Abstract
Lipin 1 regulates glycerolipid homeostasis by acting as a phosphatidic acid phosphohydrolase (PAP) enzyme in the triglyceride-synthesis pathway and by regulating transcription factor activity. Mutations in human lipin 1 are a common cause of recurrent rhabdomyolysis in children. Mice with constitutive whole-body lipin 1 deficiency have been used to examine mechanisms connecting lipin 1 deficiency to myocyte injury. However, that mouse model is confounded by lipodystrophy not phenocopied in people. Herein, 2 muscle-specific mouse models were studied: 1) Lpin1 exon 3 and 4 deletion, resulting in a hypomorphic protein without PAP activity, but which preserved transcriptional coregulatory function; and 2) Lpin1 exon 7 deletion, resulting in total protein loss. In both models, skeletal muscles exhibited a chronic myopathy with ongoing muscle fiber necrosis and regeneration and accumulation of phosphatidic acid and, paradoxically, diacylglycerol. Additionally, lipin 1–deficient mice had abundant, but abnormal, mitochondria likely because of impaired autophagy. Finally, these mice exhibited increased plasma creatine kinase following exhaustive exercise when unfed. These data suggest that mice lacking lipin 1–mediated PAP activity in skeletal muscle may serve as a model for determining the mechanisms by which lipin 1 deficiency leads to myocyte injury and for testing potential therapeutic approaches.—Schweitzer, G. G., Collier, S. L., Chen, Z., McCommis, K. S., Pittman, S. K., Yoshino, J., Matkovich, S. J., Hsu, F.-F., Chrast, R., Eaton, J. M., Harris, T. E., Weihl, C. C., Finck, B. N. Loss of lipin 1–mediated phosphatidic acid phosphohydrolase activity in muscle leads to skeletal myopathy in mice.
Keywords: LPIN1, rhabdomyolysis, diacylglycerol, triacylglycerol, autophagy
Lipin 1 is an intracellular protein that controls metabolism by acting at multiple regulatory levels (1). This soluble protein acts at the endoplasmic reticulum to dephosphorylate phosphatidic acid (PA) to form diacylglycerol (DAG), the penultimate step in triacylglycerol (TAG) synthesis (2). Lipin 1 also acts in the nucleus to directly interact with DNA-bound transcription factors to regulate gene expression (3–6). Mutations in the gene encoding lipin 1 (LPIN1) are a common cause of early onset, recurrent pediatric rhabdomyolysis (7–15), a condition in which damage to skeletal myocytes results in substantial buildup of myocellular proteins in the blood. Severe rhabdomyolysis can cause acute kidney failure and may lead to death.
Most mutations in LPIN1 that have been associated with rhabdomyolysis are frameshift mutations leading to complete loss of protein expression or generation of severely truncated proteins lacking the carboxyl terminus, which contains its catalytic site and transcription factor–interacting domains. However, other mutations are single amino acid substitutions that we have recently shown (11) to cause a loss of intrinsic phosphatidic acid phosphohydrolase (PAP) activity, but which does not affect transcriptional regulatory function. These in vitro findings suggest that defects in lipin 1–mediated PAP activity underscore rhabdomyolysis in people with LPIN1 mutations, but this has not been tested in vivo. Zhang et al. (16) recently identified previously uncharacterized skeletal muscle myocyte damage in the fatty-liver dystrophic (fld) mouse model caused by a spontaneous mutation in the mouse Lpin1 gene. Mechanistic studies suggested that myopathy in fld mice, and potentially rhabdomyolysis in people with LPIN1 mutations, may be due to impaired autophagy and lysosomal function secondary to PA accumulation and reduced abundance of DAG in skeletal muscle (16). However, fld mice also exhibit progressive lipodystrophy (17) and neuropathy (18), which complicates the interpretation of data obtained with that model. In addition, the adipocyte and neuropathic abnormalities associated with lipin 1 deficiency in fld mice are not phenocopied in humans with LPIN1 mutations (10).
To separate the myocyte-autonomous effects of lipin 1 from the neuropathic and lipodystrophic phenotype of the fld mice, we generated and studied 2 models of muscle-specific Lpin1 deficiency dependent on muscle creatine kinase (MCK)-promoter driven Cre expression. First, we crossed MCK-Cre mice with a previously described Lpin1 flox mouse that serendipitously expresses a truncated lipin 1 protein missing the N-terminal 115 aa after Cre-mediated recombination. The truncated protein lacks intrinsic PAP activity but has preserved transcriptional regulatory function (19). We also crossed MCK-Cre mice with mice harboring a novel Lpin1 flox allele that leads to complete lipin 1 protein knockout. We demonstrate that either selective loss of PAP activity or complete deletion of lipin 1 protein in skeletal muscle leads to active myopathy and myofibrillar regeneration, increased muscle PA levels, evidence for mitochondrial dysfunction, and blunted myocellular autophagy. The phenotype of these mice seems somewhat more severe but mechanistically similar to the reported phenotype of fld mice (16, 20). However, compared to the fld model, the muscle-specific modulation of lipin 1 activity shows differences in the muscle abundance of other key lipids, such as DAG and TAG, suggesting that low levels of these lipids in fld mice are due to lipodystrophy and are not copied in humans (7–9, 11). Lastly, we demonstrate that skeletal muscle lipin 1 deficiency leads to increased exercise endurance in younger mice, but that capacity to exercise declines with age and is associated with elevated plasma creatine kinase after exhaustive exercise, which parallels some reports of people with LPIN1 mutations. These data suggest that myocyte-autonomous deletion of lipin 1-mediated PAP activity leads to active myopathy and eventual decline in exercise capacity in mice. These mice may have utility for better defining mechanisms and as a model for potential therapies to prevent rhabdomyolysis in patients with LPIN1 mutations.
MATERIALS AND METHODS
Animal studies
All mouse studies were approved by the Institutional Animal Care and Use Committee of Washington University and fulfilled U.S. National Institutes of Health (NIH) requirements for humane care. All mice were in the C57BL/6J background. The generation of mice harboring an Lpin1 allele with LoxP sites flanking exons 3 and 4 (Lpin1Δ115) has been previously described (21). MCK-Lpin1Δ115 mice were generated by crossing mice harboring the Lpin1Δ115 allele with mice expressing Cre recombinase under control of the muscle creatine kinase (MCK) promoter (Fig. 1A). To generate mice with complete, but conditional, loss of lipin 1, embryonic stem cells containing a knockout allele of Lpin1 were obtained from the Knockout Mouse Project (IKMC project ID 89403; International Knockout Mouse Consortium). This construct contains a targeted allele of Lpin1 containing inserted cassettes for promoter-driven LacZ and Neo cDNAs flanked by flippase recognition target sites upstream of exon 7 of the Lpin1 gene, which are flanked by LoxP sites. Propagated embryonic stem cells were injected into developing embryos and then implanted into pseudopregnant females. Chimeric offspring were mated to establish germline transmission. Resulting heterozygous offspring were mated with C57BL/6J mice expressing Flp recombinase in a global manner (chicken α actin promoter-driven transgenic) to remove LacZ and Neo cassettes and generate mice harboring the conditional floxed allele. Lpin1 floxed mice were then crossed with hemizygous C57BL/6J mice expressing Cre recombinase under the MCK promoter to create MCK-Lpin1−/− mice (Fig. 1D). Littermates not expressing Cre [wild-type (WT) mice] were used as control mice in all experiments. All mice were group housed and maintained on standard laboratory chow diet (PicoLab Rodent Diet 5053; LabDiet, St. Louis, MO, USA) and on a 12/12-h light/dark cycle.
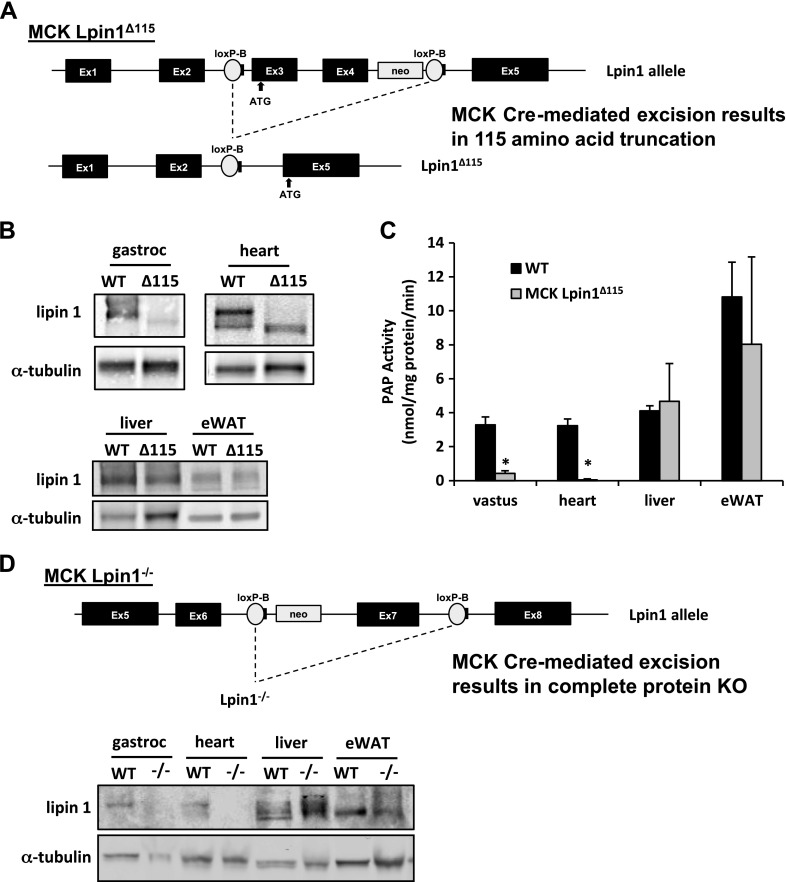
Figure 1.
Generation of mouse models with muscle-specific lipin 1 deficiency. A) Schematic showing Lpin1 gene-targeting strategy for generation of truncated lipin 1. MCK-Lpin1Δ115 animals were generated using LoxP sites flanking exons 3 and 4 of lipin 1, which encode the principal start codon. MCK-driven Cre recombination led to enforcement of an alternative start codon in exon 5. B) Western blot analysis of gastrocnemius, heart, liver, and eWAT from WT and MCK-Lpin1Δ115 mice. C) PAP activity in vastus, heart, liver, and eWAT of WT and MCK-Lpin1Δ115 mice. Data are shown as means ± sem. *P < 0.05 (Student’s t test) for differences in MCK-Lpin1Δ115 compared with WT mice. n = 5–6 and animals were 3–4 mo old. D) Schematic showing Lpin1 gene-targeting strategy for generation of full-protein knockout of lipin 1. MCK-Lpin1−/− animals were generated with LoxP sites flanking exon 7 of lipin 1, and MCK-driven Cre recombination led to complete ablation of the lipin 1 protein. Western blot analysis of gastrocnemius, heart, liver, and eWAT from WT and MCK-Lpin1−/− mice 3–4 mo old; n = 5.
Protein isolation and abundance assessment
Protein from frozen tissue was homogenized in 0.3–1 ml ice-cold homogenization buffer [25 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 150 mM NaCl, 5 mM EDTA, 1% Triton X-100, pH 8.0], supplemented with 1 mM activated Na3VO4, 1 mM phenylmethanesulfonyl fluoride, 5 mM sodium fluoride, and 1× complete protease inhibitor cocktail tablet (04693116001; F. Hoffmann-La Roche, Basel, Switzerland) using high-speed tissue disruption with the TissueLyser II (Qiagen, Hilden, Germany). For tissue used for LC3 and p62 protein assessment, tissue was homogenized in RIPA buffer [50 mM Tris, ph 8.0, 150 mM NaCl, 1% nonidet-p40 (octylphenoxypolyethoxyethanol; MilliporeSigma, Burlington, MA, USA), 0.5% sodium deoxycholate, 0.1% SDS, and 1× σ protease inhibitor tablet (S8820; MilliporeSigma)] and disrupted via glass Dounce homogenization. Tissue homogenates were subsequently solubilized by rotating at 4°C at 50 rpm for 1 h before being centrifuged (15,000 g for 15 min at 4°C) and the supernatant collected. Aliquots of the lysate from tissues were used to determine protein concentration by the bicinchoninic acid assay (BCA), according to the manufacturer’s instructions (23227; Thermo Fisher Scientific, Waltham, MA, USA). The remaining lysates were stored at −80°C until further analyzed. Mitochondrial protein was isolated by fractionation as per previous studies (22, 23). In brief, skeletal muscles (gastrocnemius and vastus tissue combined) from mice were Dounce-homogenized in Tris-sucrose buffer. Cellular debris was spun in 4°C at 1000 g for 5 min, and the supernatant containing mitochondria was spun in 4°C at 10,000 g for 10 min and washed in Tris-sucrose buffer 3 times. The final pellet was resuspended in mito buffer (14 mM NaCl, 10 mM Tris, 1 mM EDTA, 0.2% NP-40, 10% glycerol supplemented with 1 mM activated Na3VO4, 1 mM phenylmethanesulfonyl fluoride, 5 mM sodium fluoride, and 1× complete protease inhibitor cocktail tablet). The total protein content of this pellet, measured by BCA, was greater in MCK Lpin1Δ115 vs. WT littermates.
Lysates were subjected to SDS-PAGE and transferred to PVDF membranes. Blots were then rinsed with Tris-buffered saline plus Tween 20 (TBST; 0.14 µm NaCl, 0.02 µm Tris base, pH 7.6, and 0.1% Tween), blocked with 5% bovine serum albumin (BSA) in TBST for 1 h at room temperature, washed 3 times for 10 min at room temperature, and incubated with the relevant primary antibody (1:1000 for all antibodies; 1:500 for LC3) in 5% BSA overnight at 4°C. Blots were then washed 3 times for 5 min with TBST, incubated with relevant secondary antibodies for 1 h at room temperature, washed again 3 times for 10 min with TBST, and washed 2 times for 10 min with Tris-buffered saline. For lipin 1 and α-tubulin, protein bands were visualized with the Odyssey Imaging System (Li-Cor Biosciences, Lincoln, NE, USA). For p62 and LC3, protein bands were visualized by administering ECL detection reagent (RPN2209; GE Healthcare Life Sciences, Little Chalfont St. Giles, United Kingdom) and quantifying chemiluminescence with G:Box Chemi XT4 (Syngene International, Bangalore, India). Lipin 1 (sc-98450) antibody was obtained from Santa Cruz Biotechnology (Dallas, TX, USA). Anti–α-tubulin clone B-5-1-2 (T5168) and LC3 (L7543) were purchased from MilliporeSigma. Goat anti-mouse IRDye 680 (926-32220) and goat anti-rabbit IRDye 680 (926-68021) secondary antibodies were obtained from Li-Cor Biosciences. p62 (18420-1-AP) was obtained from the Proteintech Group (Rosemont, IL, USA). Anti-rabbit secondary antibody (7074) was obtained from Cell Signaling Technology (Danvers, MA, USA).
PAP assay
The method to assess PAP activity was modified from Martin et al. (24) and has also been previously described (25). Approximately 50 mg of tissue were homogenized in 1 ml of lysis buffer [0.01 M Tris-HCl, pH 7.3, 0.25 M sucrose, 0.5% Tween-20, 1 mM DTT, 1× EDTA-free protease inhibitor (04693132001; F. Hoffmann-La Roche)] using Tissue Master-125 (Omni International, Kennesaw, GA, USA). After homogenization, aliquots of the supernatant were used to determine protein concentration by BCA. The 14C-phosphatidic acid used was prepared as follows: 15 µl 0.1 mCi/ml of 14C-phosphatidic acid l-α-dioleoyl (oleoyl-1-14C) Na salt (14C -PA) (1303; American Radiolabeled Chemicals, St. Louis, MO, USA) was added to 3 mM 3-sn-phosphatidic acid sodium salt (P9511; MilliporeSigma) and 2 mM l-α-phosphatidylcholine (P3556; MilliporeSigma) in 1 ml of chloroform, evaporated under a stream of N2, and resuspended in 1 ml of ice-cold double-distilled H2O. This mixture was sonicated 3 times for 10 s and kept on ice between sonications. To run the assay reaction, 5 µl of each sample was incubated with 40 µl of assay buffer [0.1 M Tris/maleate, pH 6.9, 10 mM MgCl2, 0.2% fatty acid-free BSA, 1 mM DTT, and 1× EDTA-free protease inhibitor with or without 12.5 mM N-ethylmaleimide (E3876; MilliporeSigma)] and 5 µl of 14C-phosphatidic acid, which was incubated at 37°C for 20 min. The reaction was stopped by adding 1 ml of a 19:1 (v/v) chloroform/methanol mixture containing 0.08% olive oil to each reaction and vortexing briefly; 500 mg of activated aluminum oxide was added to the tubes, and they were capped securely. Then, samples were vortexed for 3 cycles of 30 s, followed by being left undisturbed for 10 min. Samples were then spun at 10,000 g for 5 min, and 250 µl of sample was added to the scintillation fluid to determine the 14C radioactivity.
Microarray for transcriptional profile and pathway analysis
Microarray gene expression analysis of mouse vastus tissue was performed by the Washington University Genome Technology Access Center (St. Louis, MO, USA) using the Illumina (San Diego, CA, USA) 8v2 platform. Total RNA was isolated with RNeasy Mini Kit (Qiagen).
The background-subtracted raw microarray data were subjected to quantile normalization. Parametric analysis of gene set enrichment was performed with the normalized data to determine the effect of Lpin1 deletion on skeletal muscle biologic pathways, as previously described (26, 27). Gene sets were obtained from Molecular Signatures Database (gene ontology gene sets, C5 collection; Broad Institute of Massachusetts Institute of Technology and Harvard, Cambridge, MA, USA), and z scores and significance values were calculated for each gene set. A value of P < 0.05 was considered significantly changed. All data were obtained using an R-based statistical software package (R Foundation for Statistical Computing, Vienna, Austria), Parametric Gene Set Enrichment Analysis, available at the Bioconductor (Seattle, WA, USA) website. The heat map was generated from microarray data using Partek software (St. Louis, MO, USA), and data are shown for all probes with a false-discovery rate (FDR) < 0.05. The FDR was determined using the step-up method between MCK-Lpin1Δ115 and WT littermate control animals.
Muscle histology
Isolated muscle was mounted with tragacanth gum (G1128; MilliporeSigma) and quick frozen in liquid nitrogen–cooled 2-methylbutane. Samples were stored at −80°C until sectioning into 12-µm sections. Cryostat sections were processed and fixed in acetone. Hematoxylin and eosin (H&E) staining was conducted, and muscle fiber cross-sections were visualized using EVOS FL Color Imaging system (Thermo Fisher Scientific). Succinate dehydrogenase staining of muscle fiber cross sections were visualized with an Eclipse 80i microscope (Nikon, Tokyo, Japan) with NIS-Elements AR 3.0 software (Nikon). For immunohistochemistry, the sections were fixed with 10 min incubation in ice-cold acetone, then blocked in TSA blocking reagent (FP1012; PerkinElmer, Waltham, MA, USA) for 2 h, and incubated with primary antibodies: TOM20 (sc-11415; Santa Cruz Biotechnology) 1:500, SQSTM1/p62 (H00008878; Novus Biologicals, Littleton, CO, USA) 1:100, LC3 (0231-100; NanoTools Antikoerpertechnik, Teningen, Germany) 1:250, and BNIP3 (ab10433; Abcam, Cambridge, United Kingdom) 1:200; overnight at 4°C, followed by the appropriate secondary antibody. Coverslips were mounted with Mowiol 4–88 (MilliporeSigma) + DAPI and examined with a fluorescence microscope [Nikon 80i upright+ and Scientific EZ monochrome charge-coupled device (CCD) camera (Roper Technologies, Sarasota, FL, USA) with deconvolution software analysis (NIS Elements; Nikon)]. Nonfluorescent images were taken with a 5-megapixel color CCD camera (Nikon).
mRNA isolation and gene expression quantification
For all analyses, total RNA from skeletal muscle was isolated in RNAzol (Thermo Fisher Scientific) reagent. RNA (500 ng) was subjected to high-capacity cDNA reverse transcription (Thermo Fisher Scientific) and SYBR Green RT-PCR (Thermo Fisher Scientific), following the manufacturer’s instructions. Results were corrected to the expression of Rplp0. Sequence of gene-specific primers are listed in Supplemental Table 1.
Mitochondrial DNA determination
Tissue was homogenized in RNAzol (1 ml of RNAzol/50 mg tissue), chloroform added, and the mixture was vortexed vigorously. Samples were spun at 10,000 g for 15 min at 4°C. The top aqueous phase was removed and used for RNA isolation, and 500 μl of back-extraction buffer (stock: 4 M guanidine thiocyanate, 50 mM sodium citrate, 1 M Tris-free base) was added per 1 ml remaining interphase and organic phase. Sample was mixed well and stood for 10 min at room temperature before being centrifuged at 3000 g for 30 min at 4°C. The upper phase containing DNA was transferred, and 4 μl of polyacryl carrier was added/ml RNAzol used; DNA was precipitated by adding 400 μl of isopropanol/ml of RNAzol used, mixed, left standing for 5 min at room temperature, and centrifuged at 12,000 g for 5 min at 4°C. The pellet was washed in ethanol 3 times, air dried, dissolved in 8 mM NaOH, vortexed, and heated, if needed (55°C) to dissolve; 0.34 μl of 1 M HEPES and 0.3 μl of 100 mM EDTA were added. ND1 and LPL genes were quantified for mitochondrial DNA (mtDNA) and nuclear DNA.
Mitochondrial respiration
High-resolution respirometry was conducted using a 2-chamber Oxygraph O2k (Oroboros Instruments, Innsbruck, Austria). Skeletal muscle respiration protocol was derived from Ryan et al. (28) with modification for mouse tissue. Soleus muscles were isolated and placed in muscle biopsy buffer [50 mM K-2-(N-morpholino)ethanesulfonic acid, 7.23 mM K2EGTA, 2.77 mM CaK2EGTA, 20 mM imidazole, 20 mM taurine, 5.7 mM Na2ATP, 14.3 mM phosphocreatine, 0.5 mM DTT, and 6.56 mM MgCl20 · 6H2O, pH 7.1] before further processing. Fiber strips from soleus were separated along their longitudinal axis with needle-tipped forceps under magnification (SZX7; Olympus, Tokyo, Japan) and permeabilized in muscle biopsy buffer with 0.05 mg/ml of saponin for 20 min at 4°C with gentle rocking. Fibers were transferred to MiRO5 buffer (20 mM HEPES, 0.5 mM EGTA, 10 mM K2HPO4, 3 mM MgCl20 · 6H2O, 60 mM lactobionic acid, 20 mM taurine, 110 mM d-sucrose, 1.0 mg/ml BSA, pH 7.4) and gently rocked for 15 min at 4°C. Fibers were blotted dry and weighed. Approximately 3 mg of fibers were placed in an Oxygraph chamber with MiRO5 buffer containing 3 mg/ml creatine and 10 µM blebbistatin. The respiration protocol was conducted with sequential additions of the following: 10 mM glutamate, 2 mM malate, 5 mM pyruvate (complex I substrates), 5 mM ADP, 10 mM succinate (complex II substrate), 3 titrations of 0.5 mM carbonyl cyanide‑p‑trifluoromethoxyphenylhydrazone (FCCP; uncoupling agent), 10 μM cytochrome c to test for mitochondrial membrane integrity, and 0.5 μM rotenone (complex I inhibitor). State 2 (i.e., leak) respiration was measured when oxygen flux reached a steady state after the addition of pyruvate. State 3 (i.e., OxPhos) respiration was measured when the oxygen flux reached steady state after the addition of succinate. Uncoupled respiration (i.e., electron transport system) was measured as the highest oxygen flux achieved after FCCP was added.
Autophagy assessment
The method to assess autophagy in skeletal muscle has been previously described (29). MCK-Lpin1Δ115 mice were i.p. injected with 0.4 mg/kg colchicine (1364; Bio-Techne, Minneapolis, MN, USA) on d −2 and −1. Mice were euthanized on d 0, and skeletal muscles were isolated and prepared for protein isolation, as described above.
Electron microscopy
Tissue was fixed in a modified Karnovsky’s fixative of 3% glutaraldehyde and 1% paraformaldehyde in 0.1 M sodium cacodylate buffer, postfixed in 2% osmium tetroxide in 0.1 M sodium cacodylate buffer for 1 h, en bloc stained with 3% aqueous uranyl acetate for 30 min, dehydrated in graded ethanols, and embedded in PolyBed 812 (08792-1; Polysciences, Warrington, PA, USA). Tissue blocks were sectioned at 90 nm thick, after staining with Venable’s lead citrate, and being viewed with a JEOL model 1400EX electron microscope (Jeol, Tokyo, Japan). Digital images were acquired with the AMT Advantage HR (Advanced Microscopy Technology, Woburn, MA, USA) high-definition CCD, 11-megapixel transmission electron microscopy camera. Lipid droplet area was quantified from 6 scans/mouse and analyzed with ImageJ (National Institutes of Health, Bethesda, MD, USA) software.
Lipid analysis
Electrospray ionization liquid chromatography–mass spectrometry (LC-MS) analysis of lysophosphatidic acid (LPA), PA, DAG, and TAG, phosphatidylglycerol (PG), and cardiolipin (CL) in MCK-Lpin1Δ115 and MCK-Lpin1−/− vastus and gastrocnemius muscles, was determined as described with modification (30). The LC-MS analysis was performed with either a 10A HPLC system (Shimadzu, Kyoto, Japan) or a SIL-20AC HT auto-sampler (Shimadzu) coupled to a Thermo Fisher Scientific TSQ Quantum Ultra Triple Quadrupole Mass Spectrometer operated in selected reaction monitoring mode or with a Thermo Fisher Scientific Vantage TSQ Mass Spectrometer with Thermo Fisher Scientific Accela Ultra Performance Liquid Chromatography operated by Xcalibur software (Thermo Fisher Scientific) using selected ion monitoring mode.
LPA-(17:0), PA-(14:0)2, DAG-(15:0)2, TG-(17:0)3, PG-(15:0)2, and CL-(14:0–14:0)2 were used as internal standards for LPA, PA, DG, TG, PG, and CL, respectively. Quantitation of lipids was based on the ratio of the peak area of the analyte to the internal standard. For example, the ratio of CL-(18:2–18:2)2 and CL-(14:0–14:0)2 is used for measurement of CL-(18:2–18:2)2.
Endurance exercise assessment and plasma creatine kinase measures
MCK-Lpin1Δ115 and MCK-Lpin1−/− mice with their respective WT littermate controls (3–4 mo or 1 yr old) performed a single bout of treadmill endurance exercise to exhaustion. The mice were unfed overnight (∼12 h) before the exercise session. After a 5 min familiarization period on the treadmill, the exercise protocol consisted of graduated intensity running at 5 m/min for 5 min, 10 m/min for 5 min, 15 m/min for 5 min, 20 m/min for 5 min, and 25 m/min until exhaustion. The mice were encouraged to run with a mild electric stimulus (20 V) placed at the end of the treadmill. Mice were judged to be exhausted by refusal to remain on treadmill belt for >5 s. In MCK-Lpin1−/− mice, blood was collected 3 h after completing exercise via mandibular bleed, which was spun at 8000 g for 8 min to isolate plasma. Plasma was also isolated from blood collected from those mice during a sedentary, fed state as a basal measurement. Plasma creatine kinase was determined with the Hoffmann-La Roche Creatine Kinase Kit and analyzed on the Hoffmann-La Roche Cobas c501 analyzer.
Statistical analysis
Data were analyzed via SPSS software (IBM, Armonk, NY, USA) and Excel (Microsoft, Redmond, WA, USA). Statistical comparisons were made with independent-sample Student’s t tests or 2-way ANOVA with Tukey’s post hoc testing to identify specific differences. Before analysis, normality and equal variance of samples were determined by Kolmogorov-Smirnov testing and Levene testing, respectively. If those conditions were violated, a square root transformation, intended to produce data to satisfy normality and equal variance assumptions, was used. Otherwise, nonparametric methods (e.g., Kruskal-Wallis for 1-way ANOVA with Mann-Whitney U post hoc analysis and Friedman’s test for 2-way ANOVA) were used as an alternative to the more standard analyses. All data are presented as the means ± sem, with a statistically significant difference defined as a P < 0.05.
RESULTS
Characterization of lipin 1 protein in mouse models
We generated mice lacking lipin 1–mediated PAP activity in a muscle-specific manner by crossing mice harboring a Lpin1 allele flanked by LoxP sites around exons 3 and 4 (21) with mice expressing Cre recombinase driven by the MCK gene promoter (MCK-Lpin1Δ115 mice) (Fig. 1A). Consistent with previous reports using this lipin 1 floxed allele (19, 25), Western blot analyses revealed that a truncated lipin 1 protein of diminished abundance was expressed in a skeletal muscle– and heart-specific manner in MCK-Lpin1Δ115 mice (Fig. 1B). That protein lacks 115 aa of the N terminus of lipin 1, and the resulting protein is deficient in PAP activity but retains transcriptional regulatory function (19, 25). Accordingly, compared with WT littermate controls, PAP activity in the tissues of MCK-Lpin1Δ115 mice was greatly diminished in skeletal muscle and heart (Fig. 1C). PAP activity was not affected in liver and epididymal white adipose tissue (eWAT). MCK-Lpin1Δ115 mice were viable and outwardly normal. Compared with WT littermate control mice, MCK-Lpin1Δ115 mice exhibited similar body weights and tissue masses (Supplemental Fig. 1).
Using a novel line of mice with a floxed Lpin1 exon 7 (Fig. 1D), which creates a frameshift mutation, we also generated mice completely lacking lipin 1 protein in a skeletal muscle– and heart-specific manner that retained protein in liver and eWAT (MCK-Lpin1−/− mice; Fig. 1D). Those mice were also outwardly normal, viable, and exhibited similar body weights and tissue masses (Supplemental Fig. 1). In studies described below, MCK-Lpin1Δ115 and MCK-Lpin1−/− mice are often used interchangeably, but which strain was used is always indicated.
Myocyte damage in mouse models of lipin 1 deficiency
Analysis of H&E-stained sections of MCK-Lpin1Δ115 gastrocnemius muscles and MCK-Lpin1−/− vastus muscles showed an active myopathy, with muscle fiber necrosis and regeneration that was more prominent with increasing age (Fig. 2A). Nearly all muscle fibers in 1-yr-old mice were centrally nucleated, suggesting almost complete turnover of muscle fibers. The similarity of the phenotypes of the 2 models suggests that loss of lipin 1–mediated PAP activity is sufficient to induce myopathy in vivo, whereas the transcriptional regulatory function of the protein is dispensable.
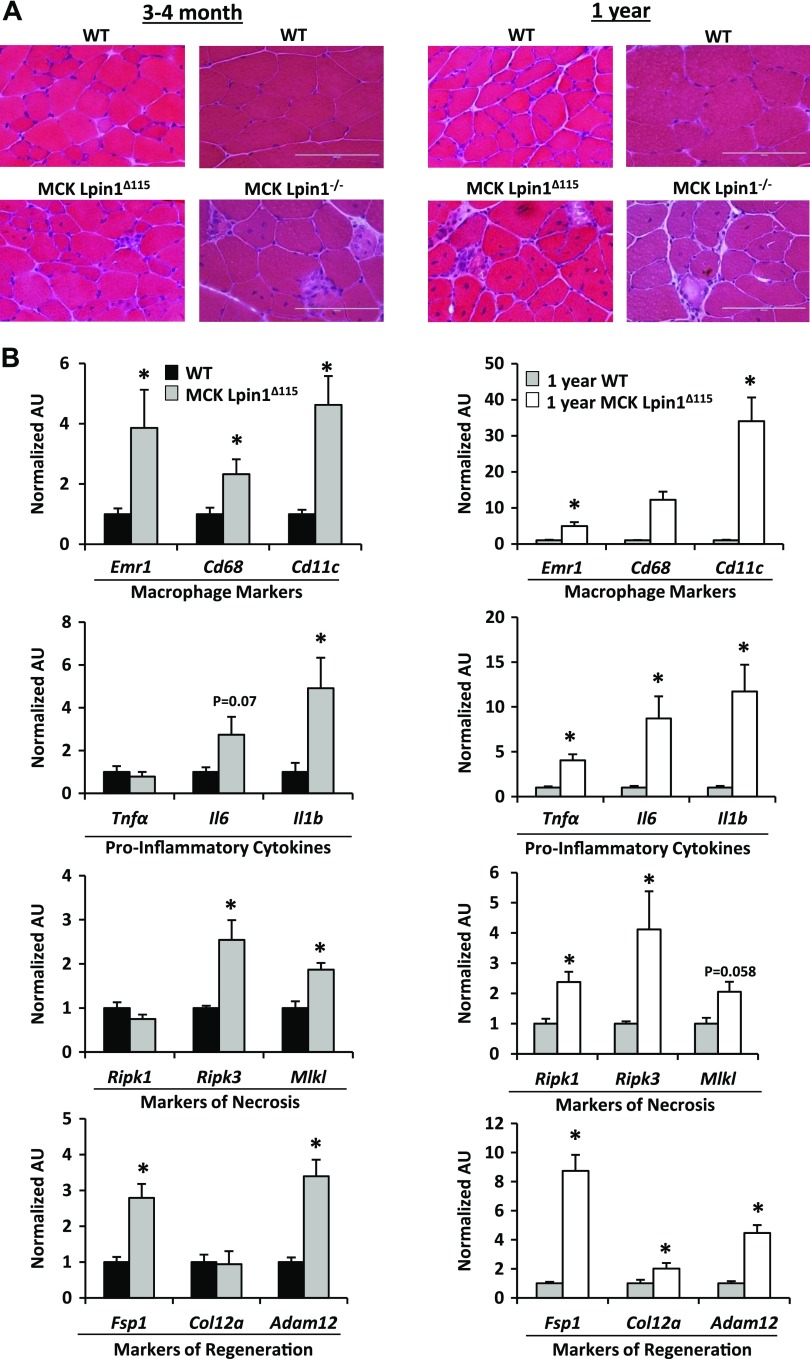
Figure 2.
Mice with muscle-specific Lpin1 deficiency exhibit active and progressive myopathy. A) H&E stains of skeletal muscle cross sections from MCK-Lpin1Δ115 and MCK-Lpin1−/− mice with respective age-matched WT controls at 3–4 mo and 1 yr old. Field width is ∼200 µm. B) Quantitative RT-PCR performed to quantify expression of the indicated genes from MCK-Lpin1Δ115 mice 3–4 mo and 1 yr old. Various markers of macrophage, proinflammatory cytokines, necrosis, and muscle regeneration were measured. Values were normalized (1.0) to age-matched WT mice, and data are shown as means ± sem. *P < 0.05 (Student’s t test) for differences in MCK-Lpin1Δ115 compared with age-matched WT mice; n = 7–9.
Consistent with those observations, quantitative RT-PCR detected increased expression of genes encoding macrophage markers (Emr1, Cd68, Cd11c), proinflammatory cytokines (Tnfa, Il6, Il1b) as well as markers of necrosis (Ripk3 and Mlkl) in MCK-Lpin1Δ115 muscles compared with WT littermate muscle at 3–4 mo and 1 yr old (Fig. 2B). Expression of regeneration markers (Fsp1, Col12a, Adam12) was observed in MCK-Lpin1Δ115 mice, compared with age-matched WT littermates. These histologic and molecular biologic findings are consistent with active and progressive myopathy with regeneration because of loss of lipin 1–mediated PAP activity in muscle.
Alterations in transcript profiles with muscle deficiency in PAP activity
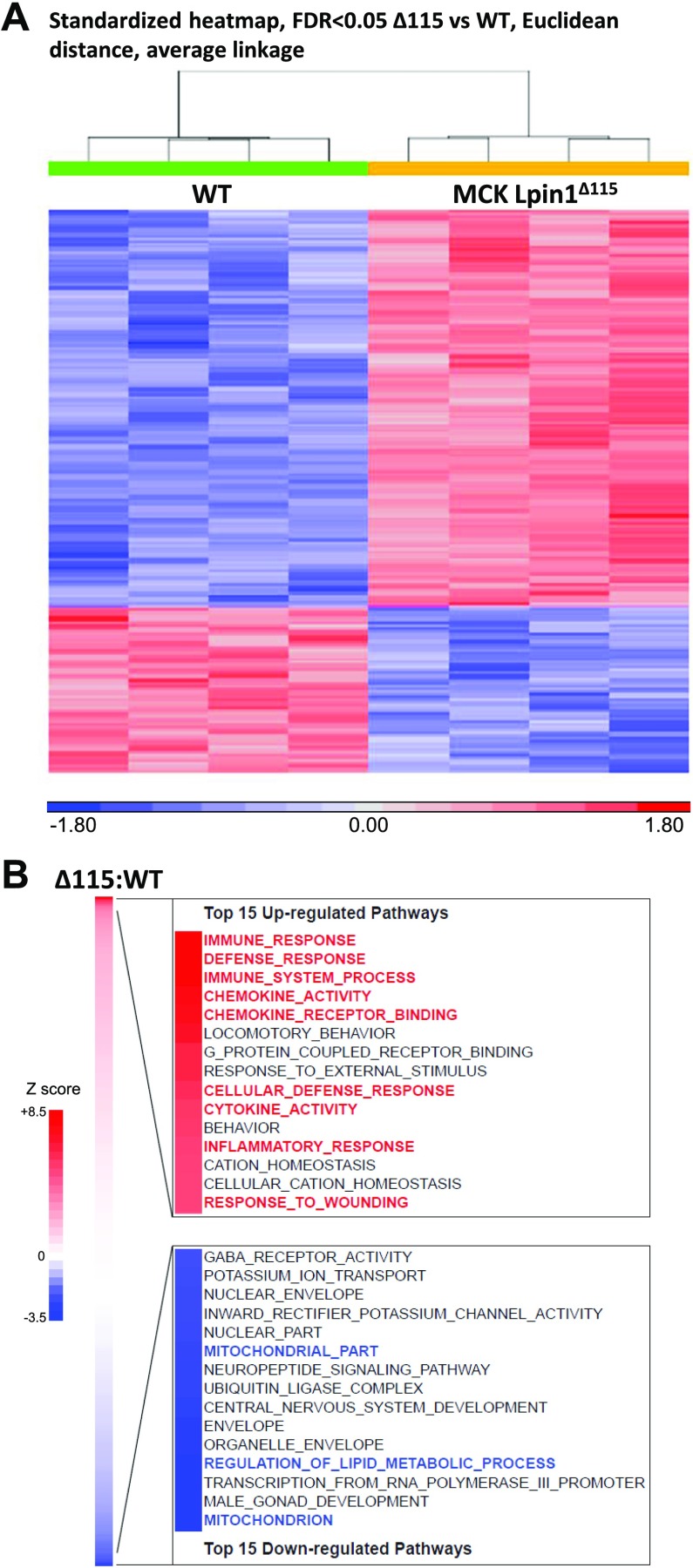
To characterize the global transcriptional differences secondary to loss of lipin 1–mediated PAP activity in muscle without the confounding effects of the loss of transcriptional regulatory function, microarray analyses were conducted with RNA from vastus muscle tissue of MCK-Lpin1Δ115 and WT mice. Unsupervised, hierarchical clustering of the data from differentially expressed mRNAs (fold change 1.8, FDR <0.05) revealed low levels of intragroup variance between samples and demonstrated that these mRNAs were sufficient to distinguish MCK-Lpin1Δ115 mice from WT control (Fig. 3A). To identify the nature of these unique genotypic signatures, analyses of the microarray data revealed an up-regulation of several cellular pathways involved in inflammatory responses, cytokine activity, and cellular defense in the MCK-Lpin1Δ115 compared with WT controls, indicating myocellular inflammation or damage (Fig. 3B, red highlighted pathways). Additionally, down-regulated pathways of MCK-Lpin1Δ115 include those involved in mitochondrial partitioning and function (Fig. 3B, blue highlighted pathways). Collectively, the transcriptomic profile suggests that loss of lipin 1–mediated PAP activity provokes an inflammatory response characterized by heightened cytokine expression and infiltration of immune cells.
Figure 3.
Microarray analysis of MCK-Lpin1Δ115. A) Standardized heat map of differentially expressed mRNAs from microarray of vastus from mice 3–4 mo old, using unsupervised hierarchical clustering selected at FDR <0.05. B) Pathway analyses of vastus muscle gene-expression microarray studies. The top 15 significantly changed cellular pathways up- and down-regulated are listed to highlight significant transcriptional alterations of MCK-Lpin1Δ115 (vs. WT) studied; n = 4.
Alterations in lipid intermediates in muscle specific lipin 1 deficiency
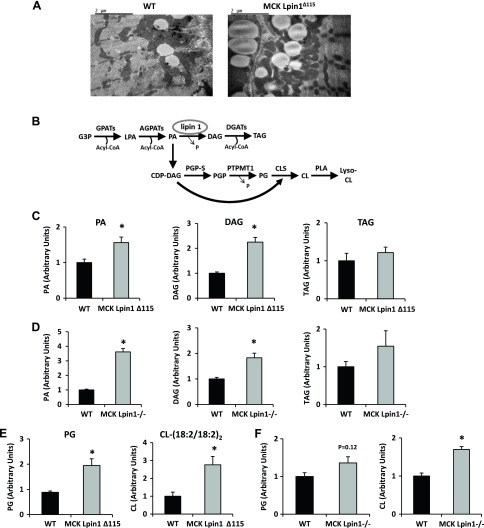
Electron microscopy revealed a 2-fold increase in lipid droplet area (WT: 4895 ± 717 pixels2, MCK-Lpin1Δ115: 10197.2 ± 2020 pixels2) in skeletal muscle of MCK-Lpin1Δ115 mice compared with normal littermates in both the intermyofibrillar and subsarcolemmal space (Fig. 4A). To characterize the nature of that lipid biochemically, we quantified triglyceride and several glycerol-3-phosphate (G3P) pathway lipid intermediates (Fig. 4B) in mouse skeletal muscle. As would be predicted, PA, the substrate of lipin 1, was significantly elevated in MCK-Lpin1Δ115 and MCK-Lpin1−/− mice vs. respective WT littermates (Fig. 4C) with contributions from many individual PA species of variable fatty acid chain composition (Supplemental Fig. 2). Curiously, DAG, the product of lipin 1–mediated PAP activity, was also significantly elevated in MCK-Lpin1Δ115 and MCK-Lpin1−/− mice compared with their respective normal littermates (Fig. 4C, D) with contributions from many individual DAG species (Supplemental Fig. 2). TAG levels were not different in either model compared with their respective WT littermate (Fig. 4C, D), and LPA levels in MCK-Lpin1Δ115 mice were also no different from WT control (Supplemental Fig. 2). These results suggest that skeletal muscle deficiency lipin 1–mediated PAP activity leads to accumulation of PA and, unexpectedly, DAG, which contradicts previous studies conducted in lipodystrophic fld mice.
Figure 4.
Mice with muscle-specific Lpin1 deficiency exhibit muscle lipid accumulation of G3P lipid and exhibit muscle accumulation of cardiolipin. A) Electron micrographs of lipid droplets in soleus muscle. A greater area of lipid droplets is present in both the contractile and sarcolemmal regions of the muscle fiber in 3–4-mo-old MCK-Lpin1Δ115 mice. Field width is ∼6 µm. B) The pathway schematic depicts the synthesis of TAG from G3P. Circled is lipin 1, the primary skeletal muscle PAP enzyme, and DAG. Also depicted is the PA branch with downstream phospholipids, including PG and CL. C, D) PA, DAG, and TAG from vastus and gastrocnemius muscles of MCK-Lpin1Δ115 and MCK-Lpin1−/− mice, respectively, were assessed via LC-MS analysis. E, F) PG and tetra linoleoyl CL (18:2/18:2)2 from vastus and gastrocnemius muscles of MCK-Lpin1Δ115 and MCK-Lpin1−/− mice with respective WT littermate controls were assessed via LC-MS analysis. Values were normalized (1.0) to age-matched WT mice (3–4 mo), and data are shown as means ± sem. *P < 0.05 (Student’s t test) for differences in MCK-Lpin1Δ115 or MCK-Lpin1−/− compared with respective WT littermate mice; n = 5–7.
Abnormal mitochondria in lipin 1 deficiency
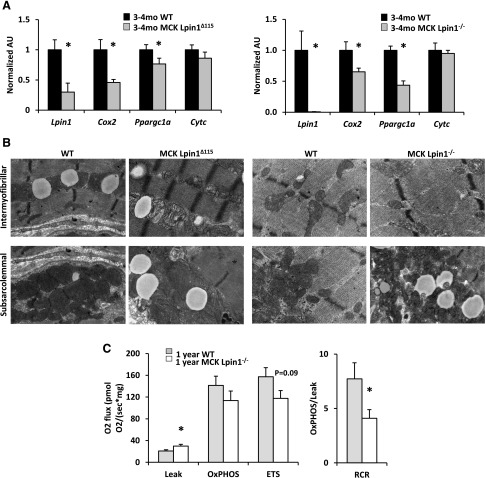
Because lipin 1 deficiency might preferentially shunt PA away from the classic G3P pathway to PG and CL (Fig. 4E, F), those lipids were also measured. In both MCK-Lpin1Δ115 and MCK-Lpin1−/− mice, increased CL abundance was detected. In MCK-Lpin1Δ115 mice, PG was significantly increased and tended to be increased in MCK-Lpin1−/− mice. Because PG and CL are phospholipids found almost exclusively in mitochondrial membranes and CL is an important and abundant membrane lipid in the inner mitochondrial membrane (31, 32), these results could also suggest an accumulation of mitochondria in skeletal muscle in these mouse models of Lpin1 deficiency.
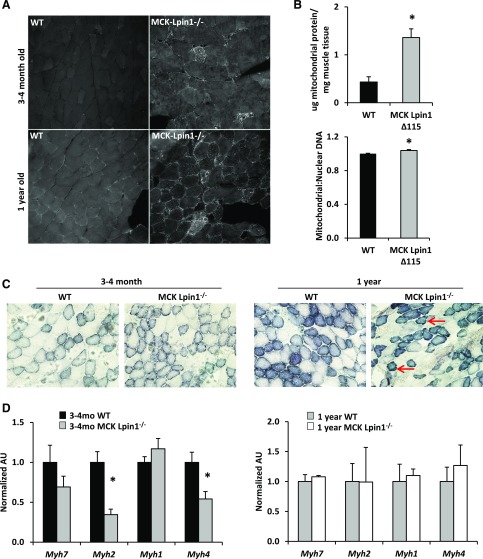
Supporting the observation of increased mitochondria in skeletal muscle-specific lipin 1–deficient animals, MCK-Lpin1−/− mice had increased staining of Tom20, a mitochondrial protein, in vastus skeletal muscle compared with WT littermate controls both in the young 3–4 mo-old and the 1-yr-old animals. Additionally, MCK-Lpin1Δ115 mice had increased mtDNA content compared with WT controls (Fig. 5B) and increased total mitochondrial protein normalized to skeletal muscle tissue weight (Fig. 5B). Further, in MCK-Lpin1−/− mice, succinate dehydrogenase staining revealed abnormal internal architecture with some muscle fibers having increased accumulation of mitochondria around the periphery compared with WT controls (Fig. 5C). Because succinate dehydrogenase is a marker of oxidative muscle fibers, we also assessed the expression of myosin isoforms associated with type 1, 2A, 2X, and 2B fibers in gastrocnemius tissue. We observed no increases in the expression of the myosin isoforms associated with the more-oxidative fiber types, MyHC-1 (Myh7), in knockout mice of any age, whereas expression of MyHC-2A (Myh2) and MyHC-2B (Myh1) were reduced in 3–4-mo-old MCK-Lpin1−/− mice compared with littermate controls by quantitative PCR (33).
Figure 5.
Mice with muscle-specific Lpin1 deficiency exhibit mitochondrial accumulation and abnormalities with noneffectual alterations in muscle fiber–type composition. A) Tom20 staining (bright punctate pattern) of vastus muscle in MCK-Lpin1−/− mice 3–4 mo and 1 yr old. B) Protein quantity per tissue weight of mitochondrial fraction and mtDNA content in MCK-Lpin1Δ115 mice and age-matched WT controls. C) Succinate dehydrogenase staining of skeletal muscle cross sections from MCK-Lpin1−/− mice with respective age-matched WT controls at 3–4 mo and 1 yr of age. Red arrows indicate examples of “ragged blue fibers,” which show intense, irregular staining of succinate dehydrogenase in the periphery of muscle fibers. Field width is ∼400 µm. D) Quantitative RT-PCR to quantify expression of muscle fiber types (type 1, 2A, 2X, and 2B) via assessing myosin heavy-chain genes in MCK-Lpin1−/− mice 3–4 mo and 1 yr old. Values were normalized (1.0) to age-matched WT mice (3–4 mo), and data are shown as means ± sem. *P < 0.05 (Student’s t test) for differences in MCK-Lpin1Δ115 or MCK-Lpin1−/− compared with respective WT littermate mice; n = 6–7 for MCK-Lpin1Δ115 and WT controls; n = 10–11 for MCK-Lpin1−/− and WT controls.
The increase in mitochondrial content in MCK-Lpin1−/− muscle could be due to enhanced mitochondrial biogenesis. However, consistent with the pathway analysis conducted with microarray data (Fig. 3B), the expression of Cox2 and the master regulator of mitochondrial biogenesis PGC-1α (Ppargc1a) was reduced in MCK-Lpin1Δ115 and MCK-Lpin1−/− mice compared with littermate controls (Fig. 6A). In contrast, Cytc expression was not affected, suggesting a partial pattern of reduced mitochondrial gene expression and mitochondrial biogenesis not underlying the observed accumulation of mitochondria. Both models of lipin 1–deficient mice had increases in swollen mitochondria with rarefied cristae in the intermyofibrillar and subsarcolemmal space (Fig. 6B). Finally, skeletal muscle respiration was altered in 1-yr-old MCK-Lpin1−/− mice compared with WT littermates with a statistically significant increase in oxygen flux in the leak state (state 2 respiration without addition of ADP), a trend toward a decrease in oxygen flux with uncoupled respiration, and overall, a significantly reduced respiratory control ratio (state 3:2). These data collectively suggest that skeletal muscle lipin 1 deficiency is associated with accumulation of mitochondria with abnormal ultrastructure and overall dysfunctional respiratory capability.
Figure 6.
Mice with muscle-specific Lpin1 deficiency display mitochondrial abnormalities and noneffectual alterations in muscle fiber–type composition. A) Quantitative RT-PCR to quantify expression of muscle fiber types (type 1, type 2A, type 2X, and type 2B) via assessing myosin heavy-chain genes in MCK-Lpin1Δ115 and MCK-Lpin1−/− mice at 3–4 mo old. Values were normalized (1.0) to age-matched WT mice, and data are shown as means ± sem. *P < 0.05 (Student’s t test) for differences in MCK-Lpin1Δ115 or MCK-Lpin1−/− compared with respective WT littermate mice. n = 10–11. B) Electron micrographs of lipid droplets in soleus muscle showing swollen and rarefied mitochondria in MCK-Lpin1Δ115 and MCK-Lpin1−/− mice in the intermyofibrillar and subsarcolemmal space. Field width is ∼3 µm. C) Oxygen flux indicating mitochondrial respiration for Leak (state 2 respiration), oxphos (state 3 respiration), electron transport system (ETS; uncoupled respiration), and respiratory control ratio (RCR), the oxphos:Leak. Values are shown as means ± sem. *P < 0.05 (Student’s t test) for differences in MCK-Lpin1−/− compared with WT littermate mice; n = 8.
Skeletal muscle autophagy
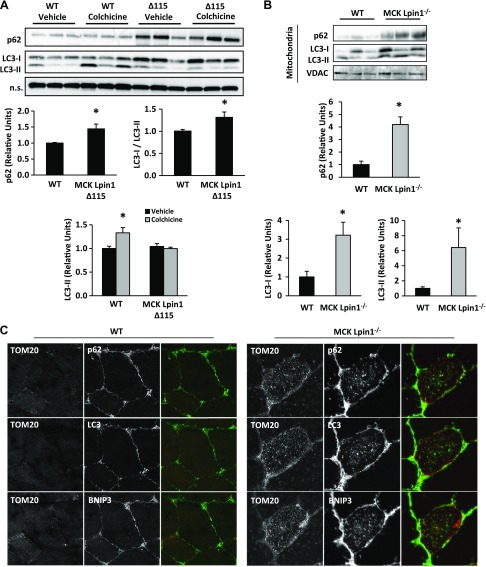
Increased abundance of abnormal mitochondria in lipodystrophic fld mice was associated with an inability to clear damaged mitochondria through the process of autophagy (16). Autophagy is an intracellular degradation system that brings targeted cellular contents to the lysosome, a membrane-bound organelle capable of degrading many biomolecules and maintaining a homeostatic environment through regulation of metabolism, cell signaling, and membrane repair (34). To determine whether autophagy-lysosomal activity might be altered in muscle-specific lipin 1-deficient mice, we assessed protein abundance of p62, LC3-I, and LC3-II in WT or MCK-Lpin1Δ115 mice after treating with vehicle or colchicine, a microtubule depolarizing agent that blocks the trafficking of autophagosomes to lysosomes resulting in autophagosome accumulation. p62 transports targeted proteins to autophagosomes for lysosomal degradation, and the p62 also becomes degraded. In whole-cell lysates, we observed increased p62 protein abundance in tibialis anterior muscle of MCK-Lpin1Δ115 mice compared with WT controls (Fig. 7A), suggesting impaired lysosomal function (35). The ratio of inactive cytosolic LC3-I to its activated form LC3-II under basal conditions was also decreased in MCK-Lpin1Δ115 mice compared with WT controls, suggesting abnormal autophagic flux (Fig. 7A). Treatment with colchicine resulted in increased LC3-II accumulation in WT mice but had no effect in MCK-Lpin1Δ115 mice. These data suggest a reduction in autophagic activity of mice with skeletal muscle-specific lipin 1 deficiency (Fig. 7A).
Figure 7.
MCK-Lpin1Δ115 mice display autophagy in skeletal muscle. A) Increased p62 protein abundance in tibialis anterior muscles of vehicle-treated MCK-Lpin1Δ115 mice compared with WT. With 2 d of intraperitoneal injections of 0.4 mg/kg colchicine, although p62 abundance is increased, there was no increase in LC3-II protein abundance detected in MCK-Lpin1Δ115 despite increased LC3-II in WT. Values were normalized (1.0) to age-matched WT mice (3–4 mo) treated with vehicle, and data are shown as means ± sem. *P < 0.05 (ANOVA post hoc) for differences in MCK-Lpin1Δ115 compared with WT littermate mice. N.s., nonspecific band. n = 5/condition. B) Increased p62, LC3-I, and LC3-II protein abundance in mitochondrial fraction from vastus muscles of MCK-Lpin1−/− of 1-yr-old mice compared with WT controls. VDAC is a mitochondrial-specific loading control. Values were normalized (1.0) to age-matched WT mice (3–4 mo) treated with vehicle, and data are shown as means ± sem. *P < 0.05 (Student’s t test) for differences in MCK-Lpin1−/− compared with WT littermate mice; n = 5–6. C) Increased abundance of Tom20, p62, LC3, and BNIP3 in vastus muscle fiber from 1-yr-old WT or MCK-Lpin1−/− mice. The third column overlay is Tom20 (green), and p62, LC3, or BNIP3 (red).
To assess the interaction of mitochondria with autophagic mediators in muscle of these mice, mitochondria were isolated from WT and MCK-Lpin1−/− mice, normalized for protein content, and then blotted for p62 and LC3. The mitochondrial fraction from gastrocnemius and vastus tissues from MCK-Lpin1−/− mice contained increased p62, LC3-I, and -II protein compared with WT littermates (Fig. 7B). Finally, skeletal muscle histology from gastrocnemius tissue revealed qualitatively increased p62, LC3, and BNIP3, another protein involved in autophagy, in the mitochondrial fraction (Fig. 7C). These findings are also consistent with impaired autophagic flux.
Reduced exercise tolerance and increased plasma creatine kinase in muscle-specific lipin 1 deficiency
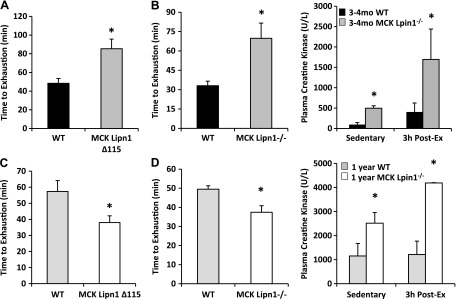
We next evaluated whether the MCK-Lpin1Δ115 and MCK-Lpin1−/− mice exhibited signs of rhabdomyolysis paralleling the phenotype of humans with LPIN1 mutations. Previous work has linked febrile illness, prolonged fasting, and intensive exercise to acute rhabdomyolysis in patients with LPIN1 mutations (11, 12). Therefore, WT and lipin 1–deficient mice were kept unfed overnight, and then, an exhaustive exercise test was conducted. Although the young 3–4-mo-old MCK-Lpin1Δ115 and MCK-Lpin1−/− mice had increased endurance during an exercise bout to exhaustion (Fig. 8A, B), the older 1-yr-old mice had significantly reduced endurance (Fig. 8C, D) compared with their littermate WT controls. Plasma creatine kinase concentration was increased in both young and older MCK-Lpin1−/− mice compared with WT controls after being unfed overnight and 3 h after the endurance exercise bout (Fig. 8B, D). During sedentary, fed conditions, those mice also had higher levels of plasma creatine kinase (Fig. 8D). Elevations of plasma creatine kinase in those basal conditions suggest a baseline level of myocyte damage with skeletal muscle–specific Lpin1 deficiency that is exacerbated with environmental stressors of being unfed and performing considerable physical activity. These findings parallel observations that fasting and/or exercise can provoke rhabdomyolysis in human patients with LPIN1 mutations.
Figure 8.
MCK-Lpin1Δ115 and MCK-Lpin1−/− mice have altered exercise endurance and increased exercise plasma creatine kinase when unfed. A, C) Exercise endurance time to exhaustion in 3–4-mo-old and 1-yr-old MCK-Lpin1Δ115, respectively. B, D) Exercise endurance time to exhaustion in 3–4-mo-old and 1-yr-old MCK-Lpin1−/−, respectively. Additionally, plasma creatine kinase is elevated in MCK-Lpin1−/− mice compared with their WT controls. Sedentary indicates that plasma creatine kinase was measured under fed and rested conditions; 3-h post-ex indicates that plasma creatine kinase was measured after being unfed overnight, and 3 h after the time to exhaustion in an acute bout of treadmill exercise. Data are shown as means ± sem. *P < 0.05 (Student’s t test) for differences in MCK-Lpin1Δ115 or MCK-Lpin1−/− compared with respective WT littermate mice; n = 7–9.
DISCUSSION
Inborn errors in intermediary metabolism are among the most common causes of unexplained episodic rhabdomyolysis in children. Among those genetic mutations, LPIN1 deficiency has garnered attention with continued reports of otherwise healthy children being afflicted with acute and serious febrile bouts with muscle pain, hyperkalemia, and ventricular tachycardia (7–9, 11, 15). Efforts to develop mouse models of Lpin1 deficiency to understand the mechanism and to test potential treatments are imperative to unraveling the pathophysiology. Because lipin 1 is bifunctional and can affect metabolism by multiple modes of action, dissecting the pathogenic mechanisms of lipin 1 deficiency is more difficult. The present study used 2 muscle-specific models of Lpin1 deficiency in mice. The first model employed a mouse with hypomorphic lipin 1 lacking PAP activity, but with retained transcriptional coregulatory function (19, 25). The second model used a novel gene-deletion strategy that resulted in complete ablation of lipin 1 protein in muscle. Herein, we report that both lines of mice with muscle lipin 1 deficiency exhibited an active and progressive myopathic phenotype and that muscle-specific loss of lipin 1–mediated PAP activity is sufficient to induce muscle injury, which may provide new clues about pathogenic mechanisms.
Recent work by Zhang et al. (16) to dissect the mechanistic aspects of myocyte injury with lipin 1 deficiency used fld mice, which are deficient in lipin 1 in all tissues because of a spontaneous mutation in Lpin1. Those studies suggested that loss of lipin 1 led to muscle injury by alterations in signaling cascades that regulate autophagy, accumulation of dysfunctional and damaged mitochondria, and regenerating muscle fibers and suggested that accumulation of PA and depletion of DAG in fld muscle were important mediators of the phenotype (16, 20). Studies were also conducted in fld mice, complemented by transgenic reexpression of lipin 1 in skeletal muscle, to show a myocyte-specific rescue could be achieved. Nevertheless, the interpretation of the data was complicated by the lipodystrophic phenotype of fld mice. Indeed, although use of MCK-Lpin1Δ115 and MCK-Lpin1−/− mice in the present study confirmed the aberrant autophagy, accumulation of abnormal mitochondria, and accumulation of PA in muscle, the steady state DAG content of muscle was actually increased compared to WT littermate controls. Previous studies have shown that human LPIN1-deficient myocytes accumulate TAG normally in vitro (36), and patients with LPIN1 mutations have lipid accumulation in muscle and heart (10); a key phenotypic difference from the fld mice. Taken together, the present data and previous studies suggest that PA accumulation, rather than depletion of downstream lipids, is linked to myocyte injury. Interestingly, PA accumulation has been linked to potentially deleterious signaling events and cytotoxicity (21, 37–40), and it is possible that multiple intracellular signaling cascades are activated.
The unexpected finding that the product of lipin 1–mediated enzymatic activity, DAG, was increased in both models of skeletal muscle lipin 1 deficiency, whereas muscle TAG abundance showed no change, remains to be explained. Whether the increase in DAG is due to reduced DAG hydrolysis by DAG lipases, such as hormone-sensitive lipase (HSL), decreased phosphorylation by DAG kinase, reduced diacylglycerol acyltransferase activity, increased monoacylglycerol acyltransferase activity, or synthesized from myriad other phospholipids is not known (41–44). We also recently found that DAG was increased and TAG was unchanged in hepatic tissue of mice with liver-specific lipin 1 deficiency using the Δ115 model (25). In addition, increased DAG in skeletal muscle has been demonstrated to result in impaired insulin sensitivity. However, compared with WT littermate controls, MCK-Lpin1Δ115 mice had normal glucose tolerance and isolated soleus and extensor digitorum longus muscles from MCK-Lpin1Δ115 animals exhibited normal insulin-stimulated glucose uptake (unpublished results). Consistent with that, previous work by Michot et al. (7) has suggested that patients with LPIN1 mutations also exhibit normal glucose tolerance and hemoglobin A1c concentrations.
The current model, based on this study and previous work by Zhang et al. (16), suggests that accumulation of mitochondria with abnormal morphology in lipin 1–deficient mice is not a consequence of increased biogenesis but, rather, is explained by impairments in autophagic degradation of mitochondria (mitophagy). Indeed, pathway analyses revealed reduced expression of genes encoding enzymes involved in mitochondrial metabolism as well as the master regulator of mitochondrial biogenesis, PGC-1α, but also accumulation of mitochondrial protein, mitochondrial DNA, and cardiolipin and increased mitochondrial staining of tissue. Furthermore, increased abundance of autophagic proteins (p62, LC3, and BNIP3) in the total cellular protein and mitochondrial fractions of lipin 1–deficient mice are consistent with impaired autophagic and mitophagic flux. It is possible that accumulation of dysfunctional mitochondria, as demonstrated by ultrastructural abnormalities and reduced respiratory control ratio, have a causative role in the myocyte dropout and myopathic phenotype observed in the mice. Strategies to enhance autophagic flux and overcome that deficit may have utility in treating this and other related disorders.
Previous work has suggested that lysosomal membrane PA content may be an important regulator of lysosome permeability, function, and dysfunction (37–39). Our work in a study published very recently (45), and another publication (16) detected a general defect in skeletal muscle autophagy in mice lacking lipin 1. In Alshudukhi et al. (45), LC3-eGFP transgenic mice were used to confirm impairments in autophagic flux and to show that loss of lipin 1 impaired Bnip3-regulated mitophagy by affecting its interaction with LC3. This is a critical step in recruiting mitochondria destined for autophagy to nascent autophagosomes. Abnormalities in lysosomal function and autophagy have been linked to several forms of muscle injury and myopathy, including muscular dystrophy (46–49). How deficits in mitophagy or macroautophagy in general lead to muscle injury and rhabdomyolysis remains to be determined. Some patients with LPIN1 deficiency exhibit mitochondrial aggregation (7), and thus, a mitochondrial phenotype may also be present in humans as well.
Rhabdomyolysis associated with lipin 1 deficiency is often induced by trauma, illness, or environmental stressors and then accompanied by extreme elevations of creatine kinase in the blood (12). The myopathy in MCK-Lpin1Δ115 and MCK-Lpin1−/− mice, although dramatic, appears to differ from severe, episodic rhabdomyolysis and the predominant lack of muscle pathology in children with LPIN1 mutations (7). The dissimilarities could be due to basic differences in muscle metabolism, physiology, or lipin function across species. However, other data suggest that some patients with LPIN1 mutations exhibit a chronic myopathy that seems to affect the oldest patients, suggesting that this phenotype may manifest with age (7). This parallels our observations that the exercise intolerance of muscle-specific lipin 1–deficient mice was apparent in 1-yr-old mice and suggests that careful follow-up examinations of older patients with LPIN1 mutations could reveal a higher incidence of myalgia, myopathy, or exercise intolerance.
In a recent study of children and young adults with LPIN1 mutations undergoing a maximal exercise tests, maximal oxygen consumption tended to be lower in subjects with LPIN1 mutations compared with healthy control subjects (12). In most of the subjects in that study, creatine kinase levels at 8, 16, and 24 h after exercise were within range of their resting, pre-exercise levels, the same resting levels as that found in healthy children. However, 1 subject who exhibited a fever had creatine kinase levels increasing 2-fold, 5-fold, and 10-fold at 8, 16, and 24 h after exercise. Additionally, other reports describe patients to be exercise intolerant between rhabdomyolytic episodes (15). It has also been reported that a high percentage of LPIN1 heterozygous carriers experience exercise-induced myalgia (7). This observation resembles the present mouse study after mice were unfed and engaged in exhaustive exercise intervention, which appears to trigger rhabdomyolysis. Future work will be needed to confirm those observations and to examine the efficacy of preventative measures to alleviate muscle injury in people affected by partial or complete LPIN1 deficiency.
In summary, our data are consistent with a role for skeletal myocyte-autonomous defects in lipin 1–mediated PAP activity in the development of skeletal muscle injury, which is likely due to autophagic and mitochondrial perturbations leading to myocyte death and development of myopathy. The muscle-specific knockout mouse models reported herein will be useful in further dissection of the pathogenic mechanisms and for testing potential therapeutic approaches.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank Dr. Alan Pestronk and Dr. Abhinav Diwan for technical assistance in interpreting histology, and Dennis J. Dietzen for determining plasma creatine kinase (all from Washington University). This work was supported by U.S. National Institutes of Health (NIH) National Institute of Diabetes and Digestive and Kidney Diseases Grant R01 DK078187 and NIH National Heart, Lung, and Blood Institute Grant R01 HL119225 (to B.N.F.). G.G.S. was supported by NIH National Heart, Lung, and Blood Institute Grants T32 HL007275, T32 HL007081, and the Washington University Institute of Clinical and Translational Sciences NIH Grant UL1 TR000448, Subaward KL2 TR000450, from the National Center for Advancing Translational Sciences (NCATS). K.S.M. was supported by NIH National Institute of Diabetes and Digestive and Kidney Diseases Grant T32-DK007120. J.Y. was supported by Washington University Institute of Clinical and Translational Sciences NIH Grant UL1 TR000448, Subaward KL2 TR000450. T.E.H. and J.M.E. were supported by NIH National Institute of Diabetes and Digestive and Kidney Diseases Grant R01 DK101946. C.C.W. and S.K.P were supported by NIH National Institute on Aging Grant R01 AG031867. This work was also supported by the cores of the Washington University Nutrition Obesity Research Center (Grant P30 DK56341), Diabetes Research Center (DRC) (Grant P30 DK020579), the Metabolomics Facility at Washington University (Grant P30 DK056341), and the Washington University NIH National Institute of General Medical Sciences Biomedical Mass Spectrometry Resource (Grant P41 GM103422) to F.-F.H.; the Washington University Genome Technology Access Center in the Department of Genetics (Grant P30 CA91842); and the Department of Pathology DRC Electron Microscopy Facility (Grant P60 DK020579). The authors declare no conflicts of interest.
Glossary
- BCA
bicinchoninic acid assay
- BSA
bovine serum albumin
- CCD
charge-coupled device
- CL
cardiolipin
- DAG
diacylglycerol
- eWAT
epididymal white adipose tissue
- FCCP
carbonyl cyanide‑p‑trifluoromethoxyphenylhydrazone
- FDR
false-discovery rate
- fld
fatty liver dystrophic
- G3P
glycerol-3-phosphate
- H&E
hematoxylin and eosin
- LC-MS
liquid chromatography–mass spectrometry
- LPA
lysophosphatidic acid
- LPIN1
lipin 1
- MCK
muscle creatine kinase
- mtDNA
mitochondrial DNA
- PA
phosphatidic acid
- PAP
phosphatidic acid phosphohydrolase
- PG
phosphatidylglycerol
- TAG
triacylglycerol
- TBST
Tris-buffered saline plus Tween 20
- WT
wild type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
G. G. Schweitzer, S. L. Collier, Z. Chen, K. S. McCommis, S. K. Pittman, J. Yoshino, S. J. Matkovich, F.-F. Hsu, J. M. Eaton, T. E. Harris, C. C. Weihl., and B. N. Finck designed and performed the experiments; G. G. Schweitzer, J. Yoshino, S. J. Matkovich, F.-F. Hsu, T. E. Harris, C. C. Weihl, and B. N. Finck analyzed the data; R. Chrast and C. C. Weihl provided mice and reagent; and G. G. Schweitzer and B. N. Finck wrote and edited the manuscript.
REFERENCES
- 1.Harris T. E., Finck B. N. (2011) Dual function lipin proteins and glycerolipid metabolism. Trends Endocrinol. Metab. 22, 226–233 10.1016/j.tem.2011.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Han G. S., Wu W. I., Carman G. M. (2006) The Saccharomyces cerevisiae lipin homolog is a Mg2+-dependent phosphatidate phosphatase enzyme. J. Biol. Chem. 281, 9210–9218 10.1074/jbc.M600425200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finck B. N., Gropler M. C., Chen Z., Leone T. C., Croce M. A., Harris T. E., Lawrence J. C., Jr., Kelly D. P. (2006) Lipin 1 is an inducible amplifier of the hepatic PGC-1alpha/PPARα regulatory pathway. Cell Metab. 4, 199–210 10.1016/j.cmet.2006.08.005 [DOI] [PubMed] [Google Scholar]
- 4.Peterson T. R., Sengupta S. S., Harris T. E., Carmack A. E., Kang S. A., Balderas E., Guertin D. A., Madden K. L., Carpenter A. E., Finck B. N., Sabatini D. M. (2011) mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway. Cell 146, 408–420 10.1016/j.cell.2011.06.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim H. B., Kumar A., Wang L., Liu G. H., Keller S. R., Lawrence J. C., Jr., Finck B. N., Harris T. E. (2010) Lipin 1 represses NFATc4 transcriptional activity in adipocytes to inhibit secretion of inflammatory factors. Mol. Cell. Biol. 30, 3126–3139 10.1128/MCB.01671-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Z., Gropler M. C., Mitra M. S., Finck B. N. (2012) Complex interplay between the lipin 1 and the hepatocyte nuclear factor 4 α (HNF4α) pathways to regulate liver lipid metabolism. PLoS One 7, e51320 10.1371/journal.pone.0051320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Michot C., Hubert L., Romero N. B., Gouda A., Mamoune A., Mathew S., Kirk E., Viollet L., Rahman S., Bekri S., Peters H., McGill J., Glamuzina E., Farrar M., von der Hagen M., Alexander I. E., Kirmse B., Barth M., Laforet P., Benlian P., Munnich A., JeanPierre M., Elpeleg O., Pines O., Delahodde A., de Keyzer Y., de Lonlay P. (2012) Study of LPIN1, LPIN2 and LPIN3 in rhabdomyolysis and exercise-induced myalgia. J. Inherit. Metab. Dis. 35, 1119–1128 10.1007/s10545-012-9461-6 [DOI] [PubMed] [Google Scholar]
- 8.Michot C., Hubert L., Brivet M., De Meirleir L., Valayannopoulos V., Müller-Felber W., Venkateswaran R., Ogier H., Desguerre I., Altuzarra C., Thompson E., Smitka M., Huebner A., Husson M., Horvath R., Chinnery P., Vaz F. M., Munnich A., Elpeleg O., Delahodde A., de Keyzer Y., de Lonlay P. (2010) LPIN1 gene mutations: a major cause of severe rhabdomyolysis in early childhood. Hum. Mutat. 31, E1564–E1573 10.1002/humu.21282 [DOI] [PubMed] [Google Scholar]
- 9.Bergounioux J., Brassier A., Rambaud C., Bustarret O., Michot C., Hubert L., Arnoux J. B., Laquerriere A., Bekri S., Galene-Gromez S., Bonnet D., Hubert P., de Lonlay P. (2012) Fatal rhabdomyolysis in 2 children with LPIN1 mutations. J. Pediatr. 160, 1052–1054 10.1016/j.jpeds.2012.02.033 [DOI] [PubMed] [Google Scholar]
- 10.Zeharia A., Shaag A., Houtkooper R. H., Hindi T., de Lonlay P., Erez G., Hubert L., Saada A., de Keyzer Y., Eshel G., Vaz F. M., Pines O., Elpeleg O. (2008) Mutations in LPIN1 cause recurrent acute myoglobinuria in childhood. Am. J. Hum. Genet. 83, 489–494 10.1016/j.ajhg.2008.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schweitzer G. G., Collier S. L., Chen Z., Eaton J. M., Connolly A. M., Bucelli R. C., Pestronk A., Harris T. E., Finck B. N. (2015) Rhabdomyolysis-associated mutations in human LPIN1 lead to loss of phosphatidic acid phosphohydrolase activity. JIMD Rep. 23, 113–122 10.1007/8904_2015_440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Legendre A., Khraiche D., Ou P., Mauvais F. X., Madrange M., Guemann A. S., Jais J. P., Bonnet D., Hamel Y., de Lonlay P. (2018) Cardiac function and exercise adaptation in 8 children with LPIN1 mutations. Mol. Genet. Metab. 123, 375–381 10.1016/j.ymgme.2017.12.429 [DOI] [PubMed] [Google Scholar]
- 13.Pichler K., Scholl-Buergi S., Birnbacher R., Freilinger M., Straub S., Brunner J., Zschocke J., Bittner R. E., Karall D. (2015) A novel therapeutic approach for LPIN1 mutation-associated rhabdomyolysis—the Austrian experience. Muscle Nerve 52, 437–439 10.1002/mus.24749 [DOI] [PubMed] [Google Scholar]
- 14.Meijer I. A., Sasarman F., Maftei C., Rossignol E., Vanasse M., Major P., Mitchell G. A., Brunel-Guitton C. (2015) LPIN1 deficiency with severe recurrent rhabdomyolysis and persistent elevation of creatine kinase levels due to chromosome 2 maternal isodisomy. Mol. Genet. Metab. Rep. 5, 85–88 10.1016/j.ymgmr.2015.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jaradat S. A., Amayreh W., Al-Qa’qa’ K., Krayyem J. (2015) Molecular analysis of LPIN1 in Jordanian patients with rhabdomyolysis. Meta Gene 7, 90–94 10.1016/j.mgene.2015.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang P., Verity M. A., Reue K. (2014) Lipin-1 regulates autophagy clearance and intersects with statin drug effects in skeletal muscle. Cell Metab. 20, 267–279 10.1016/j.cmet.2014.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reue K., Xu P., Wang X. P., Slavin B. G. (2000) Adipose tissue deficiency, glucose intolerance, and increased atherosclerosis result from mutation in the mouse fatty liver dystrophy (fld) gene. J. Lipid Res. 41, 1067–1076 [PubMed] [Google Scholar]
- 18.Langner C. A., Birkenmeier E. H., Roth K. A., Bronson R. T., Gordon J. I. (1991) Characterization of the peripheral neuropathy in neonatal and adult mice that are homozygous for the fatty liver dystrophy (fld) mutation. J. Biol. Chem. 266, 11955–11964 [PubMed] [Google Scholar]
- 19.Mitra M. S., Chen Z., Ren H., Harris T. E., Chambers K. T., Hall A. M., Nadra K., Klein S., Chrast R., Su X., Morris A. J., Finck B. N. (2013) Mice with an adipocyte-specific lipin 1 separation-of-function allele reveal unexpected roles for phosphatidic acid in metabolic regulation. Proc. Natl. Acad. Sci. USA 110, 642–647 10.1073/pnas.1213493110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang W., Zhu J., Zhuang X., Zhang X., Luo T., Esser K. A., Ren H. (2015) Lipin1 regulates skeletal muscle differentiation through extracellular signal-regulated kinase (ERK) activation and cyclin D complex-regulated cell cycle withdrawal. J. Biol. Chem. 290, 23646–23655 10.1074/jbc.M115.686519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nadra K., de Preux Charles A. S., Médard J. J., Hendriks W. T., Han G. S., Grès S., Carman G. M., Saulnier-Blache J. S., Verheijen M. H., Chrast R. (2008) Phosphatidic acid mediates demyelination in Lpin1 mutant mice. Genes Dev. 22, 1647–1661 10.1101/gad.1638008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCommis K. S., Chen Z., Fu X., McDonald W. G., Colca J. R., Kletzien R. F., Burgess S. C., Finck B. N. (2015) Loss of mitochondrial pyruvate carrier 2 in the liver leads to defects in gluconeogenesis and compensation via pyruvate-alanine cycling. Cell Metab. 22, 682–694 10.1016/j.cmet.2015.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vigueira P. A., McCommis K. S., Schweitzer G. G., Remedi M. S., Chambers K. T., Fu X., McDonald W. G., Cole S. L., Colca J. R., Kletzien R. F., Burgess S. C., Finck B. N. (2014) Mitochondrial pyruvate carrier 2 hypomorphism in mice leads to defects in glucose-stimulated insulin secretion. Cell Rep. 7, 2042–2053 10.1016/j.celrep.2014.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin A., Hales P., Brindley D. N. (1987) A rapid assay for measuring the activity and the Mg2+ and Ca2+ requirements of phosphatidate phosphohydrolase in cytosolic and microsomal fractions of rat liver. Biochem. J. 245, 347–355 10.1042/bj2450347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schweitzer G. G., Chen Z., Gan C., McCommis K. S., Soufi N., Chrast R., Mitra M. S., Yang K., Gross R. W., Finck B. N. (2015) Liver-specific loss of lipin-1-mediated phosphatidic acid phosphatase activity does not mitigate intrahepatic TG accumulation in mice. J. Lipid Res. 56, 848–858 10.1194/jlr.M055962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soufi N., Hall A. M., Chen Z., Yoshino J., Collier S. L., Mathews J. C., Brunt E. M., Albert C. J., Graham M. J., Ford D. A., Finck B. N. (2014) Inhibiting monoacylglycerol acyltransferase 1 ameliorates hepatic metabolic abnormalities but not inflammation and injury in mice. J. Biol. Chem. 289, 30177–30188 10.1074/jbc.M114.595850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mills K. F., Yoshida S., Stein L. R., Grozio A., Kubota S., Sasaki Y., Redpath P., Migaud M. E., Apte R. S., Uchida K., Yoshino J., Imai S. I. (2016) Long-term administration of nicotinamide mononucleotide mitigates age-associated physiological decline in mice. Cell Metab. 24, 795–806 10.1016/j.cmet.2016.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ryan T. E., Brophy P., Lin C. T., Hickner R. C., Neufer P. D. (2014) Assessment of in vivo skeletal muscle mitochondrial respiratory capacity in humans by near-infrared spectroscopy: a comparison with in situ measurements. J. Physiol. 592, 3231–3241 10.1113/jphysiol.2014.274456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ju J. S., Varadhachary A. S., Miller S. E., Weihl C. C. (2010) Quantitation of “autophagic flux” in mature skeletal muscle. Autophagy 6, 929–935 10.4161/auto.6.7.12785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang C., Wendel A. A., Keogh M. R., Harris T. E., Chen J., Coleman R. A. (2012) Glycerolipid signals alter mTOR complex 2 (mTORC2) to diminish insulin signaling. Proc. Natl. Acad. Sci. USA 109, 1667–1672 10.1073/pnas.1110730109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Daum G., Vance J. E. (1997) Import of lipids into mitochondria. Prog. Lipid Res. 36, 103–130 10.1016/S0163-7827(97)00006-4 [DOI] [PubMed] [Google Scholar]
- 32.Horvath S. E., Daum G. (2013) Lipids of mitochondria. Prog. Lipid Res. 52, 590–614 10.1016/j.plipres.2013.07.002 [DOI] [PubMed] [Google Scholar]
- 33.Frazier E. P., Isenberg J. S., Shiva S., Zhao L., Schlesinger P., Dimitry J., Abu-Asab M. S., Tsokos M., Roberts D. D., Frazier W. A. (2011) Age-dependent regulation of skeletal muscle mitochondria by the thrombospondin-1 receptor CD47. Matrix Biol. 30, 154–161 10.1016/j.matbio.2010.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Settembre C., Fraldi A., Medina D. L., Ballabio A. (2013) Signals from the lysosome: a control centre for cellular clearance and energy metabolism. Nat. Rev. Mol. Cell Biol. 14, 283–296 10.1038/nrm3565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma X., Liu H., Foyil S. R., Godar R. J., Weinheimer C. J., Hill J. A., Diwan A. (2012) Impaired autophagosome clearance contributes to cardiomyocyte death in ischemia/reperfusion injury. Circulation 125, 3170–3181 10.1161/CIRCULATIONAHA.111.041814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Michot C., Mamoune A., Vamecq J., Viou M. T., Hsieh L. S., Testet E., Lainé J., Hubert L., Dessein A. F., Fontaine M., Ottolenghi C., Fouillen L., Nadra K., Blanc E., Bastin J., Candon S., Pende M., Munnich A., Smahi A., Djouadi F., Carman G. M., Romero N., de Keyzer Y., de Lonlay P. (2013) Combination of lipid metabolism alterations and their sensitivity to inflammatory cytokines in human lipin-1-deficient myoblasts. Biochim. Biophys. Acta 1832, 2103–2114 10.1016/j.bbadis.2013.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao K., Zhou H., Zhao X., Wolff D. W., Tu Y., Liu H., Wei T., Yang F. (2012) Phosphatidic acid mediates the targeting of tBid to induce lysosomal membrane permeabilization and apoptosis. J. Lipid Res. 53, 2102–2114 10.1194/jlr.M027557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yi Y. P., Wang X., Zhang G., Fu T. S., Zhang G. J. (2006) Phosphatidic acid osmotically destabilizes lysosomes through increased permeability to K+ and H+. Gen. Physiol. Biophys. 25, 149–160 [PubMed] [Google Scholar]
- 39.Hu J. S., Li Y. B., Wang J. W., Sun L., Zhang G. J. (2007) Mechanism of lysophosphatidylcholine-induced lysosome destabilization. J. Membr. Biol. 215, 27–35 10.1007/s00232-007-9002-7 [DOI] [PubMed] [Google Scholar]
- 40.Repnik U., Stoka V., Turk V., Turk B. (2012) Lysosomes and lysosomal cathepsins in cell death. Biochim. Biophys. Acta 1824, 22–33 10.1016/j.bbapap.2011.08.016 [DOI] [PubMed] [Google Scholar]
- 41.Timmers S., de Vogel-van den Bosch J., Hesselink M. K., van Beurden D., Schaart G., Ferraz M. J., Losen M., Martinez-Martinez P., De Baets M. H., Aerts J. M., Schrauwen P. (2011) Paradoxical increase in TAG and DAG content parallel the insulin sensitizing effect of unilateral DGAT1 overexpression in rat skeletal muscle. PLoS One 6, e14503 10.1371/journal.pone.0014503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith S. J., Cases S., Jensen D. R., Chen H. C., Sande E., Tow B., Sanan D. A., Raber J., Eckel R. H., Farese R. V., Jr (2000) Obesity resistance and multiple mechanisms of triglyceride synthesis in mice lacking Dgat. Nat. Genet. 25, 87–90 10.1038/75651 [DOI] [PubMed] [Google Scholar]
- 43.Chibalin A. V., Leng Y., Vieira E., Krook A., Björnholm M., Long Y. C., Kotova O., Zhong Z., Sakane F., Steiler T., Nylén C., Wang J., Laakso M., Topham M. K., Gilbert M., Wallberg-Henriksson H., Zierath J. R. (2008) Downregulation of diacylglycerol kinase delta contributes to hyperglycemia-induced insulin resistance. Cell 132, 375–386 10.1016/j.cell.2007.12.035 [DOI] [PubMed] [Google Scholar]
- 44.Badin P. M., Louche K., Mairal A., Liebisch G., Schmitz G., Rustan A. C., Smith S. R., Langin D., Moro C. (2011) Altered skeletal muscle lipase expression and activity contribute to insulin resistance in humans. Diabetes 60, 1734–1742 10.2337/db10-1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alshudukhi, A. A., Zhu, J., Huang, D., Jama, A., Smith, J. D., Wang, Q. J., Esser, K. A., Ren, H. (2018) Lipin-1 regulates Bnip3-mediated mitophagy in glycolytic muscle. [E-pub ahead of print] FASEB J. 10.1096/fj.201800374
- 46.Sandri M., Coletto L., Grumati P., Bonaldo P. (2013) Misregulation of autophagy and protein degradation systems in myopathies and muscular dystrophies. J. Cell Sci. 126, 5325–5333 10.1242/jcs.114041 [DOI] [PubMed] [Google Scholar]
- 47.Grumati P., Coletto L., Sabatelli P., Cescon M., Angelin A., Bertaggia E., Blaauw B., Urciuolo A., Tiepolo T., Merlini L., Maraldi N. M., Bernardi P., Sandri M., Bonaldo P. (2010) Autophagy is defective in collagen VI muscular dystrophies, and its reactivation rescues myofiber degeneration. Nat. Med. 16, 1313–1320 10.1038/nm.2247 [DOI] [PubMed] [Google Scholar]
- 48.De Palma C., Morisi F., Cheli S., Pambianco S., Cappello V., Vezzoli M., Rovere-Querini P., Moggio M., Ripolone M., Francolini M., Sandri M., Clementi E. (2012) Autophagy as a new therapeutic target in Duchenne muscular dystrophy. Cell Death Dis. 3, e418 10.1038/cddis.2012.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.De Palma C., Perrotta C., Pellegrino P., Clementi E., Cervia D. (2014) Skeletal muscle homeostasis in duchenne muscular dystrophy: modulating autophagy as a promising therapeutic strategy. Front. Aging Neurosci. 6, 188 10.3389/fnagi.2014.00188 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.