Abstract
Keratin intermediate filaments (IFs) are the major cytoskeletal component in epithelial cells. The dynamics of keratin IFs have been described to depend mostly on the actin cytoskeleton, but the rapid transport of fully polymerized keratin filaments has not been reported. In this work, we used a combination of photoconversion experiments and clustered regularly interspaced short palindromic repeats/clustered regularly interspaced short palindromic repeats–associated protein 9 genome editing to study the role of microtubules and microtubule motors in keratin filament transport. We found that long keratin filaments, like other types of IFs, are transported along microtubules by kinesin-1. Our data revealed that keratin and vimentin are nonconventional kinesin-1 cargoes because their transport did not require kinesin light chains, which are a typical adapter for kinesin-dependent cargo transport. Furthermore, we found that the same domain of the kinesin heavy chain tail is involved in keratin and vimentin IF transport, strongly suggesting that multiple types of IFs move along microtubules using an identical mechanism.—Robert, A., Tian, P., Adam, S. A., Kittisopikul, M., Jaqaman, K., Goldman, R. D., Gelfand, V. I. Kinesin-dependent transport of keratin filaments: a unified mechanism for intermediate filament transport.
Keywords: vimentin, KIF5B, photoconversion
Depending on the tissue or cellular context, cells face different physiologic and mechanical challenges that can be overcome by the cell- and tissue-specific expression of one or a combination of some of the 70 genes encoding intermediate filament (IF) proteins in humans. For example, mesenchymal cells typically express high levels of type III vimentin IF, the assembly and disassembly of which facilitate different aspects of cell migration (1–4). In contrast, epithelial cells express a combination of type I and type II keratin IFs that are connected to desmosomes and hemidesmosomes to ensure the tight connection between cells in the epithelial sheet and between the cells and the basal membrane (5).
To accommodate constant changes of cell shape as cells contract, migrate, or invade, IFs need to undergo profound and constant reorganization [reviewed in Robert et al. (6)]. This reorganization is achieved by a combination of severing and reannealing (7, 8) and by intracellular translocation of mature IFs and their precursors. The first indication that IFs could be a cargo for microtubule-based motors came from the microinjection of a pan-kinesin antibody that induced the retraction of the vimentin network (9). With the development of fluorescent probes and advanced live cell imaging techniques, several types of cytoplasmic IFs have been observed to move along microtubule tracks. Movement of neurofilaments has been observed in axons of cultured neurons (10). Our previous studies have shown that vimentin particles, as well as mature filaments, are transported along microtubules (8, 11, 12). Recently, glial fibrillary acidic protein and nestin IFs were reported to move together with vimentin in migrating astrocytes (13). Among the 40 kinesins in mammals, the major microtubule motor kinesin-1 has been suggested to be involved in IF transport. Kinesin-1 in mammals is represented by 3 isoforms—KIF5A, KIF5B, or KIF5C—with KIF5B being the most abundant and ubiquitous version. Several reports suggested that various types of cytoplasmic IFs might be potential cargo for kinesin-1. In axons, for example, neurofilaments are transported by KIF5A (14, 15), whereas in muscle, KIF5B has been reported to be essential for the delivery of desmin and nestin IFs to the growing tip of myotubes (16). Recently, knockdown of KIF5B has been shown to reduce anterograde transport of IFs in migrating astrocytes (13).
Absent from this list are keratin IFs, which are the most abundant IFs in epithelial cells. For these filaments, a different mode of transport based on actin dynamics has been proposed. In this model, keratin IFs undergo a constant cycle of assembly and disassembly that involves actin-dependent centripetal motion of keratin particles and filaments from the cell periphery, where filament particles are formed, to the perinuclear region. When filaments reach the perinuclear region, a fraction of keratin subunits are released and returned by diffusion to the cell periphery, where another cycle of particle formation takes place (17–20). The contribution of microtubules and/or microtubule-based motors for keratin filament dynamics has been neglected because the rapid transport of mature keratin filaments has never been reported.
In this work, we used a combination of photoconversion experiments and clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein 9 (Cas9) genome editing to study the role of microtubules and microtubule motors in keratin IFs. We found that long mature keratin filaments move along microtubules mediated by kinesin-1 and that the same domain of the kinesin tail is involved in keratin and vimentin IF transport, strongly suggesting that all types of IFs move along microtubules using a similar mechanism.
MATERIALS AND METHODS
DNA constructs
mEos3.2-vimentin in pQCXIN has been described by Hookway et al. (8). To create mEos3.2-keratin 8 and mEos3.2-keratin 18, Eos3.2 was amplified from mEos3.2-vimentin by PCR with Phusion polymerase (Thermo Fisher Scientific, Waltham, MA, USA) and joined to pQCXIN cut with NotI by InFusion (Takara Bio USA, Mountain View, CA, USA). The resulting vector was cut with BamHI. The keratins were amplified from pcDNA-keratin 8 or 18 by PCR with Phusion polymerase and joined to the BamHI cut pQCXIN with InFusion.
Mouse (m)KIF5B cDNA was provided by Addgene (Cambridge, MA, USA) (pKIN1B plasmid 31604). To create mKIF5B truncations, mKIF5B was amplified by PCR using the forward primer, 5′-ATAAGAATGCGGCCGCTTCCAGAAAGATGGC-3′, together with one of the following reverse primers: for mKIF5B full length, 5′-ATGGATCCCACGACTGCTTGCCTCCACCAC-3′; mKIF5B1–773, 5′-ATGGATCCCAGTCTTGCATAACCGTGAGCT-3′; mKIF5B1–806, 5′-ATGGATCCCAGTCCTGAACAAAGAGCTTAC-3′; mKIF5B1–892, 5′-ATGGATCCCAACGGTCTCGAGATGCATTTT-3′. For mKIF5BΔ775–802, the complementary oligos 5′-CACGGTTATGCAAGACAGATTTGTTCAGGACTTGCCTACCAGGGTGAAAAAGAGCGCCGAGG-3′ and 5′-TCGACCTCGGCGCTCTTTTTCACCCTGGTAGCCAAGTCCTGAACAAATCTGTCTTGCATAACCGTGAGCT-3′ were phosphorylated, annealed, and inserted into the SacI/SalI restriction sites of the mKIF5B cDNA. The deletion mutant was amplified by PCR using the same primers as the mKIF5B full length. All the Emerald-tagged mKIF5B constructs were generated by replacing vimentin from vimentin-Emerald pQCXIN (8) with the mKIF5 constructs using the AgeI/BamHI sites.
Antibodies and reagents
Chicken polyclonal antivimentin (PCK-594P) was from BioLegend (Dedham, MA, USA), rat monoclonal anti–α-tubulin (YL1/2) was from Santa Cruz Biotechnology (Dallas, TX, USA), mouse pan-cytokeratin (C2931) was from MilliporeSigma (Burlington, MA, USA), and rabbit polyclonal kinesin light chain (KLC)1 (19028-1-AP) and KLC2 (17668-1-AP) antibodies were from Proteintech (Rosemont, IL, USA). C terminus and head domain polyclonal antibodies against kinesin were a kind gift from Fatima Gyoeva (Institute of Protein Research, Russian Academy of Sciences, Moscow, Russia). Rhodamine-conjugated phalloidin, MitoTracker Deep Red, and LysoTracker Deep Red dyes were from Invitrogen Molecular Probes (Eugene, OR, USA)
Cell lines and CRISPR/Cas9 knockout
All cells were maintained at 37°C in 5% CO2. hTERT–retinal pigment epithelial (RPE) cells were maintained in DMEM supplemented with 1 mM sodium pyruvate (Gibco, Gaithersburg, MD, USA) and 10% fetal bovine serum (Neuromics, Minneapolis, MN, USA). All vectors for CRISPR/cas9 genome editing were from GenScript (Piscataway, NJ, USA). The KIF5B knockout (KO) and klc1 KO cell lines were created by the transduction of RPE cells with lentivirus carrying LentiCRISPR V2 KIF5B guide RNA (gRNA)1 (target sequence 5′-CTATACCTTGTGCTCGAAGC-3′) or LentiCRISPR V2 KLC1 gRNA1 (target sequence 5′-GAAGCAGAAACTGCGTGCGC-3′). Lentivirus was produced in HEK 293 FT cells transfected with the LentiCRISPR V2 vector containing cas9 and KIF5B or KLC1 gRNA together with the helper plasmids pVSVG (Takara Bio USA) and pPAX2 (Imgenex, San Diego, CA, USA) encoding the gag/pol and env proteins required for virus production. Virus-containing medium was collected and filtered 48 h after the transfection of the packaging cells. Polybrene (MilliporeSigma) was added to the freshly collected virus-containing medium to 8 µg/ml, and RPE cells were incubated for 6 h with this virus/medium/polybrene mixture. Two days later, infected cells were selected using 5 µg/ml puromycin for 1 wk, and survivor cells were plated at 1 cell per well. For all CRISPR cell lines, single colonies were amplified, lysed, and tested for knockout by Western blot.
mEos3.2-vimentin and mEos3.2-keratin 8/18 were stably expressed in RPE [wild type (WT) and KIF5B KO #4] by retroviral transduction. Retroviruses were produced as described above except that the helper plasmids pVSVG (Takara Bio USA) and pCL-Eco (Imgenex) were used. Transduced cells were selected using 2 mg/ml G418 for 1 wk. Retrovirus transduction of RPE KIF5B KO was performed to replace WT KIF5B with the mouse KIF5B constructs listed above.
Enrichment of the kinesin-1 complex by pulldown using GFP-binder
KIF5B KO cells expressing mKIF5B-Emerald of mKIF5BΔ775–802-Emerald from 2 subconfluent 100 mm dishes lysed in 1 ml of ice-cold RIPA buffer [50 mM Tris (pH 7.4), 150 mM NaCl, 1% Triton, 0.5% Na-Deoxycholate, 0.1% SDS, 10 mM NaPPi, 1.5 mM NaVO3, 1 mM PMSF] supplemented with peptidase inhibitors (chymostatin, leupeptin, and pepstatin A, 20 µg/ml). The cell lysates were centrifuged at 20,000 g for 5 min. The soluble fraction was incubated for 4 h at 4°C with 30 µl Sepharose beads conjugated with single-chain GFP antibody (GFP-binder) (GFP-Trap-M; Chromotek, Hauppauge, NY, USA). Beads were washed 3 times with RIPA-base buffer [50 mM Tris (pH 7.4), 150 mM NaCl, 1% Triton X-100, 10 mM NaPPi, 1.5 mM NaVO3, 1 mM PMSF] supplemented with chymostatin, leupeptin, and pepstatin A. The kinesin-1 complex was pulled down via binding of Emerald from mKIF5B-Emerald to the GFP binder beads by centrifugation at 3000 g and resuspended in 30 µl of Laemmli buffer [5% SDS, 0.1 mM Tris (pH 6.8), 140 mM β-mercaptoethanol, 25% glycerol]. Samples were boiled for 5 min and analyzed by Western blot.
Immunostaining for widefield microscopy, confocal microscopy, and structured illumination microscopy
Cells were plated on glass coverslips to the desired confluence ∼16 h prior to fixation. For vimentin and actin costaining or keratin and actin costaining, cells were fixed with 3.7% formaldehyde in CSK buffer [100 mM NaCl, 300 mM sucrose, 3 mM MgCl2, 10 mM Pipes (pH 6.8)] supplemented with 0.1% Triton X-100 for 10 min. For tubulin and keratin or vimentin and keratin costaining, cells were fixed with ice-cold MeOH for 5 min at −20°C. Fixed cells were further extracted in 0.2% Triton X-100 in PBS before staining. Immunostaining was performed in wash buffer (Tris-buffered saline supplemented with 1% bovine serum albumin, 0.1% Triton-X100) as previously described (12).
Widefield microscopy imaging of IFs and F-actin costaining were captured on an inverted microscope (Eclipse U2000; Nikon Instruments, Melville, NY, USA) equipped with Ph3 ×40/1.0 numerical aperture (NA) objective and a CoolSnap ES CCD camera (Roper Scientific, Planegg/Martinsried, Germany) and driven by Nikon Elements software. Fluorescence excitation was achieved using a mercury lamp. Images of vimentin and keratin costaining were collected with a confocal microscope (LSM510 META; Carl Zeiss, Jena, Germany) with oil immersion objective lenses (Plan-Apochromat, ×60, 1.40 NA; Carl Zeiss). Structured illumination microscopy (SIM) images of microtubules and keratin IFs were collected on an inverted microscope (Ti-E; Nikon Instruments) with an SIM illuminator and an SIM enclosure equipped with an Apo TIRF ×100/NA 1.49 oil objective and electron-multiplying charge-coupled device camera (iXon DU897; Andor, Belfast, United Kingdom) at the Nikon Center at the Northwestern University Feinberg School of Medicine Center for Advanced Microscopy.
Keratin and vimentin distribution measurement
Widefield microscopy images of vimentin or keratin and actin costaining captured as previously described were analyzed using Fiji software v.2.0 (ImageJ; National Institutes of Health, Bethesda, MD, USA). For at least 30 cells per condition, a single line, 3 pixels in width, was traced manually from the center of the nucleus to the cell edge as delineated by F-actin staining (Supplemental Fig. 2). The intensity values along this line were obtained using the plot profile plugin from Fiji. The mean fluorescence intensity and the positions along the line for each cell were normalized from 0 to 1 using Matlab. The normalized data were transferred to Prism 7 software (GraphPad Prism; GraphPad Software, La Jolla, CA, USA) for further analyses. The percentage of signal at the edge was calculated by dividing the area under the curve between positions 0.5 and 1 by the total area under the curve between positions 0 and 1. Data are shown as the means ± sd (n > 30 cells). Statistical significance was determined using the nonparametric Mann-Whitney U test with a confidence interval of 95%. This test analysis compares the distributions of 2 unpaired groups.
Live cell imaging and photoconversion
For all live cell experiments, cells were plated on glass coverslips ∼16 h prior to imaging. Cells were maintained at 37°C in 5% CO2 during imaging using a stage-top incubator (Tokai-Hit, Fujinomiya City, Japan) and a gas mixer (Okolab, Naples, Italy).
Confocal images were collected on a Nikon Eclipse U2000 inverted microscope equipped with a spinning-disk confocal head (CSU10; Yokogawa Electric Corp., Sugar Land, TX, USA), a Plan-Apochromat ×100/1.45 NA objective, an Agilent MLC 400 laser set (including 488- and 561-nm lasers; Agilent Technologies, Wood Dale, IL, USA), an 89 North Heliophor pumped phosphor light engine at 405 nm (Chroma Technology, Bellows Falls, VT, USA) to drive photoconversion, and an electron-multiplying charge-coupled device (Evolve; Photometrics, Tucson, AZ, USA) driven by Nikon Elements software. Photoconversion mEos3.2-keratin 8/18 from green to red was performed using illumination from a Heliophor LED light source in the epifluorescence pathway filtered with a 400-nm filter and confined by a diaphragm. Photoconversion time was 5 s, and the zone was 10 μm in diameter, which was positioned near the cell center. Time-lapse sequences were acquired at 15 s intervals for 3 min using the 561-nm laser. Images were analyzed in Fiji and assembled in Adobe Illustrator (San Jose, CA, USA).
Imaging of mEos3.2-vimentin was performed using TIRF on a Nikon Eclipse U2000 inverted microscope equipped with a Plan-Apochromat TIRF ×100 1.49 NA objective and a CMOS Orca Flash 4.0 camera (Hamamatsu Photonics, Hamamatsu City, Japan) controlled by NIS-Elements AR 4.51.01 software (Nikon). The angle of a 561-nm laser was manually adjusted until near-total internal reflection was reached as judged by imaging of photoconverted mEos3.2-vimentin expressing cells. For photoconversion, cells were exposed for 3 s to UV light from a mercury arc in the epifluorescent light path filtered through a 400-nm excitation filter and spatially restricted by a pinhole in the field diaphragm position. Time-lapse sequences were acquired at 15-s intervals for 3 min using the 561-nm laser. For each photoconversion experiment, at least 10 cells were photoconverted, and each condition was repeated in at least 3 independent experiments. Representative photoconversion data are shown for each condition.
For individual keratin and vimentin filaments tracking, photoconversion was performed as previously described, and the time-lapse sequences were acquired at 2-s intervals for 2 min. Filament ends were tracked (at least 10 filaments for 15–30 s) using the Manual Tracking Plugin in Fiji (ImageJ processing package).
Image analysis of photoconverted filaments
Image analysis of photoconverted filaments was done in MatLab (MathWorks, Natick, MA, USA) using custom software including portions of U-Track and the Filament Network Analysis toolbox (Danuser and Jaqaman Laboratories, University of Texas Southwestern) (21, 22).
The empirical location of the photoconversion zone was automatically located by: 1) convolving the fluorescence intensity image of the 561 nm channel with a gaussian kernel of sd of 3 pixels (∼0.3 μm), 2) using the Otsu method to determine an automatic threshold (23), 3) binarizing the image using that threshold, 4) applying morphologic closing with a radius of 3 pixels, 5) filling holes, 6) selecting the largest connected component, and 7) dilating the largest connected component by 10 pixels (∼1.1 μm).
Keratin filaments were located using a steerable filter designed in frequency space (24, 25) to determine orientation and applying nonmaximum suppression to trace the centerline positions of individual filaments. The filter used an aspect ratio parameter of K = 8 to determine orientation, an aspect ratio parameter of K = 3 to measure orientation responses, along with bandpass (1/2/π/fc) centered around 2 pixels (0.2 μm) and a bandpass width (1/2/π/bf) of 2 pixels. The unsuppressed pixels containing the remaining steerable filter responses were binarized using an empirically determined fixed threshold of 650 arbitrary units. The resulting binary image was skeletonized using morphologic skeletonization, followed by the removal of short edges of less than 2 pixels in length and conversion of junctions to single points as previously applied to other IFs (26).
The area occupied by photoconverted keratin filaments was determined by counting pixels of the detected filaments (1 pixel = 0.0119 μm2) outside of the automatically determined photoconversion zone as described above and within manually drawn masks of the biologic cell on a per-frame basis.
The overall slope of the occupied area per second was determined using GraphPad Prism.
RESULTS
Mature keratin IFs are transported along microtubules
The role of the actin cytoskeleton in the cycle of keratin assembly/disassembly has been described in great detail by Leube et al. (27). This is remarkable because other types of IF, at least type III and type IV, were shown to move along microtubules (8, 10). However, this has yet to be investigated for keratin. We therefore decided to test if mature keratin IFs can also be transported along microtubules. As a cell model, we used hTERT-immortalized RPE cells that coexpress keratin 8 and 18 together with vimentin IFs (28) so we could compare directly the dynamics of both types of filaments.
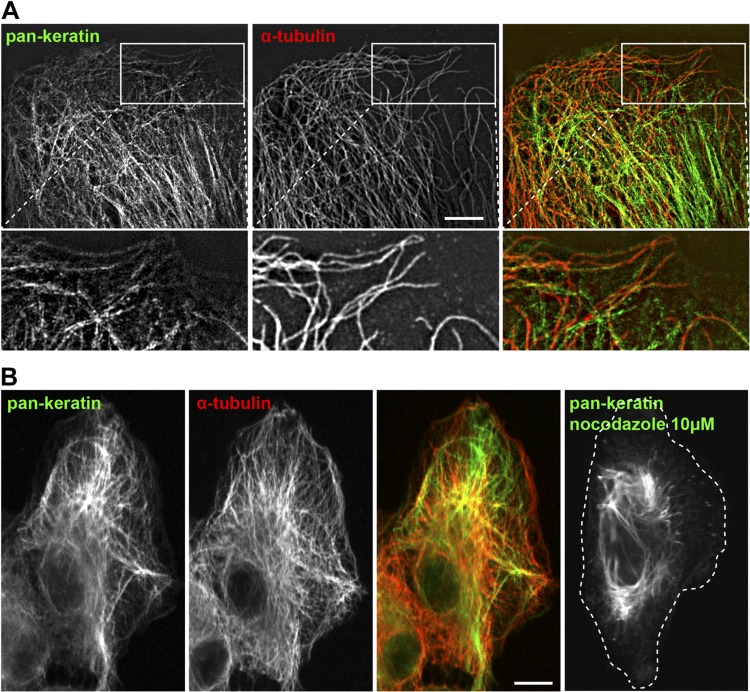
To determine if microtubules could participate in keratin IF dynamics, we first performed immunostaining using pan-keratin and α-tubulin antibodies and analyzed the interconnection between keratin filaments and microtubules in RPE cells. Super-resolution microscopy analysis using 3D SIM demonstrated clear alignment of a subpopulation of keratin filaments along microtubules (Fig. 1A). To evaluate the requirement of the microtubule network for the proper keratin filament distribution, we depolymerized microtubules with nocodazole. Confocal microscopy showed an intricate network of keratin filaments and microtubules that extended to the cell edge in control cells (Fig. 1B). However, nocodazole treatment induced a retraction of the keratin filaments from the cell periphery (Fig. 1B, right panel). These observations suggested that, at least in RPE cells, keratin filaments could potentially be transported along microtubules.
Figure 1.
Keratin filaments are associated with microtubules. A) 3D-SIM imaging of keratin and tubulin immunostaining of RPE cells. The enlargements show the alignment of keratin filaments with microtubules. Scale bar, 5 µm. B) Confocal imaging of keratin and tubulin immunostaining. In control cells, keratin filaments extend to the cell periphery, and the filaments retract into the perinuclear region after 3-h treatment with 10 µM nocodazole to depolymerize microtubules. Scale bar, 10 µm.
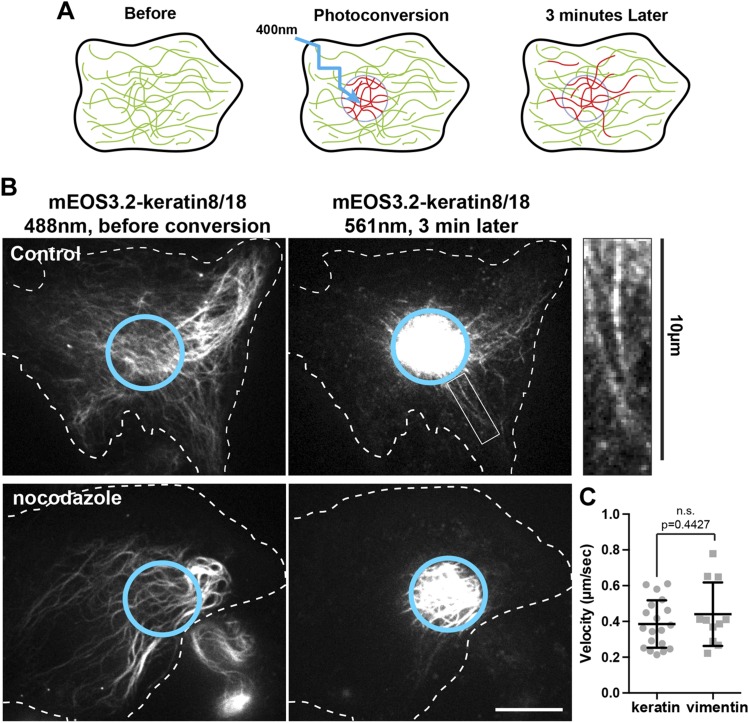
To study keratin IF dynamics in live cells, we used photoconversion of mEos3.2-keratin 8 and mEos3.2-keratin 18. The emission of mEos3.2 changes from green to red when exposed to UV light at 400 nm. By restricting photoconversion to a circular area of about 10 µm in diameter, we produced fiduciary marks on filaments, permitting us to monitor their transport in regions of cells with high filament density (Fig. 2A). Photoconverted mEos3.2-keratin 8/18 was imaged over a period of 3 min using spinning disk confocal microscopy. Remarkably, long keratin filaments (some of them longer than 8 µm) robustly moved away from the photoconverted zone during the 3 min observation period (Fig. 2B, upper panels and Supplemental Video 1). When cells were treated with 10 µM nocodazole for 3 h to depolymerize microtubules, keratin filament transport was completely inhibited (Fig. 2B, lower panels and Supplemental Video 2). Computational analyses of the area occupied by photoconverted keratin IF outside from the photoconverted zone of 10 cells per condition confirmed that nocodazole treatment inhibited the accumulation of photoconverted keratin filaments outside of the conversion zone (Fig. 3E). These results demonstrated, for the first time, the microtubule-dependent transport of fully polymerized keratin filaments.
Figure 2.
Fully polymerized keratin filaments are transported along microtubules. A) Photoconversion strategy to study keratin filament transport. A subset of mEOS3.2-keratin8/18 filaments is photoconverted from green to red using 400 nm light. Transport of the photoconverted filament outside from the photoconverted zone (cyan circle) is monitored in the red channel during 3 min. B) Photoconversion of mEos3.2-keratin 8/18 in RPE cells using spinning disk confocal microscopy. The left panels show the keratin network in the green channel (488 nm) before conversion, and the right panels show the red channel (561 nm) 3 min after photoconversion. Several photoconverted filaments were present outside of the original photoconverted zone (cyan circle) in the control cells, whereas photoconverted filaments remained inside the initial zone in nocodazole-treated cells (10 µM for 3 h). Inset enlargement shows the presence of long filaments outside from the photoconversion area 3 min after photoconversion. Scale bar, 10 µm. C) The tip of at least 10 photoconverted filaments coming form 5 different cells has been tracked for a period of 15 to 30 s. The graph compares the velocity of vimentin and keratin filament in the control condition (mean ± sd). Significance was determined using the Mann-Whitney U test.
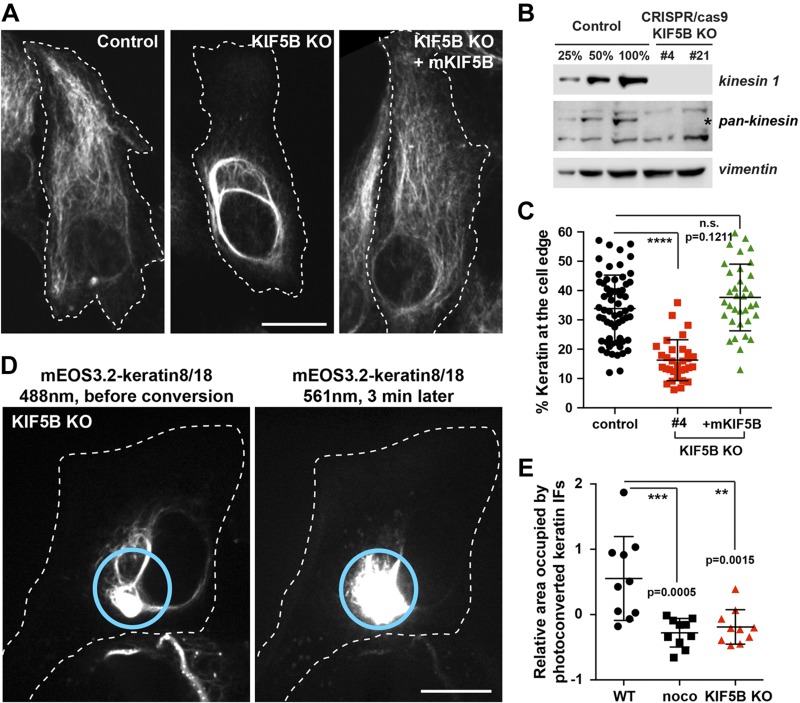
Figure 3.
KIF5B KO changes keratin filament distribution and inhibits keratin filament transport. A) Confocal imaging of keratin immunostaining in control vs. KIF5B KO cells (clone #4). The cell periphery was delineated by a dashed line to emphasize the retraction of the keratin filaments from the cell edge in KIF5b KO cells. In the last column, mKIF5B-Emerald was expressed in KIF5B KO cells to rescue keratin distribution. B) Western blot analyses using kinesin-1 antibody show the absence of kinesin-1 in 2 different KIF5B CRISPR clones. Pan-kinesin antibody shows the specificity of the KO for kinesin-1 (position marked by the star) but not the other kinesin family members. Vimentin is used as loading control. C) Graph shows the percentage of keratin at the cell edge (mean with sd; n > 30 cells). Statistical significance was determined using the Mann-Whitney U test. ****P < 0.0001. D) Photoconversion of mEos3.2-keratin 8/18 in RPE cells using spinning disk confocal microscopy in RPE KIF5B KO cells. mEos3.2-keratin was photoconverted from green to red at the cell center (cyan circle). The left panel shows keratin network in the green channel (488 nm) before conversion, and the right panel shows the red channel (561 nm) 3 min after photoconversion. Photoconverted filaments remained inside the initial zone even 3 min after photoconversion. Scale bar, 10 µm. E) Photoconverted keratin IFs outside from the conversion zone were segmented, and the pixels in segmented filaments were counted for each frame. The area occupied by photoconverted keratin IFs is the slope of the area occupied by photoconverted filaments per second. Negative values are due to photobleaching. Statistical significance was determined using the Mann-Whitney U test (n = 10 cells). **P < 0.005, ***P < 0.0005.
Keratin filaments are transported by conventional kinesin (kinesin-1)
Previous work demonstrated a role for kinesin-1 in the transport of class III and IV IFs (9, 13, 15, 16). We compared the behavior of keratin and vimentin IFs by tracking the ends of individual filaments and did not detect any significant differences between the velocity of keratin and vimentin IF transported in RPE cells (0.39 ± 0.13 and 0.44 ± 0.18 µm/s, respectively; Fig. 2C and Supplemental Video 3). This analysis suggested that class I and II keratin IFs, like class III vimentin IFs, could be transported by kinesin-1. In mammals, kinesin-1 heavy chain is encoded by 3 genes: KIF5A, KIF5B, and KIF5C. We used CRISPR/cas9 genome-editing to knock out KIF5B, the major gene encoding kinesin-1 in RPE cells. Several clones of genome-edited cells were amplified, and the KO was verified using Western blot analysis with an antibody (C terminus) directed against a peptide in the tail domain of kinesin heavy chain common to all 3 isoforms of kinesin-1. Clones 4 and 21 were selected for further analysis. The specificity of the KO was further confirmed using a blot with an antibody (HD) that recognizes the motor domains of multiple kinesins. This blot demonstrated that only the band corresponding to kinesin-1 was absent from the lysates of the KO cells (Fig. 3B). We checked the functional implications of KIF5B KO by analyzing the integrity of the microtubules network as well as the distribution and motility of known kinesin cargos. Staining of microtubules using anti–α-tubulin showed that the microtubule network is not impaired by the absence of KIF5B (Supplemental Fig. 1A). As expected, KIF5B KO induced the retraction of mitochondria from the cell periphery previously as described (29) (Supplemental Fig. 1B). In contrast, the motility of lysosomes was not affected (Supplemental Fig. 1C) because lysosome transport is driven not only by kinesin-1 but also by multiple kinesins [reviewed by Pu et al. (30)]. We also confirmed previous studies suggesting the contribution of KIF5B in vimentin transport. Vimentin immunostaining in RPE WT vs. KIF5B KO showed that, in the absence of KIF5B, the majority of the mature vimentin filaments retracted from the leading edge (Supplemental Fig. 2). To confirm that this phenotype was not due to an off-target effect, we performed a rescue experiment using the mouse ortholog of KIF5B (mKIF5B), which is insensitive to the gRNA used to knock out human KIF5B. When mKIF5B-Emerald was expressed in RPE KIF5B KO cells, the vimentin filament network distribution was fully restored, demonstrating that the retraction of the network was indeed caused by the absence of kinesin-1 [Supplemental Fig. 2A (right panel), 2E (green dots)]. Moreover, by using photoconversion of mEos3.2-vimentin, we showed that KIF5B KO abolished vimentin transport (Supplemental Videos 4 and 5), confirming that the mislocalization of vimentin filaments in KIF5B KO RPE cells was a consequence of the inhibition of anterograde transport of the filaments by kinesin-1.
We performed keratin immunostaining to determine how the absence of kinesin-1 affects keratin filament distribution. In control cells, the keratin filament network extended to the cell periphery. In contrast, in the absence of KIF5B, the majority of the mature keratin filaments retracted from the leading edge, with only a few short IF and nonfilamentous particles left behind (Fig. 3A). We quantified the results using the procedure described in Supplemental Fig. 2B–E, and confirmed the initial visual observation that the KO of KIF5B correlated with the retraction of the keratin network toward the nucleus. As was described for vimentin, the normal distribution of keratin filaments was fully restored by the expression of mKIF5B-emerald, confirming that the retraction of the keratin network was caused by the absence of kinesin-1 rather than being an off-target effect (Fig. 3A, C).
To directly demonstrate that kinesin-1 was essential for keratin filament’s anterograde transport, we performed photoconversion of mEOS3.2-keratin 8/18 in KIF5B KO cells. No fully mature filaments were observed outside the initial photoconversion zone 3 min after conversion, showing that keratin filament transport was abolished by KIF5B KO (Fig. 3D and Supplemental Video 6). Computational analyses of the area occupied by photoconverted keratin filaments outside the photoconverted zone revealed that KIF5B KO had a statistically indistinguishable effect from nocodazole treatment on keratin transport using our methods (Fig. 3E).
Keratin and vimentin filaments are transported using the same mechanism
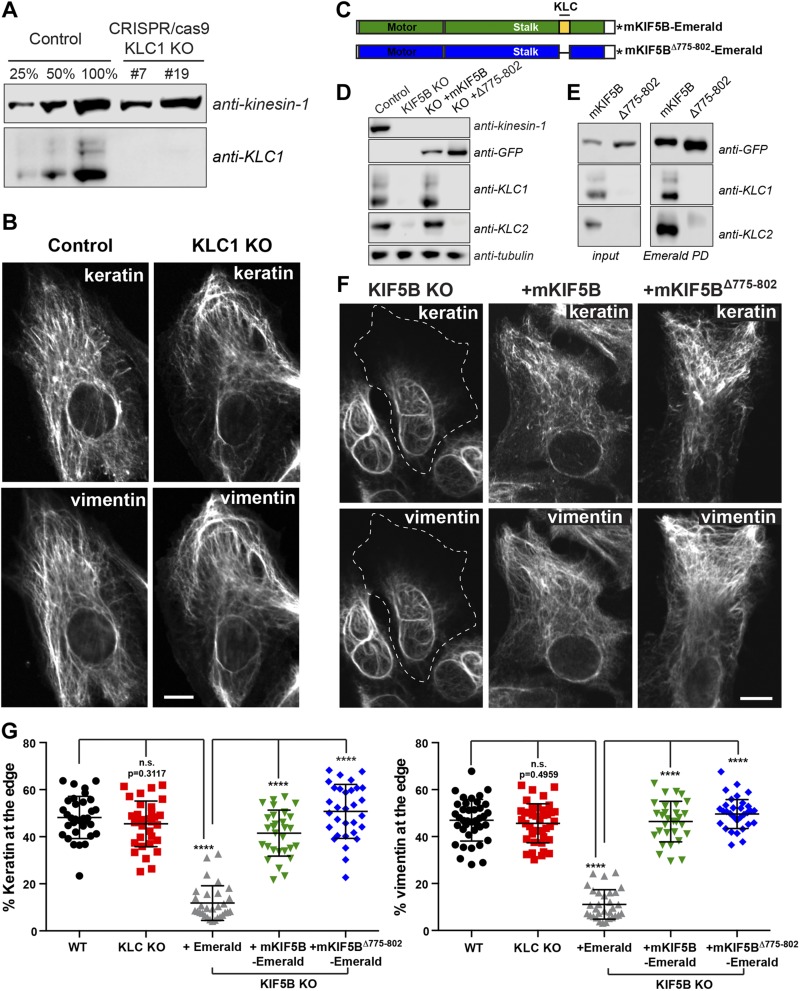
Our results have demonstrated that keratin filaments are transported along microtubules by kinesin-1, the same motor that transports vimentin filaments. Typically, kinesin-1 binds to its cargo via KLCs (31–33). In a small number of cases, some cargoes bind to the kinesin-1 tail and do not require KLC for transport (34, 35). To determine if IFs bind to KIF5B via KLC, we used genome editing to knock out KLC1, the gene encoding the predominant isoform of KLC in RPE cells (Fig. 4A). Immunostaining of keratin and vimentin filaments in RPE cells revealed that KLC1 KO did not affect the distribution of either type of IF network (Fig. 4B, G). However, the human genome contains 4 genes encoding for KLC, and their pattern of expression in RPE cells is not well established. Therefore, we used an alternative approach, replacing the WT KIF5B in RPE cells with a truncated version of the motor lacking the region that recruits KLC to the kinesin-1 complex. To accomplish this, we deleted the heptad repeats (residues 775–802) of mKIF5B responsible for KLC binding, creating (mKIF5BΔ775–802-Emerald) (Fig. 4C, blue construct). Pull-down experiments and Western blot analyses were performed to show that mKIF5BΔ775–802-Emerald did not interact with KLC. As previously described, KLC binding to kinesin-1 is required for their stability (36). Therefore, neither KLC1 nor KLC2 was detectable by Western blot analysis in crude extract of KIF5B KO cells (Fig. 4D). We found that the rescue of KIF5B KO by expression of the full-length mKIF5B-Emerald prevented degradation of KLC. In contrast, KLC1 and KLC2 remained undetectable in lysates from cells expressing mKIF5BΔ775–802-Emerald (Fig. 4D). In addition to probing crude extracts, the kinesin-1 complex was enriched by pull-down using GFP-binder, and the pellets were probed for the presence of KLC1 and KLC2. These experiments showed that the full-length mKIF5B-Emerald bound KLC1 and KLC2, whereas no KLC could be found even after enrichment of mKIF5BΔ775–802 -Emerald (Fig. 4E).
Figure 4.
Transport of keratin and vimentin filaments is independent of kinesin light chain. A) Western blot analyses using KLC-1 antibody showed the absence of KLC-1 in 2 different KLC-1 CRISPR clones (#7 and #19). KIF5 antibody was used as loading control. B) Confocal microscopy of keratin and vimentin immunostaining in RPE cells WT (control) and KLC-1 KO (clone #7). C) Schematic representation of mKIF5B-Emerald and mKIF5BΔ775–802-Emerald. The domain in blue is the motor domain of mKIF5B, and the red part is the stalk, which is comprised of the heptad repeat domain responsible for KLC binding in yellow. This KLC binding domain is absent from the mKIF5BΔ775–802-Emerald. The asterisk represents Emerald. D) Western blot analyses of kinesin-1, KLC1, and KLC2 showed that KLCs were absent from the lysate prepared from the KIF5B KO cells. Antitubulin was used as loading control. E) Endogenous KIF5B was replaced by mKIF5B-Emerald or mKIF5BΔ775–802-Emerald in RPE KIF5B KO #4. The presence of KLC1 and KLC2 was determined by Western blot of crude cell lysates (left panel) or after enrichment of the kinesin-1 complex by pulldown using GFP-binder recognizing Emerald. F) Confocal microscopy imaging of keratin and vimentin immunostaining in RPE KIF5B KO #4 cells after the expression of Emerald (KIF5B KO), mKIF5B-Emerald (+mKIF5B), or mKIF5BΔ775–802-Emerald (+Δ775–802). G) Graphs show the percentage of vimentin (left) or keratin (right) at the cell edge (mean with sd; n > 30 cells). Data are representative of at least 2 independent experiments. Statistical significance was determined using the Mann-Whitney U test. ****P < 0.0001.
Immunostaining of keratin and vimentin IFs was used to compare the efficiency of full-length mKIF5B and mKIF5BΔ775–802 in rescuing IF distribution. The images showed that removal of KLC and the region of kinesin tail that interacts with KLC had no effect on the capacity of mKIF5B to rescue keratin or vimentin distribution (Fig. 4F). This observation was reflected in the quantification of keratin and vimentin fluorescence intensity at the cell edge, confirming that both mKIF5B and mKIF5BΔ775–802 constructs rescued IF distribution to the same extent (Fig. 4G). These results demonstrate that KLCs are not involved in the kinesin-dependent transport of IFs.
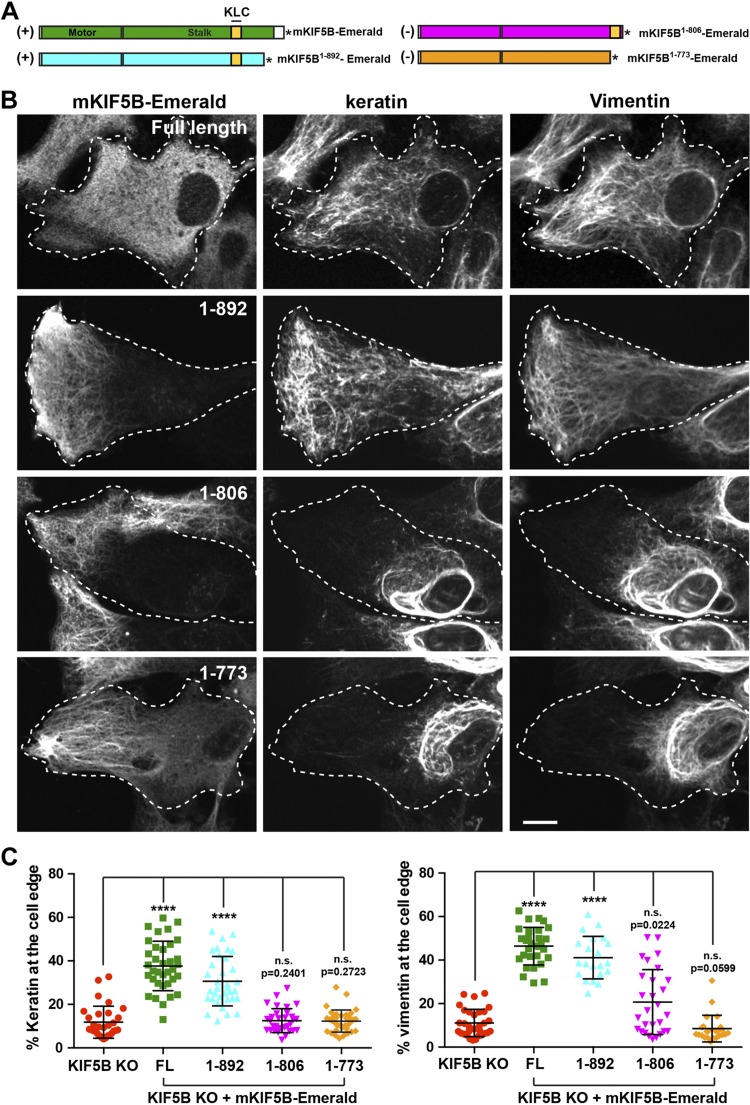
Because KLC was not required for IF transport, we concluded that a specific cargo-binding region of the kinesin tail might be involved. To determine which part of the KIF5B tail is important, we created 3 other truncated version of mKIF5B fused to Emerald: mKIF5B1–773, mKIF5B1–806, and mKIF5B1–892 (Fig. 5A). All 3 constructs were properly expressed in KIF5B KO RPE cells and are likely functionally active because they accumulated at the cell periphery, where most of the microtubule plus-ends are located (Fig. 5B, first column). This distribution was different from the distribution of full-length mKIF5B because the C-terminal truncations removed the autoinhibitory domain located at residues 937–952 of KIF5B (37), producing a constitutively active motor (Fig. 5B).
Figure 5.
Mouse KIF5B1–892 rescues IF distribution in KIF5B KO cells. A) Schematic representation of different truncations of the KIF5B tail. The mKIF5B constructs capable of rescuing keratin and vimentin distribution are marked by a (+); the one that does not is marked by a (−). The asterisk represents Emerald. B) Confocal microscopy imaging of keratin and vimentin immunostaining in RPE KIF5B KO cells (clone #4) after the expression of mKIF5B-Emerald (full length), mKIF5B1–892-Emerald (1–892), mKIF5B1–806-Emerald (1–806), or mKIF5B1–773-Emerald (1–773). C) Graphs show the percentage of vimentin (left) or keratin (right) at the cell edge (mean with sd, n > 30 cells). Data are representative of at least 2 independent experiments. Statistical significance was determined using the Mann-Whitney U test. ****P < 0.0001.
The ability of these 3 truncations to rescue keratin and vimentin IF distribution in KIF5B KO cells was tested by immunostaining. Deletion of the last 70 residues of KIF5B, creating mKIF5B1–892, did not affect the ability of mKIF5B to rescue the IF distribution. Furthermore, in some cells, the IFs are even more abundant at the periphery of the cell where the constitutively active truncated motor itself accumulated (Fig. 5B, second row and Supplemental Fig. 3G). However, more extensive truncations (mKIF5B1–773, mKIF5B1–806) inhibited the ability of mKIF5B to disperse IFs from the perinuclear location while these motors accumulated at the edge of the cell (Fig. 5B, third and fourth rows and Supplemental Fig. 3E, F). These results and the rescue by mKIF5BΔ775–802 strongly support the presence of an IF binding between residues 803 and 892 of the KIF5B tail. The constructs that rescued vimentin distribution were also able to rescue the distribution of keratin and vice versa, suggesting that both types of IFs bind (directly or indirectly) to the same region of kinesin heavy chain.
DISCUSSION
The development of fluorescent probes and advances in live cell imaging have dramatically changed our understanding of IF dynamics. Traditionally considered as mostly static rigid structures, IFs are, in fact, highly dynamic, undergoing constant rearrangement by severing and reannealing (7, 8), subunit exchange (38), and translocation of precursors and fully polymerized filaments (8, 12, 39, 40). In this paper, we showed that even fully polymerized keratin IFs undergo constant transport along microtubules (Fig. 2). We established that kinesin-1 is essential to keratin IF transport along microtubules and for vimentin IF transport. In KIF5B KO cells, both types of IFs are depleted from the cell edge (Fig. 3A–C and Supplemental Fig. 3), and active transport of keratin or vimentin filaments from the cell center to the cell periphery is no longer observed (Supplemental Videos 5 and 6). Finally, we demonstrated that KLC was not involved in keratin or vimentin IF transport by kinesin (Fig. 4) and that the same region of the kinesin tail is required for both keratin and vimentin transport (Fig. 5).
Contribution of microtubules to keratin network dynamics
The dynamics of the keratin filament network has been extensively studied (27). It has been clearly demonstrated that actin dynamics are responsible for the retrograde transport of keratin filament precursors formed at the focal adhesion and long filaments to the perinuclear region (18, 20, 41, 42). Experiments using fluorescence recovery after photobleaching have been very important in deciphering the role of subunit exchange during the cycle of assembly and disassembly of the entire keratin network (19, 43). This model was recently recapitulated in vivo in an elegant study using YFP-tagged keratin in murine embryos (44). However, even though microtubule-dependent motion of keratin particles has been observed (42, 45), the rapid transport of fully polymerized keratin filaments has never been reported.
In our study, we used photoconversion as an alternative approach to follow a small subset of individual filaments at the cell center where the filament network is especially dense. By using this technique, we observed for the first time the anterograde transport of fully polymerized keratin filaments. Our data complement very well the published work about keratin dynamics, demonstrating the contribution of microtubule-dependent transport from the cell center to the periphery. We believe that the importance of the contribution of microtubule-dependent transport to keratin IF dynamics depends on the physiologic context. For example, we studied keratin dynamics in RPE cells, which are relatively motile as compared with other epithelial cells. We speculate that anterograde transport of keratin filaments in epithelial cells delivers filaments to newly formed areas of cytoplasm in migrating epithelial cells. As previously reported (28), we observed a severe retraction of the keratin network in RPE after nocodazole treatment as compared with what was described for other epithelial cell types. This raises the possibility that epithelial cells with looser cell-cell junctions are more dependent to microtubule-dependent transport to maintain the keratin filament network extension to the cell periphery. Detailed analyses of keratin transport in other epithelial cells is required to determine the regulation of keratin dynamics.
Kinesin-1 as a universal transporter of IFs
In this paper, we demonstrated using KIF5B KO that kinesin-1 is the anterograde motor responsible for the transport of keratin IFs. We also established the essential role of kinesin-1 for vimentin transport. A role for kinesin-1 has been suggested previously using less specific or less efficient approaches, such as antibody injection that inhibits multiple kinesins (9) or short hairpin RNA that causes incomplete knock down (13). In our current study, the KO of KIF5B completely removed kinesin-1 from RPE cells, resulting in the complete inhibition of not only vimentin but also keratin transport along microtubules and the retraction of the two IF networks to the perinuclear region. This retraction is very likely caused by actin retrograde flow, as previously demonstrated during nocodazole treatment (46), or by retrograde transport along microtubules (8, 47) and is normally counteracted by kinesin-driven anterograde transport.
Kinesin-1 KO has been demonstrated to have a major impact in at least 2 different tissues by affecting different types of IFs. Conditional KO of the neuronal isoform of kinesin-1 Kif5a in mouse neurons inhibits neurofilament transport into the axon, leading to the accumulation of the 3 neurofilaments (NF-H, NF-M, and NF-L) in the cell body, thereby causing neurodegeneration and premature death of the animal (15, 48). More recently, conditional KO of KIF5B in mouse myogenic cells has been shown to prevent the recruitment of desmin and nestin IFs to the growing tip of the myotube during muscle formation associated with severe skeletal muscle abnormalities and heart failure (16). Based on our study and others, we can conclude that all types of IFs, except perhaps the nuclear lamins, are kinesin-1 cargoes.
Are all IFs transported along microtubules using the same mechanism?
Using mKIF5B truncations to rescue keratin and vimentin IF distribution in KIF5B KO cells, we determined that KLC was not involved in the transport of these 2 types of IFs and that a region between residues 803 and 892 of the kinesin tail was required (Figs. 4 and 5). These findings corroborate a previous study showing that proper localization of desmin- and nestin-containing IFs in the myofibril is independent of KLC and can be rescued with a truncated version of KIF5B lacking the last 73 residues of the kinesin tail (1–890) (16). This suggests that several types of IF probably bind to the same C-terminal region of kinesin heavy chain, reinforcing the hypothesis that all IFs are transported along microtubules by a common mechanism.
Kinesin-1 is also capable of directing transport of microtubules, another cytoskeletal structure (36). The region of the heavy chain required for microtubule transport is located between residues 892 and 914 of the heavy chain (49) close to the region required for IF transport. Although microtubules bind to the C-terminal part of the heavy chain directly, in the case of IFs it is unknown whether this binding is direct or mediated by an adaptor protein. Direct interaction between the IF protein desmin and the kinesin tail has been demonstrated in vitro (16), suggesting that it could be the case for other types of IF. If an adaptor is involved, it likely links all types of IF to the region 803–892 of the kinesin tail. Whether IF binding to kinesin-1 is direct or adaptor mediated, the region of the IF proteins responsible for the interaction with kinesin-1 needs to be identified. Because all IF types are potential kinesin-1 cargoes, it is plausible that the recognition domain for kinesin-1 is located in the central α-helical rod domain that is highly conserved in IF proteins (50).
There is compelling evidence that IF functions go well beyond the control of mechanical integrity because they are becoming key players in the signal transduction of stress-related and other cellular responses (51). In that context, the proper delivery of IFs to subcellular locations might be a key requirement for their ever-growing list of functions.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank Dr. Fatima Gyoeva (Institute of Protein Research, Russian Academy of Sciences, Moscow, Russia) for the C terminus and head domain polyclonal antibodies against kinesin, and David Kirchenbuechler (Center for Advanced Microscopy/Nikon Imaging Center, Northwestern University, Chicago, IL, USA) for help with quantification of IF distribution. Research reported in this publication was supported by the of the U.S. National Institutes of Health, National Institute of General Medical Science (P01GM09697 and R01 GM52111). The authors declare no conflicts of interest.
Glossary
- cas9
clustered regularly interspaced short palindromic repeats–associated protein 9
- CRISPR
clustered regularly interspaced short palindromic repeats
- gRNA
guide RNA
- IF
intermediate filament
- KLC
kinesin light chain
- KO
knockout
- mKIF5B
mouse KIF5B
- NA
numerical aperture
- NF
neurofilament
- RPE
retinal pigment epithelial
- SIM
structured illumination microscopy
- WT
wild type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
A. Robert and V. I. Gelfand designed the research; A. Robert and P. Tian performed research; P. Tian quantified the data; A. Robert, R. D. Goldman, and V. I. Gelfand analyzed the data; S. A. Adam contributed new reagents; M. Kittisopikul and K. Jaqaman developed and contributed the analytic tool to segment, analyze, and quantify filament transport; and A. Robert and V. I. Gelfand wrote the paper.
REFERENCES
- 1.Mendez M. G., Kojima S., Goldman R. D. (2010) Vimentin induces changes in cell shape, motility, and adhesion during the epithelial to mesenchymal transition. FASEB J. 24, 1838–1851 10.1096/fj.09-151639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rogel M. R., Soni P. N., Troken J. R., Sitikov A., Trejo H. E., Ridge K. M. (2011) Vimentin is sufficient and required for wound repair and remodeling in alveolar epithelial cells. FASEB J. 25, 3873–3883 10.1096/fj.10-170795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Helfand B. T., Mendez M. G., Murthy S. N. P., Shumaker D. K., Grin B., Mahammad S., Aebi U., Wedig T., Wu Y. I., Hahn K. M., Inagaki M., Herrmann H., Goldman R. D. (2011) Vimentin organization modulates the formation of lamellipodia. Mol. Biol. Cell 22, 1274–1289 10.1091/mbc.e10-08-0699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Costigliola N., Ding L., Burckhardt C. J., Han S. J., Gutierrez E., Mota A., Groisman A., Mitchison T. J., Danuser G. (2017) Vimentin fibers orient traction stress. Proc. Natl. Acad. Sci. USA 114, 5195–5200 10.1073/pnas.1614610114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones J. C., Kam C. Y., Harmon R. M., Woychek A. V., Hopkinson S. B., Green K. J. (2017) Intermediate filaments and the plasma membrane. Cold Spring Harb. Perspect. Biol. 9 10.1101/cshperspect.a025866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robert A., Hookway C., Gelfand V. I. (2016) Intermediate filament dynamics: what we can see now and why it matters. BioEssays 38, 232–243 10.1002/bies.201500142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uchida A., Çolakoğlu G., Wang L., Monsma P. C., Brown A. (2013) Severing and end-to-end annealing of neurofilaments in neurons. Proc. Natl. Acad. Sci. USA 110, E2696–E2705 10.1073/pnas.1221835110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hookway C., Ding L., Davidson M. W., Rappoport J. Z., Danuser G., Gelfand V. I. (2015) Microtubule-dependent transport and dynamics of vimentin intermediate filaments. Mol. Biol. Cell 26, 1675–1686 10.1091/mbc.e14-09-1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gyoeva F. K., Gelfand V. I. (1991) Coalignment of vimentin intermediate filaments with microtubules depends on kinesin. Nature 353, 445–448 10.1038/353445a0 [DOI] [PubMed] [Google Scholar]
- 10.Wang L., Ho C. L., Sun D., Liem R. K., Brown A. (2000) Rapid movement of axonal neurofilaments interrupted by prolonged pauses. Nat. Cell Biol. 2, 137–141 10.1038/35004008 [DOI] [PubMed] [Google Scholar]
- 11.Prahlad V., Yoon M., Moir R. D., Vale R. D., Goldman R. D. (1998) Rapid movements of vimentin on microtubule tracks: kinesin-dependent assembly of intermediate filament networks. J. Cell Biol. 143, 159–170 10.1083/jcb.143.1.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robert A., Herrmann H., Davidson M. W., Gelfand V. I. (2014) Microtubule-dependent transport of vimentin filament precursors is regulated by actin and by the concerted action of Rho- and p21-activated kinases. FASEB J. 28, 2879–2890 10.1096/fj.14-250019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leduc C., Etienne-Manneville S. (2017) Regulation of microtubule-associated motors drives intermediate filament network polarization. J. Cell Biol. 216, 1689–1703 10.1083/jcb.201607045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Theiss C., Napirei M., Meller K. (2005) Impairment of anterograde and retrograde neurofilament transport after anti-kinesin and anti-dynein antibody microinjection in chicken dorsal root ganglia. Eur. J. Cell Biol. 84, 29–43 10.1016/j.ejcb.2004.09.001 [DOI] [PubMed] [Google Scholar]
- 15.Uchida A., Alami N. H., Brown A. (2009) Tight functional coupling of kinesin-1A and dynein motors in the bidirectional transport of neurofilaments. Mol. Biol. Cell 20, 4997–5006 10.1091/mbc.e09-04-0304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Z., Cui J., Wong W. M., Li X., Xue W., Lin R., Wang J., Wang P., Tanner J. A., Cheah K. S., Wu W., Huang J. D. (2013) Kif5b controls the localization of myofibril components for their assembly and linkage to the myotendinous junctions. Development 140, 617–626 10.1242/dev.085969 [DOI] [PubMed] [Google Scholar]
- 17.Windoffer R., Wöll S., Strnad P., Leube R. E. (2004) Identification of novel principles of keratin filament network turnover in living cells. Mol. Biol. Cell 15, 2436–2448 10.1091/mbc.e03-09-0707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kölsch A., Windoffer R., Leube R. E. (2009) Actin-dependent dynamics of keratin filament precursors. Cell Motil. Cytoskeleton 66, 976–985 10.1002/cm.20395 [DOI] [PubMed] [Google Scholar]
- 19.Kölsch A., Windoffer R., Würflinger T., Aach T., Leube R. E. (2010) The keratin-filament cycle of assembly and disassembly. J. Cell Sci. 123, 2266–2272 10.1242/jcs.068080 [DOI] [PubMed] [Google Scholar]
- 20.Windoffer R., Kölsch A., Wöll S., Leube R. E. (2006) Focal adhesions are hotspots for keratin filament precursor formation. J. Cell Biol. 173, 341–348 10.1083/jcb.200511124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jaqaman K., Loerke D., Mettlen M., Kuwata H., Grinstein S., Schmid S. L., Danuser G. (2008) Robust single-particle tracking in live-cell time-lapse sequences. Nat. Methods 5, 695–702 10.1038/nmeth.1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gan Z., Ding L., Burckhardt C. J., Lowery J., Zaritsky A., Sitterley K., Mota A., Costigliola N., Starker C. G., Voytas D. F., Tytell J., Goldman R. D., Danuser G. (2016) Vimentin intermediate filaments template Mmcrotubule networks to enhance persistence in cell polarity and directed migration. Cell Syst. 3, 252–263.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Otsu N. (1979) A threshold selection method from gray-level histograms. IEEE Trans. Syst. Man Cybern. 9, 62–66 10.1109/TSMC.1979.4310076 [DOI] [Google Scholar]
- 24.Van Ginkel M. (2002) Image Analysis Using Orientation Space Based on Steerable Filters, Delft University of Technology, Delft, The Netherlands [Google Scholar]
- 25.Nieuwenhuizen R. P., Nahidiazar L., Manders E. M., Jalink K., Stallinga S., Rieger B. (2015) Co-orientation: quantifying simultaneous co-localization and orientational alignment of filaments in light microscopy. PLoS One 10, e0131756 10.1371/journal.pone.0131756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shimi T., Kittisopikul M., Tran J., Goldman A. E., Adam S. A., Zheng Y., Jaqaman K., Goldman R. D. (2015) Structural organization of nuclear lamins A, C, B1, and B2 revealed by superresolution microscopy. Mol. Biol. Cell 26, 4075–4086 10.1091/mbc.e15-07-0461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leube R. E., Moch M., Kölsch A., Windoffer R. (2011) “Panta rhei”: perpetual cycling of the keratin cytoskeleton. Bioarchitecture 1, 39–44 10.4161/bioa.1.1.14815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hunt R. C., Davis A. A. (1990) Altered expression of keratin and vimentin in human retinal pigment epithelial cells in vivo and in vitro. J. Cell. Physiol. 145, 187–199 10.1002/jcp.1041450202 [DOI] [PubMed] [Google Scholar]
- 29.Tanaka Y., Kanai Y., Okada Y., Nonaka S., Takeda S., Harada A., Hirokawa N. (1998) Targeted disruption of mouse conventional kinesin heavy chain, kif5B, results in abnormal perinuclear clustering of mitochondria. Cell 93, 1147–1158 10.1016/S0092-8674(00)81459-2 [DOI] [PubMed] [Google Scholar]
- 30.Pu J., Guardia C. M., Keren-Kaplan T., Bonifacino J. S. (2016) Mechanisms and functions of lysosome positioning. J. Cell Sci. 129, 4329–4339 10.1242/jcs.196287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hirokawa N., Pfister K. K., Yorifuji H., Wagner M. C., Brady S. T., Bloom G. S. (1989) Submolecular domains of bovine brain kinesin identified by electron microscopy and monoclonal antibody decoration. Cell 56, 867–878 10.1016/0092-8674(89)90691-0 [DOI] [PubMed] [Google Scholar]
- 32.Diefenbach R. J., Mackay J. P., Armati P. J., Cunningham A. L. (1998) The C-terminal region of the stalk domain of ubiquitous human kinesin heavy chain contains the binding site for kinesin light chain. Biochemistry 37, 16663–16670 10.1021/bi981163r [DOI] [PubMed] [Google Scholar]
- 33.Gyoeva F. K., Sarkisov D. V., Khodjakov A. L., Minin A. A. (2004) The tetrameric molecule of conventional kinesin contains identical light chains. Biochemistry 43, 13525–13531 10.1021/bi049288l [DOI] [PubMed] [Google Scholar]
- 34.Skoufias D. A., Cole D. G., Wedaman K. P., Scholey J. M. (1994) The carboxyl-terminal domain of kinesin heavy chain is important for membrane binding. J. Biol. Chem. 269, 1477–1485 [PubMed] [Google Scholar]
- 35.Seiler S., Kirchner J., Horn C., Kallipolitou A., Woehlke G., Schliwa M. (2000) Cargo binding and regulatory sites in the tail of fungal conventional kinesin. Nat. Cell Biol. 2, 333–338 10.1038/35014022 [DOI] [PubMed] [Google Scholar]
- 36.Jolly A. L., Kim H., Srinivasan D., Lakonishok M., Larson A. G., Gelfand V. I. (2010) Kinesin-1 heavy chain mediates microtubule sliding to drive changes in cell shape. Proc. Natl. Acad. Sci. USA 107, 12151–12156 10.1073/pnas.1004736107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hackney D. D., Baek N., Snyder A. C. (2009) Half-site inhibition of dimeric kinesin head domains by monomeric tail domains. Biochemistry 48, 3448–3456 10.1021/bi8022575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robert A., Rossow M. J., Hookway C., Adam S. A., Gelfand V. I. (2015) Vimentin filament precursors exchange subunits in an ATP-dependent manner. Proc. Natl. Acad. Sci. USA 112, E3505–E3514 10.1073/pnas.1505303112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang L., Brown A. (2001) Rapid intermittent movement of axonal neurofilaments observed by fluorescence photobleaching. Mol. Biol. Cell 12, 3257–3267 10.1091/mbc.12.10.3257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoon M., Moir R. D., Prahlad V., Goldman R. D. (1998) Motile properties of vimentin intermediate filament networks in living cells. J. Cell Biol. 143, 147–157 10.1083/jcb.143.1.147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Windoffer R., Leube R. E. (1999) Detection of cytokeratin dynamics by time-lapse fluorescence microscopy in living cells. J. Cell Sci. 112, 4521–4534 [DOI] [PubMed] [Google Scholar]
- 42.Wöll S., Windoffer R., Leube R. E. (2005) Dissection of keratin dynamics: different contributions of the actin and microtubule systems. Eur. J. Cell Biol. 84, 311–328 10.1016/j.ejcb.2004.12.004 [DOI] [PubMed] [Google Scholar]
- 43.Moch M., Herberich G., Aach T., Leube R. E., Windoffer R. (2013) Measuring the regulation of keratin filament network dynamics. Proc. Natl. Acad. Sci. USA 110, 10664–10669 10.1073/pnas.1306020110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schwarz N., Windoffer R., Magin T. M., Leube R. E. (2015) Dissection of keratin network formation, turnover and reorganization in living murine embryos. Sci. Rep. 5, 9007 10.1038/srep09007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liovic M., Mogensen M. M., Prescott A. R., Lane E. B. (2003) Observation of keratin particles showing fast bidirectional movement colocalized with microtubules. J. Cell Sci. 116, 1417–1427 10.1242/jcs.00363 [DOI] [PubMed] [Google Scholar]
- 46.Hollenbeck P. J., Bershadsky A. D., Pletjushkina O. Y., Tint I. S., Vasiliev J. M. (1989) Intermediate filament collapse is an ATP-dependent and actin-dependent process. J. Cell Sci. 92, 621–631 [DOI] [PubMed] [Google Scholar]
- 47.Helfand B. T., Mikami A., Vallee R. B., Goldman R. D. (2002) A requirement for cytoplasmic dynein and dynactin in intermediate filament network assembly and organization. J. Cell Biol. 157, 795–806 10.1083/jcb.200202027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xia C.-H., Roberts E. A., Her L.-S., Liu X., Williams D. S., Cleveland D. W., Goldstein L. S. B. (2003) Abnormal neurofilament transport caused by targeted disruption of neuronal kinesin heavy chain KIF5A. J. Cell Biol. 161, 55–66 10.1083/jcb.200301026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seeger M. A., Rice S. E. (2010) Microtubule-associated protein-like binding of the kinesin-1 tail to microtubules. J. Biol. Chem. 285, 8155–8162 10.1074/jbc.M109.068247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Herrmann H., Kreplak L., Aebi U. (2004) Isolation, characterization, and in vitro assembly of intermediate filaments. Methods Cell Biol. 78, 3–24 10.1016/S0091-679X(04)78001-2 [DOI] [PubMed] [Google Scholar]
- 51.Sanghvi-Shah R., Weber G. F. (2017) Intermediate filaments at the junction of mechanotransduction, migration, and development. Front. Cell Dev. Biol. 5, 81 10.3389/fcell.2017.00081 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.