Abstract
Metabolites are essential for the normal operation of cells and fulfill various physiological functions. It was recently found that in several metabolic disorders, the associated metabolites could self-assemble to generate amyloid-like structures, similar to canonical protein amyloids that have a role in neurodegenerative disorders. Yet, assemblies with typical amyloid characteristics are also known to have physiological function. In addition, many non-natural proteins and peptides presenting amyloidal properties have been used for the fabrication of functional nanomaterials. Similarly, functional metabolite assemblies are also found in nature, demonstrating various physiological roles. A notable example is the structural color formed by guanine crystals or fluorescent crystals in feline eyes responsible for enhanced night vision. Moreover, some metabolites have been used for the in vitro fabrication of functional materials, such as glycine crystals presenting remarkable piezoelectric properties or indigo films used to assemble organic semiconductive electronic devices. Therefore, we believe that the study of metabolite assemblies is not only important in order to understand their role in normal physiology and in pathology, but also paves a new route in exploring the fabrication of organic, bio-compatible materials.
Keywords: Metabolites, Self-assembly, Photonic crystals, Functional amyloids, Supramolecular structures, Nanostructures
Introduction
After an era of genomics and proteomics, metabolites and metabolomics represent the next stage in the exploration of biochemical diversity. Metabolites are intermediates or end products of metabolism comprising a class of small molecules, such as nucleobases, amino acids, and vitamins (James 2017). Metabolites participate in key cellular functions and fulfill various roles, including acting as fuel, maintaining cell structure, signaling, defense, and exerting stimulatory and inhibitory effects on enzymes (Harvey and Ferrier 2011; Valle et al. 2016). While often found in their soluble state, the ability of metabolites to self-assemble into distinct entities, giving rise to some of the most complex and diverse structures in nature, is recently emerging as an intriguing phenomenon with applicable implications. Moreover, the inherent ability of metabolites to self-organize goes beyond their physiological function, as it was recently found that metabolite structures may accumulate as a result of several metabolic disorders. These simple building blocks, such as phenylalanine, uric acid, uracil, orotic acid, tyrosine, cysteine, and adenine, can organize by non-covalent interactions, including hydrogen bonding, aromatic interactions, and van der Waals interactions, to form ordered supramolecular assemblies. Remarkably, some of the assembled metabolite structures present amyloid-like biophysical, biochemical, and cytotoxic properties (Adler-Abramovich et al. 2012; Shaham-Niv et al. 2015; Gazit 2016). Canonical amyloids are insoluble fibrillar protein deposits with an underlying cross-β structure initially discovered in the context of human degenerative diseases (Rochet and Lansbury Jr 2000; Makin and Serpell 2005; Chiti and Dobson 2006). Functional amyloids and amyloid-like materials were found to play various roles throughout nature (Pham et al. 2014; Wei et al. 2017), including structural (as in bacterial biofilms and hyphae) or catalytic (as in mammalian melanosomes) functions. Moreover, a wide range of unrelated peptides and proteins have been shown to form artificial amyloid-like fibrillar materials in vitro. These materials are characterized by a well-organized supramolecular structure and have led to the design of many functional nanomaterials (Knowles and Buehler 2011). Similarly, the inherent ability of metabolites to self-associate into ordered structures is now initiating the exploration of their unique properties and functionalities. Here, we describe the assembly of functional metabolites in the biological world and in material science, as well as the physicochemical properties of the assemblies. We further discuss the use and future potential of these assemblies for technological and therapeutic applications.
Naturally accruing functional metabolite assemblies
Supramolecular architectures of metabolite assemblies in biological systems are frequently found in photonic structures. Unlike pigmental colors, structural colors are formed by highly organized multiscale hierarchical architectures. The structural colors observed in biological and other natural materials are often the consequence of interference, diffraction, or selective reflection of light from photonic structures (Adepalli et al. 2017). In these optical systems, the basic metabolite units are maintained by non-covalent interactions constructing highly ordered materials with extraordinary optical properties. Although other purines and their analogues, such as uric acid and isoxathopterin, are also found as components in natural photonic-based systems, the most commonly used purine in biological structural color systems is guanine (Herring 1994; Gur et al. 2017). As a nitrogenous metabolite, guanine takes part in many cellular metabolic processes and is thus readily available. For example, pigment cells, denoted as iridophores or guanophores, consisting of anhydrous guanine crystal nanoplates, are present in the skin of a wide range of animals of very distinct taxonomic groups. One notable example is the panther chameleon (Furcifer pardalis), a lizard from Madagascar capable of dynamic and reversible color change serving for camouflage, communication, and thermoregulation when excited or relaxed (Teyssier et al. 2015). By changing the osmotic pressure in the cells, the guanine lattice is expanded or reduced, leading to a shift in reflectivity (Fig. 1). The stacked arrangement of the crystalline plates acts as a high-quality reflector that generates structural colors. This efficient color formation is based on the very high refractive index of the guanine crystals, which is 1.83 in the reflecting direction. A similar phenomenon is observed in neon tetra fish, where a variation in the guanine crystal lattice is used to modulate the color. Interestingly, in this system, light is used as the trigger to modulate the lattice spacing of the guanine crystal for color tuning (Gur et al. 2015).
Fig. 1.
The rearrangement of guanine-based photonic crystals in the panther chameleon serves as the molecular mechanism for controlled color change. Upper panel: Panther chameleon in a relaxed (a) and an excited (b) state. TEM micrograph of the guanine nanocrystals lattice from panther chameleon iridophores in a relaxed (c) and an excited (d) state, scale bar 200 nm (adapted from Teyssier et al. 2015)
Another interesting example demonstrating the importance of the guanine metabolite-based structures is the image-forming mirror in the eye of the scallop (Palmer et al. 2017b). Scallops possess a visual system comprising up to 200 eyes, each containing a concave mirror, rather than a lens, to focus the light (Fig. 2a). The mirror is composed of a multilayered structure, tiled with a mosaic of square guanine crystals tuned to reflect light penetrating the scallop’s habitat. This hierarchical organization is used to control image formation, from the guanine crystals at the nanoscale to the complex three-dimensional morphology at the millimeter level (Fig. 2b–e).
Fig. 2.
The anatomy and ultrastructure of the multilayer mirror of scallop eyes. a An image of five eyes of the scallop. b–d Cryo-SEM micrographs of high-pressure-frozen, freeze-fractured cross sections through the eye of P. maximus. b The mirror viewed perpendicular to the eye axis. White arrow indicates direction of on-axis incident light. c The tiled mirror viewed from above. d Crystals in adjacent layers, stacked directly on top of each other, viewed in a fracture through the mirror. e TEM micrograph of a single, regular square crystal extracted from the eye (adapted from Palmer et al. 2017b with permission)
Similar optical metabolite-based architectures are found in the tapeta lucida tissue of some spiders, marine fauna, crocodiles, and other animals with shiny mirror-reflecting eyes. The reflective material varies in chemical composition between different species (Vukusic 2004; Vukusic and Sambles 2003; Ollivier et al. 2004). For example, in animals such as cats and lemurs, riboflavin, the vitamin B2 metabolite, was found to be the reflecting material, forming rodlet-shaped crystals which are grouped in bundles arranged in a very regular hexagonal lattice pattern (Braekevelt 1990a, b; Coles 1971; Pirie 1959). Riboflavin absorbs light at a low wavelength and fluoresces at 520 nm, which is approximately the maximum absorption of rhodospin (500 nm). Thus, the cat and lemur tapeta lucida appear to not only reflect light but to also divert it into a more useful range by fluorescence. Therefore, the tapetum lucidum provides the light-sensitive retinal cells with a second opportunity for photoreceptor stimulation by acting as a reflecting surface. Any light not absorbed during its first passage through the light-sensitive cells is reflected back again and has a second opportunity to be absorbed, thereby enhancing visual sensitivity at low light levels, which also accounts for the phenomenon of “eye-shine” (Braekevelt 1990b). Another metabolite found to be essential for the function of the tapetum lucidum of other animals, such as dogs and ferret, is zinc-cysteine. In other cases, cholesterol, pteridine, and several lipids were identified as the reflecting materials (Ollivier et al. 2004; Palmer et al. 2017a).
The crystallization of uric acid is often related to high levels of this metabolite in the blood, resulting in its accumulation around the joints, thus underlying the cause of gout disease (Richette and Bardin 2010; Pittman and Bross 1999). Interestingly, ordered layers of uric acid were also found to play a role in reflecting layers of the iridescent cuticle (insect shell) of Rutelino scarab beetles (Fig. 3). The reflecting layers have an anticlockwise helicoidal architecture and are responsible for selectively reflecting circularly polarized light with a left-handed (anticlockwise) rotation, thereby causing the metallic-shiny appearance of the beetles. It was found that circularly polarized light is reflected far more efficiently in beetles incorporating uric acid into their reflecting layers, compared to beetles lacking this metabolite. To facilitate these optical properties of uric acid, an epitaxial incorporation into the helicoidal framework is necessary. When uric acid was removed, a significant reduction in iridescence was observed (Caveney 1971; Finlayson et al. 2017).
Fig. 3.
Enhanced iridescent of the cuticle. a–d The visual appearances of different colored jewel Chrysina scarab beetles (adapted from Vargas et al. 2016)
The above examples demonstrate how the aggregation of different metabolites is naturally exploited to form complex and sophisticated self-assembling systems. In the next section, we will explore the in vitro manipulations of metabolite assemblies aimed to fabricate functional materials.
Self-assembly of metabolites in material science
Self-assembly of supramolecular metabolite structures provides a means for bottom-up fabrication of various materials. These materials exhibit diverse functional and physiochemical properties which arise from the assembly of the simplest molecular building blocks. The assembly process is dynamic and reversible, resulting in responsiveness and tunable characteristics. Moreover, the use of organic and natural building blocks makes these materials highly suitable for green and bio-compatible applications (Zhang 2003; Lihi Adler-Abramovich and Gazit 2014). For instance, the use of nucleobases and nucleobases-containing molecules as supramolecular motifs is well established (Sivakova and Rowan 2005; Lopez and Liu 2017). Although most of the examples involve the assembly of modified nucleobases or the use of coordination chemistry, there are a few examples that demonstrate the intrinsic ability of nucleobases to self-assemble into entities with unique properties. For instance, the origin of guanine-based hydrogels can be traced back to Bang 1910, when Bang et al. reported that 5′-guanosine monophosphate (5′-GMP) or guanylic acid (a nucleotide used as a monomer in RNA) forms polycrystalline gel aggregates at millimolar concentrations. In 1962, using crystallographic methods, Gellert et al. reported that the gel network contained macrocyclic G-quartet (Hoogsteen-bonded guanosine tetramers) building blocks. The unusual ability of this guanine derivative to form planar G-quartet structures via Hoogsteen-type H bonding and their subsequent assembly into columnar aggregates by π–π stacking offer the possibility of generating tunable three-dimensional intertwined fibrillar networks. The ability to tune the properties of these assemblies has led to the finding that 5′-GMP can self-assemble into left-handed columnar aggregates which, at high concentrations, pack into left-handed cholesteric mesophases liquid crystals (Gottarelli et al. 1997; Davis 2004). The same research group also studied the capabilities of folic acid (vitamin B9) to form liquid crystals (Bonazzi et al. 1993). In this work, it was found that folic acid salts can self-organize in water and generate a supramolecular hexagonal lyotropic liquid crystalline phase with a chiral columnar shape. At a high concentration, the solution becomes birefringent and forms textures similar to those of the hexagonal mesophases formed by guanylic acid phases.
Notably, some metabolites were used for the fabrication of organic-based electronics. For example, Irimia-Vladu et al. (2012) have reported the use of indigo for the fabrication of ambipolar organic semiconductors. Indigo is a dye produced from the Indigofera tinctoria and Isatis tinctoria plants, which have been cultivated for thousands of years for coloring in the textile industry. Indigo has very low solubility and a high melting point (390–392 °C) due to stabilization by inter- and intra-molecular hydrogen bonding. These intermolecular interactions of π-domains are responsible for the efficient charge transport that was observed by the authors in highly ordered thin films of indigo, obtained by evaporation. In this work, indigo films were characterized by optical, electrical, and morphological methods, which led to the construction of organic field effect transistors (OFETs) fabricated on natural shellac resin substrates, all made completely from natural and biodegradable compounds (Fig. 4a). The authors calculated a bandgap of 1.7 eV, high and well-balanced electron and hole mobilities of 1 × 10−2 cm2 V−1 s−1, and good stability against degradation in the air. These inspiring results place the indigo structures in line with the best organic electronic materials (Fig. 4b).
Fig. 4.
Indigo field effect transistors. a Photograph of ambipolar indigo transistors on shellac. b Detailed composition of the OFET composed of completely natural and biodegradable compounds (adapted from Irimia-Vladu et al. 2012 with permission)
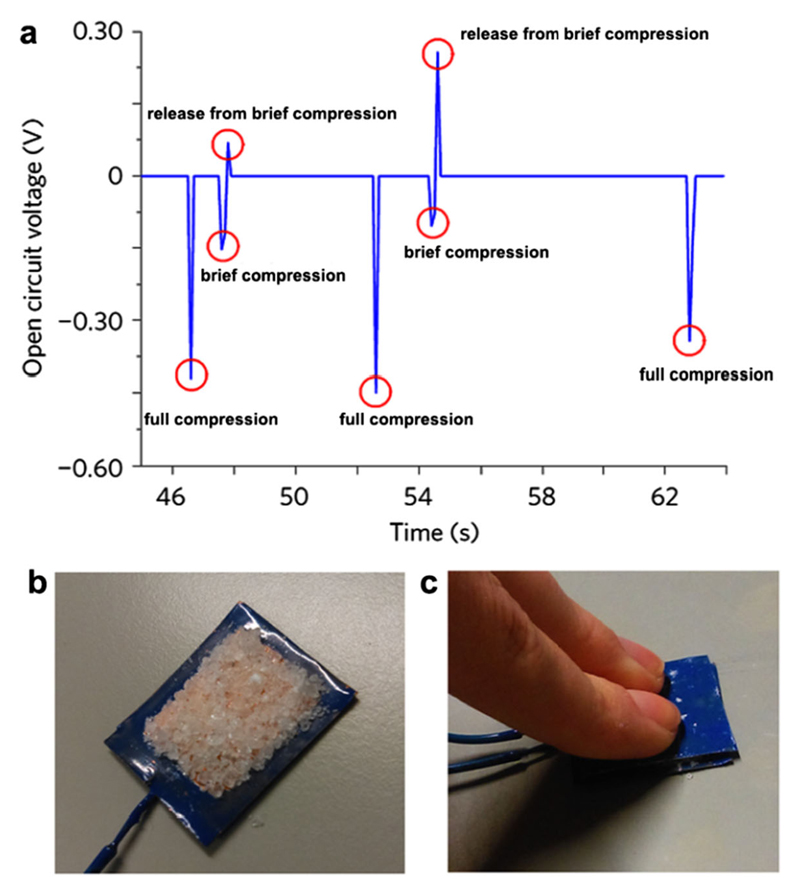
Guerin et al. (2018) have recently demonstrated high piezoelectricity values in certain polymorphs of glycine crystals by efficient packing of the molecules along specific crystallographic planes and directions. Piezoelectricity is the linear relationship between stress and induced electrical charge. Biologically based materials usually present low piezoelectricity values in the range of 0.1–10 pm V−1, which limits their technological applications, compared to 20–800 pm V−1 exhibited by inorganic materials used for piezoelectricity applications, such as lead zirconate titanate, and barium titanate. Guided by quantum mechanical calculations, the authors predicted low permittivity values for γ and β-glycine polymorphs, and assumed it will result in high voltage constants. This predicted quality could make γ and β-glycine suitable for use as sensors and energy-harvesting devices. In order to experimentally test this prediction, an energy-harvesting device was constructed, in which an open-circuit voltage of 0.45 V was obtained after applying a force of approximately 0.172 N to a 324 mm2 area electrode (Fig. 5). This is the first demonstration of an energy device composed of undoped amino acids crystals. Furthermore, the authors showed that β-glycine could exhibit substantially higher piezoelectricity coefficient than previously thought (6 pm V−1). A piezoelectric coefficient (d16) of 2 × 102 pm V−1 was experimentally measured, equivalent to standard inorganic piezoelectric materials. These results prove that glycine polymorphs have piezoelectrical properties, which render them suitable for use in energy-harvesting technologies for regenerative medicine.
Fig. 5.
Piezoelectric properties of glycine crystals polymorphs. a Open-circuit voltage response of a layer of γ-glycine seed crystals, orientated along the crystallo-graphic 2-axis. Manual force is applied along the crystallographic 2-axis over time. b A layer of γ-glycine seed crystals on a square copper 18 mm × 18 mm electrode insulated with varnish. c Manual compression of a γ-glycine seed crystal layer. Full compression of the layer as shown averaged a force of 0.172 N (adapted from Guerin et al. 2018 with permission)
A summary of the structure and function of the metabolite assemblies reviewed here is presented in Table 1.
Table 1. Summary of functional metabolites presented in the review.
| Name of metabolite | Chemical structure | Function or functional property | Reference |
|---|---|---|---|
| Guanine | 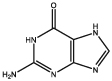 |
Reflecting crystals for structural colors and mirror-like eyes formation | Gur et al. 2015, 2017; Palmer et al. 2017b |
| Riboflavin (B2) | 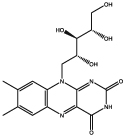 |
Reflecting and fluorescent crystals for enhanced night sight | Braekevelt 1990; Coles 1971 |
| Indigo |  |
Thin films acting as OFETs | Irimia-Vladu et al. 2011 |
| Uric acid |  |
Iridescence enhancement | Caveney 1971; Finlayson et al. 2017 |
| Folic Acid (B9) |  |
lyotropic liquid crystals | Bonazzi et al. 1993 |
| Glycine |  |
β and γ -glycerine crystals exhibiting piezoelectricity properties | Guerin et al. 2017 |
| 5′-GMP |  |
Hydrogels and cholesteric mesophases liquid crystals | Gottarelli et al. 1997; Davis 2004 |
To conclude, self-assembly of metabolites presents a new avenue in the construction of biological and bioinspired materials. Although metabolites are often overlooked as building blocks for self-assembly processes, their versatile physical properties, inherent biocompatibility, biodegradability, and low costs make them promising candidates for diverse applications, from the fabrication of optical materials to high-performance electronics. Moreover, basic research of the complex architectures spontaneously generated by various metabolites, both in health and disease, can lead to more profound understanding of their diversified roles, as well as to the development of novel therapeutics for numerous metabolic disorders.
Acknowledgements
The authors thank the members of the Gazit laboratory for helpful discussions.
Funding information This work was supported in part by grants from the European Research Council under the European Union Horizon 2020 research and innovation program (no. 694426) (E.G.). R.A. thanks financial support of the Tel Aviv University Global Research & Training Fellowship (GRTF), the Naomi foundation.
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
References
- Adepalli S, Slocik J, Gupta M, Naik RR, Singamaneni S. Bio-optics and bioinspired optical materials. Chem Rev. 2017;117:12705–12763. doi: 10.1021/acs.chemrev.7b00153. [DOI] [PubMed] [Google Scholar]
- Adler-Abramovich L, Gazit E. The physical properties of supramolecular peptide assemblies: from building block association to technological applications. Chem Soc Rev. 2014;43:6881–6893. doi: 10.1039/c4cs00164h. [DOI] [PubMed] [Google Scholar]
- Adler-Abramovich L, Vaks L, Carny O, Trudler O, Magno A, Caflisch A, Frenkel D, Gazit E. Phenylalanine assembly into toxic fibrils suggests amyloid etiology in phenylketonuria. Nat Chem Biol. 2012;8:701–706. doi: 10.1038/nchembio.1002. [DOI] [PubMed] [Google Scholar]
- Bang I. Untersuchungen über die Guanylsäure. Biochem Z. 1910;26:293–311. [Google Scholar]
- Bonazzi S, Demorais MM, Gottarelli G, Mariani P, Spada GP. Self-assembly and liquid crystal formation of folic acid salts. Angew Chem Int Ed Engl. 1993;32:248–250. doi: 10.1002/anie.199302481. [DOI] [Google Scholar]
- Braekevelt CR. Retinal epithelial fine structure in the domestic cat (Felis catus) Anat Histol Embryol. 1990a;19:58–66. doi: 10.1111/j.1439-0264.1990.tb00878.x. [DOI] [PubMed] [Google Scholar]
- Braekevelt CR. Fine structure of the feline tapetum lucidum. Anat Histol Embryol. 1990b;19:97–105. doi: 10.1111/j.1439-0264.1990.tb00892.x. [DOI] [PubMed] [Google Scholar]
- Caveney S. Cuticle reflectivity and optical activity in scarab beetles: the role of uric acid. Proc R Soc Lond B. 1971;178:205–225. doi: 10.1098/rspb.1971.0062. [DOI] [PubMed] [Google Scholar]
- Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem. 2006;75:333–366. doi: 10.1146/annurev.biochem.75.101304.123901. [DOI] [PubMed] [Google Scholar]
- Coles JA. Some reflective properties of the tapetum lucidum of the cat’s eye. J Physiol. 1971;212:393–409. doi: 10.1113/jphysiol.1971.sp009331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JT. G-quartets 40 years later: from 5′-GMP to molecular biology and supramolecular chemistry. Angew Chem Int Ed Engl. 2004;43:668–698. doi: 10.1002/anie.200300589. [DOI] [PubMed] [Google Scholar]
- Finlayson ED, McDonald LT, Vukusic P. Optically ambidextrous circularly polarized reflection from the chiral cuticle of the scarab beetle Chrysina resplendens. J R Soc Interface. 2017;14 doi: 10.1098/rsif.2017.0129. 20170129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazit E. Metabolite amyloids: a new paradigm for inborn error of metabolism disorders. J Inherit Metab Dis. 2016;39:483–488. doi: 10.1007/s10545-016-9946-9. [DOI] [PubMed] [Google Scholar]
- Gellert M, Lipsett MN, Davies DR. Helix formation by guanylic acid. Proc Natl Acad Sci U S A. 1962;48:2013–2018. doi: 10.1073/pnas.48.12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottarelli G, Proni G, Spada GP. The self-assembly and lyotropic mesomorphism of riboguanylic acids (GMP) Liq Cryst. 1997;22:563–566. doi: 10.1080/026782997208956. [DOI] [Google Scholar]
- Guerin S, Stapleton A, Chovan D, Mouras R, Gleeson M, McKeown C, Noor MR, Silien C, Rhen FM, Kholkin AL, Liu N, et al. Control of piezoelectricity in amino acids by supramolecular packing. Nat Mater. 2018;17:180–186. doi: 10.1038/nmat5045. [DOI] [PubMed] [Google Scholar]
- Gur D, Palmer BA, Leshem B, Oron D, Fratzl P, Weiner W, Addadi L. The mechanism of color change in the neon tetra fish: a light-induced tunable photonic crystal array. Angew Chem Int Ed. 2015;54:12426–12430. doi: 10.1002/anie.201502268. [DOI] [PubMed] [Google Scholar]
- Gur D, Palmer BA, Weiner S, Addadi L. Light Manipulation by Guanine Crystals in Organisms: Biogenic Scatterers, Mirrors, Multilayer Reflectors and Photonic Crystals. Adv Funct Mater. 2017;27:1603514. [Google Scholar]
- Harvey RA, Ferrier DR. Lippincott’s illustrated reviews: biochemistry. Lippincott Williams & Wilkins; Philadelphia: 2011. [Google Scholar]
- Herring P. Reflective systems in aquatic animals. Comp Biochem Physiol Part A. 1994;109:513–546. doi: 10.1016/0300-9629(94)90192-9. [DOI] [Google Scholar]
- Irimia-Vladu M, Głowacki ED, Troshin PA, Schwabegger G, Leonat L, Susarova DK, Krystal O, Ullah M, Kanbur Y, Bodea MA, Razumov VF, et al. Indigo—a natural pigment for high performance ambipolar organic field effect transistors and circuits. Adv Mater. 2012;24:375–380. doi: 10.1002/adma.201102619. [DOI] [PubMed] [Google Scholar]
- James KD. Animal metabolites: from amphibians, reptiles, aves/birds, and invertebrates. Pharmacognosy. 2017;19:401–411. doi: 10.1016/B978-0-12-802104-0.00019-6. [DOI] [Google Scholar]
- Knowles TPJ, Buehler MJ. Nanomechanics of functional and pathological amyloid materials. Nat Nanotech. 2011;6:469–479. doi: 10.1038/nnano.2011.102. [DOI] [PubMed] [Google Scholar]
- Lopez A, Liu J. Self-assembly of nucleobase, nucleoside and nucleotide coordination polymers: from synthesis to applications. ChemNanoMat. 2017;3:670–684. doi: 10.1002/cnma.201700154. [DOI] [Google Scholar]
- Makin OS, Serpell LC. Structures for amyloid fibrils. FEBS J. 2005;272:5950–5961. doi: 10.1111/j.1742-4658.2005.05025.x. [DOI] [PubMed] [Google Scholar]
- Ollivier FJ, Samuelson DA, Brookes DE, Lewis PA, Kallberg ME, Komaromy AM. Comparative morphology of the tapetum lucidum (among selected species) Vet Ophthalmol. 2004;7:11–22. doi: 10.1111/j.1463-5224.2004.00318.x. [DOI] [PubMed] [Google Scholar]
- Palmer BA, Hirsch A, Brumfeld V, Aflalo ED, Pinkas I, Sagi A, Rozenne S, Oron D, Leiserowitz L, Kronik L, Weiner W, et al. Isoxanthopterin: an optically functional biogenic crystal in the eyes of decapod crustaceans. bioRxiv. 2017a doi: 10.1101/240366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer BA, Taylor GJ, Brumfeld V, Gur D, Shemesh M, Elad N, Osherov A, Oron D, Weiner S, Addadi L. The image-forming mirror in the eye of the scallop. Science. 2017b;358:1172–1175. doi: 10.1126/science.aam9506. [DOI] [PubMed] [Google Scholar]
- Pham CL, Kwan AH, Sunde M. Functional amyloid: wide-spread in nature, diverse in purpose. Essays Biochem. 2014;56:207–219. doi: 10.1042/bse0560207. [DOI] [PubMed] [Google Scholar]
- Pirie A. Crystals of riboflavin making up the tapetum lucidum in the eye of a lemur. Nature. 1959;183:985–986. doi: 10.1038/183985a0. [DOI] [PubMed] [Google Scholar]
- Pittman JR, Bross MH. Diagnosis and management of gout. Am Fam Physician. 1999;59:1799–1806. doi: 10.1042/bse0560207. [DOI] [PubMed] [Google Scholar]
- Richette P, Bardin T. Gout. Lancet. 2010;375:318–328. doi: 10.1016/S0140-6736(09)60883-7. [DOI] [PubMed] [Google Scholar]
- Rochet JC, Lansbury PT., Jr Amyloid fibrillogenesis: themes and variations. Curr Opin Struct Biol. 2000;10:60–68. doi: 10.1016/S0959-440X(99)00049-4. [DOI] [PubMed] [Google Scholar]
- Shaham-Niv S, Adler-Abramovich L, Schnaider L, Gazit E. Extension of the generic amyloid hypothesis to nonproteinaceous metabolite assemblies. Sci Adv. 2015;1:e1500137. doi: 10.1126/sciadv.1500137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivakova S, Rowan SJ. Nucleobases as supramolecular motifs. Chem Soc Rev. 2005;34:9–21. doi: 10.1039/B304608G. [DOI] [PubMed] [Google Scholar]
- Teyssier J, Saenko SV, van der Marel D, Milinkovitch MC. Photonic crystals casue active colour change in chameleons. Nat Commun. 2015;6 doi: 10.1038/ncomms7368. 6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valle D, Beudet AL, Vogelstein B, Kinzler KW, Antonarakis SE, Ballabio A, Gibson KM, Mitchell G. The online metabolic and molecular bases of inherited disease. McGraw-Hill; New York: 2016. [Google Scholar]
- Vargas W, Hernández-Jiménez M, Libby E, Azofeifa D, Barboza C, Solis Á. Light reflection by cuticles of Chrysina jewel scarabs: optical measurements, morphology characterization, and theoretical modeling. Optics and photonics Journal. 2016;6:146–163. doi: 10.4236/opj.2016.67017. [DOI] [Google Scholar]
- Vukusic P. Natural photonics. Physics World. 2004;7:35–39. doi: 10.1088/2058-7058/17/2/34. [DOI] [Google Scholar]
- Vukusic P, Sambles JR. Photonic structures in biology. Nature. 2003;424:852–855. doi: 10.1038/nature01941. [DOI] [PubMed] [Google Scholar]
- Wei G, Su Z, Reynolds NP, Arosio P, Hamley IW, Gazit E, Mezzenga R. Self-assembling peptide and protein amyloids: from structure to tailored function in nanotechnology. Chem Soc Rev. 2017;46:4661–4708. doi: 10.1039/C6CS00542J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S. Fabrication of novel biomaterials through molecular self-assembly. Nat Biotechnol. 2003;21:1171–1178. doi: 10.1038/nbt874. [DOI] [PubMed] [Google Scholar]