Abstract
Weight management strategies during pregnancy reduce child cardiometabolic risk. However, since maternal weight has an overall positive correlation with offspring bone mass, pregnancy weight management could adversely affect child bone health. We aimed to estimate associations between gestational weight gain (GWG) and bone mineralization in the offspring at 7 years of age, and test early pregnancy body mass index (BMI) as an effect modifier. We analyzed prospective data from 2,167 mother-child pairs from the Generation XXI birth cohort who underwent whole-body dual-energy X-ray absorptiometry at 7 years of age. GWG was analyzed as a continuous measure and using the Institute of Medicine categories. In the whole sample and for each early pregnancy BMI category (under/normal weight and overweight/obese), relationships between GWG and offspring bone measures (bone mineral content, BMC, bone areal density, aBMD, size-corrected BMC, scBMC, and height) at 7 years were fitted thought local polynomial regression and smoothing splines. The magnitude of associations was estimated through linear regression coefficients (95% CIs), crude and adjusted for maternal age, height, educational level, and child gestational age. In under/normal weight mothers, GWG was associated with slightly increased bone measures at 7 years [per 5 kg of GWG, BMC: 0.07SD (95% CI: 0.01, 0.12); aBMD: 0.10SD (0.05, 0.15), scBMC: 0.11SD (0.06, 0.16), height: 0.05 SD (0.00, 0.10)], while in overweight/obese mothers no effect of GWG on bone was observed [BMC: 0.02SD (-0.04, 0.09); aBMD: 0.02SD (-0.04, 0.08), scBMC: 0.01SD (-0.06, 0.08), height: 0.02SD (-0.04; 0.08)]]. Also, no advantageous effect of gaining weight above the Institute of Medicine recommendations was observed in either early pregnancy BMI group. Our results suggest that adherence to Institute of Medicine recommendations for pregnancy weight gain is unlikely to have a negative repercussion on offspring bone health, particularly in women with excess weight in early pregnancy.
Keywords: Generation XXI, Cohort study, Bone density, Gestational weight gain, Body mass index
Introduction
The weight gained by women during pregnancy is an important and potentially modifiable determinant of short- and long-term health outcomes for both the mother and the child.(1) Since its management is a key component of prenatal care, in 2009, the United States Institute of Medicine (IOM) published revised guidelines for maternal gestational weight gain (GWG) according to early pregnancy body mass index (BMI) categories, where an overall inverse relation between baseline BMI and weight gain is recommended.(1–3) Those guidelines are now used as standard recommendations in the majority of high-income countries. Due to their policy nature, IOM guidelines overlook the heterogeneity of the associations between GWG and distinct health outcomes in terms of strength and direction.(4) For instance, while an inverted U-shaped association has been suggested for the effect of GWG on preterm birth, a monotonic dose-response association was described for GWG and birth weight, after adjustment for gestational age.(1) Heterogeneous associations have also been found for long-term child health outcomes, namely adiposity, cardiometabolic risk and asthma.(5)
With regard to bone mass, there is evidence of positive effects of GWG on offspring bone mineral content (BMC) and areal density (aBMD).(6, 7) By altering the intrauterine environment, including nutrient availability or endocrine factors such as leptin and estrogen, GWG may influence not only fetal bone formation, but also the programming of bone strength during childhood and even later in life.(8, 9) However, it seems plausible that the effect of GWG on offspring bone is qualitatively or quantitatively modified by prepregnancy maternal weight status. Particularly in view of the IOM guidelines for GWG - that recommend lower and narrower ranges of weight gain for overweight/obese women - possible implications of following those recommendations for the offspring’s bone mineralization should be assessed and compared by prepregnancy BMI groups.
By means of a longitudinal study of children and their mothers within the Generation XXI birth cohort, our objective was to quantify the associations between GWG, continuously and using IOM recommended categories, and dual-energy X-ray absorptiometry (DXA)-derived bone measures and height of the offspring at 7 years of age. Specifically, we aimed to explore a possible modification of the effect of GWG on child bone mineralization by maternal early pregnancy BMI.
Material and Methods
Generation XXI cohort assembly and follow-up
This study was based on the Generation XXI birth cohort whose full details have been published previously.(10, 11) In 2005–2006, all women hospitalized for childbirth in one of the five public maternity units of Porto, Portugal, whose obstetric outcome was a live birth with at least 24 weeks of gestation, were eligible to participate. Of the invited mothers, 91.4% (n=8,495 women) accepted to participate and their 8,647 infants were enrolled in the cohort study. In the 7-year-old follow-up wave (2012/2014), 67.6% of the cohort (5,849 children) was re-evaluated by face to face interview. This age represents as a biological milestone before the onset of puberty, when sexual development becomes a major driving force for growth and development, particularly regarding micro- and macro-architectural changes to the skeleton. Details of the cohort design and description of the cohort’s baseline characteristics were provided as Supporting Data and Supporting Table 1.
The study protocol was approved by the Ethics Committee of Hospital de São João and registered with the Portuguese Authority of Data Protection and was carried out in accordance with the principles of the Declaration of Helsinki. In all evaluations written informed consent was obtained from parents or legal guardians and oral assent from children.
Measures of early pregnancy body mass index and gestational weight gain
At baseline, within 72 hours after delivery, trained interviewers applied face-to-face structured questionnaires including data on demographic and socioeconomic characteristics, lifestyles, medical history, and anthropometrics. In the context of this large cohort comprising women recruited after delivery, the preferred method to collect weight throughout pregnancy was self-report by women. Besides feasibility, this option is supported by a previous systematic review showing that weight misreporting does not largely bias associations between pregnancy-related weight and birth outcomes.(12) To calculate early pregnancy BMI and gestational weight gain we used weight in early pregnancy as the baseline measure, defined as the self-reported weight in kilograms at the beginning of pregnancy (“What was your weight at the beginning of pregnancy?”) or on the first pre-natal medical visit (“What was your weight on the first pregnancy appointment?”) if the latter occurred before the 13th gestational week. For women with missing information on this variable (6.0%), early pregnancy weight was recovered using either obstetric clinical records (0.6%) or the proxy question “What was your weight in the 2 years preceding pregnancy?” (5.4%). Clinical record review was planned with the purpose of recovering only data that were missing from the baseline questionnaire, which is why anthropometrics from this source is available only for a small number of participants.(13) Sensitivity analysis to assess the impact of using these different sources of information is described below. Pre-delivery weight was also obtained from the questionnaire (“What was your weight at the end of pregnancy?”) or recovered from clinical records in the 3.1% of mothers with missing questionnaire information. Height was measured by the interviewer to the nearest 0.1 cm (55.4% of the mothers), abstracted from the national ID card when measurement was not possible (38.7% of the mothers) or recovered from clinical records (6.0% of the mothers). Early pregnancy BMI was calculated [weight (kg) / height2 (m)] and categorized according to the standard World Health Organization (WHO) definition: underweight (<18.5 kg/m2), normal (18.5–24.9 kg/m2), overweight (25.0–29.9 kg/m2) or obese (≥30.0 kg/m2).(14)
GWG was computed as the difference between the mother’s self-reported pre-delivery weight and her early pregnancy weight. For 0.6% of the participants, GWG was recovered from the clinical record review. Women were categorized as gaining below (insufficient GWG), as recommended (adequate GWG) or above (excessive GWG) the IOM recommendations, as detailed on the footnote to Table 1.(1)
Table 1.
Maternal and child characteristics according to groups of early pregnancy body mass index.
| Whole sample | Early pregnancy BMI groupsa | |||
|---|---|---|---|---|
| n=2,167 | Under/normal weight n=1,392) | Overweight/obese (n=775) | pb | |
| Maternal characteristics | ||||
| Early pregnancy BMI, mean (SD), kg/m2 | 24.4 (4.4) | 21.8 (1.9) | 28.9 (3.8) | <0.001 |
| Age at delivery, mean (SD), years | 29.9 (5.1) | 29.5 (5.0) | 30.7 (5.1) | <0.001 |
| No. (%) of women by educational level | ||||
| ≤9 years | 859 (39.7) | 491 (35.3) | 368 (47.5) | |
| 10-12 years | 624 (28.8) | 385 (27.7) | 239 (30.8) | |
| >12 years | 682 (31.5) | 514 (37.0) | 168 (21.7) | <0.001 |
| Missing | 2 | 2 | 0 | |
| Weight gain during pregnancy, mean (SD), kg | 13.2 (5.3) | 14.1 (4.9) | 11.7 (5.6) | <0.001 |
| No. (%) of women by categories of gestational weight gainc | ||||
| Insufficient | 513 (23.7) | 406 (29.2) | 107 (13.8) | |
| Adequate | 861 (39.7) | 614 (44.1) | 247 (31.9) | |
| Excessive | 793 (36.6) | 372 (26.7) | 421 (54.3) | <0.001 |
| No. (%) of women with gestational diabetes | 126 (5.8) | 58 (4.2) | 68 (8.8) | <0.001 |
| Missing | 6 | 4 | 2 | |
| No. (%) of women who ever smoked during pregnancy | 450 (20.9) | 313 (22.6) | 137 (17.9) | 0.010 |
| Missing | 15 | 7 | 8 | |
| Child characteristics | ||||
| Gestational age at birth, mean (SD), weeks | 38.7 (1.6) | 38.7 (1.6) | 38.8 (1.6) | 0.184 |
| Birth weight, mean (SD), g | 3,197.6 (475.8) | 3,167.0 (473.4) | 3,252.5 (475.3) | <0.001 |
| Weight at 7 years, mean (SD) | ||||
| Crude, kg | 27.3 (5.5) | 26.4 (4.8) | 29.0 (6.2) | <0.001 |
| Z-score, WHO reference d | 0.67 (1.14) | 0.48 (1.09) | 1.01 (1.17) | <0.001 |
| Z-score, Generation XXI reference e | 0.02 (0.99) | -0.15 (0.87) | 0.32 (1.10) | <0.001 |
| Height at 7 years, mean (SD) | ||||
| Crude, cm | 124.7 (5.6) | 124.4 (5.6) | 125.2 (5.6) | 0.002 |
| Z-score, WHO reference d | 0.30 (0.94) | 0.24 (0.94) | 0.40 (0.93) | 0.002 |
| Z-score, Generation XXI reference e | -0.03 (0.99) | -0.10 (0.99) | 0.08 (0.98) | <0.001 |
| BMI at 7 years, mean (SD) | ||||
| Crude, kg/m2 | 17.4 (2.6) | 17.0 (2.2) | 18.3 (3.0) | <0.001 |
| Z-score, WHO reference d | 0.68 (1.16) | 0.47 (1.08) | 1.06 (1.21) | <0.001 |
| Z-score, Generation XXI reference e | 0.05 (0.99) | -0.14 (0.84) | 0.38 (1.13) | <0.001 |
| Child DXA-derived bone measures at 7 years | ||||
| BMC, mean (SD) | ||||
| Crude, g | 597.4 (86.3) | 588.8 (84.2) | 612.7 (87.8) | <0.001 |
| Z-score, Zemel, 2011 reference f | -0.04 (0.89) | -0.14 (0.89) | 0.14 (0.86) | <0.001 |
| Z-score, Generation XXI reference g | 0.00 (1.00) | -0.10 (0.98) | 0.18 (1.02) | <0.001 |
| aBMD, mean (SD) | ||||
| Crude, g/cm2 | 0.619 (0.056) | 0.612 (0.054) | 0.631 (0.056) | <0.001 |
| Z-score, Zemel, 2011 reference f | 0.40 (1.09) | 0.26 (1.07) | 0.66 (1.08) | <0.001 |
| Z-score, Generation XXI reference g | 0.00 (1.00) | -0.12 (0.97) | 0.22 (1.02) | <0.001 |
| scBMC, mean (SD) | ||||
| Crude, g | 597.4 (39.6) | 592.9 (36.8) | 605.4 (43.1) | <0.001 |
| Z-score, Generation XXI reference g | 0.00 (1.00) | -0.12 (0.93) | 0.21 (1.09) | <0.001 |
Notes:
According to standard World Health Organization definition.
p-values obtained for comparison between early pregnancy BMI groups (Wilcoxon rank or chi-squared tests).
According to the Institute of Medicine classification (2009): Insufficient - gestational weight gain (GWG) < 12.5 kg for underweight women, < 11.5 kg for normal-weight women, <7 kg for overweight women, and <5 kg for obese women. Adequate - GWG of 12.5–18 kg for underweight women, 11.5–16 kg for normal-weight women, 7–11.5 kg for overweight women, and 5–9 kg for obese women. Excessive - GWG >18 kg for underweight women, >16 kg for normal-weight women, >11.5 kg for overweight women, and >9 kg for obese women.
Age and sex-specific z-scores computed using World Health Organization reference data(19).
Age and sex-specific z-scores computed based on the means and SDs derived from all Generation XXI participants in the 7-year-old follow-up wave with anthropometric data (n=5,838)
Age and sex-specific z-scores computed using the Bone Mineral Density in Childhood Study reference data(17).
Age and sex-specific z-scores computed based on the means and SDs derived from the study sample (n=2,167).
aBMD, areal bone mineral density; BMC, bone mineral content; BMI, body mass index; scBMC, size-corrected bone mineral content.
Children bone densitometry and anthropometric data
In the follow-up evaluation at the age of 7 years, all children assessed between 1st December 2012 and 31st August 2013 were consecutively invited to undergo a whole body dual-energy X-ray absorptiometry (DXA) scan (3,015 children, 43.8% of the participants). Scans were performed using a Hologic Discovery QDR® 4500W device (Hologic Inc., Bedford, Massachusetts, USA, software version 13.3.0.1.) according to standard manufacturer protocol. Standard quality assurance tests were performed daily using the spine phantom according to manufacturer instructions. The coefficient of variation obtained from repeated phantom measurements was below 1%. Scans were evaluated immediately after acquisition and later validated by a second technician with at least 5 years of experience. Total body less head BMC (g) and aBMD (g/cm2) were obtained.(15) Size-corrected BMC (scBMC) was derived separately for girls and boys by linear regression of BMC on bone area and addition of the residuals of the regression to the mean sample BMC.(16) Weight and height were measured according to standard procedures and BMI was calculated. Height was also used in the present study as an outcome measure, as a proxy of linear growth of long bones.
Maternal and offspring neonatal data
Maternal age at delivery was recorded. Maternal educational level at the baseline evaluation was recorded as the number of completed years of education. Clinical records were reviewed at birth to retrieve data on birth weight and gestational age of the offspring.
Study sample
Of the 3,015 children eligible for bone densitometry, 2,408 had a valid DXA scan (79.9% of the eligible subsample) after excluding participants who refused to perform the scan and those whose images had unacceptable technical quality. For the current analysis, we additionally excluded children from multiple pregnancies (n=111) and participants with no information on maternal GWG (n=115) or without information to compute maternal early pregnancy BMI (n=15) (Supporting Fig. 1). Additionally, we analyzed implausible values of GWG (less than 0 kg and more than 30 kg) and for 6 participants we recovered weights from an alternative source of information, among those previously described. The final sample comprised 2,167 mother-child pairs (46.8% girls) whose comparison with the remainder of the cohort is presented in Supporting Table 2, which shows that study participants were more likely to present higher socioeconomic position than non-participants.
Data analysis
Given the frequency of underweight (3.1%) and obese women (10.4%), we combined the four early pregnancy BMI categories into two groups: (1) under/normal weight, and (2) overweight/obese. Sensitivity analyses were conducted to test the robustness of that option. Maternal and child characteristics were compared between early pregnancy BMI groups with Wilcoxon’s rank-sum or Chi-squared tests. Age and sex-specific z-scores were computed for BMC and aBMD based on the method and reference values published by Zemel et al(17). We also computed internal z-scores based on the means and SDs derived from the study sample (n=2,167), which was essential to obtain scBMC z-scores. Age and sex-specific weight, height and BMI z-scores were obtained according to WHO growth charts(18) and also using as reference all Generation XXI participants with anthropometric data at 7 years of age (n=5,838).
The shapes of the associations between GWG and bone parameters were assessed using local polynomial regression and smoothing splines (R packages fANCOVA and splines). Since the visual inspection of plots was consistent with linear relations, estimates of the magnitude of the associations between GWG and DXA-derived bone measures and height were obtained by using multiple linear regression coefficients and respective 95% confidence intervals (95% CIs). Results are presented per 5 kg increase in GWG to improve readability.
To assess whether the relationship between GWG and offspring bone mineralization was modified by maternal weight status, we stratified regression analyses by early pregnancy BMI groups. Analyses were conducted crude and adjusted for potential confounding factors, defined as documented causes of both GWG and child’s bone mineralization and that are not likely to mediate their relationship: maternal age, height and educational level, and offspring gestational age.
Additional analyses with GWG defined as a categorical variable according to IOM categories were conducted by computing adjusted means of the offspring’s BMC, aBMD, scBMC and height using analysis of covariance (ANCOVA), adjusted for the previously defined potential confounders. The differences (95% CI) between those means in adequate vs. insufficient and excessive vs. adequate weight gain groups were estimated using Stata's postestimation command lincom.
Sensitivity analyses
To assess the effect of including the 6.0% of women whose early pregnancy weight was recovered from the alternative sources described above (clinical records or an alternative item on the questionnaire), we carried out a sensitivity analysis by recomputing estimates after excluding this group, and comparing these estimates with those obtained for the whole sample. Additionally, to assess the impact of misclassification due to self-reporting of weight, we recalculated estimates after excluding women classified as having higher susceptibility to weight under-reporting according to published literature,(19–23) i.e. participants with at least one of the following characteristics (n=641 mothers): young maternal age (<25 years old), low educational level (<6 years), unmarried, late pre-natal care (after the 12th gestational week) and higher parity (two or more previous pregnancies).
Statistical analysis and graphics were performed using Stata version 11.2 for Windows (Stata Corp. LP, College Station, Texas, USA) and R version 3.5.1 (The R Foundation for Statistical Computing, Vienna, Austria).
Results
Maternal and child characteristics are presented in Table 1. At the beginning of pregnancy, 35.8% of the mothers were overweight or obese. In the whole sample, the mean (SD) maternal GWG was 13.2 (5.3) kg, greater in under/normal weight women than in women with excess weight (14.1 vs 11.7 kg, p<0.001). More than one third (36.6%) of children were born to women who gained excessive weight during pregnancy, according to the IOM recommendations, while 23.7% to women who had insufficient GWG. Women with higher BMI in early pregnancy had children with increased BMC, aBMD and scBMC at 7 years of age.
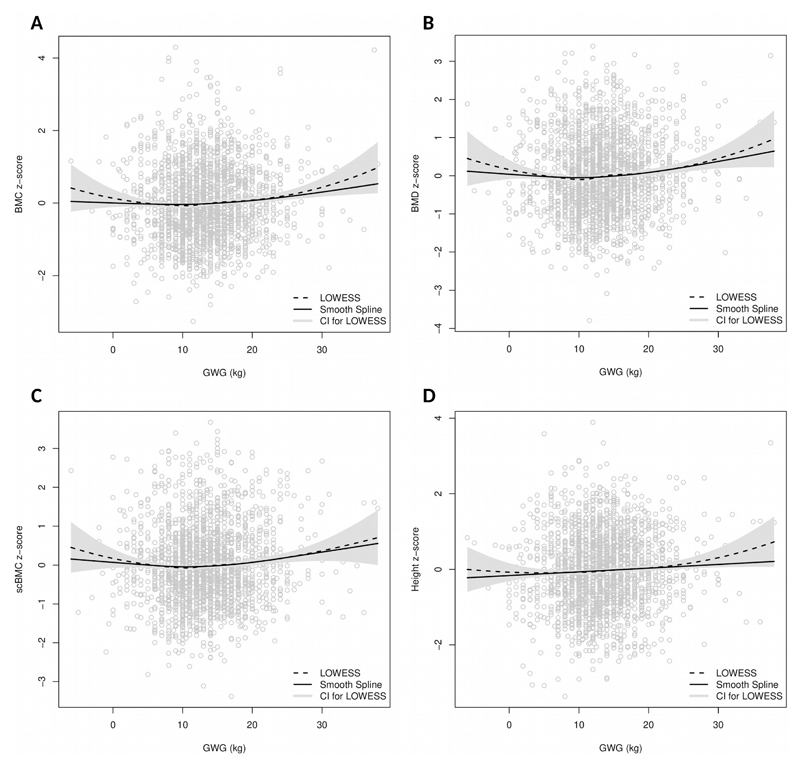
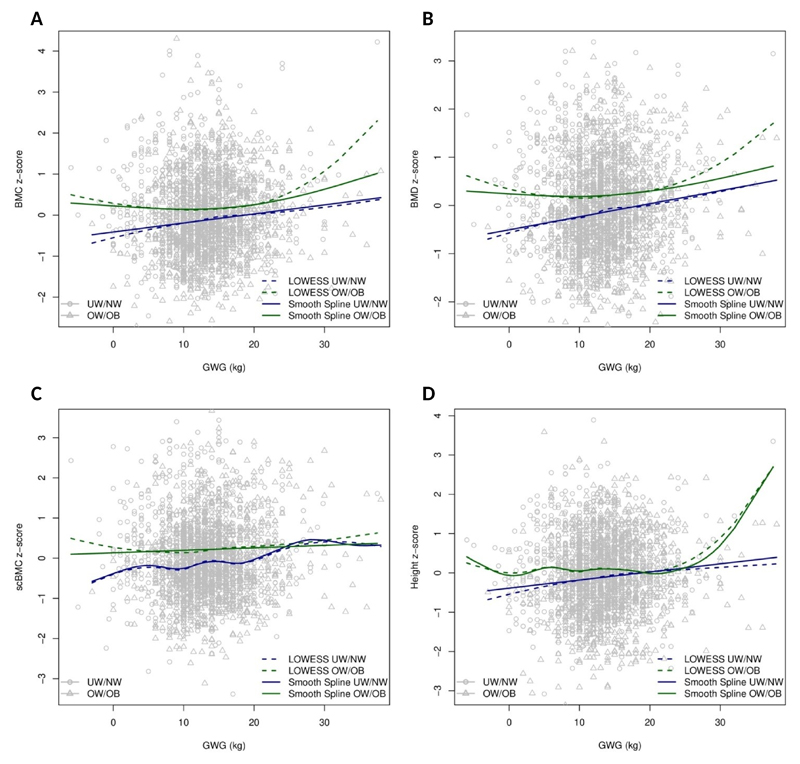
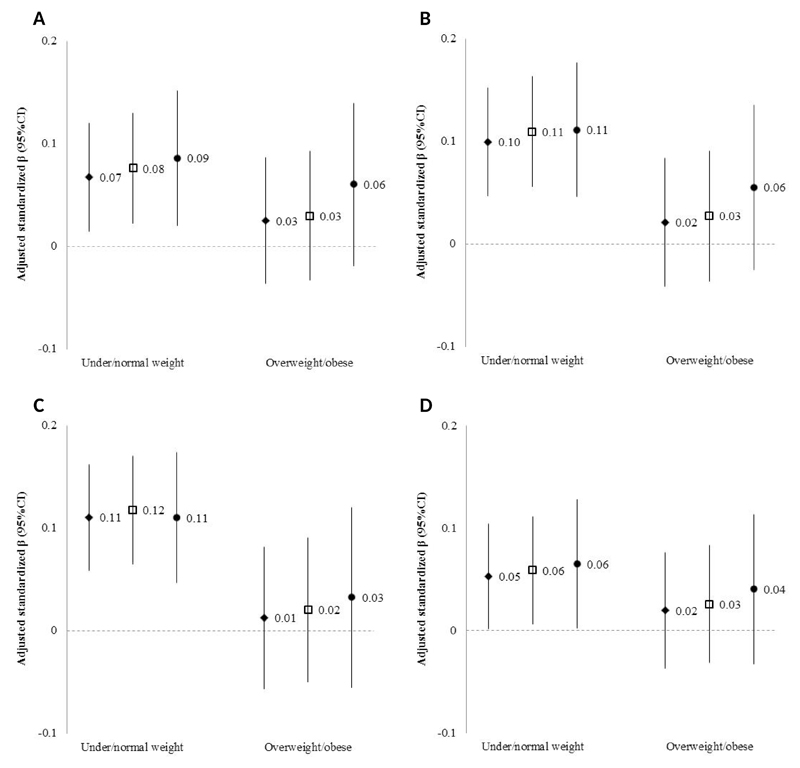
Fig. 1A-D presents the relationship between GWG and DXA-derived bone measures and height in the whole sample, including the plots of local polynomial regressions and smoothing splines, while Fig. 2A-D shows those relationships after stratification by early pregnancy BMI. When compared to smoothing splines, local polynomial regression showed higher variance in the ends of the GWG spectrum, as expected due to data sparsity. In under/normal weight mothers, visual inspection of the association between GWG and offspring bone suggests linear relations for all outcomes. After fitting linear regression models we estimated a modest positive slope, which persisted after adjustment for maternal age, height, educational level and gestational age [β (95% CI): BMC z-score= 0.07 (0.01, 0.12) per 5 kg weight gain; aBMD z-score= 0.10 (0.05, 0.15) per 5 kg; scBMC z-score= 0.11 (0.06, 0.16) per 5 kg; height z-score= 0.05 (0.00, 0.10) per 5 kg] (Fig. 3A-D). Nevertheless, the predictive performance of the model was low, with r-squared estimates between 1 and 2% for all bone measures. More importantly, however, among women with excess weight in early pregnancy, there were no linear associations between GWG and offspring DXA-derived bone measures or height (Fig. 2A-D), before or after adjustment for confounders [adjusted β (95% CI): BMC z-score= 0.02 (-0.04, 0.09) per 5 kg weight gain; aBMD z-score= 0.02 (-0.04, 0.08) per 5 kg; and scBMC z-score= 0.01 (-0.06, 0.08) per 5 kg; height z-score= 0.02 (-0.04; 0.08)] (Fig. 3A-D). As expected, the fit of the model was very low, with r-squared estimates below 1%.
Figure 1.
Local polynomial regression and smoothing splines for gestational weight gain and offspring bone measures at 7 years. BMC (A), aBMD (B), scBMC (C), height (D).
Notes:
Dashed line, local polynomial regression; Solid line, smoothing spline.
aBMD, areal bone mineral density; BMC, bone mineral content; GWG, gestational weight gain; scBMC, size-corrected bone mineral content.
Figure 2.
Local polynomial regression and smoothing splines for gestational weight gain and offspring bone measures at 7 years, in under/normal weight and overweight/obese women. BMC (A), aBMD (B), scBMC (C), height (D).
Notes:
Dashed blue line, Local polynomial regression in under/normal weight women; Solid blue line, smoothing spline in under/normal weight women; Dashed green line, Local polynomial regression in overweight/obese women; Solid green line, smoothing spline in overweight/obese women.
aBMD, areal bone mineral density; BMC, bone mineral content; GWG, gestational weight gain; scBMC, size-corrected bone mineral content; UN/NW, Under/normal weight women; OW/OB, overweight/obese women.
Figure 3.
Adjusted linear regression coefficients (95% CIs) for associations between gestational weight gain and offspring bone measures at 7 years in under/normal weight and overweight/obese women. BMC (A), aBMD (B), scBMC (C), height (D).
Notes:
Values are regression coefficients (95% CIs) that reflect the difference in standardized BMC, aBMD, scBMC or height per 5kg increase in gestational weight gain adjusted for maternal age, height and educational level and gestational age at birth of the offspring.
Diamond: Whole sample (n=1,390 in the under/normal weight group; n=775 in the overweight/obese group); Square: Only participants with information on self-reported weight in early pregnancy from the primary questionnaire source (n=1,357 in the under/normal weight group; n=751 in the overweight/obese group); Circle: Only participants with lower susceptibility to weight misreporting (n=1,001 in the under/normal weight group; n=523 in the overweight/obese group).
aBMD, areal bone mineral density; BMC, bone mineral content; GWG, gestational weight gain; scBMC, size-corrected bone mineral content.
Within the under/normal weight group, and taking women with adequate GWG as the reference category, women with insufficient GWG had children with aBMD on average 0.14 SD (95% CI: 0.02, 0.26) lower and scBMC 0.19 SD (95% CI: 0.07, 0.30) lower at 7 years of age, after adjustment for confounders presented in Table 2. In contrast, in overweight women, no such relation was observed. Interestingly, for both early pregnancy BMI groups, there were no differences in mean bone measures between children born to women with excessive when compared to adequate GWG.
Table 2.
Adjusted means (standard deviation, SD) of z-scored bone mass properties at 7 years of age, according to maternal gestational weight gain categories (Institute of Medicine, 2009), in under/normal weight and overweight/obese women.
| Adjusted means (SD) | Difference between means (95% CIs) | ||||
|---|---|---|---|---|---|
| Insufficient GWG | Adequate GWG | Excessive GWG | Adequate - Insufficient GWG | Excessive - Adequate GWG | |
| BMC z-score | |||||
| Under/normal weight | -0.19 (0.05) | -0.09 (0.04) | -0.00 (0.05) | 0.10 (-0.02, 0.22) | 0.09 (-0.03, 0.21) |
| Overweight/obese | 0.22 (0.09) | 0.16 (0.06) | 0.18 (0.05) | -0.05 (-0.28, 0.17) | 0.02 (-0.13, 0.17) |
| aBMD z-score | |||||
| Under/normal weight | -0.25 (0.05) | -0.11 (0.04) | 0.00 (0.05) | 0.14 (0.02, 0.26) | 0.12 (-0.00, 0.24) |
| Overweight/obese | 0.24 (0.10) | 0.20 (0.06) | 0.23 (0.05) | -0.04 (-0.27, 0.18) | 0.02 (-0.13, 0.18) |
| scBMC z-score | |||||
| Under/normal weight | -0.27 (0.05) | -0.08 (0.04) | 0.00 (0.05) | 0.19 (0.07, 0.30) | 0.08 (-0.04, 0.20) |
| Overweight/obese | 0.19 (0.11) | 0.20 (0.07) | 0.22 (0.05) | 0.00 (-0.24, 0.25) | 0.02 (-0.15, 0.19) |
| Height z-score | |||||
| Under/normal weight | -0.17 (0.05) | -0.11 (0.04) | 0.01 (0.05) | 0.06 (-0.05, 0.18) | 0.12 (0.00, 0.24) |
| Overweight/obese | 0.03 (0.09) | 0.07 (0.06) | 0.10 (0.04) | 0.04 (-0.16, 0.24) | 0.03 (-0.11, 0.17) |
Notes:
Means are adjusted for maternal age, height and educational level and gestational age at birth of the offspring.
aBMD, areal bone mineral density; BMC, bone mineral content; BMI, body mass index; GWG, gestational weight gain; scBMC, size-corrected bone mineral content
Sensitivity analysis
After restricting the analysis to participants whose information for weight in early pregnancy was collected from the primary source, i.e. self-reported weight at the beginning of pregnancy or at the first medical appointment in the first trimester [n=2,108 (97.3%) of all mothers, n=1,357 (97.6%) of under/normal weight mothers and n=751 (96.9%) of those overweight/obese], the associations between GWG and offspring bone properties remained similar to those obtained including participants with alternative sources of information (Fig. 3A-D). Associations between GWG and offspring bone properties also remained essentially unchanged after restricting the analysis to mothers who were classified as having lower susceptibility to weight misreporting [n=1,001 (72.0%) of under/normal weight mothers and n=523 (67.5%) of those overweight/obese] (Fig. 3A-D).
Discussion
In the present study, we found a modest direct linear association between maternal GWG and bone mineralization in the offspring of women who were under/normal weight in early pregnancy, possibly reflecting a weak biological effect. However, among overweight/obese women, increased GWG had no apparent relation with offspring bone mineralization. The corresponding causal interpretation would be that there is little or no apparent advantage of gaining excess weight during pregnancy for offspring bone health, particularly in women with excessive weight in early pregnancy. Our results were robust to different bone mineralization measures – DXA-derived or height – and different exposure definitions – GWG as a continuous variable or as IOM categories. Results remained practically unchanged after adjustment for maternal age, height and educational level, and gestational age of the offspring. Likewise, no relevant changes were found after excluding women whose weight was recovered from alternative sources or those more susceptible to weight misreporting.
Previous studies have looked at maternal body size during pregnancy and offspring bone.(6–9) Maternal fat stores during pregnancy were positively associated with offspring bone mineral and geometry at birth in the Southampton Women’s Survey, particularly in late pregnancy.(8, 9) With respect to weight changes, GWG was an independent predictor of BMD in 6-months infants from Generation R and in Chinese children aged 0 to 3 years.(6, 7) In our study, we found evidence of a weak relationship between maternal GWG and childhood bone measures only among under/normal weight women. However, when stratified by IOM categories, there were no differences between mean bone properties between women gaining excessive and adequate weight, in contrast to the comparison between women gaining adequate and insufficient weight. This may be explained by either a relative lack of precision on the higher end of the GWG spectrum or a true levelling off of the biological effect with increasing GWG.
To the best of our knowledge, no studies have looked at the potential effect modification by maternal early pregnancy BMI on GWG-bone associations, even though GWG also showed greater impact on childhood overweight when mothers had normal vs. excess weight before pregnancy.(24–27) Possible intrauterine mechanisms underlying a differential response of pediatric bone to GWG in low/normal BMI vs higher BMI in early pregnancy are several. Firstly, differences in glucose metabolism profiles may contribute to a differential effect. when compared to overweight/obese, under/normal weight women are less likely to have insulin resistance and/or impaired glucose tolerance during pregnancy(28), and may be more sensitive to weight changes during gestation, inducing a more effective decrease in insulin sensitivity, followed by an augmented supply of nutrients to the fetus and enhanced bone accretion.(29) Another possible mechanism may be related to chronic low-grade systemic inflammation associated with adiposity.(30, 31) The placenta of obese women has increased number of macrophages and enhanced expression of proinflammatory cytokines interleukin-1 (IL-1), tumor necrosis factor-alpha, and IL-6 in comparison with that of normal weight women(30) and low-grade inflammation in the higher BMI group may have restricted bone mineral accretion associated to GWG. Also, obese women are more likely to have low serum levels of vitamin D(32), whose insufficiency has been associated with reduced bone mineral accrual.(33) Additionally, differences in weight or body composition trajectories according to early pregnancy BMI may also contribute to the discrepancy: in contrast to women with excessive weight at time of conception, higher weight gains during the second and third trimesters are more frequent in normal weight women.(1, 34) Since 80% of fetal bone mineral accumulation occurs during the last trimester of pregnancy(35), greater weight gains later in pregnancy may partially explain a positive association with offspring bone mass restricted to the low/normal BMI group. Also, under/normal weight women gain more fat than obese women, causing increased nutrient availability and greater exposure to hormones such as leptin and estrogen that may explain a clearer benefit of GWG on offspring bone.(9) Finally, we should not rule out the possibility that the absence of GWG-offspring bone associations in overweight women is attributable to a ceiling effect for bone mass change in response to weight gain due to the attainment of a weight level above which GWG no longer has an effect on intrauterine bone mineral accumulation.
Two artefactual explanations for our findings should also be considered. First, the heterogeneous effect of GWG on the offspring’s bone by maternal BMI may have resulted from different GWG distributions. In comparison to overweight women, mean GWG in under/normal weight women was higher, which may account for the positive GWG-offspring bone associations found in the latter group. However, we observed that heterogeneity by early pregnancy BMI remained similar throughout a wide range of GWG, from -1 kg to 37.5 kg (Supporting Fig. 2). Alternatively, different confounding structures in different strata of maternal BMI could have contributed to the differential associations observed. To rule out this explanation we tested different sets of potential confounders separately in each BMI group - maternal age, height, smoking during pregnancy, gestational diabetes, parity, maternal educational level and employment status, household income, offspring gestational age and sex - and we found that the associations of GWG with bone measures did not change regardless of the confounding structure tested (Supporting Tables 3 and 4).
We used self-reported weight to compute BMI and GWG. This option might raise some concerns because there is evidence that women underestimate their early pregnancy weight, particularly those who are overweight before pregnancy.(36, 37) To assess the potential impact of reporting bias on our effect estimates, we performed a sensitivity analysis by restricting the sample to women less likely to under-report their weight (12, 38). GWG-offspring bone associations in women less susceptible to misreporting were essentially similar to those obtained for the whole sample. Accordingly, results from previous studies have shown that reporting error does not significantly change associations between pregnancy-related weight and birth outcomes and that there is high agreement between self-reported and measured pregnancy weights when recall occurs within 1 year after delivery for early pregnancy weight and within 6 weeks after delivery for pre-delivery weight,(12) as was the case in our study.
Although the vast majority of women (94.0%) had BMI and GWG estimated from self-reported weight in early pregnancy we opted to recover information from alternative sources for the remaining 6.0%. Our sensitivity analysis excluding the latter group showed that associations remained similar to those obtained for all participants. These results are consistent with previous evidence about agreement between data sources in Generation XXI, describing small differences between self-reported information and clinical records for pregnancy weight and height.(13)
DXA is a well validated and commonly used technique to assess bone density in children due to speed, precision, safety, low cost, and widespread availability. Since DXA does not provide a measure of true volumetric density (15), we computed and used scBMC as an approximation of volumetric BMD (39, 40), and we also tested height as an outcome. We did not collect data on in vivo reproducibility to avoid repeated exposure of children to radiation.
This study is strengthened by the use of data from a large prospective population-based birth cohort, assembled during a short period of time, thereby avoiding confounding by age or birth cohort effects. However, we observed differences in maternal characteristics between participants included and the remainder of the cohort that pose a challenge for the generalizability of the findings to the whole cohort. Nevertheless, in our setting the most important correlates of non-participation at baseline and attrition throughout follow-up are also determinants of weight misclassification recognized in published literature. Such determinants, including maternal age and educational level, have been considered in our sensitivity analyses to assess the impact of weight misclassification, which was minor. If those findings are extrapolated to the potential impact of attrition and non-participation, it seems plausible that our estimates are not substantially biased, which would be compatible with a biological rather than socially-patterned effect of GWG on child bone. A related subject is the generalizability of our findings. Generation XXI is comparatively homogeneous in terms of geographical origin, since only 4.5% of mothers are first-generation immigrants and 3.5% from non-European countries. (41) This background may limit generalizability to other settings, even though our estimates remained essentially unchanged after excluding women born outside the country (data not shown).
In this study we estimated heterogeneous associations between GWG and offspring bone mineralization according to maternal early pregnancy BMI, suggesting a biological interaction between early pregnancy nutritional status and subsequent trajectory. Given the well-known adverse implications of excessive GWG for both mother and offspring on a wide range of outcomes, our findings support that women who are encouraged to follow IOM weight gain recommendations during pregnancy should not expect a deleterious effect on the child’s skeletal health.
At the policy level, our findings reinforce IOM guidelines for weight gain. At the clinical practice level, this study supports the inclusion of bone health in the context of weight counselling during pregnancy.
Supplementary Material
Acknowledgments
The authors would like to thank the families enrolled in Generation XXI for their kindness, the members of the research team for their enthusiasm and perseverance and the participating hospitals and their staff for their help and support.
Funding Source:
This work was supported by European Regional Development Fund (ERDF) through the Operational Programme Competitiveness and Internationalization and national funding from the Foundation for Science and Technology (FCT) - Portuguese Ministry of Science, Technology and Higher Education - under the projects “BioAdversity: Como a adversidade social na infância condiciona a saúde: A biologia da adversidade social” (POCI-01-0145-FEDER-016838; Ref. FCT PTDC/DTP-EPI/1687/2014) and “PathMOB.: Risco cardiometabólico na infância: desde o início da vida ao fim da infância” (POCI-01-0145-FEDER-016837; Ref. FCT PTDC/DTP-EPI/3306/2014); by the Unidade de Investigação em Epidemiologia - Instituto de Saúde Pública da Universidade do Porto (EPIUnit) (POCI-01-0145-FEDER-006862; Ref. UID/DTP/04750/2013); by Administração Regional de Saúde Norte (Regional Department of Ministry of Health) and Fundação Calouste Gulbenkian. This research was also supported by the PhD Grant SFRH/BD/92370/2013 (Teresa Monjardino) and the Postdoc Grant SFRH/BPD/88729/2012 (Raquel Lucas) - co-funded by the FCT and Human Potential Operating Program of the European Social Fund (POPH/FSE Program) - and by the FCT Investigator contract IF/01060/2015 (Ana Cristina Santos).
This study is also a result of the project DOCnet (NORTE-01-0145-FEDER-000003), supported by Norte Portugal Regional Operational Programme (NORTE 2020), under the PORTUGAL 2020 Partnership Agreement, through the European Regional Development Fund (ERDF).
Footnotes
Authors’ roles
Study design: Study design: TM, ACS and RL. Study conduct: TM, AH, ACS and RL. Data collection: TM and ACS. Data analysis: TM, CO and RL. Data interpretation: TM, AH, CO, TR, NA, LN, CC, ACS and RL. Drafting manuscript: TM. Revising manuscript content: AH, CO, TR, NA, LN, CC, ACS and RL. Approving final version of manuscript: TM, AH, CO, TR, NA, LN, CC, ACS and RL. RL takes responsibility for the integrity of the data analysis.
Financial Disclosure
TM, AH, CM, TR, NA, LN, ACS and RL state that they have no conflicts of interest. CC has received personal fees from Alliance for Better Bone Health, Amgen, Eli Lilly, GSK, Medtronic, Merck, Novartis, Pfizer, Roche, Servier, Takeda and UCB, outside of the submitted work.
Potential Conflicts of Interest
The authors have no conflicts of interest relevant to this article to disclose.
References
- 1.Institute of Medicine, National Research Council Committee to Reexamine IOM Pregnancy Weight Guidelines. The National Academies Collection: Reports funded by National Institutes of Health. In: Rasmussen KM, Yaktine AL, editors. Weight Gain During Pregnancy: Reexamining the Guidelines. Washington (DC): National Academies Press (US), National Academy of Sciences; 2009. [Google Scholar]
- 2.World Health Organization (WHO). WHO Guidelines Approved by the Guidelines Review Committee. WHO Recommendations on Antenatal Care for a Positive Pregnancy Experience. Geneva: World Health Organization Copyright; 2016. [Google Scholar]
- 3.American Academy of Pediatrics, American College of Obstetricians and Gynecologists. Guidelines for perinatal care. 7th Edition. Elk Grove Village, IL: American Academy of Pediatrics; 2012. [Google Scholar]
- 4.Oken E, Kleinman KP, Belfort MB, Hammitt JK, Gillman MW. Associations of gestational weight gain with short- and longer-term maternal and child health outcomes. Am J Epidemiol. 2009;170(2):173–80. doi: 10.1093/aje/kwp101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Godfrey KM, Reynolds RM, Prescott SL, Nyirenda M, Jaddoe VW, Eriksson JG, et al. Influence of maternal obesity on the long-term health of offspring. Lancet Diabetes Endocrinol. 2017;5(1):53–64. doi: 10.1016/S2213-8587(16)30107-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ay L, Jaddoe VW, Hofman A, Moll HA, Raat H, Steegers EA, et al. Foetal and postnatal growth and bone mass at 6 months: the Generation R Study. Clin Endocrinol (Oxf) 2011;74(2):181–90. doi: 10.1111/j.1365-2265.2010.03918.x. [DOI] [PubMed] [Google Scholar]
- 7.Xu H, Zhao Z, Wang H, Ding M, Zhou A, Wang X, et al. Bone mineral density of the spine in 11,898 Chinese infants and young children: a cross-sectional study. PLoS One. 2013;8(12):e82098. doi: 10.1371/journal.pone.0082098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Godfrey K, Walker-Bone K, Robinson S, Taylor P, Shore S, Wheeler T, et al. Neonatal bone mass: influence of parental birthweight, maternal smoking, body composition, and activity during pregnancy. J Bone Miner Res. 2001;16(9):1694–703. doi: 10.1359/jbmr.2001.16.9.1694. [DOI] [PubMed] [Google Scholar]
- 9.Harvey NC, Javaid MK, Arden NK, Poole JR, Crozier SR, Robinson SM, et al. Maternal predictors of neonatal bone size and geometry: the Southampton Women's Survey. J Dev Orig Health Dis. 2010;1(1):35–41. doi: 10.1017/S2040174409990055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larsen PS, Kamper-Jorgensen M, Adamson A, Barros H, Bonde JP, Brescianini S, et al. Pregnancy and birth cohort resources in europe: a large opportunity for aetiological child health research. Paediatr Perinat Epidemiol. 2013;27(4):393–414. doi: 10.1111/ppe.12060. [DOI] [PubMed] [Google Scholar]
- 11.Alves E, Correia S, Barros H, Azevedo A. Prevalence of self-reported cardiovascular risk factors in Portuguese women: a survey after delivery. Int J Public Health. 2012;57(5):837–47. doi: 10.1007/s00038-012-0340-6. [DOI] [PubMed] [Google Scholar]
- 12.Headen I, Cohen AK, Mujahid M, Abrams B. The accuracy of self-reported pregnancy-related weight: a systematic review. Obes Rev. 2017;18(3):350–69. doi: 10.1111/obr.12486. [DOI] [PubMed] [Google Scholar]
- 13.Alves E, Lunet N, Correia S, Morais V, Azevedo A, Barros H. Medical record review to recover missing data in a Portuguese birth cohort: agreement with self-reported data collected by questionnaire and inter-rater variability. Gac Sanit. 2011;25(3):211–9. doi: 10.1016/j.gaceta.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 14.Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: executive summary. Expert Panel on the Identification, Evaluation, and Treatment of Overweight in Adults. Am J Clin Nutr. 1998;68(4):899–917. doi: 10.1093/ajcn/68.4.899. [DOI] [PubMed] [Google Scholar]
- 15.Crabtree NJ, Arabi A, Bachrach LK, Fewtrell M, El-Hajj Fuleihan G, Kecskemethy HH, et al. Dual-energy X-ray absorptiometry interpretation and reporting in children and adolescents: the revised 2013 ISCD Pediatric Official Positions. J Clin Densitom. 2014;17(2):225–42. doi: 10.1016/j.jocd.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Tobias JH, Cook DG, Chambers TJ, Dalzell N. A comparison of bone mineral density between Caucasian, Asian and Afro-Caribbean women. Clin Sci (Lond) 1994;87(5):587–91. doi: 10.1042/cs0870587. [DOI] [PubMed] [Google Scholar]
- 17.Zemel BS, Kalkwarf HJ, Gilsanz V, Lappe JM, Oberfield S, Shepherd JA, et al. Revised reference curves for bone mineral content and areal bone mineral density according to age and sex for black and non-black children: results of the bone mineral density in childhood study. J Clin Endocrinol Metab. 2011;96(10):3160–9. doi: 10.1210/jc.2011-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards: Length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: Methods and development. Geneve: World Health Organization; 2006. [Google Scholar]
- 19.Shin D, Chung H, Weatherspoon L, Song WO. Validity of prepregnancy weight status estimated from self-reported height and weight. Matern Child Health J. 2014;18(7):1667–74. doi: 10.1007/s10995-013-1407-6. [DOI] [PubMed] [Google Scholar]
- 20.Han E, Abrams B, Sridhar S, Xu F, Hedderson M. Validity of Self-Reported Pre-Pregnancy Weight and Body Mass Index Classification in an Integrated Health Care Delivery System. Paediatr Perinat Epidemiol. 2016;30(4):314–9. doi: 10.1111/ppe.12286. [DOI] [PubMed] [Google Scholar]
- 21.Schieve LA, Perry GS, Cogswell ME, Scanion KS, Rosenberg D, Carmichael S, et al. Validity of self-reported pregnancy delivery weight: an analysis of the 1988 National Maternal and Infant Health Survey. NMIHS Collaborative Working Group. Am J Epidemiol. 1999;150(9):947–56. doi: 10.1093/oxfordjournals.aje.a010103. [DOI] [PubMed] [Google Scholar]
- 22.Tomeo CA, Rich-Edwards JW, Michels KB, Berkey CS, Hunter DJ, Frazier AL, et al. Reproducibility and validity of maternal recall of pregnancy-related events. Epidemiology. 1999;10(6):774–7. [PubMed] [Google Scholar]
- 23.Buka SL, Goldstein JM, Spartos E, Tsuang MT. The retrospective measurement of prenatal and perinatal events: accuracy of maternal recall. Schizophr Res. 2004;71(2–3):417–26. doi: 10.1016/j.schres.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 24.Diesel JC, Eckhardt CL, Day NL, Brooks MM, Arslanian SA, Bodnar LM. Gestational weight gain and the risk of offspring obesity at 10 and 16 years: a prospective cohort study in low-income women. BJOG. 2015;122(10):1395–402. doi: 10.1111/1471-0528.13448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.von Kries R, Ensenauer R, Beyerlein A, Amann-Gassner U, Hauner H, Rosario AS. Gestational weight gain and overweight in children: Results from the cross-sectional German KiGGS study. Int J Pediatr Obes. 2011;6(1):45–52. doi: 10.3109/17477161003792564. [DOI] [PubMed] [Google Scholar]
- 26.Wrotniak BH, Shults J, Butts S, Stettler N. Gestational weight gain and risk of overweight in the offspring at age 7 y in a multicenter, multiethnic cohort study. Am J Clin Nutr. 2008;87(6):1818–24. doi: 10.1093/ajcn/87.6.1818. [DOI] [PubMed] [Google Scholar]
- 27.Sridhar SB, Darbinian J, Ehrlich SF, Markman MA, Gunderson EP, Ferrara A, et al. Maternal gestational weight gain and offspring risk for childhood overweight or obesity. Am J Obstet Gynecol. 2014;211(3):259.e1–8. doi: 10.1016/j.ajog.2014.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Torloni MR, Betran AP, Horta BL, Nakamura MU, Atallah AN, Moron AF, et al. Prepregnancy BMI and the risk of gestational diabetes: a systematic review of the literature with meta-analysis. Obes Rev. 2009;10(2):194–203. doi: 10.1111/j.1467-789X.2008.00541.x. [DOI] [PubMed] [Google Scholar]
- 29.Waters TP, Huston-Presley L, Catalano PM. Neonatal body composition according to the revised institute of medicine recommendations for maternal weight gain. J Clin Endocrinol Metab. 2012;97(10):3648–54. doi: 10.1210/jc.2012-1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Challier JC, Basu S, Bintein T, Minium J, Hotmire K, Catalano PM, et al. Obesity in pregnancy stimulates macrophage accumulation and inflammation in the placenta. Placenta. 2008;29(3):274–81. doi: 10.1016/j.placenta.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Segovia SA, Vickers MH, Reynolds CM. The impact of maternal obesity on inflammatory processes and consequences for later offspring health outcomes. J Dev Orig Health Dis. 2017:1–12. doi: 10.1017/S2040174417000204. [DOI] [PubMed] [Google Scholar]
- 32.Karlsson T, Andersson L, Hussain A, Bosaeus M, Jansson N, Osmancevic A, et al. Lower vitamin D status in obese compared with normal-weight women despite higher vitamin D intake in early pregnancy. Clin Nutr. 2015;34(5):892–8. doi: 10.1016/j.clnu.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 33.Javaid MK, Crozier SR, Harvey NC, Gale CR, Dennison EM, Boucher BJ, et al. Maternal vitamin D status during pregnancy and childhood bone mass at age 9 years: a longitudinal study. Lancet. 2006;367(9504):36–43. doi: 10.1016/S0140-6736(06)67922-1. [DOI] [PubMed] [Google Scholar]
- 34.Piccinini-Vallis H, Lee-Baggley D, Stewart M, Ryan B. Gestational weight gain trajectories in primary care. Can Fam Physician. 2016;62(7):e407–14. [Google Scholar]
- 35.Cooper C, Westlake S, Harvey N, Javaid K, Dennison E, Hanson M. Review: developmental origins of osteoporotic fracture. Osteoporos Int. 2006;17(3):337–47. doi: 10.1007/s00198-005-2039-5. [DOI] [PubMed] [Google Scholar]
- 36.Schuette SA, Kominiarek MA, Wisner KL, Massey SH. Pre-pregnancy Body Mass Index and Third-Trimester Depressive Symptoms in a Healthy Privately Insured Sample. AJP Rep. 2018;8(1):e13–7. doi: 10.1055/s-0038-1625974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gunderson EP, Abrams B. Epidemiology of gestational weight gain and body weight changes after pregnancy. Epidemiol Rev. 2000;22(2):261–74. doi: 10.1093/oxfordjournals.epirev.a018038. [DOI] [PubMed] [Google Scholar]
- 38.Brunner Huber LR. Validity of self-reported height and weight in women of reproductive age. Matern Child Health J. 2007;11(2):137–44. doi: 10.1007/s10995-006-0157-0. [DOI] [PubMed] [Google Scholar]
- 39.van den Hooven EH, Heppe DH, Kiefte-de Jong JC, Medina-Gomez C, Moll HA, Hofman A, et al. Infant dietary patterns and bone mass in childhood: the Generation R Study. Osteoporos Int. 2015;26(5):1595–604. doi: 10.1007/s00198-015-3033-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Macdonald-Wallis C, Tobias JH, Smith GD, Lawlor DA. Relation of maternal prepregnancy body mass index with offspring bone mass in childhood: is there evidence for an intrauterine effect? Am J Clin Nutr. 2010;92(4):872–80. doi: 10.3945/ajcn.2010.29501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kana MA, Correia S, Barros H. Adverse Pregnancy Outcomes: A Comparison of Risk Factors and Prevalence in Native and Migrant Mothers of Portuguese Generation XXI Birth Cohort. J Immigr Minor Health. 2018 doi: 10.1007/s10903-018-0761-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.