase activity (HDS, the enzyme that encodes the penultimate enzyme of the plastid-localized MEP pathway, Figure 1) led to accumulation of ME-cDP in the HDS-deficient mutant (hds-3 mutant) when compared to wild type plants [90]. This effect was similar to the changes in ME-cDP pool size in fosmidomycin/bisphosphonate treatment in hybrid aspen (see above and [88] for a detailed discussion). Despite the accumulation of ME-cDP, in fosmidomycin-inhibited plants, the 13C incorporation into ME-cDP in hds-3 plants is only 25% compared to 70% in wild type plants [90]. Similarly, 13CO2-labelling of ME-cDP in the DXS-upregulation lines is significantly lower than in the wild-type [92].These facts suggest an alternative pool of ME-cDP different from the MEP pathway. This hypothesis is supported by an observed residual pool of ME-cDP following dark treatment, and a build-up of ME-cDP due to downregulation of activity of MEP pathway enzymes [88,90]. However, it is generally regarded that phosphorylated intermediates such as ME-cDP are not readily taken up by chloroplasts; thus, a cytosolic flux of non-phosphorylated pentose intermediates into chloroplasts, followed by their conversion into ME-cDP might be responsible for the alternative a substrate for MEP pathway [12,40] (Figure 2).
Analysis of leaves from plants grown under nutrient deprivation or subject to other stresses, such as root oxidative stress, root wounding or biotic stress, revealed a close relationship between the levels of MEP intermediates, e.g., DXP, ME-cDP and HMBDP, and the production of hemiterpene glycosides [95,96]. In addition, significant increases in the levels of hemiterpene glycosides were found in fosmidomycin-treated hds-3 mutant plants compared to untreated wild type and no 13C label was detected at these metabolites during labeling experiments. Previous results suggest that under conditions that restrict the MEP pathway activity, the ME-cDP or HMBDP can be exported out of the plastid and then converted to hemiterpene glycosides in cytosol [95,96]. Due to the lack of a specific carrier capable of transporting these phosphorylated intermediates through the chloroplast membrane, it is likely that a dephosphorylation occurs within the chloroplasts and the glycosylation in the cytosol [96]. In addition, experimental evidence has shown that the accumulation of ME-cDP in plastids can elicit stress-signaling pathway, including changes in nuclear gene expression linked to plant defense signaling [90,93]. However, the exact nature of the signaling mechanisms coupled to ME-cDP content and gene expression as well as the mode of transport of this plastid metabolite still need to be elucidated.
Apart from ME-cDP, there is conclusive evidence of a certain bidirectional exchange of intermediates between cytosolic and plastidic isoprenoid biosynthetic pathways [78,80,97]. In particular, a plastidic membrane transporter involved in the export of phosphorylated intermediates of isoprenoid synthesis has been characterized [78]. This transporter efficiently carries IDP and GDP, but lower transport rates were observed for the substrates FDP and DMADP [78,80]. However, the way this transporter operates is not fully clear. The transport of IDP seems to occur via a proton symport mechanism driven by transmembrane pH gradient and membrane potential, and the transport rate is regulated by Ca2+ concentration [78,80]. It was further demonstrated that the transport mechanisms are different from those of the known plastidic phosphate translocator family (PT) [80,97]. The transport does not appear to be antiport in exchange of other phosphorylated compounds (e.g., inorganic phosphate) at the other side of the membrane [78]. Nevertheless, transport of IDP is strongly dependent on the presence of inorganic phosphate or small phosphorylated molecules on the opposite side of the membrane. These compounds appear to have positive control over unidirectional transport of IDP, rather than acting as substrates for an antiport exchange mechanism [80].
The cytosolic flux of pentose phosphate or its formation from the plastidic pentose phosphate pathway might represent an important entry point for the MEP pathway at DXP level (Figure 2). Experimental evidence has demonstrated that DXP can be synthesized in the cytosol from xylulose 5-phosphate (XU5P) (intermediate of the cytosolic oxidative pentose phosphate pathway) or from 1-deoxy-D-xylulose (DX), and then transported into plastid [12,40]. Identification of D-xylulose kinase (XK) in plants, an enzyme capable of phosphorylating exogenous D-xylulose to make 2C-methyl-D-erythritol 4-phosphate (MEP), has led to suggestions that MEP could be potentially formed in the cytosol [40]. In the presence of ATP as a co-substrate, XK is also able to catalyse the conversion of DX into DXP, however, with lower substrate affinity than for D-xylulose [40]. Such a phosphorylation of DX or D-xylulose in the cytosol and subsequent import into chloroplast by a transporter suggests that these substrates may be responsible for the build-up of ME-cDP in response to increases in flux through the MEP pathway or due to downregulation of activity of MEP pathway enzymes as mentioned above.
The MEP pathway includes several phosphorylated intermediates that could be transported by members of the plastidic phosphate translocator family across chloroplast membrane. A plastidic transporter for the pentose phosphate pathway, the xylulose 5-phosphate (XPT) carrier, has been identified; it belongs to the family of PT [97]. This translocator is able to transport and exchange with inorganic phosphate or triose phosphate, xylulose 5-phosphate (XU5P), DXP or DX and, to a lesser extent, ribulose 5-phosphate [98]. The transport of DXP may also be mediated, to a minor extent, by carriers such as the G6P/phosphate (GPT), triose phosphate/phosphate (TPT) and PEP/phosphate (PPT) transporters (Figure 2) [80]. Some observations suggest that cytosolic DX, XK or DXP can be phosphorylated and/or subsequently translocated to chloroplasts by the XPT carrier and subsequently enter the MEP pathway. Exogenous application of DX in the DXS mutant cla1-1 resulted in recovery of normal chloroplast development and mesophyll tissue formation [99]. A further argument supporting the transport of pentose intermediate into MEP pathway is given by the application of [1,1,1,4-2H4]DX to the tobacco Bright Yellow-2 (TBY-2) cells and labelling of all isoprenoid units in the prenyl side chain of plastoquinone and phytoene [89]. Further, feeding experiments with dideuterated deoxyxylulose (DOX-d2) caused the emitted isoprene to become rapidly labelled with deuterium, and the rate of emission of isoprene-d2 was correlated with the DMADP-d2 pool measured in the leaf, but labelling of isoprene was less pronounced when the leaves were transferred to higher temperatures. Interestingly, the growth inhibition by lovastatin is partially overcome by the supply of DX [89], suggesting a possible bidirectional exchange of pentose intermediates between the MVA and MEP pathway mediated by XPT carrier [80].
An alternative possibility might be the conversion of these C5 precursors into DMADP/IDP, followed by transportation into chloroplasts by plastidic IDP carriers [80]. This hypothesis is supported by incorporation of [4-2H]DX in the isoprenoid units derived from DMADP, as well as from IDP. However, the cross-talk between isoprenoid synthesis pathways at DMADP/IDP level typically involves relatively long time periods [100], while the production of MEP pathway intermediates requires higher carbon fluxes in isoprene-emitting species [101]. Therefore, the DMADP cannot be imported by chloroplast at rates that are significant for isoprene biosynthesis. Thus, one may speculate that pentose intermediates can be phosphorylated inside the chloroplasts and thereby increase the pool size of MEP pathway metabolites upstream of DMADP. These results further underscore the significant exchange of phosphorylated metabolites between the cytosol and chloroplast, however, the biosynthetic origin and transport characteristics of these carbon sources to isoprenoids in plant cells require further research.
Linking sources of internal CO2 release, C1pathway and isoprene emission
The feeding of 13CO2 via the xylem has indicated that a large amount of internal CO2 can be transported from the roots to the canopy via the transpiration stream [102]. Based on efflux of 13C relative to label uptake, it was estimated that up to 17% of 13C label provided at the base of the stem was assimilated in photosynthetic tissues [102]. In plants incubated with gaseous 14C-labelled formaldehyde, about 20-25% of the total 14C label was recovered from the plants, mainly from the leaves [103]. Sucrose, glucose and fructose were the main 14C-labelled metabolites found in the leaves. The formaldehyde metabolism is linked to the photosynthetic metabolism via CO2 re-assimilation in the CB cycle [104,105]; 14C labelling among sugars via primary photosynthetic products suggests that light-dependent formaldehyde assimilation in the CB cycle may be responsible. This hypothesis is supported by the high activity of formaldehyde dehydrogenase and formate dehydrogenase enzyme (enzymes responsible for the two-step oxidation of formaldehyde to CO2) in plants fed with formaldehyde.
Temperature-dependent incorporation of 13C from xylem-fed glucose (presumably from the CO2 moiety derived from degradation products), and intermediates from the C1 (e.g. methanol and formaldehyde; discussed below) and C2 pathway (Box 1) into isoprene suggests that these intermediates can provide an alternative carbon source for isoprene synthesis (Figure 1) [42,46,106]. Above the temperature optimum for photosynthesis (35-37 ºC), the transport of carbon to leaves from transpiration stream increased and the incorporation of 13C into isoprene was maximal [42]. The largest detected incorporation of 13C label was observed in only single (mass 70) and double (mass 71) 13C-labelled isoprene. The single or double labelling of isoprene may reflect the availability of different carbon sources for isoprene biosynthesis; e.g., re-assimilation of 13CO2 release from intercellular decarboxylation process or transport of carbohydrates into leaves via xylem sap or cytosolic flux of intermediate into plastids as pyruvate or PEP; Figures 1 and 2 [41,42,46,48,106,107].
Box 1. Relationship between C2 metabolism and isoprene emission.
It has been suggested that the C2 intermediates may contribute substantial carbon towards the production of key intermediates within chloroplasts and therefore contribute to the flux of carbon among different metabolic pathways [103,106]. Dynamic 13C-pulse chase experiments have also shown that leaves, when fed [2-13C]emit13C-labelled acetaldehyde, acetic acid, ethanol, isoprene and monoterpenes [114]. Additionally, [13C]isoprene emission was strongly correlated to [13C2]acetate and [13C2]glycine intermediates under sub-ambient CO2 concentration [106].
Acetic acid can be formed from the oxidation of acetaldehyde and its subsequent activation to acetyl CoA inside the chloroplasts [115–117]. Once formed, acetic acid can undergo multiple transformations within the chloroplast, including conversion of glyoxylate and acetyl-coenzyme A (acetyl-CoA) into malate and CoA, catalysed by malate synthase enzyme [118]. Once malate is formed, it may be transported into the chloroplast and oxidized via plastid-localized NAD(P)-malic enzyme to pyruvate and further to CO2 [119]; a fraction of the resulting pyruvate or CO2 released could be used as a carbon source for isoprenoid formation (Figure 2). The slower incorporation of 13C label into isoprene in [13C2]acetate-fed leaves led to speculation that this mechanism can integrate the C2 metabolism with MEP pathway via pyruvate production [106]. Previous studies have demonstrated plastidic localization of the enzymes malate synthase, aldehyde dehydrogenases and acetyl-CoA synthetase in vascular plants [115,117,118,120]. The presence of these enzymes within the chloroplast could provide a complete catabolic cycle in which C2 intermediates (e.g. acetaldehyde and acetic acid) can be recycled, with concomitant production of photosynthetic products like isoprenoid molecules. In accordance with this idea, transgenic lines co-expressing both glycolate oxidase and malate synthase within chloroplasts increased the levels of malate and carbon assimilation [121]. However, the activities of these enzymes inside the chloroplast are still uncertain, and it is unknown whether these mechanisms are widespread in plant cells.
Interestingly, it has been proposed that acetaldehyde bursts following rapid light-to-dark transition are mediated by DXS activity [34]. This hypothesis is based on the formation of the first reaction product catalysed by DXS, namely hydroxyethyl thiamine diphosphate; this same intermediate can be hydrolyzed to acetaldehyde by the action of cytosolic or plastidial pyruvate decarboxylase (PDC). Further evidence of the role of DXS as a potential source of acetaldehyde is supported by the observation of a higher burst of acetaldehyde after switching off the light in isoprene-emitting than in non-emitting species [34]. The accumulation of DMADP normally observed in non-emitting species might reduce DXS activity and, consequently, contribute to decline in acetaldehyde. Accordingly, the incorporation of 13CO2 into isoprene and acetaldehyde supports the hypothesis that both metabolites are produced from labelling pyruvate [122]. Given all this evidence, the emission of [13C]isoprene upon the supplied [13C2] metabolite labelling suggests that these C2 intermediates can undergo several reaction sequence steps in plant cells and this contributes either indirectly (via CO2 reassimilation) or directly (via substrate availability), or to a combination of both factors.
It has been suggested that the internal recycling of CO2, released by several decarboxylation processes, e.g., respiration, photorespiration, pentose phosphate pathway, glycolate cycle, among other metabolic processes, might be redirected to synthesis and emission of isoprene at conditions limiting to the photosynthesis. The internal recycling of CO2 is considered an important mechanism that might improve the overall carbon budget and help the plants cope with predicted increases in intensity and frequency of extreme events [108–110]. In C3 plants under low [CO2], the release of photorespired CO2 represented 85-89% of total decarboxylation reactions, while the release of respired CO2 mitochondrial represented only 11-15% of total decarboxylation reactions [111]. Estimates of CO2 re-fixation suggest that intracellular re-assimilation in C3 plants ranges from 24% to 50% [109,112] or more than 80% of the photorespiratory rate [110]. The highest reassimilation rates result in increased photosynthesis; the use of this mechanism is seen occurring across a number of species [109,110,112]. Dynamic 13C-pulse-chase experiments under photorespiratory CO2 concentration have demonstrated that the recycling mechanisms in the CB cycle is also linked to fluxes of C1intermediates metabolites [48,106]. Upon [2-13C] glycine feeding, the emissions of both labelled [13C1-2]isoprene and 13CO2 were strongly stimulated by increases in leaf temperature at low CO2 concentrations. By contrast, during 13CO2 labelling, emissions of unlabeled [12C]isoprene were stimulated at temperatures above the optimum for net photosynthesis [33]. Further, feeding leaves with 13C-enriched glucose under CO2-free atmosphere, both isoprene and respiratory CO2 are labeled by 13C coming from the enriched glucose [113], and under drought stress conditions, a positive relationship between isoprene emission rate and dark respiration has been observed [35]. Therefore, it is reasonable to postulate that the internal transport and the re-assimilated products of internal sources of CO2 may be channeled to the reactions of the MEP pathway, at least partially supporting the synthesis of isoprene, especially under conditions that impair photosynthetic processes [30,46,48,106].
A strong incorporation of the 13C-isoprene label was also found in branches fed with 13C-methanol (up to 12% emitted as [13C1]isoprene), 13C-formate (up to 38.5% emitted as [13C1]isoprene) and13C-formaldehyde (up to 23.8% emitted as [13C1]isoprene). During the labelling experiments, emission rates of [13C1]isoprene were positively correlated with the percentage of 13CO2 under all [13C]-labelled C1 intermediates, suggesting that the re-assimilation of C1 units and internal carbon pools in the CB cycle can provide an alternative carbon source for MEP pathway. C1 compounds, such as methanol, formaldehyde and formate, are involved in the photosynthetic process through conversion of these compounds to CO2 and its re-assimilation in the CB cycle [104,105]. The relationship between C1 metabolism and photosynthetic processes is corroborated by the identification of the enzyme formate dehydrogenase within the chloroplasts [104], where its activity appears to be stimulated in the presence of C1 metabolites. It is believed that formaldehyde could be oxidized to formate, first and subsequently consumed by formate dehydrogenase within chloroplasts. Thus, these results suggest that isoprene biosynthesis may be linked to C1 pathways via reassimilation of CO2 release in plants cells.
Partitioning carbon and energy under the influence of CO2 and temperature
A further new hypothesis has been used to explain the CO2 suppression effect on isoprene emissions is based in the triose phosphate use (TPU) limitation of photosynthesis [131]. At higher CO2 concentration, photosynthesis can be also feedback-limited by decrease of plastidial Pi pool which is determined by the restriction in triose phosphate utilization in end-product synthesis downstream of the light-dependent photosynthetic reactions [132]. It has been suggested that decrease of the stromal Pi pool cannot only not limit the ATP synthesis but, compromise the exchange of intermediates between the cytosol and chloroplast via antiporter translocator [97]. The suppression of the isoprene emission rate by elevated CO2 has also been proposed to be a result of a limited supply of the cytosolic PEP into chloroplast as result of a decrease of the stromal Pi pool, which might compromise the exchange of PEP between the cytosol and chloroplasts via the antiporter PEP/Pi translocator [107,131] (Figure 2). On one hand, the suppression of isoprene emission has also been observed even in Pi-enriched leaves at high CO2 conditions [133,134] combined with the apparent enhanced levels of pyruvate and decline of ADP levels. In the short term, the limitation of photosynthesis by the restriction of triose phosphate use (situation in which the stromal Pi pool drops), the flow of extrachloroplastic carbon may be changed, but until the restriction of triose phosphate use occurs, the yield of sugar phosphates from the chloroplast will equal the amount of PEP entering the chloroplast. However, it is possible that long-term effects of environmental factors may result in changes in Pi allocation within the plant and thus modify the relationship between photosynthetic mechanisms and the synthesis of isoprene [133]. It has been confirmed that these changes in the intracellular Pi concentrations can affect the balance in the synthesis of starch, sucrose, protein, and chlorophyll content, as well as transport of intermediate metabolites across cell membranes [97,132,135].
A strong negative correlation between isoprene emission rate and PEP carboxylase activity [43,44], combined with a large decrease in leaf DMADP content in elevated CO2 experiments has been observed [57,136]. This inverse relationship between isoprene emission rate and activity of PEP carboxylase has also been observed in a number of experiments, e.g. in drought-stress treatments, and in a free air CO2 enrichment experiment (FACE), through changes in O3, light, temperature [46,137]. Application of a PEP carboxylase inhibitor in excised leaves of Populus deltoides resulted in an increase in the isoprene emission rate and leaf DMADP content [44], supporting the hypothesis that isoprene emission responds to cytosolic PEP availability. However, this result has not been confirmed in subsequent studies, and the PEP limitation hypothesis is also not consistent with other evidence [28,36,66,131,138]. In particular, the specific inhibition of PEP carboxylase by e.g. malate, diethyloxaloacetate (DOA), or 3,3-dichloro-2-(dihydroxyphosphinoylmethyl)-propeonate (DCDP), can also reduce photosynthetic activity in both C3 and C4 species [44,66]. It has been recently observed that both isoprene emission rate and DMADP pool size decrease in malate-fed leaves at either ambient or elevated CO2 [66]. In contrast, the so-called ‘dark pool’ of MEP pathway intermediates (thought to be provided by ME-cDP [139]) was much greater in malate-inhibited leaves [66]. Inhibition of PEP carboxylase by DOA resulted in rise of leaf PEP, but did not reverse the inhibition of isoprene by high CO2. This result is inconsistent with the PEP control theory, whereby the inhibition of PEP carboxylase increases isoprene emission due to greater availability of PEP. Given that malate feeding suppresses both photosynthetic processes and isoprene emission in parallel, it is suggested that suppression under high CO2 is due to decreased availability of reductive equivalents and energy [57]. Indeed, reductions in isoprene emission rate and DMADP pool size were correlated to the observed feedback inhibition on electron transport upon malate feeding [66]. This evidence implies that isoprene emission may not only be limited by supply of PEP/pyruvate from cytosol, but also by ATP and NADPH availability.
The sensitivity of isoprene emission to high CO2 is reduced or absent as leaf temperature increases [67,138,140,141]. This is due to the fact that, at high temperatures and with high CO2, the electron transport rate is higher than with normal CO2 and at low temperatures. The maximum temperature for photosynthetic electron transport increases with CO2 levels, and this might result in a higher supply of energy for isoprene synthesis; this occurs together with increased catalytic activity of isoprene synthase in response to the increase in temperature [141]. However, as the temperature rises above the thermal optimum for photosynthesis, availability of photosynthate for the MEP pathway may decrease [142]. It is also suggested that the lack or loss of the CO2 sensitivity in isoprene emission at higher temperatures is not compatible with the hypothesis of limited transport of PEP to chloroplasts by the PEP/Pi translocator [67]. As temperature increases, an enhanced PEP carboxylase activity is expected, further reducing the cytosolic PEP pool. In addition, the increased temperature stimulates sucrose synthesis, which enhances carbon influx to the cytosol - this might relieve the Pi-limited rates of CO2 assimilation [143]. Thus, increased carbon export in order to meet the high sucrose production rates results in a continuous recycling of Pi to the chloroplast and increases both PEP/Pi transporter activity and ATP formation (Figure 3c,d). In fact, ATP limitation increases with increasing internal [CO2], but under elevated temperatures, this limitation is rather low [144]. Accordingly, the suppression of isoprene emission is reduced as temperature increases at elevated [CO2] and it may be associated with higher turnover rate of sugar-phosphate and the continuous recycling of Pi to the chloroplast. This effect may result in increased formation and supplementation of energetic cofactors for the reducing equivalent-dependent regulation of MEP enzyme activity, thus sustaining the production of DMADP [145].
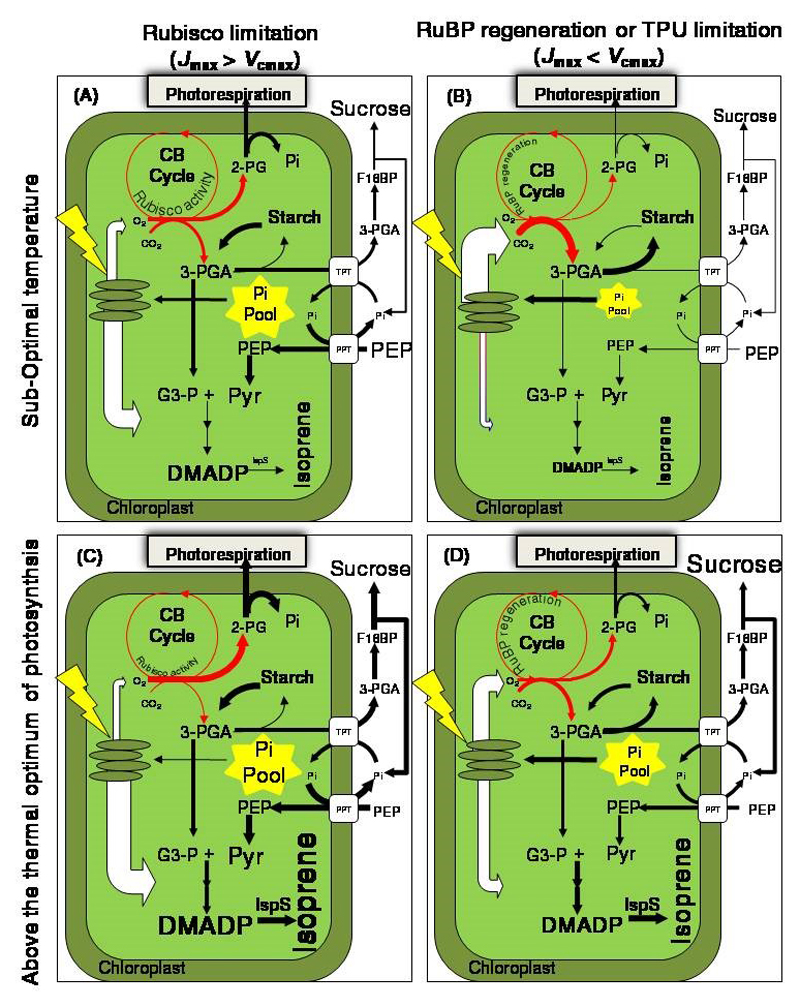
Figure 3.
Schematic representation of the processes underlying the proposed hypothesis of the temperature and CO2 effects on isoprene synthesis. (A) and (B) correspond to the sub-optimal temperature of photosynthesis, and (C) and (D) to the temperature above the thermal optimum of photosynthesis at low (A, C) and high CO2 (B, D) concentrations. Under low [CO2], the light-saturated photosynthesis is Rubisco limited (Jmax>Vcmax; see glossary for definition), while under high [CO2], photosynthesis it is limited by ribulose-1,5-bisphosphate (RuBP) regeneration or triose phosphate utilization (Jmax<Vcmax), especially under low temperatures. The energy in excess to that used for CO2 assimilation is available for isoprene production and photorespiration. The energy partitioning to CO2 and O2 reduction in the Calvin-Benson (CB) cycle, and MEP pathway is represented by white arrows. The Pi pool size is higher at low [CO2] than at high [CO2] and this could favor enhanced involvement of extrachloroplastic PEP sources via PEP/phosphate translocator (PPT). The cytosolic PEP pool size is lower at higher temperatures due to the greater activity of PEP carboxylase enzyme. However, heat stress can trigger enhanced starch and sucrose turnover and, thus, a greater net release of Pi from sugar phosphates. Under higher temperature and supra-optimum [CO2], greater availability of carbon intermediates and photosynthetic energy can increase MEP pathway activity. This results in increases in DMADP availability and, together with the strong effect of temperature on isoprene synthase activity (IspS), this enhances the rate of isoprene synthesis. Note the thicker arrows indicate a higher flow of intermediaries (black arrows) and energy (white arrows) in response to the change in temperature and CO2 concentration. Metabolites: 2-PG, 2-phosphoglycolate; F16BP, fructose 1,6-bisphosphate. Explanations of other abbreviations are given in the figures 1 and 2.
The optimal temperature threshold of leaf physiological status coupled with other strong environmental drivers (e.g. water deficit) can determine the extent of the CO2 suppression of isoprene synthesis. A species best adapted to physiologically high temperatures, subjected to rising [CO2], can increase its tolerance or even the potential for stimulation of photosynthetic processes [146]. Improved function of light reactions in parallel with increase in demand for ATP and NADPH by the reactions of the CB cycle can reduce the share of photosynthetic electron flow going to isoprene synthesis [57,68]. It is well known that the strong effect of temperature MEP pathway enzymes and especially isoprene synthase, can counterbalance the suppressive effects of CO2 [141]. However, MEP pathway enzymes exhibit slight differences in temperature dependency as well as different affinities for their respective substrates and cofactors [15,147]. In the case of extreme stress, impaired availability of cofactors can inhibit MEP pathway flux, resulting in an accumulation of MEP pathway intermediates (e.g. ME-cDP; [147]) and a decrease of carbon availability for isoprene production [24,147]. Consistent with this, under conditions that restrict photosynthesis, the use of older (stored) carbon sources can supply the required substrates for DMADP production [34]. Heat stress can trigger higher rates of sucrose and starch turnover, and thus could counterbalance a possible competition for triose phosphate by stimulating sucrose synthesis, as well as greater net release of Pi from sugar-phosphates. Long-term changes in isoprene emission in response to environmental factors can be linked to both energy cofactor availability and involvement of stored carbon sources. On the other hand, possible alternative routes for pyruvate synthesis inside the chloroplasts [148–151], or pyruvate import from the cytosol via plastid Na+-coupled pyruvate transporter [152] can provide substrates. In addition, cytosolic pyruvate can be reduced to malate, shuttled into plastids through the malate valve [119,151,153], and converted again into pyruvate, where it can be used as carbon source for isoprenoid formation (Figure 2).
Concluding Remarks and Future Prospects
We have highlighted advances in the knowledge of how carbon is allocated to isoprene and other volatile isoprenoid production in the context of chloroplast and whole cell metabolism. We reviewed the evidence suggesting that alternative, not recently-assimilated, carbon sources can contribute to isoprene synthesis. The available evidence conclusively demonstrates that the MEP pathway can operate on multiple carbon sources, provided that photochemical reactions continue to supply ATP and reducing power to the MEP pathway. Regardless of the relative proportion of these carbon sources, the substrate supply and formation of isoprene are often maintained when the rate of photosynthesis is suppressed. Among the alternative carbon sources, recent work suggests that a major part might result from the activation of carbon flux through the glucose-6P shunt around the Calvin-Benson cycle that depends on the existing pools of glucose phosphate. The relative contribution of the alternative carbon sources is greater under photosynthesis-limiting conditions, and the key question is what determines the share of these different carbon sources to the MEP pathway.
The sensitivity of isoprene emission to atmospheric [CO2] and temperature varies among species. Due to the close relationship between the MEP pathway and photosynthetic processes, it is possible that the degree of sensitivity of emission rates in response to changes in temperature and [CO2] is determined by the species capacity to maintain high pools of MEP pathway intermediates and responsiveness of partial processes of photosynthesis to atmospheric [CO2] and temperature. Furthermore, there are important interactive effects among environmental drivers operating at different time scales, and the extent to which high [CO2] can suppress the synthesis of isoprene can depend on the adaptation and acclimation responses of some species to thermally contrasting environments.
Supplementary Material
Acknowledgements
We are grateful to the National Institute of Amazonian Research (MCTI-INPA) and the National Council for Scientific and Technological Development (CNPq, Brazil) for financially supporting our work on isoprene emission. This work was also supported by funding from Coordination for the Improvement of Higher Level Personnel (CAPES, Brazil) and Amazonas State Research Support Foundation (FAPEAM, Brazil). Research fellowships granted by CNPq-Brazil to W.L.A. and J.F.C.G. are also gratefully acknowledged. C.E.V. was supported by a Queensland Government Acce Fellowship, and Ü.N. and B.R. by the European Commission through the European Research Council (advanced grant 322603, SIP VOL+), the European Regional Development Fund (Center of Excellence EcolChange) and the Estonian Ministry of Science and Education (institutional grant IUT-8-3).
Footnotes
Conflicts of Interest:
The authors declare that there are no conflicts of interest
References
- 1.Kessler A. Introduction to a Virtual Special Issue on plant volatiles. New Phytol. 2016;209:1333–1337. doi: 10.1111/nph.13854. [DOI] [PubMed] [Google Scholar]
- 2.Dudareva N, et al. Plant Volatiles : Recent Advances and Future Perspectives. Landscape. 2006 doi: 10.1080/07352680600899973. [DOI] [Google Scholar]
- 3.Guenther AB, et al. The model of emissions of gases and aerosols from nature version 2.1 (MEGAN2.1): An extended and updated framework for modeling biogenic emissions. Geosci Model Dev. 2012;5:1471–1492. [Google Scholar]
- 4.Henrot A-J, et al. Implementation of the MEGAN (v2.1) biogenic emission model in the ECHAM6-HAMMOZ chemistry climate model. Geosci Model Dev. 2017;10:903–926. [Google Scholar]
- 5.Unger N. Isoprene emission variability through the twentieth century. J Geophys Res Atmos. 2013;118:13,606–13,613. [Google Scholar]
- 6.Bregonzio-Rozier L, et al. Secondary organic aerosol formation from isoprene photooxidation during cloud condensation-evaporation cycles. Atmos Chem Phys. 2016;16:1747–1760. [Google Scholar]
- 7.Fini A, et al. Isoprene responses and functions in plants challenged by environmental pressures associated to climate change. Front Plant Sci. 2017;8:1281. doi: 10.3389/fpls.2017.01281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loivamäki M, et al. Isoprene interferes with the attraction of bodyguards by herbaceous plants. Proc Natl Acad Sci. 2008;105:17430–17435. doi: 10.1073/pnas.0804488105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rasmussen RA. Isoprene: Identified as a Forest-Type Emission to the Atmosphere. Environ Sci Technol. 1970;4:667–671. [Google Scholar]
- 10.Sharkey TD, Monson RK. Isoprene research - 60 years later, the biology is still enigmatic. Plant, Cell Environ. 2017;40:1671–2028. doi: 10.1111/pce.12930. [DOI] [PubMed] [Google Scholar]
- 11.Perez-Gil J, et al. Formation of isoprenoids. In: Geiger O, editor. Handbook of Hydrocarbon and Lipid Microbiology. Vol. 5. Springer; 2017. pp. 1–29. [Google Scholar]
- 12.Kirby J, et al. Enhancing Terpene Yield from Sugars via Novel Routes to 1-Deoxy-D-Xylulose 5-Phosphate. Appl Environ Microbiol. 2015;81:130–138. doi: 10.1128/AEM.02920-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moore BD, et al. Tansley review Explaining intraspecific diversity in plant secondary metabolites in an ecological context. New Phytol. 2014;201:733–750. doi: 10.1111/nph.12526. [DOI] [PubMed] [Google Scholar]
- 14.Pichersky E, Raguso RA. Why do plants produce so many terpenoid compounds? New Phytol. 2016 doi: 10.1111/nph.14178. [DOI] [PubMed] [Google Scholar]
- 15.Vranová E, et al. Network analysis of the MVA and MEP pathways for isoprenoid synthesis. Annu Rev Plant Biol. 2013;64:665–700. doi: 10.1146/annurev-arplant-050312-120116. [DOI] [PubMed] [Google Scholar]
- 16.Hemmerlin A, et al. A raison d’être for two distinct pathways in the early steps of plant isoprenoid biosynthesis? Prog Lipid Res. 2012;51:95–148. doi: 10.1016/j.plipres.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 17.Harrison SP, et al. Volatile isoprenoid emissions from plastid to planet. New Phytol. 2013;197:49–57. doi: 10.1111/nph.12021. [DOI] [PubMed] [Google Scholar]
- 18.Dani KGS, et al. Do cytokinins, volatile isoprenoids and carotenoids synergically delay leaf senescence? Plant Cell and Environment. 2016;39:1103–1111. doi: 10.1111/pce.12705. Wiley/Blackwell (10.1111) [DOI] [PubMed] [Google Scholar]
- 19.Owen SM, Peñuelas J. Opportunistic emissions of volatile isoprenoids. Trends Plant Sci. 2005;10:420–6. doi: 10.1016/j.tplants.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 20.Ruiz-Sola MÁ, Rodríguez-Concepción M. Carotenoid biosynthesis in Arabidopsis: a colorful pathway. Arabidopsis Book. 2012;10:e0158. doi: 10.1199/tab.0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Opitz S, et al. Both methylerythritol phosphate and mevalonate pathways contribute to biosynthesis of each of the major isoprenoid classes in young cotton seedlings. Phytochemistry. 2014;98:110–119. doi: 10.1016/j.phytochem.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 22.Kasahara H, et al. Contribution of the mevalonate and methylerythritol phosphate pathways to the biosynthesis of gibberellins in Arabidopsis. J Biol Chem. 2002;277:45188–94. doi: 10.1074/jbc.M208659200. [DOI] [PubMed] [Google Scholar]
- 23.Sharkey TD, et al. Isoprene Emission from Plants: Why and How. Ann Bot. 2008;101:5–18. doi: 10.1093/aob/mcm240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghirardo A, et al. Metabolic flux analysis of plastidic isoprenoid biosynthesis in poplar leaves emitting and nonemitting Isoprene. Plant Physiol. 2014;165:37–51. doi: 10.1104/pp.114.236018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pokhilko A, et al. Mathematical modelling of the diurnal regulation of the MEP pathway in Arabidopsis. New Phytol. 2015;206:1075–1085. doi: 10.1111/nph.13258. [DOI] [PubMed] [Google Scholar]
- 26.Niinemets Ü, et al. Controls of the quantum yield and saturation light of isoprene emission in different-aged aspen leaves. Plant, Cell Environ. 2015;38:2707–2720. doi: 10.1111/pce.12582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dani S, et al. Increased ratio of electron transport to net assimilation rate supports elevated isoprenoid emission rate in eucalypts under drought. Plant Physiol. 2014;166:1059–1072. doi: 10.1104/pp.114.246207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rasulov B, et al. Spectacular oscillations in plant isoprene emission under transient conditions explain the enigmatic CO2 Response. Plant Physiol. 2016;172:2275–2285. doi: 10.1104/pp.16.01002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Affek HP, Yakir D. Natural abundance carbon isotope composition of isoprene reflects incomplete coupling between isoprene synthesis and photosynthetic carbon flow. Plant Physiol. 2003;131:1727–1736. doi: 10.1104/pp.102.012294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loreto F, et al. 13C labeling reveals chloroplastic and extrachloroplastic pools of dimethylallyl pyrophosphate and their contribution to isoprene formation. Plant Physiol. 2004;135:1903–1907. doi: 10.1104/pp.104.039537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Funk JL, et al. Stress-induced changes in carbon sources for isoprene production in Populus deltoides. Plant Cell Environ. 2004;27:747–755. [Google Scholar]
- 32.Delwiche CF, Sharkey TD. Rapid appearance of 13C in biogenic isoprene when 13CO2 is fed to intact leaves. Plant Cell Environ. 1993;16:587–591. [Google Scholar]
- 33.Karl T, et al. On-line analysis of the 13CO2 labeling of leaf isoprene suggests multiple subcellular origins of isoprene precursors. Planta. 2002;215:894–905. doi: 10.1007/s00425-002-0825-2. [DOI] [PubMed] [Google Scholar]
- 34.Jud W, et al. Effects of heat and drought stress on post-illumination bursts of volatile organic compounds in isoprene-emitting and non-emitting poplar. Plant, Cell Environ. 2016;39:1204–1215. doi: 10.1111/pce.12643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brilli F, et al. Response of isoprene emission and carbon metabolism to drought in white poplar (Populus alba) saplings. New Phytol. 2007;175:244–254. doi: 10.1111/j.1469-8137.2007.02094.x. [DOI] [PubMed] [Google Scholar]
- 36.Trowbridge AM, et al. Contribution of various carbon sources toward isoprene biosynthesis in poplar leaves mediated by altered atmospheric CO2 concentrations. PLoS One. 2012;7 doi: 10.1371/journal.pone.0032387. e32387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schnitzler J-P, et al. Contribution of different carbon sources to isoprene biosynthesis in poplar leaves. Plant Physiol. 2004;135:152–160. doi: 10.1104/pp.103.037374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu C, et al. 13C labelling study of constitutive and stress-induced terpenoid emissions from Norway spruce and Scots pine. Biogeosciences Discuss. 2017 [Google Scholar]
- 39.Loreto F, et al. Different sources of reduced carbon contribute to form three classes of terpenoid emitted by Quercus ilex L. leaves. Proc Natl Acad Sci. 1996;93:9966–9969. doi: 10.1073/pnas.93.18.9966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hemmerlin A, et al. A cytosolic Arabidopsis D-xylulose kinase catalyzes the phosphorylation of 1-deoxy-D-xylulose into a precursor of the plastidial isoprenoid pathway. Plant Physiol. 2006;142:441–457. doi: 10.1104/pp.106.086652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mayrhofer S, et al. Carbon balance in leaves of young poplar trees. Plant Biol. 2004;6:730–739. doi: 10.1055/s-2004-821268. [DOI] [PubMed] [Google Scholar]
- 42.Kreuzwieser J, et al. Xylem-transported glucose as an additional carbon source for leaf isoprene formation in Quercus robur. New Phytol. 2002;156:171–178. doi: 10.1046/j.1469-8137.2002.00516.x. [DOI] [PubMed] [Google Scholar]
- 43.Possell M, Hewitt CN. Isoprene emissions from plants are mediated by atmospheric CO2 concentrations. Glob Chang Biol. 2011;17:1595–1610. [Google Scholar]
- 44.Rosenstiel TN, et al. Increased CO2 uncouples growth from isoprene emission in an agriforest ecosystem. Nature. 2003;421:256–259. doi: 10.1038/nature01312. [DOI] [PubMed] [Google Scholar]
- 45.Dani KGS, Loreto F. Trade-off between dimethyl sulfide and isoprene emissions from marine phytoplankton. Trends Plant Sci. 2017;22:1–12. doi: 10.1016/j.tplants.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 46.Loreto F, et al. The relationship between isoprene emission rate and dark respiration rate in white poplar (Populus alba L.) leaves. Plant Cell Environ. 2007;30:662–669. doi: 10.1111/j.1365-3040.2007.01648.x. [DOI] [PubMed] [Google Scholar]
- 47.Srikanta Dani KG, et al. De novo post-illumination monoterpene burst in Quercus ilex (holm oak) Planta. 2017;245:459–465. doi: 10.1007/s00425-016-2636-x. [DOI] [PubMed] [Google Scholar]
- 48.Jardine K, et al. Dynamic balancing of isoprene carbon sources reflects photosynthetic and photorespiratory responses to temperature stress. Plant Physiol. 2014;166:2051–2064. doi: 10.1104/pp.114.247494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Srikanta Dani KG, et al. Species-specific photorespiratory rate, drought tolerance and isoprene emission rate in plants. Plant Signal Behav. 2015;10:1–3. doi: 10.4161/15592324.2014.990830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Szecowka M, et al. Metabolic fluxes in an illuminated Arabidopsis rosette. Plant Cell. 2013;25:694–714. doi: 10.1105/tpc.112.106989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sharkey TD, Weise SE. The glucose 6-phosphate shunt around the Calvin-Benson cycle. J Exp Bot. 2016;67:4067–4077. doi: 10.1093/jxb/erv484. [DOI] [PubMed] [Google Scholar]
- 52.Neuhaus HE, Schulte N. Starch degradation in chloroplasts isolated from C3 or CAM (crassulacean acid metabolism)-induced Mesembryanthemum crystallinum L. Biochem J. 1996;318(Pt 3):945–953. doi: 10.1042/bj3180945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weise SE. Carbon Balance and Circadian Regulation of Hydrolytic and Phosphorolytic Breakdown of Transitory Starch. Plant Physiol. 2006;141:879–886. doi: 10.1104/pp.106.081174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Monson RK, et al. Why only some plants emit isoprene. Plant Cell Environ. 2013;36:503–16. doi: 10.1111/pce.12015. [DOI] [PubMed] [Google Scholar]
- 55.Li M, et al. In Planta Recapitulation of Isoprene Synthase Evolution from Ocimene Synthases. Mol Biol Evol. 2017;34:2583–2599. doi: 10.1093/molbev/msx178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Velikova V, et al. Increased thermostability of thylakoid membranes in isoprene-emitting leaves probed with three biophysical techniques. Plant Physiol. 2011;157:905–16. doi: 10.1104/pp.111.182519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rasulov B, et al. Evidence that light, carbon dioxide, and oxygen dependencies of leaf isoprene emission are driven by energy status in hybrid aspen. Plant Physiol. 2009;151:448–60. doi: 10.1104/pp.109.141978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Magel E, et al. Photosynthesis and substrate supply for isoprene biosynthesis in poplar leaves. Atmos Environ. 2006;40:138–151. [Google Scholar]
- 59.Wiberley AE, et al. Regulation of isoprene emission from poplar leaves throughout a day. Plant Cell Environ. 2009;32:939–947. doi: 10.1111/j.1365-3040.2009.01980.x. [DOI] [PubMed] [Google Scholar]
- 60.Paparelli E, et al. Nighttime sugar starvation orchestrates gibberellin biosynthesis and plant growth in Arabidopsis. Plant Cell. 2013;25:3760–3769. doi: 10.1105/tpc.113.115519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kölling K, et al. Carbon partitioning in Arabidopsis thaliana is a dynamic process controlled by the plants metabolic status and its circadian clock. Plant Cell Environ. 2015;38:1965–1979. doi: 10.1111/pce.12512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Loivamäki M, et al. Circadian rhythms of isoprene biosynthesis in grey poplar leaves. Plant Physiol. 2007;143:540–551. doi: 10.1104/pp.106.092759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mayrhofer S, et al. Diurnal and seasonal variation of isoprene biosynthesis-related genes in grey poplar leaves. Plant Physiol. 2005;139:474–484. doi: 10.1104/pp.105.066373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Keenan TF, Niinemets Ü. Circadian control of global isoprene emissions. Nat Geosci. 2012;5:435. [Google Scholar]
- 65.Peñuelas J, et al. Sensing the energetic status of plants and ecosystems. Trends Plant Sci. 2015;20:528–530. doi: 10.1016/j.tplants.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 66.Rasulov B, et al. Evidence that isoprene emission is not limited by cytosolic metabolites. Exogenous malate does not invert the reverse sensitivity of isoprene emission to high [CO2] Plant Physiol. 2018;176:1–14. doi: 10.1104/pp.17.01463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sun Z, et al. Elevated [CO2] magnifies isoprene emissions under heat and improves thermal resistance in hybrid aspen. J Exp Bot. 2013;64:5509–5523. doi: 10.1093/jxb/ert318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Morfopoulos C, et al. A model of plant isoprene emission based on available reducing power captures responses to atmospheric CO2. New Phytol. 2014;203:125–139. doi: 10.1111/nph.12770. [DOI] [PubMed] [Google Scholar]
- 69.Rosenstiel TN, et al. Induction of poplar leaf nitrate reductase: A test of extrachloroplastic control of isoprene emission rate. Plant Biol. 2004;6:12–21. doi: 10.1055/s-2003-44722. [DOI] [PubMed] [Google Scholar]
- 70.Peñuelas J, Llusià J. Linking photorespiration, monoterpenes and thermotolerance in Quercus. New Phytol. 2002;155:227–237. [Google Scholar]
- 71.Harris A, et al. Constitutive changes in pigment concentrations: Implications for estimating isoprene emissions using the photochemical reflectance index. Physiol Plant. 2016;156:190–200. doi: 10.1111/ppl.12361. [DOI] [PubMed] [Google Scholar]
- 72.Télef N, et al. Sucrose deficiency delays lycopene accumulation in tomato fruit pericarp discs. Plant Mol Biol. 2006;62:453–469. doi: 10.1007/s11103-006-9033-y. [DOI] [PubMed] [Google Scholar]
- 73.Flores-Pérez U, et al. Pleiotropic regulatory locus 1 (PRL1) integrates the regulation of sugar responses with isoprenoid metabolism in Arabidopsis. Mol Plant. 2010;3:101–112. doi: 10.1093/mp/ssp100. [DOI] [PubMed] [Google Scholar]
- 74.Hemmerlin A. Post-translational events and modifications regulating plant enzymes involved in isoprenoid precursor biosynthesis. Plant Sci. 2013;203–204:41–54. doi: 10.1016/j.plantsci.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 75.Rodríguez-Concepción M. Supply of precursors for carotenoid biosynthesis in plants. Arch Biochem Biophys. 2010;504:118–22. doi: 10.1016/j.abb.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 76.Nemeth K, et al. Pleiotropic control of glucose and hormone responses by PRL1, a nuclear WD protein, in Arabidopsis. Genes Dev. 1998;12:3059–3073. doi: 10.1101/gad.12.19.3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bhalerao RP, et al. Regulatory interaction of PRL1 WD protein with Arabidopsis SNF1-like protein kinases. Proc Natl Acad Sci. 1999;96:5322–5327. doi: 10.1073/pnas.96.9.5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bick JA, Lange BM. Metabolic cross talk between cytosolic and plastidial pathways of isoprenoid biosynthesis: unidirectional transport of intermediates across the chloroplast envelope membrane. Arch Biochem Biophys. 2003;415:146–154. doi: 10.1016/s0003-9861(03)00233-9. [DOI] [PubMed] [Google Scholar]
- 79.Laule O, et al. Crosstalk between cytosolic and plastidial pathways of isoprenoid biosynthesis in Arabidopsis thaliana. Proc Natl Acad Sci U S A. 2003;100:6866–6871. doi: 10.1073/pnas.1031755100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Flügge U-I, Gao W. Transport of isoprenoid intermediates across chloroplast envelope membranes. Plant Biol. 2005;7:91–97. doi: 10.1055/s-2004-830446. [DOI] [PubMed] [Google Scholar]
- 81.Sanmiya K, et al. Localization of farnesyl diphosphate synthase in chloroplasts. Plant Cell Physiol. 1999;40:348–354. doi: 10.1093/oxfordjournals.pcp.a029549. [DOI] [PubMed] [Google Scholar]
- 82.Pazouki L, Niinemets Ü. Multi-Substrate Terpene Synthases: Their Occurrence and Physiological Significance. Front Plant Sci. 2016;7:1–16. doi: 10.3389/fpls.2016.01019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Thabet I, et al. The subcellular localization of periwinkle farnesyl diphosphate synthase provides insight into the role of peroxisome in isoprenoid biosynthesis. J Plant Physiol. 2011;168:2110–2116. doi: 10.1016/j.jplph.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 84.Vickers CE, et al. A unified mechanism of action for volatile isoprenoids in plant abiotic stress. Nat Chem Biol. 2009;5:283–91. doi: 10.1038/nchembio.158. [DOI] [PubMed] [Google Scholar]
- 85.Bonk M, et al. Chloroplast Import of Four Carotenoid Biosynthetic Enzymes In Vitro Reveals Differential Fates Prior to Membrane Binding and Oligomeric Assembly. Eur J Biochem. 1997;247:942–950. doi: 10.1111/j.1432-1033.1997.00942.x. [DOI] [PubMed] [Google Scholar]
- 86.Gutensohn M, et al. Cytosolic monoterpene biosynthesis is supported by plastid-generated geranyl diphosphate substrate in transgenic tomato fruits. Plant J. 2013;75:351–363. doi: 10.1111/tpj.12212. [DOI] [PubMed] [Google Scholar]
- 87.Skorupinska-Tudek K, et al. Contribution of the mevalonate and methylerythritol phosphate pathways to the biosynthesis of dolichols in plants. J Biol Chem. 2008;283:21024–21035. doi: 10.1074/jbc.M706069200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rasulov B, et al. Bisphosphonate inhibitors reveal a large elasticity of plastidic isoprenoid synthesis pathway in isoprene-emitting hybrid aspen. Plant Physiol. 2015;168:532–548. doi: 10.1104/pp.15.00470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hemmerlin A, et al. Cross-talk between the cytosolic mevalonate and the plastidial methylerythritol phosphate pathways in tobacco bright yellow-2 cells. J Biol Chem. 2003;278:26666–26676. doi: 10.1074/jbc.M302526200. [DOI] [PubMed] [Google Scholar]
- 90.González-Cabanelas D, et al. The diversion of 2- C -methyl- d -erythritol-2,4-cyclodiphosphate from the 2- C -methyl- d -erythritol 4-phosphate pathway to hemiterpene glycosides mediates stress responses in Arabidopsis thaliana. Plant J. 2015;82:122–137. doi: 10.1111/tpj.12798. [DOI] [PubMed] [Google Scholar]
- 91.Rasulov B, et al. Competition between isoprene emission and pigment synthesis during leaf development in aspen. Plant, Cell Environ. 2014;37:724–741. doi: 10.1111/pce.12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wright LP, et al. Deoxyxylulose 5-Phosphate Synthase Controls Flux through the Methylerythritol 4-Phosphate Pathway in Arabidopsis. Plant Physiol. 2014;165:1488–1504. doi: 10.1104/pp.114.245191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xiao Y, et al. Retrograde signaling by the plastidial metabolite MEcPP regulates expression of nuclear stress-response genes. Cell. 2012;149:1525–35. doi: 10.1016/j.cell.2012.04.038. [DOI] [PubMed] [Google Scholar]
- 94.Lemos M, et al. The plastidial retrograde signal methyl erythritol cyclopyrophosphate is a regulator of salicylic acid and jasmonic acid crosstalk. J Exp Bot. 2016;67:1557–1566. doi: 10.1093/jxb/erv550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ward JL, et al. Metabolomic analysis of Arabidopsis reveals hemiterpenoid glycosides as products of a nitrate ion-regulated, carbon flux overflow. Proc Natl Acad Sci. 2011;108:10762–10767. doi: 10.1073/pnas.1018875108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.González-Cabanelas D, et al. The diversion of 2-C-methyl-D-erythritol-2,4-cyclodiphosphate from the 2-C-methyl-D-erythritol 4-phosphate pathway to hemiterpene glycosides mediates stress responses in Arabidopsis thaliana. Plant J. 2015;82:122–137. doi: 10.1111/tpj.12798. [DOI] [PubMed] [Google Scholar]
- 97.Flügge U-I. Phosphate Translocators in Platids. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:27–45. doi: 10.1146/annurev.arplant.50.1.27. [DOI] [PubMed] [Google Scholar]
- 98.Eicks M, et al. The Plastidic Pentose Phosphate Translocator Represents a Link between the Cytosolic and the Plastidic Pentose Phosphate Pathways in Plants. Plant Physiol. 2002;128:512–522. doi: 10.1104/pp.010576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Estévez JM, et al. Analysis of the expression of CLA1, a gene that encodes the 1-deoxyxylulose 5-phosphate synthase of the 2-C-methyl-D-erythritol-4-phosphate pathway in Arabidopsis. Plant Physiol. 2000;124:95–104. doi: 10.1104/pp.124.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Laule O, et al. Crosstalk between cytosolic and plastidial pathways of isoprenoid biosynthesis in Arabidopsis thaliana. Proc Natl Acad Sci U S A. 2003;100:6866–71. doi: 10.1073/pnas.1031755100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ghirardo A, et al. Metabolic flux analysis of plastidic isoprenoid biosynthesis in poplar leaves emitting and nonemitting isoprene. Plant Physiol. 2014;165:37–51. doi: 10.1104/pp.114.236018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bloemen J, et al. Transport of root-respired CO2 via the transpiration stream affects aboveground carbon assimilation and CO2 efflux in trees. New Phytol. 2013;197:555–565. doi: 10.1111/j.1469-8137.2012.04366.x. [DOI] [PubMed] [Google Scholar]
- 103.Schmitz H, et al. Assimilation and metabolism of formaldehyde by leaves appear unlikely to be of value for indoor air purification. New Phytol. 2000;147:307–315. [Google Scholar]
- 104.Olson BJSC, et al. Formate dehydrogenase in Arabidopsis thaliana: Characterization and possible targeting to the chloroplast. Plant Sci. 2000;159:205–212. doi: 10.1016/s0168-9452(00)00337-x. [DOI] [PubMed] [Google Scholar]
- 105.Song Z-B, et al. C1 metabolism and the Calvin cycle function simultaneously and independently during HCHO metabolism and detoxification in Arabidopsis thaliana treated with HCHO solutions. Plant Cell Environ. 2013;36:1490–506. doi: 10.1111/pce.12079. [DOI] [PubMed] [Google Scholar]
- 106.Jardine K, et al. Integration of C1 and C2 metabolism in trees. Int J Mol Sci. 2017;18:2045. doi: 10.3390/ijms18102045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fischer K, et al. A new class of plastidic phosphate translocators: a putative link between primary and secondary metabolism by the phosphoenolpyruvate/phosphate antiporter. Plant Cell. 1997;9:453–62. doi: 10.1105/tpc.9.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bloemen J, et al. Internal recycling of respired CO2 may be important for plant functioning under changing climate regimes. Plant Signal Behav. 2013;8:197–555. doi: 10.4161/psb.27530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Busch Fa, et al. C3 plants enhance rates of photosynthesis by reassimilating photorespired and respired CO2. Plant Cell Environ. 2013;36:200–212. doi: 10.1111/j.1365-3040.2012.02567.x. [DOI] [PubMed] [Google Scholar]
- 110.Loreto F, et al. Estimation of photorespiratory carbon dioxide recycling during photosynthesis. Aust J Plant Physiol. 1999;26:733–736. [Google Scholar]
- 111.Pärnik T, et al. Photorespiratory and respiratory decarboxylations in leaves of C3 plants under different CO2 concentrations and irradiances. Plant Cell Environ. 2007;30:1535–1544. doi: 10.1111/j.1365-3040.2007.01725.x. [DOI] [PubMed] [Google Scholar]
- 112.Pärnik T, Keerberg O. Advanced radiogasometric method for the determination of the rates of photorespiratory and respiratory decarboxylations of primary and stored photosynthates under steady-state photosynthesis. Physiol Plant. 2007;129:34–44. [Google Scholar]
- 113.Affek HP, Yakir D. Natural Abundance Carbon Isotope Composition of Isoprene Reflects Incomplete Coupling between Isoprene Synthesis and Photosynthetic Carbon Flow. Plant Physiol. 2013;131:1727–1736. doi: 10.1104/pp.102.012294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jardine KJ, et al. Gas phase measurements of pyruvic acid and its volatile metabolites. Environ Sci Technol. 2010;44:2454–60. doi: 10.1021/es903544p. [DOI] [PubMed] [Google Scholar]
- 115.Jimenez-Lopez JC, et al. The maize ALDH protein superfamily: Linking structural features to functional specificities. BMC Struct Biol. 2010;10 doi: 10.1186/1472-6807-10-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lin M, Oliver DJ. The role of acetyl-coenzyme a synthetase in Arabidopsis. Plant Physiol. 2008;147:1822–1829. doi: 10.1104/pp.108.121269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kotchoni SO, et al. Over-expression of different aldehyde dehydrogenase genes in Arabidopsis thaliana confers tolerance to abiotic stress and protects plants against lipid peroxidation and oxidative stress. Plant, Cell Environ. 2006;29:1033–1048. doi: 10.1111/j.1365-3040.2005.01458.x. [DOI] [PubMed] [Google Scholar]
- 118.Peterhansel C, et al. Photorespiratory bypasses: how can they work? J Exp Bot. 2013;64:709–715. doi: 10.1093/jxb/ers247. [DOI] [PubMed] [Google Scholar]
- 119.Zhao Y, et al. Malate transported from chloroplast to mitochondrion triggers production of ROS and PCD in Arabidopsis thaliana. Cell Res. 2018;28:448–461. doi: 10.1038/s41422-018-0024-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Murphy DJ, Walker DA. Acetyl coenzyme A biosynthesis in the chloroplast - What is the physiological precursor? Planta. 1982;156:84–88. doi: 10.1007/BF00393446. [DOI] [PubMed] [Google Scholar]
- 121.Maier A, et al. Transgenic Introduction of a Glycolate Oxidative Cycle into A. thaliana Chloroplasts Leads to Growth Improvement. Front Plant Sci. 2012;3:1–12. doi: 10.3389/fpls.2012.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Karl T, et al. Transient releases of acetaldehyde from tree leaves-products of a pyruvate overflow mechanism? Plant, Cell Environ. 2002;25:1121–1131. [Google Scholar]
- 123.Jardine KJ, et al. Methanol and isoprene emissions from the fast growing tropical pioneer species Vismia guianensis (Aubl.) Pers. (Hypericaceae) in the central Amazon forest. Atmos Chem Phys. 2016;16:6441–6452. [Google Scholar]
- 124.Owen SM, Peñuelas J. Volatile isoprenoid emission potentials are correlated with essential isoprenoid concentrations in five plant species. Acta Physiol Plant. 2013;35:3109–3125. [Google Scholar]
- 125.Massad TJ, et al. Costs of defense and a test of the carbon-nutrient balance and growth-differentiation balance hypotheses for two co-occurring classes of plant defense. PLoS One. 2012;7 doi: 10.1371/journal.pone.0047554. e47554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Vickers CE, et al. Isoprene synthesis in plants: Lessons from a transgenic tobacco model. Plant, Cell Environ. 2011;34:1043–1053. doi: 10.1111/j.1365-3040.2011.02303.x. [DOI] [PubMed] [Google Scholar]
- 127.Kuhn U, et al. Strong correlation between isoprene emission and gross photosynthetic capacity during leaf phenology of the tropical tree species Hymenaea courbaril with fundamental changes in volatile organic compounds emission composition during early leaf development. Plant, Cell Environ. 2004;27:1469–1485. [Google Scholar]
- 128.Weise SE, et al. Measuring dimethylallyl diphosphate available for isoprene synthesis. Anal Biochem. 2013;435:27–34. doi: 10.1016/j.ab.2012.11.031. [DOI] [PubMed] [Google Scholar]
- 129.Rasulov B, et al. Postillumination isoprene emission: in vivo measurements of dimethylallyldiphosphate pool size and isoprene synthase kinetics in aspen leaves. Plant Physiol. 2009;149:1609–1618. doi: 10.1104/pp.108.133512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sun Z, et al. Enhanced isoprene emission capacity and altered light responsiveness in aspen grown under elevated atmospheric CO2 concentration. Glob Chang Biol. 2012;18:3423–3440. [Google Scholar]
- 131.Li Z, Sharkey T. Molecular and Pathway Controls on Biogenic Volatile Organic Compound Emissions. In: Niinemets Ü, Monson R, editors. Biology, Controls and Models of Tree Volatile Organic Compound Emissions. Vol. 5. Springer; Berlin: 2013. pp. 119–151. [Google Scholar]
- 132.Yang JT, et al. Triose phosphate use limitation of photosynthesis: short-term and long-term effects. Planta. 2016;243:687–698. doi: 10.1007/s00425-015-2436-8. [DOI] [PubMed] [Google Scholar]
- 133.Fares S, et al. Isoprene emission and primary metabolism in Phragmites australis grown under different phosphorus levels. Plant Biol. 2008;10:38–43. doi: 10.1055/s-2007-965429. [DOI] [PubMed] [Google Scholar]
- 134.Fernández-Martínez M, et al. Nutrient-rich plants emit a less intense blend of volatile isoprenoids. New Phytol. 2017 doi: 10.1111/nph.14889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Warren CR. How does P affect photosynthesis and metabolite profiles of Eucalyptus globulus? Tree Physiol. 2011;31:727–739. doi: 10.1093/treephys/tpr064. [DOI] [PubMed] [Google Scholar]
- 136.Niinemets U, Sun Z. How light, temperature, and measurement and growth [CO2] interactively control isoprene emission in hybrid aspen. J Exp Bot. 2011;66:841–851. doi: 10.1093/jxb/eru443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fortunati A, et al. Isoprene emission is not temperature-dependent during and after severe drought-stress: A physiological and biochemical analysis. Plant J. 2008;55:687–697. doi: 10.1111/j.1365-313X.2008.03538.x. [DOI] [PubMed] [Google Scholar]
- 138.Potosnak MJ, et al. Increasing leaf temperature reduces the suppression of isoprene emission by elevated CO2 concentration. Sci Total Environ. 2014;481:352–359. doi: 10.1016/j.scitotenv.2014.02.065. [DOI] [PubMed] [Google Scholar]
- 139.Li Z, Sharkey TD. Metabolic profiling of the methylerythritol phosphate pathway reveals the source of post-illumination isoprene burst from leaves. Plant Cell Environ. 2013;36:429–37. doi: 10.1111/j.1365-3040.2012.02584.x. [DOI] [PubMed] [Google Scholar]
- 140.Monson RK, et al. Interactions between temperature and intercellular CO2 concentration in controlling leaf isoprene emission rates. Plant Cell Environ. 2016;39:2404–2413. doi: 10.1111/pce.12787. [DOI] [PubMed] [Google Scholar]
- 141.Rasulov B, et al. Temperature response of isoprene emission in vivo reflects a combined effect of substrate limitations and isoprene synthase activity: a kinetic analysis. Plant Physiol. 2010;154:1558–1570. doi: 10.1104/pp.110.162081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Li Z, et al. Effect of temperature on postillumination isoprene emission in oak and poplar. Plant Physiol. 2011;155:1037–46. doi: 10.1104/pp.110.167551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sage RF, Sharkey TD. The Effect of Temperature on the Occurrence of O2 and CO2 Insensitive Photosynthesis in Field Grown Plants. Plant Physiol. 1987;84:658–664. doi: 10.1104/pp.84.3.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Loreto F, Sharkey TD. A gas-exchange study of photosynthesis and isoprene emission in Quercus rubra L. Planta. 1990;182:523–531. doi: 10.1007/BF02341027. [DOI] [PubMed] [Google Scholar]
- 145.Frank A, Groll M. The Methylerythritol Phosphate Pathway to Isoprenoids. Chem Rev. 2017;117:5675–5703. doi: 10.1021/acs.chemrev.6b00537. [DOI] [PubMed] [Google Scholar]
- 146.Lloyd J, Farquhar GD. Effects of rising temperatures and [CO2] on the physiology of tropical forest trees. Philos Trans R Soc Lond B Biol Sci. 2008;363:1811–7. doi: 10.1098/rstb.2007.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rivasseau C, et al. Accumulation of 2-C-methyl-D-erythritol 2,4-cyclodiphosphate in illuminated plant leaves at supraoptimal temperatures reveals a bottleneck of the prokaryotic methylerythritol 4-phosphate pathway of isoprenoid biosynthesis. Plant, Cell Environ. 2009;32:82–92. doi: 10.1111/j.1365-3040.2008.01903.x. [DOI] [PubMed] [Google Scholar]
- 148.Prabhakar V, et al. Phosphoenolpyruvate provision to plastids is essential for gametophyte and sporophyte development in Arabidopsis thaliana. Plant Cell. 2010;22:2594–617. doi: 10.1105/tpc.109.073171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tcherkez G, et al. Experimental evidence of phosphoenolpyruvate resynthesis from pyruvate in illuminated leaves. Plant Physiol. 2011;157:86–95. doi: 10.1104/pp.111.180711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Selinski J, et al. The plastid-localized NAD-dependent malate dehydrogenase is crucial for energy homeostasis in developing Arabidopsis thaliana seeds. Mol Plant. 2014;7:170–186. doi: 10.1093/mp/sst151. [DOI] [PubMed] [Google Scholar]
- 151.Kinoshita H, et al. The chloroplastic 2-oxoglutarate/malate transporter has dual function as the malate valve and in carbon/nitrogen metabolism. Plant J. 2011;65:15–26. doi: 10.1111/j.1365-313X.2010.04397.x. [DOI] [PubMed] [Google Scholar]
- 152.Furumoto T, et al. A plastidial sodium-dependent pyruvate transporter. Nature. 2011;476:472–5. doi: 10.1038/nature10250. [DOI] [PubMed] [Google Scholar]
- 153.Taniguchi M, et al. Identifying and characterizing plastidic 2-oxoglutarate/malate and dicarboxylate transporters in Arabidopsis thaliana. Plant Cell Physiol. 2002;43:706–717. doi: 10.1093/pcp/pcf109. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.