Abstract
Tetrabromobisphenol A (TBBPA), the most common industrial brominated flame retardant, acts as a cytotoxic, neurotoxic, and immunotoxicant, causing inflammation and tumors. However, the mechanism of TBBPA-induced matrix metalloproteinase-9 (MMP-9) expression in human breast cancer cells is not clear. In human breast cancer MCF-7 cells, treatment with TBBPA significantly induced the expression and promoter activity of MMP-9. Transient transfection with MMP-9 mutation promoter constructs verified that NF-κB and AP-1 response elements are responsible for the effects of TBBPA. Furthermore, TBBPA-induced MMP-9 expression was mediated by NF-κB and AP-1 transcription activation as a result of the phosphorylation of the Akt and MAPK signaling pathways. Moreover, TBBPA-induced activation of Akt/MAPK pathways and MMP-9 expression were attenuated by a specific NADPH oxidase inhibitor, and the ROS scavenger. These results suggest that TBBPA can induce cancer cell metastasis by releasing MMP-9 via ROS-dependent MAPK, and Akt pathways in MCF-7 cells.
Keywords: Tetrabromobisphenol A, MMP-9, ROS, MAPK, Akt
INTRODUCTION
Tetrabromobisphenol A (TBBPA) is the most important industrial brominated flame retardant (BFR) (1). BFRs are organobromine compounds that exert an inhibitory effect on combustion chemistry and tend to reduce the flammability of polymeric materials. BFRs are emitted through local sewage treatment systems that spread to homes, industries, or the environment, such that humans are readily exposed to BFRs in underwater environments. TBBPA and its derivatives have been detected in human blood samples and in eggs from predatory bird species (2,3). Additionally, their presence in sediment and mussels indicates that TBBPA may impact on aquatic organisms (4,5). Previous studies on the toxicity of BFRs have mainly focused on polybrominated diphenyl ethers (3,6). However, previous studies reported that TBBPA plays role as a cytotoxicant, neurotoxicant, and thyroid hormone agonist, and shows weak estrogenic activity in mammalian cells (3,7). Moreover, TBBPA was recently reported for its immunotoxic potential (8,9).
The regulation of matrix metalloproteinase-9 (MMP-9) plays a key role in the inflammatory response, angiogenesis, wound healing, and the differentiation of human embryonic stem cells (10,11). However, high expression of MMP-9 increases tumorigenesis and metastasis (12,13). In particular, an elevated level of MMP-9 induces the metastasis and invasion of breast cancer, leading to death in patients with breast cancer (14). Overexpression of MMP-9 has been implicated in breast cancer development, angiogenesis, invasion, and metastasis (15,16). Accumulating evidence has shown the critical role of both mitogen-activated protein kinase (MAPK) and PI3-K/Akt pathways in the regulation of MMP-9 production (17,18). Activated MAPKs and Akt induce transcription factors, including nuclear factor-kappaB (NF-κB) and activator protein-1 (AP-1), which regulate MMP-9 expression by an interaction with transcription factor-binding sites in MMP promoters (19). These transcription factors also regulate the expression of various genes, which are involved in tumorigenesis (20).
Reactive oxygen species (ROS) have emerged as an important proinflammatory mediator in various inflammatory diseases. For the activation of MMP-9 by ROS, various intracellular signaling pathways have been identified (21,22). The main sources of ROS in cells, beside the respiratory chain, are NADPH oxidases (NOX). NOX is an important cellular source of ROS production under many pathologic conditions involved in superoxide generation from NADPH (23). The physiological functions of NOX play various roles in cell signaling, gene expression regulation, cell growth, differentiation, and death (24). NOX-derived ROS have been reported to activate various signaling pathways, such as Akt and MAPK (25), and the activation of transcription factors, such as NF-κB and AP-1 (26,27). However, scant information on the regulation of MMP-9 expression by TBBPA is available. In this study, we investigated the effect of TBBPA on the regulatory mechanism of MMP-9 expression in human breast cancer MCF-7 cells.
MATERIALS AND METHODS
Materials
The TBBPA (97%) was obtained from Sigma-Aldrich (St. Louis, MO, USA). RPMI 1640, fetal bovine serum (FBS), and penicillin-streptomycin were obtained from Gibco BRL (Grand Island, NY, USA). Lipofectamine 2000TM was obtained from Life Technologies, Inc (Carlsbad, CA, USA). A dual-luciferase assay system was obtained from Promega (Madison, WI, USA). Inhibitors against LY294002, PD98059, SB203580, and SP600125 were obtained from Calbiochem (La Jolla, CA, USA). Primary antibodies against phospho-Akt, phospho-ERKl/2, phospho-p38 MAPK, phospho-JNK1/2, ERKl/2, p38 MAPK, JNK1/2, and secondary antibodies against horseradish peroxidase (HRP)-linked anti-mouse or antirabbit IgG were obtained from Cell Signaling Technologies (Beverly, MA, USA). Primary antibodies against β-actin, Lamin B1, NF-κB p65, c-Fos, c-Jun, Akt, and PKCα were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Polymerase chain reaction (PCR) oligonucleotide primers were custom synthesized by Bioneer Co. (Daejeon, Korea). Polyvinylidene fluoride (PVDF) membranes and enhanced chemiluminescence (ECL) system were obtained from Amersham Pharmacia Biotech (Piscataway, NJ, USA).
Cell cultures
The human breast carcinoma MCF-7 cell line was obtained from the American Type Culture Collection (Manassas, VA, USA). The MCF-7 cells were cultured in RPMI 1640 supplemented with 10% FBS, 100 μg/mL streptomycin, and 100 U/mL penicillin in a humidified atmosphere of 5% CO2 at 37°C. To avoid the interference of growth factors in serum, MCF-7 cells were serumstarved for 24 hr and then stimulated with TBBPA.
Measurement of cell viability
Conventional MTT reduction and lactate dehydrogenase (LDH) leakage assays were used to determine MCF-7 cell viability following treatment with TBBPA. To measure cell viability, cells were seeded onto 48-well plates and treated with TBBPA (1–50 μM) for 24 hr. After incubation, MTT solution (0.5 mg/mL) was added to the 48-well plates for 1 hr, and formazan crystals were solubilized with dimethylsulfoxide. The absorbance was measured at 550 nm in a BioTek Synergy HT microplate reader (BioTek Instruments, Winooski, VT, USA). The media were measured using an LDH kit (Cayman, Ann Arbor, MI, USA) at 490 nm on a BioTek Synergy HT microplate reader (BioTek Instruments).
RNA preparation and real-time PCR (qRT-PCR)
Total RNA was isolated from untreated and TBBPA-treated MCF-7 cells with RNAiso-plus Reagent (Takara, Tokyo, Japan). RNA was converted to cDNA using a reverse transcription kit (Promega). qRT-PCR was performed as MMP-9 gene expression analysis by monitoring increases in SYBR reporter dye fluorescence. Primer sequences were as follows: MMP-9 forward, 5′-GTCATCCAGTTTGGTGTCGC-3′; MMP-9 reverse, 5′-GGACCACAACTCGTCATCGT-3′; GAPDH forward, 5′-CCCTTCATTGACCTCAACTA-3′and GAPDH reverse, 5′-CCAAAGTTGTCATGGATGAC-3′. Expression values were normalized with GAPDH.
Transient transfection and luciferase activity assay
Promoter activity was determined using a dual-luciferase reporter assay system (Promega). MCF-7 cells were seeded onto 48-well plates overnight and transiently transfected with the MMP-9 promoter vector, the NF-κB or the AP-1 vector reporter vector, and the Renilla luciferase reporter vector using Lipofectamine. Renilla luciferase expression was used to normalized the luciferase activity.
Gelatin zymography
MCF-7 cells were seeded and incubated for 24 hr and were then maintained in serum-free medium. After 24 hr cell culture supernatants were collected, and loaded onto a 10% polyacrylamide gel containing 0.1% gelatin. Gels were washed with wash buffer (50 mM Tris-HCl pH 7.5, and 2.5% Triton X-100) and incubated with zymography reaction buffer (50 mM Tris-HCl pH 7.5, 1 mM ZnCl2, 5 mM CaCl2, 150 mM NaCl, and 40 mM NaN3) at 37°C for 24 hr. The gel was then stained with Coomassie brilliant blue in a solution of 45% methanol and 1% acetic acid, and then destained for 1 hr. Area of MMP-9 activity appeared as transparent bands against the black background.
Westernblot analysis
After treatment, cell lysates were loaded onto sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). The gel was electrophoretically transferred to PVDF membranes, and blocked with 5% skim milk. The PVDF membranes were incubated with overnight at 4°C with primary antibodies and then incubated with HRP-conjugated anti-IgG secondary antibodies at room temperature for 2 hr. Membranes were then visualized using an ECL detection kit.
ROS production
The production of ROS by TBBPA in MCF-7 cells was using the redox-sensitive fluorescent dye H2DCFDA. After treatment with TBBPA or vehicle, cells were probed with 2 μM H2DCFDA at 37°C for 20 min and washed with PBS. The fluorescence intensity was measured using a BioTek Synergy HT microplate reader (BioTek Instruments; excitation, 490 nm; emission, 530 nm).
Determination of NADPH oxidase activity
The activity of NADPH oxidase by TBBPA in MCF-7 cells was determined using the lucigenin chemiluminescence method. After treatment with TBBPA or vehicle for the indicated period of time, cells were gently scraped and centrifuged for 10 min at 4°C. The cell pellets were then incubated with pre-warmed medium containing either 20 μM lucigenin or 1 μM NADPH, to initiate the reaction, followed by immediate measurement of chemiluminescence using a luminometer (Thermo Scientific, Waltham, MA, USA).
Statistical analysis
All data were demonstrated as means ± SD from at least three representative tests. Statistical significance was assessed by one-way analysis of variance (ANOVA) followed by the Tukey-Kramer test. A value of p < 0.01 was considered significantly different.
RESULTS
Induction of MMP-9 expression and activity by TBBPA in MCF-7 cells
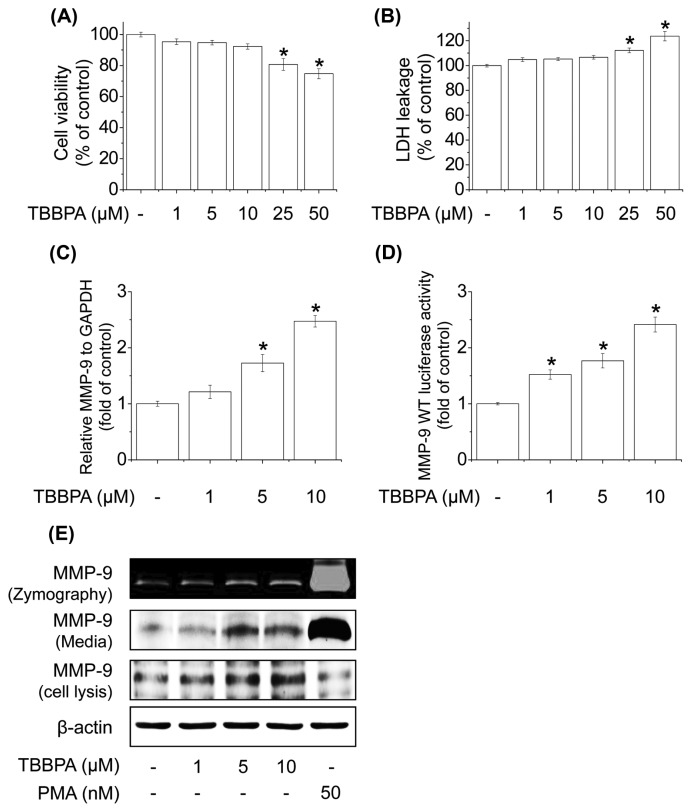
We first measured the cytotoxicity of TBBPA by treating MCF-7 cells with the indicated concentrations of TBBPA for 24 hr followed by the MTT and LDH assays. TBBPA (at concentrations ranging from 1 to 10 μM) had no cytotoxic effect on MCF-7 cells (Fig. 1A, 1B). To determine the effect of TBBPA on MMP-9 expression in MCF-7 cells, cells were treated with TBBPA at the indicated concentrations. As shown in Fig. 1C, MMP-9 mRNA expression was significantly increased by TBBPA treatment in a concentration-dependent manner. Next, the effect of TBBPA on MMP-9 promoter activity was examined. MCF-7 cells were transiently transfected with the luciferase reporter gene and treated with TBBPA for 24 hr. TBBPA significantly increased MMP-9 luciferase activity in a concentration-dependent manner (Fig. 1D). TBBPA also concentration-dependently increased MMP-9 protein expression, as well as enzyme activity in MCF-7 cells (Fig. 1E). These results indicate that TBBPA increases MMP-9 expression and activity in MCF-7 cells at nontoxic concentrations.
Fig. 1.
Effects of TBBPA on MMP-9 expression and transcriptional activity. Cell viability was estimated by the (A) MTT and (B) LDH release assays. (C) Cells were treated with 1, 5, or 10 μM TBBPA for 24 hr. Cells were then lysed and total RNA was prepared to analyze MMP-9 gene expression. (D) Cells were transfected with MMP-9-Luc for 4 hr and then treated with 1, 5, or 10 μM TBBPA for 24 hr. Cells were then harvested and assayed for luciferase activity. (E) Effect of TBBPA on MMP-9 activity levels in MCF-7 cells. Cells were treated with 1, 5, or 10 μM TBBPA for 24 hr. MMP-9 activity in MCF-7 cells was determined by gelatin zymography and Western blot analysis. Each bar represents the mean ± SD calculated from three independent experiments. *Significantly different from the control at p < 0.01.
Involvement of Akt and MAPKs in TBBPA-induced MMP-9 expression
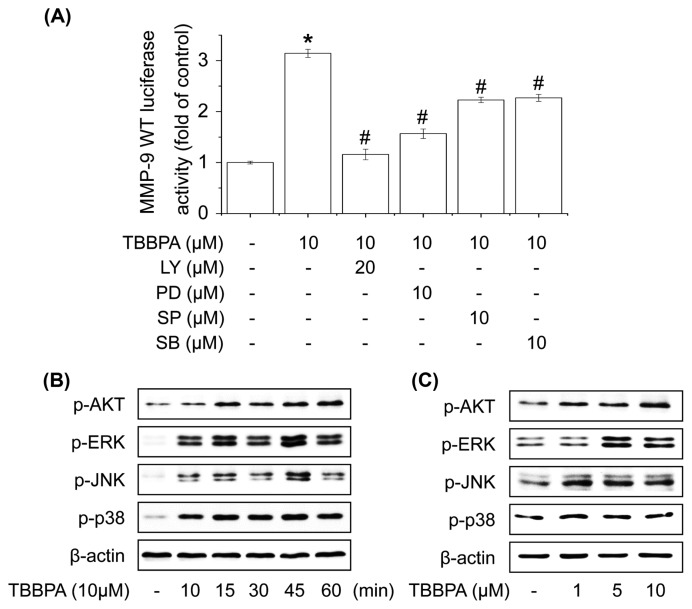
Several studies have reported that Akt and MAPKs are upstream modulators of NF-κB or AP-1, which regulates MMP-9 expression in cells (10,28). We examined whether the activation of Akt, ERK1/2, JNK1/2, and/or p38 MAPK is involved in TBBPA-increased MMP-9 promoter activity using each upstream inhibitor (LY, PD, SP, and SB). MCF-7 cells were transiently transfected with the luciferase reporter gene, pretreated with the upstream inhibitor for 1 hr, and then stimulated with TBBPA for 24 hr. As shown in Fig. 2A, TBBPA-increased MMP-9 luciferase activity was suppressed by pretreatment with LY, PD, SP, and SB in MCF-7 cells. To examine these upstream pathways of NF-κB or AP-1, cells were treated with TBBPA for 1 hr, and then upstream proteins were analyzed by Western blot. The phosphorylation of Akt, ERK1/2, JNK1/2, and p38 MAPK increased significantly following TBBPA treatment in a concentration- and time-dependent manner (Fig. 2B, 2C). These results indicate that TBBPA-increased MMP-9 expression is mediated by the activation of Akt and MAPKs in MCF-7 cells.
Fig. 2.
Effect of TBBPA on the activation of Akt and MAPK signaling pathways in human breast cancer MCF-7 cells. (A) Cells were transfected with MMP-9-Luc and then pretreated with the indicated inhibitor for 60 min followed by 10 μM TBBPA 24 hr. Cells were then harvested and assayed for luciferase activity. (B) Cells were treated with 10 μM TBBPA for 10, 15, 30, 45, or 60 min or (C) 1, 5, or 10 μM TBBPA for 30 min, and the phosphorylation levels of Akt, ERK1/2, JNK, and p38 MAPK were measured by Western blot analysis. Each bar represents the mean ± SD calculated from three independent experiments. *Significantly different from the control at p<0.01. #Significantly different from the TBBPA at p<0.01.
Activation of transcription factors NF-κB and AP-1 in TBBPA-induced MMP-9 expression
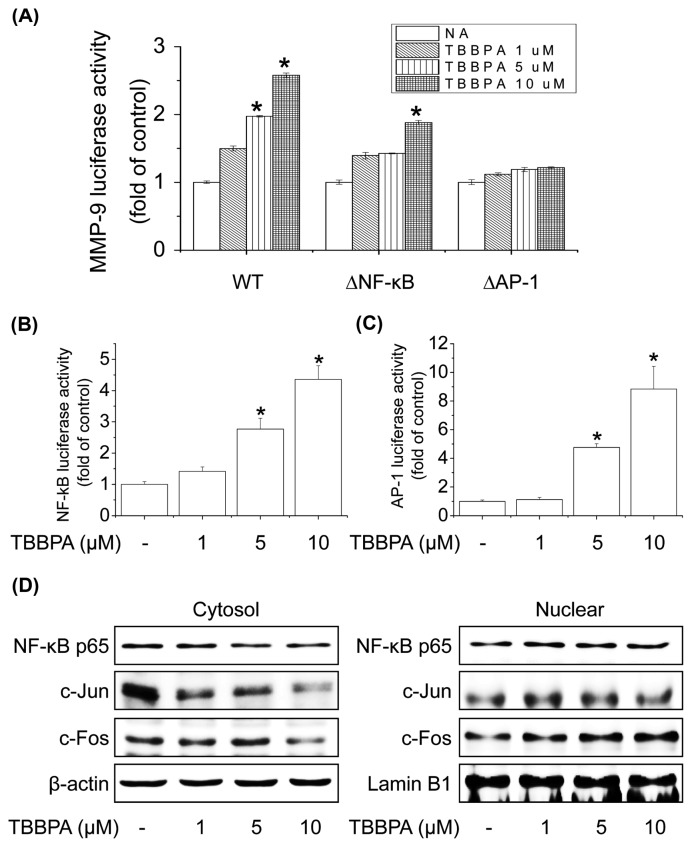
NF-κB and AP-1 are transcription factors of MMP-9 that play a central role in the regulation of MMP-9 mRNA expression in different cell types (29). To investigate which of these transcription factors regulates MMP-9 expression in MCF-7 cells, cells were transiently transfected with reporter genes that contain the wild-type MMP-9 promoter, an NF-κB site-mutated promoter, or an AP-1 site-mutated promoter. Cells were treated with TBBPA (1, 5, or 10 μM), and MMP-9 transcriptional activity was analyzed according to luciferase activity. Treatment with TBBPA induced the transcriptional activity of the wild-type MMP-9 promoter, but had no effect on the site-mutated promoter of NF-κB or AP-1 (Fig. 3A). To further determine the transcription factor involved in MMP-9 promoter activity induced by TBBPA, MCF-7 cells were transiently transfected with the respective reporter vector containing tandem repeats of the NF-κB- or AP-1-binding sites. TBBPA significantly increased NF-κB- or AP-1-responsive luciferase activity in a concentration-dependent manner (Fig. 3B, 3C). To examine which of these transcription factors is involved in the activation of MMP-9 transcription by TBBPA, we examined the effect of TBBPA on the nuclear translocation of NF-κB p65 (a major subunit of NF-κB) or c-Jun and c-Fos (major subunits of AP-1), which are required for their respective transcriptional activities. The nuclear translocation of NF-κB p65, c-Jun, and c-Fos was significantly increased by TBBPA treatment in a concentration-dependent manner (Fig. 3D). These results demonstrate that TBBPA-stimulated MMP-9 induction is mediated by the transcriptional activation of both NF-κB and AP-1 in MCF-7 cells.
Fig. 3.
Effect of TBBPA on the activity and expression of MMP-9 via NF-κB and AP-1 pathways in human breast cancer MCF-7 cells. (A) Mutations were introduced into the NF-κB- or AP-1-binding sites of pGL2-MMP-9WT. Cells were transfected with wild-type, Δ NF-κB, or Δ AP-1 of MMP-9-Luc, and then treated with TBBPA (1–10 μM) for 24 hr. Cells were then harvested and assayed for luciferase activity. (B, C) Cells were transfected with NF-κB or AP-1-Luc and then treated with TBBPA (1–10 μM) for 24 hr. Cells were harvested and assayed for luciferase activity. (D) Effects of TBBPA on NF-κB or AP-1 translocation. Cells were treated with TBBPA (1–10 μM) for 6 hr. Cytosolic or nuclear extracts were subjected to SDS-PAGE followed by Western blot analysis with anti-NF-κB, anti-c-Jun, anti-c-Fos, and anti-Lamin B antibodies. Each bar represents the mean ± SD calculated from three independent experiments. *Significantly different from the control at p < 0.01.
NADPH oxidase-derived reactive oxygen species induced by TBBPA control the activation of Akt and MAPK signaling pathways
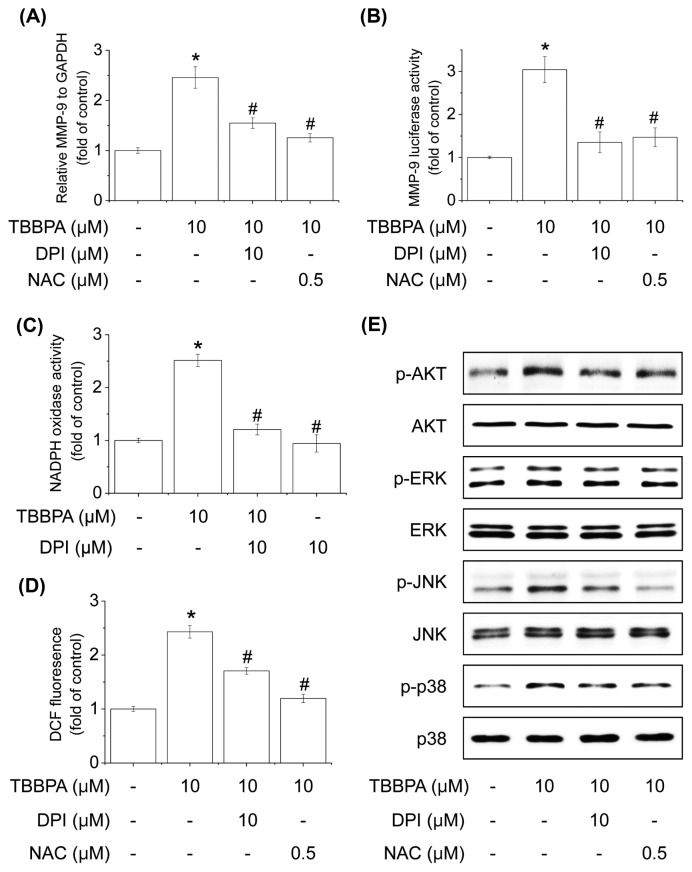
ROS have been demonstrated to induce the expression of inflammatory proteins in various cell types and stimulate Akt and MAPKs (25). The regulation of ROS activation is involved in NADPH oxidase (NOX) under various pathological conditions (30). Thus, we investigated whether TBBPA-induced MMP-9 expression is involved in NOX-dependent ROS generation. As shown in Fig. 4A, 4B, a NOX inhibitor (DPI) or an ROS scavenger (NAC) significantly suppressed TBBPA-induced MMP-9 gene expression and promoter activity. Additionally, TBBPA-induced NOX activity and ROS generation were inhibited by pre-treatment with NAC or DPI (Fig. 4C, 4D). These results indicate that NOX-dependent ROS generation plays a critical role in TBBPA-induced MMP-9 expression. Further, we determined whether TBBPA-induced phosphorylation of Akt and MAPKs was mediated through ROS generation. As shown in Fig. 4E, NAC or DPI significantly decreased the phosphorylation of Akt and MAPKs stimulated by TBBPA. These results indicate that NOX-derived ROS play a key role in TBBPA-induced MMP-9 expression in MCF-7 cells.
Fig. 4.
Effects of NOX-derived ROS generation on TBBPA-induced MMP-9 expression. (A) Cells were treated with 1, 5, or 10 μM TBBPA. Cells were lysed and total RNA was prepared for MMP-9 analysis. (B) Cells were transfected with MMP9-Luc and then pretreated with inhibitors (NAC or DPI) for 30min followed by TBBPA for 24 hr. Cells were then harvested and assayed for luciferase activity. (C) Cells were pre-treated with DPI for 30 min and TBBPA was added for an additional 10 min. Cells were harvested to measure NADPH activity as described above. (D) Cells were pre-treated with NAC or DPI for 30 min, and TBBPA was added for an additional 20min, followed by H2DCFDA for 30 min. Each bar represents the mean ± SD calculated from three independent experiments. (E) Cells were pre-treated with NAC or DPI for 30 min and TBBPA was added. The extracts were analyzed for Akt and MAPK activation using antibodies against phosphorylated Akt, ERK1/2, JNK, and p38 MAPK by Western blot analysis. *Significantly different from the control at p< 0.01. #Significantly different from the TBBPA at p< 0.01.
DISCUSSION
Our findings establish that TBBPA increases MMP-9 expression via NF-κB and AP-1 activation through ROS-dependent Akt/MAPK signaling pathways in human breast cancer MCF-7 cells.
The activation of transcription factors, such as NF-κB and AP-1, is a necessary step in MMP-9 expression, leading to remodeling of the extracellular matrix, as well as membrane degradation and the induction of angiogenesis (19). In the present study, TBBPA increased the transcriptional activation of the MMP-9 promoter. Moreover, promoter mutation analysis indicated that the major targets of TBBPA were the binding sites of both NF-κB and AP-1. These results are consistent with our pervious study, in which TBBPA increased NF-κB- or AP-1-responsive luciferase activity in Raw 264.7 cells (31). NF-κB is a transcriptional activator of MMP-9 and is combined with inhibitory molecules, such as the IκB family, and are sequestered in the cytosol. Stimulation of cells via various signaling pathway triggers the phosphorylation of IκB, leading to its proteasome-mediated degradation and translocation into the nuclei (32). AP-1 is a transcriptional activator of MMP-9 that is composed of a dimer of c-Jun and c-Fos protein families (33,34). AP-1 is activated by modulation with other transcriptional regulators and is further regulated by upstream kinases, such as MAPKs, to induce various signal transduction pathways (35). In the present study, TBBPA increased the nuclear translocation of NF-κB p65, c-Jun, and c-Fos. These results indicate that TBBPA increases MMP-9 expression in MCF-7 cells by enhancing the activity of the transcription factors NF-κB and AP-1. Several studies have shown that activation of NF-κB and AP-1 is triggered by Akt and MAPK signaling pathways (36). In the present study, TBBPA increased the phosphorylation of Akt, ERK1/2, JNK1/2, and p38 MAPK. Additionally, treatment with LY (Akt inhibitor), PD (ERK1/2 inhibitor), SP (JNK1/2 inhibitor), or SB (p38 inhibitor) reduced the TBBPA-increased promoter activity of MMP-9. These results suggest that TBBPA increases MMP-9 expression in MCF-7 cells by enhancing the activation of Akt and MAPKs.
Recent studies have reported that ROS generation can activate various signaling pathways, such as Akt and MAPK (25). Additionally, Akt and MAPK signaling pathways are activated in response to oxidant injury. Therefore, we further examined the mechanism of MMP-9 signal transduction by TBBPA-induced ROS generation in MCF-7 cells. In the present study, TBBPA-induced MMP-9 expression was attenuated by the NOX inhibitor, DPI, and the ROS scavenger, NAC. Additionally, our data also showed that the TBBPA-increased phosphorylation of Akt and MAPK was inhibited by treatment with DPI and NAC. These results suggest that ROS generation by TBBPA-induced NOX plays an important role in MMP-9 expression. However, whether TBBPA-induced ROS production is mediated by other sources remains an interesting topic to be addressed in future studies.
TBBPA is toxic to a variety of mammalian cell lines, as well as primary cells including cerebellar granule cells, splenocytes, and hepatocytes (37,38). However, the effect of TBBPA on MMP-9 expression in breast cancer cells is unknown, which served as the basis for the present study. In conclusion, our study demonstrated that TBBPA induced MMP-9 expression via ROS-dependent Akt/MAPK signaling pathways, which was mediated via increased NF-κB and AP-1 activation in MCF-7 cells. Our findings indicate that TBBPA may affect cancer development, invasion, and metastasis in spite of the lack of toxicity data for TBBPA in MCF-7 cells.
ACKNOWLEDGMENTS
This work was supported by research fund of Chungnam National University.
Footnotes
CONFLICT OF INTEREST
None of the authors declares a conflict of interest.
REFERENCES
- 1.de Wit CA. An overview of brominated flame retardants in the environment. Chemosphere. 2002;46:583–624. doi: 10.1016/S0045-6535(01)00225-9. [DOI] [PubMed] [Google Scholar]
- 2.Berger U, Herzke D, Sandanger TM. Two trace analytical methods for determination of hydroxylated PCBs and other halogenated phenolic compounds in eggs from Norwegian birds of prey. Anal Chem. 2004;76:441–452. doi: 10.1021/ac0348672. [DOI] [PubMed] [Google Scholar]
- 3.Birnbaum LS, Staskal DF. Brominated flame retardants: cause for concern? Environ Health Perspect. 2004;112:9–17. doi: 10.1289/ehp.6559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morris S, Allchin CR, Zegers BN, Haftka JJ, Boon JP, Belpaire C, Leonards PE, Van Leeuwen SP, De Boer J. Distribution and fate of HBCD and TBBPA brominated flame retardants in North Sea estuaries and aquatic food webs. Environ Sci Technol. 2004;38:5497–5504. doi: 10.1021/es049640i. [DOI] [PubMed] [Google Scholar]
- 5.Saint-Louis R, Pelletier E. LC-ESI-MS-MS method for the analysis of tetrabromobisphenol A in sediment and sewage sludge. Analyst. 2004;129:724–730. doi: 10.1039/b400743n. [DOI] [PubMed] [Google Scholar]
- 6.Darnerud PO. Toxic effects of brominated flame retardants in man and in wildlife. Environ Int. 2003;29:841–853. doi: 10.1016/S0160-4120(03)00107-7. [DOI] [PubMed] [Google Scholar]
- 7.Kitamura S, Suzuki T, Sanoh S, Kohta R, Jinno N, Sugihara K, Yoshihara S, Fujimoto N, Watanabe H, Ohta S. Comparative study of the endocrine-disrupting activity of bisphenol A and 19 related compounds. Toxicol Sci. 2005;84:249–259. doi: 10.1093/toxsci/kfi074. [DOI] [PubMed] [Google Scholar]
- 8.Pullen S, Boecker R, Tiegs G. The flame retardants tetrabromobisphenol A and tetrabromobisphenol Abisallylether suppress the induction of interleukin-2 receptor alpha chain (CD25) in murine splenocytes. Toxicology. 2003;184:11–22. doi: 10.1016/S0300-483X(02)00442-0. [DOI] [PubMed] [Google Scholar]
- 9.Reistad T, Mariussen E, Fonnum F. The effect of a brominated flame retardant, tetrabromobisphenol-A, on free radical formation in human neutrophil granulocytes: the involvement of the MAP kinase pathway and protein kinase C. Toxicol Sci. 2005;83:89–100. doi: 10.1093/toxsci/kfh298. [DOI] [PubMed] [Google Scholar]
- 10.Bruschi F, Bianchi C, Fornaro M, Naccarato G, Menicagli M, Gomez-Morales MA, Pozio E, Pinto B. Matrix metalloproteinase (MMP)-2 and MMP-9 as inflammation markers of Trichinella spiralis and Trichinella pseudospiralis infections in mice. Parasite Immunol. 2014;36:540–549. doi: 10.1111/pim.12138. [DOI] [PubMed] [Google Scholar]
- 11.Jadhav U, Chigurupati S, Lakka SS, Mohanam S. Inhibition of matrix metalloproteinase-9 reduces in vitro invasion and angiogenesis in human microvascular endothelial cells. Int J Oncol. 2004;25:1407–1414. [PubMed] [Google Scholar]
- 12.Roy R, Yang J, Moses MA. Matrix metalloproteinases as novel biomarkers and potential therapeutic targets in human cancer. J Clin Oncol. 2009;27:5287–5297. doi: 10.1200/JCO.2009.23.5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu W, Liu J, Xiong X, Ai Y, Wang H. Expression of MMP9 and CD147 in invasive squamous cell carcinoma of the uterine cervix and their implication. Pathol Res Pract. 2009;205:709–715. doi: 10.1016/j.prp.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 14.Scully OJ, Bay BH, Yip G, Yu Y. Breast cancer metastasis. Cancer Genomics Proteomics. 2012;9:311–320. [PubMed] [Google Scholar]
- 15.Blanckaert V, Ulmann L, Mimouni V, Antol J, Brancquart L, Chenais B. Docosahexaenoic acid intake decreases proliferation, increases apoptosis and decreases the invasive potential of the human breast carcinoma cell line MDA-MB-231. Int J Oncol. 2010;36:737–742. doi: 10.3892/ijo_00000549. [DOI] [PubMed] [Google Scholar]
- 16.McGowan PM, Duffy MJ. Matrix metalloproteinase expression and outcome in patients with breast cancer: analysis of a published database. Ann Oncol. 2008;19:1566–1572. doi: 10.1093/annonc/mdn180. [DOI] [PubMed] [Google Scholar]
- 17.Genersch E, Hayess K, Neuenfeld Y, Haller H. Sustained ERK phosphorylation is necessary but not sufficient for MMP-9 regulation in endothelial cells: involvement of Ras-dependent and -independent pathways. J Cell Sci. 2000;113(Pt 23):4319–4330. doi: 10.1242/jcs.113.23.4319. [DOI] [PubMed] [Google Scholar]
- 18.Hong S, Park KK, Magae J, Ando K, Lee TS, Kwon TK, Kwak JY, Kim CH, Chang YC. Ascochlorin inhibits matrix metalloproteinase-9 expression by suppressing activator protein-1-mediated gene expression through the ERK1/2 signaling pathway: inhibitory effects of ascochlorin on the invasion of renal carcinoma cells. J Biol Chem. 2005;280:25202–25209. doi: 10.1074/jbc.M413985200. [DOI] [PubMed] [Google Scholar]
- 19.Mook OR, Frederiks WM, Van Noorden CJ. The role of gelatinases in colorectal cancer progression and metastasis. Biochim Biophys Acta. 2004;1705:69–89. doi: 10.1016/j.bbcan.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 20.Garg A, Aggarwal BB. Nuclear transcription factor-kappaB as a target for cancer drug development. Leukemia. 2002;16:1053–1068. doi: 10.1038/sj.leu.2402482. [DOI] [PubMed] [Google Scholar]
- 21.Maraldi T, Angeloni C, Giannoni E, Sell C. Reactive oxygen species in stem cells. Oxid Med Cell Longev. 2015;2015 doi: 10.1155/2015/159080. 159080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Panday A, Sahoo MK, Osorio D, Batra S. NADPH oxidases: an overview from structure to innate immunity-associated pathologies. Cell Mol Immunol. 2015;12:5–23. doi: 10.1038/cmi.2014.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000;87:840–844. doi: 10.1161/01.RES.87.10.840. [DOI] [PubMed] [Google Scholar]
- 24.Brown DI, Griendling KK. Nox proteins in signal transduction. Free Radic Biol Med. 2009;47:1239–1253. doi: 10.1016/j.freeradbiomed.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giles GI. The redox regulation of thiol dependent signaling pathways in cancer. Curr Pharm Des. 2006;12:4427–4443. doi: 10.2174/138161206779010549. [DOI] [PubMed] [Google Scholar]
- 26.Manea SA, Constantin A, Manda G, Sasson S, Manea A. Regulation of Nox enzymes expression in vascular pathophysiology: Focusing on transcription factors and epigenetic mechanisms. Redox Biol. 2015;5:358–366. doi: 10.1016/j.redox.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morgan MJ, Liu ZG. Crosstalk of reactive oxygen species and NF-kappaB signaling. Cell Res. 2011;21:103–115. doi: 10.1038/cr.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reddy SA, Huang JH, Liao WS. Phosphatidylinositol 3-kinase as a mediator of TNF-induced NF-kappa B activation. J Immunol. 2000;164:1355–1363. doi: 10.4049/jimmunol.164.3.1355. [DOI] [PubMed] [Google Scholar]
- 29.Woo JH, Lim JH, Kim YH, Suh SI, Min DS, Chang JS, Lee YH, Park JW, Kwon TK. Resveratrol inhibits phorbol myristate acetate-induced matrix metalloproteinase-9 expression by inhibiting JNK and PKC delta signal transduction. Oncogene. 2004;23:1845–1853. doi: 10.1038/sj.onc.1207307. [DOI] [PubMed] [Google Scholar]
- 30.Jiang F, Zhang Y, Dusting GJ. NADPH oxidase-mediated redox signaling: roles in cellular stress response, stress tolerance, and tissue repair. Pharmacol Rev. 2011;63:218–242. doi: 10.1124/pr.110.002980. [DOI] [PubMed] [Google Scholar]
- 31.Han EH, Park JH, Kang KW, Jeong TC, Kim HS, Jeong HG. Risk assessment of tetrabromobisphenol A on cyclooxygenase-2 expression via MAP kinase/ NF-kappaB/AP-1 signaling pathways in murine macrophages. J Toxicol Environ Health A. 2009;72:1431–1438. doi: 10.1080/15287390903212873. [DOI] [PubMed] [Google Scholar]
- 32.Perkins ND. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol. 2007;8:49–62. doi: 10.1038/nrm2083. [DOI] [PubMed] [Google Scholar]
- 33.Sato T, Koike L, Miyata Y, Hirata M, Mimaki Y, Sashida Y, Yano M, Ito A. Inhibition of activator protein-1 binding activity and phosphatidylinositol 3-kinase pathway by nobiletin, a polymethoxy flavonoid, results in augmentation of tissue inhibitor of metalloproteinases-1 production and suppression of production of matrix metalloproteinases-1 and -9 in human fibrosarcoma HT-1080 cells. Cancer Res. 2002;62:1025–1029. [PubMed] [Google Scholar]
- 34.Cho HJ, Kang JH, Kwak JY, Lee TS, Lee IS, Park NG, Nakajima H, Magae J, Chang YC. Ascofuranone suppresses PMA-mediated matrix metalloproteinase-9 gene activation through the Ras/Raf/MEK/ERK- and Ap1-dependent mechanisms. Carcinogenesis. 2007;28:1104–1110. doi: 10.1093/carcin/bgl217. [DOI] [PubMed] [Google Scholar]
- 35.Karin M, Liu Z, Zandi E. AP-1 function and regulation. Curr Opin Cell Biol. 1997;9:240–246. doi: 10.1016/S0955-0674(97)80068-3. [DOI] [PubMed] [Google Scholar]
- 36.Chang YC, Li PC, Chen BC, Chang MS, Wang JL, Chiu WT, Lin CH. Lipoteichoic acid-induced nitric oxide synthase expression in RAW 264.7 macrophages is mediated by cyclooxygenase-2, prostaglandin E2, protein kinase A, p38 MAPK, and nuclear factor-kappaB pathways. Cell Signal. 2006;18:1235–1243. doi: 10.1016/j.cellsig.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 37.Fonnum F, Mariussen E, Reistad T. Molecular mechanisms involved in the toxic effects of polychlorinated biphenyls (PCBs) and brominated flame retardants (BFRs) J Toxicol Environ Health A. 2006;69:21–35. doi: 10.1080/15287390500259020. [DOI] [PubMed] [Google Scholar]
- 38.Reistad T, Mariussen E, Ring A, Fonnum F. In vitro toxicity of tetrabromobisphenol-A on cerebellar granule cells: cell death, free radical formation, calcium influx and extracellular glutamate. Toxicol Sci. 2007;96:268–278. doi: 10.1093/toxsci/kfl198. [DOI] [PubMed] [Google Scholar]