Abstract
VEGF signaling via VEGF receptor-2 (VEGFR2) is a major regulator of endothelial cell (EC) functions, including angiogenesis. Although most studies of angiogenesis focus on soluble VEGF signaling, mechanical signaling also plays a critical role. Here, we examined the consequence of disruption of mechanical signaling on soluble signaling pathways. Specifically, we observed that small interfering RNA (siRNA) knockdown of a mechanosensitive ion channel, transient receptor potential vanilloid 4 (TRPV4), significantly reduced perinuclear (Golgi) VEGFR2 in human ECs with a concomitant increase in phosphorylation at Y1175 and membrane translocation. TRPV4 knockout (KO) ECs exhibited increased plasma membrane localization of phospho-VEGFR2 compared with normal ECs. The knockdown also increased phospho-VEGFR2 in whole cell lysates and membrane fractions compared with control siRNA-treated cells. siRNA knockdown of TRPV4 enhanced nuclear localization of mechanosensitive transcription factors, yes-associated protein/transcriptional coactivator with PDZ-binding motif via rho kinase, which were shown to increase VEGFR2 trafficking to the plasma membrane. Furthermore, TRPV4 deletion/knockdown enhanced VEGF-mediated migration in vitro and increased expression of VEGFR2 in vivo in the vasculature of TRPV4 KO tumors compared with wild-type tumors. Our results thus show that TRPV4 channels regulate VEGFR2 trafficking and activation to identify novel cross-talk between mechanical (TRPV4) and soluble (VEGF) signaling that controls EC migration and angiogenesis.—Kanugula, A. K., Adapala, R. K., Midha, P., Cappelli, H. C., Meszaros, J. G., Paruchuri, S., Chilian, W. M., Thodeti, C. K., Novel noncanonical regulation of soluble VEGF/VEGFR2 signaling by mechanosensitive ion channel TRPV4.
Keywords: angiogenesis, endothelial cell, phosphorylation
Angiogenesis is required for normal function of the cardiovascular system; however, excessive or insufficient angiogenesis causes a variety of vascular diseases, including ischemic coronary heart diseases, cancer, diabetic/proliferative retinopathy, and age-related macular degeneration (1–3). Although angiogenesis has become an ideal target for therapeutic intervention in these diseases, most studies on angiogenesis focus on soluble angiogenic factors, such as VEGF. VEGF signals via VEGF receptor-2 (VEGFR2) to initiate endothelial cell (EC) proliferation and migration and also regulates vascular patterning by controlling delta/notch signaling (4). Despite the development of several antiangiogenic drugs based on anti-VEGF strategies, there are still challenges (e.g., development of resistance of tumor ECs to anti-VEGF therapy over time) that have resulted in the exploration of new avenues to treat angiogenesis (5–8).
The mechanical forces generated by hemodynamic forces can activate complex, yet largely unidentified, signaling pathways that control angiogenic processes such as EC growth, migration, and survival (9–11). However, it is unclear how mechanotransduction pathways integrate or cross-talk with soluble (growth factor) signaling in angiogenesis. We have previously identified transient receptor potential vanilloid 4 (TRPV4) as a mechanosensor that mediates integrin-to-integrin signaling during cyclic strain-induced EC reorientation (12). We and others have further shown that TRPV4 plays an important role in regulating normal EC physiology via mechanotransduction (13–16). Although our latest findings revealed that TRPV4 negatively regulates angiogenesis by modulating the Rho/Rho kinase pathway (17), the nexus between TRPV4 mechanotransduction and soluble VEGFR2 signaling and their regulation in angiogenesis is not yet known.
VEGF/VEGFR signaling is tightly controlled at various levels that include receptor expression, availability, recycling, localization, and phosphorylation (18). In the current study, we investigated the cross-talk between mechanosensitive ion channel TRPV4 and the VEGF/VEGFR2 axis in ECs by focusing on its localization and phosphorylation. We believe this cross-talk is imperative for the coordination of mechanical and soluble (growth factor) signals in angiogenesis.
MATERIALS AND METHODS
Cell culture
Human microvascular endothelial cells (HMECs-1) were obtained from ATCC (Manassas, VA, USA). HUVECs, normal endothelial cells (NECs), and TRPV4 knockout (KO) ECs were cultured as previously described (13, 17, 19).
Transfection
Human ECs were transiently transfected with either 50 nM of TRPV4 small interfering RNA (siRNA) or control siRNA (Dharmacon, Lafayette, CO, USA) in the presence of 0.3% serum using siLentiFect (Bio-Rad, Hercules, CA, USA) for 48 h. TRPV4 knockdown was measured by using PCR and Western blot analysis after 48 h of siRNA transfection (i.e., silencing). In some experiments, cells were treated with Y-27632 (MilliporeSigma, Burlington, MA, USA) 30 h after the siRNA transfection for another 12 h.
RT-PCR and quantitative PCR
RNA was extracted from human ECs by using the RNeasy Mini Kit (Qiagen, Hilden, Germany) and quantified with the NanoDrop 2000 UV-Vis Spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). cDNA was synthesized with the RevertAid first strand cDNA synthesis kit, and the Fast SYBR green master mix was used for quantitative PCR analysis on the Fast Real-Time PCR system (all from Thermo Fisher Scientific).
The following real-time primers were obtained from Integrated DNA Technologies (Coralville, IA, USA): TRPV4 (forward-5′-TCACTCTCACCGCCTACTACCA-3′, reverse: 5′-CCCAGTGAAGAGCGTAATGACC-3′) and β-actin (forward-5′-ACGTTGCTATCCAGGCTGTG-3′, reverse: 5′-GAGGGCATACCCCTCGT-AGA-3′). Gene expression was made relative to the loading control (β-actin), and ΔΔCt values were expressed as fold change over control siRNA.
Western blot and membrane fractionation
Cells were either directly lysed in RIPA buffer containing protease and phosphatase inhibitor cocktails (MilliporeSigma and Roche, Basel, Switzerland) or subjected to subcellular fractionation to extract the membrane fraction (Thermo Fisher Scientific). Lysates were loaded onto 7.5% precast polyacrylamide gels (Bio-Rad) for separation via electrophoresis and transferred onto a PVDF membrane. The membrane was blocked in 5% bovine serum albumin in Tris-buffered saline (TBS) with 0.1% Tween-20, followed by overnight incubation with primary antibodies: phospho-VEGFR2 (p-VEGFR2) Y1175 (1:1000; Cell Signaling Technology, Danvers, MA, USA); total VEGFR2 (1:1000; Cell Signaling Technology); glyceraldehyde-3-phosphate dehydrogenase (1:5000; Cell Signaling Technology); anti-TRPV4 (1:300; Alomone Labs, Jerusalem, Israel); anti–β-1 integrin (1:1000; MilliporeSigma); and tubulin (1:5000; Abcam, Cambridge, United Kingdom). After washing 3 times with TBS–Tween-20, membranes were incubated with the appropriate secondary antibodies, goat anti-rabbit (1: 20,000) or goat anti-mouse (1: 20,000), conjugated with horseradish peroxidase (The Jackson Laboratory, West Grove, PA, USA). Signals were detected with Luminata Forte Western HRP substrate (MilliporeSigma) and developed with a FluorChem M Simple Imager (Protein Simple, San Jose, CA, USA).
Calcium imaging
ECs cultured on MatTek glass bottom dishes (MatTek, Ashland, MA, USA) were loaded with Fluo-4/AM (4 µM) for 20 min, and the calcium influx was monitored as previously described (12, 13, 20, 21) by using the Olympus FluoView 300 confocal microscope (Olympus, Shinjuku, Tokyo, Japan). Cells were stimulated with a TRPV4 agonist, GSK1016790A (GSK101; 1 nM), in the presence and absence of a TRPV4 antagonist, GSK2193874 (GSK2). In the indicated experiments, cells were pretreated with TRPV4 antagonist GSK2 (50 nM) for 20 min.
Immunocytochemistry
ECs were fixed in 4% paraformaldehyde (20 min), washed with PBS, and permeabilized with 0.25% Triton X-100 (15 min), followed by blocking with 5% bovine serum albumin for 30 min at room temperature. Cells were then incubated with total VEGFR2, p-VEGFR2 Y1175 (1:100; Cell Signaling Technology), yes-associated protein (YAP) (1:100; Santa Cruz Biotechnology, Dallas, TX, USA), or Golgi marker TGN46 (1:20; Abcam) antibodies for 1 h at room temperature. After washing, cells were incubated with Alexa Fluor conjugated secondary antibody (1:500; Thermo Fisher Scientific) and mounted with DAPI containing mounting medium (Vector Laboratories, Burlingame, CA, USA). For F-actin staining, cells were incubated with Alex Fluor-Phalloidin (1:500; Thermo Fisher Scientific). Images were acquired by using an Olympus IX-71 fluorescence microscope (Olympus).
Scratch-wound migration assay
NECs and TRPV4KO ECs were plated on a 0.1% gelatin-coated 6-well plate and grown until cells formed a confluent monolayer. Cells were thoroughly washed with PBS to remove traces of growth factors. Cells were serum starved overnight and then a scratch was made in the EC monolayer by using a 200 μl tip; migration was monitored in the presence of VEGF (50 ng/ml) by acquiring images at 0 and 24 h by using an Olympus IX-51 brightfield microscope (Olympus). Percent migration was quantified from the acquired images by using ImageJ software (National Institutes of Health, Bethesda, MD, USA), as previously described (17).
Immunohistochemistry of VEGFR2 in tumors
In vivo tumor experiments were performed according to an approved protocol by Northeast Ohio Medical University’s Institutional Animal Care and Use Committee. Syngeneic tumors were generated by subcutaneously injecting mouse Lewis lung carcinoma cells (2 × 106) in the flank region of wild-type (WT) and TRPV4KO mice (C57BL/6 background), as previously described (13). Mice were euthanized on d 21, and tumor tissues were extracted and frozen in optimum cutting temperature compound (Tissue-Tek, Sakura Finetek USA, Torrance, CA, USA). Frozen sections (10 μm; collected from both the central and peripheral regions of the tumor) were fixed and permeabilized in ice-cold acetone (20 min), washed with TBS, and incubated with rat–anti-CD31 (1:50; Thermo Fisher Scientific) and anti-VEGFR2 (1:50; Cell Signaling Technology) antibodies overnight. Sections were then washed with TBS (3 times, 5 min) and incubated for 1 h with appropriate secondary antibodies coupled to Alexa Fluor-488 or Alexa Fluor-594 (Thermo Fisher Scientific) and mounted with DAPI (Vector Laboratories). Images were acquired with an Olympus Epifluorescence Microscope (IX71) (Olympus) using Metamorph (Molecular Devices, San Jose, CA, USA) and quantified by using ImageJ software. The total number of CD31+ stained vessels and the number of vessels that colocalized with VEGFR2 were counted and expressed as a percentage of VEGFR2 vessels (CD31+ + VEGFR2+/CD31+).
RESULTS
TRPV4 knockdown/deletion reduces perinuclear (Golgi) VEGFR2 and induces its membrane translocation
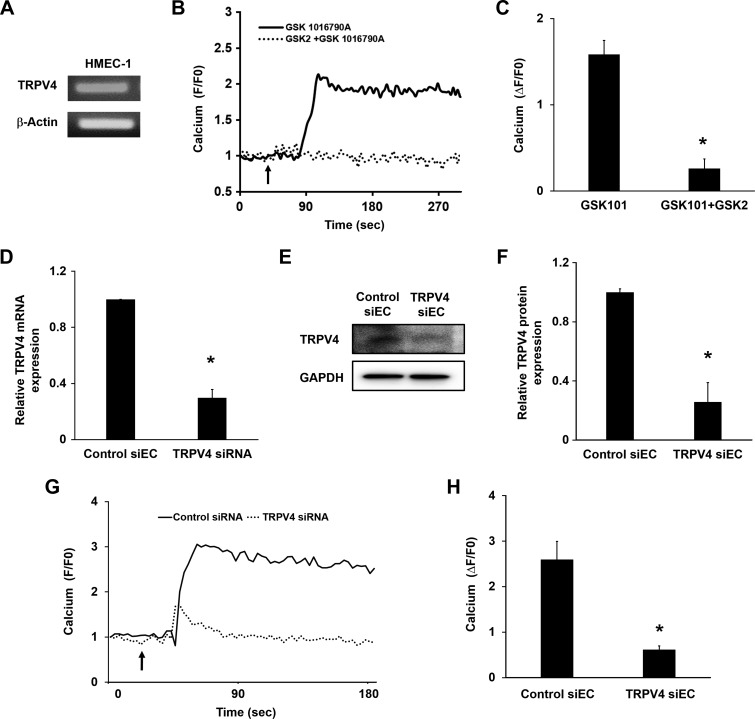
To determine whether cross-talk exists between VEGFR2 and TRPV4, we used both human (HMECs-1) and mouse (NECs, TRPV4KO ECs) microvascular ECs, with HUVECs as a control. HMECs-1 have not been well characterized for TRPV4 expression and function to this point, and we confirmed TRPV4 expression by using RT-PCR and Western blot analysis in HMECs-1 (Fig. 1A, E). In addition, the TRPV4 selective agonist GSK1016790A (GSK101; 1 nM) robustly induced calcium influx in Fluo-4–loaded cells, suggesting that TRPV4 is functionally active in HMECs-1. Notably, this calcium influx was blunted when the cells were pretreated with the TRPV4-specific antagonist GSK2193874 (GSK2; 50 nM) (Fig. 1B, C). To independently confirm the functional expression of TRPV4 in this cell type, we knocked down TRPV4 by using specific siRNA. Both RNA and protein expression of TRPV4 were significantly reduced (70 ± 5%; P < 0.05) compared with control siRNA (Fig. 1D–F) in HMEC-1. Furthermore, siRNA knockdown of TRPV4 abolished GSK-induced calcium influx in these cells (Fig. 1G, H). Although HUVECs express functional TRPV4 (22), we also confirmed functional expression of TRPV4 channels by using quantitative PCR, Western blot, and calcium imaging (Supplemental Fig. 1). These data confirm that both macrovascular and microvascular ECs (HUVECs and HMECs-1) express functional TRPV4.
Figure 1.
TRPV4 is functionally expressed in HMECs-1. A) RT-PCR analysis of TRPV4 gene expression in HMECs-1. B) Representative traces display relative changes in cytosolic calcium in response to a selective TRPV4 agonist, GSK1016790A (GSK101; 1 nM), and antagonist, GSK2193874 (GSK2; 50 nM), in Fluo-4–loaded cells (n = 60). Arrow denotes the time when the cells were stimulated with the TRPV4 agonist. C) Quantitative analysis of cytosolic calcium influx induced by GSK101 and GSK101 + GSK2 in HMECs-1 (F/F0 = ratio of normalized Fluo-4 fluorescence intensity relative to time 0). D–F) Quantitative RT-PCR and Western blot analysis showing relative TRPV4 expression in control (control siEC) and TRPV4 siRNA transfected (TRPV4 siEC) siECs after 48 h. G) Representative traces display relative changes in cytosolic calcium in response to a selective GSK101 in Fluo-4– loaded control siECs and TRPV4 siECs (n = 60). Arrow denotes the time when the cells were stimulated with the TRPV4 agonist. H) Quantitative analysis of cytosolic calcium influx induced by GSK101 in control siECs and TRPV4 siECs (F/F0 = ratio of normalized Fluo-4 fluorescence intensity relative to time 0). Data presented are the means ± sem from at least 3 independent experiments. The significance was calculated by using Student’s t test. *P ≤ 0.05.
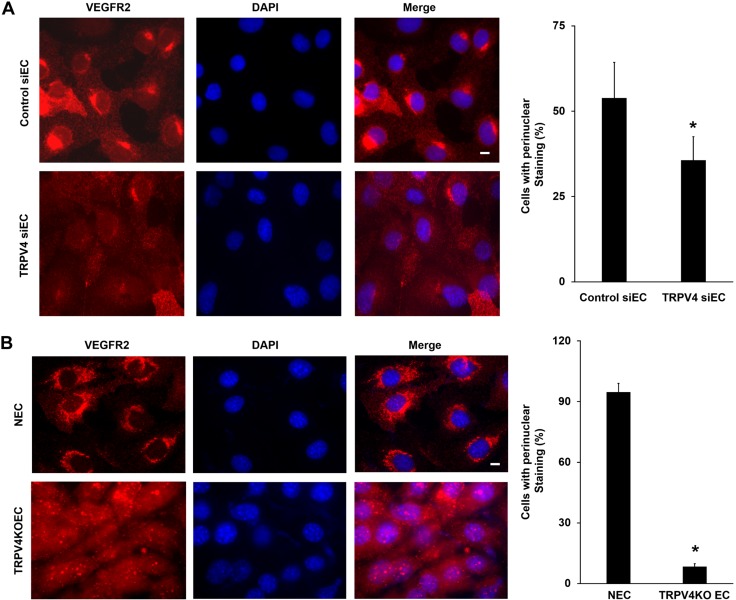
We next measured total VEGFR2 expression and localization in control siRNA-treated ECs (siECs) and TRPV4 siRNA knockdown HMECs-1 (TRPV4 siECs) by immunofluorescence. Immunostaining revealed that control siECs exhibit strong perinuclear (Golgi) staining of VEGFR2 (Fig. 2A and Supplemental Fig. 2) as well as diffuse cytoplasmic staining. Surprisingly, quantitative analysis revealed a significant (P < 0.05) decrease in the percentage of cells that displayed perinuclear (Golgi) VEGFR2 staining in TRPV4 siECs (35.6 ± 11%) compared with control siECs (53.8 ± 18%). Furthermore, measurement of perinuclear VEGFR2 intensity revealed significant reduction (1.35-fold; P < 0.05) in TRPV4 siECs compared with control siECs (Supplemental Fig. 3). To confirm this observation, we measured VEGFR2 localization in NECs and compared it with TRPV4KO ECs. Notably, predominant perinuclear VEGFR2 localization was found in NECs (Fig. 2B). Quantitative analysis revealed a low percentage of cells that displayed perinuclear VEGFR2 staining in TRPV4KO ECs (8.4 ± 1.3%) compared with NECs (94.7 ± 4%). Interestingly, VEGFR2 is found mainly in the cytoplasm in TRPV4KO ECs, with almost no perinuclear staining. Importantly, we found that VEGF stimulation, similar to the absence of TRPV4, significantly attenuated perinuclear localization of VEGFR2 in HMECs-1 (Supplemental Fig. 4), suggesting that TRPV4 may modulate VEGFR2 trafficking/localization akin to VEGF.
Figure 2.
TRPV4 knockdown/deletion reduces perinuclear (Golgi) VEGFR2 expression. A) Immunofluorescence images of control siECs and TRPV4 siECs of HMECs-1 showing localization of total VEGFR2 (red). Nuclei were stained with DAPI (blue). The graph represents the quantitative analysis of the percentage of cells with perinuclear localization of VEGFR2. B) Immunofluorescence images of total VEGFR2 (red) in NECs and TRPV4KOECs. Nuclei were stained with DAPI (blue). The graph depicts the quantitative analysis of the percentage of cells with perinuclear localization of VEGFR2. Data presented are means ± sem of 3 independent experiments. *P ≤ 0.05. Scale bars, 10 μm.
TRPV4 knockdown/deletion increases p-VEGFR2 Y1175 levels in the plasma membrane
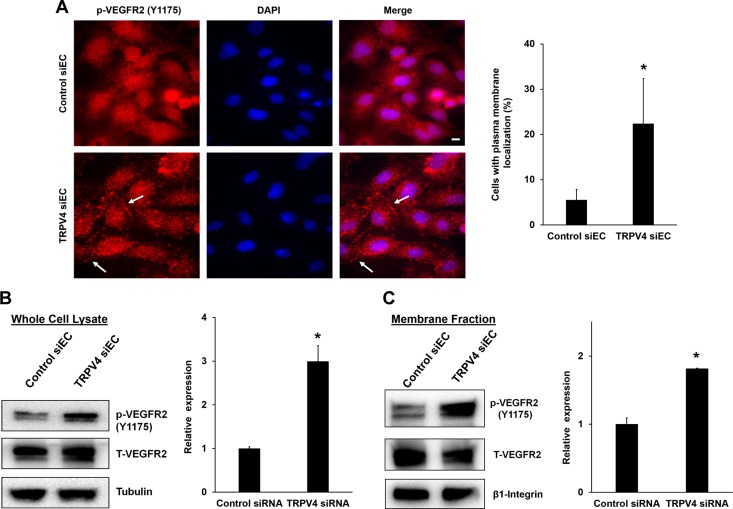
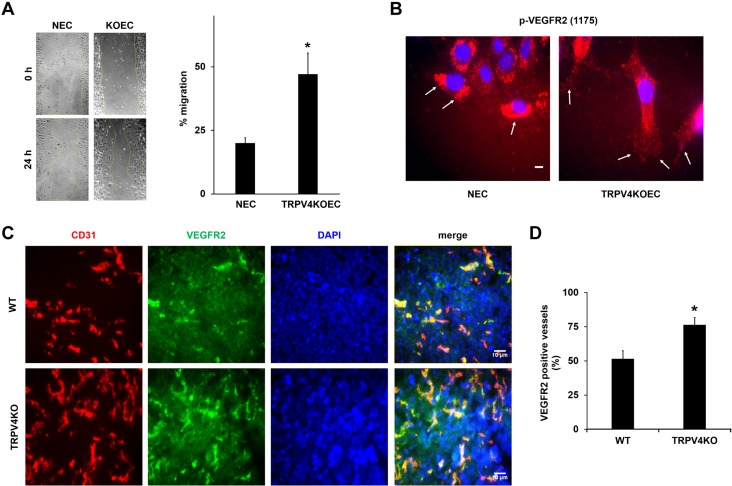
To investigate the altered VEGFR2 localization in TRPV4 knocked down or deleted cells, and determine the state of phosphorylation, p-VEGFR2 was measured at Y1175 by using phospho-specific antibodies [Y1173, in case of mouse ECs (however, for brevity we described it as Y1175)]. In control cells, the majority of p-VEGFR2 staining was found around the nucleus, with a diffused pattern throughout the cytoplasm. However, in TRPV4 siECs, p-VEGFR2 staining was evident throughout the cytoplasm, with some distinct localization at the plasma membrane. Quantitative analysis (Fig. 3A) revealed a 3-fold increase in the percentage of cells that exhibited plasma membrane localization of p-VEGFR2 in TRPV4 siECs (22.3 ± 9.9%) compared with control cells (5.5 ± 2.3%). Furthermore, we found that TRPV4KO ECs exhibited more p-VEGFR2 at the plasma membrane, whereas NECs displayed predominantly perinuclear localization (Supplemental Fig. 5).
Figure 3.
TRPV4 knockdown/deletion increases phosphorylation (Y1175) and membrane translocation of VEGFR2. A) Immunofluorescence images of p-VEGFR2 (red) in control siECs and TRPV4 siECs. Arrows denote membrane localization of p-VEGFR2. Nuclei were stained with DAPI (blue). The graph depicts the quantitative analysis of percent cells with membrane localization of p-VEGFR2 from control and TRPV4 siECs. Data presented are means ± sem of 3 independent experiments. B) Western blot analysis represents the expression levels of total and p-VEGFR2 in whole cell lysates from control siEC and TRPV4 siEC. Relative p-VEGFR2 expression was quantified by normalizing with total VEGFR2. The graph represents the quantitative analysis of relative p-VEGFR2 expression from the whole cell lysates of control and TRPV4 siEC. C) Western blots demonstrating the expression levels of total and p-VEGFR2 in membrane fractions from control and TRPV4 siEC. β1-integrin expression was used as a loading control for plasma membrane fractions. The graph depicts the quantitative analysis of relative p-VEGFR2 expression from the membrane fractions of control and TRPV4 siEC. The given results are means ± sem from 3 independent experiments. *P ≤ 0.05. Scale bar, 10 μm.
To further confirm the VEGFR2 membrane translocation and phosphorylation, Western blotting analysis was performed in whole cell lysates and membrane fractions from control siECs and TRPV4 siECs. We found that p-VEGFR2 expression was significantly (∼2.5 fold) higher in TRPV4 siECs compared with control ECs (Fig. 3B). Notably, there was no change in total VEGFR2 expression. Subcellular fractionation revealed that a significant (2-fold) proportion of p-VEGFR2 was localized in the membrane fraction of TRPV4 siECs compared with control siECs (Fig. 3C). β1-integrin expression was used as plasma membrane loading control which was unchanged.
TRPV4 knockdown/deletion increases nuclear translocation of YAP via Rho kinase pathway
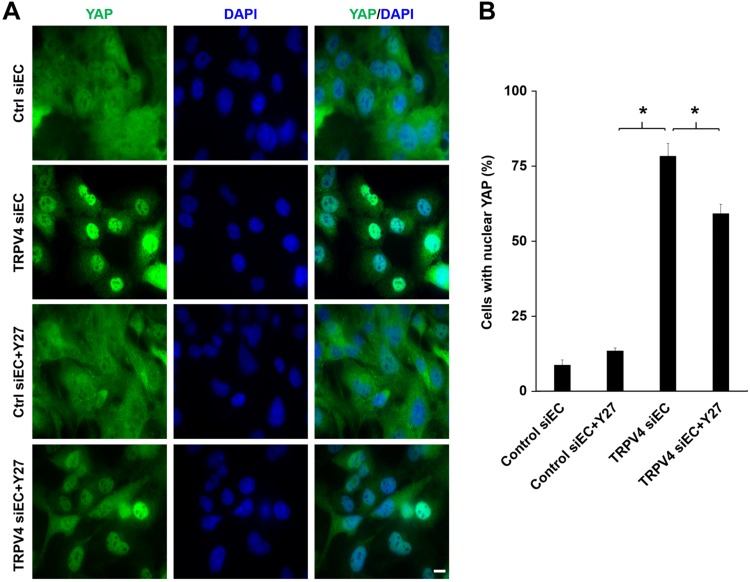
To delineate the molecular mechanism behind the VEGFR2 translocation, we focused on the mechanotranscription factors YAP/transcriptional coactivator with PDZ-binding motif (TAZ), which were shown to regulate VEGFR2 trafficking to the plasma membrane (23). To achieve this goal, we knocked down TRPV4 by using siRNA in HMECs-1 and HUVECs (data not shown) and measured YAP nuclear localization. We found that in control siECs, YAP staining was evident throughout the cell with no specific localization (Fig. 4). Notably, knock down of TRPV4 induced significant (control siECs vs. TRPV4 siEC: 8.7 ± 1.6% vs. 78.3 ± 4.2%; P < 0.05) translocation of YAP to the nucleus. Next, we questioned if TRPV4 knockdown–induced YAP nuclear localization is mediated through the Rho/Rho kinase pathway. To answer this question, we treated both TRPV4 siECs and control siECs with the Rho kinase inhibitor Y-27632 and measured YAP nuclear translocation. We found that Y-27632 significantly reduced (∼20%) YAP nuclear localization in TRPV4 siECs but not in control siECs. Importantly, Y-27632 alone did not change YAP nuclear translocation. Taken together, these results suggest that knockdown or deletion of TRPV4 regulates VEGFR2 localization and phosphorylation potentially through the activation of the transcription factor YAP/TAZ, at least in part via the Rho/Rho kinase pathway.
Figure 4.
TRPV4 knockdown increases YAP nuclear translocation in HMECs-1 via the rho kinase pathway. A) Immunofluorescence images of YAP (green) in control siECs and TRPV4 siECs in the presence or absence of Y-27632 (Y27; 10 μM). Nuclei were stained with DAPI (blue). Scale bar, 10 μm. B) The graph represents the quantitative analysis of the percentage of cells with nuclear localization of YAP from control siEC, TRPV4 siEC, control siEC + Y27, and TRPV4 siEC + Y27. Data presented are means ± sem of 3 independent experiments. *P ≤ 0.05.
Deletion of TRPV4 increases VEGF-induced EC migration in vitro and VEGFR2 expression in tumor vasculature in vivo
Previous studies have shown that VEGF/VEGFR2 signaling critically regulates EC migration and angiogenesis (24, 25). To explore the functional consequence of increased activation of VEGFR2 in the absence of TRPV4, VEGF-induced EC migration was measured by using a scratch-wound assay, and we found that cell migration was significantly more in TRPV4KO ECs (47.0 ± 8%; P < 0.05) than in NECs (20.0 ± 2%) (Fig. 5A). Immunostaining revealed that p-VEGFR2 is localized to the plasma membrane at the leading edge (wound) of TRPV4KO ECs (Fig. 5B). However, NECs exhibited a distinct perinuclear staining at the wound edge. To further confirm that the absence of TRPV4 induces this localization of p-VEGFR2, we stained control and TRPV4 siRNA knocked down ECs with phospho-specific VEGFR2 antibody. We found that p-VEGFR2 is localized to the plasma membrane in the TRPV4 siECs at the wound edge (Supplemental Fig. 6). In contrast, control siRNA-treated ECs exhibited p-VEGFR2 staining throughout the cell. These findings confirm that the absence of TRPV4 enhances VEGF signaling in ECs via VEGFR2 translocation and phosphorylation.
Figure 5.
Deletion of TRPV4 increases VEGF-induced migration in vitro and VEGFR2-positive vessels in tumors in vivo. A) Migration of serum-starved NECs and TRPV4KOECs was measured by using a scratch-wound migration assay. The graph represents the percent migration of NECs and TRPV4KOECs. B) Immunofluorescence images of p-VEGFR2 (red) in migrating NECs and TRPV4KOECs. Arrows depict membrane localization of p-VEGFR2 in ECs at the wound edge. Nuclei were stained with DAPI (blue). C) Tumors were implanted into WT and TRPV4KO mice by subcutaneously injecting Lewis lung carcinoma cells, as previously described (13), and isolated on day 21. Representative images (20×) of the tumor tissue stained with CD31 (red), VEGFR2 (green), and DAPI (nuclei) were used to quantify the VEGFR2-positive vessels. D) Quantitative analysis demonstrating a significant increase in the percentage of VEGFR2-positive vessels in tumors from TRPV4KO mice compared with WT mice. Data presented are means ± sem from 3 independent experiments. *P ≤ 0.05. Scale bars, 10 μm.
Finally, to determine the functional significance of TRPV4 in the regulation of VEGF/VEGFR2 signaling in vivo, Lewis lung carcinoma tumors were induced in WT and TRPV4KO mice. Consistent with our previous findings (13), tumor growth was significantly higher in TRPV4KO mice at 21 d compared with WT mice (Supplemental Fig. 7). Frozen sections of tumors, harvested from WT and TRPV4KO mice, were immunostained with the specific EC marker CD31 (red), VEGFR2 (green), and nuclei (DAPI; blue). VEGFR2-positive vessels (both CD31/VEGFR2 positive) were found in WT tumors, which were markedly enhanced in TRPV4KO tumors (Fig. 5C). Quantitative analysis revealed a significant increase (WT −51.5 ± 5.8% vs. TRPV4KO −76.5 ± 5.3%; P < 0.05) in the number of VEGFR2-positive vessels in tumors from TRPV4KO mice compared with tumors from WT mice (Fig. 5D).
DISCUSSION
VEGF/VEGFR2 signaling is a central regulator of angiogenesis (4, 25). Although many studies have investigated the downstream signaling effects of VEGF toward EC proliferation, migration, and angiogenesis, very few have focused on mechanosensors or integrators of soluble (growth factor) and mechanical signaling in angiogenesis. In the present study, we report novel cross-talk between mechanosensitive ion channel TRPV4 and VEGF/VEGFR2. This conclusion is based on the findings that: 1) siRNA knockdown or deletion of TRPV4 reduced the predominantly localized perinuclear (Golgi) pool of VEGFR2 in ECs; 2) TRPV4 down-regulation induced membrane localization and phosphorylation of VEGFR2 at Y1175; and 3) absence of TRPV4 enhanced VEGF-dependent EC migration in vitro and VEGFR2-positive vessels in tumors in vivo.
In addition to soluble VEGF signaling, mechanical forces play an important role in EC function, including angiogenesis (9–11, 13, 14, 26). Fluid shear stress was shown to regulate EC function by activating a mechanosensory complex that includes platelet endothelial cell adhesion molecule, vascular endothelial-cadherin, and VEGFR2. Platelet endothelial cell adhesion molecule-1 can directly transduce mechanical forces to activate vascular endothelial–cadherin, which in turn recruits VEGFR2 to cell–cell junctions (27). Studies indicate that another VEGF receptor, VEGFR3, can also participate in this mechanosensory complex upon stimulation with fluid shear stress (28). However, this signaling pathway was shown to regulate arterial lumen and atherogenesis, not angiogenesis (27–30). Direct evidence for cross-talk between VEGFR2 and mechanical signaling in angiogenesis has been reported by Mammoto et al. (26). They showed that mechanical force–dependent (matrix stiffness) activation of p190RhoGAP regulates VEGFR2 expression via modulation of transcriptional factors GATA2 and TFII-I. However, the upstream mechanosensor that transduces mechanical signaling to p190RhoGAP is not yet known. Our findings in the present study show that mechanosensitive ion channel TRPV4 regulates VEGFR2 localization and activation in ECs and suggest that TRPV4 acts upstream of mechanical regulation of VEGFR2.
One of the salient features of VEGF signaling is the trafficking/localization of VEGFR2. In contrast to the common belief that VEGFR2 presents exclusively at the plasma membrane, we found a significant portion of VEGFR2 in the perinuclear Golgi region in ECs (31). Recent evidence shows that VEGFR2 signaling can be regulated via both canonical (ligand/VEGF-dependent; VEGF/VEGFR2) and noncanonical (ligand/VEGF-independent) pathways (32). Importantly, VEGFR2 is activated in perinuclear Golgi/endosome compartments without a need for its interaction with VEGF at the plasma membrane. In fact, VEGF secreted within the cells (intracrine) is associated with VEGFR2 in these compartments. Although TRP channels have been previously implicated in VEGF signaling, those findings were mostly confined to VEGF-induced calcium signaling (22, 24, 33–36). For example, TRPC3 and TRPC6 channels were shown to mediate VEGF-induced calcium signaling in ECs (24, 34). Later studies revealed that VEGF-evoked calcium signaling is actually mediated through ORAI/CRAC channels, not TRPC6 (22). TRPV4 channels seem to play no role in VEGF-induced calcium signaling, as these channels are expressed and mediate calcium influx at the plasma membrane, and are not involved in calcium release from internal endoplasmic reticulum stores (20, 21). Unlike other TRP channels, TRPV4 is identified as a mechanosensor of shear stress, cyclic strain, and matrix stiffness in ECs (12, 13, 15). Previous studies have shown that TRPV4 can modulate angiogenesis via the Rho/Rho kinase pathway (17), which is regulated by p190RhoGAP. Because p190RhoGAP was linked to regulating VEGFR2 expression in ECs in a matrix stiffness–dependent manner, it is conceivable that TRPV4 could act as an upstream regulator of this cross-talk. Our study revealed that knockdown or deletion of TRPV4 significantly reduced the perinuclear VEGFR2 with an increase in phosphorylation, suggesting noncanonical regulation of VEGFR2 signaling by TRPV4. We also found membrane localization of phosphorylated VEGFR2 in TRPV4 knocked down/deleted EC and that these cells exhibit hallmarks of enhanced VEGFR2 function. These findings suggest that TRPV4/mechanical forces regulate activation of VEGFR2, which is critical for angiogenesis, although the molecular mechanisms are not known.
Previous studies have implicated Rho as a mediator of VEGFR2 membrane localization. Furthermore, p190RhoGAP, an inhibitor of Rho activation, modulates VEGFR2 expression through novel mechanotransduction mechanisms (26). We have previously shown that TRPV4 deletion or deficiency increases basal Rho activity in ECs (13, 17). It is therefore plausible that increased Rho activity in TRPV4KO EC mediates the observed reduction in perinuclear compartment, membrane translocation, and phosphorylation of VEGFR2. Rho activation and dependent reorganization of cytoskeleton have been implicated in the activation/nuclear translocation of YAP/TAZ. Indeed, we found that a Rho kinase inhibitor, Y27632, inhibited YAP translocation in TRPV4 siEC. The latest findings revealed that YAP/TAZ silencing impairs VEGFR2 cellular distribution, trafficking to the plasma membrane, and VEGFR2 downstream signaling in ECs (23). We found that TRPV4 knockdown induces YAP nuclear translocation as well as reductions in perinuclear VEGFR2 and its activation (translocation to membrane and phosphorylation at Y1175), suggesting that TRPV4 is an upstream mechanosensor that regulates VEGF/VEGFR2 signaling through YAP. Notably, deletion or knockdown of TRPV4 resulted in increased VEGF-induced EC migration in vitro and VEGFR2 expression in tumor vasculature. These findings, coupled with enhanced tumor angiogenesis and tumor growth in TRPV4KO mice (13), indicate that deregulation of mechanical control of VEGFR2 localization and activation by TRPV4 deletion induces pathologic angiogenesis by augmenting VEGFR2 expression/signaling in tumors.
In summary, our findings have immense clinical significance, as most of the other studies have focused on curtailing positive regulators of angiogenesis. Our results reinforce TRPV4 as a negative regulator of angiogenesis, most likely by controlling VEGFR2 localization/activation, indicating that angiogenesis is regulated by a tight co-ordination between mechanical and soluble (growth factor) signaling. In contrast to the current strategy of inhibiting angiogenesis by targeting VEGF and its receptors (1, 3–5), our study proposes that TRPV4 is an alternate VEGF-independent strategy to target pathologic angiogenic disorders, such as cancer and retinopathy, which are usually characterized by enhanced VEGFR2 expression/activity. Importantly, targeting a negative regulator of angiogenesis, such as TRPV4, could also offer a potential therapeutic mechanism to enhance angiogenesis in conditions such as ischemic heart disease to improve revascularization.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
This work was supported by U.S. National Institutes of Health (NIH)–National Cancer Institute (R15CA202847) and National Heart, Lung, and Blood Institute (R01HL119705). The authors declare no conflicts of interest.
Glossary
- EC
endothelial cell
- HMEC-1
human microvascular endothelial cell
- KO
knockout
- NEC
normal endothelial cell
- p-VEGFR2
phospho-VEGF receptor-2
- siEC
siRNA treated endothelial cell
- siRNA
small interfering RNA
- TAZ
transcriptional coactivator with PDZ-binding motif
- TBS
tris-buffered saline
- TRPV4
transient receptor potential vanilloid 4
- VEGFR2
VEGF receptor-2
- WT
wild-type
- YAP
yes-associated protein
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
A. K. Kanugula, R. K. Adapala, P. Midha, and H. C. Cappelli performed research, analyzed the data, and edited the manuscript; J. G. Meszaros, S. Paruchuri, and W. M. Chillian edited the manuscript; and C. K. Thodeti designed, interpreted and analyzed data as well as wrote the manuscript.
REFERENCES
- 1.Carmeliet P. (2003) Angiogenesis in health and disease. Nat. Med. 9, 653–660 10.1038/nm0603-653 [DOI] [PubMed] [Google Scholar]
- 2.Carmeliet P., Jain R. K. (2011) Molecular mechanisms and clinical applications of angiogenesis. Nature 473, 298–307 10.1038/nature10144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Folkman J. (1971) Tumor angiogenesis: therapeutic implications. N. Engl. J. Med. 285, 1182–1186 10.1056/NEJM197111182852108 [DOI] [PubMed] [Google Scholar]
- 4.Shibuya M., Claesson-Welsh L. (2006) Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Exp. Cell Res. 312, 549–560 10.1016/j.yexcr.2005.11.012 [DOI] [PubMed] [Google Scholar]
- 5.Caporarello N., Lupo G., Olivieri M., Cristaldi M., Cambria M. T., Salmeri M., Anfuso C. D. (2017) Classical VEGF, Notch and Ang signalling in cancer angiogenesis, alternative approaches and future directions (Review). Mol. Med. Rep. 16, 4393–4402 10.3892/mmr.2017.7179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khan K., Cunningham D., Chau I. (2017) Targeting angiogenic pathways in colorectal cancer: complexities, challenges and future directions. Curr. Drug Targets 18, 56–71 10.2174/1389450116666150325231555 [DOI] [PubMed] [Google Scholar]
- 7.Lupo G., Caporarello N., Olivieri M., Cristaldi M., Motta C., Bramanti V., Avola R., Salmeri M., Nicoletti F., Anfuso C. D. (2017) Anti-angiogenic therapy in cancer: downsides and new pivots for precision medicine. Front. Pharmacol. 7, 519 10.3389/fphar.2016.00519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tamura R., Tanaka T., Miyake K., Yoshida K., Sasaki H. (2017) Bevacizumab for malignant gliomas: current indications, mechanisms of action and resistance, and markers of response. Brain Tumor Pathol. 34, 62–77 10.1007/s10014-017-0284-x [DOI] [PubMed] [Google Scholar]
- 9.Ingber D. E. (1992) Extracellular matrix as a solid-state regulator in angiogenesis: identification of new targets for anti-cancer therapy. Semin. Cancer Biol. 3, 57–63 [PubMed] [Google Scholar]
- 10.Ingber D. E. (2002) Mechanical signaling and the cellular response to extracellular matrix in angiogenesis and cardiovascular physiology. Circ. Res. 91, 877–887 10.1161/01.RES.0000039537.73816.E5 [DOI] [PubMed] [Google Scholar]
- 11.Morrow D., Cullen J. P., Cahill P. A., Redmond E. M. (2007) Cyclic strain regulates the Notch/CBF-1 signaling pathway in endothelial cells: role in angiogenic activity. Arterioscler. Thromb. Vasc. Biol. 27, 1289–1296 10.1161/ATVBAHA.107.142778 [DOI] [PubMed] [Google Scholar]
- 12.Thodeti C. K., Matthews B., Ravi A., Mammoto A., Ghosh K., Bracha A. L., Ingber D. E. (2009) TRPV4 channels mediate cyclic strain-induced endothelial cell reorientation through integrin-to-integrin signaling. Circ. Res. 104, 1123–1130 10.1161/CIRCRESAHA.108.192930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adapala R. K., Thoppil R. J., Ghosh K., Cappelli H. C., Dudley A. C., Paruchuri S., Keshamouni V., Klagsbrun M., Meszaros J. G., Chilian W. M., Ingber D. E., Thodeti C. K. (2016) Activation of mechanosensitive ion channel TRPV4 normalizes tumor vasculature and improves cancer therapy. Oncogene 35, 314–322 10.1038/onc.2015.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baratchi S., Almazi J. G., Darby W., Tovar-Lopez F. J., Mitchell A., McIntyre P. (2016) Shear stress mediates exocytosis of functional TRPV4 channels in endothelial cells. Cell. Mol. Life Sci. 73, 649–666 10.1007/s00018-015-2018-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kohler R., Hoyer J. (2007) Role of TRPV4 in the mechanotransduction of shear stress in endothelial cells. In TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades (Liedtke W. B., and Heller S., eds.), CRC Press/Taylor & Francis, Boca Raton, FL, USA: [PubMed] [Google Scholar]
- 16.Liedtke W. (2007) TRPV channels’ role in osmotransduction and mechanotransduction. Handb. Exp. Pharmacol. 179, 473–487 10.1007/978-3-540-34891-7_28 [DOI] [PubMed] [Google Scholar]
- 17.Thoppil R. J., Cappelli H. C., Adapala R. K., Kanugula A. K., Paruchuri S., Thodeti C. K. (2016) TRPV4 channels regulate tumor angiogenesis via modulation of Rho/Rho kinase pathway. Oncotarget 7, 25849–25861 10.18632/oncotarget.8405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koch S., Tugues S., Li X., Gualandi L., Claesson-Welsh L. (2011) Signal transduction by vascular endothelial growth factor receptors. Biochem. J. 437, 169–183 10.1042/BJ20110301 [DOI] [PubMed] [Google Scholar]
- 19.Thoppil R. J., Adapala R. K., Cappelli H. C., Kondeti V., Dudley A. C., Gary Meszaros J., Paruchuri S., Thodeti C. K. (2015) TRPV4 channel activation selectively inhibits tumor endothelial cell proliferation. Sci. Rep. 5, 14257 10.1038/srep14257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adapala R. K., Talasila P. K., Bratz I. N., Zhang D. X., Suzuki M., Meszaros J. G., Thodeti C. K. (2011) PKCα mediates acetylcholine-induced activation of TRPV4-dependent calcium influx in endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 301, H757–H765 10.1152/ajpheart.00142.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adapala R. K., Thoppil R. J., Luther D. J., Paruchuri S., Meszaros J. G., Chilian W. M., Thodeti C. K. (2013) TRPV4 channels mediate cardiac fibroblast differentiation by integrating mechanical and soluble signals. J. Mol. Cell. Cardiol. 54, 45–52 10.1016/j.yjmcc.2012.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li J., Cubbon R. M., Wilson L. A., Amer M. S., McKeown L., Hou B., Majeed Y., Tumova S., Seymour V. A., Taylor H., Stacey M., O’Regan D., Foster R., Porter K. E., Kearney M. T., Beech D. J. (2011) Orai1 and CRAC channel dependence of VEGF-activated Ca2+ entry and endothelial tube formation. Circ. Res. 108, 1190–1198 10.1161/CIRCRESAHA.111.243352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X., Freire Valls A., Schermann G., Shen Y., Moya I. M., Castro L., Urban S., Solecki G. M., Winkler F., Riedemann L., Jain R. K., Mazzone M., Schmidt T., Fischer T., Halder G., Ruiz de Almodovar C. (2017) YAP/TAZ orchestrate VEGF signaling during developmental angiogenesis. Dev. Cell 42, 462–478.e7 [DOI] [PubMed] [Google Scholar]
- 24.Hamdollah Zadeh M. A., Glass C. A., Magnussen A., Hancox J. C., Bates D. O. (2008) VEGF-mediated elevated intracellular calcium and angiogenesis in human microvascular endothelial cells in vitro are inhibited by dominant negative TRPC6. Microcirculation 15, 605–614 10.1080/10739680802220323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsumoto T., Claesson-Welsh L. (2001) VEGF receptor signal transduction. Sci. STKE 2001, re21. [DOI] [PubMed] [Google Scholar]
- 26.Mammoto A., Connor K. M., Mammoto T., Yung C. W., Huh D., Aderman C. M., Mostoslavsky G., Smith L. E., Ingber D. E. (2009) A mechanosensitive transcriptional mechanism that controls angiogenesis. Nature 457, 1103–1108 10.1038/nature07765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tzima E., Irani-Tehrani M., Kiosses W. B., Dejana E., Schultz D. A., Engelhardt B., Cao G., DeLisser H., Schwartz M. A. (2005) A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature 437, 426–431 10.1038/nature03952 [DOI] [PubMed] [Google Scholar]
- 28.Coon B. G., Baeyens N., Han J., Budatha M., Ross T. D., Fang J. S., Yun S., Thomas J. L., Schwartz M. A. (2015) Intramembrane binding of VE-cadherin to VEGFR2 and VEGFR3 assembles the endothelial mechanosensory complex. J. Cell Biol. 208, 975–986 10.1083/jcb.201408103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Conway D., Schwartz M. A. (2012) Lessons from the endothelial junctional mechanosensory complex. F1000 Biol. Rep. 4, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harry B. L., Sanders J. M., Feaver R. E., Lansey M., Deem T. L., Zarbock A., Bruce A. C., Pryor A. W., Gelfand B. D., Blackman B. R., Schwartz M. A., Ley K. (2008) Endothelial cell PECAM-1 promotes atherosclerotic lesions in areas of disturbed flow in ApoE-deficient mice. Arterioscler. Thromb. Vasc. Biol. 28, 2003–2008 10.1161/ATVBAHA.108.164707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamada K. H., Nakajima Y., Geyer M., Wary K. K., Ushio-Fukai M., Komarova Y., Malik A. B. (2014) KIF13B regulates angiogenesis through Golgi to plasma membrane trafficking of VEGFR2. J. Cell Sci. 127, 4518–4530 10.1242/jcs.156109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Domigan C. K., Ziyad S., Iruela-Arispe M. L. (2015) Canonical and noncanonical vascular endothelial growth factor pathways: new developments in biology and signal transduction. Arterioscler. Thromb. Vasc. Biol. 35, 30–39 10.1161/ATVBAHA.114.303215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng H. W., James A. F., Foster R. R., Hancox J. C., Bates D. O. (2006) VEGF activates receptor-operated cation channels in human microvascular endothelial cells. Arterioscler. Thromb. Vasc. Biol. 26, 1768–1776 10.1161/01.ATV.0000231518.86795.0f [DOI] [PubMed] [Google Scholar]
- 34.Dragoni S., Laforenza U., Bonetti E., Lodola F., Bottino C., Guerra G., Borghesi A., Stronati M., Rosti V., Tanzi F., Moccia F. (2013) Canonical transient receptor potential 3 channel triggers vascular endothelial growth factor-induced intracellular Ca2+ oscillations in endothelial progenitor cells isolated from umbilical cord blood. Stem Cells Dev. 22, 2561–2580 10.1089/scd.2013.0032 [DOI] [PubMed] [Google Scholar]
- 35.Moccia F., Lucariello A., Guerra G. (2018) TRPC3-mediated Ca2+ signals as a promising strategy to boost therapeutic angiogenesis in failing hearts: The role of autologous endothelial colony forming cells. J. Cell. Physiol. 233, 3901–3917 10.1002/jcp.26152 [DOI] [PubMed] [Google Scholar]
- 36.Pocock T. M., Foster R. R., Bates D. O. (2004) Evidence of a role for TRPC channels in VEGF-mediated increased vascular permeability in vivo. Am. J. Physiol. Heart Circ. Physiol. 286, H1015–H1026 10.1152/ajpheart.00826.2003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.