Abstract
Background
IL-23 is the key cytokine for generation of pathogenic IL-17–producing helper T (TH17) cells, which contribute critically to autoimmune diseases. However, how IL-23 generates pathogenic TH17 cells remains to be elucidated.
Objectives
We sought to examine the involvement, molecular mechanisms, and clinical implications of prostaglandin (PG) E2–EP2/EP4 signaling in induction of IL-23–driven pathogenic TH17 cells.
Methods
The role of PGE2 in induction of pathogenic TH17 cells was investigated in mouse TH17 cells in culture in vitro and in an IL-23–induced psoriasis mouse model in vivo. Clinical relevance of the findings in mice was examined by using gene expression profiling of IL-23 and PGE2-EP2/EP4 signaling in psoriatic skin from patients.
Results
IL-23 induces Ptgs2, encoding COX2 in TH17 cells, and produces PGE2, which acts back on the PGE receptors EP2 and EP4 in these cells and enhances IL-23–induced expression of an IL-23 receptor subunit gene, Il23r, by activating signal transducer and activator of transcription (STAT) 3, cAMP-responsive element binding protein 1, and nuclear factor κ light chain enhancer of activated B cells (NF-κB) through cyclic AMP–protein kinase A signaling. This PGE2 signaling also induces expression of various inflammation-related genes, which possibly function in TH17 cell–mediated pathology. Combined deletion of EP2 and EP4 selectively in T cells suppressed accumulation of IL-17A+ and IL-17A+IFN-γ+ pathogenic Th17 cells and abolished skin inflammation in an IL-23–induced psoriasis mouse model. Analysis of human psoriatic skin biopsy specimens shows positive correlation between PGE2 signaling and the IL-23/TH17 pathway.
Conclusions
T cell–intrinsic EP2/EP4 signaling is critical in IL-23–driven generation of pathogenic TH17 cells and consequent pathogenesis in the skin.
Key words: Psoriasis, pathogenic TH17 cells, IL-23 receptor, prostaglandin E2, prostaglandin E receptor EP2, prostaglandin E receptor EP4, signal transducer and activator of transcription 3, cAMP-responsive element binding protein 1, nuclear factor κ light chain enhancer of activated B cells
Abbreviations used: cAMP, Cyclic AMP; CREB, cAMP-responsive element binding protein; db-cAMP, Dibutyryl cAMP; Epac, Exchange factor directly activated by cyclic AMP; IL-12R, IL-12 receptor; IL-23R, IL-23 receptor; JAK, Janus kinase; KO, Knockout; mPGES, Membrane-associated PGE synthase; NF-κB, Nuclear factor κ light chain enhancer of activated B cells; PG, Prostaglandin; PKA, Protein kinase A; STAT, Signal transducer and activator of transcription; WT, Wild-type
Graphical abstract
CD4+ T cells differentiate into TH1, TH2, and TH17 cells in response to the specific cytokine milieu present in the microenvironment of inflammation and mediate immune inflammatory responses in respective settings.1, 2, 3, 4 Among these TH subsets, TH17 cells mediate inflammatory responses in patients with many autoimmune diseases, including multiple sclerosis; inflammatory bowel diseases, such as Crohn disease; psoriasis; and rheumatoid arthritis. The importance of TH17 cells in these processes was suggested first in animal models of these diseases, including experimental autoimmune encephalomyelitis, and an IL-23– or imiquimod-induced psoriasis model,5, 6, 7, 8, 9 and was validated recently by clinical effectiveness of antibodies targeting IL-23 in patients with psoriasis.10, 11, 12, 13, 14
Differentiation of TH17 cells from naive CD4+ T cells is driven by the combined actions of IL-6 and TGF-β1.15, 16, 17, 18, 19 However, differentiated TH17 cells have little capacity to induce autoimmune and inflammatory pathology.20 It should be noted that these TH17 cells exhibit plasticity and could transdifferentiate into other effector T-cell types or even regulatory T cells under certain contexts, such as inflammation or autoimmune disease.21, 22, 23
Accumulating evidence suggests that T cell–intrinsic IL-23 signaling not only increases IL-17 production of TH17 cells but also plays a crucial role in inducing and stabilizing their pathogenicity.20, 24, 25, 26, 27 It is known that IL-23 acts on IL-23 receptor (IL-23R) complex composed of IL-23R and IL-12 receptor (IL-12R) β1, activates signal transducer and activator of transcription (STAT) 3, and induces expression of Il23r, thus forming the self-amplification loop. The pathophysiologic importance of this IL-23–IL-23R signaling has been indicated by several genomic studies that showed a positive correlation between single nucleotide polymorphisms of genes involved in this pathway, such as IL23R, IL12B (p40), Janus kinase 2 (JAK2), and STAT3, and a wide range of IL-17–dependent autoimmune diseases.28, 29, 30
Although it was shown that IL-23 signaling induces expression of TH17 pathogenic signature genes through activation of STAT3,31, 32 transcription factors other than STAT3 are also implicated for induction of pathogenic TH17 cells because IL-6, which activates STAT3 similarly to IL-23, cannot induce IL-23R gene expression.32 Therefore, the identity of additional transcriptional factors and regulatory mechanisms are important issues to be defined. Moreover, how IL-23 cooperates with other inflammatory factors formed in the disease microenvironment and the importance of such cooperation for pathogenic conversion of TH17 cells and overall pathology remain largely obscure. Clarification of these points could provide a new opportunity to develop small-molecule drugs as therapeutic alternatives to anti–IL-23 antibodies without systemic immune suppression. Biological agents might additionally cause unpredictable adverse events33 and can be costly on long-term use.34 It should also be mentioned that JAK inhibitors that are now being evaluated for their efficacy in patients with autoimmune diseases are presumably not free from adverse effects either because of their effects on general immune suppression.35
Prostanoids, including prostaglandin (PG) D2, PGE2, PGF2α, PGI2, and thromboxane A2, are oxygenated metabolites of arachidonic acid produced by sequential actions of COX and respective synthases and act on their cognate receptors, DP for PGD2, EP1 to 4 for PGE2, IP for PGI2, FP for PGF2α, and TP for thromboxane A2, to exert their actions.36 Although prostanoids were regarded previously as immunosuppressants,37, 38 recent studies have revealed their immunostimulatory actions in processes such as cytokine production, dendritic cell maturation, macrophage activation, and differentiation and expansion of TH cell subsets.39, 40, 41 Indeed, PGE2-EP2 and EP4 (EP2/EP4) signaling enhances TH1 differentiation by inducing expression of the IL-12R subunit Il12rb2 and the IFN-γ receptor Ifngr1, thus facilitating IL-12 signaling and TH1 differentiation.42, 43 Notably, this PGE2-EP2/EP4 signaling was also reported to synergize with IL-23 to facilitate TH17 cell expansion both in murine and human T cells.43, 44, 45 However, whether and how PGE2-EP2/EP4 signaling is involved in induction of pathogenic TH17 cells remain unknown.
In this study we have examined how PGE2-EP2/EP4 signaling and IL-23 stimulation together regulate the generation of pathogenic TH17 cells. Through this analysis, we have identified the transcription mechanisms in addition to STAT3 that regulate Il23r expression and TH17 pathogenicity. We have further clarified the importance of PGE2 signaling in TH17-mediated immune inflammation in vivo and found a correlation between PGE2-EP2/EP4 signaling and IL-23–IL-23R signaling in biopsy samples from patients with psoriasis.
Methods
Mice
All animal experiments were approved by the Institutional Animal Care and Use Committee of Kyoto University Graduate School of Medicine and complied with the National Institutes of Health's “Guide for the care and use of laboratory animals”. C57BL/6NCrSlc mice were purchased from Shimizu laboratory, and Lck-Cre and B6.Cg-Nfkb1tm1Bal/J mice were purchased from the Jackson Laboratory (Bar Harbor, Me). Mice deficient in Ptger246 and mice with floxed Ptger247 were established in our laboratory. Mice with floxed Ptger4 were a kind gift of Richard Breyer.48
Psoriasis models
Mice were injected subcutaneously with IL-23 (500 ng; #130-096-677; Miltenyi Biotec, Bergisch Gladbach, Germany) once a day in one ear and with PBS in the contralateral ear as a control to induce psoriasis-like lesions in the ear in an IL-23–induced psoriasis mouse model. In an imiquimod-induced psoriasis mouse model, Baselna cream containing 10% imiquimod was applied onto the ears of mice once a day. Ear thickness was then measured with a digital micrometer (#KM-BMB1-25; Mitutoyo, Kawasaki, Japan) every other day. In some experiments an antagonist for EP4, AS1954813,49 suspended in 0.5% methylcellulose was administered orally twice a day, or indomethacin and SC-236 were administered in drinking water during the experimental period.
See the Methods section in this article's Online Repository at www.jacionline.org for further details.
Results
IL-23 mobilizes the endogenous COX2-PGE2-EP2/EP4 signaling that enhances induction of Il23r expression in TH17 cells
Given the previous findings43, 44, 45 that PGE2-EP2/EP4 signaling enhances IL-23–induced TH17 cell expansion, we questioned whether and how this signaling contributes to pathogenic TH17 cell generation by IL-23. To investigate this issue, we first cultured CD4+ T cells from mouse spleens under TH17-skewing conditions (IL-6 plus TGF-β1) for 4 days and then incubated with IL-23 for an additional 3 days. Consistent with our previous findings,43 addition of PGE2 to the latter culture significantly enhanced IL-23–induced expansion and Il17a expression of TH17 cells (Fig 1, A and B). Interestingly, we also noted that PGE2 markedly upregulated IL-23–induced expression of Il23r, which was mimicked by both EP2- and EP4-selective agonists (Fig 1, C). Because both EP2 and EP4 activate protein kinase A (PKA) and exchange factor directly activated by cyclic AMP (Epac) by increasing levels of intracellular cyclic AMP (cAMP),36 we examined effects of compounds acting on these signaling and found that the cAMP analogue dibutyryl cAMP (db-cAMP), forskolin (FSK), and the phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine all synergized with IL-23 and significantly amplified IL-23–induced Il23r expression and IL-17A production in these cells (Fig 1, D and E). Furthermore, enhancement of Il23r expression was reproduced by a PKA agonist (N6-Bnz-cAMP, 300 μmol/L) but not an Epac activator (8-pCTP-2′-O-Me-cAMP, 300 μmol/L; Fig 1, F) and was ameliorated consistently by treatment with the PKA inhibitor H-89 (10 μmol/L; Fig 1, G).
Fig 1.
IL-23 mobilizes the endogenous PGE2-EP2/EP4-cAMP-PKA pathway to facilitate TH17 expansion through synergistic Il23r induction. A and B, Expansion of the TH17 population by PGE2 and IL-23. CD4+ T cells were differentiated with TGF-β1 and IL-6 to TH17 cells for 4 days and then stimulated with 100 nmol/L PGE2 in the absence or presence of IL-23 (10 ng/mL) for an additional 3 days. The cells were examined by using fluorescence-activated cell sorting for IL-17A and IFN-γ (Fig 1, A) and by using quantitative RT-PCR for Il17a expression (Fig 1, B). C-E, Effects of PGE2, 100 μM of an agonist selective to each EP subtype, and related compounds on Il23r expression. TH17 cells were incubated with 100 nmol/L PGE2, an agonist selective to each EP subtype, ONO-DI-004 (EP1), ONO-AE1-259 (EP2), ONO-AE-248 (EP3), or ONO-AE1-329 (EP4), 100 μmol/L db-cAMP, 10 μmol/L forskolin (FSK), or 100 μmol/L 3-isobutyl-1-methylxanthine (IBMX) with or without IL-23. Il23r expression (Fig 1, C and D) or IL-17A concentrations in culture supernatants (Fig 1, E) were examined. F and G, Expression of Il23r in TH17 cells stimulated with 100 μmol/L db-cAMP, 300 μmol/L N6-Bnz-cAMP (a PKA agonist), 300 μmol/L 8-pCTP-2′-O-Me-cAMP (an Epac activator; Fig 1, F), or 10 μM H-89 (a PKA inhibitor; Fig 1, G) with or without IL-23. All bars indicate means ± SEMs (n = 3). *P < .05, **P < .01, and ***P < .001.
Notably, IL-23 stimulation significantly increased Ptgs2 (COX2) gene expression in TH17 cells (Fig 2, A) and produced subnanomolar concentrations of PGE2 in culture medium (Fig 2, B). Moreover, incubation with a nonselective COX inhibitor (indomethacin, 100 μmol/L) or a selective COX2 inhibitor (SC-236, 100 μmol/L) but not a selective COX-1 inhibitor (SC-560, 100 μmol/L) significantly blocked induction of Il23r expression in response to both IL-23 alone and IL-23 and PGE2 in combination (Fig 2, C, and see Fig E1, A, in this article's Online Repository at www.jacionline.org). In addition, antagonists selective to EP2 (PF-04418948) or EP4 (ONO-AE3-208) also suppressed Il23r expression (Fig 2, D). Intriguingly, indomethacin and SC-236 suppressed expression of Il23r induced by IL-23 and PGE2 to the level that these inhibitors achieved in the presence of IL-23 alone, suggesting that they canceled the effect of exogenously added PGE2 (Fig 2, D, and see Fig E1, A). Given that PGE2 added to the culture medium degrades time dependently,50 these results suggest that exogenously added PGE2 induces COX2 and produces PGE2 endogenously and continuously, as we reported previously,51 which makes more contribution to Il23r induction, and that indomethacin and COX2 inhibitor block this process. Indeed, the addition of stable EP2 and EP4 agonists overcame the Il23r suppression by indomethacin (see Fig E1, B). Therefore, these data together suggest that IL-23 stimulates TH17 cells to produce PGE2, which acts back to EP2 and EP4 on these cells to augment Il23r expression in a positive feedback manner.
Fig 2.
IL-23 self-amplifies its own signaling through a T cell–intrinsic positive feedback COX2–PGE2–cAMP–IL-23R loop. A, Expression of COX2 mRNA (Ptgs2) in TH17 cells or TH17 cells cultured further in the presence or absence of IL-23 for 3 days, as determined by using quantitative RT-PCR. B, Concentrations of PGE2 in culture supernatants of TH17 cells in the presence or absence of IL-23 and indomethacin determined by means of ELISA. n.d., Not detected. C,Il23r expression in TH17 cells stimulated with PGE2 and IL-23 in the presence or absence of 100 μM indomethacin for 3 days. D,Il23r expression in TH17 cells stimulated with PGE2 and IL-23 in the presence or absence of EP2 (PF-04418948, 300 μM) and/or EP4 (ONO-AE3-208, 100 μM) antagonists for 3 days. All bars indicate means ± SEMs (n = 3). *P < .05, **P < .01, and ***P < .001.
Induction of Il23r expression by IL-23 and PGE2-cAMP signaling is mediated through not only STAT3 but also cAMP-responsive element binding protein 1 and nuclear factor κ light chain enhancer of activated B cells
We then investigated transcription factors responsible for induction of Il23r expression in TH17 cells by IL-23 and PGE2-EP2/EP4 signaling. Because IL-23 activates STAT3 to induce Il23r expression,52 we first examined the effect of a STAT3 inhibitor. Addition of STAT3 inhibitor VII suppressed Il23r expression not only by IL-23 but also by db-cAMP and their combination (Fig 3, A), indicating that db-cAMP action was also mediated by STAT3. Consistently, Y705 phosphorylation of STAT3 was increased by db-cAMP at 5 and 30 minutes (see Fig E2, A, in this article's Online Repository at www.jacionline.org), which was ameliorated not only by addition of STAT3 inhibitor VII but also by H-89 (Fig 3, B), indicating the involvement of PKA in db-cAMP–mediated Y705 phosphorylation of STAT3. Intriguingly, Y1007/1008 phosphorylation of JAK2, a kinase responsible for STAT3 Y705 phosphorylation in TH17 cells, was enhanced by db-cAMP, and this enhancement was suppressed by Src kinase inhibitor I (see Fig E2, B), indicating cAMP-PKA activates STAT3 through the c-Src–JAK2 pathway.
Fig 3.
STAT3, CREB1, and NF-κB mediate cAMP- and IL-23–induced Il23r expression in TH17 cells. A, Effect of STAT3 inhibitor VII on Il23r expression in TH17 cells stimulated with db-cAMP, IL-23, or both for 3 days. B, Western blot analysis of phospho-Y705 STAT3 and α-tubulin as a loading control in TH17 cells cultured as described in the Methods section in this article's Online Repository. Representative images from 2 independent experiments are shown. C, Effect of KG-501 on Il23r expression in TH17 cells stimulated by using db-cAMP, IL-23, or both. D, Effects of RNA interference for CREB1 on Il23r expression (left) and Creb1 expression to confirm CREB knockdown efficiency (right). RNA interference, subsequent culture, and stimulation of TH17 cells were performed, as described in the Methods section in this article's Online Repository. E, Western blot analysis of phospho-S536 NF-κB p65 (pp65), phospho-S933 NF-κB p105 (pp105), p65, p105/p50, and α-tubulin in TH17 cells stimulated as described in the Methods section in this article's Online Repository. Representative images from 2 independent experiments are shown. F and G, Effects of p105 KO (Fig 3, F) or 50 μM CTP-NBD (Fig 3, G) on Il23r expression in TH17 cells stimulated with db-cAMP, IL-23, or both for 3 days. All bars indicate means ± SEMs (n = 3 for Fig 3, A, C, F, and G; n = 18 in Fig 3, B). ***P < .001.
Although the above findings demonstrated that IL-23 and PGE2-cAMP signaling converge at STAT3 activation, it is well known that other STAT3 activators, such as IL-6 and IL-21, cannot substitute for IL-23 in expansion of the TH17 population,32 indicating that STAT3 is not the sole transcription factor regulating expression of Il23r.
Because PKA activates cAMP-responsive element binding protein (CREB) 1,36 we investigated the involvement of CREB1 in Il23r expression. Both KG-501, a CREB1 inhibitor,53 and RNA interference for CREB1 suppressed Il23r induction in response to db-cAMP, IL-23, or both, suggesting the involvement of CREB1 in Il23r expression in TH17 cells (Fig 3, C and D). Because IL-23 signaling enhances endogenous PGE2 production through induction of COX2 expression in TH17 cells (Fig 2, A and B), suppression of Il23r expression by inhibition or depletion of CREB1 could be due to inhibition of this endogenous PGE2 signaling for Il23r induction.
Furthermore, we detected an increase in S536 phosphorylation of NF-κB p65 (pp65) in response to db-cAMP, IL-23, or both at 24 hours (Fig 3, E) and an increase in S933 phosphorylation of NF-κB p105 subunit, a precursor of p50, in response to db-cAMP alone and its combination with IL-23 in TH17 cells (Fig 3, E). The latter is consistent with our previous finding in dendritic cells that PGE2-cAMP signaling activates the p50 subunit54 and a report that phosphorylation of p105 S933 is PKA dependent.55 Therefore we examined the involvement of NF-κB in Il23r induction by using Nfkb1-deficient mice (p105 knockout [KO]) or CTP-NBD, an NF-κB inhibitor. Interestingly, both genetic deficiency and pharmacologic inhibition of NF-κB suppressed Il23r induction in response to db-cAMP, IL-23, and their combination (Fig 3, F and G).
These results together suggest that PGE2-EP2/EP4-cAMP-PKA signaling works together with IL-23 signaling to activate STAT3, CREB1, and NF-κB for induction of Il23r expression in TH17 cells.
Gene signature induced by PGE2-EP2/EP4-cAMP signaling in CD4+ T-cell populations primed with IL-6 and TGF-β1
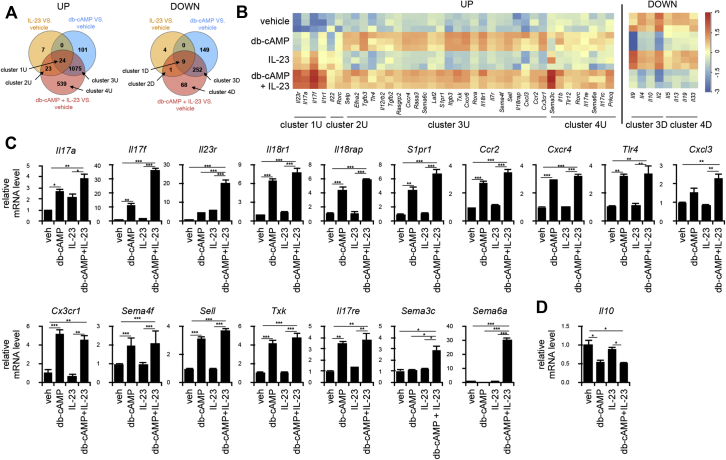
Because pathogenic TH17 cells should express various molecules in addition to IL-23R to exert their pathogenicity, we next examined how PGE2-EP2/EP4-cAMP signaling contributes to expression of such pathogenic genes in TH17 cells. CD4+ T cells were cultured under the TH17-skewing conditions with IL-6 and TGF-β1 for 3 days and then incubated with db-cAMP alone, IL-23 alone, or both for 24 hours and subjected to microarray analysis. The numbers of genes upregulated/downregulated greater than 2-fold by each stimulation were examined by using Venn diagrams (Fig 4, A), and the genes expressed in each cluster (see Tables E1 to E8 in this article's Online Repository at www.jacionline.org) were subjected to heat map analysis (see Fig E3, A, in this article's Online Repository at www.jacionline.org) and gene ontology analysis (see Fig E3, B, and Tables E9-E11 in this article's Online Repository at www.jacionline.org). Expression of representative genes in each cluster is shown in the heat map (Fig 4, B). Cluster 1U included genes (eg, Il17a, Il17f, Il1r1, and Il23r) that were upregulated by db-cAMP, IL-23, or both in combination (Fig 4, B, left). Cluster 2U included genes (eg, Il22) with expression that was increased by IL-23 alone or its combination with db-cAMP (Fig 4, B, left). Cluster 3U encompasses various genes that were upregulated by db-cAMP alone or its combination with IL-23 but not IL-23 alone. They include genes involved in cell migration and adhesion, such as Ccr2, Cxcr4, Cx3cr1, Ccr6, S1pr1, Sema4f, Sema6c, Efna2, Sell, Selp, and Itgb3; those involved in induction of IFN-γ, such as Il12rb2, Il18r1, and Il18rap; and those involved in cell activation, such as Tlr4, Tgfb3, Rasa, Rasgrp2, Lat2, Txk, and Rora (Fig 4, B, left). Cluster 4U includes genes, such as Il1b, Il17rc, Il17re, Prkcq, Sema3c, Sema6a, and Tlr12, which are upregulated by the combination of IL-23 and db-cAMP only (Fig 4, B, left). On the other hand, genes in clusters 3D and 4D were downregulated by db-cAMP and contained Il10, Il2, Il4, Il5, Il13, and Il9, which are known as suppressive factors of inflammation (Fig 4, B, right).
Fig 4.
Activation of the COX2-PGE2-EP2/EP4-cAMP pathway confers a pathogenic TH17 phenotype. A, Microarray analysis of gene expression profiles in TH17 cells stimulated with db-cAMP, IL-23, or both. Venn diagram analysis of 2-fold upregulated or downregulated genes compared with the vehicle control (Veh) on each stimulus (P < .05, 1-way ANOVA; n = 3; left and right, respectively). B, Heat map analysis of expression of selected genes from each cluster. C, Quantitative RT-PCR analysis of expression of representative genes of TH17 signature and immune activation in response to db-cAMP, IL-23, or db-cAMP and IL-23 in combination. D, Quantitative RT-PCR analysis of expression of a representative inflammation suppressor gene, Il10, in response to db-cAMP, IL-23, or db-cAMP and IL-23 in combination. All bars in Fig 4, C and D, indicate means ± SEMs (n = 3). *P < .05, **P < .01, and ***P < .001.
Expression of the representative genes was then confirmed by using quantitative RT-PCR analysis. Expression of Il17a, Il17f, and Il23r in cluster 1U; Il18r1, Il18rap, S1pr1, Ccr2, Cxcr4, Tlr4, Cxcl3, Cx3cr1, Sema4f, Sell, and Txk in cluster 3U; and Il17re, Sema3c, and Sema6a in cluster 4U was upregulated (Fig 4, C), and expression of Il10 in cluster 3D was downregulated by addition of db-cAMP compared with incubation with IL-23 alone (Fig 4, D). Thus, signaling through cAMP regulates expression of various genes that are not regulated by IL-23 alone and might confer pathogenic property to TH17 cells.
T cell–intrinsic PGE2-EP2/EP4 signaling is critical in IL-23–mediated psoriatic skin inflammation in vivo
Accumulating evidence suggests that TH17 cells become pathogenic through the IL-23–IL-23R axis and play crucial roles in development of various autoimmune diseases, including psoriasis.8, 56, 57 However, how these TH17 cells acquired the pathogenicity in vivo and to what extent the microenvironment of diseases contributes to this process remain to be defined. In the IL-23–induced psoriasis mouse model, gene expression of enzymes involved in PGE2 biosynthesis, including Ptgs2 encoding COX2, Ptges encoding membrane-associated PGE synthase (mPGES) 1, and Ptges2 encoding mPGES2, were all upregulated by IL-23 administration into the skin (see Fig E4, A, in this article's Online Repository at www.jacionline.org), which is consistent with the clinical observation that local PGE2 levels are increased in blister fluids from human psoriatic skin.58 Therefore, we hypothesized that IL-23 possibly activates PGE2–EP2/EP4 signaling, which can contribute to psoriasis pathogenesis.
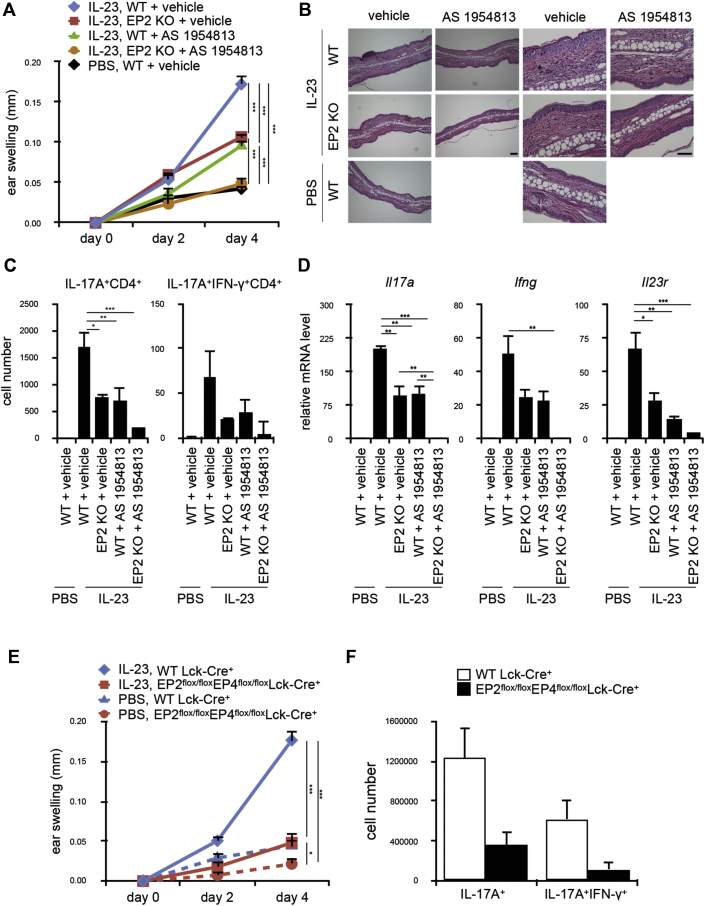
To test this hypothesis, we injected IL-23 into the skin of wild-type (WT) C57BL/6N mice, as well as EP2 KO mice,46 with or without administration with the EP4 antagonist AS195481349 and assessed skin inflammation based on ear thickness and histology. EP2 deficiency or EP4 antagonism alone reduced IL-23–induced ear swelling by half and attenuated edema and cell infiltration and, when combined, led to nearly complete suppression of IL-23–dependent skin inflammation (Fig 5, A and B). Blockade of EP2, EP4, or both caused no alteration in a PBS-injected control ear (see Fig E3, B).
Fig 5.
PGE2-EP2/EP4 signaling in T cells is required for IL-23–driven psoriatic skin inflammation. A-D, Ear swelling (n = 16-17; Fig 5, A), representative hematoxylin and eosin staining of the histologic section of the ear (n = 3-4; Fig 5, B), number of IL-17A+ and IL-17A+IFN-γ+CD4+ T cells of the ear (Fig 5, C), and gene expression of Il17a, Ifng, and Il23r in whole ear tissue (Fig 5, D) of WT or EP2 KO mice subcutaneously injected with IL-23 or PBS into the ear daily. An EP4 antagonist (AS1954813, 100 mg/kg) or vehicle was orally administered twice a day to the indicated mice. Bars in Fig 5, B = 50 μm. Representative quantification results of the cell number in each population from 4 independent fluorescence-activated cell sorting experiments are shown in Fig 5, C (n = 3). Gene expression was indicated as fold change compared with PBS-injected ears in Fig 5, D (n = 3). E and F, EP2flox/floxEP4flox/floxLck-Cre+ mice and control WT Lck-Cre+ mice were subjected to an IL-23–induced psoriasis model, and ear swelling (n = 11 and 7, respectively; Fig 5, E) and numbers of IL-17A+ and IL-17A+IFN-γ+CD4+ T cells in the ear (n = 7 and 3, respectively; Fig 5, F) were analyzed. All bars indicate means ± SEMs. *P < .05, **P < .01, and ***P < .001.
To examine at which step of inflammation EP2 deficiency and EP4 antagonism exert their effects and whether it is related to generation of pathogenic TH17 cells, we digested ear tissues and analyzed CD4+ T-cell populations in the skin by using flow cytometry. Although there were few numbers of cells producing IL-17A or IFN-γ in PBS-injected control ears, significant accumulations of the IL-17A+ and IL-17A+IFN-γ+CD4+ T-cell populations were observed in the IL-23–injected ear, as observed in psoriatic dermis of patients with psoriasis.59 The IL-17A+IFN-γ+CD4+ T-cell population is a suggested population of pathogenic TH17 cells.60 This CD4+ T-cell population was shown to arise in an IL-23–dependent manner from adoptively transferred T cells in transfer colitis26 and might reflect the TH17 to TH1 reprogramming at inflammatory sites as shown for antigen-specific TH17 cells transferred to NOD mice.22 This accumulation was significantly reduced by blockade of either EP2 or EP4 alone and nearly completely suppressed by blockade of both EP2 and EP4 (Fig 5, C, and see Fig E4, C-E). Consistently, expression of Il17a and Ifng that was upregulated in the IL-23–injected ear was also reduced to negligible levels by combined EP2 and EP4 blockade (Fig 5, D, left and middle). Notably, EP2 and EP4 blockade also markedly inhibited enhanced expression of Il23r by IL-23 injection (Fig 5, D, right). These findings together indicate that EP2/EP4 signaling is indeed involved in generation of pathogenic TH17 cells and elicitation of inflammation in this model.
We then investigated whether T cell–intrinsic EP2/EP4 signaling is responsible for these IL-23–induced phenotypes. To this end, we used EP2flox/flox mice47 and EP4flox/flox mice48 and generated EP2flox/flox EP4flox/floxLck-Cre+ mice. EP2flox/floxEP4flox/floxLck-Cre+ mice showed no significant differences in numbers of total cells, B cells, T cells, CD4 T cells, CD8 T cells, TH1 cells, TH17 cells, and regulatory T cells in the thymus, spleen, lymph node, and peripheral blood compared with control WT Lck-Cre+ mice (see Fig E5, A, in this article's Online Repository at www.jacionline.org). However, deficiency of both EP2 and EP4 selectively in T cells prevented accumulation of TH17 cells in the ear and almost completely attenuated IL-23–induced skin inflammation (Fig 5, E and F). Therefore these results together suggest that the T cell–intrinsic PGE2-EP2/EP4 signaling is critical for generation of pathogenic TH17 cells in a psoriasis model.
We also performed an imiquimod-induced psoriasis model,8 in which we applied imiquimod to the ears of WT or EP2 KO mice with or without EP4 antagonist for 6 days (see Fig E6, A, in this article's Online Repository at www.jacionline.org). We found that ear swelling was also reduced significantly by EP2 deficiency and EP4 antagonism and additively in combination, which was similar to the results in an IL-23–induced psoriasis model.
Given the above findings, we next examined the effects of COX inhibitors on skin inflammation in an IL-23–induced psoriasis model (see Fig E6, B and C). Treatment with indomethacin and SC-236 significantly suppressed the IL-23–induced ear swelling with concomitant suppression of IL-17A+ and IL-17A+IFN-γ+ cells in the skin (see Fig E6, B and C). These findings together suggest that COX inhibitors are as potent as EP2 and EP4 antagonists in suppressing skin inflammation, at least in this model.
PGE2 signaling positively correlates with the IL-23/TH17 pathway in human psoriatic skin biopsy specimens
Finally, to extrapolate our findings in mice to human subjects, we analyzed a public microarray data set on gene expression profiles in skin biopsy specimens from patients with psoriasis and healthy control subjects,61 with a particular interest in correlation of PGE2 signaling and the IL-23/TH17 pathway. As expected, psoriatic lesional skin overexpressed TH17 signature genes (including IL23A, IL12B, IL23R, IL17A, IL17F, and IL22), STAT3, and NFKB1 (encoding NF-κB p105; Fig 6, A). Moreover, psoriatic lesional skin overexpressed enzymes in PGE2 biosynthesis (eg, PTGS2, PTGES, and PTGES2 and the EP4 receptor PTGER4) but underexpressed the PGE2 degrading enzyme 15-PGDH (encoded by HPGD; Fig 6, A). Interestingly, expression of TH17 signature genes correlated positively with those involved in PGE2 biosynthesis (eg, PTGES and PTGES2) and receptor (eg, PTGER4) but correlated negatively with HPGD (Fig 6, B). In addition, the clinically effective anti–IL-23 therapy62 downregulated gene expression of not only the IL-23/IL-17 pathway (eg, IL23A, IL23R, and IL17A) but also expression of genes involved in PGE synthesis like PTGES (Fig 6, C and D). These findings support a potential crosstalk between the PGE2 and IL-23/IL-17 pathways also in human psoriatic skin inflammation.
Fig 6.
PGE2 signaling positively correlates with the IL-23/TH17 pathway in human psoriatic skin biopsy specimens. A, Expression profiles of genes related to PGE2 signaling and TH17 signature genes in human nonlesional (NL) or lesional (PL) skin biopsy specimens from patients with psoriasis (n = 58) and skin samples from healthy control subjects (HC; n = 64). The z score transformed values of the microarray gene expression data set GSE13355 were used. The TH17 score was generated based on the average expression level of IL23A, IL12B, IL23R, IL17A, IL17F, and IL22 genes. B, Correlations of PTGES, PTGES2, HPGD, and PTGER4 gene expression versus those of the TH17 score. Black, green, and red dots indicate healthy control, nonlesional, and lesional psoriatic biopsy specimens, respectively. C, Expression profiles of genes related to PGE2 synthases and TH17 signature genes in human lesional skin biopsy specimens from patients with moderate-to-severe psoriasis before (Baseline, n = 22) or 12 weeks after treatment with the IL-23–specific mAb guselkumab (n = 8). The z score transformed values of the microarray gene expression data set GSE51440 were used. D, Correlations of gene expression of PTGS2 and PTGES versus that of IL23R. P values were calculated by using nonparametric Wilcoxon-Mann-Whitney tests (Fig 6, A and C) or the nonparametric Spearman correlation test (Fig 6, B and D).
Discussion
IL-23–IL-23R signaling plays a critical role in generation of pathogenic TH17 cells in autoimmunity.5, 6, 7, 8, 9 However, there remain several issues to be solved on this action: How does this signaling get promoted. What transcriptional mechanisms other than STAT3 are involved? What, along with IL-23 signaling, makes TH17 cells pathogenic? Does such a mechanism operate in vivo and, if so, how much? How relevant are the findings obtained in mice to human subjects? Given the previously reported action of PGE2 on TH17 expansion,43, 44, 45 we focused here on PGE2 action in TH17 pathogenicity to address these issues.
We first found that PGE2 synergizes with IL-23 and enhances Il23r expression through EP2 and EP4, a finding consistent with findings in human TH17 cells.44 We then found that IL-23 stimulation induces PGE2 production in TH17 cells and that IL-23–induced Il23r expression was attenuated by treatment of cells with indomethacin or EP2/EP4 antagonists. Thus, these results suggest a previously unsuspected intrinsic amplification mechanism mediated by PGE2-EP2/EP4 signaling in TH17 cells that helps trigger the initial IL-23 response in premature TH17 cells.
We further analyzed the transcriptional mechanisms underlying the synergistic action of IL-23 and PGE2 and found that this action is mediated by not only STAT3 but also CREB1 and NF-κB. Involvement of CREB1 is analogous to that in the PGE2-EP2/EP4–mediated Il12rb2 induction during TH1 cell differentiation42 and might be consistent with the findings by Hernandez et al63 showing that the CREB1/CRTC2 pathway regulates expression of IL-17A and IL-17F and that TH17 differentiation is defective in CRTC2 mutant mice. IL-23R and IL-12Rβ2 make a pair with the same molecule, IL-12Rβ1, to form IL-23R and IL-12R, respectively. It is interesting that the same pathway regulates expression of these 2 genes. We have also used T cells from p105 NF-κB1–deficient mice and CTP-NBD and unraveled the involvement of NF-κB in the IL-23/cAMP–induced Il23r expression in TH17 cells. Consistent with these findings, we previously found that PGE2, through EP2 or EP4, activates NF-κB1 containing NF-κB in various types of cells, including macrophages and dendritic cells, and induces expression of inflammation-related genes, including COX2, which then produces PGE2 and amplifies this process.47, 54, 64 Thus our present findings further extend the importance of this COX2–PGE2–EP2/EP4–NF-κB loop to generation of TH17 cell pathogenicity.
On the other hand, Boniface et al44 suggested that PGE2-induced enhancement of Il23R expression in human TH17 cells was mediated by the IL-1β–IL-1 receptor pathway. This is also a possibility in mice because upregulated expression of Il1r1 and Il1b was detected in clusters 1U and 4U by using our microarray analysis (Fig 4, B, left). However, we assume that this mechanism is not critical in our experiment because addition of anti–IL-1β antibody to the medium did not reduce Il23r induction (see Fig E7 in this article's Online Repository at www.jacionline.org).
In addition to Il23r, our microarray analysis has revealed that stimulation of EP2/EP4 signaling together with IL-23 facilitates expression of a variety of pathogenic TH17 signature genes (ie, Il17a, Il17f, Il18r1, and Tgfb3). Interestingly, PGE2-EP2/EP4 signaling also upregulated expression of various genes related to chemotaxis and migration, such as S1pr1, Ccr2, Cxcl3, Cx3cr1, Cxcr4, Sema4f, Sell, Sema3c, and Sema6a (Fig 4, B, left). These results suggest that PGE2-EP2/EP4 signaling can contribute to migration, infiltration, and accumulation of TH17 cells into the inflammatory lesion. On the other hand, the addition of db-cAMP downregulated expression of Il10, Il2, Il4, and Il9, which are known as suppressive factors for TH17 cells. Although some of these results, such as IL-17A, are consistent with the previous findings in human TH17 cells,44 our study did not detect induction of IFN-γ and T-bet in cultured TH17 cells, which might reflect the stages of TH17 cells examined in each study.20, 24, 65 It should also be noted that our analysis was carried out on the whole CD4+ T-cell population pretreated with IL-6 and TGF-β1 and stimulated with each stimulus, in which IL-17A+ cells comprised about 10% of cells. Therefore single-cell RNA sequencing analysis is desired to establish gene expression signatures specific to TH17 cells matured with each stimulus.
Nonetheless, the most important point in our study was that the EP2/EP4 signaling in TH17 cells identified here is critical in eliciting their pathogenicity in vivo in immune inflammation. We tested this issue in an IL-23–induced mouse psoriasis model. Intriguingly, not only the systemic inhibition of EP2/EP4 signaling with the EP4 antagonist in EP2 KO mice but also selective loss of EP2 and EP4 in T cells almost completely suppressed inflammation induced by IL-23. This was accompanied by suppression of accumulation of IL-17A+ and IL-17A+IFN-γ+ T cells and suppression of expression of Il17a, Ifng, and Il23r genes in the lesion. These results suggest that PGE2-EP2/EP4 signaling functions is critical to generation of pathogenic TH17 cells induced by IL-23 in situ. Of those TH17 cells, antigen-specific TH17 cells were shown to be involved specifically in the pathogenesis of mouse models of autoimmune inflammation, including experimental autoimmune encephalomyelitis,66 type 1 diabetes,22 and psoriasis.67 Quite recently, it was also reported that mPGES1 is involved in generation of antigen-specific TH17 cells by regulating PGE2 production in a T-cell autocrine and paracrine manner.68 Our present findings combined with these findings suggest that PGE2 plays an important role in psoriasis through regulation of antigen-specific pathogenic TH17 cells.
The present study also showed that EP2 deficiency and EP4 antagonism significantly suppressed psoriatic inflammation in an imiquimod model. Notably, however, the combined EP2 deficiency and EP4 antagonism did not completely suppress ear swelling in this model, possibly because there is the IL-17–independent component in skin inflammation in this model.8
In this study we also tested the effect of COX inhibitors in an IL-23–induced psoriasis model and found that COX inhibitors are as potent as EP2 and EP4 antagonists in suppressing psoriasis-like skin inflammation in this model. The question is whether COX inhibitors are beneficial in TH17-driven human autoimmune diseases. COX inhibitors, particularly celecoxib, are used for treatment of the early stage of rheumatoid arthritis and in patients with mild psoriatic arthritis.69 In these cases COX inhibitors produce good symptomatic relief. Although this effect is ascribed to their analgesic and general anti-inflammatory actions, our study suggests that it might be derived at least in part from their suppressive action on TH17-mediated pathology, a possibility that should be tested in the future.
On the other hand, COX inhibitors have less appreciable therapeutic benefits in patients with established psoriasis and advanced rheumatoid arthritis in human patients. There are several plausible reasons. A PG-mediated process might be critical in triggering pathogenic TH17 cell generation but not so in advanced stage of diseases that might be regulated dominantly by established TH17 cells. Another reason might be the fact that PGs cause immune inflammation not by acting alone but by working with cytokines and boosting and modifying their actions. Therefore, COX inhibitors might exert therapeutic benefits more effectively when combined with anticytokine drugs and lessen the dose of the latter. Finally, COX inhibitors might divert arachidonate metabolism to leukotriene. Recent studies suggest that leukotrienes facilitate maturation and migration of TH17 cells.70, 71 Further studies need to be conducted to unravel these issues.
Another topic to be discussed on use of PGE2 in patients with psoriasis is its facilitative action in UV irradiation therapy, which at a glance contradicts our present findings on the facilitative action of PGE2 on TH17 pathogenicity. UVB irradiation is an effective therapeutic treatment of psoriasis by inducing immunosuppression.72 We previously showed that UVB induces PGE2 in the epidermis and PGE2-EP4 signaling mediates systemic immunosuppression through upregulation of receptor activator of NF-κB ligand in keratinocytes and inducing regulatory T cells.73 Thus the PGE2-EP4 signaling in this case facilitates immunosuppression and not immune activation. One point is that UVB does not penetrate to the dermis and the events it causes are within the epidermis, whereas IL-23–induced inflammatory events occur in the dermis. Another point is a difference in context, UVB irradiation in the UV therapy and IL-23 in psoriatic inflammation. PGE2 alone does not induce either effect but functions directionally dependent on the context.
Finally, we examined the relevance of our findings to human disease by analyzing biopsy samples from patients with psoriasis. Psoriatic lesional skin overexpressed not only TH17 signature genes, including IL23A, IL12B, IL23R, IL17A, IL17F, IL22, STAT3, and NFKB1, but also those involved in PGE2 biosynthesis and function, such as PTGS2, PTGES, PTGES2, and PTGER4. Expression of TH17 signature genes shows positive correlation with PTGES, PTGES2, and PTGER4 and negative correlation with HPGD and the anti–IL-23 therapy downregulated expression of not only genes in the IL-23/IL-17 pathway (eg, IL23A, IL23R, and IL17A) but also those in PGE2 synthesis, suggesting that these 2 are functionally linked. These findings together with the finding by Kofler et al74 that EP2 is expressed in TH17 cells from patients with multiple sclerosis and that forced expression of EP2 in healthy TH17 cells triggers expression of pathogenic genes indicate that T cell–intrinsic EP2/EP4 signaling is critical in IL-23–driven TH17 cell pathogenesis also in human subjects and support a view that the combined inhibition of EP2 and EP4 is of value in therapeutic intervention of various TH17-mediated diseases.
Key messages.
-
•
IL-23 triggers T cell–intrinsic PGE2-EP2/EP4 signaling that is critical in TH17 cell–mediated immune pathogenesis.
-
•
PGE2-EP2/EP4 signaling functions synergistically with IL-23 and not only amplifies Il23r expression but also induces a unique pathogenic gene expression signature by activating STAT3, CREB1, and NF-κB.
-
•
This PGE2 signaling can be a therapeutic target of TH17 cell–mediated diseases because combined blockade of EP2 and EP4 suppresses IL-23–induced pathogenic TH17 cell generation and consequent psoriatic skin inflammation.
Acknowledgments
We thank A. Kakizuka for suggestions and encouragements, T. Arai for secretarial assistance, and K. Naruo for assistance with animal experiments.
Footnotes
Supported in part by the Core Research for Evolutional Science and Technology Program on Chronic Inflammation (15gm0410006h0006) from the Japan Agency for Medical Research and Development (to S.N.); a Coordination Fund from the Japanese Ministry for Education, Culture, Sports, Science and Technology (MEXT) and Astellas Pharma to Kyoto University (to S.N.); a collaborative grant to Kyoto University from Ono Pharmaceuticals (to S.N.); and Medical Research Council UK (MR/R008167/1 to C.Y.), Cancer Research UK (C63480/A25246 to C.Y.), and Wellcome Trust Institutional Strategic Support Fund (to C.Y.).
Disclosure of potential conflict of interest: T. Aoki, D. Thumkeo, and S. Narumiya were supported by the Coordination Fund from MEXT and Astellas Pharma. S. Narumiya is a scientific advisor to Astellas Pharma. The rest of the authors declare that they have no relevant conflicts of interest.
Contributor Information
Chengcan Yao, Email: Chengcan.Yao@ed.ac.uk.
Shuh Narumiya, Email: snaru@mfour.med.kyoto-u.ac.jp.
Supplementary data
References
- 1.Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat Rev Immunol. 2008;8:337–348. doi: 10.1038/nri2295. [DOI] [PubMed] [Google Scholar]
- 2.Kemper C., Atkinson J.P. T-cell regulation: with complements from innate immunity. Nat Rev Immunol. 2007;7:9–18. doi: 10.1038/nri1994. [DOI] [PubMed] [Google Scholar]
- 3.Korn T., Bettelli E., Oukka M., Kuchroo V.K. IL-17 and Th17 cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 4.Zhu J., Paul W.E. Peripheral CD4+ T-cell differentiation regulated by networks of cytokines and transcription factors. Immunol Rev. 2010;238:247–262. doi: 10.1111/j.1600-065X.2010.00951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cua D.J., Tato C.M. Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol. 2010;10:479–489. doi: 10.1038/nri2800. [DOI] [PubMed] [Google Scholar]
- 6.Murphy C.A., Langrish C.L., Chen Y., Blumenschein W., McClanahan T., Kastelein R.A. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med. 2003;198:1951–1957. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Torchinsky M.B., Blander J.M. T helper 17 cells: discovery, function, and physiological trigger. Cell Mol Life Sci. 2010;67:1407–1421. doi: 10.1007/s00018-009-0248-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Fits L., Mourits S., Voerman J.S., Kant M., Boon L., Laman J.D. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J Immunol. 2009;182:5836–5845. doi: 10.4049/jimmunol.0802999. [DOI] [PubMed] [Google Scholar]
- 9.Yen D., Cheung J., Scheerens H., Poulet F., McClanahan T., McKenzie B. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116:1310–1316. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kopp T., Riedl E., Bangert C., Bowman E.P., Greisenegger E., Horowitz A. Clinical improvement in psoriasis with specific targeting of interleukin-23. Nature. 2015;521:222–226. doi: 10.1038/nature14175. [DOI] [PubMed] [Google Scholar]
- 11.Krueger J.G., Ferris L.K., Menter A., Wagner F., White A., Visvanathan S. Anti-IL-23A mAb BI 655066 for treatment of moderate-to-severe psoriasis: safety, efficacy, pharmacokinetics, and biomarker results of a single-rising-dose, randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol. 2015;136:116–124. doi: 10.1016/j.jaci.2015.01.018. [DOI] [PubMed] [Google Scholar]
- 12.Leonardi C.L., Kimball A.B., Papp K.A., Yeilding N., Guzzo C., Wang Y. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1) Lancet. 2008;371:1665–1674. doi: 10.1016/S0140-6736(08)60725-4. [DOI] [PubMed] [Google Scholar]
- 13.Papp K.A., Langley R.G., Lebwohl M., Krueger G.G., Szapary P., Yeilding N. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2) Lancet. 2008;371:1675–1684. doi: 10.1016/S0140-6736(08)60726-6. [DOI] [PubMed] [Google Scholar]
- 14.Teng M.W., Bowman E.P., McElwee J.J., Smyth M.J., Casanova J.L., Cooper A.M. IL-12 and IL-23 cytokines: from discovery to targeted therapies for immune-mediated inflammatory diseases. Nat Med. 2015;21:719–729. doi: 10.1038/nm.3895. [DOI] [PubMed] [Google Scholar]
- 15.Ivanov, McKenzie B.S., Zhou L., Tadokoro C.E., Lepelley A., Lafaille J.J. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 16.Morishima N., Mizoguchi I., Takeda K., Mizuguchi J., Yoshimoto Y. TGF-beta is necessary for induction of IL-23R and Th17 differentiation by IL-6 and IL-23. Biochem Biophys Res Commun. 2009;386:105–110. doi: 10.1016/j.bbrc.2009.05.140. [DOI] [PubMed] [Google Scholar]
- 17.Yang X.O., Pappu B.P., Nurieva R., Akimzhanov A., Kang H.S., Chung Y. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou L., Ivanov, Spolski R., Min R., Shenderov K., Egawa T. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 19.Zhou L., Lopes J.E., Chong M.M., Ivanov, Min R., Victora G.D. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453:236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee Y., Awasthi A., Yosef N., Quintana F.J., Xiao S., Peters A. Induction and molecular signature of pathogenic TH17 cells. Nat Immunol. 2012;13:991–999. doi: 10.1038/ni.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gagliani N., Amezcua Vesely M.C., Iseppon A., Brockmann L., Xu H., Palm N.W. Th17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature. 2015;523:221–225. doi: 10.1038/nature14452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li C.R., Mueller E.E., Bradley L.M. Islet antigen-specific Th17 cells can induce TNF-α dependent autoimmune diabetes. J Immunol. 2014;192:1425–1432. doi: 10.4049/jimmunol.1301742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stockinger B., Omenetti S. The dichotomous nature of T helper 17 cells. Nat Rev Immunol. 2017;17:535–544. doi: 10.1038/nri.2017.50. [DOI] [PubMed] [Google Scholar]
- 24.Wu C., Yosef N., Thalhamer T., Zhu C., Xiao S., Kishi Y. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature. 2013;496:513–517. doi: 10.1038/nature11984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horste G.M., Wu C., Wang C., Cong L., Pawlak M., Lee Y. RBPJ controls development of pathogenic Th17 cells by regulating IL-23 receptor expression. Cell Rep. 2016;16:392–404. doi: 10.1016/j.celrep.2016.05.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahern P.P., Schiering C., Buonocore S., McGeachy M.J., Cua D.J., Maloy K.J. Interleukin-23 drives intestinal inflammation through direct activity on T cells. Immunity. 2010;33:279–288. doi: 10.1016/j.immuni.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harbour S.N., Maynard C.L., Zindl C.L., Schoeb T.R., Weaver C.T. Th17 cells give rise to Th1 cells that are required for the pathogenesis of colitis. Proc Natl Acad Sci U S A. 2015;112:7061–7066. doi: 10.1073/pnas.1415675112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anderson C.A., Boucher G., Lees C.W., Franke A., D'Amato M., Taylor K.D. Meta-analysis identifies 29 additional ulcerative colitis risk loci, increasing the number of confirmed associations to 47. Nat Genet. 2011;43:246–252. doi: 10.1038/ng.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Franke A., McGovern D.P., Barrett J.C., Wang K., Radford-Smith G.L., Ahmad T. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn's disease susceptibility loci. Nat Genet. 2010;42:1118–1125. doi: 10.1038/ng.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stuart P.E., Nair R.P., Tsoi L.C., Tejasvi T., Das S., Kang H.M. Genome-wide association analysis of psoriatic arthritis and cutaneous psoriasis reveals differences in their genetic architecture. Am J Hum Genet. 2015;97:816–836. doi: 10.1016/j.ajhg.2015.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burkett P.R., Meyer zu Horste G., Kuchroo V.K. Pouring fuel on the fire: Th17 cells, the environment, and autoimmunity. J Clin Invest. 2015;125:2211–2219. doi: 10.1172/JCI78085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gaffen S.L., Jain R., Garg A.V., Cua D.J. The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nat Rev Immunol. 2014;14:585–600. doi: 10.1038/nri3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boyman O., Comte D., Spertini F. Adverse reactions to biologic agents and their medical management. Nat Rev Rheumatol. 2014;10:612–627. doi: 10.1038/nrrheum.2014.123. [DOI] [PubMed] [Google Scholar]
- 34.Hawkes J.E., Chan T.C., Krueger J.G. Psoriasis pathogenesis and the development of novel targeted immune therapies. J Allergy Clin Immunol. 2017;140:645–653. doi: 10.1016/j.jaci.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sandborn W.J., Ghosh S., Panes J., Vranic I., Su C., Rousell S. Tofacitinib, an oral janus kinase inhibitor, in active ulcerative colitis. N Engl J Med. 2012;367:616–624. doi: 10.1056/NEJMoa1112168. [DOI] [PubMed] [Google Scholar]
- 36.Hirata T., Narumiya S. Prostanoid receptors. Chem Rev. 2011;111:6209–6230. doi: 10.1021/cr200010h. [DOI] [PubMed] [Google Scholar]
- 37.Harris S.G., Padilla J., Koumas L., Ray D., Phipps R.P. Prostaglandins as modulators of immunity. Trends Immunol. 2002;23:144–150. doi: 10.1016/s1471-4906(01)02154-8. [DOI] [PubMed] [Google Scholar]
- 38.Sreeramkumar V., Fresno M., Cuesta N. Prostaglandin E2 and T cells: friends or foes? Immunol Cell Biol. 2012;90:579–586. doi: 10.1038/icb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aoki T., Narumiya S. Prostaglandins and chronic inflammation. Trends Pharmacol Sci. 2012;33:304–311. doi: 10.1016/j.tips.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 40.Hirata T., Narumiya S. Prostanoids as regulators of innate and adaptive immunity. Adv Immunol. 2012;116:143–174. doi: 10.1016/B978-0-12-394300-2.00005-3. [DOI] [PubMed] [Google Scholar]
- 41.Sakata D., Yao C., Narumiya S. Prostaglandin E2, an immunoactivator. J Pharmacol Sci. 2010;112:1–5. doi: 10.1254/jphs.09r03cp. [DOI] [PubMed] [Google Scholar]
- 42.Yao C., Hirata T., Soontrapa K., Ma X., Takemori H., Narumiya S. Prostaglandin E(2) promotes Th1 differentiation via synergistic amplification of IL-12 signalling by cAMP and PI3-kinase. Nat Commun. 2013;4:1685. doi: 10.1038/ncomms2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yao C., Sakata D., Esaki Y., Li Y., Matsuoka T., Kuroiwa K. Prostaglandin E2-EP4 signaling promotes immune inflammation through Th1 cell differentiation and Th17 cell expansion. Nat Med. 2009;15:633–640. doi: 10.1038/nm.1968. [DOI] [PubMed] [Google Scholar]
- 44.Boniface K., Bak-Jensen K.S., Li Y., Blumenschein W.M., McGeachy M.J., McClanahan T.K. Prostaglandin E2 regulates Th17 cell differentiation and function through cyclic AMP and EP2/EP4 receptor signaling. J Exp Med. 2009;206:535–548. doi: 10.1084/jem.20082293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Napolitani G., Acosta-Rodriguez E.V., Lanzavecchia A., Sallusto F. Prostaglandin E2 enhances Th17 responses via modulation of IL-17 and IFN-gamma production by memory CD4+ T cells. Eur J Immunol. 2009;39:1301–1312. doi: 10.1002/eji.200838969. [DOI] [PubMed] [Google Scholar]
- 46.Hizaki H., Segi E., Sugimoto Y., Hirose M., Saji T., Ushikubi F. Abortive expansion of the cumulus and impaired fertility in mice lacking the prostaglandin E receptor subtype EP(2) Proc Natl Acad Sci U S A. 1999;96:10501–10506. doi: 10.1073/pnas.96.18.10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aoki T., Frosen J., Fukuda M., Bando K., Shioi G., Tsuji K. Prostaglandin E2-EP2-NF-kappaB signaling in macrophages as a potential therapeutic target for intracranial aneurysms. Sci Signal. 2017;10 doi: 10.1126/scisignal.aah6037. [DOI] [PubMed] [Google Scholar]
- 48.Schneider A., Guan Y., Zhang Y., Magnuson M.A., Pettepher C., Loftin C.D. Generation of a conditional allele of the mouse prostaglandin EP4 receptor. Genesis. 2004;40:7–14. doi: 10.1002/gene.20048. [DOI] [PubMed] [Google Scholar]
- 49.Zenkoh T., Nozawa E., Matsuura M., Seo R. Ornithine derivative. Google Patents. 2008 [Google Scholar]
- 50.Ohno K., Fujiwara M., Fukushima M., Narumiya S. Metabolic dehydration of prostaglandin E2 and cellular uptake of the dehydration product: correlation with prostaglandin E2-induced growth inhibition. Biochem Biophys Res Commun. 1986;139:808–815. doi: 10.1016/s0006-291x(86)80062-6. [DOI] [PubMed] [Google Scholar]
- 51.Aoki T., Nishimura M., Matsuoka T., Yamamoto K., Furuyashiki T., Kataoka H. PGE2-EP2 signalling in endothelium is activated by haemodynamic stress and induces cerebral aneurysm through an amplifying loop via NF-κB. Br J Pharmacol. 2011;163:1237–1249. doi: 10.1111/j.1476-5381.2011.01358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Che Mat N.F., Zhang X., Guzzo C., Gee K. Interleukin-23-induced interleukin-23 receptor subunit expression is mediated by the Janus kinase/signal transducer and activation of transcription pathway in human CD4 T cells. J Interferon Cytokine Res. 2011;31:363–371. doi: 10.1089/jir.2010.0083. [DOI] [PubMed] [Google Scholar]
- 53.Best J.L., Amezcua C.A., Mayr B., Flechner L., Murawsky C.M., Emerson B. Identification of small-molecule antagonists that inhibit an activator: coactivator interaction. Proc Natl Acad Sci U S A. 2004;101:17622–17627. doi: 10.1073/pnas.0406374101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ma X., Aoki T., Narumiya S. Prostaglandin E2-EP4 signaling persistently amplifies CD40-mediated induction of IL-23 p19 expression through canonical and non-canonical NF-kappaB pathways. Cell Mol Immunol. 2016;13:240–250. doi: 10.1038/cmi.2015.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Christian F., Smith E.L., Carmody R.J. The regulation of NF-kappaB subunits by phosphorylation. Cells. 2016;5:12. doi: 10.3390/cells5010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lowes M.A., Russell C.B., Martin D.A., Towne J.E., Krueger J.G. The IL-23/T17 pathogenic axis in psoriasis is amplified by keratinocyte responses. Trends Immunol. 2013;34:174–181. doi: 10.1016/j.it.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rizzo H.L., Kagami S., Phillips K.G., Kurtz S.E., Jacques S.L., Blauvelt A. IL-23-mediated psoriasis-like epidermal hyperplasia is dependent on IL-17A. J Immunol. 2011;186:1495–1502. doi: 10.4049/jimmunol.1001001. [DOI] [PubMed] [Google Scholar]
- 58.Reilly D.M., Parslew R., Sharpe G.R., Powell S., Green M.R. Inflammatory mediators in normal, sensitive and diseased skin types. Acta Derm Venereol. 2000;80:171–174. doi: 10.1080/000155500750042907. [DOI] [PubMed] [Google Scholar]
- 59.Lowes M.A., Kikuchi T., Fuentes-Duculan J., Cardinale I., Zaba L.C., Haider A.S. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol. 2008;128:1207–1211. doi: 10.1038/sj.jid.5701213. [DOI] [PubMed] [Google Scholar]
- 60.R1 Duhen, Glatigny S., Arbelaez C.A., Blair T.C., Oukka M., Bettelli E. Pathogenicity of IFN-γ-producing Th17 cells is independent of T-bet. J Immunol. 2013;190:4478–4482. doi: 10.4049/jimmunol.1203172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nair R.P., Duffin K.C., Helms C., Ding J., Stuart P.E., Goldgar D. Genome-wide scan reveals association of psoriasis with IL-23 and NF-kappaB pathways. Nat Genet. 2009;41:199–204. doi: 10.1038/ng.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sofen H., Smith S., Matheson R.T., Leonardi C.L., Calderon C., Brodmerkel C. Guselkumab (an IL-23-specific mAb) demonstrates clinical and molecular response in patients with moderate-to-severe psoriasis. J Allergy Clin Immunol. 2014;133:1032–1040. doi: 10.1016/j.jaci.2014.01.025. [DOI] [PubMed] [Google Scholar]
- 63.Hernandez J.B., Chang C., LeBlanc M., Grimm D., Le Lay J., Kaestner K.H. The CREB/CRTC2 pathway modulates autoimmune disease by promoting Th17 differentiation. Nat Commun. 2015;6:7216. doi: 10.1038/ncomms8216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ma X., Aoki T., Tsuruyama T., Narumiya S. Definition of prostaglandin E2-EP2 signals in the colon tumor microenvironment that amplify inflammation and tumor growth. Cancer Res. 2015;75:2822–2832. doi: 10.1158/0008-5472.CAN-15-0125. [DOI] [PubMed] [Google Scholar]
- 65.McGeachy M.J., Chen Y., Tato C.M., Laurence A., Joyce-Shaikh B., Blumenschein W.M. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol. 2009;10:314–324. doi: 10.1038/ni.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Korn T., Mitsdoerffer M., Croxford A.L., Awasthi A., Dardalhon V.A., Galileos G. IL-6 controls Th17 immunity in vivo by inhibiting the conversion of conventional T cells into Foxp3+ regulatory T cells. Proc Natl Acad Sci U S A. 2008;105:18460–18465. doi: 10.1073/pnas.0809850105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nishimoto S., Kotani H., Tsuruta S., Shimizu N., Ito M., Shichita T. Th17 cells carrying TCR recognizing epidermal autoantigen induce psoriasis-like skin inflammation. J Immunol. 2013;191:3065–3072. doi: 10.4049/jimmunol.1300348. [DOI] [PubMed] [Google Scholar]
- 68.Maseda D., Johnson E.M., Nyhoff L.E., Baron B., Kojima F., Wilhelm A.J. mPGES1-dependent prostaglandin E2 (PGE2) controls antigen-specific Th17 and Th1 responses by regulating T autocrine and paracrine PGE2 production. J Immunol. 2018;200:725–736. doi: 10.4049/jimmunol.1601808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Menter A., Gottlieb A., Feldman S.R., Van Voorhees A.S., Leonardi C.L., Gordon K.B. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826–850. doi: 10.1016/j.jaad.2008.02.039. [DOI] [PubMed] [Google Scholar]
- 70.Lee W., Kim H.S., Lee G.R. Leukotrienes induce the migration of Th17 cells. Immunol Cell Biol. 2015;93:472–479. doi: 10.1038/icb.2014.104. [DOI] [PubMed] [Google Scholar]
- 71.Chen H., Qin J., Wei P., Zhang J., Li Q., Fu L. Effects of leukotriene B4 and prostaglandin E2 on the differentiation of murine Foxp3+ T regulatory cells and Th17 cells. Prostaglandins Leukot Essent Fatty Acids. 2009;80:195–200. doi: 10.1016/j.plefa.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 72.Nakamura M., Farahnik B., Bhutani T. Recent advances in phototherapy for psoriasis. F1000 Res. 2016;5:1684. doi: 10.12688/f1000research.8846.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Soontrapa K., Honda T., Sakata D., Yao C., Hirata T., Hori S. Prostaglandin E2–prostoglandin E receptor subtype 4 (EP4) signaling mediates UV irradiation-induced systemic immunosuppression. Proc Natl Acad Sci U S A. 2011;108:6668–6673. doi: 10.1073/pnas.1018625108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kofler D.M., Marson A., Dominguez-Villar M., Xiao S., Kuchroo V.K., Hafler D.A. Decreased RORC-dependent silencing of prostaglandin receptor EP2 induces autoimmune Th17 cells. J Clin Invest. 2014;124:2513–2522. doi: 10.1172/JCI72973. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.