Abstract
Background
Germline and tumor pharmacogenomics impact drug responses, but germline markers less commonly guide oncology prescribing. We hypothesized that a critical number of clinically actionable germline pharmacogenomic associations exist, representing clinical implementation opportunities.
Methods
We analyzed 125 oncology drugs for positive germline pharmacogenomic associations in journals with impact factors ≥5. Studies were assessed for design and genotyping quality, clinically-relevant outcomes, statistical rigor, and evidence of drug-gene effects. Associations from studies of high methodologic quality were deemed potentially clinically actionable, and translational summaries were written as point-of-care clinical decision support (CDS) tools and formally evaluated using the Appraisal of Guidelines for Research and Evaluation (AGREE) II instrument.
Results
We identified germline pharmacogenomic results for 56/125 (45%) oncology drugs across 173 publications. Actionable associations were detected for 12 drugs, including six with germline pharmacogenomic information within Food and Drug Administration labels or published guidelines (capecitabine/fluorouracil/DPYD, irinotecan/UGT1A1, mercaptopurine/thioguanine/TPMT, tamoxifen/CYP2D6), while six others were novel (asparaginase/NFACT2/HLA-DRB1, cisplatin/ACYP2, doxorubicin/ABCC2/RAC2, lapatinib/HLA-DQA1, sunitinib/CYP3A5, vincristine/CEP72). Using AGREE II, developed CDS summaries had high scores (mean ± standard deviation [SD]; maximum score=100) for Scope and Purpose (92.7 ± 5.1) and Rigour of Development (87.6 ± 7.4) and moderate, yet robust scores for Clarity of Presentation (58.6 ± 25.1) and Applicability (55.9 ± 24.6). Overall mean guideline quality score was 5.2 ± 1.0 (maximum score=7). Germline pharmacogenomic CDS summaries for these 12 drugs were recommended for implementation.
Conclusion
A number of oncology drugs have actionable germline pharmacogenomic information, justifying delivery through institutional pharmacogenomic implementations, to determine clinical utility.
Keywords: clinical decision support, germline mutation, pharmacogenetics, pharmacogenomic variants, precision medicine
Introduction
The discipline of pharmacogenomics aims to identify genetic variants that contribute to individual drug response in order to reduce adverse drug reactions and increase drug efficacy. Pharmacogenomic information plays a unique role in cancer therapy because both the tumor (somatic) genome and the patient’s germline genome can impact drug response1. Further, as the consequences of drug toxicity can sometimes be particularly life-threatening in oncology, using pharmacogenomics to prevent such events is desirable2. There is, indeed, a rich history in oncology of germline pharmacogenomic variants playing a role in serious and life-threatening toxicities. Traditionally, germline variants of interest include those of drug-metabolizing enzymes, transporters, and other proteins involved in a drug’s mechanism of action3, as such proteins are critical in determining the efficacy and toxicity of many chemotherapeutic agents4. Such germline pharmacogenomic information has been incorporated into Food and Drug Administration (FDA) labels for capecitabine5, fluorouracil6, irinotecan7, 6-mercaptopurine8, and thioguanine9, and Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines have been written for capecitabine/fluorouracil10, mercaptopurine/thioguanine11, and tamoxifen12. While these well-recognized examples already have established clinical implementation guidelines, other potential germline pharmacogenomic associations with strong supporting evidence have yet to be translated into clinical practice.
Increasingly, tumor pharmacogenomic information is incorporated into oncologic clinical decision-making13, and in some cases the use of oncology drugs is restricted to patients carrying explicit tumor mutations14,15. Germline pharmacogenomic variants, in the more traditional sense (those involved in drug metabolism or mechanisms of action), less commonly guide oncology prescribing. Despite fervent, ongoing discovery research in the field, the current number of high-level, actionable germline pharmacogenomic markers in oncology is less clear than that for tumor genomics16, and has either been outpaced by or overshadowed by the routine clinical utilization of somatic markers. Regardless, incorporating actionable information about both tumor and germline genomics into cancer treatment plans has the potential to improve patient outcomes, yet clear recommendations or standardized guidance is rarely available.
We aimed to critically appraise the current germline pharmacogenomic discoveries in oncology using a prospective methodology to discover whether additional germline pharmacogenomic markers have sufficient evidence for clinical implementation. We sought to identify replicated, high-level evidence associations for which translation into clinical decision support (CDS) guidelines is warranted. Simultaneously, we aimed to identify associations for which intriguing germline pharmacogenomic data exist, yet evidence in support of implementation may be limited due to methodological limitations of current studies. We hypothesized that the findings will enable clinical consideration of germline variants and facilitate future examinations of clinical utility in practice.
Methods
Data Collection
A total of 125 commonly prescribed cancer drugs were included17 (Supplementary Table 1). An automated search algorithm of “[drug name] polymorphism” in PubMed was used to identify pharmacogenomic publications for each drug. Articles examining the association between a germline genetic variant and a pharmacogenomic outcome were included. Specific exclusion criteria have been previously published18 and are described in the Supplementary Methods. Drug-genetic associations reported as being nominally statistically significant by the authors were recorded at first-pass as “positive” in the database, while non-significant associations were labeled “negative.”
All positive associations in a journal with a five-year impact factor (IF)≥5 were taken forward for critical analysis (described below). To ensure that no important publications were missed by the automated PubMed algorithm, in February 2017 each drug was also manually searched in PubMed using our updated search string18 (see Supplementary Methods). The same process of manual inclusion/exclusion was performed for any additionally identified studies from journals with IF ≥8.5 since the purpose of this second search was to identify any additional major findings (emphasizing specificity, whereas the first search emphasized sensitivity).
Data Analysis
Each positive drug-genetic association from the included articles was assessed using our previously described methodology19 to determine whether the association was potentially clinically actionable (see also Supplementary Methods). Studies employing multi-drug regimens were generally excluded from critical analysis, since direct attribution of a genetic association with a single drug could not be made. Exceptions were made for publications with specific drug-genetic pairs for which the variant or gene was implicated in a particular drug’s activity or if a toxicity outcome studied was known to be associated with one particular drug. Similarly, studies reporting only associations with multi-variant genetic haplotypes were excluded from our analysis, as we were interested in identifying single polymorphisms that could be clinically assessed. However, gene-level associations employed for CYP2D6 or other similar genes with known enzymatic phenotypes (e.g., “poor metabolizer”/”rapid metabolizer”) were included.
The presence of large cohort sizes, control populations, high quality phenotype measurements, treatment homogeneity amongst study subjects, and appropriate statistical measures all increased support for clinical actionability. Drug-genetic pairs that were not statistically significant after multiple testing corrections were not deemed clinically actionable unless another well-performed study supported the same genotype-phenotype association. Generally, drug-genetic pairs were also deemed not actionable if associated with only prognostic outcomes. Publications that studied response but included stable disease in the definition of clinical response were deemed not actionable. Associations from genome-wide association studies that did not meet genome-wide significance were not deemed actionable unless convincing replication or functional data existed.
Two independent reviewers considered the resulting drug-genetic pairs from the above analysis. Dedicated, manual “[drug name] [variant rs number]” PubMed searches were separately conducted for each as part of this final step to ensure that no studies were missed. Complementary to this comprehensive analysis, consideration of FDA label information, CPIC guidelines, and other published guidance (e.g., PharmGKB, Dutch Pharmacogenomics Working Group) was given. Capecitabine5, fluorouracil6, irinotecan7, mercaptopurine8, and thioguanine9 have germline pharmacogenomic information containing a recommended clinical action already incorporated into FDA labels. Capecitabine, fluorouracil, mercaptopurine, tamoxifen, and thioguanine have published CPIC guidelines10–12. Clinically actionable information for these six drugs, along with potentially actionable information for any other drugs that emerged from our comprehensive analysis, was taken forward for development into draft CDS summaries.
Finally, we considered germline genetic findings that represent gene-disease associations but which may also directly guide the prescribing of certain oncology drugs. The FDA Table of Pharmacogenomic Biomarkers in Drug Labeling14 was analyzed for oncology drugs with germline gene-disease information included in the package labeling. We reviewed each drug label for actionable prescribing recommendations based on germline disease variants.
CDS Summary Development
CDS summaries that translated genetic information into point-of-care guidance were independently written by a member or members of the CDS development team using methods previously described18 (see also Supplementary Methods). Resulting draft CDS were independently reviewed by two members of the evidence evaluation team (R.W. and P.H.O.) and were then subjected to formal Appraisal of Guidelines for Research and Evaluation (AGREE) II scoring.
AGREE II Scoring
We used a modified AGREE II20 scoring instrument to determine whether each draft CDS summary warranted clinical implementation (see Supplementary Methods for full details). Our modified AGREE II instrument included the specific items from the domains of Scope and Purpose, Rigour of Development, Clarity of Presentation, and Applicability. Four independent appraisers (R.N., B.P., W.M.S., M.J.R.) rated each draft summary on all four domains, gave each an overall score, and voted (independently) whether the summary deserved deployment as a clinical guideline. The AGREE appraisal of whether to recommend or not was used as the final determination for inclusion into our institutional pharmacogenomic program21. Unless a summary received unanimous agreement in favor of clinical deployment, it was not clinically implemented.
Results
Study Demographics
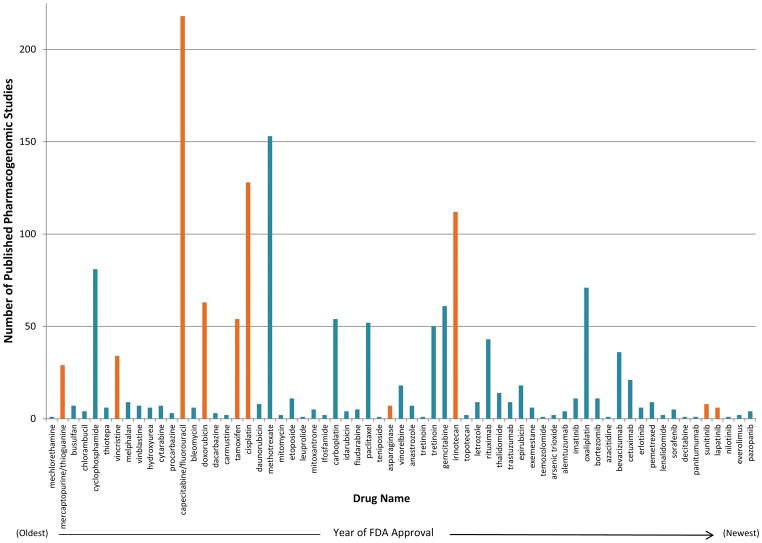
Of the 125 drugs evaluated, 67 (53.6%) had ≥1 published pharmacogenomic study, regardless of journal IF. We first examined the number of pharmacogenomic publications/drug according to drug approval year (Figure 1). We did not detect any trends suggesting that time since FDA approval was correlated with number of pharmacogenomic publications. Instead, there are a relatively small number of oncology drugs for which a large amount of pharmacogenomic research has been performed. In total, 19/67 drugs (28.4%) have >20 published pharmacogenomic studies. Publications describing ≥1 positive genetic association vastly outweighed the number of publications reporting only negative associations (Supplementary Figure 1).
Figure 1. Number of Published Pharmacogenomic Studies per Drug in Order of FDA Approval Year.
Of the 125 oncology drugs evaluated, 67 (53.6%) had at least 1 published pharmacogenomic study, regardless of journal impact factor. In total, 19 of the 67 drugs (28.4%) have more than 20 published pharmacogenomic studies. We did not detect any trends suggesting that time since FDA approval is correlated with the amount of pharmacogenomic data published. Instead, the data suggest that there are a relatively small number of oncology drugs for which a large amount of pharmacogenomic research has been performed. Drugs deemed as being potentially clinically actionable (with a clinical decision support summary sent for AGREE scoring) are shown in orange.
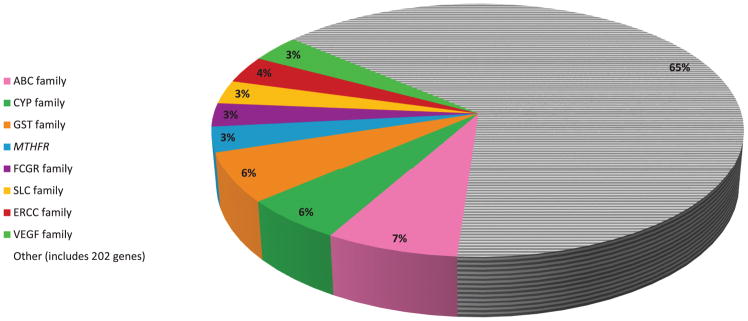
Of the drugs evaluated, 56 (44.8%) were reported to have positive oncologic pharmacogenomic associations in journals with IF ≥5 (Supplementary Table 2). These 56 drugs were supported by an average of 8 publications/drug (range: 1–72), representing 173 unique high-impact publications initially critically appraised. In total, 154 were brought forward for further analyses (see Supplementary Results). Overall, 246 genes were reported as positive pharmacogenomic findings in the included publications, comprising 436 unique gene-publication pairs. Many genes were studied in multiple publications. In fact, we found that 35.1% of these unique gene-publication pairs were comprised of a relatively small list of key pharmacogenes (Figure 2). These included the ABC, CYP, GST, FCGR, SLC, ERCC, and VEGF gene families22, as well as the MTHFR gene.
Figure 2. Genes/Gene Families Represented in Positive Pharmacogenomic Associations.
Overall, 246 genes were found to have positive pharmacogenomic associations in our 154 critically analyzed publications, comprising 436 unique gene-publication pairs. Many genes were studied in multiple publications. In fact, we found that over a third (35.1%) of the unique gene-publication pairs were comprised of a relatively small list of key pharmacogenes. These included the ABC, CYP, GST, FCGR, SLC, ERCC, and VEGF gene families, as well as the MTHFR gene. Each gene/gene family on the figure is represented as a percentage of the 436 total gene-publication pairs.
Supplementary Figure 2 displays the 6 most common clinical outcomes analyzed for pharmacogenomic association. Progression-free survival (including disease-free, event-free, recurrence-free, and relapse-free survival) was the most common clinical outcome studied across the critically analyzed studies [in 56/154 publications (36.4%)]. Overall survival and response rate were analyzed in 29.2% and 27.3% of studies, respectively (see Supplementary Results for additional details).
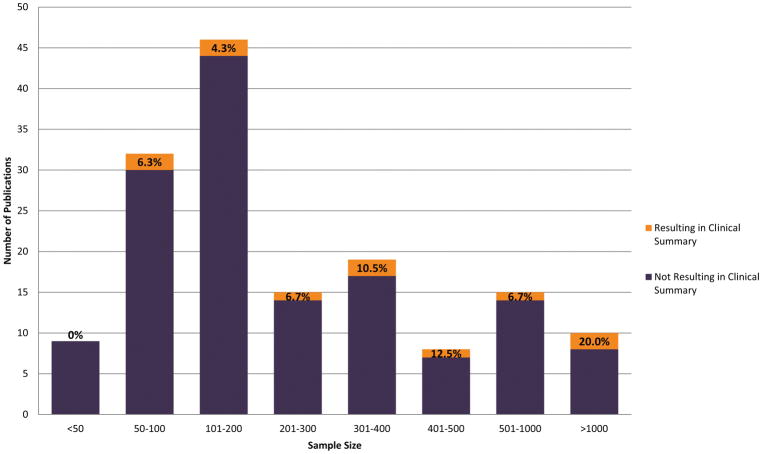
The sample size distribution of the publications analyzed is displayed in Figure 3. Sizes ranged from 6–4925 (median=179 patients). The highest percentage of studies (29.9%) had sample sizes between 101–200; interestingly, only 4.3% of studies in this range resulted in a draft CDS summary (see below). In contrast, only 6.5% of analyzed studies had sample sizes >1000 patients, and 20.0% of these resulted in a draft CDS summary.
Figure 3. Sample Size Distribution of 154 Publications Analyzed.
Sample sizes ranged from 6 to 4925, and the median sample size was 179. The highest percentage of studies (29.9%) had sample sizes in the range between 101 and 200; only 4.3% of studies in this range resulted in an ultimate draft clinical decision support summary. On the contrary, only 6.5% of analyzed studies had sample sizes greater than 1000 patients, and 20.0% of these resulted in a draft clinical decision support summary. Orange shading highlights the percentage of studies for each sample size group (i.e. 101–200, 201–300, etc.) that resulted in a clinical decision support summary.
Out of the publications critically assessed, 11/154 (7.1%) ultimately described drug-genetic pairs that resulted in draft CDS summaries. Detailed reasons explaining why the remaining 143 publications were deemed not actionable (and did not result in a summary) are available in the Supplementary Results.
Potentially Clinically Actionable Associations
Our critical analysis resulted in 12 drugs with genetic information deemed potentially clinically actionable. These highest level pharmacogenomic results are shown in Table 1. Six unique drugs–asparaginase, cisplatin, doxorubicin, lapatinib, sunitinib, and vincristine–were identified as having novel, potentially clinically actionable pharmacogenomic information through our analysis. An additional 13 drugs not currently clinically actionable were deemed to be deserving of future follow-up (see Supplementary Table 3 and Supplementary Results). Consideration of germline gene-disease (as distinct from the above gene-drug) associations that also directly impact oncology prescribing revealed three additional drugs with actionable prescribing information included in their FDA labeling: olaparib/BRCA, rucaparib/BRCA, and dabrafenib/G6PD (Supplementary Table 4).
Table 1.
Clinically Actionable Associations Detected in Critical Analysis
| Drug(s) | No. of PGx Publication s |
No. of Positive Publications in High IF Journals |
Clinically Actionable Gene(s)/Variant(s) |
Gene(s) | Phenotype | Supporting Publication(s) |
Sample Size |
Effect Size |
Recommended Clinical Action |
|---|---|---|---|---|---|---|---|---|---|
| asparaginase | 7 | 5 | rs6021191, rs17885382 | NFATC2, HLA-DRB1 | hypersensitivity | Fernandez et al. Blood. 2015;126(1):69–75 | 589 | OR=3.1 | • Closer monitoring for hypersensitivity is strongly recommended for those carrying any risk alleles at either of the specified loci. |
| capecitabine/fluorouracil | 218 | 72 | DPD deficient | DPYD | systemic toxicity | FDA label5,6/CPIC guideline10 | • For those with decreased DPD activity, decrease dose by 25–50%. • For those with complete DPD deficiency, avoid use of fluoropyrimidine drugs. |
||
| cisplatin | 128 | 28 | rs1872328 | ACYP2 | ototoxicity | Xu et al. Nat Genet. 2015;47(3):263–266, Vos et al. Pharmacogenet Genomics. 2016;26(5):243–247 | 306, 156 | HR=4.5 | • Closer monitoring for ototoxicity is strongly recommended for patients carrying the risk allele (heterozygotes and homozygotes). |
| doxorubicin | 63 | 19 | rs1883112 | NCF4 | cardiotoxicity | Wojnowski et al. Circulation. 2005;112(24):3754–3762, Rossi et al. Leukemia. 2009;23(6):1118–1126 | 450, 106 | OR=2.5 | • For carriers of one risk allele, no modifications are warranted. • For carriers of two risks alleles, closer monitoring for cardiotoxicity is strongly recommended. |
| rs8187710 | ABCC2 | cardiotoxicity | Wojnowski et al. Circulation. 2005;112(24):3754–3762, Armenian et al. Br J Haematol. 2013;163(2):205–213 | 450, 255 | OR=4.3 | • Closer monitoring for cardiotoxicity is strongly recommended for patients carrying the risk allele (heterozygotes and homozygotes). | |||
| rs13058338 | RAC2 | OR=2.8 | • Closer monitoring for cardiotoxicity is strongly recommended for patients carrying the risk allele (heterozygotes and homozygotes). | ||||||
| irinotecan | 112 | 38 | UGT1A1*28 | UGT1A1 | toxicity (primarily diarrhea and neutropenia) | FDA label7 | • For those carrying one risk allele, monitor closely for toxicities. • For those homozygous for the risk allele, reduce starting dose. |
||
| lapatinib | 6 | 4 | HLA-DQA1*02:01 | HLA | ALT elevation | Spraggs et al. J Clin Oncol. 2011;29(6):667–673, Schaid et al. J Clin Oncol. 2014;32(22):2296–2303; see also FDA label† | 1275, 1194 | OR=14.1 | • Closer monitoring for hepatotoxicity is strongly recommended for patients carrying the risk allele (heterozygotes and homozygotes). |
| mercaptopurine/thioguanine | 29 | 6 | IM/PM | TPMT | myelosuppression | FDA label8,9/CPIC guideline11 | • For intermediate metabolizers, decrease starting dose by 30–70% and adjust doses based on degree of myelosuppression. • For poor metabolizers, drastically decrease starting dose and dosing frequency and adjust doses based on degree of myelosuppression. |
||
| sunitinib | 8 | 4 | rs307826 | VEGFR3 | PFS, RR | Garcia-Donas et al. Lancet Oncol. 2011;12(12):1143–1150 | 95 | HR=8.8 | • Not recommended for clinical implementation at this time. |
| rs776746 | CYP3A5 | toxicity-induced dose reduction | HR=3.8 | • For those carrying the risk allele, closer monitoring for toxicities is strongly recommended. Earlier dose reduction may be required. | |||||
| tamoxifen | 54 | 22 | UM/NM/IM/PM | CYP2D6 | recurrence, DFS, RFS, DRFS, BCSS, OS | CPIC guideline12 | • For intermediate metabolizers, consider an alternative drug (e.g., an aromatase inhibitor), or an increased tamoxifen dose if aromatase inhibitors are contraindicated. • For poor metabolizers, an alternative drug is recommended (e.g., an aromatase inhibitor). An increased tamoxifen dose could be considered if aromatase inhibitors are contraindicated. |
||
| vincristine | 34 | 13 | rs924607 | CEP72 | peripheral neuropathy | Diouf et al. JAMA. 2015;313(8):815–823, Stock et al. Clin Pharmacol Ther. 2017;101(3):391–395 | 321, 96 | OR=4.3 | • For carriers of one risk allele, no modifications are warranted. • For carriers of two risk alleles, closer monitoring for neuropathy is strongly recommended. |
PGx=pharmacogenomics/IF=impact factor/OR=odds ratio/HR=hazard ratio/PFS=progression-free survival/RR=response rate/DFS=disease-free survival/RFS=recurrence-free survival/DRFS=distant relapse-free survival/BCSS=breast cancer-specific survival/OS=overall survival/UM=ultrarapid metabolizer/NM=normal metabolizer/IM=intermediate metabolizer/PM=poor metabolizer
The Food and Drug Administration label for lapatinib indicates that HLA-DQA1*02:01 has been associated with hepatotoxicity, but a specific clinical recommendation/action is not provided in the FDA label.
AGREE II Analysis Results
Draft CDS summaries for the potentially clinical actionable drug-genetic pairs were developed and then subjected to final, formal AGREE appraisal. The AGREE scores for each CDS summary, the overall mean ± SD scores for each domain, and the ultimate determination surrounding clinical actionability are displayed in Table 2. Of the CDS guidelines that were written for the six drug-genetic pairs that were a priori denoted as deserving CDS based on FDA/CPIC designations, the mean ± SD scores were: Domain 1 (Scope and Purpose): 97.6 ± 1.9 (range, 95.8–100.0); Domain 3 (Rigour of Development): 93.2 ± 9.1 (range, 79.6–98.6); Domain 4 (Clarity of Presentation): 90.9 ± 8.0 (range, 81.5–98.6); and Domain 5 (Applicability): 86.5 ± 4.0 (range, 80.6–88.9). Additionally, the mean ± SD overall quality score for these CDS guidelines was 6.5 ± 0.6 (range 5.7–7.0). Notably, the summaries for capecitabine/fluorouracil (DPD) and mercaptopurine/thioguanine (TPMT) received the maximum possible mean overall quality score of 7.
Table 2.
AGREE Scores for Draft Clinical Decision Support Summaries
| Drug/genetic pair | Identified a priori or novel finding? | Domain 1 (Scope and Purpose) | Domain 3 (Rigour of Development) | Domain 4 (Clarity of Presentation) | Domain 5 (Applicability) | Overall mean ± SD quality score (averaged across reviewers) | Guideline recommended for clinical deployment? |
|---|---|---|---|---|---|---|---|
| asparaginase/rs6021191/rs17885382 | novel | 93.1 | 86.1 | 29.2 | 29.2 | 4.5±0.6 | Yes |
| capectabine/fluorouracil/DPYD | a priori | 98.1 | 96.3 | 96.3 | 88.9 | 7±0.0 | Yes |
| cisplatin/rs1872328 | novel | 88.9 | 81.9 | 37.5 | 41.7 | 4.5±0.6 | Yes |
| doxorubicin/rs1883112 | novel | 86.1 | 83.3 | 44.4 | 27.1 | 4.5±1.3 | Yes |
| doxorubicin/rs8187710 | novel | 86.1 | 83.3 | 44.4 | 33.3 | 4.5±1.3 | Yes |
| doxorubicin/rs13058338 | novel | 86.1 | 83.3 | 44.4 | 33.3 | 4.5±1.3 | Yes |
| irinotecan/UGT1A1*28 | a priori | 96.3 | 79.6 | 81.5 | 80.6 | 5.7±0.6 | Yes |
| lapatanib/HLA-DQA1*02:01 | novel | 91.7 | 90.3 | 38.9 | 33.3 | 4.3±1.0 | Yes |
| mercaptopurine/thioguanine/TPMT | a priori | 95.8 | 98.6 | 98.6 | 87.5 | 7±0.0 | Yes |
| sunitinib/rs307826 | novel | 93.1 | 80.6 | 41.7 | 60.4 | 4.5±1.3 | No |
| sunitinib/rs776746 | novel | 90.3 | 80.6 | 41.7 | 54.2 | 4.8±1.3 | Yes |
| tamoxifen/CYP2D6 | a priori | 100.0 | 98.1 | 87.0 | 88.9 | 6.3±0.6 | Yes |
| vincristine/rs924607 | novel | 100.0 | 97.2 | 76.4 | 68.8 | 5.8±1.3 | Yes |
| Overall mean ± SD scores | 92.7±5.1 | 87.6±7.4 | 58.6±25.1 | 55.9±24.6 | 5.2±1.0 |
SD=standard deviation
Scores for our six novel drugs were similarly high for Domain 1 (Scope and Purpose) and Domain 3 (Rigour of Development), while scores for Domain 4 (Clarity of Presentation) and Domain 5 (Applicability) were lower. The mean ± SD scores were: Domain 1: 90.6 ± 4.5 (range, 86.1–100.0); Domain 3: 85.2 ± 5.4 (range, 80.6–97.2); Domain 4: 44.3 ± 13.0 (range, 29.2–76.4); and Domain 5: 42.4 ± 15.1 (range, 27.1–68.8). The mean overall quality score for these six drugs was 4.6 ± 0.4 (range, 4.3–5.8). Of these drugs, the summary for vincristine/rs924607 scored the highest, with an overall mean quality score (± SD) of 5.8 ± 1.3.
We chose a standard of requiring 100% consensus among AGREE scorers on the “recommend/do not recommend” assessment before affirming a summary as ultimately actionable for clinical implementation. The CDS summaries for all but one of the draft drug-genetic pairs (sunitinib/rs307826) attained 100% agreement among scorers.
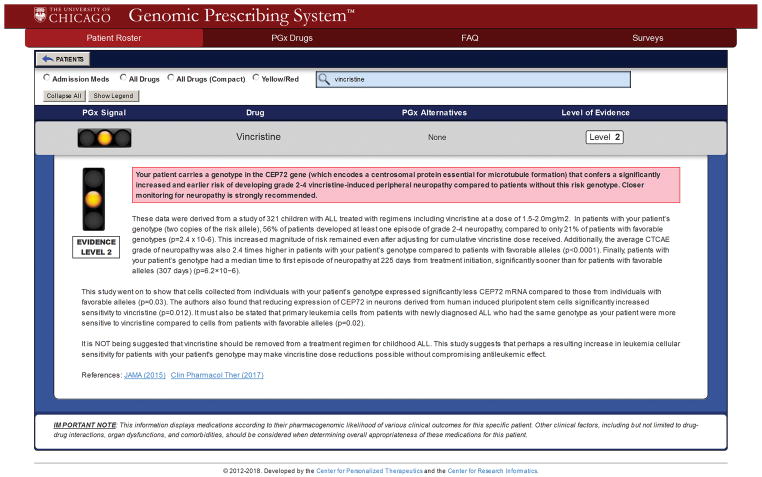
As an illustrative example, Figure 4 displays the CDS summary for vincristine that was recommended for implementation. This CDS guideline is currently delivered to institutional oncologists through our Genomic Prescribing System (GPS)18,21. Details regarding the evidence supporting this specific summary, and the development of its CDS language, are available in the Supplementary Results. CDS summaries exist in GPS for all 12 drugs that were deemed clinically actionable through this study.
Figure 4. Example of Clinical Decision Support Summary Written for Vincristine/rs924607 TT and Deployed in the Genomic Prescribing System.
This summary received scores of 100.0 for Domain 1 (Scope and Purpose), 97.2 for Domain 3 (Rigour of Development), 76.4 for Domain 4 (Clarity of Presentation), and 68.8 for Domain 5 (Applicability) on our modified AGREE II scoring instrument. Further, the overall mean quality score for this summary was 5.8. The unanimous recommendation was in support of clinical deployment of this association. It is therefore now being delivered to clinicians (for genotyped patients) using our institutional Genomic Prescribing System.
Discussion
Clinical use of genomic information in oncology has become commonplace, with the vast amount of actionable information consisting of somatic alterations from tumor sequencing. However, for the comprehensive clinical care of oncology patients in the precision medicine era, somatic information might also be integrated with patient-specific germline information. To our knowledge, ours is the first study to comprehensively and critically appraise the available evidence for utilizing germline information during the prescribing of oncology drugs, and to propose actionable CDS summaries based on published evidence. We found that there is now a critical mass of clinically actionable germline pharmacogenomic associations, with half of these well-known for decades and the other half previously unrecognized but recently identified based on discovery research. In total, we identified 12 drugs for which consistent germline pharmacogenomic information has sufficient evidence to deserve point-of-care clinical consideration. Deployment of CDS tools for these germline variants within ongoing institutional implementation efforts (coupled with appropriate genotyping) will permit future studies of clinical utility for these germline pharmacogenomic biomarkers.
Oncologists are uniquely primed for the idea of assimilating pharmacogenomic information into treatment decision-making. A survey of over 10,000 United States physicians found that oncologists were >5 times more likely to have ordered a pharmacogenomic test in the past six months when compared to general or family practitioners23. Perhaps this should not be surprising, given that oncologists are well-versed in making decisions about oncologic therapies based on tumor genomics24,25. Indeed, oncology practices have had to already solve many of the barriers of genomic clinical implementation. These include finding trusted laboratories to perform the testing, managing cost hurdles and insurance coverage questions, pursuing results in a timely fashion, storing results within the electronic health record, and communicating results with patients25–27. Changes in FDA labeling only comprise a small part of the key step of learning about important genomic information, and most oncologists probably depend on other sources to develop and hone this proficiency (e.g., national meetings, American Society of Clinical Oncology guidelines, local/institutional genomic ‘tumor boards’)28–30. Further, oncologists may find pharmacogenomics to be useful in multiple facets of oncologic care. Utility of certain results may, in fact, change based on the disease setting. For example, if the treatment goal is to cure the patient, guidelines for gene-drug interactions that predict a greater response to a drug for those carrying a certain genotype may be more desirable than guidelines that warn of a modest toxicity risk. Conversely, in the palliative treatment setting, avoiding toxicity may be considered more important; therefore, guidelines indicating that patients carry increased risk of side effects may allow providers to successfully avoid a harmful drug altogether, or adopt upfront dose-reduction.
In order to translate germline genomic findings into clinical practice, one has to first define and characterize what information is potentially ready for consideration of implementation. Several gene/drug examples have been described for decades1, with various degrees of implementation across oncology institutions and practices31–33. We utilized a previously-published methodology19 to critically assess the vast number of germline pharmacogenomic studies about oncology drugs. Interestingly, the majority of drugs have had positive pharmacogenomics associations described about them (67 of the 125 drugs), with published associations for 12 drugs withstanding rigorous evidence standards required for clinical actionability. Our data, perhaps not surprisingly, also suggest that the more pharmacogenomic publications a drug-genetic pair has, the higher the likelihood that the reported association is truly clinically actionable, illustrating the importance of replication34. We interestingly found that very few of the genomic polymorphisms supporting the associations for these 12 drugs are currently reported alongside somatic markers on our institutional Clinical Laboratory Improvement Amendments-accredited laboratory OncoPanel (J. Segal, personal communication), highlighting the need for more comprehensive or additional genotyping in order to make implementation of such germline markers a reality.
In determining which pharmacogenomic information should be implemented, we posit that a number of factors should be carefully considered. We were unable to detect clinically actionable associations for 11 of the top 20 drugs with the highest number of pharmacogenomic publications, despite there being many more studies reporting positive associations than negative associations for these drugs. It seems obvious that this may be due to publication bias, and one must therefore remember that the quality of published studies (and quality of replication), not the total number of studies, should drive actionability determinations. Additionally, progression-free survival and overall survival necessarily encapsulate prognostic information about the disease, and therefore published ‘pharmacogenomic studies’ examining these phenotypes may in fact be describing disease-related genetic associations not pharmacogenomic associations. If more oncologic pharmacogenomic studies in the future were to include control groups and/or would analyze non-prognostic outcomes (like response), an increased number of truly pharmacogenomic associations could potentially be identified. Increasing the power of future studies, through analysis of larger samples sizes, will also be essential.
In conjunction with a rigorous assessment of what to implement, it is of great importance to also critically appraise proposed clinical guidelines prior to implementation. Scores from a modified version of a well-established, validated tool—the AGREE II instrument—were, in general, high for our proposed germline pharmacogenomic CDS summaries. Importantly, we adopted a stringent requirement of unanimous recommendation for clinical deployment among our four independent AGREE scorers for guideline implementation. Although certain CDS summaries scored lower on the domains of Clarity of Presentation and Applicability, a number of previous studies have placed a particular emphasis on the Rigour of Development domain, suggesting that this domain is indicative of high quality guidelines35–38. Notably, all of our guidelines scored well above the common “high quality” threshold of 60% on this domain. Further, many past studies have classified guidelines as “recommended” for implementation if the overall quality score exceeded 50%39,40. All of our proposed guidelines exceeded this threshold. Finally, both the domain and overall quality scores of our CDS recommendations were very similar to, and in many cases higher than, AGREE II scores for guidelines currently implemented in clinical practice40–43.
Our study had limitations. Because we used the criterion of only evaluating studies published in journals with IF≥5, it is possible that we missed other potentially actionable germline associations, although this would seem unlikely. Additionally, our analyses were limited to unique drug-genetic associations where the genetic association could confidently be attributed to a clinical outcome from a specific oncology drug. We therefore did not include genetic signals associated with outcomes from multi-drug regimens where the phenotype of interest may have represented a composite drug outcome (‘regimen effect’). Finally, our comprehensive critical appraisal process of the published literature specifically excluded publications for which the studied germline genomic associations represented gene-disease, as opposed to gene-drug, interactions. Nevertheless, given the recent impact of several germline gene-disease relationships to directly impact the prescribing of certain oncology drugs, we comprehensively analyzed FDA labels for gene-disease interactions with actionable prescribing recommendations and included the findings in our results. We acknowledge that some other potentially relevant or emerging germline associations may have been missed. For example, the EGFR T790M germline mutation is a predisposing factor for lung cancer and frequently confers resistance to tyrosine kinase inhibitors when present in the tumor genome44,45. As a non-oncologic example, some diseases that are caused by germline mutations may be exacerbated by cancer medications, as is the case with Charcot-Marie-Tooth neuropathy and vincristine46,47. These examples represent additional important considerations during oncologic prescribing.
The direct application of this study’s findings will be actualized through clinical implementations that are now ongoing at many institutions, including ours. We have designed a pharmacogenomic CDS system that allows for the availability of preemptive germline results at the point-of-care18. The goal of these efforts is to permit eventual realization of a clinical care model that allows consideration of both germline and somatic genomic information at the time of prescribing. Clinicians will then be able to test the hypothesis that doing so ultimately improves clinical decision-making, aids in prescribing, reduces toxicities, improves response rates, and benefits patients. This of course represents both the challenge and the promise of precision medicine in the genomic era.
Supplementary Material
Publications describing at least one positive genetic association vastly outweighed the number of publications that described only negative genetic associations. For example, of the 61 pharmacogenomic studies for the drug gemcitabine, 53 of them (86.9%) reported at least one positive pharmacogenomic association.
Abbreviations: PGx – pharmacogenomic.
The 6 Most Common Outcomes Analyzed for a Pharmacogenomic Association. Percentage shown is out of 154 publications. Progression-free survival (including disease-free survival, event-free survival, recurrence-free survival, and relapse-free survival) was the most common clinical outcome studied across the critically analyzed oncologic pharmacogenomic studies, as it was the primary outcome analyzed in 56 of the 154 publications (36.4%). Overall survival and response rate were analyzed in 29.2% and 27.3% of the studies, respectively. Further, hematologic toxicities, including leukopenia, neutropenia, and thrombocytopenia were studied in 16.9% of publications, while neurotoxicity, including peripheral neuropathy, was studied in 11.7% of publications. Finally, hepatotoxicity was studied in 8.4% of publications.
Acknowledgments
The authors thank Drs. Gavin Hougham, William Meadow, Jeremy Segal, and Milda Saunders, as well as The University of Chicago Summer Research Program staff for their helpful assistance with this manuscript.
Footnotes
Financial support/Disclosures:
-Supported by the University of Chicago Bucksbaum Institute for Clinical Excellence (R.W.), K23GM100288-01A1 (P.H.O.), NIH/NHLBI 5U01HL105198-09 (M.J.R., P.H.O.), and The Conquer Cancer Foundation of the American Society for Clinical Oncology (M.J.R.).
-K.D., M.J.R., and P.H.O. are co-inventors on a pending patent application for the Genomic Prescribing System. M.J.R. is a co-inventor holding patents related to pharmacogenetic diagnostics and receives royalties related to UGT1A1 genotyping. No royalties were received from this work.
Conflicts of Interest
Related to this work, Keith Danahey, Peter H. O’Donnell, and Mark J. Ratain report a pending patent for the Genomic Prescribing System. Peter H. O’Donnell reports grants received from the National Institutes of Health and Mark J. Ratain from the The Conquer Cancer Foundation of the American Society for Clinical Oncology for work performed as part of the current study. Mark J. Ratain is a co-inventor holding patents related to pharmacogenetic diagnostics and receives royalties related to UGT1A1 genotyping, though no royalties were received from this work. Dr. Ratain also reports receiving personal fees from AbbVie, Amgen, Ascentage, Circle Pharma, Cyclacel, Drais Pharmaceuticals, Elion Oncology, Genentech, Shionogi, and multiple generic pharmaceutical companies, in addition to grant funding from Abbvie and Dicerna, for work unrelated to the current study. Dr. Ratain is the Director and Treasurer of the Value in Cancer Care Consortium.
Author Contributions:
R.W.: Conceptualization/methodology/investigation/analysis/writing–original draft&preparation/writing–review&editing/funding
B.A.B.: Methodology/investigation/analysis/writing–original draft&preparation/writing–review&editing
K.D.: Software/investigation/writing–review&editing
R.N.: Investigation/writing–review&editing
B.P.: Investigation/writing–review&editing
W.M.S.: Investigation/writing–review&editing
M.J.R.: Conceptualization/methodology/software/investigation/writing–review&editing/funding
P.H.O.: Conceptualization/methodology/software/investigation/analysis/writing–original draft&preparation/writing–review&editing/funding
References
- 1.Relling MV, Evans WE. Pharmacogenomics in the clinic. Nature. 2015;526:343–350. doi: 10.1038/nature15817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Donnell PH, Ratain MJ. Germline pharmacogenomics in oncology: decoding the patient for targeting therapy. Mol Oncol. 2012;6:251–259. doi: 10.1016/j.molonc.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Savonarola A, Palmirotta R, Guadagni F, Silvestris F. Pharmacogenetics and pharmacogenomics: role of mutational analysis in anti-cancer targeted therapy. Pharmacogenomics J. 2012;12:277–286. doi: 10.1038/tpj.2012.28. [DOI] [PubMed] [Google Scholar]
- 4.Hertz DL, McLeod HL. Using pharmacogene polymorphism panels to detect germline pharmacodynamic markers in oncology. Clin Cancer Res. 2014;20:2530–2540. doi: 10.1158/1078-0432.CCR-13-2780. [DOI] [PubMed] [Google Scholar]
- 5.DailyMed. [accessed December 13, 2017];Label: Capecitabine - capecitabine tablet. Available from URL: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=30830d24-1421-4c25-9b6b-c15705aa4357.
- 6.DailyMed. [accessed December 13, 2017];Label: Fluorouracil - fluorouracil injection, solution. Available from URL: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=21af1777-87f7-4e40-9917-f1677e7ff1a5.
- 7.DailyMed. [accessed December 13, 2017];Label: Irinotecan Hydrochloride - irinotecan hydrochloride injection, solution. Available from URL: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=9ef76dc4-ab02-4a45-ab36-00a14ddfc4ef.
- 8.DailyMed. [accessed December 13, 2017];Label: Mercaptopurine - mercaptopurine tablet. Available from URL: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=9001c081-f455-4fb0-aef0-175d41810c12.
- 9.DailyMed. [accessed December 13, 2017];Label: Tabloid - thioguanine tablet. Available from URL: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=4490128b-e73f-4849-9d6e-e8591639d771.
- 10.Caudle KE, Thorn CF, Klein TE, et al. Clinical Pharmacogenetics Implementation Consortium guidelines for dihydropyrimidine dehydrogenase genotype and fluoropyrimidine dosing. Clin Pharmacol Ther. 2013;94:640–645. doi: 10.1038/clpt.2013.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Relling MV, Gardner EE, Sandborn WJ, et al. Clinical Pharmacogenetics Implementation Consortium guidelines for thiopurine methyltransferase genotype and thiopurine dosing. Clin Pharmacol Ther. 2011;89:387–391. doi: 10.1038/clpt.2010.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goetz MP, Sangkuhl K, Guchelaar HJ, et al. Clinical Implementation Pharmacogenetics Consortium (CPIC) Guideline for CYP2D6 and Tamoxifen. [accessed January 16, 2018]; doi: 10.1002/cpt.1007. Available from URL: https://cpicpgx.org/guidelines/cpic-guideline-for-tamoxifen-based-on-cyp2d6-genotype/ [DOI] [PMC free article] [PubMed]
- 13.Mendelsohn J. Personalizing oncology: perspectives and prospects. J Clin Oncol. 2013;31:1904–1911. doi: 10.1200/JCO.2012.45.3605. [DOI] [PubMed] [Google Scholar]
- 14.U.S. Food and Drug Administration. [accessed Feb 28, 2018];Table of Pharmacogenomic Biomarkers in Drug Labeling. Available from URL: https://www.fda.gov/drugs/scienceresearch/researchareas/pharmacogenetics/ucm083378.htm.
- 15.Robson M, Im SA, Senkus E, et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N Engl J Med. 2017;377:523–533. doi: 10.1056/NEJMoa1706450. [DOI] [PubMed] [Google Scholar]
- 16.Pesenti C, Gusella M, Sirchia SM, Miozzo M. Germline oncopharmacogenetics, a promising field in cancer therapy. Cell Oncol (Dordr) 2015;38:65–89. doi: 10.1007/s13402-014-0214-4. [DOI] [PubMed] [Google Scholar]
- 17.American Cancer Society. [accessed July 10, 2014];Guide to Cancer Drugs. Available from URL: http://www.cancer.org/treatment/treatmentsandsideeffects/guidetocancerdrugs/index?utm_source=fullserve-20130801-corpcenter-healthyliving-healthyliving&utm_content=acxiom.
- 18.Danahey K, Borden BA, Furner B, et al. Simplifying the use of pharmacogenomics in clinical practice: Building the genomic prescribing system. J Biomed Inform. 2017;75:110–121. doi: 10.1016/j.jbi.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 19.Kaufman AL, Spitz J, Jacobs M, et al. Evidence for Clinical Implementation of Pharmacogenomics in Cardiac Drugs. Mayo Clin Proc. 2015;90:716–729. doi: 10.1016/j.mayocp.2015.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brouwers MC, Kho ME, Browman GP, et al. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010;182:E839–842. doi: 10.1503/cmaj.090449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Donnell PH, Wadhwa N, Danahey K, et al. Pharmacogenomics-Based Point-of-Care Clinical Decision Support Significantly Alters Drug Prescribing. Clin Pharmacol Ther. 2017;102:859–869. doi: 10.1002/cpt.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.U.S. National Library of Medicine. [accessed Aug 22, 2014];Gene Families - Genetics Home Reference. Available from URL: http://ghr.nlm.nih.gov/geneFamily.
- 23.Stanek EJ, Sanders CL, Taber KA, et al. Adoption of pharmacogenomic testing by US physicians: results of a nationwide survey. Clin Pharmacol Ther. 2012;91:450–458. doi: 10.1038/clpt.2011.306. [DOI] [PubMed] [Google Scholar]
- 24.Gray SW, Kim B, Sholl L, et al. Medical Oncologists’ Experiences in Using Genomic Testing for Lung and Colorectal Cancer Care. J Oncol Pract. 2017;13:e185–e196. doi: 10.1200/JOP.2016.016659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bertier G, Carrot-Zhang J, Ragoussis V, Joly Y. Integrating precision cancer medicine into healthcare-policy, practice, and research challenges. Genome Med. 2016;8:108. doi: 10.1186/s13073-016-0362-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Warner JL, Jain SK, Levy MA. Integrating cancer genomic data into electronic health records. Genome Med. 2016;8:113. doi: 10.1186/s13073-016-0371-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roychowdhury S, Chinnaiyan AM. Translating cancer genomes and transcriptomes for precision oncology. CA Cancer J Clin. 2016;66:75–88. doi: 10.3322/caac.21329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walko C, Kiel PJ, Kolesar J. Precision medicine in oncology: New practice models and roles for oncology pharmacists. Am J Health Syst Pharm. 2016;73:1935–1942. doi: 10.2146/ajhp160211. [DOI] [PubMed] [Google Scholar]
- 29.Haspel RL, Ali AM, Huang GC. Using a Team-Based Learning Approach at National Meetings to Teach Residents Genomic Pathology. J Grad Med Educ. 2016;8:80–84. doi: 10.4300/JGME-D-15-00221.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Allegra CJ, Jessup JM, Somerfield MR, et al. American Society of Clinical Oncology provisional clinical opinion: testing for KRAS gene mutations in patients with metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy. J Clin Oncol. 2009;27:2091–2096. doi: 10.1200/JCO.2009.21.9170. [DOI] [PubMed] [Google Scholar]
- 31.Lunenburg CA, van Staveren MC, Gelderblom H, Guchelaar HJ, Swen JJ. Evaluation of clinical implementation of prospective DPYD genotyping in 5-fluorouracil- or capecitabine-treated patients. Pharmacogenomics. 2016;17:721–729. doi: 10.2217/pgs-2016-0013. [DOI] [PubMed] [Google Scholar]
- 32.Harada T, Saito H, Karino F, et al. Clinical usefulness of testing for UDP glucuronosyltransferase 1 family, polypeptide A1 polymorphism prior to the inititation of irinotecan-based chemotherapy. Mol Clin Oncol. 2014;2:737–743. doi: 10.3892/mco.2014.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Donnan JR, Ungar WJ, Mathews M, Hancock-Howard RL, Rahman P. A cost effectiveness analysis of thiopurine methyltransferase testing for guiding 6-mercaptopurine dosing in children with acute lymphoblastic leukemia. Pediatr Blood Cancer. 2011;57:231–239. doi: 10.1002/pbc.22936. [DOI] [PubMed] [Google Scholar]
- 34.Ventola CL. Pharmacogenomics in clinical practice: reality and expectations. P T. 2011;36:412–450. [PMC free article] [PubMed] [Google Scholar]
- 35.Arevalo-Rodriguez I, Pedraza OL, Rodriguez A, et al. Alzheimer’s disease dementia guidelines for diagnostic testing: a systematic review. Am J Alzheimers Dis Other Demen. 2013;28:111–119. doi: 10.1177/1533317512470209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith CA, Toupin-April K, Jutai JW, et al. A Systematic Critical Appraisal of Clinical Practice Guidelines in Juvenile Idiopathic Arthritis Using the Appraisal of Guidelines for Research and Evaluation II (AGREE II) Instrument. PLoS One. 2015;10:e0137180. doi: 10.1371/journal.pone.0137180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brosseau L, Rahman P, Poitras S, et al. A systematic critical appraisal of non-pharmacological management of rheumatoid arthritis with Appraisal of Guidelines for Research and Evaluation II. PLoS One. 2014;9:e95369. doi: 10.1371/journal.pone.0095369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bekkering GE, Aertgeerts B, Asueta-Lorente JF, et al. Practitioner review: evidence-based practice guidelines on alcohol and drug misuse among adolescents: a systematic review. J Child Psychol Psychiatry. 2014;55:3–21. doi: 10.1111/jcpp.12145. [DOI] [PubMed] [Google Scholar]
- 39.Birken SA, Ellis SD, Walker JS, et al. Guidelines for the use of survivorship care plans: a systematic quality appraisal using the AGREE II instrument. Implement Sci. 2015;10:63. doi: 10.1186/s13012-015-0254-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cassis L, Cortes-Saladelafont E, Molero-Luis M, et al. Review and evaluation of the methodological quality of the existing guidelines and recommendations for inherited neurometabolic disorders. Orphanet J Rare Dis. 2015;10:164. doi: 10.1186/s13023-015-0376-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bragge P, Pattuwage L, Marshall S, et al. Quality of guidelines for cognitive rehabilitation following traumatic brain injury. J Head Trauma Rehabil. 2014;29:277–289. doi: 10.1097/HTR.0000000000000066. [DOI] [PubMed] [Google Scholar]
- 42.Alonso-Coello P, Irfan A, Sola I, et al. The quality of clinical practice guidelines over the last two decades: a systematic review of guideline appraisal studies. Qual Saf Health Care. 2010;19:e58. doi: 10.1136/qshc.2010.042077. [DOI] [PubMed] [Google Scholar]
- 43.Huang TW, Lai JH, Wu MY, Chen SL, Wu CH, Tam KW. Systematic review of clinical practice guidelines in the diagnosis and management of thyroid nodules and cancer. BMC Med. 2013;11:191. doi: 10.1186/1741-7015-11-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu SG, Shih JY. Management of acquired resistance to EGFR TKI-targeted therapy in advanced non-small cell lung cancer. Mol Cancer. 2018;17:38. doi: 10.1186/s12943-018-0777-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oxnard GR, Miller VA, Robson ME, et al. Screening for germline EGFR T790M mutations through lung cancer genotyping. J Thorac Oncol. 2012;7:1049–1052. doi: 10.1097/JTO.0b013e318250ed9d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nakamura T, Hashiguchi A, Suzuki S, Uozumi K, Tokunaga S, Takashima H. Vincristine exacerbates asymptomatic Charcot-Marie-tooth disease with a novel EGR2 mutation. Neurogenetics. 2012;13:77–82. doi: 10.1007/s10048-012-0313-1. [DOI] [PubMed] [Google Scholar]
- 47.Orejana-Garcia AM, Pascual-Huerta J, Perez-Melero A. Charcot-Marie-Tooth disease and vincristine. J Am Podiatr Med Assoc. 2003;93:229–233. doi: 10.7547/87507315-93-3-229. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Publications describing at least one positive genetic association vastly outweighed the number of publications that described only negative genetic associations. For example, of the 61 pharmacogenomic studies for the drug gemcitabine, 53 of them (86.9%) reported at least one positive pharmacogenomic association.
Abbreviations: PGx – pharmacogenomic.
The 6 Most Common Outcomes Analyzed for a Pharmacogenomic Association. Percentage shown is out of 154 publications. Progression-free survival (including disease-free survival, event-free survival, recurrence-free survival, and relapse-free survival) was the most common clinical outcome studied across the critically analyzed oncologic pharmacogenomic studies, as it was the primary outcome analyzed in 56 of the 154 publications (36.4%). Overall survival and response rate were analyzed in 29.2% and 27.3% of the studies, respectively. Further, hematologic toxicities, including leukopenia, neutropenia, and thrombocytopenia were studied in 16.9% of publications, while neurotoxicity, including peripheral neuropathy, was studied in 11.7% of publications. Finally, hepatotoxicity was studied in 8.4% of publications.