Abstract
The Slc9 family of Na+/H+ exchangers (NHEs) plays a critical role in electroneutral exchange of Na+ and H+ in the mammalian intestine as well as other absorptive and secretory epithelia of digestive organs. These transport proteins contribute to the transepithelial Na+ and water absorption, intracellular pH and cellular volume regulation as well as the electrolyte, acid-base, and fluid volume homeostasis at the systemic level. They also influence the function of other membrane transport mechanisms, affect cellular proliferation and apoptosis as well as cell migration, adherence to the extracellular matrix, and tissue repair. Additionally, they modulate the extracellular milieu to facilitate other nutrient absorption and to regulate the intestinal microbial microenvironment. Na+/H+ exchange is inhibited in selected gastrointestinal diseases, either by intrinsic factors (e.g., bile acids, inflammatory mediators) or infectious agents and associated bacterial toxins. Disrupted NHE activity may contribute not only to local and systemic electrolyte imbalance but also to the disease severity via multiple mechanisms. In this review, we describe the cation proton antiporter superfamily of Na+/H+ exchangers with a particular emphasis on the eight SLC9A isoforms found in the digestive tract, followed by a more integrative description in their roles in each of the digestive organs. We discuss regulatory mechanisms that determine the function of Na+/H+ exchangers as pertinent to the digestive tract, their regulation in pathological states of the digestive organs, and reciprocally, the contribution of dysregulated Na+/H+ exchange to the disease pathogenesis and progression.
Introduction
Since the original descriptions of the Na+/H+ exchange mechanism by Mitchell and Moyle (196) and by Brierley et al. (37) in rat liver and cow heart mitochondria, respectively, the field has quickly expanded to describe Na+/H+ exchange in both prokaryotic, plant, and other eukaryotic organisms, and to clone and molecularly and functionally characterize multiple isoforms of Na+/H+ exchangers. While the basic principle of Na+/H+ exchange is consistent across domains, procaryotes, plants, and yeasts evolved to utilize Na+/H+ exchange to cope with extreme hypersaline or hyperalkaline conditions, where Na+/H+ exchange mechanisms lead to the net uptake of protons and net loss of sodium. The same mechanism in higher organisms (metazoa) is primarily utilized in reverse mode, allowing for transepithelial Na+ transport and tight regulation of intracellular pH (pHi), which is crucial for cell function and survival. In this overview, we will briefly describe the families of mammalian Na+/H+ exchangers that belong to the monovalent cation proton antiporter (CPA) superfamily, with a particular emphasis on the CPA1 family containing all of the SLC9 genes coding for Na+/H+ exchangers with most prominent roles in the physiology of the gastrointestinal (GI) tract. We follow with detailed integrative description of their physiological roles in the function of key organs of the mammalian digestive system: salivary gland, esophagus, liver, pancreas, and small and large intestine.
Mammalian Monovalent Cation Proton Antiporter Superfamily
The classification of genes-encoding membrane transport proteins is an evolving process that changes with the improved bioinformatics tools and our understanding of evolutionary biology. Based on the most recent classification described by Chen et al. (45) and by Saier et al. (262) and available online at http://www.tcdb.org/, the monovalent CPA superfamily can be divided into three CPA families: CPA1 (TC# 2.A.36), CPA2 (TC# 2.A.37), and CPA3 (TC# 2.A.63). According to the most current classification, the CPA2 family includes primarily bacterial, fungal, and plant transporter proteins, with one exception, human transmembrane and coiled-coil domain 3 (TMCO3) expressed in the human cornea, lens capsule, and choroid-retinal pigment epithelium (46). The CPA3 family of the monovalent cation (K+ or Na+):proton antiporters is currently composed of only six bacterial proteins. The CPA1 family is the largest of the three and includes proteins derived from Gram-positive and Gram-negative bacteria, blue-green bacteria, archaea, yeast, plants, and animals. CPA1 transporters from eukaryotes have been functionally characterized as Na+/H+ exchangers. This family will be the main focus of this review, with particular emphasis of the SCL9A subfamily.
CPA1 family of Na+/H+ exchangers
CPA1 family is the largest member of the CPA super family. It includes transport proteins expressed by Gram-positive and Gram-negative bacteria, blue-green bacteria, archaea, yeast, plants, and animals. It is comprised of three subfamilies: SLC9A subfamily of nine mammalian NHE paralogs (NHE1–9), SLC9B containing NHA1 and NHA2, and SLC9C (previously categorized in the NaT-DC family) (35,263), with two sperm-specific NHEs (NHE10 and NHE11). The latter family will not be discussed in this focused review. Mammalian Na+/H+ exchangers of the SLC9A family are widely expressed and involved in numerous essential physiological processes. Their primary function is to transport Na+ into cells by exporting H+ out of cells at an exchange ratio of 1:1. These proteins play central roles in intracellular/organellar pH and cell-volume regulation. Their function is tightly regulated throughout developmental stages via mechanisms involving transcriptional and posttranslational modifications. The SLC9A family members also display different tissue/cellular localization and varying sensitivity to known NHE inhibitors.
SLC9A subfamily
Following the cloning of the prototypical mammalian Na+/H+ exchanger NHE1 (265, 266), eight other isoforms have been described using various methodological approaches and in a range of species. All but NHE5 have been detected in the GI tract with segmental differences, crypt-villus gradients of expression, and different cellular localizations. An additional isoform of a chloride-dependent Na+/H+ exchanger has been cloned from the rat colon (264), but it was not independently verified since. The nine mammalian members of the SLC9A subfamily of Na+/H+ exchangers differ significantly in their protein sequence, with amino acid identity varying from under 12% (hNHE1 vs. hNHE9) to over 70% (hNHE6 vs. hNHE7). Despite those differences, a very similar structural arrangement for all nine isoforms has been suggested by the in silico prediction transmembrane protein domains, whereby approximately 60% of the N-terminal of the protein is amphipathic and contains 10 to 12 membrane-spanning α-helices. The much more hydrophilic and less conserved carboxyl-terminus faces the cytoplasm. This C-terminal cytoplasmic tail contains multiple phosphorylation sites and sites responsible for interaction with scaffolding and regulatory proteins. It is important to note that the empirical knowledge of NHE membrane topology comes exclusively from studies on NHE1 and NHE3, it includes alternative or contentious models (21, 141, 142, 178), and their generalization to other isoforms may not always be accurate. Below, we briefly introduce the eight isoforms found in the digestive tract, followed by a more integrative description of their roles in each of the digestive organs. Regulatory mechanisms, which determine their function as pertinent to the digestive tract, will also be discussed, and we will conclude this review with the description of pathological states affecting Na+/H+ exchange in the gut, and reciprocally, the contribution of dysregulated Na+/H+ exchange to the disease pathogenesis and progression. For additional reviews, the reader is referred to Donowitz et al. (78), and Orlowski and Grinstein (224,225).
NHE1
NHE1 is expressed at the plasma membrane in almost all mammalian cell types. In the intestine, NHE1 is expressed at the basolateral membrane where it contributes to cell-volume and pHi regulation (339). NHE1 protein has 813 to 822 amino acids with a calculated molecular mass of ~91 kDa. N-linked glycosylation is the likely explanation for the apparent size of mature NHE1 of 110 kDa in Western blotting (62, 178). Its membrane topology has been extensively studied. Although NHE1 primarily plays a role in regulating cell volume and pHi, its activation also affects a number of downstream cellular events. A transient increase in pHi induced by NHE1-activation participates in cell proliferation through the G2-M checkpoint of the cell cycle (241). NHE1 may also be involved in cellular differentiation (304) and regulation of apoptosis (242). Additionally, NHE1 function affects cytoskeletal organization and cell migration. Studies have shown that the cytoplasmic C-terminal domain of NHE1 physically, but indirectly associates with the actin filaments with ezrin, radixin, and moesin (ERM) proteins as intermediaries. Disruption of these scaffolding complexes or NHE1 inhibition results in the impairment of focal adhesion formation and cell migration (242). Importantly, the vast majority of our knowledge about NHE1 expression, activity, regulation, and function is provided by studies in cells and tissues not directly related to the GI tract function. Thus, their translation to GI physiology and pathophysiology has to be approached with some reservations. Despite the rapid turnover of intestinal epithelial cells, Nhe1−/− mice had no reported intestinal defect, suggesting a minor role of NHE1 in the gut. The reader can find more information in focused reviews discussing various aspects of NHE1 biology (7,90,186,269,286).
NHE2
NHE2 has been cloned from the rat, rabbit, and human (56, 101, 184, 297, 306). In the intestine, NHE2 protein is located at the apical membrane of enterocytes (339). The expression of NHE2 along the crypt-villus axis in the intestine has some species-dependent differences. In rabbits, NHE2 is present in the brush-border of the entire villus of the small intestine, in colonic surface cells, and in the apical membrane of the upper half of the crypt (125). In the mouse colon, however, NHE2 is predominantly expressed in the crypt cells (12,53). Out of nine NHEs, NHE2 protein has higher similarity with NHE4, especially within the cytoplasmic C-terminus. Interestingly, in the human, rat, and mouse, the SLC9A2 and SLC9A4 genes cosegregate on chromosomes 2, 9, and 1, respectively (235,290), suggesting they may have originated from gene duplication early in their evolution. The predicted molecular weight of NHE2 protein in rat, rabbit, and human is ~91 kDa, although its mobility on SDS-PAGE gels does not confirm these calculations. Rabbit NHE2 expressed in PS120 fibroblasts was described as an O-linked sialoglycoprotein since neuraminidase treatment shifted the mobility of NHE2 protein from 85 to 81 kDa, and O-glycanase further shifted the mobility of the 81 kDa protein to 75 kDa (296). In NHE2 stably transfected Chinese hamster ovary AP-1 cells, NHE2 displays a relatively high affinity for amiloride and its analogues, with potencies in decreasing order of 5-(N-ethyl-N-isopropyl) amiloride (EIPA; IC50 = 79 nmol/L) > 5-(N,N-dimethyl) amiloride hydrochloride (DMA; IC50 = 250 nmol/L) > amiloride (IC50 = 1.4 μmol/L) > benzamil (IC50 = 320 μmol/L). Non-amiloride compounds also inhibit NHE2 with the following order of potency: clonidine (IC50 = 42 μmol/L) > harmaline and cimetidine (both with IC50 = 330 μmol/L). Kinetic analyses of NHE2 showed a simple, saturating Michaelis-Menten kinetics with an apparent affinity constant for Na+ (KNa) ~ 50 mmol/L. NHE2 function could be activated by low intracellular H+ with an apparent half-maximal activation value of pK 6.90.(332) The same study showed that Li+ and H+ could inhibit NHE2-mediated Na+ influx, but extracellular K+ had no effect on NHE2 activity. The physiological role of NHE2 remains elusive despite its relatively wide expression pattern. In fact, NHE2 has little role in intestinal Na+ absorption, and its intestinal crypt localization suggest a role for pHi and volume homeostasis in the crypt. NHE2 deletion in mice had no effect on intestinal sodium absorption, but led to abnormal parietal cell viability, impaired recovery of barrier function, and enhanced colonic NHE3 expression (12,198,270).
NHE3
NHE3 was cloned first in rabbit and rat (226, 295), followed by human cDNA cloning (34). Human SLC9A3 gene encoding NHE3 was mapped to chromosome 5p15.3 (33). NHE3 protein contains 831 to 834 amino acids with a calculated molecular weight of ~93 kDa. NHE3 protein has a higher homology to NHE5 (51.3%) and NHE2 (33.4%). Although N-glycosylation sites were predicted in the NHE3 protein, the glycosylation of NHE3 is species-specific. Renal NHE3 from rabbit and pig is a glycosylated protein and is sensitive to the treatment by glycopeptidase F and general N-linked glycosylation inhibitor, tunicamycin (25,280). In rat or canine NHE3, glycosylation was absent (25, 62). The physiological significance of NHE3 glycosylation is unclear. In porcine LLC-PK cells, inhibition of N-glycosylation by tunicamycin significantly decreased NHE3 activity, demonstrated by reduced pH-dependent 22Na uptake and Na-dependent pHi recovery from an acid load (280). On the other hand, deglycosylation of rabbit renal brush-border protein has no effect on acid-stimulated, amiloride-sensitive 22Na influx into the vesicles (25). The kinetics of NHE3 has been studied in NHE3-expressing fibroblasts and in intestinal epithelial cells (172, 190). There, NHE3 displayed Na+/H+ exchange function with simple Michaelis-Menten kinetics for extracellular Na+ (Km ~ 17 mmol/L). NHE3 activity was sensitive to phorbol esters and ATP depletion. Among the known NHE inhibitors, amiloride and its analogues have limited effect on NHE3 activity, while S3226 significantly inhibits NHE3 activity with IC50 of 0.02 μmol/L). NHE3 protein is detected at the apical membrane in intestinal epithelial cells. Interestingly, the expression of NHE3 in rabbits and humans is higher in the ileum than in other intestinal segments (79, 125). In the small intestinal epithelium, NHE3 may be considered a marker for the absorptive epithelial cells, since it is expressed only in the villus or surface epithelium, and not in the crypts (28,125). NHE3 plays an important role in transepithelial Na+ absorption in the mammalian GI tract (339). This concept is supported by the fact that mice carrying Nhe3 deletion exhibit a moderate to severe sodium absorptive defect in the intestine and mild diarrhea (97,271).
NHE4
NHE4 was initially cloned from the rat stomach cDNA library by Orlowski et al. (226). The rat NHE4 protein contains 717 amino acids with a calculated molecular weight of ~81.5 kDa. A 100 kDa protein was detected using a polyclonal antibody against fusion protein containing amino acids 393 to 625 in rat NHE4-expressed PS120 fibroblast cells (30), and a predominant band of ~65 to 70 kDa with two minor bands at 45 to 50 kDa, ~75 kDa using a monoclonal antibody raised against a similar fragment (565–675 AA) of rat NHE4 (237). The discrepancies observed in NHE4 molecular weight remain unresolved. A limited tissue distribution was seen in NHE4. With the highest expression in the stomach (237), and lower levels in the kidney (30), pancreas (8, 256), salivary glands (234), hippocampus (29), and endometrium (305), the expression of NHE4 in the intestine is uncertain. Although an initial study reported NHE4 transcript in these tissues (226), later studies with more specific cDNA probes found no expression of this isoform in the rat jejunum or colon (31, 59). Therefore, NHE4 plays little to no role in the small intestine.
NHE6
Initially cloned by Nagase et al. (209) from the human myeloid cell line KG-1 and termed KIAA0267, it was later identified by Numata et al. as NHE6 (SLC9A6) (215). Human NHE6 protein shares the highest homology with NHE7 and NHE9 (71% and 61%, respectively). NHE6 transcript was found to be most abundant in mitochondrion-rich tissues such as brain, skeletal muscle, and heart, but detectable expression was also described in the liver and pancreas. Although NHE6 expression has not been experimentally confirmed in other GI organs, it cannot be excluded. NHE6 has a putative N-terminal mitochondrial inner membrane targeting signal. Indeed, initial fluorescence microscopy studies showed colocalization with MitoTracker in HeLa cells (215). This suggested that NHE6 is the putative mitochondrial Na+/H+ exchanger regulating intramitochondrial Na+ and H+ levels as postulated by Garlid (95). Other studies, however, indicated that the positive charge-rich N-terminal segment is not a mitochondrial-targeting sequence, and that NHE6 accumulates in the sorting and recycling compartment of the endoplasmic reticulum where it may contribute to establishment of organelle-specific pH and in ion homeostasis (36, 197). NHE6 has also been shown to transiently appear on the cytoplasmic membrane (36). In hepatoma HepG2 cells, NHE6 was shown to regulate recycling endosome pH, and to modulate the maintenance of apical canalicular plasma membranes and cell polarity in general (219). Beyond this finding, the role of NHE6 in the pancreas and liver has not been extensively investigated.
NHE7
NHE7 was cloned in 2001 by Numata and Orlowski using a combination of computational and molecular biology approaches (215). NHE7 has a ubiquitous expression pattern with the most prominent expression in the putamen and occipital lobe of the brain, in skeletal muscle, and in a number of secretory tissues such as prostate, stomach, pancreas, pituitary gland, adrenal gland, thyroid gland, salivary gland, and mammary gland, but also in the liver, small intestine, and colon (215). Dual-labeling experiments indicated that NHE7 protein is accumulated in a juxtanuclear compartment partially overlapping with α-mannosidase II-positive medial and trans-cisternae of the Golgi apparatus, in the trans-Golgi network and in the mid-trans-Golgi stacks (92,215). NHE7 was shown to function primarily as a K+/H+ exchanger in regulating organelle’s volume through transmembrane K+ flux (215), and the role of NHE7 in the intestinal tract has not been studied.
NHE8
NHE8 was cloned from a mouse kidney cDNA library by Goyal et al. (107), and rat intestine by Xu et al. (313). The rodent NHE8 protein contains 576 amino acids, with very high amino-acid identity to its human ortholog. Human NHE8 protein shares less than 24% homology with other known NHE isoforms. The predicted molecular weight of NHE8 protein is ~64 kDa. Western blot detected a ~65 kDa band in mouse and rat intestine and in human intestinal epithelial cells (Caco2) (301, 313), while an 85 kDa protein was detected in mouse kidney (107). The discrepancy of NHE8 protein size might be the result of posttranslational modification in different tissues, since inhibition of glycosylation with tunicamycin reduced the size of NHE8 protein (107). In rat NHE8-expressing PS120 cells, kinetic analysis showed that NHE8 had a Km for pHi of ~ pH 6.5, and a Km for sodium of ~ 23 mmol/L. HOE694 (1 μmol/L) had no effect on NHE8 activity, but at 10 μmol/L, it significantly reduced the activity (312). S3226, typically considered as a NHE3-specific inhibitor, also inhibited NHE8 activity at 80 μmol/L (312). NHE8 is broadly distributed. In mice, NHE8 is predominantly expressed in the liver, skeletal muscle, kidney, and testes (107). In humans, NHE8 was detected in almost all the tissues in a 76-tissue array (313). In the intestinal tract, NHE8 is expressed throughout the GI tract (312,313). NHE8 expression also displays segmental differences. Higher NHE8 expression was seen in the stomach, duodenum, and ascending colon in humans, while in mice, higher NHE8 expression was seen in the jejunum, ileum, and colon (312). Moreover, the expression level of NHE8 is much higher in the stomach and jejunum in young mice compared with adult mice (312). In the stomach, NHE8 is expressed on the apical membrane in the epithelial cells of fundic and pyloric glands (315). In the intestine, NHE8 is also expressed on the brush-border membrane of intestinal epithelial cells (313). Studies have revealed the role of NHE8 in sodium absorption in early life and in mucosal protection in adult life. In rats and mice, NHE8 protein expression is the highest before weaning and is reduced by 44% in adults, whereas NHE8 mRNA abundance is similar in suckling and weanling rats, but decreased twofold in adult rats (312, 313). In the mouse colon, NHE8 expression is low in the suckling period and it reaches plateau after weaning (321). NHE8 may compensate for the loss of NHE2 and NHE3 in knockout mice, suggesting the role of NHE8 in the intestinal sodium absorption in early life, when NHE2 ND NHE3 are at very low levels (316). Targeted ablation of the Slc9a8 gene in mice results in a higher incidence of gastric ulcer formation, impaired mucosal protection, and altered gut microbiota (177, 300, 315, 317, 321), pointing to the role of NHE8 in mucosal protection in the gut.
NHE9
NHE9 was first identified by de Silva et al. as one of two genes associated with attention-deficit hyperactivity disorder (68). This isoform has high similarity to human NHE6 (61.2% at the protein level). The predicted NHE9 protein has 645 amino acids with a molecular weight of 72.6 kDa. NHE9 expression is fairly widespread, with the highest expression in the heart, skeletal muscle, and brain. NHE9 is expressed in the liver and to a smaller extent in the small intestine, but was below the detection limit in the colon (68). The homology of NHE6 and NHE9 proteins suggested that both of these iso-forms are located in the intracellular compartments. In NHE9-expressing COS-7 cells, NHE9 was primarily detected in the late recycling endosomes, with a small pool of NHE9 colocalized to with EEA1-positive early endosomes (210). Functional studies of NHE9 in COS-7 cells confirmed its function as a Na+/H+ exchanger. In COS-7 cells, overexpression of NHE9 resulted in luminal alkalinization to near cytosolic pH in the organelles positive for NHE9 (210).
SLC9B subfamily
Two members of this family have been described, NHA1 (encoded by the Slc9b1 gene) and NHA2 (Slc9b2) and are best known for controlling sperm motility (47). Within the digestive organs, NHA1 protein has been detected in the pancreatic β cells, but its role has not been extensively studied (71). NHA2 (also known as Na+/H+ exchanger domain-containing protein 2) is expressed more broadly in the digestive tract, where it was detected in the stomach, jejunum, and colon (94). Along with NHA1, NHA2 is also present in the pancreatic β cells, where it contributes to insulin secretion as described in more detail in the section on endocrine pancreas below. The roles of NHA2 in the mammalian stomach or intestine have not been investigated to date. In the gut epithelium of Drosophila, both Nha1 and Nha2 were required for survival via protecting against high luminal Na+ load, thus implicating its role in response to osmotic stress and Na+ tolerance (49). This was an interesting finding since fruit fly NHA1 and NHA2 utilized different substrates (H+-Cl− cotransporter and an Na+/H+ exchanger, respectively) (49). NHA2 was also found in the osteoclasts where it contributes to their differentiation and resorptive activity (16). Its subcellular localization in osteoclasts is controversial, with mitochondrial expression reported by Battaglino et al. (16), and exclusive enrichment at the basolateral membrane and endosomes/lysosomes (121). Functionally, human NHA2 was described as an amiloride-insensitive Li+-Na+/H+ exchanger (155). The same study demonstrated functional coupling of NHA2 to the V-type H+-ATPase at the plasma membrane in the renal MDCK cell line to create a virtual Na+ efflux pump, and suggested that NHA2 functionally recapitulates the ancient bacterial NhaA. Endogenous NHA2 was also described recently in the renal distal convoluted and connecting tubules where it was upregulated in response to a high-Na+ diet (156), a physiological response consistent with its contribution to Na+ efflux rather than reabsorption. The cellular localization of NHA2 and directionality of NHA2-mediated Na+ transport in the mammalian intestinal epithelia is not known.
Na+/H+ Exchange in Salivary Gland Physiology
The main functions of the salivary gland are to provide hydration and protection from mechanical and chemical insults for oral mucosa, oropharynx, and esophagus; to initiate digestion; provide antimicrobial defense; and as a source of luminal growth/trophic factors for the intestine. The process of saliva formation can be considered in two stages (Fig. 1). The first stage consists of isotonic fluid secretion by the specialized acinar cells through the coordinated net transepithelial Cl− movement and HCO3− efflux. In the second stage, ductal cells modify acinar secretions primarily by reabsorbing NaCl. Because the apical surfaces of salivary ducts are relatively water-impermeable, the resulting saliva is generally hypotonic. Six NHE isoforms have been found in the salivary glands either at the mRNA or both mRNA and protein levels. The roles for the basolateral (NHE1 and NHE4) and apical (NHE2 and NHE3) NHEs have been investigated to some extent and are described below. Two other NHE isoforms found in the salivary glands, NHE7 and NHE8 remain to be studied.
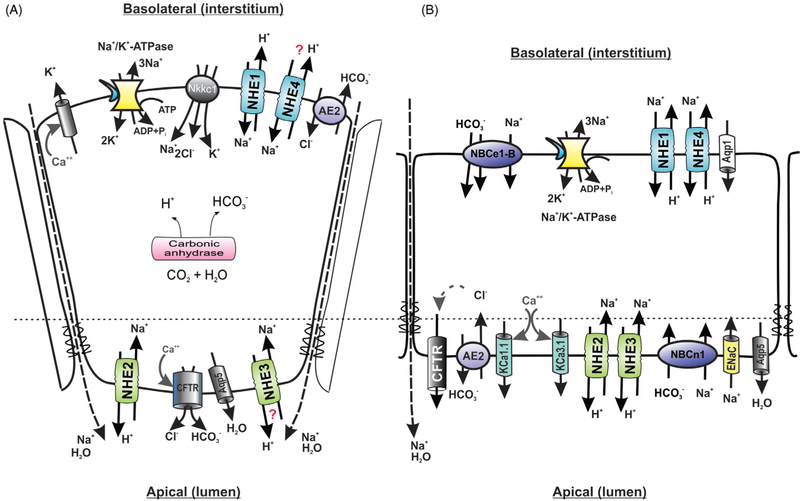
Figure 1.
Simplified model of basolateral and apical electrolyte transport in the (A) acinar and (B) ductal cells of the salivary (parotid) gland. NKCC1, Na-K-Cl cotransporter, SLC12A2; NHE1–4, Na+/H+ exchangers 1 to 4, SLC9A1-A4; AE2, anion exchanger 2, SLC4A2; CFTR, cystic fibrosis transmembrane conductance regulator; AQP1, aquaporin 1; AQP5, aquaporin 5; NBCe1-B, major splice variant of the electrogenic Na+/HCO3− cotransporter (NBCe1, SLC4A4); NBCn1, electroneutral sodium/bicarbonate cotransporter, SLC4A7; ENac, electrogenic Na+ channels, SCNN1; KCa1.1 and KCa3.1, calcium-activated potassium channels, KCNMA1 and KCNN4, respectively. For detailed reviews of the key players and mechanism involved, see Ohana (218) and Roussa et al. (255). Question marks indicate controversial contribution of individual transport proteins.
Contribution of Na+/H+ exchange to membrane transport in the salivary acinar cells
In the acinar cells, a 10- to 15-fold inward-directed Na+ gradient is provided by the basolateral Na+/K+ ATPase. Several transport proteins utilize this gradient to increase intracellular Cl− levels. One of them is the basolatoral electroneutral Na+/K+/2Cl− cotransporter (NKCC1). The other Cl− uptake pathway is provided by the coupled basolateral Cl−/HCO−3 and Na+/H+ exchanges. These two Na+-dependent Cl− uptake mechanisms lead to intracellular Cl− concentrations exceeding its electrochemical gradient five times, which is required to trigger Cl− efflux via Cl− channels. Cl− channels are activated along with K+ channels, a response necessary to maintain the electrochemical driving force for Cl− exit. Rapid efflux of intracellular Cl− and K+ results in the transepithelial potential difference that leads to paracellular passive cation movement, which creates a transepithelial osmotic gradient to drive water drag. Apical Cl− efflux is coupled with HCO3− secretion, primarily mediated by Cl− channels. Intracellular HCO3− is the product of carbonic anhydrases. Intracellular proton load, a by-product of HCO3− synthesis has to be rapidly eliminated via increased Na+/H+ exchange (193). In the salivary acinar cells, this process is primarily mediated by basolateral NHE1 (252). Expression of NHE4 on the basolateral membranes has also been described (217), but pH recovery studies suggested that it plays no active role in regulating pHi in the acinar cells under physiological conditions (234). On the other hand, ablation of the NHE1 gene significantly reduced pH recovery from an acid load in resting or stimulated acinar cells (81) and the salivary secretion in Nhe1−/− was reduced by 30% to 40% compared to wild-type mice, despite a compensatory increase in expression of basolateral NKCC1 (233). Apical expression of NHE2 and NHE3 iso-forms has been described in rodents, albeit with some species differences (NHE3 is expressed in mice but not rats). Park et al. (233) showed apical NHE2 expression in the mouse parotic glands in both acinar and ductal cells, whereas NHE3 was present only in the duct cells, this suggesting no significant role for NHE3 in salivary secretion by the acinar cells. Consistent with this hypothesis, Nhe3 gene knockout did not affect the rate of salivary secretion after muscarinic stimulation in vivo (233). Acid-loaded acinar cells from Nhe2−/− mice had a similar pH recovery rate as cells from WT mice (81), suggesting a similarly limited role for this isoform in the pHi regulation in these cells.
Na+/H+ exchange in the salivary ducts
Efficient mechanisms of NaCl conservation in the salivary ducts contribute to the final product, hypotonic saliva. Two mechanisms contribute to Na+ reabsorption at the luminal membrane of the salivary duct epithelia: Na+/H+ exchange and amiloride-sensitive Na+ channel, possibly ENaC (41,61). Ductal epithelial cells express the same four NHE isoforms as acinar cells, with NHE1 and NHE4 located on the basolateral membrane and NHE2 and NHE3 located apically (116, 170, 234). Logically, NHE2 and NHE3 were the most likely candidates for Na+ absorption in the salivary ducts, but studies with Nhe2−/− and Nhe3−/− null mice surprisingly showed no difference in salivary concentrations of Na+, K+, and Cl−, or osmolarity in these mice compared to wild-type littermates (233). These observations suggested that apical Na+/H+ exchangers do not play a major role in NaCl reabsorption in ductal epithelia of the salivary gland. Their minor contribution may have been missed, however, due a compensatory increase in the expression of ENac (epithelial Na+ channel) subunits α, β, and γ in both knockout lines, Nhe2−/− and Nhe3−/− mice (233). On the other hand, Nhe1−/− mice had higher osmolality and Na+, K+, and Cl− content in the saliva (233), an observation difficult to reconcile since salivary secretion is significantly decreased in Nhe1−/− mice (see paragraph on acinar transport above) and other studies showed that Na+ reabsorption in the salivary ducts is flow-rate dependent (191). The precise mechanism of NHE1 contribution to the biology of ductal epithelial cells remains unclear.
Na+/H+ Exchange in Esophageal Physiology
NHE1 is the only NHE isoform described and studied in the esophagus (274) and due to its prominent role in pHi regulation. Intracellular acidification activates NHE1 via a proton-sensing domain located within the fifth intracellular loop and the 11th transmembrane domain (299), a phenomenon further modified by regulatory elements located in the C-terminal tail. Activation of NHE1 during acid exposure has been implicated in cytoprotection during gastroesophageal reflux. Epidermal growth factor (EGF), abundant in the saliva, exerts a protective effect in acid-exposed cells via Ca2+/calmodulinand protein kinase C (PKC)-dependent activation of NHE1 (91). This finding is consistent with the role of PKC in the allosteric activation of NHE1 by reduced pHi (186). Low salivary EGF levels in patients with gastroesophageal reflux disease (GERD) were found to correlate with more severe esophageal damage, and conferred high-risk for development of Barrett’s esophagus, a precancerous condition defined by the metaplasia of columnar epithelium with goblet cells that replace the normal squamous stratified epithelium (108,187). NHE1 expression increases in GERD patients (276) and in Barrett’s esophagus (105), and may be considered as an adaptive response and a cellular defensive mechanism to manage the acute and chronic acid overload. The protective effects of increased NHE1 activity are compromised when bile acids are present in reflux chime. Bile acids inhibit NHE1 activity via a nitric oxide-mediated mechanism, and lead to increased DNA damage and potentially to mutations and progression to cancer (105). The role of NHE1 in the esophagus should not, however, be viewed in simplistic terms. In addition to controlling pHi and cell volume, NHE1 activity controls cell proliferation. The transiently elevated pHi that results from NHE1 activation promotes transit through the G2-M checkpoint of the cell cycle (241). It also influences cell differentiation (304), migration (242), and apoptosis (242), and contributes to pathological processes such as cancer cell invasion and heart failure (111, 139). In a Barrett’s adenocarcinoma cell line, activation of NHE1 by acid pulse correlated with increased proliferation, which could be reduced by inhibition of NHE1 or PKC (88). Considering the earlier description of cytoprotective effects of NHE1 activation, the conclusion of the latter study that NHE1 inhibition may be of therapeutic value in Barrett’s esophagus and prevention of its progression to cancer seems paradoxical.
Na+/H+ Exchange in Liver Physiology
Relatively little is known about the role of Na+/H+ exchangers in hepatic functions. Some of the earliest evidence came from Koch and Leffert (154), who demonstrated an involvement of Na+/H+ exchange in hepatocyte proliferation in response to trophic stimuli. Shortly after, Arias and Forgac (10) described Na+/H+ exchange at the sinusoidal domain of the hepatocyte plasma membrane in rats and suggested that this transport mechanism plays a role in the regulation of pHi in hepatocytes. An increase in hepatic plasma membrane NHE activity was seen in hepatectomized rats (200) and in neonatal rats (106), implicating Na+/H+ exchange in the mechanisms of liver regeneration and development. In growth factor-treated hepatocytes, stimulated DNA synthesis coincided with Na+/H+ exchange activation (136,293). In rat hepatocytes, basolateral Na+/H+ exchange has been implicated in the tumor-promoting effects of carcinogens (168).
Resting perisinusoidal hepatic stellate cells (HSCs) constitute 5% to 8% of the total number of liver cells. When activated, they act as liver-resident antigen-presenting cells, and contribute to liver fibrosis via collagen secretion. Na+/H+ exchange also functions as the main pHi regulator in HSCs. Elevated pHi in these cells is associated with activation and proliferative response to platelet-derived growth factor (PDGF) was linked to Na+/H+ antiport activation (presumably NHE1) (73). The promotion of fibrosis by oxidative stress has been attributed in part to NHE activation in HSCs, and inhibition of Na+/H+ exchange with amiloride to reduce collagen synthesis and cell proliferation was suggested as a potential therapy in liver fibrosis (289). It was experimentally confirmed that in in vitro, in HSCs under oxidative stress or treated with PDGF, an amiloride analogue EIPA could reduce type I collagen accumulation and cell proliferation (19). Similarly, amiloride treatment in rats that underwent bile duct ligation or treated with dimethylnitrosamine considerably reduced fibrosis. Although the resulting plasma concentration of amiloride was much lower than that expected to inhibit Na+/H+ exchange (113), a follow-up study with cariporide, a specific NHE1 inhibitor, seemed to confirm the initial results (74). Nhe1−/− mice showed decreased lipid accumulation in the liver induced by a high-fat diet (239). These findings strongly suggest that Na+/H+ exchange inhibition may be beneficial in chronic liver diseases leading to steatosis and fibrosis, and perhaps in the treatment of an already established hepatic steatosis or nonalcoholic steatohepatitis. This suggestion was additionally supported by reduction of de novo hepatic lipogenesis and of the HSC activation in Nhe1-deficient mice (238). Additionally, reminiscent of the reported roles of NHE1 in the cardiac ischemia/reperfusion injury, beneficial results of the NHE inhibition by EIPA in a rat model of partial hepatic ischemia suggest not only an active role that NHE1 plays in oxidative liver damage but also implicates inhibition of NHE as potential strategy aimed at prevention or reduction of ischemic liver injury (302).
NHE1 has been suggested to represent a potential therapeutic target in hepatocellular carcinoma (HCC). Increased NHE1 expression has been associated with HCC in human patients, where NHE1 expression levels correlated with the size of the tumors, their invasiveness, and the survival time (326). siRNA-mediated NHE1 knockdown or NHE1 inhibition reduced HCC growth and induced apoptosis in vitro (327, 328), while in vivo, EIPA treatment inhibited tumor growth in nude mouse xenografts of HCC cells (327).
Cholangiocytes form the biliary tract epithelia and contribute to bile secretion via net release of bicarbonate and water. They also contribute to ductal bile modifications by absorption of ions, bile acids, amino acids, glucose, and other molecules (291). The primary apical Na+/H+ exchanger was identified as NHE3 in mice, and its deletion (i.e., Nhe3−/−) resulted in inhibition of fluid reabsorption in isolated bile duct units following forskolin stimulation (194). In rats with bile duct ligation, a model of obstructive jaundice resulting in cholestasis, liver cell apoptosis, and fibrosis, Nhe3 mRNA and protein expression were significantly reduced in cholangiocytes (257). These observations suggest that reduced NHE3 activity may contribute to a diminished absorptive capacity in cholangiocytes during cholestatic disease.
Na+/H+ Exchange Physiology in the Gallbladder
Na+/H+ exchange, and NHE3 in particular, has also been detected in the gallbladder epithelium in human (277), calf (17), and prairie dog (1). Primary epithelial cells from the prairie dog gallbladder showed H+ gradient-dependent 22Na uptake mediated by NHE1 (6%), NHE2 (66%), and NHE3 (~28% of total uptake), as determined by studies with DMA and HOE-694 inhibitors (211). It has been hypothesized that apically expressed Na+/H+ exchangers may participate in the pathogenesis of gallstone formation. Indeed, early stages of gallstone formation were associated with increased gallbladder Na+ and fluid absorption (60,104), but further experimental evidence is required to address causality.
Na+/H+ Exchange in the Exocrine and Endocrine Pancreas
The pancreas is a glandular organ and a part of the vertebrate digestive and endocrine systems. As a digestive organ, the pancreas secretes pancreatic juice containing digestive enzymes that assist digestion and absorption of nutrients in the small intestine. As an endocrine organ, the pancreas produces several important hormones, including insulin, glucagon, somatostatin, and pancreatic peptide.
Exocrine pancreas
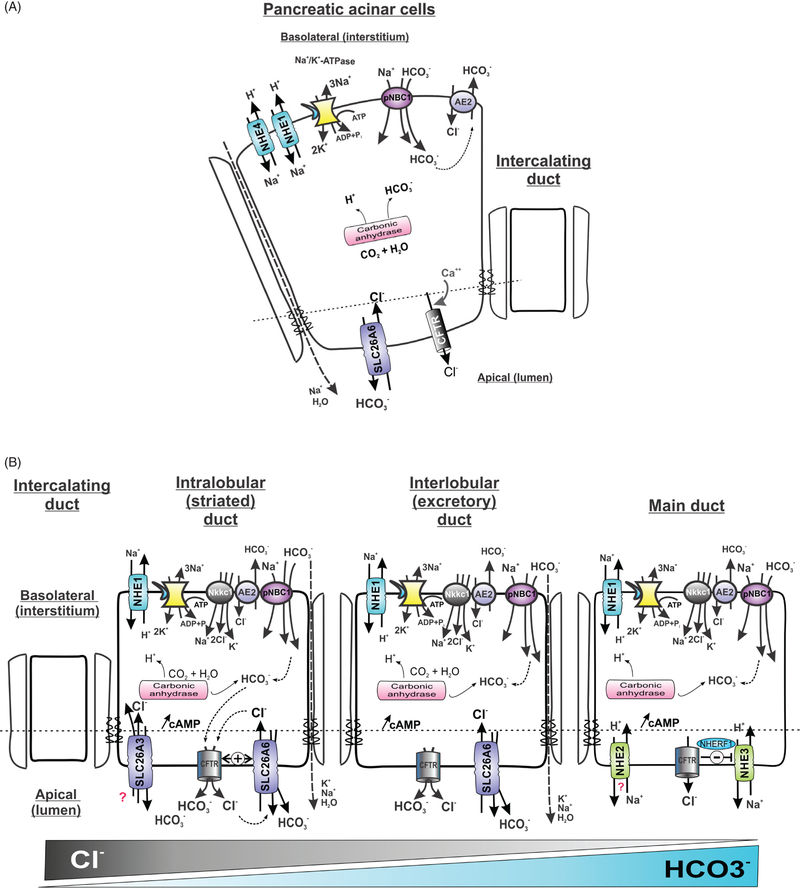
Pancreatic secretion is carried out by morphologically and functionally different epithelial cells of the acini and of the ducts. Pancreatic acinar cells synthesize, package, and secrete zymogens in response to extracellular cues. Pancreatic acinar cells also secrete a NaCl-rich primary fluid, which is modified downstream by the duct epithelial cells to generate the pancreatic juice rich in bicarbonate. Electrolyte fluxes contributing to the acinar and ductal pancreatic juice secretion are similar to those observed in the salivary glands (Fig. 2). Acinar cells secrete a near pH-neutral primary fluid thanks to the Na+-coupled secondary active Cl− transport resulting from the combined Na+/H+ exchange, Na+/K+-ATPase, and carbonic anhydrase activities. A variant of the electrogenic basolateral Na+ bicarbonate cotransporter (pNBC1) provides an additional route for intracellular accumulation of HCO3− (258), which ultimately exits the cell to the interstitium via basolateral Cl−/HCO3− exchange. Cl− exits the cell via either apical cystic fibrosis transmembrane regulator (CFTR) Cl− channel or Ca2+-activated Cl− (CaCC) channels upon secretagogue (cAMP/Ca2+) stimulation. This Cl− flux is complemented with a paracellular Na+ movement to preserve electroneutrality.
Figure 2.
Simplified model of basolateral and apical electrolyte transport in the (A) acinar and (B) ductal cells of the exocrine pancreas. NHE1, NHE4, Na+/H+ exchangers 1 and 4; SLC9A1 and SLC9A4, respectively; pNBC1, electrogenic Na+/HCO3− cotransporter, SLC4A4, AE2, anion exchanger 2, SLC4A2; CFTR, cystic fibrosis transmembrane conductance regulator; SLC26A6 (apical Cl−/HCO3− exchanger). For detailed review of the key players and mechanism involved, see excellent reviews by Steward and Ishiguro (282), Steward et al. (284), and Ishiguro et al. (130)
Several NHE isoforms (except NHE5 and NHE9) have been detected in the pancreas, although their distribution at the cellular, membrane domains, or organellar levels have not been extensively studied. NHE1 is expressed on the plasma membrane of pancreatic acinar cells (256) and accounts for a majority of Na+/H+ exchange activity in mouse pancreatic acinar cells, evidenced by the fact that the pHi in Nhe1−/− acinar cells failed to recover from an acid load (38). The acinar cells were also unable to recover from the acid challenge in the absence or presence of extracellular HCO3−, which suggested that basolateral Na+/HCO3− cotransport was not sufficient to compensate for the loss of NHE1 to buffer the change in pHi. NHE4 is also expressed at the plasma membrane in pancreatic acinar cells, but it plays no role in pHi regulation (256). Although Nhe4 knockout mice have been described (96), the role of this isoform in pancreatic physiology has not been studied. The roles of NHE2 and NHE3 in the pancreatic acinar cells appear to be insignificant since acini isolated from either wild-type, Nhe3−/− or Nhe2−/− mice displayed no difference in the kinetics of pHi recovery from intracellular acidification (38). NHE1 and NHE4 are also present on the zymogen granules, but their function in this secretory compartment remains uncertain (8). Since cholecystokinin-mediated amylase release was inhibited with 50 μmol/L EIPA and a high extracellular concentration of Na+ (~130 mmol/L) and stimulated by low extracellular Na+ concentration (30 mmol/L), Na+/H+ exchange in the zymogen granules may contribute to their membrane fusion and exocytosis of the pancreatic enzymes (9). In this scenario, secretagogue-stimulated pancreatic acinar cells would increase basolateral NHE1 activity, which would lead to a transient increase in the cytoplasmic Na+ concentration from resting level of 10 mmol/L up to 50 to 60 mmol/L. In turn, this would provide the necessary Na+ gradient for the organellar NHEs to increase the pH of the intragranular space (223), thus facilitating fusion and secretion of the zymogen-stored enzymes (166).
Bicarbonate secretion in pancreatic ducts is induced by secretagogue secretion. This process is mediated by moving HCO3− from the blood into the luminal pancreatic fluid through duct cells. The mechanisms of regulating bicarbonate secretion is a complex process involving neural, hormonal, and intracellular signal transduction in ductal and acinar pancreatic cells (89). Steward et al. (282, 283) provide excellent reviews on the molecular mechanisms of bicarbonate secretion in the pancreatic ducts. In the more traditional model, CO2 that diffuses from the interstitial space into the duct cell across the basolateral membrane is enzymatically hydrated to HCO3− by carbonic anhydrase, while the produced protons are extruded by the basolateral Na+/H+ exchange. Bicarbonate leaves the cell at the apical membrane via Cl−/HCO3− exchange mediated by SLC26A6, which acts with 1Cl−/2HCO3− stoichiometry (275). The resulting transepithelial potential difference drives paracellular Na+ flow, followed by osmotically driven water secretion. This model has been amended recently by demonstrating an additional pathway for HCO3− entry into pancreatic duct cells, involving basolateral HCO3− absorption via an electrogenic Na+/HCO3− cotransporter, pNBC1 (294). Like in acinar cells, NHE1 and NHE4 are present at the basolateral membrane of the duct cells, but their contribution to HCO3− secretion is less certain. In the guinea pig, measurements of the pHi decline in isolated pancreatic ducts following the application of amiloride and DIDS (4,4’-diisothiocyanatostilbene-2,2’-disulfonate; NBC1 inhibitor) suggested a similar contribution of the two transport systems to basal secretion and pHi regulation (129). After stimulation with secretin, a hormone produced by duodenal and jejunal enteroendocrine S-cells, the activity of pNBC1 increases and contributes ~75% of the basolateral HCO3− uptake. Bicarbonate secretion from the duct cells is also reduced by the inhibition of pNCB1 (by ~56%) and to a lesser extent (18%) by NHE inhibition (128).
Juice collected from resting pancreatic ducts is relatively acidic, chloride-rich, and has relatively high pCO2, suggesting active acidification of the ductal lumen. Apical Na+/H+ exchange has been implicated in this phenomenon in a murine (341) and bovine pancreas (188), and initially attributed to the apically expressed NHE2 and NHE3 (169). However, the luminal Na+-dependent H+ efflux in Nhe2−/− ducts is normal, suggesting NHE2 plays no role in HCO3− salvage in the ducts. On the other hand, pancreatic duct cells isolated from Nhe3−/− mice displayed 45% reduced luminal Na+-dependent H+ efflux (169), indicating an important role of NHE3 in HCO3− salvage under resting, low-flow conditions, especially in larger pancreatic ducts. The finding that CFTR enhances the cAMP-induced inhibition of NHE3, a phenomenon likely dependent on the physical interaction of the two transport proteins mediated through a scaffolding protein EBP50 (NHERF1) (2) supports this hypothesis.
Endocrine pancreas
The role of Na+/H+ exchange in the endocrine pancreatic function received relatively less attention. In murine islets, mRNA of eight NHE isoforms has been found: NHE1, NHE5 through NHE9, as well as NHA1 and NHA2, although only the roles of NHE1 and NHA2 have been studied in detail, beyond the expression level (72, 201, 285). The involvement of NHE1 and NHA2 in β-cell functions have been discussed in detail by Deisl et al. (71). In brief, glucose uptake into β cells followed by its metabolism lead to an increase in cellular ATP/ADP ratio which triggers the closure of ATP-sensitive potassium (KATP) channels, a main determinant of the β-cell membrane potential. The resulting membrane depolarization directly activates voltage-sensitive L-type Ca2+ channels to increase intracellular Ca2+ levels, which then drives the exocytosis of insulin-containing large dense core vesicles (LDCVs). Following glucose metabolism, NHE1 activity was initially believed to be responsible for cytosolic alkalinization and to facilitate insulin secretion (176). Later studies showed that the contribution of NHE1 to intracellular alkalinization could only be demonstrated in the absence of HCO3−/CO2, and that under physiological conditions, it was entirely dependent on bicarbonate production (285). A shorter splice variant of NHE1 was also found in the insulin containing LDCVs in β cells, but its role has not been investigated. The role of NHA2 in insulin secretion is less controversial. Deisl et al. (72) showed that NHA2 protein is localized to transferrin-positive endosomes and synaptic-like microvesicles (SLMVs), but not in insulin-containing LDCVs in β cells. The same report showed that NHA2 knockout mice exhibited a pathological glucose tolerance with diminished insulin secretion and islets from NHA2 knockout or heterozygous mice showed decreased insulin secretion after stimulation with glucose or tolbutamide, a first-generation potassium channel blocker and oral hypoglycemic drug from sulfonylurea class. More detailed studies by this group indicated an indirect role for NHA2 by facilitating clathrin-dependent endocytosis and speculate that defective endoexocytosis coupling may be the mechanism for the secretory deficit observed in NHA2-deficient mice. The precise function and role of NHA2 in the endosomes and SLMV remains unclear.
Na+/H+ Exchange in Gastric Physiology
Gastric acid secretion provides means for breaking solid foods down to smaller absorbable units. The production of acid by the stomach is tightly controlled by neural and hormonal mechanisms and with the input of gastric epithelial cells. Due to the periodically very low pH of the gastric lumen, the control of the pHi in the gastric epithelial cells, and the contribution of Na+/H+ exchange to this process are of significant interest. Paradiso et al. (231, 232) provided the first descriptions of Na+/H+ exchange in gastric mucosa, a transport mechanism that has since been implicated in gastric epithelial cell pHi homeostasis (137), volume regulation during secretory stimulation (281), and gastric epithelial restitution after injury (133,324). Several NHE isoforms (except NHE5, NHE6, and NHE9) have been described in the gastric epithelium, with the strongest evidence for NHE1, NHE2, NHE3, and NHE4.
NHE1 is the basolateral isoform present in the surface and neck mucous cells, chief cells, and to a lesser extent in the parietal cells (254, 287). Histological analysis of the gastric epithelium of Nhe1−/−mice showed thinning of the glandular epithelium and a widening of the interstitial space between gastric glands (18). These morphological changes could not be attributed to inflammatory changes, and they did not seem to be associated with any perturbations in systemic acid-base homeostasis (18).
NHE2 has a tissue distribution similar to that of NHE1 in the gastric mucosa (254). NHE2 was believed to be located on the basolateral membrane, although no immunohistochemical staining supports this. NHE2 may be involved in basolateral alkalization during stimulated acid secretion by parietal cells. Increased NHE2 activity in response to increased interstitial pH could, in theory, contribute to the net acid secretion, affect the parietal cell viability, and promote mucosal protection. Indeed, Nhe2-deficient mice had significantly reduced numbers of parietal and chief cells, and decreased net acid secretion (270). The reduction of the number of parietal cells in Nhe2−/− mice may be secondary to their decreased viability. These cells develop normally in the absence of NHE2, but undergo premature necrosis (270) that coincides with progressive diffuse corporal gastritis with symptoms ranging from transmural neutrophilic infiltration to a profound atrophic gastritis with chronic achlorhydria (27). However, Hue et al. (323) reported that NHE2 protein is localized to apical membranes of gastric surface epithelium, and that trefoil factor 3 (TFF3) failed to induce mucosal restitution of microscopic lesions (wound healing) when NHE2 was inhibited. More recently, in vitro studies with normal rat gastric mucous surface cell line RGM1, showed unexpectedly that forced expression of NHE2 significantly slowed down the restitution velocity after acid preincubation (230). The discrepancy between the earlier in vivo and these in vitro studies remains to be addressed.
NHE3 expression in the stomach is somewhat controversial. Although documented in the rat (226), human, and guinea pig (159), it has not been detected in rabbit gastric mucosa (254,295). According to Kulaksiz et al. (159), NHE3 protein expression was confined to the basolateral membrane of surface mucous cells in both human and guinea pig specimens. This surprising subcellular distribution is likely to be true, since the same antibodies properly stained the brush-border membrane of the duodenal enterocytes (159). These findings were later challenged by functional studies in perfused isolated rat gastric glands, which demonstrated NHE3-like activity in the parietal cell apical membrane (153). Since no gastric abnormalities were reported in Nhe3−/− mice (271), the expression and physiological role of NHE3 in the gastric epithelium still remain obscure.
NHE4 is abundantly expressed in the gastric gland epithelium, with protein located on the basolateral membrane of parietal and chief cells, and to a lesser extent in mucous cells (237, 254). Similar to the putative function of NHE2 in the basolateral membrane of parietal cells, NHE4 is also likely an important player in maintaining pHi homeostasis and in acid secretion. Young Nhe2−/− mice maintain a normal gastric acidity (270) suggesting the presence of a different basolateral NHE isoform. NHE1 expression in parietal cells is low, thus NHE4 (Slc9a4) became a possible candidate for the key basolateral Na+/H+ exchanger in the parietal cell. Targeted ablation of the Slc9a4 gene in mice indeed led to hypochlorhydria in the early postnatal life and was not progressing with age (96).
Apical expression of NHE8 was described in the mouse fundic and pyloric glands by Xu et al. (315). Mice with targeted ablation of the Slc9a8 gene showed that gastric expression of NHE8 was critical for the maintenance of bicarbonate secretion by the Cl−/HCO3− exchanger downregulated-in-adenoma (DRA; Slc26a3), and that compared with their wild-type littermates, Nhe8-deficient mice had a higher incidence of gastric ulcer formation (315). Although the mechanism responsible for downregulation of DRA expression remains unknown, it appears to be a phenomenon consistent across organs as it was also described in the colon (321), conjunctiva, cornea, and the lacrimal glands (322).
Small Intestinal Na+/H+ Exchange
Overall Na+ absorptive mechanisms in the jejunum are primarily driven by the Na+ cotransport with other nutrients, including Na+-linked D-glucose/D-galactose and L-amino acid transporters, H+-dipeptide cotransport, and by electroneutral epithelial Na+ transport mediated by Na+/H+ exchange linked to Cl−/HCO3− exchangers (particularly in the ileum). The latter mechanism is thought to account for the majority of Na+ absorption both between meals and post-prandially (181,182). A simplified model of electrolyte transport mechanisms in the intestinal epithelial cell is depicted in Figure 3.
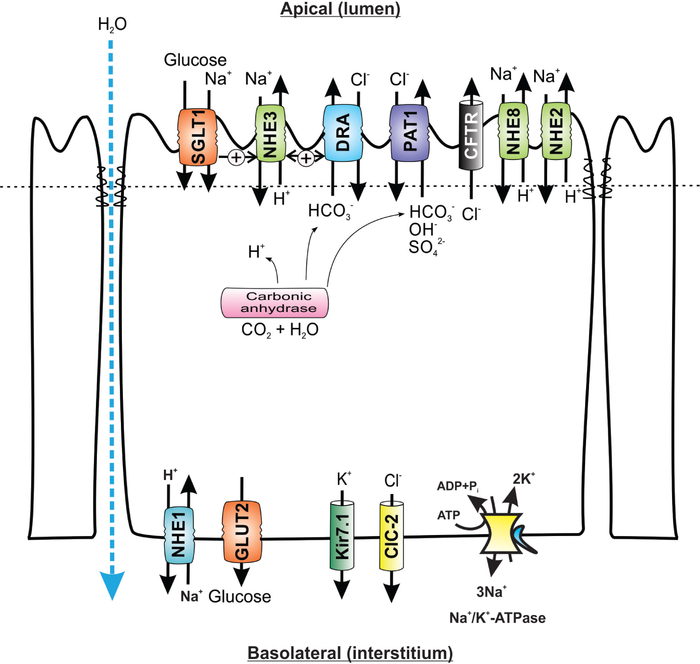
Figure 3.
Simplified cellular model of the role of apical Na+/H+ exchange in transepithelial sodium, glucose, and water absorption in small intestinal epithelial cells. SGLT1, sodium/glucose cotransporter 1, SLC5A1; GLUT2, glucose transporter 2, SLC2A2; DRA, downregulated in adenoma, SLC26A3; PAT1, putative anion transporter 1, SLC26A6; CFTR, cystic fibrosis transmembrane conductance regulator, Kir7.1, potassium inwardly rectifying channel, subfamily J, member 13, KCNJ13; ClC-2, chloride channel protein 2, CLCN2. For detailed reviews about the physiology and pathophysiology of intestinal electrolyte transport and Na+/H+ exchange in particular, see Fuster and Alexander (93), He and Yun (115), Zachos et al. (339), and Gurney et al. (109)
The role of Na+/H+ exchange in the intestinal sodium and water absorption
The average daily luminal load of water and sodium in the GI tract of an adult human amounts to ~9 L and 800 mEq of Na+. Thus, a major function of the GI tract is to absorb virtually all water and Na+ to maintain overall body water and Na+ homeostasis. Healthy gut is capable of absorbing more than 98% of this load, resulting in ~200 g daily stool output (subject to dietary influences). The intestinal water absorption is closely coupled with solute transport in the intestinal epithelium and is attributed to the influence of hydrostatic and osmotic pressures and passive paracellular diffusion. Aquaporins (AQPs), the water channel proteins, also play important roles in water transport. At least six AQP isoforms (AQP1, 3, 4, 5, 8, and 9) are identified in the digestive system (189). More recently, an alternative mode of water transport has been proposed, in which an active apical Na+/glucose cotransporter SGLT1 serves as a water channel with stoichiometry of 220 to 400 molecules of water per one molecule of glucose and two molecules of sodium (340). The latter model is still a subject of scientific debate as reviewed by Lapointe et al. (162). Transport of water across the intestinal epithelium is beyond the scope of this article and is only referred to as a secondary phenomenon related to NHE-mediated sodium absorption.
The transport functions in the neonatal intestine are not fully developed in mammals, therefore the colon appears to have a significant role in water and sodium absorption in the maintenance of normal fluid and electrolyte homeostasis. High Na+ absorption in the neonatal period and the reduced Na+ absorption in postnatal life are observed in the colon of rat, rabbit, and human (86, 132, 216), and also in the rat small intestine (227). The Na+ absorptive pathway in the colon is mainly mediated by amiloride-sensitive Na+ channels (216, 228, 229). In agreement with this, the abundance of colonic Na+ channels increases from fetal life through the suckling period before which it begins to decline (65, 307, 308). Interestingly, the developmental changes in colonic Na+ transport vary among species. The colonic Na+ transport persists until adulthood in rabbits (216), while the colonic Na+ transport in rats ceases after weaning (228, 229) and is replaced by Na+/H+ exchange (227). In pigs, Na+ absorption changes from an electroneutral mechanism in the perinatal period to an electrogenic process later in postnatal development (63). The sodium absorption in the small intestine involves carrier-mediated pathways. The sodium-hydrogen exchangers (NHEs) play an integral role in electroneutral sodium absorption in the mammalian intestine. Na+/H+ exchange activity increases during development and reaches its maximum after weaning (57, 58, 152). In rats, the lowest Na+ uptake is seen in the suckling period, a higher Na+ uptake is observed in weanlings and adults, and the highest Na+ uptake is detected in adolescents (58,152).
Among the nine identified NHE isoforms (NHE 1–9), only four NHE isoforms (NHE1, 2, 3, and 8) are expressed at the plasma membrane of the mammalian intestinal epithelial cells, and two isoforms (NHE7 and 9) are limited to the intracellular compartments. The expression and activity of the apical isoforms NHE2, NHE3, and NHE8 are regulated during development (56–58, 312, 313). The functional coupling between Na+/H+ and Cl−/HCO3− exchange in the small intestinal epithelium may also be regulated during ontogeny with correlated patterns of expression with Cl−/HCO3− exchangers (52, 250, 313). NHE2 was shown to be the dominant Na+/H+ exchanger in the chicken ileum and large intestine (76). In the dog ileum, NHE3 is responsible for the majority of basal and postprandial Na+ absorption (181, 182, 329). In the rabbit ileum, NHE2 and NHE3 are equal contributors to the unstimulated Na+/H+ exchange, but the contribution of NHE3 outweighs that of NHE2 after glucocorticoid treatment [~68% of total; (310)]. During postnatal development in rats, the contribution of NHE3 to total small intestinal Na+/H+ exchange gradually increases from 59% at 2 to 3 weeks of age to ~92% at 6 weeks (58). Based on these results, we initially postulated that NHE2 is the dominant contributor to the small intestinal Na+ absorption before weaning. However, with the later identification and characterization of NHE8 expression pattern and NHE inhibitor sensitivity, particularly with its declining small intestinal expression during postnatal development (57, 58, 313), the contribution of NHE2 and NHE3 to intestinal Na+ absorption in early life may need to be reconsidered. The relevance of NHE2 as an intestinal Na+/H+ exchange was brought into question by the observations with Nhe2−/− mice, which had no identifiable Na+ or water absorptive defect (270) and no apparent compensatory increase in NHE3 expression (97). On the other hand, Nhe3−/− mice developed moderate diarrhea, intestinal distention, alkalinization of the intestinal fluid, mild metabolic acidosis, lower blood pressure, reduced body fat stores, and increased mortality when placed on low Na+ diet (97,271). Mucosal hypertrophy and increased length of the small and large intestines may serve compensatory function to increase the absorptive surface, although the mechanism for this phenomenon is not known. NHE2 also does not appear to compensate for the loss of NHE3 (97). Its mRNA expression does not change in Nhe3−/− mice, and the residual, EIPA-sensitive Na+ absorption that remained is unlikely to be mediated by NHE2 since it was reduced by elevated cAMP (97). Also, double knockout (DKO) mice lacking both Nhe2 and Nhe3 genes, there was no further reduction of viability, no additional effect on the systemic acid-base balance, and the symptoms of diarrhea that were initially attributed to NHE3 loss were not worsened (167). The residual NHE activity observed in Nhe3−/− and in DKO mice may have been mediated by NHE8, but the overlap in sensitivity of this isoform to common NHE inhibitors complicate any experimental design that could verify it (312). The response of NHE8 to elevated cAMP has not been tested in intestinal epithelial cells. Interestingly, Nhe3−/− mice have elevated expression of IFNγ in the small intestinal mucosa and a fivefold increase in the cytokine’s serum levels (150,165,309). Consistent with these observations, a number of known IFNγ-target genes were activated in the small intestine based on microarray analysis (309). Without histological evidence of inflammation in Nhe3−/− jejunum, Woo et al. (309) hypothesized that increased IFNγ might help compensate for the defect in Na+ absorption via its known antisecretory effects, for example, by decreased CFTR expression. However, our group later demonstrated that changes in the small intestinal epithelial homeostasis are detrimental and lead to unusual sensitivity to small intestinal mucosal injury by dextran sulfate (DSS), a compound that does not affect this segment of the gut in wild-type mice (150).
Colonic Na+/H+ Exchange
Intestinal nutrient transporters are expressed during gestation and become especially important during the third trimester to facilitate nutrient absorption from the amniotic fluid. The postnatal pattern of nutrient transporter expression reflects the increasing need to assimilate nutrients indispensable to sustain rapid growth and metabolic activity. Intestinal transport capacity grows as a function of increased intestinal mass, changing properties of the epithelial cell membrane, and the types and levels of transporters expressed as well as the kinetics of solute transport specific to each of them. During suckling period, colon participates in nutrient absorption to a larger extent. In healthy humans, approximately 2 L of electrolyte-rich fluid flows daily across the ileocecal valve. Of this amount, 1.5 to 1.9 L will be absorbed in the colon, but the maximal capacity to absorb fluid in the human colon may be as high 5 to 6 L/day (70). Thus, healthy colon can compensate for a significantly increased ileocecal fluid flow in cases of absorptive deficits in the small intestine. Exceeding this maximal capacity, and/or coinciding colonic absorptive defect will lead to diarrhea. On the other hand, in colonic disease with unaffected small intestine, even relatively small changes in water and electrolyte transport will lead to a significant increase in stool output, highlighting the importance of fine tuning of epithelial transport in the large intestine. The majority of the electrolyte absorption in the proximal and to a lesser extent distal colon occurs via electroneutral NaCl transport in the crypt and surface epithelium. This process is mediated by coupled apical Na+/H+ and Cl−/HCO3− exchange. ENaC and transcellular and/or para-cellular Cl− absorption provide the alternative pathways for NaCl absorption. Figure 4 depicts a simple model of electrolyte transport and the role for the Na+/H+ exchange in the colonocyte, and reviews by Kunzelmann and Mall (160), Geibel (98), and Kiela and Ghishan (146) provide more extensive descriptions of mechanisms governing colonic electrolyte absorption.
Figure 4.
Colonic epithelial Na+/H+ exchange in relation to other electrolyte fluxes and short-chain fatty acid absorption and metabolism. NHE1, NHE2, NHE3, and NHE8, Na+/H+ exchangers; SLC9A, SLC9A2, SLC9A3, and SLC9A8, respectively; DRA, downregulated in adenoma, SLC26A3; CFTR, cystic fibrosis transmembrane conductance regulator; AE1, anion exchanger 1, SLC4A4; MCT1, monocarboxylate transporter 1, SLC16A1. Apical SCFA−/Cl− exchange indicated with question mark depicts described transport modality without identified transport protein/gene responsible. SCFA-H, protonated short-chain fatty acids; SCFA-, ionized forms of short-chain fatty acids. For additional sources, the reader is referred to reviews listed in Figure 3, and Binder (22) and Kunzelmann and Mall (160).
Segmental differences related to the primary mode of Na+ absorption in the colon can also be species-specific. Generally, in the proximal colon, Na+ transport is thought to be dominated by Na+/H+ exchange. In the distal segment of rat and mouse it is mixed, but in rabbit, human, and guinea pig’s descending colon, Na+ absorption occurs predominantly via electrogenic ENaC-mediated transport. NHE1 to 4, NHE7, and NHE8 have all been detected in the colon. The expression of the basolateral NHE1 is not significantly different in colonic segments and is not regulated by luminal (dietary) Na+ load (79,127). NHE2 and NHE3 are coexpressed on the apical membrane of the colonocytes (125). While NHE3 has been clearly described in the surface epithelial cells (28, 79), NHE2 expression pattern has been less consistent. Studies with human colonic biopsies showed uniform distribution of NHE2 mRNA along the crypt-surface axis (79). Studies in mice later showed that in the distal colon, NHE2 functions primarily in the crypts (12, 53), and contributed to the growing body of evidence that contrary to the textbook dogma, colonic crypts are also an important site of electrolyte and water absorption (278). Cl−-dependent Na+/H+ exchange mechanism has also been described in the of rat distal colonic crypts (24, 245, 264), but not in the same segment of the mouse large intestine (12,53). The reasons for this discrepancy are not known. Despite the high colonic NHE2 expression, and the known contribution of this isoform to pHi and cell-volume regulation (12), Nhe2−/− mice had no apparent intestinal absorptive defect (97, 270), although ectopic expression of NHE3 in colonic crypts of Nhe2−/− mice could compensate for the lack of the latter (12). In birds, NHE2 mediates the majority (~85%) of colonic Na+/H+ exchange both under unstimulated conditions and under Na+ depletion (76). In contrast, in mice, NHE3 appears to provide the major Na+ transport route in the proximal colon (271), and was estimated to contribute to ~70% of basal net Na+ absorption (15). As described earlier in this review, Nhe3−/− mice exhibit moderate diarrhea with colonic distention and alkaline fluid accumulation, mild metabolic acidosis, lower blood pressure, and a high mortality when deprived of dietary Na+ (97, 271). To counteract the loss of NHE3, knockout mice develop mucosal hypertrophy and have elevated expression and activity of the apical sodium channels in the distal colon and a dramatic induction of H+/K+-ATPase (cHKA) mRNA, which may provide a K+ sparing mechanism in the state of increased electrogenic Na+ absorption (271). The increases in ENaC and cHKA expression may be a direct result of an approximately fivefold increase in circulating aldosterone in Nhe3−/− mice (271).
Electroneutral NaCl absorption is thought to result from coupled activities of the apical Na+/H+ and Cl−/HCO3− exchanges. The HCO3−-dependent constituent of electroneutral NaCl absorption is stimulated by adrenergic agonists and reduced by cAMP, aldosterone, and by intracellular Ca2+ (160). DRA (downregulated in adenoma; SLC26A3) may be the most important anion exchanger responsible for the coupled colonic NaCl absorption. Mutations in the human SLC26A3 gene are responsible for congenital chloride diarrhea (122, 192, 199), also developed by DRA knockout mice (272). Musch et al. (205) showed in vitro (in colonic epithelial Caco2BBE) that expression of DRA increases Cl− uptake by a mechanism dependent on carbonic anhydrase and apical Na+/H+ exchange. These observations supported the hypothesis that apical Na+/H+ exchange and DRA, when coexisting, mediate coupled electroneutral NaCl absorption in the colon. Talbot and Lytle (292) suggested however, that this coexpression and functional coupling may be limited only to the mid-section of the large intestine. Considering on the expression patterns of NHE3 and DRA in rodents and their analysis of mucosal surface pH measurements, this group suggested a model in which uncoupled DRA activity in the cecum helps maintain an alkaline mucosal surface that could neutralize H+ from microbial fermentation. The uncoupled NHE3 activity in the proximal colon maintains an acidic mucosal surface, which facilitates short-chain fatty acid (SCFA) absorption, and coupled NHE3/DRA activities in the mid-colon permit an efficient NaCl absorption at closer to neutral mucosal pH (292).
SCFA, and especially butyrate, enhance colonic NaCl absorption. Several mechanisms have been implicated in this phenomenon. Cytosolic acidification as a direct result of SCFA uptake may stimulate electroneutral Na+ uptake through the activation of apical Na+/H+ exchangers (273). Increased HCO3− production during colonocyte SCFA metabolism stimulates Cl− absorption via apical Cl−/HCO3− exchange (Fig. 4). In another, complementary model butyrate uptake via nonionic diffusion or MCT1 (monocarboxylate transporter 1)-mediated SCFA/HCO3− exchange (64) activated electroneutral NaCl absorption by Cl−/butyrate and Na+/H+ exchanges operating in parallel (244). SCFA uptake stimulates basolateral volume-sensitive Cl− channels, while inhibiting basal and cAMP-activated, CFTR-mediated Cl− secretion (66,75). Na+/H+ exchange activation and inhibition of Cl− secretion are likely the two key components of the antidiarrheal effects of butyrate (39,160,220). In the rat distal colon in vitro, butyrate was shown to stimulate Na+ absorption through both NHE2 and NHE3 activities (157). In C2bbe cells, however, only NHE3 and NHE8 but not NHE2 were stimulated by butyrate (207, 318). The observed increase in NHE3 activity in response to SCFA is at least in part due to transcriptional activation of NHE3 gene promoter, as both protein and mRNA were induced by SCFAs in vitro and in the colon of rats fed diet supplemented with 5% pectin (207). This hypothesis was further supported by strong stimulation of the rat Nhe3 promoter activity by butyrate in transient transfection assays (148, 149). NHE8 is also regulated transcriptionally by butyrate via enhanced binding of Sp3 transcription factor to the proximal gene promoter (318). The concept of SCFA-activated colonic Na+/H+ exchange has been explored to propose an alternative composition of the oral rehydration solution (ORS). Although conventional ORS targets primarily small intestinal Na+ and water absorption, an improved formulation has been proposed to target the colon as well (247). In this approach, D-glucose is replaced with a relatively amylase-resistant cornstarch. A proportion of the starch is broken down enzymatically in the small intestine to stimulate Na+ absorption, while most of it would enter the colon where it could be fermented to SCFAs such as propionate, butyrate, and acetate by the resident bacteria. Butyrate increases the expression and/or activity of apical NHEs (4, 149, 157, 318) and aids in transepithelial Na+ absorption via a neutral linked Na+ absorptive process that exchanges SCFAs for OH− ions along with the apical Na+/H+ exchange. In a small randomized clinical trial, this ORS formulation performed better than standard ORS by reducing both the duration and volume loss of severe acute diarrhea (247). Two other randomized controlled trials with the addition of high-amylose maize starch to ORS performed in South India have shown a substantial decrease in diarrhea duration in both adults and children hospitalized for acute diarrhea (243,248).
Regulation of the Na+/H+ Exchangers Expressed in the Digestive Tract
NHE1
NHE1 expression in rodents and humans is similar throughout the intestine, with only a slight reduction along the crypt-villus axis in the jejunum (28, 79). This relatively uniform expression of NHE1 is consistent with its role as a “housekeeping isoform” participating in pHi and cell-volume regulation. Although Na+/H+ activity at the basolateral membrane of enterocytes increases with age (59, 67), expression of NHE1 mRNA remains unchanged.
The promoter region of NHE1 has been cloned and characterized in human, mouse, rabbit, and pig (26, 80, 82, 195), but only the mouse NHE1 promoter was analyzed in more detail. The mouse NHE1 promoter is activated by AP-2-like transcription factors (80), as well as a poly (dA:dT) region of the promoter interacting with an unidentified nuclear protein (325). Serum and growth factors have been shown to stimulate NHE1 promoter activity in cardiomyocytes and fibroblasts through more distal regions of the NHE1 promoter (0.8–1.1 kb) by interacting with chicken ovalbumin upstream promoter (COUP) transcription factors (20,85). However, the in vitro findings do not correlate with the in vivo observations obtained from transgenic mice bearing the NHE1 gene promoter reporter construct. In the latter studies, crossing these mice with AP-2α or COUP-TFI knockout mice did not change the reporter gene expression in embryonic mouse tissue (251). The transcriptional regulation of NHE1 expression in the intestine has not been extensively studied, although limited data suggest that NHE1 expression is unlikely to be regulated at the mRNA level, which is consistent with its housekeeping role.
Although NHE1 transcription is not highly regulated, extensive posttranslational mechanisms of NHE1 regulation have been described. Many functionally distinct signaling molecules, including phosphatidylinositol 4,5-bisphosphate, calcineurin homologous protein (CHP)1, and actin-binding proteins of the ERM family, are associated with the cytoplasmic C-terminal regulatory domain of NHE1 protein. The distal C-terminal region of NHE1 protein also contains a number of serine residues that can be phosphorylated by ERK-regulated kinase p90RSK and Ste20-like Nck-interacting kinase upon activation of growth-factor receptors, and by Rho kinase 1 (ROCK1) upon activation of integrin receptors and G protein-coupled receptors for thrombin and lysophosphatidic acid (LPA). NHE1 activity could be increased by C-terminal serine phosphorylation. p90RSK phosphorylates NHE1 at Ser703 and promotes direct binding of the adaptor protein 14-3-3, which is thought to provide a “nucleus” for the assembly of other signaling molecules. HSP70 heat shock protein and carbonic anhydrase II also bind within this regulatory domain of NHE1. The interaction between NHE1 and carbonic anhydrase II may explain increased NHE1 activity upon phosphorylation. It has been shown that serum-induced phosphorylation within the last C-terminal 178 amino acids facilitates binding of carbonic anhydrase II through catalysis of CO2 hydration causes local acidification and an increase in NHE1 activity (173). It is important to note that despite ubiquitous expression of NHE1, it is not known whether the described mechanisms involved in NHE1 regulation are universal to all cell types, and whether they contribute to the physiology of digestive tissues.
NHE2
Unlike intestinal NHE1, the expression of intestinal NHE2 is modulated during postnatal development. NHE2 protein expression levels are lowest in suckling rats and higher in weanling and adult rats (fourfold to sixfold increase), which is consistent with NHE2 mRNA expression where the lowest expression is seen in suckling rats and higher expression is seen after weaning (threefold to fivefold increase) (57). The mechanism of this ontogenic regulation on NHE2 function is thought to be at the transcriptional level, as nuclear run-on analyses showed about twofold increases in transcription rate in adolescent rats compared to suckling rats (57).
The NHE2 gene promoter has been cloned and characterized in rat and human (183, 204). The proximal promoters from both species lack canonical TATA and CAAT boxes and are GC-enriched. These proximal promoter regions share ~59% homology with a number of conserved, predicted, and regulatory elements. Simple analysis on the human NHE2 promoter suggested putative binding sites for the trans-acting factors: Sp1, AP-2, Egr-1, p300, NF-κB, Oct-1, zinc finger protein-1, MyoD, two caudal-related homeobox (Cdx) family members, CdxA and Cdx-2, glucocorticoid receptor, thyroid hormone receptor, a CACCC site, and several polyoma viral enhancer sites (183). Of all these sites, only Sp1, AP-2, CACCC, NF-κB, and Oct-1 were conserved in human and rat NHE2 promoters. A minimal promoter analysis on rat NHE2 identified Sp transcription factors that were required to activate or inhibit NHE2 transcription. In NHE2 promoter DNA transfected renal epithelial cells, Sp1 activates NHE2 promoter while Sp3 and Sp4 inhibit NHE2 promoter activity (14). In intestinal epithelial cells transfected with rat and human NHE2 promoter, both Sp1 and Sp3 transcription factors activate the NHE2 promoter (126,236).
Similar to NHE1, serum and EGF also activate NHE2 (124, 297). Interestingly, a transcriptional mechanism is involved in EGF-mediated NHE2 activation. EGF is well known to play important roles during gut maturation. It is secreted primarily by salivary glands and binds to its receptors expressed along the GI tract (43). In EGF-treated rat hepatocytes, the pHi recovery rate from an intracellular acid load, as a measurement of NHE activity, was significantly faster (110). EGF stimulates Na+ absorption in the GI tract (221, 222), and this effect can also be traced to stimulation of Na+/H+ exchange. In isolated rat enterocytes, EGF at 200 ng/mL stimulated Na+/H+ exchange activity 1.8-fold (100). In in vivo studies, EGF treatment increases jejunal NHE2 activity by almost twofold in suckling rats. The increase of NHE activity correlates with the increase of NHE2 mRNA abundance. EGF-mediated NHE2 activation was not seen in the kidney (314). This finding was confirmed by in vitro study in rat intestinal epithelial (RIE) cells. In vitro studies show that EGF also stimulates pHi recovery in cultured RIE cells by twofold, and a similar induction is observed in Nhe2 gene promoter activity (314). However, neither exogenous EGF nor salivarectomy affected intestinal NHE2 mRNA expression in adult mice (83), suggesting that EGF regulation may be species and/or age dependent. Growth factors could induce the production of 1,2 diacylglycerol (DAG), an activator of PKC. Using DAG structural analog, phorbol 12-myristate 13-acetate (PMA), elevated NHE2 activity and NHE2 mRNA expression could be detected (135). Later study showed that PMA triggers phosphorylation of nPKCδ, activation of extra-cellular signal-regulated protein kinase-1 and −2 (ERK1/2) and subsequent nuclear translocation of Egr-1 transcription factor. Egr-1 then binds to the human NHE2 promoter and promotes NHE2 gene transcription (208). It remains unknown whether this mechanism of NHE2 transcriptional regulation is related to the differential effects of EGF during growth/aging.
Osmolality regulation of NHE2 has been described. Hyperosmolarity inhibited NHE2 activity in NHE2-expressing PS120 cells, (212), but it activates NHE2 in mouse inner medullary collecting duct (mIMCD-3) cells (279), AP-1 cells (138), and colonic crypt cells (12). In mIMCD cells, hyperosmotic stress stimulated NHE2 mRNA expression (13, 279). In studies with the rat NHE2 promoter, our group identified a TonE-like element and a novel cis-element, named OsmoE, as directly responsible for the enhanced NHE2 gene transcription under hyperosmotic stress (13). Transcription factors interacting with these two cis-elements have not been identified, and it is not known if the same mechanism is also present in the colonic crypt epithelium.
NHE2 activity is also regulated by intracellular ATP levels. ATP depletion reduces NHE2 activity by a dramatic decrease in H+ affinity as well as Vmax, with virtual elimination of the allosteric effect of H+ (172). The fact that ATP depletion eliminates the stimulatory effect of serum suggests that growth factor-stimulated NHE2 activity is mediated via its pH-sensing mechanism. In fibroblasts, thrombin treatment increased the Vmax of NHE2 without altering the Hill coefficient (172), although it is not clear if this could be attributed to the elevated intracellular Ca++.
NHE3
Similar to NHE2, NHE3 expression is also subject to developmental regulation. At the functional level, NHE3-mediated Na+ uptake in rat jejuna is similar between suckling and wean-ling rats, higher in adult rats, and the highest in adolescent rats (58). At the protein level, NHE3 expression is lowest in suckling rats and increased up to sevenfold after weaning. NHE3 mRNA expression is also lowest in suckling rats and increased approximately twofold after weaning (58). Therefore, the ontogenic regulation of NHE3 expression likely involves both transcriptional and posttranscriptional mechanisms.
The rat Nhe3 gene promoter was first cloned by Tse et al. and by Orlowski et al. with a discrepancy in the transcriptional start site (134) (40). Later, our group resolved this discrepancy by showing that the atypical TATA box located at bp −26/−31 upstream of the transcription start site mapped by Tse et al. (134) was not necessary and even detrimental for Nhe3 promoter activity, and that a −20/+8-bp fragment represents a functional, albeit atypical, initiator (151). Three Sp transcription factor binding sites within the −81-bp upstream region of Nhe3 promoter were critical because mutation at these sites drastically reduced NHE3 promoter activity. Electromobility shift assay and forced expression of Sp1 and Sp3 in SL2 cells further demonstrated the roles of Sp1 and Sp3 in transactivation of the Nhe3 promoter. Both of Sp1 and Sp3 acted synergistically with GATA-5 bound to a GATA box within exon 1 (+20/+23 bp). These experiments showed that rat Nhe3 promoter is initiator-driven and controlled primarily by Sp1/Sp3 and their interaction with GATA-5. This interaction may also be responsible for the gradient of NHE3 expression along the crypt-villus/surface axis (151). The human NHE3 promoter was also cloned and a maximal promoter activity in the −95/+5 nt region was also defined. The sequence in the human NHE3 maximal promoter has very high homology with the proximal promoter of the rat Nhe3, with overlapping and functional regulatory elements for Sp and AP-2 transcription factors (185).
The expression of NHE3 is subject to hormonal regulation. Studies have showed that glucocorticoids regulate NHE3 function at mRNA transcription and NHE3 activation mediated by serum- and glucocorticoid-induced protein kinase (SGK1). Yun et al. showed that rabbit intestinal NHE3 mRNA expression could be altered by glucocorticoid treatment (335). Similarly, glucocorticoids also regulate NHE3 expression in rats. Dexamethasone exposure increased NHE3 mRNA expression in the ileum (but not jejunum) and proximal colon (but not distal colon) in adult rats (50). As a confirmation, the expression of NHE3 in the ileum and proximal colon was reduced in adrenalectomy rat. Glucocorticoids mediated NHE3 regulation in the intestine is segment-specific and age-dependent. The response in the proximal small intestine was greatest in suckling rats and decreased with age to no detectable change in adults, while ileal NHE3 was induced by methylprednisolone only in adults (147). This age- and segment-specific glucocorticoid response correlated with the expression and ligand binding capacity of the glucocorticoid receptor in the enterocytes (147). Glucocorticoids-mediated transcriptional regulation of the Nhe3 gene was further confirmed in cells transfected with rat Nhe3 promoter reporter constructs by Cano (40) and Kandasamy and Orlowski (134).
SCFAs are produced by colonic bacterial flora fermentation of dietary carbohydrates, and they are important to maintain gut health. They are potent stimuli of intestinal sodium and water absorption (23, 51, 158, 246, 259), with butyrate being the most effective molecule (158). SCFA-mediated increase in Na+ absorption is considered as the collaborative work between Na+/H+ and SCFA−/Cl− exchange. The use of amylase-resistant starch as an additive to oral rehydration solution proved effective in reducing diarrheal stool output in cholera patients (248), indicating a potential use of SCFAs as antidiarrheal agents. The mechanism of SCFA-mediated NHE activation appears to involve transcriptional induction of NHE3 gene expression. Rats fed a 5% pectin-supplemented diet for two days displayed an increase in NHE3 mRNA, protein and activity in the colonic epithelial cells (207). Similar results were also seen in SCFAs-treated Caco-2/bbe cells (207). Our own work also showed that rat Nhe3 promoter activity is significantly induced by SCFAs, especially butyrate, in Caco-2 cells (148). This mechanism involves Ser/Thr kinase activity with a likely permissive role for protein kinase A (PKA), as the butyrate-mediated Nhe3 promoter activation could be blocked by H-7, Rp-cAMPS, and H-89 inhibitors as well as by overexpression of a dominant-negative mutant form of the regulatory subunit of PKA (148). Subsequent studies revealed that butyrate could induce Sp1 phosphorylation and Sp3 acetylation, and a shift in their interaction with the Nhe3 promoter in favor of Sp3 (149). Since Sp3 is a more potent inducer of NHE3 transcription, such shift would result in increased NHE3 promoter activity.
Neural transmitters also regulate NHE3 function. Serotonin (5-hydroxytryptamine), largely produced in the gut, plays an important role in regulating GI motility, secretion, and absorption. Increased serotonin levels have been detected in carcinoid syndrome associated diarrhea, in ulcerative colitis (UC) and in irritable bowel syndrome. Studies by Gill et al. (103) has showed that serotonin acutely decreases NHE3 activity via 5-HT4 receptors in Caco2, and that serotonin transiently inhibits NHE3 transcription by reducing the interaction among Sp1, Sp3, and the proximal NHE3 gene promoter via a PKCα-dependent pathway (5).
Systemic acid-base imbalance also affects intestinal Na+ absorption (44) through its effect on intestinal NHEs. When rats were fed with 5% NH4+Cl water, the expression of NHE2 and NHE3 mRNA, protein, and their activities were increased in the ileum (179). The mechanism of this regulation was later studied in Nhe3 promoter construct transfected opossum kidney cells. The Nhe3 gene promoter activity was enhanced after prolonged (24-h) exposure to acidified media (40).
NHE3 expression is also enhanced after intestinal resection, as an adaptive response to enhance the intestinal absorptive capacity (260). In rats that had 50% proximal small bowel resection, the expression of NHE3 mRNA and protein is increased approximately threefold (206). The increase was only observed in the ileal segment distal from the anastomosis site, suggesting that dietary rather than humoral factors might be responsible. Similar observations were also seen in enterectomized mice (83).
NHE3 activity is subject to regulation at the protein level. The vast majority of knowledge on acute regulation of NHE3 activity comes from heterologous cell expression systems such as PS120 fibroblasts, or from renal epithelial cells, or colon cancer cells. However, the described mechanisms may also apply to the epithelial cells with the digestive tract. Several detailed reviews have been published on the topic of NHE3 regulation (3,78,115,338). The key elements for NHE3 protein modification are located within the C-terminal regulatory domain (78). The C-terminus of NHE3 binds to several scaffolding proteins to form higher-order multiprotein complexes, and in some respect, NHE3 itself can be considered as a scaffold. A small, putative α-helical domain of NHE3, located between amino acids 586 to 605 was suggested to act as a “switch domain” to interact with at least seven other proteins to activate or inhibit NHE3. The dynamic complex assembly, association with the cytoskeleton, endosomal recycling, and protein phosphorylation/dephosphorylation, all act in concert to provide a mechanism for tightly controlled and fine-tuned NHE3 protein turnover and activity (339).
cAMP regulates NHE3 activity via PKA-mediated phosphorylation mechanism. NHE3 protein was phosphorylated by PKA in response to elevated intracellular cAMP, at Ser552 and Ser605 (or Ser554 and Ser607 in the extensively studied rabbit NHE3 protein) in vitro, and only at Ser605in vivo (342). In this study, phosphorylation of Ser552 was shown to participate in the NHE3 response to cAMP, but this site was not involved in another study by Kurashima et al. (161). NHE3 phosphorylation by PKA results in reduced Vmax and decreased surface NHE3 protein distribution, which is presumably due to increased endocytosis, and decreased exocytosis. During PKA activation, a multiprotein complex was recruited to the C-terminus of NHE3. This complex contains NHERF1 (SLC9A3R1), NHERF2 (E3KARP, SLC9A3R2) and a scaffolding protein ezrin. When overexpressed in NHERF-deficient cells, both NHERF1 and NHERF2 restitute PKA-dependent NHE3 inhibition (337). NHERF1 and NHERF2 interact through their C-terminal 29 amino acids with cytoskeleton-associated ezrin (249, 336), which functions as A kinase anchoring protein (AKAP). Phosphorylation of NHE3 by PKA is therefore facilitated by bringing the catalytic subunit of PKA to the vicinity of the NHE3 cytoplasmic tail by a protein complex containing either of the two NHERF factors and cytoskeleton-associated AKAP protein, ezrin. PDZ-binding protein PDZK1 (NHERF3/CAP70/PDZ-dc-1) was also necessary for the cAMP- and Ca2+-mediated NHE3 regulation in mouse colonocytes (54).
Similar to cAMP regulation on NHE3, cGMP also inhibits NHE3 activity via the cGMP-dependent type II protein kinase cGKII (PRKG2) pathway. Chen et al. (48) showed that cGMP/cGKII-mediated rapid inhibition of rabbit NHE3 was associated with decreased NHE3 plasma membrane abundance and required phosphorylation of all three serines (Ser554, Ser607, and Ser663, equivalent to mouse Ser552, Ser605, and Ser659).
Phosphorylation mediated by casein kinase 2 (CK2) and Ca2+/calmodulin-dependent protein kinase II (CaM KII) has been shown to modulate NHE3 function. CK2-mediated phosphorylation at Ser719 is required for ~40% of rabbit basal NHE3 activity and mutation of this residue led to decreased NHE3 exocytosis and reduced NHE3 plasma membrane abundance (268). The follow-up study also showed that Ser719 phosphorylation is part of the PI3-K/AKT-dependent pathway (267). Mutation of Ser719 led to a reduced NHE3 complex size, reduced expression in lipid rafts, increased brush border mobile fraction of NHE3, and reduced binding of multiple proteins to the C-terminus, including CHP, the NHERF family proteins, and SNX27 (related PDZ domains) (267). CaM KII binds to the same α-helical domain as CK2 in NHE3 protein, and results in basal NHE3 activity inhibition in both fibroblasts and colonic epithelial cells (343). Physical interaction between CaMKIIγ subunit and NHE3 protein was inversely related to intracellular Ca2+ concentration. It occurred between aa 586 and 605 in the NHE3 C terminus, but phosphorylation occurred downstream of aa 690. CaM KII-mediated NHE3 inhibition was not associated with a change in NHE3 plasma membrane expression but, rather via a decreased NHE3 turnover number (343).
LPA stimulates NHE3 activity via the G-protein coupled LPA5 receptor (175). Phosphorylation at Ser719 of NHE3 protein is required (267). An alternative or complementary pathway for LPA stimulation was also proposed whereby LPA transactivates EGFR, which results in the parallel activation of RhoA-Rho-associated kinase-proline-rich tyrosine kinase 2 (Pyk2) cascade and the MEK-ERK pathway (331). The results of this study suggested that Pyk2 and ERK1/2 may converge on the same effector, which was later confirmed to be the p90 ribosomal S6 kinase RSK2 (214). RSK2 was shown to physically interact with the NHE3 C-terminus in LPA-treated cells, resulting in phosphorylation at Ser663 (human NHE3), and increased brush-border membrane delivery of NHE3 (214).
Glucocorticoids also affect NHE3 activity. The similar scaffolding mechanism is involved with glucocorticoid-stimulated NHE3 activity. In this case, however, the mediating kinase is serum and glucocorticoid inducible kinase, SGK1. SGK1 directly and specifically interacts with NHERF2, acting as a bridge between the kinase and NHE3, to stimulate activity of the latter (333). The second PDZ domain of NHERF2 binds both NHE3 (aa 585 and 660) and SGK1. A model facilitating this assembly was proposed (334), in which NHERF2 dimerizes. It has also been postulated that the mechanism of posttranscriptional regulation of NHE3 by glucocorticoids is biphasic, with an initial phase involving phosphorylation of the preexisting membrane NHE3, and a later phase in which SGK1 and NHERF2 facilitate translocation of the newly synthesized NHE3 to the cytoplasmic membrane (333). Ser663 of NHE3 appears to be the major site of phosphorylation by SGK1 and its mutation blocks the effect of dexamethasone (303). Ser663 phosphorylation precedes the changes in NHE3 activity, which is associated with an increased amount of NHE3 proteins at the surface membrane. However, in vivo, SGK1 or NHERF2 deletion, while significantly attenuating the effect of dexamethasone on NHE3 activity, did not completely abolish the stimulation (114). The authors showed that the SGK3 isoform also contributes to the end result at the endosomal level. Dexamethasone activated SGK3 and NHE3 activities via a mechanism dependent on phosphoinositide 3-kinase and phosphoinositide-dependent kinase 1 (PDK1). Dexamethasone induced translocation of PDK1 to endosomes, the primary location of SGK3; Arg90 mutation of SGK3 disrupted its endosomal localization and delayed NHE3 activation (114).
NHE8
NHE8 expression in the intestine is regulated by intestinal maturation. In rodents, NHE8 protein expression is the highest before weaning and is reduced almost by half in adults, while NHE8 mRNA abundance is twofold higher in sucklings and weanlings compared with in adults (312, 313). In the colon, low NHE8 expression is detected in suckling mice and high expression is seen after weaning in mice (321). In the absence of NHE2 and NHE3, NHE8 expression is increased to compensate the loss of NHE2 and NHE3 function (316).
NHE8 expression is subject to regulation by growth hormones. Unlike the stimulatory role of EGF on intestinal NHE2 expression, EGF actually inhibits NHE8 expression in the ileum in suckling rats at both protein and mRNA levels. A similar observation was also made in Caco-2 cells. EGF inhibits NHE8 expression by reducing Sp3 transcriptional factor binding to the NHE8 basal promoter (319). These observations suggest that the effect of EGF on NHE gene expression is isoform and segment-specific, and that this regulation occurs at the gene transcriptional level.
Glucocorticoids, another important regulator of intestinal maturation, also affect NHE8 expression in the intestine. Endogenous glucocorticoid hormone levels surge after weaning (119), this coincidentally correlates with a decrease of NHE8 expression in the intestine. Studies showed that NHE8 expression in young rats is inhibited by methylprednisone treatment. Methylprednisone administration reduced jejunal NHE8 protein abundance by 30% and ileal NHE8 protein abundance by 90%. It also inhibited NHE8 mRNA synthesis by 28% in the jejunum and 45% in the ileum. Further study identified that NHE8 expression inhibition mediated by glucocorticoids involves enhanced Pax5 transcriptional factor binding to the NHE8 gene promoter (320).
SCFAs also play a role in regulating NHE8 expression in the intestine. In Caco2, butyrate treatment strongly induced NHE8 protein (threefold) and NHE8 mRNA expression (2.3-fold). Transfection with the human NHE8 promoter reporter constructs in Caco2 cells showed that butyrate treatment stimulated NHE8 promoter activity at an amount comparable with its stimulation of NHE8 mRNA expression. Mechanistic study revealed an enhanced Sp3 protein binding on the human NHE8 basal promoter region upon butyrate stimulation, and that the effect of butyrate on NHE8 expression involves the acetylation of Sp3 transcriptional factor, a key protein regulating NHE8 basal transcription activation (318).
Somatostatin, an important neuropeptide produced by D cells in the GI tract, is a proabsorptive and antisecretory molecule. Studies have shown that somatostatin could stimulate sodium absorption through its effect on NHE8 expression. Octreotide, an analog of somatostatin, increased brush-border membrane distribution of NHE8 protein by ~62% in the small intestine. In human intestinal cells (Caco-2), somatostatin treatment increased the plasma membrane NHE8 protein expression by ~86% and NHE8 activity by ~80%. The somatostatin-mediated increase in NHE8 expression required p38 mitogen-activated protein kinase (MAPK) activation via somatostatin receptor SSTR2 (301). A follow-up study also showed that octreotide treatment stimulates colonic NHE8 expression in colitic mice. Both somatostatin receptor 2 (SSTR2) and somatostatin receptor 5 (SSTR5) were involved in restoring NHE8 expression via their roles in suppressing ERK1/2 phosphorylation (174).
Na+/H+ Exchange in Pathological States
Na+/H+ exchangers are frequent targets of inhibition in GI diseases, either by the host factors (e.g., bile acids, inflammatory mediators) or by selected infectious agents and/or the associated bacterial toxins. Moreover, disruption of Na+/H+ exchange activity via impaired expression or function of respective isoforms may contribute not only to local and systemic electrolyte imbalance but may also modify the course and severity of disease via the effects on resident gut microbiota or via intrinsic defects in the epithelial cell functions.
NHE1
NHE1 expression in the colonic mucosa has been studied in animal and in vitro models of intestinal inflammation. In acetic acid or 2,4,6-trinitrobenzenesulfonic acid (TNBS)-induced rat models of colitis, Khan et al. (143) described increased NHE1 mRNA expression in the colonic mucosa. In TNBS-treated rats, amiloride administered orally at 3 mg/kg body weight attenuated the symptoms of colitis, decreased neutrophilic infiltration in the colonic mucosa, and reduced the level of IL-1β and ERK, but not of p38 in inflamed colonic smooth muscle and ERK activity in the colonic smooth muscle (144). In vitro, in Caco-2 and HT-29 cells, inhibition of Na+/H+ exchange with amiloride and other related and unrelated NHE inhibitors has been shown to reduce IL1β-, TNFα-, and LPS-stimulated IL-8 production, IL-1β-induced NF-κB activation and phosphorylation of ERK (extracellular signal-regulated kinase) (213). It is not clear which NHE isoform (if any) was targeted in this study, since amiloride concentrations required for inhibition of IL-8 production were significantly greater than those needed to inhibit NHE1 and NHE2, the two isoforms likely to be expressed in the utilized cells. The same report demonstrated the effectiveness of amiloride in vivo in murine DSS colitis, where it reduced disease activity score and mucosal production of MIP-2 (mouse ortholog of human IL-8) (213). Consistent with these and other reports of NHE1 contribution to inflammatory response (69), a report in humans also confirmed sixfold elevated expression and activity of NHE1 mRNA in UC patients (84). Other human studies do not confirm this observation, however. A decreased NHE1 mRNA expression was observed in colonic biopsies from Crohn’s disease (CD) and UC patients, as compared to healthy colon (145). Similarly, the expression of NHE1 was also reduced in the sigmoid mucosa of CD and UC patients (288). Further work showed that NHE1 activity could be inhibited by acute or chronic IFNγ treatment in Caco-2 cells (180). Therefore, the involvement of NHE1 in the pathogenesis of inflammatory bowel disease and its potential as a therapeutic target remains unclear. In a rat model of necrotizing enterocolitis, the expression of NHE1 protein was not altered, but in vitro analysis in IEC-6 cells showed LPS-mediated inhibition of NHE1 activity and intracellular acidification, without concomitant changes in protein expression or cellular localization (42).
NHE2
As we described in the section on the expression, regulation, and roles of Na+/H+ exchangers in gastric physiology, based on murine studies, NHE2 plays a significant role in the gastric epithelium. However, alterations in NHE2 expression or activity in gastric disorders in human patients have not been documented. Interferon downregulated NHE2 expression and activity, implicating a role for NHE2 in inflammation-associated diarrhea (253), although normal intestinal Na+ and water homeostasis in Nhe2−/− mice argue against the role of NHE2 in the pathogenesis of diarrhea associated with inflammatory bowel disease (IBD). Surprisingly, enteropathogenic Escherichia coli infection significantly increased NHE2 activity via a PKCε-dependent mechanism in intestinal epithelial cells, while reducing the activities of NHE3 and Cl−/OH− exchange (117, 120). This study suggested a potential compensatory role of NHE2 in luminal fluid absorption during bacterial infection. On the other hand, TNFα also inhibits NHE2 expression through a NF-κB-dependent mechanism, a phenomenon postulated to contribute to inflammation-associated diarrhea in IBD (6). Although our group has not demonstrated any differences between wild-type and Nhe2−/− mice in their susceptibility to DSS-induced mucosal injury (150), Moeser et al. showed that Nhe2-deficiency delays the recovery from mesenteric ischemia with increased mucosal permeability and disruption in the localization of the tight junctions proteins occludin and claudin-1 (198).
NHE3
A form of congenital secretory diarrhea (CSD) associated with defective Na+/H+ exchange has been described by Holm-berg and Perheentupa (123) and Booth et al. (32). Based on a close phenotypical resemblance between this rare disease and symptoms displayed by Nhe3−/− mice, NHE3 became a likely candidate. However, homozygosity mapping and multipoint linkage analysis in four candidate regions known to contain NHE1, NHE2, NHE3, and NHE5 genes showed that CSD is not associated with mutations in these genes (203). More recent genomic studies revised the idea of reduced NHE3 activity as a contributor to the classic or nonsyndromic form of CSD. Using a cohort of 18 patients from 16 families, Janecke et al. detected a variety of mutations (point, missense, and truncation) in the NHE3-encoding SLC9A3 gene in half of the studied CSD cases (131). The identified SLC9A3 mutations included one whole-gene deletion, one splicing, and two frameshift mutations, all of which were expected to have abolished NHE3 protein production. Four missense mutations/variants, p.Arg382Gln, p.Ala311Val, p.Ala269Thr, and p.Ala127Thr, were tested in vitro, and all but p.Ala127Thr (benign variant) conferred decreased basal Na+/H+ exchange activity (131). In a recent genome-wide SNP analysis of syndromic CSD patients, Heinz-Erian et al. identified loss-of function mutations in SPINT2 gene encoding a Kunitz-type serine-protease inhibitor (118). These mutations are believed to be responsible for a third of CSD cases described as syndromic CSD form. The physiological target(s) of SPINT2, the molecular pathology caused by SPINT2 deficiency, and its relationship with Na+/H+ exchange remains unknown. In addition, an activating mutation in the catalytic domain of the guanylate cyclase 2C gene (GUCY2C, which also serves as a heat-stable enterotoxin receptor) may account for the additional 20% of sporadic CSD cases (87,202). It is thought that this mutation is mechanistically linked to hyperactivation of the CFTR and to inhibit NHE3 function via increased intracellular cGMP and a cGKII kinase-dependent mechanism (11,48,87,109).
NHE3 expression and activity are altered in primary and diabetes-related hypertension, implying a potential role of NHE3 in the development of high blood pressure. Spontaneously hypertensive rats have elevated NHE3 activity in both the ileal brush-border membranes (298) and the renal proximal tubules (140), suggesting an increased intestinal and renal sodium absorption which may contribute to systemic sodium retention and the pathogenesis of hypertension. In streptozotocin-induced diabetic rats and in BB/W autoimmune diabetic rats, the renal cortex brush-border Na+/H+ exchange (presumably mediated by NHE3) was significantly increased, likely due to acidosis but not hypoinsulinemia (112). In streptozotocin-induced diabetic rats, twofold increase in NHE3 mRNA expression was detected in the ileum (102). However, the observed change was most likely due to vitamin D deficiency, as repletion of diabetic mice with 1,25(OH)2D3 restored NHE3 mRNA expression to control levels.
Diarrhea is common in patients with IBDs. It is the result of perturbations in intestinal absorptive and secretory processes (99, 240). Studies have showed that proinflamma-tory mediator IFNγ downregulates NHE3 mRNA and protein expression both in vivo and in vitro (253). IL-2 knockout mice display a disease resembling human UC (261), along with a dramatic reduction in colonic transepithelial Na+ flux, which has been associated with a reduction in electroneutral NaCl absorption and reduced NHE3 mRNA and protein expression in the proximal colon. In the distal colon of IL-2−/− mice, aldosterone-stimulated electrogenic Na+ transport was also reduced with decreased ENac expression (15). This suggests NHE3 involvement in the pathogenesis of UC-associated diarrhea and reduced capacity of the distal colon to compensate via electrogenic Na+ transport. Two other studies with UC patients showed reduced NHE3 activity in colonic biopsies (84,330). NHE3 inhibition could not be explained by changes in the expression level or cellular localization of NHE3 (330), an observation in contrast with an earlier study by Sullivan et al. (288), in which NHE3 expression at protein and RNA levels were decreased in the biopsies of UC and CD patients as well as in DSS colitis in mice. NHE3 activity was significantly decreased in isolated colonic crypts in IL10−/− mice, although NHE3 expression and localization were not affected (171).
In an in vivo model of diarrhea mediated by anti-CD3 monoclonal antibody-induced T-cell activation, tumor necrosis factor induced NHE3 internalization in the jejunum via PKCα activation and resulted in Na+ malabsorption (55). In a rat model of necrotizing enterocolitis, ileal NHE3 protein expression was not affected (42). These observations implied the differential regulation of NHE3 expression and activity by inflammatory mediators. Although it is clear that the NHE3-mediated apical Na+/H+ exchange and epithelial Na+ absorption are inhibited in IBD, the exact mechanism remains unclear and may depend on the specific disease, segment involved, and/or the severity of inflammation.
Chronic NHE3 inhibition in intestinal inflammation may have consequences going beyond alteration in ion transport. Observations from Nhe3-deficient mice showed a significant upregulation of IFNγ and IFNγ-inducible genes in the jejunal mucosa (165,309), and the infiltrating CD8+ and asialoGM1+ NK cells as the primary sources of IFNγ (150). Our group has also demonstrated that Nhe3−/− mice spontaneously develop distal, neutrophilic colitis that could be alleviated with broad-spectrum antibiotics (165). Nhe3−/− mice were also dramatically susceptible to DSS-induced epithelial injury. DSS in concentrations insufficient to induce histological changes in wild-type mice were lethal in Nhe3-deficient littermates (150). Moreover, NHE3 deficiency exacerbated colitis in IL10−/− mice (163), and dramatically accelerated and exacerbated disease in response to adoptive naïve CD4+ T cell transfer in Rag2 mice (164). Thus, NHE3 may serve as a modifier gene determining the extent of the gut innate inflammatory responses in the face of intestinal injury (150). The inhibition of NHE3 and NHE8 (discussed below) in the course of IBD may be one of the factors contributing to microbial dysbiosis and exacerbated inflammatory response.
NHE8
In TNBS-treated rats, the expression of NHE8 mRNA and protein in the ileum (tissue not directly exposed to the haptenizing agent) was reduced by 35% and 41%, respectively (311). In LPS-treated rats, the expression of NHE8 mRNA and protein in the ileum was reduced by 35% and 44%, respectively (311). In DSS-induced colitis in mice, the colonic NHE8 expression was reduced by 52% at the mRNA level and by 68% at the protein level (177). Recently, the expression of NHE8 in human UC patients has also been studied. In active UC patients, the expression of NHE8 protein was significantly reduced by ~55% (174). Similar findings were also observed in TNFα-treated Caco-2 cells, where TNFα treatment at 10 ng/mL reduced NHE8 mRNA expression by 40% (311). In human intestinal goblet cells (HT29-MTX), TNFα treatment at 30 ng/mL reduced NHE8 mRNA expression by 54% and protein by 35% (317). The mechanism of TNFα-mediated NHE8 expression inhibition was further investigated in both of these cell lines, and determined to be transcriptionally mediated via decreased Sp3 interaction with the human proximal NHE8 promoter (311,317). Although the overall contribution of NHE8 to the intestinal Na+ absorption is still unclear (compensatory increase in NHE2 and NHE3 expression in the small intestine of NHE8−/− mice may mask the true role of NHE8) (321), considering the reported simultaneous inhibition of NHE3 in UC, it was postulated that reduced NHE8 expression and activity inhibition might contribute to the inflammation-associated diarrhea.
Recent studies have also suggested important roles for NHE8 in mucosal homeostasis through its modulation of goblet cell function. Goblet cells express NHE8, which is downregulated in rodent experimental colitis and in UC patients, and its deletion confers higher susceptibility with an enhanced T-helper 2–like response (174, 177, 311). Reduced expression or absence of NHE8 also leads to decreased Muc2 mRNA expression in goblets cells, and decreased mucosal expression of antimicrobial peptides (300). Reduced Muc2 expression is also detected in NHE8−/− goblet cells and in intestinal organoids from Nhe8-deficient mice (Xu et al., unpublished data). These findings suggest that loss of NHE8 expression may be responsible for the reduced inner mucous layer and closer proximity of luminal bacteria to the brush border membrane in Nhe8−/− mice (177). In support of this hypothesis, deletion of NHE8 led to increased mucosal adhesion of Salmonella typhimurium in vivo, with similar effects seen in vitro in epithelial cells with siRNA-mediated NHE8 knockdown (177). Interestingly, NHE8 knockdown did not affect the adhesion of a probiotic strain Lactobacillus plan-tarum JDM1, thus suggesting at least some degree of selectivity that could not be explained by mucus production alone (177).
Conclusion
Na+/H+ exchange is an evolutionarily conserved and key membrane transport mechanism involved in a wide array of processes on cellular, tissue, and systemic levels. The majority of current knowledge is limited to the function of the isoforms expressed on the plasma membrane, and to a much lesser degree on the intracellular NHEs. Molecular physiology of this transporter family has been extensively studied in a range of invertebrate and vertebrate organisms and their expression, regulation, and physiological importance in the digestive tract has been highlighted in this review. More recently, a number of observations with genetically modified mice underscored the potentially detrimental (NHE1) or protective roles (NHE3, NHE8) that NHEs play during the pathogenesis of diarrheal and inflammatory disorders of the GI tract. Future studies may bring new approaches that selectively target NHEs in the gut to enhance or inhibit their function for the benefit of patients.
Didactic Synopsis.
Sodium is one of the five major elements in the human body. It is essential to maintain normal physiological functions involving body fluid volume, blood pressure, osmotic equilibrium, neuronal excitability, and nutrient transport, among others. The mechanisms of Na+ flux across biological membranes include Na+/H+ exchange (NHE), a process crucial for Na+ absorption in the gastrointestinal tract and kidney. Defective NHE may contribute to the pathogenesis of acute and chronic diseases, such as hypotension or hypertension, diarrhea, or intestinal inflammation. In this review, we discuss the following:
Distribution and function of the SLC9 gene family members in the gastrointestinal tract.
Emphasize the roles of Na+/H+ exchange in intracellular pH regulation, gastric acid secretion, salivary and pancreatic secretion, hepatic physiology, epithelial sodium absorption, and mucosal protection in the gastrointestinal tract.
Transcriptional and posttranslational mechanisms of regulation of Na+/H+ exchangers during development, by physiological stimuli, and in pathophysiological conditions.
Acknowledgements
NIH grants R01DK041274 and R01DK113754 are acknowledged for financial support. We thank Mrs. Trudy Meckler for the editorial help during preparation of this manuscript.
References
- 1.Abedin MZ, Giurgiu DI, Abedin ZR, Peck EA, Su X, Smith PR. Characterization of Na+/H+ exchanger isoform (NHE1, NH32 and NHE3) expression in prairie dog gallbladder. J Membr Biol 182: 123–134, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Ahn W, Kim KH, Lee JA, Kim JY, Choi JY, Moe OW, Milgram SL, Muallem S, Lee MG. Regulatory interaction between the cystic fibrosis transmembrane conductance regulator and HCO3− salvage mechanisms in model systems and the mouse pancreatic duct. J Biol Chem 276: 17236–17243, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Alexander RT, Grinstein S. Tethering, recycling and activation of the epithelial sodium-proton exchanger, NHE3. J Exp Biol 212: 1630–1637, 2009. [DOI] [PubMed] [Google Scholar]
- 4.Amin MR, Dudeja PK, Ramaswamy K, Malakooti J. Involvement of Sp1 and Sp3 in differential regulation of human NHE3 promoter activity by sodium butyrate and IFN-gamma/TNF-alpha. Am J Physiol Gastrointest Liver Physiol 293: G374–G382, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Amin MR, Ghannad L, Othman A, Gill RK, Dudeja PK, Ramaswamy K, Malakooti J. Transcriptional regulation of the human Na+/H+ exchanger NHE3 by serotonin in intestinal epithelial cells. Biochem Biophys Res Commun 382: 620–625, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amin MR, Orenuga T, Tyagi S, Dudeja PK, Ramaswamy K, Malakooti J. Tumor necrosis factor-alpha represses the expression of NHE2 through NF-kappaB activation in intestinal epithelial cell model, C2BBe1. Inflamm Bowel Dis 17(3): 720–731, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amith SR, Fong S, Baksh S, Fliegel L. Na (+)/H (+)exchange in the tumour microenvironment: does NHE1 drive breast cancer carcinogen-esis? Int J Dev Biol 59: 367–377, 2015. [DOI] [PubMed] [Google Scholar]
- 8.Anderie I, Blum R, Haase W, Grinstein S, Thevenod F. Expression of NHE1 and NHE4 in rat pancreatic zymogen granule membranes. Biochem Biophys Res Commun 246: 330–336, 1998. [DOI] [PubMed] [Google Scholar]
- 9.Anderie I, Thevenod F. Evidence for involvement of a zymogen granule Na+/H+ exchanger in enzyme secretion from rat pancreatic acinar cells. J Membr Biol 152: 195–205, 1996. [DOI] [PubMed] [Google Scholar]
- 10.Arias IM, Forgac M. The sinusoidal domain of the plasma membrane of rat hepatocytes contains an amiloride-sensitive Na+/H+ antiport. J Biol Chem 259: 5406–5408, 1984. [PubMed] [Google Scholar]
- 11.Arshad N, Visweswariah SS. The multiple and enigmatic roles of guanylyl cyclase C in intestinal homeostasis. FEBS Lett 586: 2835–2840, 2012. [DOI] [PubMed] [Google Scholar]
- 12.Bachmann O, Riederer B, Rossmann H, Groos S, Schultheis PJ, Shull GE, Gregor M, Manns MP, Seidler U. The Na+/H+ exchanger isoform 2 is the predominant NHE isoform in murine colonic crypts and its lack causes NHE3 upregulation. Am J Physiol Gastrointest Liver Physiol 287: G125–G133, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Bai L, Collins JF, Muller YL, Xu H, Kiela PR, Ghishan FK. Characterization of cis-elements required for osmotic response of rat Na(+)/H(+) exchanger-2 (NHE-2) gene. Am J Physiol 277: R1112–R1119, 1999. [DOI] [PubMed] [Google Scholar]
- 14.Bai L, Collins JF, Xu H, Ghishan FK. Transcriptional regulation of rat Na(+)/H(+) exchanger isoform-2 (NHE-2) gene by Sp1 transcription factor. Am J Physiol Cell Physiol 280: C1168–C1175, 2001. [DOI] [PubMed] [Google Scholar]
- 15.Barmeyer C, Harren M, Schmitz H, Heinzel-Pleines U, Mankertz J, Seidler U, Horak I, Wiedenmann B, Fromm M, Schulzke JD. Mechanisms of diarrhea in the interleukin-2-deficient mouse model of colonic inflammation. Am J Physiol Gastrointest Liver Physiol 286: G244–G252, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Battaglino RA, Pham L, Morse LR, Vokes M, Sharma A, Odgren PR, Yang M, Sasaki H, Stashenko P. NHA-oc/NHA2: A mitochondrial cation-proton antiporter selectively expressed in osteoclasts. Bone 42: 180–192, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bazzini C, Botta G, Meyer G, Baroni MD, Paulmichl M. The presence of NHE1 and NHE3 Na+-H+ exchangers and an apical cAMP-independent Cl− channel indicate that both absorptive and secretory functions are present in calf gall bladder epithelium. Exp Physiol 86: 571–583, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Bell SM, Schreiner CM, Schultheis PJ, Miller ML, Evans RL, Vorhees CV, Shull GE, Scott WJ. Targeted disruption of the murine Nhe1 locus induces ataxia, growth retardation, and seizures. Am J Physiol 276: C788–C795, 1999. [DOI] [PubMed] [Google Scholar]
- 19.Benedetti A, Di Sario A, Casini A, Ridolfi F, Bendia E, Pigini P, Tonnini C, D’Ambrosio L, Feliciangeli G, Macarri G, Svegliati-Baroni G. Inhibition of the NA(+)/H(+) exchanger reduces rat hepatic stellate cell activity and liver fibrosis: An in vitro and in vivo study. Gastroenterology 120: 545–556, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Besson P, Fernandez-Rachubinski F, Yang W, Fliegel L. Regulation of Na+/H+ exchanger gene expression: Mitogenic stimulation increases NHE1 promoter activity. Am J Physiol 274: C831–C839, 1998. [DOI] [PubMed] [Google Scholar]
- 21.Biemesderfer D, DeGray B, Aronson PS. Membrane topology of NHE3. Epitopes within the carboxyl-terminal hydrophilic domain are exoplasmic. J Biol Chem 273: 12391–12396, 1998. [DOI] [PubMed] [Google Scholar]
- 22.Binder HJ. Role of colonic short-chain fatty acid transport in diarrhea. Annu Rev Physiol 72: 297–313, 2010. [DOI] [PubMed] [Google Scholar]
- 23.Binder HJ, Mehta P. Short-chain fatty acids stimulate active sodium and chloride absorption in vitro in the rat distal colon. Gastroenterology 96: 989–996, 1989. [DOI] [PubMed] [Google Scholar]
- 24.Binder HJ, Singh SK, Geibel JP, Rajendran VM. Novel transport properties of colonic crypt cells: Fluid absorption and Cl-dependent Na-H exchange. Comp Biochem Physiol A Physiol 118: 265–269, 1997. [DOI] [PubMed] [Google Scholar]
- 25.Bizal GL, Howard RL, Bookstein C, Rao MC, Chang EB, Soleimani M. Glycosylation of the Na+/H+ exchanger isoform NHE-3 is species specific. J Lab Clin Med 128: 304–312, 1996. [DOI] [PubMed] [Google Scholar]
- 26.Blaurock MC, Reboucas NA, Kusnezov JL, Igarashi P. Phylogenetically conserved sequences in the promoter of the rabbit sodium-hydrogen exchanger isoform 1 gene (NHE1/SLC9A1). Biochim Biophys Acta 1262: 159–163, 1995. [DOI] [PubMed] [Google Scholar]
- 27.Boivin GP, Schultheis PJ, Shull GE, Stemmermann GN. Variant form of diffuse corporal gastritis in NHE2 knockout mice. Comp Med 50: 511–515, 2000. [PubMed] [Google Scholar]
- 28.Bookstein C, DePaoli AM, Xie Y, Niu P, Musch MW, Rao MC, Chang EB. Na+/H+ exchangers, NHE-1 and NHE-3, of rat intestine. Expression and localization. J Clin Invest 93: 106–113, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bookstein C, Musch MW, DePaoli A, Xie Y, Rabenau K, Villereal M, Rao MC, Chang EB. Characterization of the rat Na+/H+ exchanger isoform NHE4 and localization in rat hippocampus. Am J Physiol 271: C1629–C1638, 1996. [DOI] [PubMed] [Google Scholar]
- 30.Bookstein C, Musch MW, DePaoli A, Xie Y, Villereal M, Rao MC, Chang EB. A unique sodium-hydrogen exchange isoform (NHE-4) of the inner medulla of the rat kidney is induced by hyperosmolarity. J Biol Chem 269: 29704–29709, 1994. [PubMed] [Google Scholar]
- 31.Bookstein C, Xie Y, Rabenau K, Musch MW, McSwine RL, Rao MC, Chang EB. Tissue distribution of Na+/H+ exchanger isoforms NHE2 and NHE4 in rat intestine and kidney. Am J Physiol 273: C1496–C1505, 1997. [DOI] [PubMed] [Google Scholar]
- 32.Booth IW, Stange G, Murer H, Fenton TR, Milla PJ. Defective jejunal brush-border Na+/H+ exchange: A cause of congenital secretory diarrhoea. Lancet 1: 1066–1069, 1985. [DOI] [PubMed] [Google Scholar]
- 33.Brant SR, Bernstein M, Wasmuth JJ, Taylor EW, McPherson JD, Li X, Walker S, Pouyssegur J, Donowitz M, Tse CM. Physical and genetic mapping of a human apical epithelial Na+/H+ exchanger (NHE3) iso-form to chromosome 5p15.3. Genomics 15: 668–672, 1993. [DOI] [PubMed] [Google Scholar]
- 34.Brant SR, Yun CH, Donowitz M, Tse CM. Cloning, tissue distribution, and functional analysis of the human Na+/N+ exchanger isoform, NHE3. Am J Physiol 269: C198–C206, 1995. [DOI] [PubMed] [Google Scholar]
- 35.Brett CL, Donowitz M, Rao R. Evolutionary origins of eukaryotic sodium/proton exchangers. Am J Physiol Cell Physiol 288: C223–C239, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Brett CL, Wei Y, Donowitz M, Rao R. Human Na(+)/H(+) exchanger isoform 6 is found in recycling endosomes of cells, not in mitochondria. Am J Physiol Cell Physiol 282(5): C1031–C1041, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Brierley GP, Settlemire CT, Knight VA. Ion transport by heart mitochondria. XI. The spontaneous and induced permeability of heart mitochondria to cations. Arch Biochem Biophys 126: 276–288, 1968. [DOI] [PubMed] [Google Scholar]
- 38.Brown DA, Melvin JE, Yule DI. Critical role for NHE1 in intracellular pH regulation in pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol 285: G804–G812, 2003. [DOI] [PubMed] [Google Scholar]
- 39.Canani RB, Terrin G, Cirillo P, Castaldo G, Salvatore F, Cardillo G, Coruzzo A, Troncone R. Butyrate as an effective treatment of congenital chloride diarrhea. Gastroenterology 127: 630–634, 2004. [DOI] [PubMed] [Google Scholar]
- 40.Cano A Characterization of the rat NHE3 promoter. Am J Physiol 271: F629–F636, 1996. [DOI] [PubMed] [Google Scholar]
- 41.Catalan MA, Nakamoto T, Gonzalez-Begne M, Camden JM, Wall SM, Clarke LL, Melvin JE. Cftr and ENaC ion channels mediate NaCl absorption in the mouse submandibular gland. J Physiol 588: 713–724, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cetin S, Dunklebarger J, Li J, Boyle P, Ergun O, Qureshi F, Ford H, Upperman J, Watkins S, Hackam DJ. Endotoxin differentially modulates the basolateral and apical sodium/proton exchangers (NHE) in enterocytes. Surgery 136: 375–383, 2004. [DOI] [PubMed] [Google Scholar]
- 43.Chailler P, Menard D. Ontogeny of EGF receptors in the human gut. Front Biosci 4: D87–D101, 1999. [DOI] [PubMed] [Google Scholar]
- 44.Charney AN, Dagher PC. Acid-base effects on colonic electrolyte transport revisited. Gastroenterology 111: 1358–1368, 1996. [DOI] [PubMed] [Google Scholar]
- 45.Chen JS, Reddy V, Chen JH, Shlykov MA, Zheng WH, Cho J, Yen MR, Saier MH Jr Phylogenetic characterization of transport protein superfamilies: Superiority of SuperfamilyTree programs over those based on multiple alignments. J Mol Microbiol Biotechnol 21: 83–96, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen P, Hao X, Li W, Zhao X, Huang Y. Mutations in the TMCO3 gene are associated with cornea guttata and anterior polar cataract. Sci Rep 6: 31021, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen SR, Chen M, Deng SL, Hao XX, Wang XX, Liu YX. Sodium-hydrogen exchanger NHA1 and NHA2 control sperm motility and male fertility. Cell Death Dis 7: e2152, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen T, Kocinsky HS, Cha B, Murtazina R, Yang J, Tse CM, Singh V, Cole R, Aronson PS, de Jonge H, Sarker R, Donowitz M. Cyclic GMP kinase II (cGKII) inhibits NHE3 by altering its trafficking and phosphorylating NHE3 at three required sites: Identification of a multifunctional phosphorylation site. J Biol Chem 290: 1952–1965, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chintapalli VR, Kato A, Henderson L, Hirata T, Woods DJ, Overend G, Davies SA, Romero MF, Dow JA. Transport proteins NHA1 and NHA2 are essential for survival, but have distinct transport modalities. Proc Natl Acad Sci USA 112: 11720–11725, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cho JH, Musch MW, DePaoli AM, Bookstein CM, Xie Y, Burant CF, Rao MC, Chang EB. Glucocorticoids regulate Na+/H+ exchange expression and activity in region- and tissue-specific manner. Am J Physiol 267: C796–C803, 1994. [DOI] [PubMed] [Google Scholar]
- 51.Choshniak I, Mualem R. SCFA and electrolyte absorption in the colon of three rodent species. Comp Biochem Physiol A Physiol 118: 381–384, 1997. [DOI] [PubMed] [Google Scholar]
- 52.Chow A, Zhou W, Jacobson R. Regulation of AE2 Cl−/HCO3− exchanger during intestinal development. Am J Physiol 271: G330–G337, 1996. [DOI] [PubMed] [Google Scholar]
- 53.Chu J, Chu S, Montrose MH. Apical Na+/H+ exchange near the base of mouse colonic crypts. Am J Physiol Cell Physiol 283: C358–C372, 2002. [DOI] [PubMed] [Google Scholar]
- 54.Cinar A, Chen M, Riederer B, Bachmann O, Wiemann M, Manns M, Kocher O, Seidler U. NHE3 inhibition by cAMP and Ca2+ is abolished in PDZ-domain protein PDZK1-deficient murine enterocytes. J Physiol 581: 1235–1246, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Clayburgh DR, Musch MW, Leitges M, Fu YX, Turner JR. Coordinated epithelial NHE3 inhibition and barrier dysfunction are required for TNF-mediated diarrhea in vivo. J Clin Invest 116: 2682–2694, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Collins JF, Honda T, Knobel S, Bulus NM, Conary J, DuBois R, Ghishan FK. Molecular cloning, sequencing, tissue distribution, and functional expression of a Na+/H+ exchanger (NHE-2). Proc Natl Acad Sci U S A 90: 3938–3942, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Collins JF, Kiela PR, Xu H, Zeng J, Ghishan FK. Increased NHE2 expression in rat intestinal epithelium during ontogeny is transcriptionally mediated. Am J Physiol 275: C1143–C1150, 1998. [DOI] [PubMed] [Google Scholar]
- 58.Collins JF, Xu H, Kiela PR, Zeng J, Ghishan FK. Functional and molecular characterization of NHE3 expression during ontogeny in rat jejunal epithelium. Am J Physiol 273: C1937–C1946, 1997. [DOI] [PubMed] [Google Scholar]
- 59.Collins JF, Xu H, Kiela PR, Zeng J, Ghishan FK. Ontogeny of basolateral membrane sodium-hydrogen exchange (NHE) activity and mRNA expression of NHE-1 and NHE-4 in rat kidney and jejunum. Biochim Biophys Acta 1369: 247–258, 1998. [DOI] [PubMed] [Google Scholar]
- 60.Conter RL, Roslyn JJ, Porter-Fink V, DenBesten L. Gallbladder absorption increases during early cholesterol gallstone formation. Am J Surg 151: 184–191, 1986. [DOI] [PubMed] [Google Scholar]
- 61.Cook DI, Dinudom A, Komwatana P, Kumar S, Young JA. Patch-clamp studies on epithelial sodium channels in salivary duct cells. Cell Biochem Biophys 36: 105–113, 2002. [DOI] [PubMed] [Google Scholar]
- 62.Counillon L, Pouyssegur J, Reithmeier RA. The Na+/H+ exchanger NHE-1 possesses N- and O-linked glycosylation restricted to the first N-terminal extracellular domain. Biochemistry 33: 10463–10469, 1994. [DOI] [PubMed] [Google Scholar]
- 63.Cremaschi D, Ferguson DR, Henin S, James PS, Meyer G, Smith MW. Post-natal development of amiloride sensitive sodium transport in pig distal colon. J Physiol 292: 481–494, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cuff MA, Shirazi-Beechey SP. The importance of butyrate transport to the regulation of gene expression in the colonic epithelium. Biochem Soc Trans 32: 1100–1102, 2004. [DOI] [PubMed] [Google Scholar]
- 65.Dagenais A, Kothary R, Berthiaume Y. The alpha subunit of the epithelial sodium channel in the mouse: Developmental regulation of its expression. Pediatr Res 42: 327–334, 1997. [DOI] [PubMed] [Google Scholar]
- 66.Dagher PC, Egnor RW, Taglietta-Kohlbrecher A, Charney AN. Short-chain fatty acids inhibit cAMP-mediated chloride secretion in rat colon. Am J Physiol 271: C1853–C1860, 1996. [DOI] [PubMed] [Google Scholar]
- 67.Daher M, Acra S, Ghishan FK. Ontogeny of the Na(+)-H+ exchanger in rat ileal basolateral membrane vesicles. J Dev Physiol 15: 175–181, 1991. [PubMed] [Google Scholar]
- 68.de Silva MG, Elliott K, Dahl HH, Fitzpatrick E, Wilcox S, Delatycki M, Williamson R, Efron D, Lynch M, Forrest S. Disruption of a novel member of a sodium/hydrogen exchanger family and DOCK3 is associated with an attention deficit hyperactivity disorder-like phenotype. J Med Genet 40: 733–740, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.De Vito P The sodium/hydrogen exchanger: A possible mediator of immunity. Cell Immunol 240: 69–85, 2006. [DOI] [PubMed] [Google Scholar]
- 70.Debongnie JC, Phillips SF. Capacity of the human colon to absorb fluid. Gastroenterology 74: 698–703, 1978. [PubMed] [Google Scholar]
- 71.Deisl C, Albano G, Fuster DG. Role of Na/H exchange in insulin secretion by islet cells. Curr Opin Nephrol Hypertens 23: 406–410, 2014. [DOI] [PubMed] [Google Scholar]
- 72.Deisl C, Simonin A, Anderegg M, Albano G, Kovacs G, Ackermann D, Moch H, Dolci W, Thorens B, M AH, Fuster DG. Sodium/hydrogen exchanger NHA2 is critical for insulin secretion in beta-cells. Proc Natl Acad Sci USA 110: 10004–10009, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Di Sario A, Baroni GS, Bendia E, D’Ambrosio L, Ridolfi F, Marileo JR, Jezequel AM, Benedetti A. Characterization of ion transport mechanisms regulating intracellular pH in hepatic stellate cells. Am J Physiol 273: G39–G48, 1997. [DOI] [PubMed] [Google Scholar]
- 74.Di Sario A, Bendia E, Taffetani S, Marzioni M, Candelaresi C, Pigini P, Schindler U, Kleemann HW, Trozzi L, Macarri G, Benedetti A. Selective Na+/H+ exchange inhibition by cariporide reduces liver fibrosis in the rat. Hepatology 37: 256–266, 2003. [DOI] [PubMed] [Google Scholar]
- 75.Diener M, Scharrer E. Effects of short-chain fatty acids on cell volume regulation and chloride transport in the rat distal colon. Comp Biochem Physiol A Physiol 118: 375–379, 1997. [DOI] [PubMed] [Google Scholar]
- 76.Donowitz M, De La Horra C, Calonge ML, Wood IS, Dyer J, Gribble SM, De Medina FS, Tse CM, Shirazi-Beechey SP, Ilundain AA. In birds, NHE2 is major brush-border Na+/H+ exchanger in colon and is increased by a low-NaCl diet. Am J Physiol 274: R1659–R1669, 1998. [DOI] [PubMed] [Google Scholar]
- 77.Donowitz M, Ming Tse C, Fuster D. SLC9/NHE gene family, a plasma membrane and organellar family of Na(+)/H(+) exchangers. Mol Aspects Med 34: 236–251, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Donowitz M, Mohan S, Zhu CX, Chen TE, Lin R, Cha B, Zachos NC, Murtazina R, Sarker R, Li X. NHE3 regulatory complexes. J Exp Biol 212: 1638–1646, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dudeja PK, Rao DD, Syed I, Joshi V, Dahdal RY, Gardner C, Risk MC, Schmidt L, Bavishi D, Kim KE, Harig JM, Goldstein JL, Layden TJ, Ramaswamy K. Intestinal distribution of human Na+/H+ exchanger isoforms NHE-1, NHE-2, and NHE-3 mRNA. Am J Physiol 271: G483–G493, 1996. [DOI] [PubMed] [Google Scholar]
- 80.Dyck JR, Silva NL, Fliegel L. Activation of the Na+/H+ exchanger gene by the transcription factor AP-2. J Biol Chem 270: 1375–1381, 1995. [DOI] [PubMed] [Google Scholar]
- 81.Evans RL, Bell SM, Schultheis PJ, Shull GE, Melvin JE. Targeted disruption of the Nhe1 gene prevents muscarinic agonist-induced up-regulation of Na(+)/H(+) exchange in mouse parotid acinar cells. J Biol Chem 274: 29025–29030, 1999. [DOI] [PubMed] [Google Scholar]
- 82.Facanha AL, dos Reis MC, Montero-Lomeli M. Structural study of the porcine Na+/H+ exchanger NHE1 gene and its 5’-flanking region. Mol Cell Biochem 210: 91–99, 2000. [DOI] [PubMed] [Google Scholar]
- 83.Falcone RA Jr., Shin CE, Stern LE, Wang Z, Erwin CR, Soleimani M, Warner BW. Differential expression of ileal Na(+)/H(+) exchanger isoforms after enterectomy. J Surg Res 86: 192–197, 1999. [DOI] [PubMed] [Google Scholar]
- 84.Farkas K, Yeruva S, Rakonczay Z Jr. , Ludolph L, Molnar, Nagy F, Szepes Z, Schnur, Wittmann T, Hubricht J, Riederer B, Venglovecz V, Lazar G, Kiraly M, Zsembery A, Varga G, Seidler U, Hegyi P. New therapeutic targets in ulcerative colitis: The importance of ion transporters in the human colon. Inflamm Bowel Dis 17(4): 884–898, 2011. [DOI] [PubMed] [Google Scholar]
- 85.Fernandez-Rachubinski F, Fliegel L. COUP-TFI and COUP-TFII regulate expression of the NHE through a nuclear hormone responsive element with enhancer activity. Eur J Biochem 268: 620–634, 2001. [DOI] [PubMed] [Google Scholar]
- 86.Finkel Y, Eklof AC, Aperia A. Mechanisms for colonic sodium transport during ontogeny: Loss of an amiloride-sensitive sodium pathway. Pediatr Res 24: 46–49, 1988. [DOI] [PubMed] [Google Scholar]
- 87.Fiskerstrand T, Arshad N, Haukanes BI, Tronstad RR, Pham KD, Johansson S, Havik B, Tonder SL, Levy SE, Brackman D, Boman H, Biswas KH, Apold J, Hovdenak N, Visweswariah SS, Knappskog PM. Familial diarrhea syndrome caused by an activating GUCY2C mutation. N Engl J Med 366: 1586–1595, 2012. [DOI] [PubMed] [Google Scholar]
- 88.Fitzgerald RC, Omary MB, Triadafilopoulos G. Altered sodium-hydrogen exchange activity is a mechanism for acid-induced hyperproliferation in Barrett’s esophagus. Am J Physiol 275: G47–G55, 1998. [DOI] [PubMed] [Google Scholar]
- 89.Flemstrom G Gastric and duodenal mucosal bicarbonate secretion In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. New York: Raven Press, 1994, [Google Scholar]
- 90.Fliegel L Molecular biology of the myocardial Na+/H+ exchanger. J Mol Cell Cardiol 44: 228–237, 2008. [DOI] [PubMed] [Google Scholar]
- 91.Fujiwara Y, Higuchi K, Takashima T, Hamaguchi M, Hayakawa T, Tominaga K, Watanabe T, Oshitani N, Shimada Y, Arakawa T. Roles of epidermal growth factor and Na+/H+ exchanger-1 in esophageal epithelial defense against acid-induced injury. Am J Physiol Gastroin-test Liver Physiol 290: G665–G673, 2006. [DOI] [PubMed] [Google Scholar]
- 92.Fukura N, Ohgaki R, Matsushita M, Nakamura N, Mitsui K, Kanazawa H. A membrane-proximal region in the C-terminal tail of NHE7 is required for its distribution in the trans-Golgi network, distinct from NHE6 localization at endosomes. J Membr Biol 234: 149–158, 2010. [DOI] [PubMed] [Google Scholar]
- 93.Fuster DG, Alexander RT. Traditional and emerging roles for the SLC9 Na+/H+ exchangers. Pflugers Arch 466: 61–76, 2014. [DOI] [PubMed] [Google Scholar]
- 94.Fuster DG, Zhang J, Shi M, Bobulescu IA, Andersson S, Moe OW. Characterization of the sodium/hydrogen exchanger NHA2. J Am Soc Nephrol 19: 1547–1556, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Garlid KD. Mitochondrial cation transport: a progress report. J Bioenerg Biomembr 26(5): 537–542, 1994. [DOI] [PubMed] [Google Scholar]
- 96.Gawenis LR, Greeb JM, Prasad V, Grisham C, Sanford LP, Doetschman T, Andringa A, Miller ML, Shull GE. Impaired gastric acid secretion in mice with a targeted disruption of the NHE4 Na+/H+ exchanger. J Biol Chem 280(13): 12781–12789, 2005. [DOI] [PubMed] [Google Scholar]
- 97.Gawenis LR, Stien X, Shull GE, Schultheis PJ, Woo AL, Walker NM, Clarke LL. Intestinal NaCl transport in NHE2 and NHE3 knockout mice. Am J Physiol Gastrointest Liver Physiol 282: G776–G784, 2002. [DOI] [PubMed] [Google Scholar]
- 98.Geibel JP. Secretion and absorption by colonic crypts. Annu Rev Physiol 67: 471–490, 2005. [DOI] [PubMed] [Google Scholar]
- 99.Ghishan FK, Kiela PR. Epithelial transport in inflammatory bowel diseases. Inflamm Bowel Dis 20: 1099–1109, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ghishan FK, Kikuchi K, Riedel B. Epidermal growth factor up-regulates intestinal Na+/H+ exchange activity. Proc Soc Exp Biol Med 201: 289–295, 1992. [DOI] [PubMed] [Google Scholar]
- 101.Ghishan FK, Knobel SM, Summar M. Molecular cloning, sequencing, chromosomal localization, and tissue distribution of the human Na+/H+ exchanger (SLC9A2). Genomics 30: 25–30, 1995. [DOI] [PubMed] [Google Scholar]
- 102.Gill R, Nazir TM, Wali R, Sitrin M, Brasitus TA, Ramaswamy K, Dudeja PK. Regulation of rat ileal NHE3 by 1,25(OH)2-vitamin D3. Dig Dis Sci 47: 1169–1174, 2002. [DOI] [PubMed] [Google Scholar]
- 103.Gill RK, Saksena S, Tyagi S, Alrefai WA, Malakooti J, Sarwar Z, Turner JR, Ramaswamy K, Dudeja PK. Serotonin inhibits Na+/H+ exchange activity via 5-HT4 receptors and activation of PKC alpha in human intestinal epithelial cells. Gastroenterology 128: 962–974, 2005. [DOI] [PubMed] [Google Scholar]
- 104.Giurgiu DI, Saunders-Kirkwood KD, Roslyn JJ, Abedin MZ. Sequential changes in biliary lipids and gallbladder ion transport during gallstone formation. Ann Surg 225: 382–390, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Goldman A, Shahidullah M, Goldman D, Khailova L, Watts G, Delamere N, Dvorak K. A novel mechanism of acid and bile acid-induced DNA damage involving Na+/H+ exchanger: Implication for Barrett’s oesophagus. Gut 59: 1606–1616, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Goodrich AL, Suchy FJ. Na(+)-H+ exchange in basolateral plasma membrane vesicles from neonatal rat liver. Am J Physiol 259: G334–G339, 1990. [DOI] [PubMed] [Google Scholar]
- 107.Goyal S, Vanden Heuvel G, Aronson PS. Renal expression of novel Na+/H+ exchanger isoform NHE8. Am J Physiol Renal Physiol 284: F467–F473, 2003. [DOI] [PubMed] [Google Scholar]
- 108.Gray MR, Donnelly RJ, Kingsnorth AN. Role of salivary epidermal growth factor in the pathogenesis of Barrett’s columnar lined oesophagus. Br J Surg 78: 1461–1466, 1991. [DOI] [PubMed] [Google Scholar]
- 109.Gurney MA, Laubitz D, Ghishan FK, Kiela PR. Pathophysiology of Intestinal Na+/H+ exchange. Cell Mol Gastroenterol Hepatol 3: 27–40, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Haimovici J, Beck JS, Molla-Hosseini C, Vallerand D, Haddad P. Different modulation of hepatocellular Na+/H+ exchange activity by insulin and EGF. Am J Physiol 267: G364–G370, 1994. [DOI] [PubMed] [Google Scholar]
- 111.Harguindey S, Orive G, Luis Pedraz J, Paradiso A, Reshkin SJ. The role of pH dynamics and the Na+/H+ antiporter in the etiopathogenesis and treatment of cancer. Two faces of the same coin—One single nature. Biochim Biophys Acta 1756: 1–24, 2005. [DOI] [PubMed] [Google Scholar]
- 112.Harris RC, Brenner BM, Seifter JL. Sodium-hydrogen exchange and glucose transport in renal microvillus membrane vesicles from rats with diabetes mellitus. J Clin Invest 77: 724–733, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Haussinger D Liver fibrosis and NA(+)/H(+) exchange. Gastroenterology 120: 572–575, 2001. [DOI] [PubMed] [Google Scholar]
- 114.He P, Lee SJ, Lin S, Seidler U, Lang F, Fejes-Toth G, Naray-Fejes-Toth A, Yun CC. Serum- and glucocorticoid-induced kinase 3 in recycling endosomes mediates acute activation of Na+/H+ exchanger NHE3 by glucocorticoids. Mol Biol Cell 22: 3812–3825, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.He P, Yun CC. Mechanisms of the regulation of the intestinal Na+/H+ exchanger NHE3. J Biomed Biotechnol 2010: 238080, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.He X, Tse CM, Donowitz M, Alper SL, Gabriel SE, Baum BJ. Polarized distribution of key membrane transport proteins in the rat submandibular gland. Pflugers Arch 433: 260–268, 1997. [DOI] [PubMed] [Google Scholar]
- 117.Hecht G, Hodges K, Gill RK, Kear F, Tyagi S, Malakooti J, Ramaswamy K, Dudeja PK. Differential regulation of Na+/H+ exchange isoform activities by enteropathogenic E. coli in human intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol 287(2): G370–G378, 2004. [DOI] [PubMed] [Google Scholar]
- 118.Heinz-Erian P, Muller T, Krabichler B, Schranz M, Becker C, Ruschendorf F, Nurnberg P, Rossier B, Vujic M, Booth IW, Holmberg C, Wijmenga C, Grigelioniene G, Kneepkens CM, Rosipal S, Mistrik M, Kappler M, Michaud L, Doczy LC, Siu VM, Krantz M, Zoller H, Utermann G, Janecke AR. Mutations in SPINT2 cause a syndromic form of congenital sodium diarrhea. Am J Hum Genet 84: 188–196, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Henning SJ. Plasma concentrations of total and free corticosterone during development in the rat. Am J Physiol 235: E451–E456, 1978. [DOI] [PubMed] [Google Scholar]
- 120.Hodges K, Gill R, Ramaswamy K, Dudeja PK, Hecht G. Rapid activation of Na+/H+ exchange by EPEC is PKC mediated. Am J Physiol Gastrointest Liver Physiol 291: G959–G968, 2006. [DOI] [PubMed] [Google Scholar]
- 121.Hofstetter W, Siegrist M, Simonin A, Bonny O, Fuster DG. Sodium/hydrogen exchanger NHA2 in osteoclasts: Subcellular localization and role in vitro and in vivo. Bone 47: 331–340, 2010. [DOI] [PubMed] [Google Scholar]
- 122.Hoglund P, Haila S, Socha J, Tomaszewski L, Saarialho-Kere U, Karjalainen-Lindsberg ML, Airola K, Holmberg C, de la Chapelle A, Kere J. Mutations of the down-regulated in adenoma (DRA) gene cause congenital chloride diarrhoea. Nat Genet 14: 316–319, 1996. [DOI] [PubMed] [Google Scholar]
- 123.Holmberg C, Perheentupa J. Congenital Na+ diarrhea: A new type of secretory diarrhea. J Pediatr 106: 56–61, 1985. [DOI] [PubMed] [Google Scholar]
- 124.Honda T, Knobel SM, Bulus NM, Ghishan FK. Kinetic characterization of a stably expressed novel Na+/H+ exchanger (NHE-2). Biochim Biophys Acta 1150: 199–202, 1993. [DOI] [PubMed] [Google Scholar]
- 125.Hoogerwerf WA, Tsao SC, Devuyst O, Levine SA, Yun CH, Yip JW, Cohen ME, Wilson PD, Lazenby AJ, Tse CM, Donowitz M. NHE2 and NHE3 are human and rabbit intestinal brush-border proteins. Am J Physiol 270: G29–G41, 1996. [DOI] [PubMed] [Google Scholar]
- 126.Hua P, Xu H, Uno JK, Lipko MA, Dong J, Kiela PR, Ghishan FK. Sp1 and Sp3 mediate NHE2 gene transcription in the intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol 293: G146–G153, 2007. [DOI] [PubMed] [Google Scholar]
- 127.Ikuma M, Kashgarian M, Binder HJ, Rajendran VM. Differential regulation of NHE isoforms by sodium depletion in proximal and distal segments of rat colon. Am J Physiol 276: G539–G549, 1999. [DOI] [PubMed] [Google Scholar]
- 128.Ishiguro H, Naruse S, Steward MC, Kitagawa M, Ko SB, Hayakawa T, Case RM. Fluid secretion in interlobular ducts isolated from guinea-pig pancreas. J Physiol 511 (Pt 2): 407–422, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ishiguro H, Steward MC, Lindsay AR, Case RM. Accumulation of intracellular HCO3− by Na(+)-HCO3− cotransport in interlobular ducts from guinea-pig pancreas. J Physiol 495 (Pt 1): 169–178, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ishiguro H, Yamamoto A, Nakakuki M, Yi L, Ishiguro M, Yamaguchi M, Kondo S, Mochimaru Y. Physiology and pathophysiology of bicarbonate secretion by pancreatic duct epithelium. Nagoya J Med Sci 74: 1–18, 2012. [PMC free article] [PubMed] [Google Scholar]
- 131.Janecke AR, Heinz-Erian P, Muller T. Congenital sodium diarrhea: A form of intractable diarrhea, with a link to inflammatory bowel disease. J Pediatr Gastroenterol Nutr 63: 170–176, 2016. [DOI] [PubMed] [Google Scholar]
- 132.Jenkins HR, Fenton TR, McIntosh N, Dillon MJ, Milla PJ. Development of colonic sodium transport in early childhood and its regulation by aldosterone. Gut 31: 194–197, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Joutsi T, Paimela H, Bhowmik A, Kiviluoto T, Kivilaakso E. Role of Na(+)-H(+)-antiport in restitution of isolated guinea pig gastric epithelium after superficial injury. Dig Dis Sci 41: 2187–2194, 1996. [DOI] [PubMed] [Google Scholar]
- 134.Kandasamy RA, Orlowski J. Genomic organization and glucocorticoid transcriptional activation of the rat Na+/H+ exchanger Nhe3 gene. J Biol Chem 271: 10551–10559, 1996. [DOI] [PubMed] [Google Scholar]
- 135.Kandasamy RA, Yu FH, Harris R, Boucher A, Hanrahan JW, Orlowski J. Plasma membrane Na+/H+ exchanger isoforms (NHE-1, −2, and −3) are differentially responsive to second messenger agonists of the protein kinase A and C pathways. J Biol Chem 270: 29209–29216, 1995. [DOI] [PubMed] [Google Scholar]
- 136.Kaneko A, Hayashi N, Tanaka Y, Horimoto M, Ito T, Sasaki Y, Fusamoto H, Kamada T. Activation of Na+/H+ exchanger by hepatocyte growth factor in hepatocytes. Hepatology 22: 629–636, 1995. [PubMed] [Google Scholar]
- 137.Kaneko K, Guth PH, Kaunitz JD. Na+/H+ exchange regulates intracellular pH of rat gastric surface cells in vivo. Pflugers Arch 421: 322–328, 1992. [DOI] [PubMed] [Google Scholar]
- 138.Kapus A, Grinstein S, Wasan S, Kandasamy R, Orlowski J. Functional characterization of three isoforms of the Na+/H+ exchanger stably expressed in Chinese hamster ovary cells. ATP dependence, osmotic sensitivity, and role in cell proliferation. J Biol Chem 269: 23544–23552, 1994. [PubMed] [Google Scholar]
- 139.Karmazyn M, Sawyer M, Fliegel L. The Na(+)/H(+) exchanger: A target for cardiac therapeutic intervention. Curr Drug Targets Cardiovasc Haematol Disord 5: 323–335, 2005. [DOI] [PubMed] [Google Scholar]
- 140.Kelly MP, Quinn PA, Davies JE, Ng LL. Activity and expression of Na(+)-H+ exchanger isoforms 1 and 3 in kidney proximal tubules of hypertensive rats. Circ Res 80: 853–860, 1997. [DOI] [PubMed] [Google Scholar]
- 141.Kemp G, Young H, Fliegel L. Structure and function of the human Na(+)/H(+) exchanger isoform 1. Channels (Austin) 2: 329–336, 2008. [DOI] [PubMed] [Google Scholar]
- 142.Khan I Topology of the C-terminus of sodium hydrogen exchanger isoform-1: Presence of an extracellular epitope. Arch Biochem Biophys 391: 25–29, 2001. [DOI] [PubMed] [Google Scholar]
- 143.Khan I, al-Awadi FM, Abul H. Colitis-induced changes in the expression of the Na+/H+ exchanger isoform NHE-1. J Pharmacol Exp Ther 285: 869–875, 1998. [PubMed] [Google Scholar]
- 144.Khan I, Oriowo MA, Anim JT. Amelioration of experimental colitis by Na-H exchanger-1 inhibitor amiloride is associated with reversal of IL-1ss and ERK mitogen-activated protein kinase. Scand J Gastroenterol 40: 578–585, 2005. [DOI] [PubMed] [Google Scholar]
- 145.Khan I, Siddique I, Al-Awadi FM, Mohan K. Role of Na+/H+ exchanger isoform-1 in human inflammatory bowel disease. Can J Gastroenterol 17: 31–36, 2003. [DOI] [PubMed] [Google Scholar]
- 146.Kiela PR, Ghishan FK. Ion transport in the intestine. Curr Opin Gastroenterol 25: 87–91, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kiela PR, Guner YS, Xu H, Collins JF, Ghishan FK. Age- and tissue-specific induction of NHE3 by glucocorticoids in the rat small intestine. Am J Physiol Cell Physiol 278: C629–C637, 2000. [DOI] [PubMed] [Google Scholar]
- 148.Kiela PR, Hines ER, Collins JF, Ghishan FK. Regulation of the rat NHE3 gene promoter by sodium butyrate. Am J Physiol Gastrointest Liver Physiol 281: G947–G956, 2001. [DOI] [PubMed] [Google Scholar]
- 149.Kiela PR, Kuscuoglu N, Midura AJ, Midura-Kiela MT, Larmonier CB, Lipko M, Ghishan FK. Molecular mechanism of rat NHE3 gene promoter regulation by sodium butyrate. Am J Physiol Cell Physiol 293: C64–C74, 2007. [DOI] [PubMed] [Google Scholar]
- 150.Kiela PR, Laubitz D, Larmonier CB, Midura-Kiela MT, Lipko MA, Janikashvili N, Bai A, Thurston R, Ghishan FK. Changes in mucosal homeostasis predispose NHE3 knockout mice to increased susceptibility to DSS-induced epithelial injury. Gastroenterology 137: 965–975, 975e961–910, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kiela PR, LeSueur J, Collins JF, Ghishan FK. Transcriptional regulation of the rat NHE3 gene. Functional interactions between GATA-5 and Sp family transcription factors. J Biol Chem 278: 5659–5668, 2003. [DOI] [PubMed] [Google Scholar]
- 152.Kikuchi K, Kikuchi T, Ghishan FK. Ontogeny of the Na(+)-H+ exchanger in rat ileal brush-border membrane vesicles. J Membr Biol 114: 257–265, 1990. [DOI] [PubMed] [Google Scholar]
- 153.Kirchhoff P, Wagner CA, Gaetzschmann F, Radebold K, Geibel JP. Demonstration of a functional apical sodium hydrogen exchanger in isolated rat gastric glands. Am J Physiol Gastrointest Liver Physiol 285: G1242–G1248, 2003. [DOI] [PubMed] [Google Scholar]
- 154.Koch KS, Leffert HL. Increased sodium ion influx is necessary to initiate rat hepatocyte proliferation. Cell 18: 153–163, 1979. [DOI] [PubMed] [Google Scholar]
- 155.Kondapalli KC, Kallay LM, Muszelik M, Rao R. Unconventional chemiosmotic coupling of NHA2, a mammalian Na+/H+ antiporter, to a plasma membrane H+ gradient. J Biol Chem 287: 36239–36250, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kondapalli KC, Todd Alexander R, Pluznick JL, Rao R. NHA2 is expressed in distal nephron and regulated by dietary sodium. J Physiol Biochem 73(2): 199–205, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Krishnan S, Rajendran VM, Binder HJ. Apical NHE isoforms differentially regulate butyrate-stimulated Na absorption in rat distal colon. Am J Physiol Cell Physiol 285: C1246–C1254, 2003. [DOI] [PubMed] [Google Scholar]
- 158.Krishnan S, Ramakrishna BS, Binder HJ. Stimulation of sodium chlo-ride absorption from secreting rat colon by short-chain fatty acids. Dig Dis Sci 44: 1924–1930, 1999. [DOI] [PubMed] [Google Scholar]
- 159.Kulaksiz H, Bektas H, Cetin Y. Expression and cell-specific and membrane-specific localization of NHE-3 in the human and guinea pig upper gastrointestinal tract. Cell Tissue Res 303: 337–343, 2001. [DOI] [PubMed] [Google Scholar]
- 160.Kunzelmann K, Mall M. Electrolyte transport in the mammalian colon: Mechanisms and implications for disease. Physiol Rev 82: 245–289, 2002. [DOI] [PubMed] [Google Scholar]
- 161.Kurashima K, Yu FH, Cabado AG, Szabo EZ, Grinstein S, Orlowski J. Identification of sites required for down-regulation of Na+/H+ exchanger NHE3 activity by cAMP-dependent protein kinase. Phosphorylation-dependent and -independent mechanisms. J Biol Chem 272: 28672–28679, 1997. [DOI] [PubMed] [Google Scholar]
- 162.Lapointe JY, Gagnon MP, Gagnon DG, Bissonnette P. Controversy regarding the secondary active water transport hypothesis. Biochem Cell Biol 80: 525–533, 2002. [DOI] [PubMed] [Google Scholar]
- 163.Larmonier CB, Laubitz D, Thurston RD, Bucknam AL, Hill FM, Midura-Kiela M, Ramalingam R, Kiela PR, Ghishan FK. NHE3 modulates the severity of colitis in IL-10-deficient mice. Am J Physiol Gastrointest Liver Physiol 300: G998–G1009, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Laubitz D, Harrison CA, Midura-Kiela MT, Ramalingam R, Larmonier CB, Chase JH, Caporaso JG, Besselsen DG, Ghishan FK, Kiela PR. Reduced epithelial Na+/H+ exchange drives gut microbial dysbiosis and promotes inflammatory response in T cell-mediated murine colitis. PLoS One 11: e0152044, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Laubitz D, Larmonier CB, Bai A, Midura-Kiela MT, Lipko MA, Thurston RD, Kiela PR, Ghishan FK. Colonic gene expression profile in NHE3-deficient mice: Evidence for spontaneous distal colitis. Am J Physiol Gastrointest Liver Physiol 295: G63–G77, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Leblond FA, Viau G, Laine J, Lebel D. Reconstitution in vitro of the pH-dependent aggregation of pancreatic zymogens en route to the secretory granule: Implication of GP-2. Biochem J 291 (Pt 1): 289–296, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ledoussal C, Woo AL, Miller ML, Shull GE. Loss of the NHE2 Na(+)/H(+) exchanger has no apparent effect on diarrheal state of NHE3-deficient mice. Am J Physiol Gastrointest Liver Physiol 281: G1385–G1396, 2001. [DOI] [PubMed] [Google Scholar]
- 168.Lee CH, Cragoe EJ Jr., Edwards AM. Control of hepatocyte DNA synthesis by intracellular pH and its role in the action of tumor promoters. J Cell Physiol 195: 61–69, 2003. [DOI] [PubMed] [Google Scholar]
- 169.Lee MG, Ahn W, Choi JY, Luo X, Seo JT, Schultheis PJ, Shull GE, Kim KH, Muallem S. Na(+)-dependent transporters mediate HCO(3)(−) salvage across the luminal membrane of the main pancreatic duct. J Clin Invest 105: 1651–1658, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lee MG, Schultheis PJ, Yan M, Shull GE, Bookstein C, Chang E, Tse M, Donowitz M, Park K, Muallem S. Membrane-limited expression and regulation of Na+-H+ exchanger isoforms by P2 receptors in the rat submandibular gland duct. J Physiol 513 (Pt 2): 341–357, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lenzen H, Lunnemann M, Bleich A, Manns MP, Seidler U, Jorns A. Downregulation of the NHE3-binding PDZ-adaptor protein PDZK1 expression during cytokine-induced inflammation in interleukin-10-deficient mice. PLoS One 7: e40657, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Levine SA, Montrose MH, Tse CM, Donowitz M. Kinetics and regulation of three cloned mammalian Na+/H+ exchangers stably expressed in a fibroblast cell line. J Biol Chem 268: 25527–25535, 1993. [PubMed] [Google Scholar]
- 173.Li X, Alvarez B, Casey JR, Reithmeier RA, Fliegel L. Carbonic anhydrase II binds to and enhances activity of the Na+/H+ exchanger. J Biol Chem 277: 36085–36091, 2002. [DOI] [PubMed] [Google Scholar]
- 174.Li X, Cai L, Xu H, Geng C, Lu J, Tao L, Sun D, Ghishan FK, Wang C. Somatostatin regulates NHE8 protein expression via the ERK1/2 MAPK pathway in DSS-induced colitis mice. Am J Physiol Gastrointest Liver Physiol 311: G954–G963, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lin S, Yeruva S, He P, Singh AK, Zhang H, Chen M, Lamprecht G, de Jonge HR, Tse M, Donowitz M, Hogema BM, Chun J, Seidler U, Yun CC. Lysophosphatidic acid stimulates the intestinal brush border Na(+)/H(+) exchanger 3 and fluid absorption via LPA(5) and NHERF2. Gastroenterology 138: 649–658, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lindstrom P, Sehlin J. Effect of glucose on the intracellular pH of pancreatic islet cells. Biochem J 218: 887–892, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Liu C, Xu H, Zhang B, Johansson ME, Li J, Hansson GC, Ghishan FK. NHE8 plays an important role in mucosal protection via its effect on bacterial adhesion. Am J Physiol Cell Physiol 305: C121–C128, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Liu Y, Basu A, Li X, Fliegel L. Topological analysis of the Na+/H+ exchanger. Biochim Biophys Acta 1848: 2385–2393, 2015. [DOI] [PubMed] [Google Scholar]
- 179.Lucioni A, Womack C, Musch MW, Rocha FL, Bookstein C, Chang EB. Metabolic acidosis in rats increases intestinal NHE2 and NHE3 expression and function. Am J Physiol Gastrointest Liver Physiol 283: G51–G56, 2002. [DOI] [PubMed] [Google Scholar]
- 180.Magro F, Fraga S, Soares-da-Silva P. Signaling of short- and long-term regulation of intestinal epithelial type 1 Na+/H+ exchanger by interferon-gamma. Br J Pharmacol 145: 93–103, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Maher MM, Gontarek JD, Bess RS, Donowitz M, Yeo CJ. The Na+/H+ exchange isoform NHE3 regulates basal canine ileal Na+ absorption in vivo. Gastroenterology 112: 174–183, 1997. [DOI] [PubMed] [Google Scholar]
- 182.Maher MM, Gontarek JD, Jimenez RE, Donowitz M, Yeo CJ. Role of brush border Na+/H+ exchange in canine ileal absorption. Dig Dis Sci 41: 651–659, 1996. [DOI] [PubMed] [Google Scholar]
- 183.Malakooti J, Dahdal RY, Dudeja PK, Layden TJ, Ramaswamy K. The human Na(+)/H(+) exchanger NHE2 gene: Genomic organization and promoter characterization. Am J Physiol Gastrointest Liver Physiol 280: G763–G773, 2001. [DOI] [PubMed] [Google Scholar]
- 184.Malakooti J, Dahdal RY, Schmidt L, Layden TJ, Dudeja PK, Ramaswamy K. Molecular cloning, tissue distribution, and functional expression of the human Na(+)/H(+) exchanger NHE2. Am J Physiol 277: G383–G390, 1999. [DOI] [PubMed] [Google Scholar]
- 185.Malakooti J, Memark VC, Dudeja PK, Ramaswamy K. Molecular cloning and functional analysis of the human Na(+)/H(+) exchanger NHE3 promoter. Am J Physiol Gastrointest Liver Physiol 282: G491–G500, 2002. [DOI] [PubMed] [Google Scholar]
- 186.Malo ME, Fliegel L. Physiological role and regulation of the Na+/H+ exchanger. Can J Physiol Pharmacol 84: 1081–1095, 2006. [DOI] [PubMed] [Google Scholar]
- 187.Marcinkiewicz M, Grabowska SZ, Czyzewska E. Role of epidermal growth factor (EGF) in oesophageal mucosal integrity. Curr Med Res Opin 14: 145–153, 1998. [DOI] [PubMed] [Google Scholar]
- 188.Marteau C, Silviani V, Ducroc R, Crotte C, Gerolami A. Evidence for apical Na+/H+ exchanger in bovine main pancreatic duct. Dig Dis Sci 40: 2336–2340, 1995. [DOI] [PubMed] [Google Scholar]
- 189.Matsuzaki T, Tajika Y, Ablimit A, Aoki T, Hagiwara H, Takata K. Aquaporins in the digestive system. Med Electron Microsc 37: 71–80, 2004. [DOI] [PubMed] [Google Scholar]
- 190.McSwine RL, Musch MW, Bookstein C, Xie Y, Rao M, Chang EB. Regulation of apical membrane Na+/H+ exchangers NHE2 and NHE3 in intestinal epithelial cell line C2/bbe. Am J Physiol 275: C693–C701, 1998. [DOI] [PubMed] [Google Scholar]
- 191.Melvin JE. Chloride channels and salivary gland function. Crit Rev Oral Biol Med 10: 199–209, 1999. [DOI] [PubMed] [Google Scholar]
- 192.Melvin JE, Park K, Richardson L, Schultheis PJ, Shull GE. Mouse down-regulated in adenoma (DRA) is an intestinal Cl(−)/HCO(3)(−) exchanger and is up-regulated in colon of mice lacking the NHE3 Na(+)/H(+) exchanger. J Biol Chem 274: 22855–22861, 1999. [DOI] [PubMed] [Google Scholar]
- 193.Melvin JE, Yule D, Shuttleworth T, Begenisich T. Regulation of fluid and electrolyte secretion in salivary gland acinar cells. Annu Rev Physiol 67: 445–469, 2004. [DOI] [PubMed] [Google Scholar]
- 194.Mennone A, Biemesderfer D, Negoianu D, Yang CL, Abbiati T, Schultheis PJ, Shull GE, Aronson PS, Boyer JL. Role of sodium/hydrogen exchanger isoform NHE3 in fluid secretion and absorption in mouse and rat cholangiocytes. Am J Physiol Gastroin-test Liver Physiol 280: G247–G254, 2001. [DOI] [PubMed] [Google Scholar]
- 195.Miller RT, Counillon L, Pages G, Lifton RP, Sardet C, Pouyssegur J. Structure of the 5’-flanking regulatory region and gene for the human growth factor-activatable Na/H exchanger NHE-1. J Biol Chem 266: 10813–10819, 1991. [PubMed] [Google Scholar]
- 196.Mitchell P, Moyle J. Acid-base titration across the membrane system of rat-liver mitochondria. Catalysis by uncouplers. Biochem J 104: 588–600, 1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Miyazaki E, Sakaguchi M, Wakabayashi S, Shigekawa M, Mihara K. NHE6 protein possesses a signal peptide destined for endoplasmic reticulum membrane and localizes in secretory organelles of the cell. J Biol Chem 276(52): 49221–49227, 2001. [DOI] [PubMed] [Google Scholar]
- 198.Moeser AJ, Nighot PK, Ryan KA, Simpson JE, Clarke LL, Blikslager AT. Mice lacking the Na+/H+ exchanger 2 have impaired recovery of intestinal barrier function. Am J Physiol Gastrointest Liver Physiol 295: G791–G797, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Moseley RH, Hoglund P, Wu GD, Silberg DG, Haila S, de la Chapelle A, Holmberg C, Kere J. Downregulated in adenoma gene encodes a chloride transporter defective in congenital chloride diarrhea. Am J Physiol 276: G185–G192, 1999. [DOI] [PubMed] [Google Scholar]
- 200.Moseley RH, Barrett C, Boyer JL. Sodium-proton exchanged activity is enhanced in liver plasma membrane vesicles derived from partially hepatectomized rats. Gastroenterology 90: 1749, 1986. [Google Scholar]
- 201.Moulin P, Guiot Y, Jonas JC, Rahier J, Devuyst O, Henquin JC. Identification and subcellular localization of the Na+/H+ exchanger and a novel related protein in the endocrine pancreas and adrenal medulla. J Mol Endocrinol 38: 409–422, 2007. [DOI] [PubMed] [Google Scholar]
- 202.Muller T, Rasool I, Heinz-Erian P, Mildenberger E, Hulstrunk C, Muller A, Michaud L, Koot BG, Ballauff A, Vodopiutz J, Rosipal S, Petersen BS, Franke A, Fuchs I, Witt H, Zoller H, Janecke AR, Visweswariah SS. Congenital secretory diarrhoea caused by activating germline mutations in GUCY2C. Gut 65: 1306–1313, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Muller T, Wijmenga C, Phillips AD, Janecke A, Houwen RH, Fischer H, Ellemunter H, Fruhwirth M, Offner F, Hofer S, Muller W, Booth IW, Heinz-Erian P. Congenital sodium diarrhea is an autosomal recessive disorder of sodium/proton exchange but unrelated to known candidate genes. Gastroenterology 119: 1506–1513, 2000. [DOI] [PubMed] [Google Scholar]
- 204.Muller YL, Collins JF, Bai L, Xu H, Ghishan FK. Molecular cloning and characterization of the rat NHE-2 gene promoter. Biochim Biophys Acta 1442: 314–319, 1998. [DOI] [PubMed] [Google Scholar]
- 205.Musch MW, Arvans DL, Wu GD, Chang EB. Functional coupling of the downregulated in adenoma Cl-/base exchanger DRA and the apical Na+/H+ exchangers NHE2 and NHE3. Am J Physiol Gastrointest Liver Physiol 296: G202–G210, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Musch MW, Bookstein C, Rocha F, Lucioni A, Ren H, Daniel J, Xie Y, McSwine RL, Rao MC, Alverdy J, Chang EB. Region-specific adaptation of apical Na/H exchangers after extensive proximal small bowel resection. Am J Physiol Gastrointest Liver Physiol 283: G975–G985, 2002. [DOI] [PubMed] [Google Scholar]
- 207.Musch MW, Bookstein C, Xie Y, Sellin JH, Chang EB. SCFA increase intestinal Na absorption by induction of NHE3 in rat colon and human intestinal C2/bbe cells. Am J Physiol Gastrointest Liver Physiol 280: G687–G693, 2001. [DOI] [PubMed] [Google Scholar]
- 208.Muthusamy S, Shukla S, Amin MR, Cheng M, Orenuga T, Dudeja PK, Malakooti J. PKCdelta-dependent activation of ERK1/2 leads to upregulation of the human NHE2 transcriptional activity in intestinal epithelial cell line C2BBe1. Am J Physiol Gastrointest Liver Physiol 302: G317–G325, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Nagase T, Seki N, Ishikawa K, Ohira M, Kawarabayasi Y, Ohara O, et al. Prediction of the coding sequences of unidentified human genes. VI. The coding sequences of 80 new genes (KIAA0201-KIAA0280) deduced by analysis of cDNA clones from cell line KG-1 and brain. DNA Res 3(5): 321–329, 41–54, 1996. [DOI] [PubMed] [Google Scholar]
- 210.Nakamura N, Tanaka S, Teko Y, Mitsui K, Kanazawa H. Four Na+/H+ exchanger isoforms are distributed to Golgi and post-Golgi compartments and are involved in organelle pH regulation. J Biol Chem 280: 1561–1572, 2005. [DOI] [PubMed] [Google Scholar]
- 211.Narins SC, Park EH, Ramakrishnan R, Garcia FU, Diven JN, Balin BJ, Hammond CJ, Sodam BR, Smith PR, Abedin MZ. Functional characterization of Na(+)/H(+) exchangers in primary cultures of prairie dog gallbladder. J Membr Biol 197: 123–134, 2004. [DOI] [PubMed] [Google Scholar]
- 212.Nath SK, Hang CY, Levine SA, Yun CH, Montrose MH, Donowitz M, Tse CM. Hyperosmolarity inhibits the Na+/H+ exchanger isoforms NHE2 and NHE3: An effect opposite to that on NHE1. Am J Physiol 270: G431–G441, 1996. [DOI] [PubMed] [Google Scholar]
- 213.Nemeth ZH, Deitch EA, Szabo C, Mabley JG, Pacher P, Fekete Z, Hauser CJ, Hasko G. Na+/H+ exchanger blockade inhibits enterocyte inflammatory response and protects against colitis. Am J Physiol Gastrointest Liver Physiol 283: G122–G132, 2002. [DOI] [PubMed] [Google Scholar]
- 214.No YR, He P, Yoo BK, Yun CC. Regulation of NHE3 by lysophosphatidic acid is mediated by phosphorylation of NHE3 by RSK2. Am J Physiol Cell Physiol 309: C14–C21, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Numata M, Orlowski J. Molecular cloning and characterization of a novel (Na+,K+)/H+ exchanger localized to the trans-Golgi network. J Biol Chem 276: 17387–17394, 2001. [DOI] [PubMed] [Google Scholar]
- 216.O’Loughlin EV, Hunt DM, Kreutzmann D. Postnatal development of colonic electrolyte transport in rabbits. Am J Physiol 258: G447–G453, 1990. [DOI] [PubMed] [Google Scholar]
- 217.Oehlke O, Sprysch P, Rickmann M, Roussa E. Na(+)/H(+) exchanger isoforms are differentially regulated in rat submandibular gland during acid/base disturbances in vivo. Cell Tissue Res 323: 253–262, 2006. [DOI] [PubMed] [Google Scholar]
- 218.Ohana E Transepithelial ion transport across duct cells of the salivary gland. Oral Dis 21: 826–835, 2015. [DOI] [PubMed] [Google Scholar]
- 219.Ohgaki R, Matsushita M, Kanazawa H, Ogihara S, Hoekstra D, van Ijzendoorn SC. The Na+/H+ exchanger NHE6 in the endosomal recycling system is involved in the development of apical bile canalicular surface domains in HepG2 cells. Mol Biol Cell 21: 1293–1304, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Oldfield EC III. Antibiotic-associated diarrhea: It’s all about the butyrate. Rev Gastroenterol Disord 4: 216–217, 2004. [PubMed] [Google Scholar]
- 221.Opleta-Madsen K, Hardin J, Gall DG. Epidermal growth factor upregulates intestinal electrolyte and nutrient transport. Am J Physiol 260: G807–G814, 1991. [DOI] [PubMed] [Google Scholar]
- 222.Opleta-Madsen K, Meddings JB, Gall DG. Epidermal growth factor and postnatal development of intestinal transport and membrane structure. Pediatr Res 30: 342–350, 1991. [DOI] [PubMed] [Google Scholar]
- 223.Orci L, Ravazzola M, Anderson RG. The condensing vacuole of exocrine cells is more acidic than the mature secretory vesicle. Nature 326: 77–79, 1987. [DOI] [PubMed] [Google Scholar]
- 224.Orlowski J, Grinstein S. Diversity of the mammalian sodium/proton exchanger SLC9 gene family. Pflugers Arch 447: 549–565, 2004. [DOI] [PubMed] [Google Scholar]
- 225.Orlowski J, Grinstein S. Na+/H+ exchangers. Compr Physiol 1: 2083–2100, 2011. [DOI] [PubMed] [Google Scholar]
- 226.Orlowski J, Kandasamy RA, Shull GE. Molecular cloning of putative members of the Na/H exchanger gene family. cDNA cloning, deduced amino acid sequence, and mRNA tissue expression of the rat Na/H exchanger NHE-1 and two structurally related proteins. J Biol Chem 267: 9331–9339, 1992. [PubMed] [Google Scholar]
- 227.Pacha J Development of intestinal transport function in mammals. Physiol Rev 80: 1633–1667, 2000. [DOI] [PubMed] [Google Scholar]
- 228.Pacha J, Pohlova I, Karen P. Regulation of amiloride-sensitive Na+ transport in immature rat distal colon by aldosterone. Pediatr Res 38: 356–360, 1995. [DOI] [PubMed] [Google Scholar]
- 229.Pacha J, Popp M, Capek K. Amiloride-sensitive sodium transport of the rat distal colon during early postnatal development. Pflugers Arch 409: 194–199, 1987. [DOI] [PubMed] [Google Scholar]
- 230.Paehler Vor der Nolte A, Chodisetti G, a Z, Busch F, Riederer B, Luo M, Yu Y, Menon MB, Schneider A, Stripecke R, Nikolovska K, Yeruva S, Seidler U. Na+/H+ exchanger NHE1 and NHE2 have opposite effects on migration velocity in rat gastric surface cells. J Cell Physiol 232: 1669–1680, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Paradiso AM, Negulescu PA, Machen TE. Na+-H+ and Cl(−)-OH-(HCO3−) exchange in gastric glands. Am J Physiol 250: G524–G534, 1986. [DOI] [PubMed] [Google Scholar]
- 232.Paradiso AM, Tsien RY, Machen TE. Na+-H+ exchange in gastric glands as measured with a cytoplasmic-trapped, fluorescent pH indicator. Proc Natl Acad Sci U S A 81: 7436–7440, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Park K, Evans RL, Watson GE, Nehrke K, Richardson L, Bell SM, Schultheis PJ, Hand AR, Shull GE, Melvin JE. Defective fluid secretion and NaCl absorption in the parotid glands of Na+/H+ exchangerdeficient mice. J Biol Chem 276: 27042–27050, 2001. [DOI] [PubMed] [Google Scholar]
- 234.Park K, Olschowka JA, Richardson LA, Bookstein C, Chang EB, Melvin JE. Expression of multiple Na+/H+ exchanger isoforms in rat parotid acinar and ductal cells. Am J Physiol 276: G470–G478, 1999. [DOI] [PubMed] [Google Scholar]
- 235.Pathak BG, Shull GE, Jenkins NA, Copeland NG. Mouse chromosomal location of four Na/H exchanger isoform genes. Genomics 31: 261–263, 1996. [DOI] [PubMed] [Google Scholar]
- 236.Pearse I, Zhu YX, Murray EJ, Dudeja PK, Ramaswamy K, Malakooti J. Sp1 and Sp3 control constitutive expression of the human NHE2 promoter by interactions with the proximal promoter and the transcription initiation site. Biochem J 407: 101–111, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Pizzonia JH, Biemesderfer D, Abu-Alfa AK, Wu MS, Exner M, Isenring P, Igarashi P, Aronson PS. Immunochemical characterization of Na+/H+ exchanger isoform NHE4. Am J Physiol 275: F510–F517, 1998. [DOI] [PubMed] [Google Scholar]
- 238.Prasad V, Chirra S, Kohli R, Shull GE. NHE1 deficiency in liver: Implications for non-alcoholic fatty liver disease. Biochem Biophys Res Commun 450: 1027–1031, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Prasad V, Lorenz JN, Miller ML, Vairamani K, Nieman ML, Wang Y, Shull GE. Loss of NHE1 activity leads to reduced oxidative stress in heart and mitigates high-fat diet-induced myocardial stress. J Mol Cell Cardiol 65: 33–42, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Priyamvada S, Gomes R, Gill RK, Saksena S, Alrefai WA, Dudeja PK. Mechanisms underlying dysregulation of electrolyte absorption in inflammatory bowel disease-associated diarrhea. Inflamm Bowel Dis 21: 2926–2935, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Putney LK, Barber DL. Na-H exchange-dependent increase in intra-cellular pH times G2/M entry and transition. J Biol Chem 278: 44645–44649, 2003. [DOI] [PubMed] [Google Scholar]
- 242.Putney LK, Denker SP, Barber DL. The changing face of the Na+/H+ exchanger, NHE1: Structure, regulation, and cellular actions. Annu Rev Pharmacol Toxicol 42: 527–552, 2002. [DOI] [PubMed] [Google Scholar]
- 243.Raghupathy P, Ramakrishna BS, Oommen SP, Ahmed MS, Priyaa G, Dziura J, Young GP, Binder HJ. Amylase-resistant starch as adjunct to oral rehydration therapy in children with diarrhea. J Pediatr Gastroenterol Nutr 42: 362–368, 2006. [DOI] [PubMed] [Google Scholar]
- 244.Rajendran VM, Binder HJ. Apical membrane Cl-butyrate exchange: Mechanism of short chain fatty acid stimulation of active chloride absorption in rat distal colon. J Membr Biol 141: 51–58, 1994. [DOI] [PubMed] [Google Scholar]
- 245.Rajendran VM, Geibel J, Binder HJ. Characterization of apical membrane Cl-dependent Na/H exchange in crypt cells of rat distal colon. Am J Physiol Gastrointest Liver Physiol 280: G400–G405, 2001. [DOI] [PubMed] [Google Scholar]
- 246.Ramakrishna BS, Nance SH, Roberts-Thomson IC, Roediger WE. The effects of enterotoxins and short-chain fatty acids on water and electrolyte fluxes in ileal and colonic loops in vivo in the rat. Digestion 45: 93–101, 1990. [DOI] [PubMed] [Google Scholar]
- 247.Ramakrishna BS, Subramanian V, Mohan V, Sebastian BK, Young GP, Farthing MJ, Binder HJ. A randomized controlled trial of glucose versus amylase resistant starch hypo-osmolar oral rehydration solution for adult acute dehydrating diarrhea. PLoS One 3: e1587, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Ramakrishna BS, Venkataraman S, Srinivasan P, Dash P, Young GP, Binder HJ. Amylase-resistant starch plus oral rehydration solution for cholera. N Engl J Med 342: 308–313, 2000. [DOI] [PubMed] [Google Scholar]
- 249.Reczek D, Bretscher A. The carboxyl-terminal region of EBP50 binds to a site in the amino-terminal domain of ezrin that is masked in the dormant molecule. J Biol Chem 273: 18452–18458, 1998. [DOI] [PubMed] [Google Scholar]
- 250.Riedel BD, Ghishan FK. Maturation of chloride-bicarbonate exchange in rat ileal brush border membrane vesicles. Pediatr Res 25: 189–193, 1989. [DOI] [PubMed] [Google Scholar]
- 251.Rieder CV, Fliegel L. Transcriptional regulation of Na+/H+ exchanger expression in the intact mouse. Mol Cell Biochem 243: 87–95, 2003. [DOI] [PubMed] [Google Scholar]
- 252.Robertson MA, Woodside M, Foskett JK, Orlowski J, Grinstein S. Muscarinic agonists induce phosphorylation-independent activation of the NHE-1 isoform of the Na+/H+ antiporter in salivary acinar cells. J Biol Chem 272: 287–294, 1997. [PubMed] [Google Scholar]
- 253.Rocha F, Musch MW, Lishanskiy L, Bookstein C, Sugi K, Xie Y, Chang EB. IFN-gamma downregulates expression of Na(+)/H(+) exchangers NHE2 and NHE3 in rat intestine and human Caco-2/bbe cells. Am J Physiol Cell Physiol 280: C1224–C1232, 2001. [DOI] [PubMed] [Google Scholar]
- 254.Rossmann H, Sonnentag T, Heinzmann A, Seidler B, Bachmann O, Vieillard-Baron D, Gregor M, Seidler U. Differential expression and regulation of Na(+)/H(+) exchanger isoforms in rabbit parietal and mucous cells. Am J Physiol Gastrointest Liver Physiol 281: G447–G458, 2001. [DOI] [PubMed] [Google Scholar]
- 255.Roussa E. Channels and transporters in salivary glands. Cell Tissue Res 343: 263–287, 2011. [DOI] [PubMed] [Google Scholar]
- 256.Roussa E, Alper SL, Thevenod F. Immunolocalization of anion exchanger AE2, Na(+)/H(+) exchangers NHE1 and NHE4, and vacuolar type H(+)-ATPase in rat pancreas. J Histochem Cytochem 49: 463–474, 2001. [DOI] [PubMed] [Google Scholar]
- 257.Roussa E, Bertram J, Berge KE, Labori KJ, Thevenod F, Raeder MG. Differential regulation of vacuolar H+-ATPase and Na+/H+ exchanger 3 in rat cholangiocytes after bile duct ligation. Histochem Cell Biol 125: 419–428, 2006. [DOI] [PubMed] [Google Scholar]
- 258.Roussa E, Nastainczyk W, Thevenod F. Differential expression of electrogenic NBC1 (SLC4A4) variants in rat kidney and pancreas. Biochem Biophys Res Commun 314: 382–389, 2004. [DOI] [PubMed] [Google Scholar]
- 259.Ruppin H, Bar-Meir S, Soergel KH, Wood CM, Schmitt MG, Jr. Absorption of short-chain fatty acids by the colon. Gastroenterology 78: 1500–1507, 1980. [PubMed] [Google Scholar]
- 260.Sacks AI, Acra SA, Dykes W, Polk DB, Barnard JA, Pietsch J, Ghishan FK. Intestinal Na+/H+ exchanger activity is up-regulated by bowel resection in the weanling rat. Pediatr Res 33: 215–220, 1993. [DOI] [PubMed] [Google Scholar]
- 261.Sadlack B, Merz H, Schorle H, Schimpl A, Feller AC, Horak I. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell 75: 253–261, 1993. [DOI] [PubMed] [Google Scholar]
- 262.Saier MH Jr., Reddy, Tsu BV, Ahmed MS, Li C, Moreno-Hagelsieb G. The Transporter Classification Database (TCDB): Recent advances. Nucleic Acids Res 44: D372–D379, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Saier MH Jr., Yen MR, Noto K, Tamang DG, Elkan C. The Transporter Classification Database: Recent advances. Nucleic Acids Res 37: D274–D278, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Sangan P, Rajendran VM, Geibel JP, Binder HJ. Cloning and expression of a chloride-dependent Na+-H+ exchanger. J Biol Chem 277: 9668–9675, 2002. [DOI] [PubMed] [Google Scholar]
- 265.Sardet C, Franchi A, Pouyssegur J. Molecular cloning of the growth-factor-activatable human Na+/H+ antiporter. Cold Spring Harb Symp Quant Biol 53 (Pt 2): 1011–1018, 1988. [DOI] [PubMed] [Google Scholar]
- 266.Sardet C, Franchi A, Pouyssegur J. Molecular cloning, primary structure, and expression of the human growth factor-activatable Na+/H+ antiporter. Cell 56: 271–280, 1989. [DOI] [PubMed] [Google Scholar]
- 267.Sarker R, Cha B, Kovbasnjuk O, Cole R, Gabelli S, Tse CM, Donowitz M. Phosphorylation of NHE3-S719 regulates NHE3 activity through the formation of multiple signaling complexes. Mol Biol Cell 28(13): 1754–1767, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Sarker R, Gronborg M, Cha B, Mohan S, Chen Y, Pandey A, Litchfield D, Donowitz M, Li X. Casein kinase 2 binds to the C terminus of Na+/H+ exchanger 3 (NHE3) and stimulates NHE3 basal activity by phosphorylating a separate site in NHE3. Mol Biol Cell 19: 3859–3870, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Schelling JR, Abu Jawdeh BG. Regulation of cell survival by Na+/H+ exchanger-1. Am J Physiol Renal Physiol 295: F625–F632, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Schultheis PJ, Clarke LL, Meneton P, Harline M, Boivin GP, Stem-mermann G, Duffy JJ, Doetschman T, Miller ML, Shull GE. Targeted disruption of the murine Na+/H+ exchanger isoform 2 gene causes reduced viability of gastric parietal cells and loss of net acid secretion. J Clin Invest 101: 1243–1253, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Schultheis PJ, Clarke LL, Meneton P, Miller ML, Soleimani M, Gawenis LR, Riddle TM, Duffy JJ, Doetschman T, Wang T, Giebisch G, Aronson PS, Lorenz JN, Shull GE. Renal and intestinal absorptive defects in mice lacking the NHE3 Na+/H+ exchanger. Nat Genet 19: 282–285, 1998. [DOI] [PubMed] [Google Scholar]
- 272.Schweinfest CW, Spyropoulos DD, Henderson KW, Kim JH, Chapman JM, Barone S, Worrell RT, Wang Z, Soleimani M. slc26a3 (dra)-deficient mice display chloride-losing diarrhea, enhanced colonic proliferation, and distinct up-regulation of ion transporters in the colon. J Biol Chem 281: 37962–37971, 2006. [DOI] [PubMed] [Google Scholar]
- 273.Sellin JH, De Soignie R. Short-chain fatty acids have polarized effects on sodium transport and intracellular pH in rabbit proximal colon. Gastroenterology 114: 737–747, 1998. [DOI] [PubMed] [Google Scholar]
- 274.Shallat S, Schmidt L, Reaka A, Rao D, Chang EB, Rao MC, Ramaswamy K, Layden TJ. NHE-1 isoform of the Na+/H+ antiport is expressed in the rat and rabbit esophagus. Gastroenterology 109: 1421–1428, 1995. [DOI] [PubMed] [Google Scholar]
- 275.Shcheynikov N, Wang Y, Park M, Ko SB, Dorwart M, Naruse S, Thomas PJ, Muallem S. Coupling modes and stoichiometry of Cl−/HCO3−exchange by slc26a3 and slc26a6. J Gen Physiol 127: 511–524, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Siddique I, Khan I. Regulation of Na/H exchanger-1 in gastroesophageal reflux disease: Possible interaction of histamine receptor. Dig Dis Sci 48: 1832–1838, 2003. [DOI] [PubMed] [Google Scholar]
- 277.Silviani V, Gastaldi M, Planells R, Marteau C, Massacrier A, Cohen P, Cau P, Gerolami A. NHE-3 isoform of the Na+/H+ exchanger in human gallbladder. Localization of specific mRNA by in situ hybridization. J Hepatol 26: 1281–1286, 1997. [DOI] [PubMed] [Google Scholar]
- 278.Singh SK, Binder HJ, Boron WF, Geibel JP. Fluid absorption in isolated perfused colonic crypts. J Clin Invest 96: 2373–2379, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Soleimani M, Singh G, Bizal GL, Gullans SR, McAteer JA. Na+/H+ exchanger isoforms NHE-2 and NHE-1 in inner medullary collecting duct cells. Expression, functional localization, and differential regulation. J Biol Chem 269: 27973–27978, 1994. [PubMed] [Google Scholar]
- 280.Soleimani M, Singh G, Bookstein C, Rao MC, Chang EB, Dominguez JH. Inhibition of glycosylation decreases Na+/H+ exchange activity, blocks NHE-3 transport to the membrane, and increases NHE-3 mRNA expression in LLC-PK1 cells. J Lab Clin Med 127: 565–573, 1996. [DOI] [PubMed] [Google Scholar]
- 281.Sonnentag T, Siegel WK, Bachmann O, Rossmann H, Mack A, Wagner HJ, Gregor M, Seidler U. Agonist-induced cytoplasmic volume changes in cultured rabbit parietal cells. Am J Physiol Gastrointest Liver Physiol 279: G40–G48, 2000. [DOI] [PubMed] [Google Scholar]
- 282.Steward MC, Ishiguro H. Molecular and cellular regulation of pancreatic duct cell function. Curr Opin Gastroenterol 25: 447–453, 2009. [DOI] [PubMed] [Google Scholar]
- 283.Steward MC, Ishiguro H, Case RM. Mechanisms of bicarbonate secretion in the pancreatic duct. Annu Rev Physiol 67: 377–409, 2004. [DOI] [PubMed] [Google Scholar]
- 284.Steward MC, Ishiguro H, Case RM. Mechanisms of bicarbonate secretion in the pancreatic duct. Annu Rev Physiol 67: 377–409, 2005. [DOI] [PubMed] [Google Scholar]
- 285.Stiernet P, Nenquin M, Moulin P, Jonas JC, Henquin JC. Glucose-induced cytosolic pH changes in beta-cells and insulin secretion are not causally related: Studies in islets lacking the Na+/H+ exchanger NHE1. J Biol Chem 282: 24538–24546, 2007. [DOI] [PubMed] [Google Scholar]
- 286.Stock C, Schwab A. Role of the Na/H exchanger NHE1 in cell migration. Acta Physiol (Oxf) 187: 149–157, 2006. [DOI] [PubMed] [Google Scholar]
- 287.Stuart-Tilley A, Sardet C, Pouyssegur J, Schwartz MA, Brown D, Alper SL. Immunolocalization of anion exchanger AE2 and cation exchanger NHE-1 in distinct adjacent cells of gastric mucosa. Am J Physiol 266: C559–C568, 1994. [DOI] [PubMed] [Google Scholar]
- 288.Sullivan S, Alex P, Dassopoulos T, Zachos NC, Iacobuzio-Donahue C, Donowitz M, Brant SR, Cuffari C, Harris ML, Datta LW, Conklin L, Chen Y, Li X. Downregulation of sodium transporters and NHERF proteins in IBD patients and mouse colitis models: Potential contributors to IBD-associated diarrhea. Inflamm Bowel Dis 15: 261–274, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Svegliati-Baroni G, Di Sario A, Casini A, Ferretti G, D’Ambrosio L, Ridolfi F, Bolognini L, Salzano R, Orlandi F, Benedetti A. The Na+/H+ exchanger modulates the fibrogenic effect of oxidative stress in rat hepatic stellate cells. J Hepatol 30: 868–875, 1999. [DOI] [PubMed] [Google Scholar]
- 290.Szpirer C, Szpirer J, Riviere M, Levan G, Orlowski J. Chromosomal assignment of four genes encoding Na/H exchanger isoforms in human and rat. Mamm Genome 5: 153–159, 1994. [DOI] [PubMed] [Google Scholar]
- 291.Tabibian JH, Masyuk AI, Masyuk TV, O’Hara SP, LaRusso NF. Physiology of cholangiocytes. Compr Physiol 3: 541–565, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Talbot C, Lytle C. Segregation of Na/H exchanger-3 and Cl/HCO3 exchanger SLC26A3 (DRA) in rodent cecum and colon. Am J Physiol Gastrointest Liver Physiol 299: G358–G367, 2010. [DOI] [PubMed] [Google Scholar]
- 293.Tanaka Y, Hayashi N, Kaneko A, Ito T, Horimoto M, Sasaki Y, Kasahara A, Fusamoto H, Kamada T. Characterization of signaling pathways to Na+/H+ exchanger activation with epidermal growth factor in hepatocytes. Hepatology 20: 966–974, 1994. [DOI] [PubMed] [Google Scholar]
- 294.Thevenod F, Roussa E, Schmitt BM, Romero MF. Cloning and immunolocalization of a rat pancreatic Na(+) bicarbonate cotransporter. Biochem Biophys Res Commun 264: 291–298, 1999. [DOI] [PubMed] [Google Scholar]
- 295.Tse CM, Brant SR, Walker MS, Pouyssegur J, Donowitz M. Cloning and sequencing of a rabbit cDNA encoding an intestinal and kidney-specific Na+/H+ exchanger isoform (NHE-3). J Biol Chem 267: 9340–9346, 1992. [PubMed] [Google Scholar]
- 296.Tse CM, Levine SA, Yun CH, Khurana S, Donowitz M. Na+/H+ exchanger-2 is an O-linked but not an N-linked sialoglycoprotein. Biochemistry 33: 12954–12961, 1994. [DOI] [PubMed] [Google Scholar]
- 297.Tse CM, Levine SA, Yun CH, Montrose MH, Little PJ, Pouyssegur J, Donowitz M. Cloning and expression of a rabbit cDNA encoding a serum-activated ethylisopropylamiloride-resistant epithelial Na+/H+ exchanger isoform (NHE-2). J Biol Chem 268: 11917–11924, 1993. [PubMed] [Google Scholar]
- 298.Vazquez CM, Coleto R, Zanetti R, Ruiz-Gutierrez V. Increased Na(+)-H+ exchanger activity in the ileal brush-border membrane of spontaneously hypertensive rats. Cell Mol Life Sci 53: 442–446, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Wakabayashi S, Hisamitsu T, Pang T, Shigekawa M. Mutations of Arg440 and Gly455/Gly456 oppositely change pH sensing of Na+/H+ exchanger 1. J Biol Chem 278: 11828–11835, 2003. [DOI] [PubMed] [Google Scholar]
- 300.Wang A, Li J, Zhao Y, Johansson ME, Xu H, Ghishan FK. Loss of NHE8 expression impairs intestinal mucosal integrity. Am J Physiol Gastrointest Liver Physiol 309: G855–G864, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Wang C, Xu H, Chen H, Li J, Zhang B, Tang C, Ghishan FK. Somatostatin stimulates intestinal NHE8 expression via p38 MAPK pathway. Am J Physiol Cell Physiol 300: C375–C382, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Wang D, Dou K, Song Z, Liu Z. The Na+/H+ exchange inhibitor: A new therapeutic approach for hepatic ischemia injury in rats. Transplant Proc 35: 3134–3135, 2003. [DOI] [PubMed] [Google Scholar]
- 303.Wang D, Sun H, Lang F, Yun CC. Activation of NHE3 by dexamethasone requires phosphorylation of NHE3 at Ser663 by SGK1. Am J Physiol Cell Physiol 289: C802–C810, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Wang H, Singh D, Fliegel L. The Na+/H+ antiporter potentiates growth and retinoic acid-induced differentiation of P19 embryonal carcinoma cells. J Biol Chem 272: 26545–26549, 1997. [DOI] [PubMed] [Google Scholar]
- 305.Wang XF, Yu MK, Lam SY, Leung KM, Jiang JL, Leung PS, Ko WH, Leung PY, Chew SB, Liu CQ, Tse CM, Chan HC. Expression, immunolocalization, and functional activity of Na+/H+ exchanger iso-forms in mouse endometrial epithelium. Biol Reprod 68: 302–308, 2003. [DOI] [PubMed] [Google Scholar]
- 306.Wang Z, Orlowski J, Shull GE. Primary structure and functional expression of a novel gastrointestinal isoform of the rat Na/H exchanger. J Biol Chem 268: 11925–11928, 1993. [PubMed] [Google Scholar]
- 307.Wang ZM, Yasui M, Celsi G. Differential effects of glucocorticoids and mineralocorticoids on the mRNA expression of colon ion transporters in infant rats. Pediatr Res 38: 164–168, 1995. [DOI] [PubMed] [Google Scholar]
- 308.Westrom BR, Ohlsson BG, Svendsen J, Tagesson C, Karlsson BW. Intestinal transmission of macromolecules (BSA and FITC-dextran) in the neonatal pig: Enhancing effect of colostrum, proteins and proteinase inhibitors. Biol Neonate 47: 359–366, 1985. [DOI] [PubMed] [Google Scholar]
- 309.Woo AL, Gildea LA, Tack LM, Miller ML, Spicer Z, Millhorn DE, Finkelman FD, Hassett DJ, Shull GE. In vivo evidence for interferon-gamma-mediated homeostatic mechanisms in small intestine of the NHE3 Na+/H+ exchanger knockout model of congenital diarrhea. J Biol Chem 277: 49036–49046, 2002. [DOI] [PubMed] [Google Scholar]
- 310.Wormmeester L, Sanchez de Medina F, Kokke F, Tse CM, Khurana S, Bowser J, Cohen ME, Donowitz M. Quantitative contribution of NHE2 and NHE3 to rabbit ileal brush-border Na+/H+ exchange. Am J Physiol 274: C1261–C1272, 1998. [DOI] [PubMed] [Google Scholar]
- 311.Xu H, Chen H, Dong J, Li J, Chen R, Uno JK, Ghishan FK. Tumor necrosis factor-{alpha} reducing downregulates intestinal NHE8 expression by basal promoter activity. Am J Physiol Cell Physiol 296: C489–C497, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Xu H, Chen H, Dong J, Lynch R, Ghishan FK. Gastrointestinal distribution and kinetic characterization of the sodium-hydrogen exchanger isoform 8 (NHE8). Cell Physiol Biochem 21: 109–116, 2008. [DOI] [PubMed] [Google Scholar]
- 313.Xu H, Chen R, Ghishan FK. Subcloning, localization and expression of the rat intestinal sodium-hydrogen exchanger isoform 8 (NHE-8). Am J Physiol Gastrointest Liver Physiol 289: G36–G41, 2005. [DOI] [PubMed] [Google Scholar]
- 314.Xu H, Collins JF, Bai L, Kiela PR, Lynch RM, Ghishan FK. Epidermal growth factor regulation of rat NHE2 gene expression. Am J Physiol Cell Physiol 281: C504–C513, 2001. [DOI] [PubMed] [Google Scholar]
- 315.Xu H, Li J, Chen H, Wang C, Ghishan FK. NHE8 plays important roles in gastric mucosal protection. Am J Physiol Gastrointest Liver Physiol 304: G257–G261, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Xu H, Li J, Chen R, Zhang B, Wang C, King N, Chen H, Ghishan FK. NHE2×3 DKO mice exhibit gender-specific NHE8 compensation. Am J Physiol Gastrointest Liver Physiol 300: G647–G653, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Xu H, Li Q, Zhao Y, Li J, Ghishan FK. Intestinal NHE8 is highly expressed in goblet cells and its expression is subject to TNF-alpha regulation. Am J Physiol Gastrointest Liver Physiol 310: G64–G69, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Xu H, McCoy A, Li J, Zhao Y, Ghishan FK. Sodium butyrate stimulates NHE8 expression via its role on activating NHE8 basal promoter activity. Am J Physiol Gastrointest Liver Physiol 309: G500–G505, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Xu H, Zhang B, Li J, Chen H, Tooley J, Ghishan FK. Epidermal growth factor inhibits intestinal NHE8 expression via reducing its basal transcription. Am J Physiol Cell Physiol 299: C51–C57, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Xu H, Zhang B, Li J, Chen H, Wang C, Ghishan FK. Transcriptional inhibition of intestinal NHE8 expression by glucocorticoids involves Pax5. Am J Physiol Gastrointest Liver Physiol 299: G921–G927, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Xu H, Zhang B, Li J, Wang C, Chen H, Ghishan FK. Impaired mucin synthesis and bicarbonate secretion in the colon of NHE8 knockout mice. Am J Physiol Gastrointest Liver Physiol 303: G335–G343, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Xu H, Zhao Y, Li J, Wang M, Lian F, Gao M, Ghishan FK. Loss of NHE8 expression impairs ocular surface function in mice. Am J Physiol Cell Physiol 308: C79–C87, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Xue L, Aihara E, Wang TC, Montrose MH. Trefoil factor 2 requires Na/H exchanger 2 activity to enhance mouse gastric epithelial repair. J Biol Chem 286: 38375–38382, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Yanaka A, Suzuki H, Shibahara T, Matsui H, Nakahara A, Tanaka N. EGF promotes gastric mucosal restitution by activating Na(+)/H(+) exchange of epithelial cells. Am J Physiol Gastrointest Liver Physiol 282: G866–G876, 2002. [DOI] [PubMed] [Google Scholar]
- 325.Yang W, Wang H, Fliegel L. Regulation of Na+/H+ exchanger gene expression. Role of a novel poly(dA.dT) element in regulation of the NHE1 promoter. J Biol Chem 271: 20444–20449, 1996. [DOI] [PubMed] [Google Scholar]
- 326.Yang X, Wang D, Dong W, Song Z, Dou K. Expression and modulation of Na(+)/H(+) exchanger 1 gene in hepatocellular carcinoma: A potential therapeutic target. J Gastroenterol Hepatol 26: 364–370, 2011. [DOI] [PubMed] [Google Scholar]
- 327.Yang X, Wang D, Dong W, Song Z, Dou K. Over-expression of Na+/H+ exchanger 1 and its clinicopathologic significance in hepatocellular carcinoma. Med Oncol 27: 1109–1113, 2010. [DOI] [PubMed] [Google Scholar]
- 328.Yang X, Wang D, Dong W, Song Z, Dou K. Suppression of Na+/H+ exchanger 1 by RNA interference or amiloride inhibits human hepatoma cell line SMMC-7721 cell invasion. Med Oncol 28: 385–390, 2011. [DOI] [PubMed] [Google Scholar]
- 329.Yeo CJ, Barry K, Gontarek JD, Donowitz M. Na+/H+ exchange mediates meal-stimulated ileal absorption. Surgery 116: 388–394; discussion 394–385, 1994. [PubMed] [Google Scholar]
- 330.Yeruva S, Farkas K, Hubricht J, Rode K, Riederer B, Bachmann O, Cinar A, Rakonczay Z, Molnar T, Nagy F, Wedemeyer J, Manns M, Raddatz D, Musch MW, Chang EB, Hegyi P, Seidler U. Preserved Na(+)/H(+) exchanger isoform 3 expression and localization, but decreased NHE3 function indicate regulatory sodium transport defect in ulcerative colitis. Inflamm Bowel Dis 16: 1149–1161, 2010. [DOI] [PubMed] [Google Scholar]
- 331.Yoo BK, He P, Lee SJ, Yun CC. Lysophosphatidic acid 5 receptor induces activation of Na(+)/H(+) exchanger 3 via apical epidermal growth factor receptor in intestinal epithelial cells. Am J Physiol Cell Physiol 301: C1008–C1016, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Yu FH, Shull GE, Orlowski J. Functional properties of the rat Na/H exchanger NHE-2 isoform expressed in Na/H exchanger-deficient Chinese hamster ovary cells. J Biol Chem 268: 25536–25541, 1993. [PubMed] [Google Scholar]
- 333.Yun CC. Concerted roles of SGK1 and the Na+/H+ exchanger regulatory factor 2 (NHERF2) in regulation of NHE3. Cell Physiol Biochem 13: 29–40, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Yun CC, Chen Y, Lang F. Glucocorticoid activation of Na(+)/H(+) exchanger isoform 3 revisited. The roles of SGK1 and NHERF2. J Biol Chem 277: 7676–7683, 2002. [DOI] [PubMed] [Google Scholar]
- 335.Yun CH, Gurubhagavatula S, Levine SA, Montgomery JL, Brant SR, Cohen ME, Cragoe EJ Jr., Pouyssegur J, Tse CM, Donowitz M. Glucocorticoid stimulation of ileal Na+ absorptive cell brush border Na+/H+ exchange and association with an increase in message for NHE-3, an epithelial Na+/H+ exchanger isoform. J Biol Chem 268: 206–211, 1993. [PubMed] [Google Scholar]
- 336.Yun CH, Lamprecht G, Forster DV, Sidor A. NHE3 kinase A regulatory protein E3KARP binds the epithelial brush border Na+/H+ exchanger NHE3 and the cytoskeletal protein ezrin. J Biol Chem 273: 25856–25863, 1998. [DOI] [PubMed] [Google Scholar]
- 337.Yun CH, Oh S, Zizak M, Steplock D, Tsao S, Tse CM, Weinman EJ, Donowitz M. cAMP-mediated inhibition of the epithelial brush border Na+/H+ exchanger, NHE3, requires an associated regulatory protein. Proc Natl Acad Sci U S A 94: 3010–3015, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Zachos NC, Kovbasnjuk O, Donowitz M. Regulation of intestinal electroneutral sodium absorption and the brush border Na+/H+ exchanger by intracellular calcium. Ann N Y Acad Sci 1165: 240–248, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Zachos NC, Tse M, Donowitz M. Molecular physiology of intestinal Na+/H+ exchange. Annu Rev Physiol 67: 411–443, 2005. [DOI] [PubMed] [Google Scholar]
- 340.Zeuthen T, Meinild AK, Loo DD, Wright EM, Klaerke DA. Isotonic transport by the Na+-glucose cotransporter SGLT1 from humans and rabbit. J Physiol 531: 631–644, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Zhao H, Star RA, Muallem S. Membrane localization of H+ and HCO3− transporters in the rat pancreatic duct. J Gen Physiol 104: 57–85, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Zhao H, Wiederkehr MR, Fan L, Collazo RL, Crowder LA, Moe OW. Acute inhibition of Na/H exchanger NHE-3 by cAMP. Role of protein kinase a and NHE-3 phosphoserines 552 and 605. J Biol Chem 274: 3978–3987, 1999. [DOI] [PubMed] [Google Scholar]
- 343.Zizak M, Chen T, Bartonicek D, Sarker R, Zachos NC, Cha B, Kovbasnjuk O, Korac J, Mohan S, Cole R, Chen Y, Tse CM, Donowitz M. Calmodulin kinase II constitutively binds, phosphorylates, and inhibits brush border Na+/H+ exchanger 3 (NHE3) by a NHERF2 protein-dependent process. J Biol Chem 287: 13442–13456, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]