Abstract
Helicobacter pylori infection is a major risk factor for the development of gastric cancer. Aberrant expression of microRNAs is strongly implicated in gastric tumorigenesis; however, their contribution in response to H. pylori infection has not been fully elucidated. In this study, we evaluated the expression of miR-135b-5p and its role in gastric cancer. We describe the overexpression of miR-135b-5p in human gastric cancer tissue samples compared with normal tissue samples. Furthermore, we found that miR-135b-5p is also up-regulated in gastric tumors from the trefoil factor 1–knockout mouse model. Infection with H. pylori induced the expression of miR-135b-5p in the in vitro and in vivo models. miR-135b-5p induction was mediated by NF-κB. Treatment of gastric cancer cells with TNF-α induced miR-135b-5p in a NF-κB–dependent manner. Mechanistically, we found that miR-135b-5p targets Krüppel-like factor 4 (KLF4) and binds to its 3′ UTR, leading to reduced KLF4 expression. Functionally, high levels of miR-135b-5p suppress apoptosis and induce cisplatin resistance. Our results uncovered a mechanistic link between H. pylori infection and miR-135b-5p–KLF4, suggesting that targeting miR-135b-5p could be a potential therapeutic approach to circumvent resistance to cisplatin.—Shao, L., Chen, Z., Soutto, M., Zhu, S., Lu, H., Romero-Gallo, J., Peek, R., Zhang, S., El-Rifai, W. Helicobacter pylori-induced miR-135b-5p promotes cisplatin resistance in gastric cancer.
Keywords: microRNA, tff1 knockout, inflammation, KLF4, H. pylori
Gastric cancer is one of the most common malignancies worldwide. Although gastric cancer incidence and mortality rates have declined in recent years, it remains the third leading cause of cancer-associated deaths worldwide (1, 2). Infection with Helicobacter pylori is a major risk factor for the development of gastric cancer. H. pylori colonizes to the gastric mucosa to induce a progressive cascade of histologic lesions from chronic gastritis, atrophic gastritis, intestinal metaplasia, dysplasia, and, ultimately, intestinal-type gastric adenocarcinoma (3–5). Cytokines, including TNF-α, IL-1β, and TGF-β, are known to be up-regulated in response to chronic infection with H. pylori (6–8). TNF-α recognizes and binds to 2 receptors (TNF-αR-1 and TNF-αR-2), which recruit the TNFRSF1A Associated Via Death Domain (TRADD) (9) through heterotypic interactions with the death domain of the TNF receptor. This action leads to activation of NF-κB proinflammatory signaling (10), which regulates several important biologic functions such as cell survival (11), apoptosis (12, 13), and metabolism (14).
MicroRNAs (miRNAs) are short, noncoding RNAs containing ∼22 nt that predominantly interact with other genes by binding to complementary sequences in the 3′ UTR of target mRNAs, thereby adversely regulating mRNA translation (15–17). Numerous studies have shown deregulation of miRNAs expression profiles in various diseases (18), including cancer (17, 19, 20). miRNAs play crucial roles in all physiologic and pathologic processes such as cell proliferation, apoptosis, invasion, and chemoresistance. Recent discoveries have shed light on miRNA dysregulation in response to H. pylori infection, playing an important role in regulating response to H. pylori by altering normal biologic processes, such as apoptosis, proliferation, and host inflammatory immune response (3). High-throughput sequencing data indicate that miR-135b-5p is up-regulated in gastric cancer (21, 22).
Krüppel-like factor 4 (KLF4), also known as gut-enriched KLF or epithelial zinc finger, is a transcription factor that can exert either oncogenic or tumor suppressor functions in a cell-specific and context-dependent manner (23–26). Recent data have illustrated the emerging role of KLF4 in human gastric cancer (27, 28), including proliferation, metastasis, and invasion.
Chemotherapy remains the main treatment option for patients with gastric cancer diagnosed at advanced stages (29). Cisplatin (cis-diamminedichloroplatinum) is a chemotherapeutic drug predominantly used in the treatment of advanced and recurrent gastric cancer (29, 30). However, chemotherapeutic resistance is a challenging clinical problem in the treatment of patients with gastric cancer. There are multiple molecular mechanisms that can lead to cisplatin resistance, including increased DNA damage repair, increased drug inactivation, reduced intracellular drug accumulation, and suppression of apoptotic pathways (31). Although there are pieces of evidence showing a role of several miRNAs in cisplatin resistance in cancer, the role of miRNAs in cisplatin resistance in gastric cancer remains understudied.
In the present study, we report overexpression of miR-135b-5p in gastric cancer. We describe its role in suppressing the expression of KLF4, leading to resistance to cisplatin. We also demonstrate that H. pylori can induce the expression of miR-135b-5p through NF-κB, providing a novel link between infection, inflammation, miRNA, and cisplatin resistance in gastric cancer.
MATERIALS AND METHODS
Tissue samples
All gastric tissue samples were obtained de-identified from the archives of pathology, in accordance with the institutional review board. For DNA and mRNA analysis, 81 frozen tissue samples (54 normal stomach samples and 27 gastric cancer samples) were collected. All adenocarcinomas were classified according to recent guidelines of the Union for International Cancer Control TNM classification system. The trefoil factor 1 (Tff1) knockout mice can exhibit histologic changes in the stomach, progressing from gastritis to malignant adenocarcinoma (32). Gastric tissues from the Tff1-knockout mice were used for analysis of miR-135b-5p expression, in accordance with a protocol approved by the Institutional Animal Care and Use Committees.
Cell culture and reagents
Human gastric cancer cell lines were cultured in F-12 medium (MKN45 and SNU1) (Thermo Fisher Scientific, Waltham, MA USA) and 1640 medium (SNU601) (Thermo Fisher Scientific), supplemented with 10% fetal bovine serum (Thermo Fisher Scientific) and 1% penicillin/streptomycin (Thermo Fisher Scientific) at 37°C in an atmosphere containing 5% CO2. MKN45 cells were purchased from the Japanese Collection of Research Bioresources Cell Bank (Osaka, Japan). SNU1 and SNU601 were purchased from American Type Culture Collection (Manassas, VA, USA). All cell lines were ascertained to conform to the original in vitro morphologic characteristics and were authenticated by using short tandem repeat profiling (Genetica DNA Laboratories, Burlington, NC, USA).
Reconstitution of P65, miR-135b-5p, and KLF4 expression in cell lines
For the transient expression of P65 in MKN45 and SNU1, gastric cancer cells were transfected with a mammalian expression plasmid, pCMV, in frame with P65 or empty vector with PolyJet reagent (SignaGen Laboratories, Rockville, MD, USA) for 48 h. To study the function of miR-135b-5p, MKN45, SNU1, and SNU601 cells were transfected with miR-135b-5p mimic 30 pM or inhibitor 90 pM (Applied Biological Materials, Richmond, BC, Canada) with LipoJet (SignaGen Laboratories) for 48 h. pcDNA3.1-HA-KLF4 FL was a gift from Michael Ruppert (plasmid 34593; Addgene, Cambridge, MA, USA) (33). KLF4 small interfering RNA (siRNA) was purchased from Santa Cruz Biotechnology (Dallas, TX, USA). KLF4 FL plasmid transfection was performed by using PolyJet reagent; KLF4 siRNA was transfected by using LipoJet reagent. The expression levels of P65, miR-135b-5p, and KLF4 were confirmed by using Western blot analysis or quantitative RT-PCR (qRT-PCR).
Drugs, recombinant proteins, and antibodies
Recombinant human TNF-α was purchased from PeproTech (Rocky Hill, NJ, USA). The NF-kB inhibitor Bay 11-7082 was obtained from MilliporeSigma (Burlington, MA, USA). miR-135b-5p mimic and inhibitor were obtained from Applied Biological Materials. Horseradish peroxidase (HRP)-conjugated mouse and rabbit secondary antibodies, phosopho-P65 (S536), P65, KLF4, and β-actin antibodies were purchased from Cell Signaling Technology (Danvers, MA, USA). The cytotoxin-associated gene A (CagA) antibody was purchased from MilliporeSigma.
H. pylori strains and infection
Wild-type CagA+ H. pylori strain 7.13 and the rodent-adapted CagA+ H. pylori strain PMSS1 were used in this study. The H. pylori bacteria were cultured on trypticase soy agar with 5% sheep blood agar plates (BD Biosciences, San Jose, CA, USA) for in vitro passage, as previously described (34). For in vitro studies, the bacteria were cocultured with gastric cancer cells at a multiplicity of infection of 100:1. MKN45 and SUN1 cells were incubated with TNF-α neutralizing antibody (100 ng/ml; R&D Systems, Minneapolis, MN, USA) for 3 h before infection with H. pylori strain 7.13 (35). Incubation with TNF-α antibody and H. pylori infection were continued for 24 h. For in vivo studies, H. pylori PMSS1 strain liquid cultures for mouse inoculation were grown in Brucella broth (BD Biosciences) with 5% newborn calf serum and antibiotic supplementation for ∼24 h, pelleted by centrifugation, and suspended in Brucella broth. C57BL/6 mice were purchased from Charles River Laboratories (Wilmington, MA, USA). Using orogastric gavage, mice (10 mice/group) were challenged with Brucella broth, as an uninfected control or with the mouse-adapted wild-type H. pylori strain PMSS1 (109 CFU/mouse). Mice were euthanized at 2 wk and 6 mo postchallenge, and gastric tissues were harvested for real-time PCR analysis.
qRT-PCR
To detect miRNA, total RNA was isolated by using the miRNeasy Kit (Qiagen, Germantown, MD, USA), and single-stranded cDNA was subsequently synthesized. miRNA cDNA was synthesized by using a 3-step protocol that includes poly (A) tail synthesis using 2 μg RNA in the presence of 1.5 units of poly (A) polymerase, 10 times poly (A) buffer, and 10 times ATP in a reaction volume of 15 µl incubated at 37°C for 30 min, followed by the annealing of a universal poly (dT)-adaptor at 60°C for 5 min. Reverse transcription was conducted by means of RevertAid Reverse Transcriptase (Thermo Fisher Scientific) following the manufacturer’s instructions. To detect mRNA, total RNA was purified by using the RNeasy Mini Kit (Qiagen). Total RNA (1 µg) was reverse transcribed by using an iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA). The qRT-PCR was performed by using a Bio-Rad CFX Connect Real-time System (Bio-Rad), with the threshold cycle number determined by Bio-Rad CFX manager software v.3.0. mRNA expression was normalized to HPRT1, and miRNA expression was normalized to miR-16-5p (36, 37). The following primers were used for PCR analysis: miR-135b-5p 5′-GGTATGGCTT T TCAT TCCT-3′ (sense) and 5′-GCGAGCACAGAATTAATACGAC-3′ (anti-sense), and miR-16-5p 5′-TAGCAGCACGTAAATATTGGCG-3′ (sense), and 5′-GCGAGCACAGAATTAATACGAC-3′ (anti-sense), PMSS1 5′-CGTCCGGCAATAGCTGCCATAGT-3′ (sense) and 5′-GTAGGTCCTGCTACTGAAGCCTTA-3′ (anti-sense), and HPRT1 5′-TATGCCGAGGATTTGGAAAA-3′ (sense) and 5′-ACAGAGGGCCACAATGTGAT-3′ (anti-sense).
Luciferase reporter assay
We used the luciferase reporter construct, KLF4 wild-type 3′UTR lenti-luc vector, purchased from Applied Biological Materials. KLF4 mutant 3′UTR lenti-luc vector (without KLF4 3′UTR binding site) was generated by using the QuikChange Multi Site-Directed Mutagenesis Kit (Agilent, Santa Clara, CA, USA). The removal of the KLF4 3′UTR binding site on the KLF4 mutant 3′UTR lenti-luc vector was confirmed by sequencing. PolyJet reagent was used for transfection as directed by the manufacturer’s protocol. After transfection with miR-135b-5p mimic or control for 6 h, cells were transfected with KLF4 3′UTR luciferase reporter and β-galactosidase. Forty-eight hours later, luciferase and β-galactosidase activity were measured, as previously described (38). The firefly luciferase activity was normalized to β-galactosidase activity and expressed as relative luciferase units ± sem.
Western blot analysis
Cell lysates were lysed in RIPA buffer (Santa Cruz Biotechnology) containing Halt Protease and Phosphatase Inhibitors Cocktail (Thermo Fisher Scientific) and centrifuged at 13,000 rpm for 10 min at 4°C. Protein concentration was measured by using a Pierce BCA Protein Assay (ThermoFisher Scientific). Proteins were subjected to SDS/PAGE and transferred onto nitrocellulose membranes. Membranes were probed with specific antibodies and subsequently with HRP-conjugated secondary antibodies. β-actin was used as the loading control. Protein bands were detected by a commercial Immobilon Western Chemiluminescent HRP Substrate detection reagent (MilliporeSigma).
Public databases and survival data
KLF4 expression in human nontumor normal gastric tissues (n = 71), unclassified gastric adenocarcinomas (n = 27), diffuse-type gastric adenocarcinomas (n = 17), intestinal-type gastric adenocarcinomas (n = 89), and mixed-type gastric adenocarcinomas (n = 10) were analyzed from 3 different datasets, including GSE2109 (http://www.ncbi.nlm.nih.gov/geo), Chen et al. (39), and Wang et al. (40). Using Kaplan-Meier survival plots, KLF4 expression level and association with overall survival in 876 gastric cancer patients were analyzed using 6 different online datasets [http://kmplot.com/(410)]. These included GSE14210 (n = 146), GSE15459 (n = 200), GSE22377 (n = 43), GSE29272 (n = 268), GSE51105 (n = 94), and GSE62254 (n = 300).
Statistical analyses
All data are expressed as means ± sd from 3 independent experiments. Using GraphPad Prism software (GraphPad Software, San Diego, CA, USA), statistical significance was analyzed by using 1-way ANOVA, Student’s t test, and Kaplan-Meier analysis. Differences with values of P ≤ 0.05 were considered significant.
RESULTS
miR-135b-5p expression is up-regulated in human and mouse gastric cancer tissues
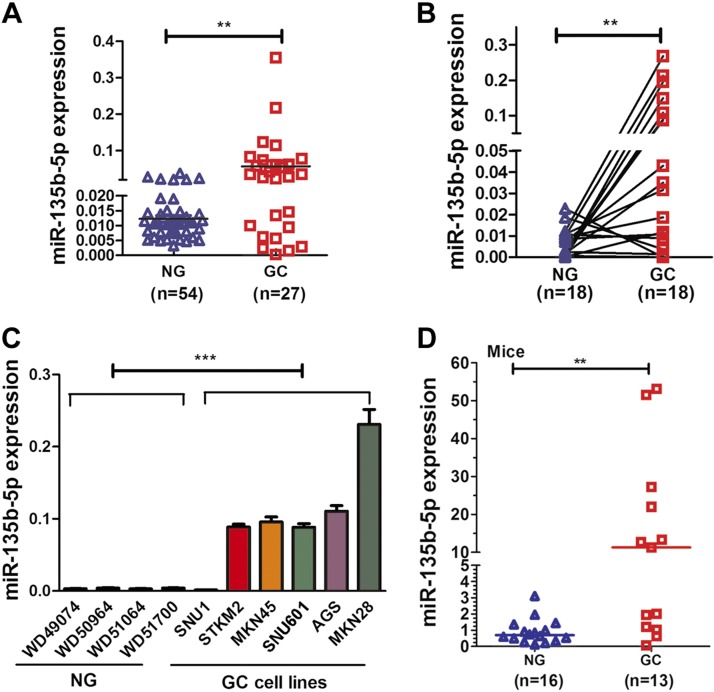
To investigate whether miR-135b-5p is aberrantly expressed in human gastric cancers, qRT-PCR was performed by using human gastric tissue samples. Significant overexpression of miR-135b-5p was detected in human gastric tissue samples (n = 27), compared with nontumor normal gastric tissue samples (n = 54) (P < 0.01) (Fig. 1A). Using paired tissue samples, there was a significant increase in the expression of miR-135b-5p in the cancerous tissue compared with its adjacent nontumor tissue (P < 0.01) (Fig. 1B). We also examined miR-135b-5p in 6 human gastric cancer cell lines, which exhibited overexpression of miR-135b-5p compared with normal gastric tissue samples (Fig. 1C). In addition, using gastric tissues from the Tff1-knockout mouse model, overexpression of miR-135b-5p was detected in gastric cancer tissue samples compared with normal gastric tissue samples (P < 0.01) (Fig. 1D). Collectively, these results indicate that miR-135b-5p up-regulation is a frequent finding in gastric cancer, suggesting its role in gastric tumorigenesis.
Figure 1.
miR-135b-5p expression levels are increased in gastric cancer (GC) tissue samples and cell lines. A) qRT-PCR data showing miR-135b-5p expression levels in 27 human GC and 54 nontumor normal gastric (NG) tissues samples. Scatter plots of gene expression levels with a bar indicating the median. B) qRT-PCR result of miR-135b-5p expression level in 18 pairs of matched human NG and GC samples. C) qRT-PCR data of miR-135b-5p expression in 4 human NG samples and 6 GC cell lines. D) qRT-PCR analysis of miR-135b-5p expression in wild-type normal (NG) and Tff1-knockout GC tissue samples. Mann-Whitney test. **P < 0.01, ***P < 0.001.
H. pylori infection enhances miR-135b-5p expression through activation of a NF-κB–signaling pathway
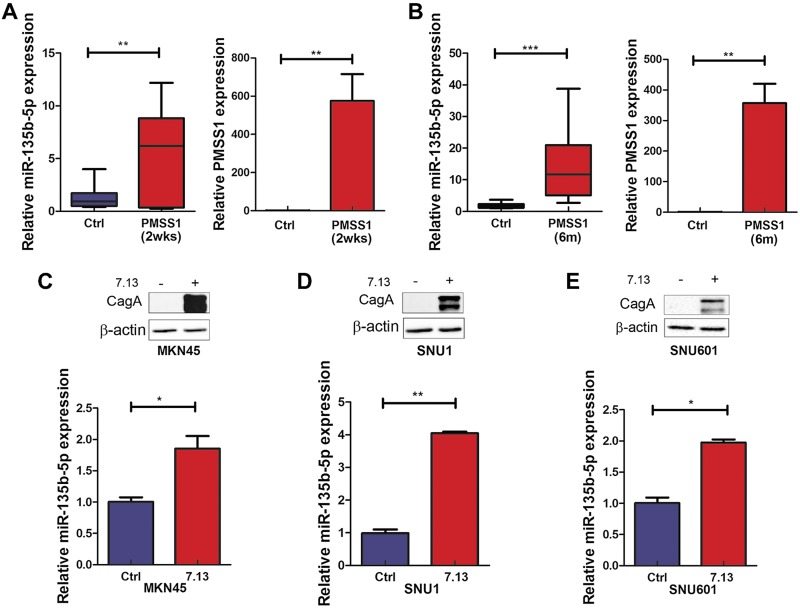
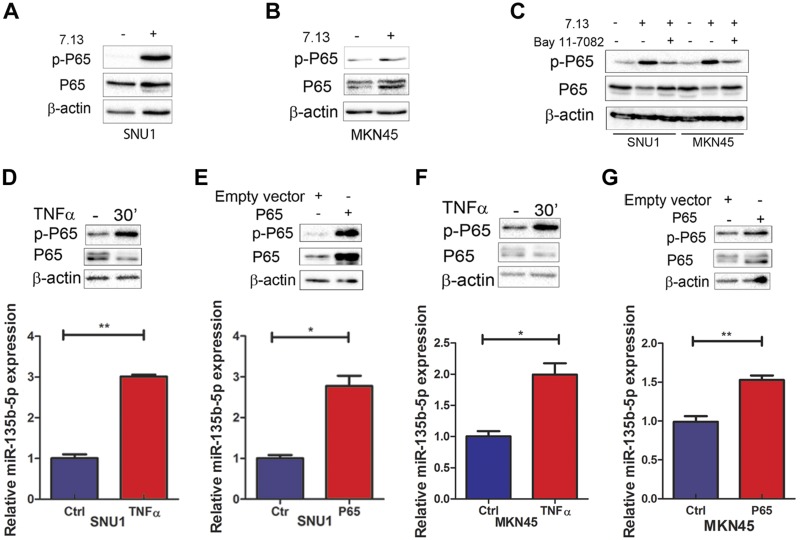
Because H. pylori infection plays a vital role in gastric carcinogenesis through colonizing the gastric mucosa and triggering chronic inflammation (6), we investigated if H. pylori and inflammatory signaling played a role in the induction of miR-135b-5p. Significant up-regulation of miR-135b-5p was detected in gastric tissues of the C57BL/6 mice, following infection with mouse-adapted H. pylori strain PMSS1 for 2 wk and 6 mo, compared with noninfected controls (Fig. 2A, B). Using in vitro cell models (MKN45, SNU1, and SNU601), we also found that miR-135b-5p was induced in response to infection with H. pylori strain 7.13 for 6 h (Fig. 2C–E). The H. pylori infection was confirmed by determining the levels of CagA using Western blot analysis. Cytokines affect tumor initiation and progression by activating proinflammatory signaling pathways and the regulation of host immune response to gastric cancer (6, 42). Infection with H. pylori is known to induce high levels of several cytokines such as TNF-α (7). Furthermore, the Tff1-knockout mouse model of gastric cancer has high levels of TNF-α with constitutive activation of NF-κB–p65 in gastric tissues (43). Indeed, H. pylori infection for 6 h promoted P65 phosphorylation (S536) in SNU1 and MKN45 (Fig. 3A, B). The NF-κB inhibitor Bay 11-7082 effectively abrogated the induction of P65 phosphorylation by H. pylori (Fig. 3C). Similar effects were obtained after treatment of cells with TNF-α or P65 overexpression vector (Fig. 3D–G). The treatment of cells with recombinant TNF-α or transient expression of the P65 subunit led to significant overexpression of miR-135b-5p, consistent with the levels of phosopho-P65.
Figure 2.
H. pylori infection induces miR-135b-5p expression in vitro and in vivo. A, B) qRT-PCR analysis of miR-135b-5p and PMSS1 expression levels with or without H. pylori strain PMSS1 infection for 2 wk (A) or 6 mo (B) in C57BL/6 mouse gastric tissue samples. C–E) Upper panels: Western blotting data of CagA and β-actin protein expression levels with or without H. pylori strain 7.13 infection for 6 h. Lower panels: miR-135b-5p expression with or without H. pylori strain 7.13 infection for 6 h in MKN45, SNU1, and SNU601, respectively. *P < 0.05, **P < 0.01, ***P < 0.001.
Figure 3.
H. pylori infection induces miR-135b-5p expression through activating the NF-κB–signaling pathway in human gastric cancer cells. A, B) Western blot analysis of phosphorylated P65 (S536), total P65, and β-actin protein expression levels in SNU1 (A) or MKN45 (B) cells, with or without H. pylori strain 7.13 infection for 24 h. C) Phosphorylated P65 (S536), total P65, and β-actin protein expression levels in control or after H. pylori 7.13 strain infection (24 h) in SNU1 or MKN45 cells with or without Bay 11-7082 treatment. D, E) Upper panels: Phosphorylated P65 (S536), total P65, and β-actin protein expression levels in SNU1 cells, 30 min after TNF-α treatment (D) or 48 h after transient overexpression of P65 vector (E). Lower panels: qRT-PCR analysis of miR-135b-5p expression levels in SNU1 cells with or without 6 h of TNF-α treatment (D) or 48 h after transient overexpression of P65 (E). F, G) Similar results in MKN45 cells, as in D and E. *P < 0.05, **P < 0.01.
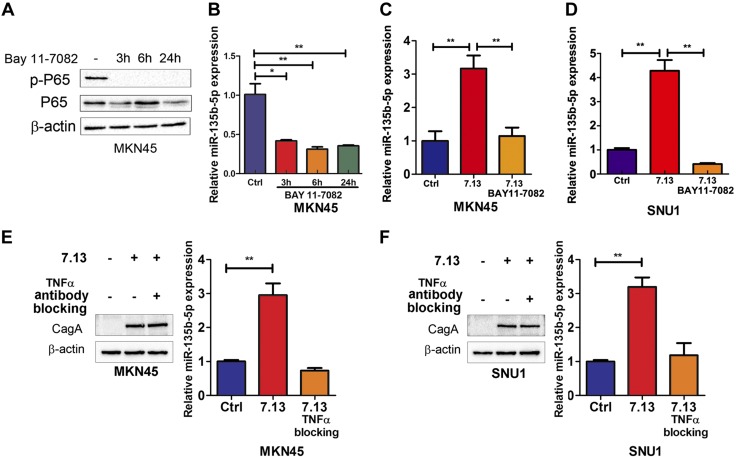
To determine whether activation of NF-κB signaling is an essential step in induction of miR-135b-5p, we treated MKN45 cells with Bay 11-7082, a specific inhibitor of NF-κB signaling (3, 6, and 24 h). Treatment with Bay 11-7082 significantly suppressed P65-induced expression of miR-135b-5p (Fig. 4A, B). Furthermore, Bay 11-7082 significantly abrogated miR-135b-5p induction in SNU1 and MKN45 cells after H. pylori infection for 24 h (Fig. 4C, D). Collectively, miR-135b-5p is induced by H. pylori or TNF-α through activation of a NF-κB–p65 signaling pathway. To further investigate whether miR-135b-5p induction is dependent on TNF-α, a neutralizing antibody of TNF-α was added to and incubated with MKN45 or SUN1 cells. Western blot data showed that CagA protein was clearly detected in MKN45 cells after H. pylori strain 7.13 infection for 24 h with or without TNF-α neutralizing antibody incubation (Fig. 4E, left panel). However, the induction of miR-135b-5p was abrogated by TNF-α neutralizing antibody incubation in MKN45 cells (Fig. 4E, right panel). Similar results were detected in SNU1 cells (Fig. 4F). These results suggest that miR-135b-5p expression is dependent on TNF-α and activation of NF-κB signaling in gastric cancer cells.
Figure 4.
NF-κB–signaling inhibition or TNF-α blocking abrogates H. pylori-induced miR-135b-5p expression in gastric cancer cells. A) The levels of phosphorylated P65 (S536), total P65, and β-actin are shown in MKN45 cells with or without Bay 11-7082 treatment (3, 6, and 24 h). B) qRT-PCR data of miR-135b-5p expression in the same cells as in A. C, D) miR-135b-5p expression levels are shown after H. pylori strain 7.13 infection for 24 h with or without BAY 11-7082 treatment in MKN45 (C) and SNU1 (D) cells. E) Left panel: Western blot analysis of CagA and β-actin in MKN45 cells incubated with TNF-α neutralizing antibody (100 ng/ml, 3 h before H. pylori infection) with or without H. pylori strain 7.13 infection for 24 h. Right panel: RT-PCR analysis of miR-135b-5p expression in MKN45 cells as in left panel. F) Similar results as in E are shown in SNU1 cells. *P < 0.05, **P < 0.01.
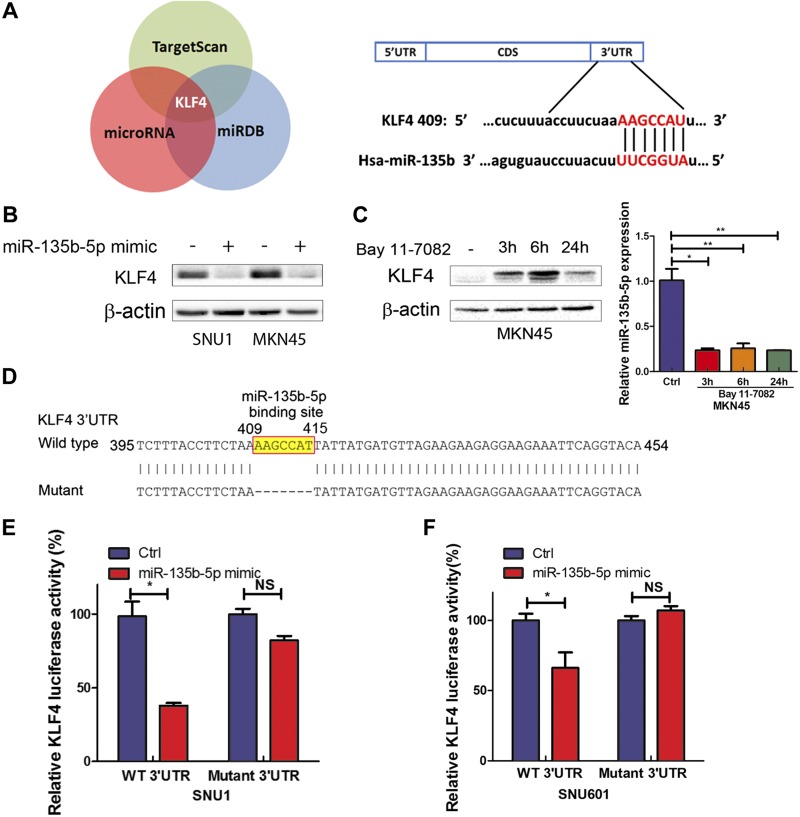
miR-135b-5p down-regulates KLF4 expression by directly targeting its 3′UTR in gastric cancer cells
miRNAs control biologic processes in cancer cells by negatively regulating expression of their target genes. To search miR-135b-5p targets, 3 online databases (TargetScan.org, microRNA.org, and miRDB.org) were analyzed. KLF4 was identified as a potential target for miR-135b-5p, containing a specific binding site in its 3′UTR region (Fig. 5A). We next investigated if miR-135b-5p can indeed suppress the levels of KLF4. As shown in Fig. 5B, transient transfection with miR-135b-5p mimic (30 pM for 48 h) led to notable down-regulation of KLF4 protein levels in SNU1 and MKN45. To confirm the link between NF-κB, miR-135b-5p, and KLF4, MKN45 cells were treated with Bay 11-7082. This treatment led to an increase in the levels of KLF4 (Fig. 5C, left panel) with down-regulation of miR-135b-5p (Fig. 5C, right panel). To specifically investigate miR-135b-5p binding on KLF4, the binding site of miR-135b-5p on KLF4 3′UTR was deleted from 409 to 415 (Fig. 5D) and cloned into pLenti-Luc to generate a mutant luciferase reporter. Cotransfection of miR-135b-5p mimic with KLF4 wild-type 3′UTR led to a significant reduction in luciferase levels (P < 0.05), indicating the binding of miR-135b-5p to KLF4 3′UTR (Fig. 5E, F). Conversely, transfection of miR-135b-5p with mutant 3′UTR luciferase reporter did not produce significant changes in luciferase activity. These results support the notion that this KLF4 3′UTR region is indeed a functional binding site for miR-135b-5p.
Figure 5.
miR-135b-5p down-regulates KLF4 expression by directly targeting its 3′UTR in gastric cancer cells. A) KLF4 is a predicted target gene of miR-135b-5p in 3 different databases (left panel); a schematic drawing shows the specific binding sites of miR-135b-5p on KLF4 3′UTR (right panel). B) KLF4 and β-actin protein expression levels in control or after transient transfection of miR-135b-5p mimic in SNU1 or MKN45 cells. C) KLF4 and β-actin protein levels in MKN45 cells treated with control or Bay 11-7082 (3, 6, or 24 h). D) A schematic drawing shows miR-135b-5p binding sites that were deleted on KLF4 3′UTR (from 409 to 415 bp) to construct a 3′UTR mutant reporter. E, F) KLF4 3′UTR luciferase activity reporter assay analysis in SNU1 (E) or SNU601 (F) cells with wild-type or mutant KLF4 3′UTR reporters. NS, not significant. *P < 0.05, **P < 0.01.
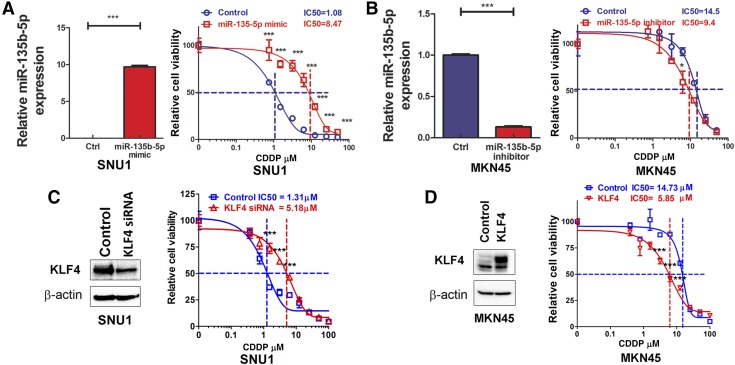
miR-135b-5p overexpression or KLF4 down-regulation lead to cisplatin resistance in gastric cancer cells
Because gastric cancers are frequently resistant to chemotherapy, we postulated that overexpression of miR-135b-5p could be one of the molecular changes that promote chemotherapeutic resistance. To test this hypothesis, we investigated the role of miR-135b-5p expression in conferring cisplatin resistance in gastric cancer cells. SNU1 gastric cancer cells had the lowest level of miR-135b-5p expression (Fig. 1C). We tested the sensitivity of these cells to cisplatin by using a range from 0 to 50 µM (0, 0.78, 1.56, 3.125, 6.25, 12.5, 25, and 50 µM), after overexpression miR-135b-5p mimic 30 pM, compared with the control. Cell viability was determined by CellTiter-Glo Luminescent cell viability assay, and qRT-PCR was performed to confirm miR-135b-5p expression. Overexpression of miR-135b-5p in SNU1 led to an 8-fold increase in the IC50 value for cisplatin, compared with the control (P < 0.001) (Fig. 6A). To further corroborate these findings, we used MKN45 cells, which have high levels of miR-135b-5p (Fig. 1C). Indeed, MKN45 cells were more resistant to cisplatin than SNU1 (IC50: 14.5 µM vs. 1.08 µM). The use of the miR-135b-5p inhibitor 90 pM led to a significant reduction in cisplatin IC50 in MKN45, from 14.5 to 9.4 µM (Fig. 6B). To further investigate the potential role of KLF4 in cisplatin resistance, the direct downstream target of miR-135b-5p, cisplatin IC50 values were analyzed in SUN1 cells with or without KLF4 knockdown. Our Western blot data validated the knock down of KLF4 with siRNA transfection (Fig. 6C, left panel). In the meantime, the ATP-GLO assay demonstrated a dramatic increase in cisplatin IC50 in SNU1 cells from 1.31 to 5.18 µM (Fig. 6C, right panel). Conversely, KLF4 transient overexpression obviously decreased cisplatin IC50 in MKN45 cells, from 14.73 to 5.85 µM (Fig. 6D). Taken together, these results indicate that the miR-135b-5p–KLF4 axis plays an important role in cisplatin resistance in gastric cancer cells.
Figure 6.
miR-135b-5p overexpression or KLF4 down-regulation leads to cisplatin resistance in gastric cancer cells. A) Left panel: qRT-PCR analysis of miR-135b-5p expression levels in SNU1 control or after transient transfection with miR-135b-5p mimic. Right panel: ATP-Glo cell viability assay data of cells in the left panel following treatment with cisplatin (CDDP). B) Left panel, qRT-PCR analysis of miR-135b-5p expression level in MKN45 control or after transient transfection with miR-135b-5p inhibitor. Right panel, ATP-Glo cell viability assay data of cells in the left panel, following treatment with CDDP. C) Left panel, Western blot analysis of KLF4 and actin expression in SNU1 cells with or without KLF4 siRNA knockdown. Right panel, ATP-Glo cell viability assay data of the cells in left panel following treatment with CDDP. D) Left panel, Western blot analysis of KLF4 and actin expression in MKN45 cells with or without KLF4 transient overexpression. Right panel, ATP-Glo cell viability assay data of cells in the left panel following treatment with CDDP. *P < 0.05, ***P < 0.001.
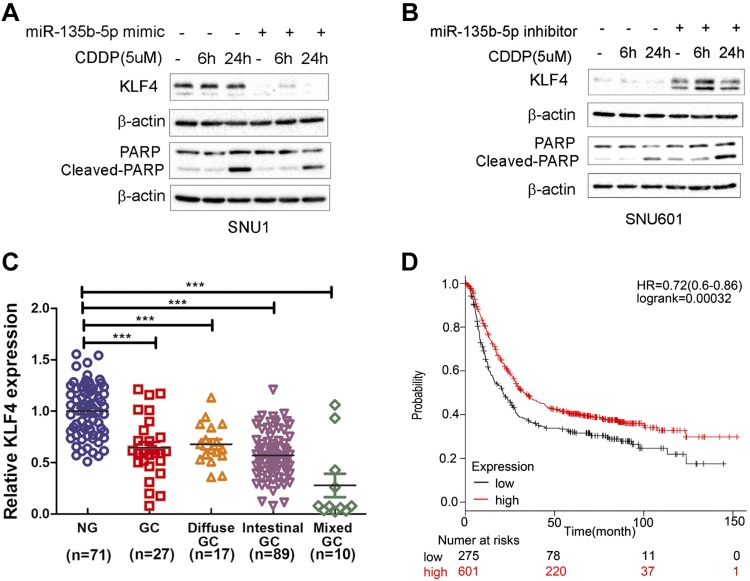
miR-135b-5p protects gastric cancer cells from cisplatin-induced apoptosis
Drug resistance often prevents tumor cells from undergoing programmed cell death or apoptosis (44, 45). We investigated the role of miR-135b-5p in suppression of apoptosis by measuring the levels of cleaved poly(ADP-ribose) polymerase (PARP). After treatment with cisplatin for 6 and 24 h, overexpression of miR-135b-5p mimic reduced the levels of KLF4 and led to a notable reduction in the levels of cleaved PARP (Fig. 7A). We observed no significant changes in the level of cleaved-PARP after overexpression of miR-135b-5p in SNU1 cells. Conversely, the use of the miR-135b-5p inhibitor led to an increase in KLF4 protein levels with a notable increase in the levels of cleaved PARP after treatment with cisplatin (Fig. 7B). These results suggest that miR-135b-5p affects drug resistance through regulation of the levels of KLF4 and cleaved PARP.
Figure 7.
miR-135b-5p promotes drug resistance through regulation of the levels of KLF4 and cleaved PARP. High expression level of KLF4 predicts favorable prognostic outcome. A) Western blot data of KLF4, cleaved PARP, PARP, and β-actin protein expression levels in SNU1 control or after transient transfection with miR-135b-5p mimic and cisplatin (CDDP) treatment (0, 6, and 24 h). B) KLF4, cleaved PARP, PARP, and β-actin protein expression levels in SNU601 control or after transient transfection with miR-135b-5p inhibitor and CDDP treatment (0, 6, and 24 h). C) qRT-PCR analysis of KLF4 expression in human nontumor normal gastric tissues, unclassified gastric cancer (GC), diffuse-type GC, intestinal-type GC, and mixed-type GC. D) Kaplan-Meier curves of 876 GC patients’ survival data with low (n = 275) or high (n = 601) expression levels of KLF4 mRNA (P < 0.001, log-rank test). KLF4 mRNA low or high expression levels were determined by using the median of all samples’ KLF4 mRNA expression level. Original data sources are listed under “Public databases and survival data” in the Materials and Methods section. ***P < 0.001.
KLF4 down-regulation is observed in gastric cancer tissue samples, correlating with poor patient survival
We analyzed the levels of KLF4 using 3 public datasets of gastric cancer (as described in Materials and Methods). KLF4 expression was low in the 3 datasets; unclassified gastric adenocarcinoma (n = 27), diffuse-type gastric adenocarcinoma (n = 17), intestinal-type gastric adenocarcinoma (n = 89), and mixed-type gastric adenocarcinoma (n = 10), compared with normal gastric tissue samples (n = 71) (Fig. 7C). We also found that low expression levels of KLF4 in human primary gastric cancers correlated with poor survival (P < 0.001) (Fig. 7D). We therefore concluded that regulation of KLF4 expression by miR-135b-5p may have a strong impact on cisplatin resistance and chemotherapeutic response.
DISCUSSION
In the present study, we investigated the molecular and biologic functions of miR-135b-5p in gastric cancer using human tissues, Tff1- knockout mouse samples, and in vitro cell models of gastric cancer. We showed that overexpression of miR-135b-5p can be induced by proinflammatory signaling as a result of H. pylori infection or TNF-α. We also found that miR-135b-5p can directly bind to 3′ UTR and suppress KLF4 expression, leading to resistance to cisplatin. These findings suggest a novel link between miR-135b-5p expression, infection, inflammation, and cisplatin resistance.
H. pylori is a gram-negative, helical-shaped bacteria classified by the World Health Organization as a type 1 carcinogen since 1994 (46). H. pylori infection is considered a major risk factor and an underlying cause for gastric cancer. H. pylori bacteria have the ability to specifically colonize the human gastric epithelium and trigger inflammatory responses, characterized by increased expression of proinflammatory cytokines, including TNF-α (6). TNF-α is an essential cytokine involved in the activation of NF-κB signaling that promotes gastric cancer development and progression (47, 48). Earlier reports have shown that H. pylori infection can regulate miRNA expression (49) and promote gastric cancer cell proliferation and invasion (21, 22). One study indicated that miR-135b-5p can promote proliferation and migration of endothelial cells and vascular smooth muscle cells (50). We found that miR-135b-5p is induced by H. pylori infection through activation of NF-κB signaling. In addition, gastric tissues from the Tff1-knockout mouse model that developed chronic inflammation, high levels of TNF-α, and activation of NF-κB in gastric mucosa exhibited high levels of miR-135b-5p in neoplastic gastric glands. These data suggest that NF-κB is also mediating miR-135b-5p expression in vivo. The pharmacologic inhibition of NF-κB by Bay 11-7082 in gastric cancer cells suppressed the expression levels of miR-135b-5p, confirming the link between NF-κB and miR-135b-5p. We further confirmed that TNF-α is essential for miR-135b-5p induction by H. pylori in gastric cancer cells, indicating a key role of TNF-α in regulating miR-135b-5p. These in vitro and in vivo data support the notion that expression of miR-135b-5p is regulated by inflammatory response and activation of NF-κB in gastric cancer cells.
Resistance to chemotherapeutic interventions is a challenging clinical problem in gastric cancer. Earlier studies have shown that activation of NF-κB is associated with resistance to therapy and poor clinical outcome (51, 52). Previous studies have suggested that overexpression of miR-135b-5p in non–small cell lung cancer and colorectal cancer can lead to chemotherapeutic resistance to cisplatin and 5-FU (19, 53). Our findings indicate that high levels of miR-135b-5p promote resistance to cisplatin treatment in gastric cancer cells. Of note, in support of our findings, a log-rank analysis indicated that low expression of miR-135b-5p is associated with a better prognosis (54). We investigated the downstream effector of miR-135-5p in gastric cancer. An analysis of online databases revealed that miR-135b-5p harbors complementary sequences to the 3′UTR of KLF4, suggesting KLF4 as a downstream target. KLF4 expression is down-regulated in gastric cancers compared with normal tissues (27, 28, 55, 56). We confirmed that miR-135b-5p directly binds to KLF4 3′UTR between 409 and 415 bp. In addition, we showed that high expression of KLF4 predicted a better survival outcome in patients with gastric cancer. We further confirmed the functions of KLF4 in cisplatin resistance, suggesting its role as a potential tumor suppressor gene in gastric cancer cells. It is worth mentioning that recent studies suggest the presence of additional miRNAs, which can target KLF4 expression in gastric cancer cells (23, 56, 57). Taken together, these findings indicate that regulation of KLF4 is a critical step in gastric cancer which can be mediated by several coexisting molecular mechanisms in gastric cancer cells. We must acknowledge that miR-135b-5p, similar to all other miRNAs, has several additional downstream targets that may augment its oncogenic activity and therapeutic resistance.
In summary, we propose a novel signaling axis between H. pylori infection, inflammation, and resistance to therapy, highly relevant to the etiology of gastric cancer. We showed that infection with H. pylori promotes inflammation and activation of NF-κB, which in turn induces miR-135b-5p expression. High levels of miR-135b-5p suppress KLF4 and mediate cisplatin resistance. This novel H. pylori–NF-κB–miR-135b-5p–KLF4 axis provides a new paradigm in gastric carcinogenesis. Targeting miR-135b-5p could therefore be a novel therapeutic opportunity to overcome cisplatin resistance in patients with gastric cancer.
ACKNOWLEDGMENTS
This study was supported by the U.S. National Institutes of Health, National Cancer Institute (R01CA93999 and R01CA177372), a Research Career Scientist Award (1IK6BX003787), and a merit award (I01BX001179) from the U.S. Department of Veterans Affairs (to W.E.-R.). The results were also supported by the Biostatistics and Bioinformatics Shared Resource at the Sylvester Comprehensive Cancer Center, University of Miami. The contents of this work are solely the responsibility of the authors and do not necessarily represent the official views of the Department of Veterans Affairs, the U.S. National Institutes of Health, or the University of Miami. The authors declare no conflicts of interest.
Glossary
- CagA
cytotoxin-associated gene A
- HRP
horseradish peroxidase
- KLF4
Krüppel-like factor 4
- miRNA
microRNA
- PARP
poly(ADP-ribose) polymerase
- qRT-PCR
quantitative RT-PCR
- siRNA
small interfering RNA
- Tff1
trefoil factor 1
AUTHORS CONTRIBUTIONS
S. Zhang and W. El-Rifai designed the study concept, supervised the research, and provided the resources; S. Zhu and H. Lu assisted in troubleshooting the experiments and interpretation of results; L. Shao and Z. Chen conducted the research experiments and contributed to the manuscript preparation; M. Soutto provided mouse tissue samples and assisted in mouse experiments; and J. Romero-Gallo and R. Peek assisted in design of the H. pylori experiments and provided the H. pylori bacteria.
REFERENCES
- 1.Zhu S., Soutto M., Chen Z., Peng D., Romero-Gallo J., Krishna U. S., Belkhiri A., Washington M. K., Peek R., El-Rifai W. (2017) Helicobacter pylori-induced cell death is counteracted by NF-κB-mediated transcription of DARPP-32. Gut 66, 761–762 10.1136/gutjnl-2016-312141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferlay J., Soerjomataram I., Dikshit R., Eser S., Mathers C., Rebelo M., Parkin D. M., Forman D., Bray F. (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 136, E359–E386 10.1002/ijc.29210 [DOI] [PubMed] [Google Scholar]
- 3.Noto J. M., Peek R. M. (2012) The role of microRNAs in Helicobacter pylori pathogenesis and gastric carcinogenesis. Front. Cell. Infect. Microbiol. 1, 21 10.3389/fcimb.2011.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moss S. F. (2016) The clinical evidence linking Helicobacter pylori to gastric cancer. Cell. Mol. Gastroenterol. Hepatol. 3, 183–191 10.1016/j.jcmgh.2016.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamajima N., Ito H., Matsuo K., Tajima K., Tominaga S. (2002) Helicobacter pylori seropositivity, the interleukin 1B polymorphism, and smoking among first-visit outpatients. Asian Pac. J. Cancer Prev. 3, 23–28 [PubMed] [Google Scholar]
- 6.Bagheri V., Memar B., Momtazi A. A., Sahebkar A., Gholamin M., Abbaszadegan M. R. (2018) Cytokine networks and their association with Helicobacter pylori infection in gastric carcinoma. J. Cell. Physiol. 233, 2791–2803https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28121015&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 7.Rad R., Gerhard M., Lang R., Schöniger M., Rösch T., Schepp W., Becker I., Wagner H., Prinz C. (2002) The Helicobacter pylori blood group antigen-binding adhesin facilitates bacterial colonization and augments a nonspecific immune response. J. Immunol. 168, 3033–3041 10.4049/jimmunol.168.6.3033 [DOI] [PubMed] [Google Scholar]
- 8.Rossi A. F., Cadamuro A. C., Biselli-Périco J. M., Leite K. R., Severino F. E., Reis P. P., Cordeiro J. A., Silva A. E. (2016) Interaction between inflammatory mediators and miRNAs in Helicobacter pylori infection. Cell. Microbiol. 18, 1444–1458 10.1111/cmi.12587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsu H., Xiong J., Goeddel D. V. (1995) The TNF receptor 1-associated protein TRADD signals cell death and NF-kappa B activation. Cell 81, 495–504 10.1016/0092-8674(95)90070-5 [DOI] [PubMed] [Google Scholar]
- 10.Hayden M. S., Ghosh S. (2014) Regulation of NF-κB by TNF family cytokines. Semin. Immunol. 26, 253–266 10.1016/j.smim.2014.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dou L., Wang S., Sun L., Huang X., Zhang Y., Shen T., Guo J., Man Y., Tang W., Li J. (2017) Mir-338-3p mediates Tnf-A-induced hepatic insulin resistance by targeting PP4r1 to regulate PP4 expression. Cell. Physiol. Biochem. 41, 2419–2431 [DOI] [PubMed] [Google Scholar]
- 12.Yang L., Tang L., Dai F., Meng G., Yin R., Xu X., Yao W. (2017) Raf-1/CK2 and RhoA/ROCK signaling promote TNF-α-mediated endothelial apoptosis via regulating vimentin cytoskeleton. Toxicology 389, 74–84 10.1016/j.tox.2017.07.010 [DOI] [PubMed] [Google Scholar]
- 13.Yu Y., Cao F., Ran Q., Wang F. (2017) Long non-coding RNA Gm4419 promotes trauma-induced astrocyte apoptosis by targeting tumor necrosis factor α. Biochem. Biophys. Res. Commun. 491, 478–485 10.1016/j.bbrc.2017.07.021 [DOI] [PubMed] [Google Scholar]
- 14.Tse M. C. L., Herlea-Pana O., Brobst D., Yang X., Wood J., Hu X., Liu Z., Lee C. W., Zaw A. M., Chow B. K. C., Ye K., Chan C. B. (2017) Tumor necrosis factor-α promotes phosphoinositide 3-kinase enhancer A and AMP-activated protein kinase interaction to suppress lipid oxidation in skeletal muscle. Diabetes 66, 1858–1870 10.2337/db16-0270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krol J., Loedige I., Filipowicz W. (2010) The widespread regulation of microRNA biogenesis, function and decay. Nat. Rev. Genet. 11, 597–610 10.1038/nrg2843 [DOI] [PubMed] [Google Scholar]
- 16.Hayes J., Peruzzi P. P., Lawler S. (2014) MicroRNAs in cancer: biomarkers, functions and therapy. Trends Mol. Med. 20, 460–469 10.1016/j.molmed.2014.06.005 [DOI] [PubMed] [Google Scholar]
- 17.Liu L., He J., Wei X., Wan G., Lao Y., Xu W., Li Z., Hu H., Hu Z., Luo X., Wu J., Xie W., Zhang Y., Xu N. (2017) MicroRNA-20a-mediated loss of autophagy contributes to breast tumorigenesis by promoting genomic damage and instability. Oncogene 36, 5874–5884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gulyaeva L. F., Kushlinskiy N. E. (2016) Regulatory mechanisms of microRNA expression. J. Transl. Med. 14, 143 10.1186/s12967-016-0893-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Su W., Mo Y., Wu F., Guo K., Li J., Luo Y., Ye H., Guo H., Li D., Yang Z. (2016) miR-135b reverses chemoresistance of non-small cell lung cancer cells by downregulation of FZD1. Biomed. Pharmacother. 84, 123–129 [DOI] [PubMed] [Google Scholar]
- 20.Nezu Y., Hagiwara K., Yamamoto Y., Fujiwara T., Matsuo K., Yoshida A., Kawai A., Saito T., Ochiya T. (2016) miR-135b, a key regulator of malignancy, is linked to poor prognosis in human myxoid liposarcoma. Oncogene 35, 6177–6188 10.1038/onc.2016.157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu M., Huang Y., Sun W., Li P., Li L., Li L. (2018) miR-135b-5p promotes gastric cancer progression by targeting CMTM3. Int. J. Oncol. 52, 589–598 [DOI] [PubMed] [Google Scholar]
- 22.Darnet S., Moreira F. C., Hamoy I. G., Burbano R., Khayat A., Cruz A., Magalhães L., Silva A., Santos S., Demachki S., Assumpção M., Assumpção P., Ribeiro-Dos-Santos Â. (2015) High-throughput sequencing of miRNAs reveals a tissue signature in gastric cancer and suggests novel potential biomarkers. Bioinform. Biol. Insights 9(Suppl 1), 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng J., Liu Y., Qiao Y., Zhang L., Lu S. (2017) miR-103 promotes proliferation and metastasis by targeting KLF4 in gastric cancer. Int. J. Mol. Sci. 18 10.3390/ijms18050910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu S., Yang H., Chen Y., He B., Chen Q. (2016) Krüppel-like factor 4 enhances sensitivity of cisplatin to lung cancer cells and inhibits regulating epithelial-to-mesenchymal transition. Oncol. Res. 24, 81–87 10.3727/096504016X14597766487717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen H. F., Wu K. J. (2016) Endothelial transdifferentiation of tumor cells triggered by the Twist1-Jagged1-KLF4 axis: relationship between cancer stemness and angiogenesis. Stem Cells Int. 2016, 6439864 10.1155/2016/6439864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leng Z., Tao K., Xia Q., Tan J., Yue Z., Chen J., Xi H., Li J., Zheng H. (2013) Krüppel-like factor 4 acts as an oncogene in colon cancer stem cell-enriched spheroid cells. PLoS One 8, e56082 10.1371/journal.pone.0056082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hashimoto I., Nagata T., Sekine S., Moriyama M., Shibuya K., Hojo S., Matsui K., Yoshioka I., Okumura T., Hori T., Shimada Y., Tsukada K. (2017) Prognostic significance of KLF4 expression in gastric cancer. Oncol. Lett. 13, 819–826 10.3892/ol.2016.5499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei D., Gong W., Kanai M., Schlunk C., Wang L., Yao J. C., Wu T. T., Huang S., Xie K. (2005) Drastic down-regulation of Krüppel-like factor 4 expression is critical in human gastric cancer development and progression. Cancer Res. 65, 2746–2754 10.1158/0008-5472.CAN-04-3619 [DOI] [PubMed] [Google Scholar]
- 29.Mo D., Fang H., Niu K., Liu J., Wu M., Li S., Zhu T., Aleskandarany M. A., Arora A., Lobo D. N., Madhusudan S., Balajee A. S., Chi Z., Zhao Y. (2016) Human helicase RECQL4 drives cisplatin resistance in gastric cancer by activating an AKT-YB1-MDR1 signaling pathway. Cancer Res. 76, 3057–3066 10.1158/0008-5472.CAN-15-2361 [DOI] [PubMed] [Google Scholar]
- 30.Tanida S., Mizoshita T., Ozeki K., Tsukamoto H., Kamiya T., Kataoka H., Sakamuro D., Joh T. (2012) Mechanisms of cisplatin-induced apoptosis and of cisplatin sensitivity: potential of BIN1 to act as a potent predictor of cisplatin sensitivity in gastric cancer treatment. Int. J. Surg. Oncol. 2012, 862879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gately D. P., Howell S. B. (1993) Cellular accumulation of the anticancer agent cisplatin: a review. Br. J. Cancer 67, 1171–1176 10.1038/bjc.1993.221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Soutto M., Belkhiri A., Piazuelo M. B., Schneider B. G., Peng D., Jiang A., Washington M. K., Kokoye Y., Crowe S. E., Zaika A., Correa P., Peek R. M., Jr., El-Rifai W. (2011) Loss of TFF1 is associated with activation of NF-κB-mediated inflammation and gastric neoplasia in mice and humans. J. Clin. Invest. 121, 1753–1767 10.1172/JCI43922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin C. C., Liu L. Z., Addison J. B., Wonderlin W. F., Ivanov A. V., Ruppert J. M. (2011) A KLF4-miRNA-206 autoregulatory feedback loop can promote or inhibit protein translation depending upon cell context. Mol. Cell. Biol. 31, 2513–2527 10.1128/MCB.01189-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Brien D. P., Romero-Gallo J., Schneider B. G., Chaturvedi R., Delgado A., Harris E. J., Krishna U., Ogden S. R., Israel D. A., Wilson K. T., Peek R. M., Jr (2008) Regulation of the Helicobacter pylori cellular receptor decay-accelerating factor. J. Biol. Chem. 283, 23922–23930 10.1074/jbc.M801144200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamashita A., Soga Y., Iwamoto Y., Yoshizawa S., Iwata H., Kokeguchi S., Takashiba S., Nishimura F. (2007) Macrophage-adipocyte interaction: marked interleukin-6 production by lipopolysaccharide. Obesity (Silver Spring) 15, 2549–2552 10.1038/oby.2007.305 [DOI] [PubMed] [Google Scholar]
- 36.Rinnerthaler G., Hackl H., Gampenrieder S. P., Hamacher F., Hufnagl C., Hauser-Kronberger C., Zehentmayr F., Fastner G., Sedlmayer F., Mlineritsch B., Greil R. (2016) miR-16-5p is a stably-expressed housekeeping microRNA in breast cancer tissues from primary tumors and from metastatic sites. Int. J. Mol. Sci. 17 10.3390/ijms17020156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Floris I., Billard H., Boquien C. Y., Joram-Gauvard E., Simon L., Legrand A., Boscher C., Rozé J. C., Bolaños-Jiménez F., Kaeffer B. (2015) MiRNA analysis by quantitative PCR in preterm human breast milk reveals daily fluctuations of hsa-miR-16-5p. PLoS One 10, e0140488 10.1371/journal.pone.0140488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smale S. T. (2010) Beta-galactosidase assay. Cold Spring Harb. Protoc. 2010, pdb.prot5423. [DOI] [PubMed] [Google Scholar]
- 39.Chen X., Leung S. Y., Yuen S. T., Chu K. M., Ji J., Li R., Chan A. S., Law S., Troyanskaya O. G., Wong J., So S., Botstein D., Brown P. O. (2003) Variation in gene expression patterns in human gastric cancers. Mol. Biol. Cell 14, 3208–3215 10.1091/mbc.e02-12-0833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Q., Wen Y. G., Li D. P., Xia J., Zhou C. Z., Yan D. W., Tang H. M., Peng Z. H. (2012) Upregulated INHBA expression is associated with poor survival in gastric cancer. Med. Oncol. 29, 77–83 10.1007/s12032-010-9766-y [DOI] [PubMed] [Google Scholar]
- 41.Lánczky A., Nagy Á., Bottai G., Munkácsy G., Szabó A., Santarpia L., Győrffy B. (2016) miRpower: a web-tool to validate survival-associated miRNAs utilizing expression data from 2178 breast cancer patients. Breast Cancer Res. Treat. 160, 439–446 10.1007/s10549-016-4013-7 [DOI] [PubMed] [Google Scholar]
- 42.Seruga B., Zhang H., Bernstein L. J., Tannock I. F. (2008) Cytokines and their relationship to the symptoms and outcome of cancer. Nat. Rev. Cancer 8, 887–899 10.1038/nrc2507 [DOI] [PubMed] [Google Scholar]
- 43.Soutto M., Chen Z., Katsha A. M., Romero-Gallo J., Krishna U. S., Piazuelo M. B., Washington M. K., Peek R. M., Jr., Belkhiri A., El-Rifai W. M. (2015) Trefoil factor 1 expression suppresses Helicobacter pylori-induced inflammation in gastric carcinogenesis. Cancer 121, 4348–4358 10.1002/cncr.29644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilson T. R., Johnston P. G., Longley D. B. (2009) Anti-apoptotic mechanisms of drug resistance in cancer. Curr. Cancer Drug Targets 9, 307–319 10.2174/156800909788166547 [DOI] [PubMed] [Google Scholar]
- 45.Hanahan D., Weinberg R. A. (2000) The hallmarks of cancer. Cell 100, 57–70 10.1016/S0092-8674(00)81683-9 [DOI] [PubMed] [Google Scholar]
- 46.Bateson M. C. (1994) Helicobacter pylori infection. Gut 35, 716–717 10.1136/gut.35.5.716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oshima H., Ishikawa T., Yoshida G. J., Naoi K., Maeda Y., Naka K., Ju X., Yamada Y., Minamoto T., Mukaida N., Saya H., Oshima M. (2014) TNF-α/TNFR1 signaling promotes gastric tumorigenesis through induction of Noxo1 and Gna14 in tumor cells. Oncogene 33, 3820–3829 10.1038/onc.2013.356 [DOI] [PubMed] [Google Scholar]
- 48.Chen G., Tang N., Wang C., Xiao L., Yu M., Zhao L., Cai H., Han L., Xie C., Zhang Y. (2017) TNF-α-inducing protein of Helicobacter pylori induces epithelial-mesenchymal transition (EMT) in gastric cancer cells through activation of IL-6/STAT3 signaling pathway. Biochem. Biophys. Res. Commun. 484, 311–317 10.1016/j.bbrc.2017.01.110 [DOI] [PubMed] [Google Scholar]
- 49.Matsushima K., Isomoto H., Inoue N., Nakayama T., Hayashi T., Nakayama M., Nakao K., Hirayama T., Kohno S. (2011) MicroRNA signatures in Helicobacter pylori-infected gastric mucosa. Int. J. Cancer 128, 361–370 10.1002/ijc.25348 [DOI] [PubMed] [Google Scholar]
- 50.Xu Z., Han Y., Liu J., Jiang F., Hu H., Wang Y., Liu Q., Gong Y., Li X. (2015) MiR-135b-5p and MiR-499a-3p promote cell proliferation and migration in atherosclerosis by directly targeting MEF2C. Sci. Rep. 5, 12276 10.1038/srep12276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huan Y., Wu D., Zhou D., Sun B., Li G. (2015) DBC1 promotes anoikis resistance of gastric cancer cells by regulating NF-κB activity. Oncol. Rep. 34, 843–849 10.3892/or.2015.4007 [DOI] [PubMed] [Google Scholar]
- 52.Kwon O. H., Kim J. H., Kim S. Y., Kim Y. S. (2014) TWEAK/Fn14 signaling mediates gastric cancer cell resistance to 5-fluorouracil via NF-κB activation. Int. J. Oncol. 44, 583–590 10.3892/ijo.2013.2211 [DOI] [PubMed] [Google Scholar]
- 53.Liu B., Liu Y., Zhao L., Pan Y., Shan Y., Li Y., Jia L. (2017) Upregulation of microRNA-135b and microRNA-182 promotes chemoresistance of colorectal cancer by targeting ST6GALNAC2 via PI3K/AKT pathway. Mol. Carcinog. 56, 2669–2680 [DOI] [PubMed] [Google Scholar]
- 54.Gao S., Zhou F., Zhao C., Ma Z., Jia R., Liang S., Zhang M., Zhu X., Zhang P., Wang L., Su F., Zhao J., Liu G., Peng B., Feng X. (2016) Gastric cardia adenocarcinoma microRNA profiling in Chinese patients. Tumour Biol. 37, 9411–9422 10.1007/s13277-016-4824-5 [DOI] [PubMed] [Google Scholar]
- 55.Yan C., Yu J., Liu Y., Kang W., Ma Z., Zhou L. (2015) MiR-32 promotes gastric carcinoma tumorigenesis by targeting Kruppel-like factor 4. Biochem. Biophys. Res. Commun. 467, 913–920 10.1016/j.bbrc.2015.10.044 [DOI] [PubMed] [Google Scholar]
- 56.Zhao L., Han T., Li Y., Sun J., Zhang S., Liu Y., Shan B., Zheng D., Shi J. (2017) The lncRNA SNHG5/miR-32 axis regulates gastric cancer cell proliferation and migration by targeting KLF4. FASEB J. 31, 893–903 10.1096/fj.201600994R [DOI] [PubMed] [Google Scholar]
- 57.Ma Z., Chen Y., Min L., Li L., Huang H., Li J., Yan Q., Song P., Dai L., Yao X. (2015) Augmented miR-10b expression associated with depressed expression of its target gene KLF4 involved in gastric carcinoma. Int. J. Clin. Exp. Pathol. 8, 5071–5079 [PMC free article] [PubMed] [Google Scholar]