Abstract
Coordinated changes in signaling pathways and gene expression in hearts subjected to prolonged stress maintain cardiac function. Loss of steroid receptor coactivator-2 (SRC-2) results in a reversal to the fetal gene program and disrupts the response to pressure overload, accompanied by prominent effects on metabolism and growth signaling, including increased AMPK activation. We proposed that early metabolic stress driven by AMPK activation induces contractile dysfunction in mice lacking SRC-2. We used 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR) to activate AMPK transiently before transverse aortic constriction (TAC) in wild-type and cardiomyocyte-specific SRC-2 knockout (CKO) animals. In contrast to AMPK activities during stress, in unstressed hearts, AICAR induced a mild activation of Akt signaling, and, in SRC-2–CKO mice, partially relieved an NAD+ deficiency and increased antioxidant signaling. These molecular changes translated to a mild hypertrophic response to TAC with decreased maladaptive remodeling, including markedly decreased fibrosis. Additionally, preactivation of AMPK in SRC-2–CKO mice was accompanied by a dramatic improvement in cardiac function compared with saline-treated SRC-2–CKO mice. Our results show that altered molecular signaling before stress onset has extended effects on sustained cardiac stress responses, and prestress modulation of transient growth and metabolism pathways may control those effects.—Nam, D. H., Kim, E., Benham, A., Park, H.-K., Soibam, B., Taffet, G. E., Kaelber, J. T., Suh, J. H., Taegtmeyer, H., Entman, M. L., Reineke, E. L. Transient activation of AMPK preceding left ventricular pressure overload reduces adverse remodeling and preserves left ventricular function.
Keywords: cardiac metabolism, cardiac stress, hypertrophy, fibrosis, heart failure
Steroid receptor coactivator-2 (SRC-2) is a transcriptional coactivator important in regulation and amplification of activity for a diverse number of transcription factors. In the heart, SRC-2 controls the activation and/or expression of several critical regulators of cardiac function, including GATA binding protein 4, myocyte enhancer factor 2, T-box 5, serum response factor, heart and neural crest derivatives expressed 2, and peroxisome proliferator-activated receptor-α (PPARα) (1). These pleiotropic activities position SRC-2 at the fulcrum of a number of stress-regulated cardiac pathways. As such, loss of SRC-2 results in a cardiac gene expression profile matching that of a stressed heart. In addition, SRC-2 deletion results in accelerated functional decline of the heart in response to transverse aortic constriction (TAC), manifested by severe maladaptive remodeling associated with both metabolic and growth abnormalities. That decline was associated with increased activation of AMPK, suggesting a role for disrupted regulation of metabolic and growth responses to stress in SRC-2 knockout animals (1, 2). AMPK is commonly described as a cellular energy sensor, being activated by increased AMP and ADP to ATP ratios during times of environmental stress. AMPK acts on several targets to decrease catabolic, and to increase anabolic, processes, ultimately restoring steady-state ATP levels (3). In the heart, AMPK targets include regulators of metabolism, protein translation, and protein degradation (4).
To investigate a role for AMPK controlling SRC-2-regulated cardiac stress responses, we studied the effects of AMPK stimulation through pretreatment with the AMP analog 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR). As such, we investigated changes in SRC-2–CKO and control animals after administration in a prestress state (where there is little AMPK stimulation). The results suggest that AMPK stimulation before stress markedly reduces pathologic remodeling subsequent to aortic constriction in both control and SRC-2 cardiomyocyte-specific knockout (CKO) mice. Moreover, treatment with AICAR in the SRC-2–CKO mouse was even more dramatic, suggesting that the effects of SRC-2 on protective pathways may largely involve AMPK-sensitive pathways.
MATERIALS AND METHODS
Animals
All experiments were approved by the Institutional Animal Care Research Committee at Baylor College of Medicine (protocols AN-544 and AN-124). The generation of the SRC-2–CKO mice has been previously described (1). Only male, age-matched mice littermates (3–6 mo old) were used. Animals were kept in a temperature-controlled (23°C) facility with a 12/12-h light/dark cycle. Mice on normal chow were fed a 2920X Teklad Global rodent normal chow diet (Envigo, Huntingdon, United Kingdom) ad libitum. AICAR (Toronto Research Chemicals, Toronto, ON, Canada) was administered through an intraperitoneal injection once daily for 6 d at 0.5 mg/g body weight in saline, immediately before TAC surgery. Controls received saline injections only.
Gene expression analysis
RNA was isolated from left ventricular sections of frozen heart tissue using the Fibrous Tissue RNeasy RNA Isolation Kit (Qiagen, Hilden, Germany), according to manufacturer’s instructions. cDNA was made using iScript (Bio-Rad Laboratories, Hercules, CA, USA) according to manufacturer’s instructions. Quantitative PCR (qPCR) was performed with sequence-specific primers and the iTaq Universal SYBR Mix (Bio-Rad Laboratories) on a Light Cycler (F. Hoffmann-La Roche, Basel, Switzerland). All genes tested are listed in Supplemental Table S1. Primer sequences are included in Supplemental Table S2.
Western blotting
Tissue lysates were made from left ventricular sections by homogenizing in 1× heart lysis buffer [25 mM Tris-HCl (pH 7.4), 250 mM NaCl, 5 mM EDTA, 1% Triton X-100, 0.1% SDS, 0.1% sodium deoxycholate, 1 mM DTT, 1× protease inhibitor cocktail (MilliporeSigma, Burlington, MA, USA), 1× phosphatase inhibitor cocktail (F. Hoffman-La Roche)]. SDS-PAGE and protein transfer to PVDF were performed following standard procedures. Immunoblotting was performed by standard methods with the antibodies indicated [anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH, ab2302; MilliporeSigma); anti-p-pyruvate dehydrogenase (ABS203; MilliporeSigma), anti-p-AMPK (sc-33524; Santa Cruz Biotechnology, Dallas, TX, USA), anti-p-eukaryotic elongation factor 2 (eEF2, Thr56, 2331S; Cell Signaling Technology, Danvers, MA, USA), anti-eEF2 (2332S; Cell Signaling Technology), anti-p-Akt (Ser473, 9271S; Cell Signaling Technology), anti-Akt (9272S; Cell Signaling Technology), anti-p-p85 (4228S; Cell Signaling Technology), anti-p-ERK-1/2 (9101S; Cell Signaling Technology), anti-ERK (9102S; Cell Signaling Technology), anti-p-S6 (Ser235/236, 4858S; Cell Signaling Technology), anti-S6 (2317S; Cell Signaling Technology), anti-p70 ribosomal S6 kinase (9202S; Cell Signaling Technology), anti-p-eukaryotic translation initiation factor 4E-binding protein 1 (Ser 65, 9456S; Cell Signaling Technology), anti-IGF1 (sc-9013; Santa Cruz Biotechnology), anti-superoxide dismutase 3 (sc-271170; Santa Cruz Biotechnology), anti-carbonic anhydrase 9 (bs-4029R; Bioss Antibodies, Woburn, MA, USA), anti-p-NOS3 (sc-81510; Santa Cruz Biotechnology), anti-p65 (8242S; Cell Signaling Technology), and anti–forkhead box O 3a (FOXO3a, 12829S; Cell Signaling Technology)]. Secondary antibodies were either fluorophore- or horseradish peroxidase–conjugated, and detection was performed on an Odyssey imaging system (Li-Cor Biosciences, Lincoln, NE, USA). Unless otherwise noted, each band represents 3 pooled heart lysates for each experimental group. Total protein stain was performed with Revert Total Protein Stain, according to manufacturer’s instructions (Li-Cor Biosciences).
Nuclear-cytoplasmic isolation
Nuclear-cytoplasmic fractionation was conducted with the Ne-Per Nuclear and Cytoplasmic Extraction Reagents (Thermo Fisher Scientific, Waltham, MA, USA), according to the manufacturer’s protocol.
TAC and cardiac monitoring
TAC surgeries, echocardiography, and cardiac Doppler measurements were performed at the Cardiovascular Sciences Core at Baylor College of Medicine (Houston, TX, USA), as previously described (5–7). For all time-point analyses, numbers of animals and animal groupings are shown in Table 1.
TABLE 1.
Animal groups and numbers for time point analyses before and after intervention
| Mouse group | Mice (n) | |||
|---|---|---|---|---|
| Before TAC surgery | After TAC surgery | |||
| 1 wk | 2 wk | 4 wk | ||
| WT + saline | 15 | 11 | 10 | 10 |
| WT + AICAR | 12 | 8 | 5 | 8 |
| CKO + saline | 12 | 9 | 9 | 7 |
| CKO + AICAR | 18 | 12 | 12 | 11 |
Histologic analyses
Cross sections from the ventricle of animals harvested at 4 wk after banding were fixed with Z-Fix (Anatech, Battle Creek, MI, USA) overnight and then processed, embedded in paraffin, and sectioned for histology. Masson’s trichrome staining was performed by standard techniques. Briefly, after deparaffinization, sections were treated with Bouin’s solution, followed by serial staining in the following solutions: Weigert’s iron hematoxylin, Biebrich scarlet-acid fuchsin solution (1% Biebrich scarlet, 1% acid fuchsin, and 0.1% acetic acid), phosphomolybdic-phosphotungstic acid (5% phosphomolybdic acid, 5% phosphotungstic acid), aniline blue (2.5% aniline blue by weight, 2% acetic acid), and a 1% acetic acid solution. Solutions and chemicals were purchased through MilliporeSigma or Thermo Fisher Scientific. Sections were then dehydrated and mounted for imaging. Wheat germ agglutinin (WGA) staining was performed using WGA tetramethylrhodamine conjugate (Thermo Fisher Scientific) at 100 μg/ml in 1× PBS for 60 min at 37°C. Imaging was performed with a BX41 microscope (Olympus, Tokyo, Japan) fitted with a DFC300 FX camera (Leica Microsystems, Wetzlar, Germany).
Image analysis
The signals for trichrome staining and cardiac cell area were deconvolved as previously described (8). Slides were stained in 3 groups, and a constant threshold was applied to all slides within a staining group, regardless of condition. For each micrograph, the number of pixels positive for blue trichrome straining was divided by the number of pixels positive for cell staining. That procedure normalizes the statistic to the fraction of the field of view occupied by tissue. For WGA staining, to enhance the contrast of cell membranes and eliminate intensity variation caused by thickness differences in the section, uneven illumination, or other low-frequency signals, a high-pass filter with Gaussian falloff was applied in Fourier space. This preprocessing was implemented in the Eman2-extensible processing suite (9) and subsequent operations were implemented in CellProfiler (10). Areas of each micrograph without tissue were excluded from analysis using a binary mask from the unpreprocessed image. Incorporating the prior biologic knowledge that a cell membrane should be a closed polygon having no gaps and be of approximately constant width, the binary operations of dilation, erosion, and skeletonization were chained to track the cell membrane.
RNA sequencing
RNA sequencing reads were aligned to the mouse genome using TopHat Software (11) with the following parameters: maximum mismatches of 2, seed length of 20, maximum allowed alignments for a single read of 5, and number of threads of 8. Reads that mapped to multiple locations of the genome were discarded before downstream analysis. An annotated list of known transcripts (mm9 University of California, Santa Cruz, RefSeq genes; http://genome.ucsc.edu/cgi-bin/hgGateway?db=mm9) was used for the final set of the transcriptome. The abundance and number of raw fragments aligned to each gene were computed using the Cufflinks suite (12). The abundance of each gene was expressed as fragments/kb/million mapped reads (FPKM).
To obtain differentially expressed genes between 2 conditions, we used DESeq software (13). The raw counts mapped to a transcript were obtained using the HTSeq script (14) and were used as inputs to the DESeq tool. To remove genes with low-expression profiles, we included only those with a total aligned fragment count across all the samples >5. We used an adjusted value of P < 0.05 as the criteria to determine differentially expressed genes between 2 conditions. Enriched gene ontology (GO) terms were obtained from the Database for Annotation, Visualization, and Integrated Discovery (DAVID) (15).
Tissue analyses
All tissue analyses were performed on freshly isolated tissue. Heart and lung weights were taken immediately after euthanasia and excision. Total peroxidase activity was analyzed with the Amplex Red Hydrogen Peroxide/Peroxidase Assay Kit (Promega, Madison, WI, USA), according to manufacturer’s instructions. The reduced glutathione to oxidized glutathione (GSH:GSSG) ratios were measured with the Glutathione (GSSG:GSH) Detection Kit (Enzo Life Sciences, Farmingdale, NY, USA), according to manufacturer’s instructions. The NAD:NADH ratios were measured with the NAD:NADH Quantitation Colorimetric Kit (BioVision, Milpitas, CA, USA) according to the manufacturer’s instructions. All results were normalized to total protein levels.
Statistical analysis
All statistical comparisons were performed with a 2-way ANOVA and multiple comparisons and with Tukey’s post hoc analysis. Differences of P ≤ 0.05 were considered significant. Results are presented as the means ± sem. Group sizes for mouse experiments are shown in Table 2.
TABLE 2.
Group sizes for mouse heart tissue experiments
| Mouse group | Mice (n) |
|---|---|
| WT + saline | 10–15 |
| WT + AICAR | 4–12 |
| SRC-2–CKO + saline | 7–12 |
| SRC-2–CKO + AICAR | 11–18 |
For qPCR analysis, n = 4–6 for all groups.
RESULTS
AICAR minimally affects metabolic gene expression in unstressed mice
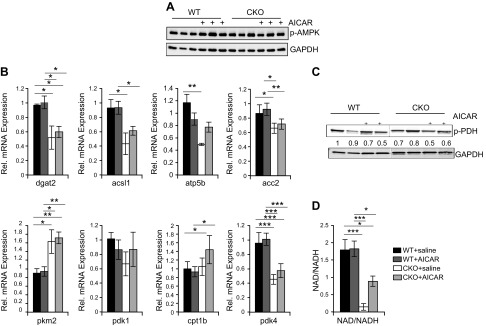
Because metabolic pathways are the main targets of AMPK and we already know that SRC- CKO animals have a metabolic gene-expression profile that mimics a stressed heart, we investigated effects for AICAR treatment on metabolic pathways. As previously shown, intraperitonal injections of AICAR resulted in activation of cardiac AMPK as observed by phosphorylation of its catalytic subunit (4, 16–18). At the dose used for these experiments, activation of cardiac AMPK was observed at 24 h and decreased between 36 and 48 h after injection (Supplemental Fig. S1). We chose to inject mice for 6 d to allow their hearts to adjust to changes in steady-state signaling and then harvested animals on d 7. As expected, 6 d of AICAR treatment resulted in a modest increase in activated AMPK in both wild-type (WT) and SRC-2–CKO mice (Fig. 1A). We found that AICAR treatment did not significantly affect metabolic gene expression in WT mice, and it did not affect the metabolic gene changes observed in SRC-2–CKO mouse hearts (Fig. 1B). However, there were small changes in phosphorylation of metabolic regulator pyruvate dehydrogenase, suggesting possible changes in transient metabolic flux (Fig. 1C) and a partial rescue of depleted NAD+ levels in SRC-2–CKO heart tissues in AICAR-treated mice (Fig. 1D).
Figure 1.
AICAR activates metabolic AMPK targets but largely does not affect metabolic gene expression. A, C) Immunoblotting on whole heart extracts from left ventricle heart sections after 6 d of saline or AICAR injections. B) RNA was isolated from left ventricle heart sections and qPCR was run from unstressed-saline or AICAR-injected animals after 6 d of injections. Expression is normalized to GAPDH. n = 4–6. D) NAD/NADH ratios were calculated from freshly harvested whole heart tissue from mice subjected to saline or AICAR injections for 6 d; n = 5 (WT + saline), 6 (WT + AICAR), 3 (CKO + saline, 3 were under the detection limit), and 6 (CKO + AICAR). Results are presented as the means ± sem analyzed by 2-way ANOVA with multiple comparisons and with a Tukey’s post hoc analysis. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
AICAR activates Akt in unstressed mice
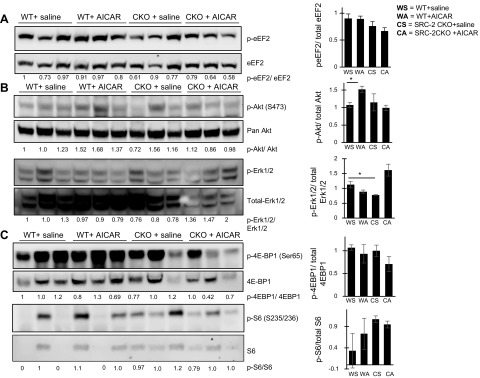
AMPK can directly inhibit stimulators of protein translation during times of stress (3, 19); therefore, we next investigated direct effects on the growth and protein translation pathways from AICAR in unstressed animals. Interestingly, we found no significant changes in either WT or SRC-2–CKO mice in phosphorylation of translation regulator eEF2, which is commonly phosphorylated during stress in response to AMPK phosphorylation of upstream eEF2 kinase (Fig. 2A and Supplemental Fig. S4). Furthermore, instead of inhibition of Akt and downstream pathways, which usually accompanies AMPK activation during stress, we observed a significant increase in Akt activation accompanied by a trend toward increased phosphorylation of downstream pathway component p-S6, supporting a mild, positive effect on protein translation of pre-TAC in both WT and SRC-2–CKO mice (Fig. 2B, C and Supplemental Fig. S4). Another regulator of protein translation downstream of Akt, eukaryotic translation initiation factor 4E-binding protein 1, had both an increase in total and phosphorylated levels in AICAR-treated samples, thus increasing the potential for it to be regulated, but with no net change in amount of activated protein. AICAR treatment additionally resulted in decreased activation of growth suppressors Erk 1/2 (Fig. 2C) in WT, but not SRC-2–CKO, mice, suggesting that the effects of AICAR on translation are through regulation of multiple intracellular kinase pathways. No activation of upstream PI3K subunit p85 or PI3K/Akt activator IGF-1 was observed (Supplemental Fig. S3).
Figure 2.
AICAR activates Akt and downstream protein translation regulators. Immunoblotting in total heart lysate made from left ventricle sections. Densitometry analysis was performed normalizing phosphoprotein to total target protein levels: peEF2/total eEF2 (A), p-Akt/total Akt and p-Erk1/2/total Erk1/2 (B), and p-4EBP1/total 4EBP1 and p-S6/total S6 (C). Total protein staining for all gels can be found in the Supplemental Data.
AICAR up-regulates antioxidant defenses in pre-TAC SRC-2-CKO hearts
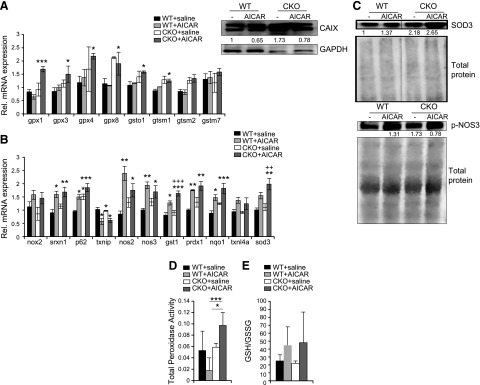
Because AICAR treatment appeared to have different effects on cell signaling in unstressed mice compared with what is known for AMPK activation under stressed conditions, we performed RNA sequencing on saline- or AICAR-treated hearts to investigate whether other possible pathways were altered. Similar to metabolic gene changes, we observed very few changes (Supplemental Fig. S2A). Pathway analysis highlighted the variation among groups and supported WT and SRC-2–CKO hearts having very different profiles at rest, with small changes induced by AICAR treatment in both models (Supplemental Fig. S2B). However, of several direct comparisons, only SRC-2–CKO mice treated with AICAR, compared with WT mice treated with saline, gave significant GO analysis enrichment. Interestingly, for the pathways modified, in addition to general metabolic changes, there was an enrichment of genes involved with oxidation/reduction activities with AICAR treatment, resulting in increased expression of several antioxidant genes, especially in the GST family (Fig. 3A and Table 3). Further analysis of major antioxidant regulators revealed a similar pattern to that observed with the RNA sequencing in which AICAR treatment resulted in increased antioxidant gene expression (Fig. 3B), correlating with increased protein expression of superoxide dismutase 3, active extracellular NOS, and decreased oxidative stress marker carbonic anhydrase 9 (Fig. 3C and Supplemental Fig. S3). AICAR treatment resulted in increased peroxidase activity in SRC-2–CKO hearts and trended toward an increase in the GSH:GSSG ratio, suggesting functional effects of increased antioxidant expression in SRC-2–CKO hearts (Fig. 3D, E).
Figure 3.
AICAR up-regulates cardiac antioxidant programs. A) Gene-specific expression of a subset of genes is shown relative to WT + saline controls for antioxidant genes from RNA sequencing analyses of total hearts before TAC. B) qRT-PCR on total heart RNA from pre-TAC hearts normalized to Rpl32 expression. n = 3–4. C) Immunoblotting in total heart lysates before TAC (3 hearts pooled/sample). Total protein or GAPDH was used for normalization, as indicated. D) Total peroxidase activity measured using Amplex Red in total hearts before TAC; n = 4 (WT + saline and WT + AICAR), 3 (CKO + saline), and 6 (CKO + AICAR). E) GSH:GSSG ratios measured in total heart lysates before TAC. n = 3 (WT + saline, WT + AICAR, and CKO + saline) and n = 4 (CKO + AICAR). Results are presented as the means ± sem analyzed by 2-way ANOVA with multiple comparisons and with a Tukey’s post hoc analysis. An asterisk (*) indicates comparison to WT + saline, whereas as a plus (+) indicates comparison to SRC-2–CKO + saline. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, +P ≤ 0.05, ++P ≤ 0.01.
TABLE 3.
GO analysis of genes significantly up-regulated
| GO ID | Description | P | FDR q |
|---|---|---|---|
| 0044710 | Single-organism metabolic process | 2.77E−05 | 1.52E−01 |
| 0006091 | Generation of precursor metabolites and energy | 7.32E−05 | 2.00E−01 |
| 0008152 | Metabolic process | 2.86E−04 | 5.22E−01 |
| 0006518 | Peptide metabolic process | 6.46E−04 | 8.85E−01 |
| 0055114 | Oxidation–reduction process | 9.36E−04 | 1.00E+00 |
The ontology analysis shows genes significantly up-regulated ≥2-fold in SRC-2–CKO + AICAR mice vs. WT + saline controls from RNA sequencing on total heart RNA before TAC surgery. FDR, false-discovery rate.
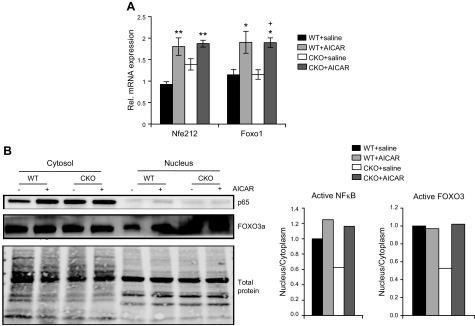
Antioxidant gene expression and pathways involved with cellular responses to oxidant stress are controlled through the activity of several transcription factors, including FOXO family members, nuclear factor erythroid 2-related factor 2, and NF-κB. AICAR treatment induced increased gene expression of both nuclear factor erythroid 2-related factor 2 and FOXO1 in both WT and SRC-2–CKO mice hearts (Fig. 4A). Furthermore, comparison of the fraction of nuclear localization of NF-κB and FOXO3a, as an indication of activity in heart lysates, were decreased in all 3 of these factors in SRC-2–CKO hearts, which was alleviated in hearts pretreated with AICAR (Fig. 4B). Together, these data suggest up-regulation of mechanisms that clear oxidative stress products in AICAR-treated hearts.
Figure 4.
AICAR increases activation of antioxidant transcription factor regulators in SRC-2–CKO mice. A) qRT-PCR on total heart RNA before TAC. Expression is normalized to rpl32 expression and is shown relative to WT + saline levels. Rel., relative. B) Pre-TAC heart lysates were subcellular fractionated and immunoblotted (3 hearts pooled/sample). Densitometry analysis compares nuclear vs. cytoplasmic protein normalized to total protein and relative to WT saline hearts. An asterisk (*) indicates comparison to WT + saline, whereas as a plus (+) indicates comparison to SRC-2–CKO + saline. *P ≤ 0.05, **P ≤ 0.01, +P ≤ 0.05.
Pretreatment with AICAR blunts TAC-induced hypertrophy in control mice
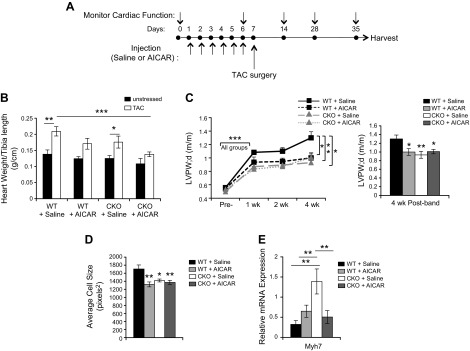
Despite our initial goal in activating AMPK before stress onset to mimic a stressed heart to compare with changes from SRC-2 loss, we found that many classic stress-activated AMPK targets were largely unaffected, and hearts appear to have increased translational capacity with additional effects on antioxidant capacity in SRC-2–CKO mice. We hypothesized that the observed molecular profile of hearts receiving AICAR may actually have a beneficial effect during cardiac stress. Therefore, we tested effects of transient pretreatment of mice with AICAR only before TAC surgery. Similar to the testing above, animals were injected once daily for 6 d. On d 7, mice were subjected to TAC surgery and then monitored by echocardiography and cardiac Doppler at 1, 2, and 4 wk after TAC; at which points, animals were euthanized, and tissues were collected and analyzed (Fig. 5A). As expected from our previous work (2), SRC-2–CKO mice had a blunted hypertrophic response (Fig. 5B–D). Pretreatment with AICAR was accompanied by some hypertrophy; however, there was significantly reduced TAC-induced growth in WT animals 4 wk after TAC surgery. Although wall thickness and cell size in SRC-2–CKO animals were not affected by AICAR, we observed decreased expression of hypertrophic marker Myh7 as compared to SRC-2–CKO saline controls (Fig. 5E), suggesting a decrease in maladaptive remodeling.
Figure 5.
AICAR injections blunt hypertrophic response to TAC. A) Schematic of experimental time line for AICAR injections, TAC surgery, and cardiac monitoring performed on WT and SRC-2–CKO mice. B) Hearts were excised, weighed, and compared with tibia length. Before surgery: n = 4 (WT + saline), n = 5 (WT + AICAR), n = 7 (CKO + saline), and n = 6 (CKO + AICAR); after surgery: n = 8 (WT + saline), n = 6 (WT + AICAR), n = 6 (CKO + saline), and n = 10 (CKO + AICAR) C) Echocardiography measurements of left ventricular posterior wall thickness in diastole (LVPWd). Right: 4-wk time point (n = 5 − 18; see Table 1 for n at each time point). WT + saline data after TAC have been previously published (28). D) Average cardiomyocyte size calculated from cross sections of left ventricles stained with WGA; ≥4 sections/animal with >100 cells/section were analyzed; n = 7–10. WT + saline data after TAC have been previously published (28). E) qPCR for Myh7 expression in total heart RNA from left ventricular sections 4 wk after TAC surgery. Expression is normalized to GAPDH. n = 3–4 Results are presented as the means ± sem analyzed by 2-way ANOVA with multiple comparisons and with a Tukey’s post hoc analysis. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
AICAR pretreatment averts the severe dysfunction seen in SRC-2–CKO mice during TAC
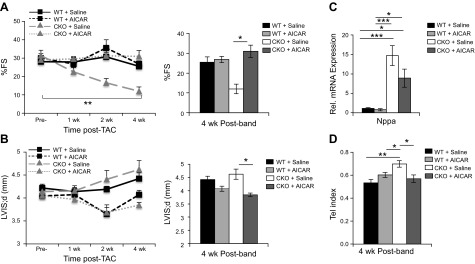
In our previous experiments, attenuated hypertrophy with full-body SRC-2 loss was coupled to decreased cardiac function and increased expression of molecular markers of cardiac stress, Myh7 and Nppa (2). That phenotype is recapitulated in the SRC-2–CKO mice, which had markedly decreased fractional shortening by 2 wk after TAC surgery, which was maintained at 4 wk (Fig. 6A). In contrast, despite attenuated hypertrophy (as shown in Fig. 5), AICAR pretreatment averted the severe dysfunction seen in SRC-2–CKO mice and sustained cardiac function in WT mice (Fig. 6A). Furthermore, although WT mice showed modest left ventricular dilation, AICAR treatment protected both WT and SRC-2–CKO mice from dilatation (Fig. 6B), which was associated with moderately decreased expression of the stress marker Nppa (Fig. 6C). Tei index measurements for all mice suggest decreased left ventricular function in SRC-2–CKO mice, which is corrected by pretreatment with AICAR (Fig. 6D).
Figure 6.
AICAR rescues cardiac decline from TAC in SRC-2–CKO animals. A, B) Echocardiography measurements for the percentage of fractional shortening (%FS; A) and left ventricular interior diameter in diastole (LVIDd; B); n = 5–18 (see Table 1 for n at each time point.) WT + saline data after TAC have been previously published (28). C) qPCR for atrial natriuretic factor in RNA in gene Nppa isolated from the left ventricle regions of WT and SRC-2–CKO hearts at 4 wk after TAC surgery. Expression is normalized to GAPDH; n = 5–6. D) Tei index calculated from cardiac Doppler measurements at 4 wk after TAC; n = 10 (WT + saline), n = 8 (WT + AICAR), and n = 7 (CKO + saline and CKO + AICAR) Results are presented as the means ± sem analyzed by 2-way ANOVA with multiple comparisons and with a Tukey’s post hoc analysis. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
AICAR pretreatment attenuates adverse remodeling in response to TAC
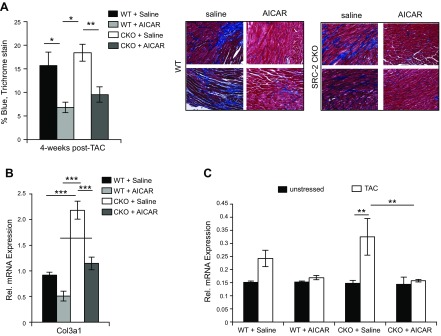
Downstream of hypertrophy, several other pathologic changes accompany decreases in cardiac function during TAC, including fibrosis, vascular impairment, and pleural edema. Examination of hearts from WT and SRC-2–CKO mice 4 wk after TAC for collagen deposition showed that AICAR treatment was able to almost completely prevent fibrosis in both WT and SRC-2–CKO mice (Fig. 7A). Decreased gene expression of collagen III was observed in left ventricular heart sections 4 wk after TAC in AICAR-treated mice (Fig. 7B). Additionally, AICAR pretreatment prevented an increase in lung weight in both genotypes of mice, suggesting prevention of pleural edema (Fig. 7C).
Figure 7.
AICAR reduces maladaptive remodeling events after TAC surgery. A) Left ventricle sections 4 wk after TAC were stained with trichrome and the percentage of blue staining was analyzed. Four pictures/section for ≥4 sections were analyzed/animal. The panels on the right are representative images for each group; n = 8 (WT + saline), n = 6 (WT + AICAR and CKO + saline), and n = 9 (CKO + AICAR). B) qPCR for collagen III, as described in Fig. 2C; n = 4–7. C) Lungs were excised and weighed at 4 wk after TAC, as described in Fig. 2A. Before surgery (unstressed): n = 4 (WT + saline), n = 5 (WT + AICAR), n = 7 (CKO + saline), and n = 6 (CKO + AICAR); after TAC surgery: n = 8 (WT + saline), n = 8 (WT + AICAR), n = 8(CKO + saline), and n = 10 (CKO + AICAR). Results are presented as the means ± sem analyzed by 2-way ANOVA with multiple comparisons and with a Tukey’s post hoc analysis. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
DISCUSSION
The cardiac response to pressure overload induced by TAC follows a sequence of events beginning with metabolic responses (20), followed by ventricular hypertrophy, then fibrotic accumulation, resulting wall stiffening, decreased pumping efficacy, ventricle dilation, and eventually failure. Each of those physical changes is accompanied by molecular signaling and in most cases genetic remodeling; the coordination of which, we hypothesized, is paramount to maintenance of function.
This study was prompted by our previous observations in which we demonstrated a critical role for SRC-2 in mediation of both metabolic and growth responses to cardiac stress and an association of loss of SRC-2 during stress with highly increased levels of active AMPK (1, 2). Here, we used SRC-2–CKO and control hearts to restrict effects on stress to cardiomyocyte-specific changes. Work by Li et al. (17) and Meng et al. (18) showed that stimulation of AMPK with AICAR treatment in WT hearts at the start of aortic-constriction onset and proceeding for its duration restricted hypertrophy and offered modest protection against failure. In those studies, well-known stress-activated AMPK targets were activated and associated with decreased protein translation (17, 18). In contrast, our study found that AMPK activation in the absence of stress results in a different molecular profile, with up-regulation of Akt activity and a modest corresponding increase in metabolic flux. In the SRC-2–CKO mice, there was also increased antioxidant capacity. To test the effects of this different molecular signature on the cardiac response to stress, we then performed TAC surgery on AICAR- or saline-pretreated hearts. Our studies confirmed that SRC-2–CKO mice manifested exaggerated sensitivity to TAC; however, pretreatment with AICAR largely prevented the functional decline. We were unprepared for the striking, long-lasting effects of AICAR; such that 4 wk after TAC surgery, both WT and SRC-2–CKO mice treated with AICAR had markedly reduced fibrosis and preserved function. The present results show that transient, pharmacologic pretreatment of mice genetically predisposed to TAC-induced failure (SRC-2–CKO mice) with AICAR can delay maladaptive remodeling and cardiac decline. Our experiments were designed to examine the possibility that prestimulating several mechanisms known to be involved with the response to stress through activation of AMPK activity might influence the outcome of cardiac stress. Those results further supported our previous studies indicating that SRC-2 controls a vital adaptive response central to cardiomyocytes and critical for maintaining cardiac function. The effects of AICAR pretreatment in the SRC-2–CKO mice further suggests the important role of SRC-2 in AMPK-modulated adaptation, possibly through coordinated regulation of overlapping pathways because we have previously shown an important role for SRC-2 in control of cardiac transcription factors, which regulate both growth and metabolic pathways (1), similar to the pathways targeted by AMPK.
Although the interactive pathways are not completely defined by this study, it is clear that early adaptation markedly mitigates the pathology. The targets of AMPK activation are multiple, but often rapidly reversed in the absence of AMPK activity, strongly suggesting that modulation of targets either preceding or during the first few days of stress can profoundly affect the magnitude and duration of the heart’s ability to prevent pathologic remodeling. Before TAC, hearts receiving AICAR show a mild activation of both metabolic and growth pathways. As AMPK is well characterized to activate aerobic respiration of fat and carbohydrate sources as well as increase glucose uptake, activation of metabolic pathways is an expected response to AICAR administration. We suggest that this metabolic boost allows the heart to more easily respond to the ATP demand created at TAC onset. The ability of AICAR to limit, but not prevent, hypertrophic growth associated with sustained function for several weeks after injection was unexpected. Previous in vitro studies suggest a direct role in AMPK inhibition of cardiomyocyte growth (21–23), although how AICAR activation of AMPK during unstressed conditions translates into in vivo responses to stress is unknown. AMPK is known to directly inhibit protein translation in an eEF2-dependent manner and can oppose Akt signaling through TSC2 regulation (3, 19); however, we did not observe any changes in p-eEF2 levels and observed downstream activation of S6 kinase consistent with active mammalian target of rapamycin signaling (24). Therefore, AICAR administered according to our protocol results in pre-TAC activation of AMPK, Akt, and the signaling pathways downstream of Akt that promote growth. This is coupled with decreased activity of growth suppressors Erk 1/2, which can mediate inhibition of hypertrophy associated with AMPK activation during TAC (22, 25). Collectively, these signaling events resulted in an attenuated pathologic hypertrophic response to TAC, suggesting that modulation of the cellular profile during hypertrophy may be a better determinant of effect on cardiomyocyte function than the degree of cardiomyocyte growth. AMPK also affects protein degradation pathways, and maintaining a balance of protein synthesis and degradation is important during cardiac remodeling (4, 26). These parallel activities may also contribute to the attenuated hypertrophy in our experiments. Further experiments will be needed to understand how protein flux downstream of TAC is affected in our model.
The idea of pharmacologic preconditioning is largely untested in pressure-overload models. After publication of the benefits of AICAR in murine skeletal muscle (27), increased AICAR use in athletes in many venues was observed, especially during endurance exercise. It is tempting to speculate that AICAR dosing before these events may be protective to the adverse events that can occur from the prolonged increased workload on the heart during endurance events. In our experiments, AICAR activation of AMPK was cleared within 1.5 d (Supplemental Fig. S1), strongly suggesting that there are very early changes during the cardiac response to TAC that can have lasting effects on the trajectory of the cardiac stress response. We suggest that SRC-2–CKO mice represent a novel model of potential importance in investigating and defining the mechanisms of cardioprotection to increased workload. Further experiments may help define the SRC-2–dependent, protective mechanisms. Understanding the key targets and pathways driving these very early changes during pressure overload will be a first step to translating those results into clinical applications.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank Hong Chen (Houston Methodist Research Institute), and, Katherina Marcelo, Jennifer Pocius, Thuy Pham, and Alejandro Granillo Ibanez (all from Baylor College of Medicine) for technical assistance with surgery and cardiac imaging and molecular analysis. Histology was performed at the Pathology and Histology Core at Baylor College of Medicine with the assistance of Zahida Sayeeduddin. This work was supported by the U.S. National Institutes of Health/National Heart, Lung, and Blood Institute (NIH/NHLBI; Grant R00-HL118159-01 to E.L.R.), the Souki Mitochondrial Project Fund (Grants R01-HL061483 to H.T. and R01-HL089792 to M.L.E.), and the U.S. Army Department of Defense (Grant 68847MAREP/W911NF1610480 to B.S.). The Pathology and Histology Core at Baylor College of Medicine is supported by funding from the NIH National Cancer Institute (Grant P30-CA125123). The authors declare no conflicts of interest.
Glossary
- AICAR
5-aminoimidazole-4-carboxamide ribonucleotide
- CKO
cardiomyocyte-specific knockout
- eEF2
eukaryotic elongation factor 2
- FOXO
forkhead box O
- GAPDH
glyceraldehyde 3-phosphatase dehydrogenase
- GO
gene ontology
- GSH
reduced glutathione
- GSSG
oxidized glutathione
- PPARα
peroxisome proliferator-activated receptor-α
- qPCR
quantitative PCR
- S6
S6 ribosomal protein
- SRC-2
steroid receptor coactivator-2
- TAC
transverse aortic constriction
- WGA
wheat germ agglutinin
- WT
wild type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
D. H. Nam, A. Benham, G. E. Taffet, H. Taegtmeyer, M. L. Entman, and E. L. Reineke all designed experiments; D. H. Nam, E. Kim, A. Benham, H. Park, J. H. Suh, and E. L. Reineke performed research; A. Benham, B. Soibam, G. E. Taffet, J. T. Kaelber, H. Taegtmeyer, and M. L. Entman all aided in data analysis and interpretation; and H. Taegtmeyer, M. L. Entman, and E. L. Reineke wrote and revised the manuscript.
REFERENCES
- 1.Reineke E. L., Benham A., Soibam B., Stashi E., Taegtmeyer H., Entman M. L., Schwartz R. J., O’Malley B. W. (2014) Steroid receptor coactivator-2 is a dual regulator of cardiac transcription factor function. J. Biol. Chem. 289, 17721–17731 10.1074/jbc.M113.539908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reineke E. L., York B., Stashi E., Chen X., Tsimelzon A., Xu J., Newgard C. B., Taffet G. E., Taegtmeyer H., Entman M. L., O’Malley B. W. (2012) SRC-2 coactivator deficiency decreases functional reserve in response to pressure overload of mouse heart. PLoS One 7, e53395 10.1371/journal.pone.0053395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zaha V. G., Young L. H. (2012) AMP-activated protein kinase regulation and biological actions in the heart. Circ. Res. 111, 800–814 10.1161/CIRCRESAHA.111.255505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baskin K. K., Taegtmeyer H. (2011) AMP-activated protein kinase regulates E3 ligases in rodent heart. Circ. Res. 109, 1153–1161 10.1161/CIRCRESAHA.111.252742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cieslik K. A., Taffet G. E., Carlson S., Hermosillo J., Trial J., Entman M. L. (2011) Immune-inflammatory dysregulation modulates the incidence of progressive fibrosis and diastolic stiffness in the aging heart. J. Mol. Cell. Cardiol. 50, 248–256 10.1016/j.yjmcc.2010.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Y. H., Reddy A. K., Taffet G. E., Michael L. H., Entman M. L., Hartley C. J. (2003) Doppler evaluation of peripheral vascular adaptations to transverse aortic banding in mice. Ultrasound Med. Biol. 29, 1281–1289 10.1016/S0301-5629(03)00986-4 [DOI] [PubMed] [Google Scholar]
- 7.Taffet G. E., Hartley C. J., Wen X., Pham T., Michael L. H., Entman M. L. (1996) Noninvasive indexes of cardiac systolic and diastolic function in hyperthyroid and senescent mouse. Am. J. Physiol. 270, H2204–H2209 [DOI] [PubMed] [Google Scholar]
- 8.Ruifrok A. C., Johnston D. A. (2001) Quantification of histochemical staining by color deconvolution. Anal. Quant. Cytol. Histol. 23, 291–299 [PubMed] [Google Scholar]
- 9.Tang G., Peng L., Baldwin P. R., Mann D. S., Jiang W., Rees I., Ludtke S. J. (2007) EMAN2: an extensible image processing suite for electron microscopy. J. Struct. Biol. 157, 38–46 10.1016/j.jsb.2006.05.009 [DOI] [PubMed] [Google Scholar]
- 10.Kamentsky L., Jones T. R., Fraser A., Bray M. A., Logan D. J., Madden K. L., Ljosa V., Rueden C., Eliceiri K. W., Carpenter A. E. (2011) Improved structure, function and compatibility for CellProfiler: modular high-throughput image analysis software. Bioinformatics 27, 1179–1180 10.1093/bioinformatics/btr095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trapnell C., Pachter L., Salzberg S. L. (2009) TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 25, 1105–1111 10.1093/bioinformatics/btp120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mortazavi A., Williams B. A., McCue K., Schaeffer L., Wold B. (2008) Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 5, 621–628 10.1038/nmeth.1226 [DOI] [PubMed] [Google Scholar]
- 13.Anders S., Huber W. (2010) Differential expression analysis for sequence count data. Genome Biol. 11, R106 10.1186/gb-2010-11-10-r106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anders S., Pyl P. T., Huber W. (2015) HTSeq--a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169 10.1093/bioinformatics/btu638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang W., Sherman B. T., Lempicki R. A. (2009) Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 37, 1–13 10.1093/nar/gkn923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cieslik K. A., Taffet G. E., Crawford J. R., Trial J., Mejia Osuna P., Entman M. L. (2013) AICAR-dependent AMPK activation improves scar formation in the aged heart in a murine model of reperfused myocardial infarction. J. Mol. Cell. Cardiol. 63, 26–36 10.1016/j.yjmcc.2013.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li H. L., Yin R., Chen D., Liu D., Wang D., Yang Q., Dong Y. G. (2007) Long-term activation of adenosine monophosphate-activated protein kinase attenuates pressure-overload-induced cardiac hypertrophy. J. Cell. Biochem. 100, 1086–1099 10.1002/jcb.21197 [DOI] [PubMed] [Google Scholar]
- 18.Meng R. S., Pei Z. H., Yin R., Zhang C. X., Chen B. L., Zhang Y., Liu D., Xu A. L., Dong Y. G. (2009) Adenosine monophosphate-activated protein kinase inhibits cardiac hypertrophy through reactivating peroxisome proliferator-activated receptor-alpha signaling pathway. Eur. J. Pharmacol. 620, 63–70 10.1016/j.ejphar.2009.08.024 [DOI] [PubMed] [Google Scholar]
- 19.Leprivier G., Remke M., Rotblat B., Dubuc A., Mateo A. R., Kool M., Agnihotri S., El-Naggar A., Yu B., Somasekharan S. P., Faubert B., Bridon G., Tognon C. E., Mathers J., Thomas R., Li A., Barokas A., Kwok B., Bowden M., Smith S., Wu X., Korshunov A., Hielscher T., Northcott P. A., Galpin J. D., Ahern C. A., Wang Y., McCabe M. G., Collins V. P., Jones R. G., Pollak M., Delattre O., Gleave M. E., Jan E., Pfister S. M., Proud C. G., Derry W. B., Taylor M. D., Sorensen P. H. (2013) The eEF2 kinase confers resistance to nutrient deprivation by blocking translation elongation. Cell 153, 1064–1079 10.1016/j.cell.2013.04.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taegtmeyer H., Golfman L., Sharma S., Razeghi P., van Arsdall M. (2004) Linking gene expression to function: metabolic flexibility in the normal and diseased heart. Ann. N. Y. Acad. Sci. 1015, 202–213 10.1196/annals.1302.017 [DOI] [PubMed] [Google Scholar]
- 21.Chan A. Y., Soltys C. L., Young M. E., Proud C. G., Dyck J. R. (2004) Activation of AMP-activated protein kinase inhibits protein synthesis associated with hypertrophy in the cardiac myocyte. J. Biol. Chem. 279, 32771–32779 10.1074/jbc.M403528200 [DOI] [PubMed] [Google Scholar]
- 22.Du J., Guan T., Zhang H., Xia Y., Liu F., Zhang Y. (2008) Inhibitory crosstalk between ERK and AMPK in the growth and proliferation of cardiac fibroblasts. Biochem. Biophys. Res. Commun. 368, 402–407 10.1016/j.bbrc.2008.01.099 [DOI] [PubMed] [Google Scholar]
- 23.Stuck B. J., Lenski M., Böhm M., Laufs U. (2008) Metabolic switch and hypertrophy of cardiomyocytes following treatment with angiotensin II are prevented by AMP-activated protein kinase. J. Biol. Chem. 283, 32562–32569 10.1074/jbc.M801904200 [DOI] [PubMed] [Google Scholar]
- 24.Wullschleger S., Loewith R., Hall M. N. (2006) TOR signaling in growth and metabolism. Cell 124, 471–484 10.1016/j.cell.2006.01.016 [DOI] [PubMed] [Google Scholar]
- 25.Meng R., Pei Z., Zhang A., Zhou Y., Cai X., Chen B., Liu G., Mai W., Wei J., Dong Y. (2011) AMPK activation enhances PPARα activity to inhibit cardiac hypertrophy via ERK1/2 MAPK signaling pathway. Arch. Biochem. Biophys. 511, 1–7 10.1016/j.abb.2011.04.010 [DOI] [PubMed] [Google Scholar]
- 26.Baskin K. K., Taegtmeyer H. (2011) An expanded role for AMP-activated protein kinase: regulator of myocardial protein degradation. Trends Cardiovasc. Med. 21, 124–127 10.1016/j.tcm.2012.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Narkar V. A., Downes M., Yu R. T., Embler E., Wang Y. X., Banayo E., Mihaylova M. M., Nelson M. C., Zou Y., Juguilon H., Kang H., Shaw R. J., Evans R. M. (2008) AMPK and PPARδ agonists are exercise mimetics. Cell 134, 405–415 10.1016/j.cell.2008.06.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suh J. H., Lai L., Nam D., Kim J., Jo J., Taffet G. E., Kim E., Kaelber J. T., Lee H. K., Entman M. L., Cooke J. P., Reineke E. L. (2017) Steroid receptor coactivator-2 (SRC-2) coordinates cardiomyocyte paracrine signaling to promote pressure overload-induced angiogenesis. J Biol Chem 292, 21643–21652 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.