Abstract
Hepatitis E virus (HEV) infection has emerged as a global health problem. However, no approved medication is available, and the infection biology remains largely elusive. Electron transport chain (ETC), a key component of the mitochondria, is the main site that produces ATP and reactive oxygen species (ROS). By profiling the role of the different complexes of the mitochondrial ETC, we found that pharmacological inhibition of complex III, a well-defined drug target for the treatment of malaria and Pneumocystis pneumonia, potently restricts HEV replication. This effect demonstrated in our HEV models is equivalent to the anti-HEV potency of ribavirin, a widely used off-label treatment for patients with chronic HEV. Mechanistically, we found that this effect is independent of ATP production, ROS level, and pyridine depletion. By using pharmacological inhibitors and genetic approaches, we found that mitochondrial permeability transition pore (MPTP), a newly identified component of ETC, provides basal defense against HEV infection. HEV interferes with the opening of the MPTP. Furthermore, inhibition of the MPTP attenuated the anti-HEV effect of complex III inhibitors, suggesting that the MPTP mediates the antiviral effects of these inhibitors. These findings reveal new insights on HEV–host interactions and provide viable anti-HEV targets for therapeutic development.—Qu, C., Zhang, S., Wang, W., Li, M., Wang, Y., van der Heijde-Mulder, M., Shokrollahi, E., Hakim, M. S., Raat, N. J. H., Peppelenbosch, M. P., Pan, Q. Mitochondrial electron transport chain complex III sustains hepatitis E virus replication and represents an antiviral target.
Keywords: MPTP, HEV, virus-host interaction, ETC
Hepatitis E virus (HEV) infection is the major cause of acute viral hepatitis worldwide. Although the infection is asymptomatic and self-limiting in healthy populations, it often causes high mortality in pregnant women and chronic hepatitis in recipients of organ transplants (1). More recently, extrahepatic manifestations of HEV infection in particular neurologic disorders have been widely reported (2). Although ribavirin is effective as an off-label treatment for some cases of chronic hepatitis E, a substantial proportion of patients are not eligible for or do not tolerate the treatment. Furthermore, development of resistance mutations may result in failure of viral clearance (3, 4). Further study of HEV–host interactions is essential for understanding the pathogenesis, revealing novel antiviral targets, and developing new anti-HEV therapies (5).
Mitochondria are unique double-membraned organelles present in all eukaryotic organisms. The number of mitochondria in a cell varies widely, but liver cells are rich in these organelles, containing more than 2000 mitochondria per cell (6). Dysfunctions of mitochondrial are implicated in various liver diseases—in particular, viral hepatitis. Acting as a powerhouse to generate energy, it plays essential roles in physiology to sustain life. Electron transport chain (ETC), a key component of mitochondria, is the main site of production of ATP and reactive oxygen species (ROS). The ETC is located in the inner mitochondrial membrane and consists of 4 multisubunit enzyme complexes (complexes I–IV). Electrons harvested from NADH through complex I or reduced flavin adenine dinucleotide-2 through complex II are received by ubiquinol. Ubiquinol subsequently transfers the electrons to cytochrome c through complex III and is oxidized to ubiquinone, which is required for the de novo biosynthesis of pyrimidines by dihydro-orotate dehydrogenase (7). Cytochrome c then passes the electrons to complex IV, which uses the electrons and hydrogen ions to reduce molecular oxygen to H2O (8, 9). During this process, the high energy of the electron is converted to the electrochemical proton gradient across the inner membrane, which drives the synthesis of ATP by ATP synthase. Meanwhile, leakage of electrons from the ETC leads to the production of ROS.
Accumulating evidence has shown that mitochondria serves as a signaling hub for the innate immune response and facilitate downstream signaling leading to IFN synthesis. On the other hand, virus strategically alters mitochondrial function to influence the energy production, metabolism, and immune signaling (10). With respect to defending pathogen invasion and maintaining homeostasis, mitochondria constantly communicate with cytosol to initiate biologic events. Mitochondrial permeability transition pore (MPTP), a newly identified component of ETC (11, 12), is a nonselective channel that facilitates the exchange of molecules between the mitochondrial matrix and cytoplasm. Most of the RNA viruses, including hepatitis C virus (HCV) and HEV, develop solely in the cytoplasm. HCV has been shown to trigger mitochondrial permeability transition to establish chronic liver disease (13). HEV has been shown to protect cells from mitochondria depolarization (14). However, whether MPTP is involved in this process remains unknown. In this study, we investigated the role of the mitochondrial ETC in HEV infection and explored the potential of therapeutic targeting.
MATERIALS AND METHODS
Reagents and antibodies
Rotenone (ROT), 2-thenoyltrifluoroacetone (TTFA), antimycin A (AMA), myxothiazol (MYXO), potassium cyanide, oligomycin A (OLM), ribavirin, vitamin E acetate (VE), ethidium bromide (EB), 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR), uridine, cytidine, cyclosporin A (CsA), bongkrekic acid (BKA), and H2O2 were purchased from MilliporeSigma (Burlington, MA, USA). The mouse mAb against cyclophilin D (CypD) was purchased from Santa Cruz Biotechnology (Dallas, TX, USA). The HEV-specific antibody against open reading frame (ORF)-2 was purchased from MilliporeSigma.
Cell culture and establishment of ETC-deficient cell culture model
The human hepatoma (Huh7.5) cell line, Hep3B cell line, and human glioblastoma U87 cell line were kindly provided by the Department of Viroscience (Erasmus Medical Center). Huh7.5, Hep3B, and U87 cells were cultured in DMEM (Lonza Biowhittaker, Verviers, Belgium) supplemented with 10% (v/v) heat-inactivated fetal calf serum (Thermo Fisher Scientific), 100 IU/ml penicillin, and 100 μg/ml streptomycin. An ETC-deficient cell culture model was established as follows: cells harboring the p6 infectious clone were cultured in the presence of medium EB (50 ng/ml), pyruvate (100 μg/ml), and uridine (50 μg/ml) for 96 h, as described in refs. 15 and 16.
HEV cell culture models
A plasmid construct containing subgenomic HEV sequence coupled with a Gaussia luciferase reporter gene (p6-Luc) and a construct containing the full-length HEV genome (Kernow-C1 p6 clone, GenBank: JQ679013; National Center for Biotechnology Information, Bethesda, MD, USA; https://www.ncbi.nlm.nih.gov/genbank/) was used to generate HEV genomic RNA by using the mMessage mMachine In Vitro RNA Transcription Kit (Thermo Fisher Scientific, Waltham, MA, USA) (17, 18). Cells were electroporated with p6-Luc subgenomic HEV RNA and p6 full-length HEV RNA to generate replication luciferase and infection models, respectively (2). For the HEV genotype, a replicon model or viral RNA was generated from a Sar55/S17/luc-encoding plasmid. Huh7.5 cells were electroporated with Sar55/S17/luc HEV RNA to generate a genotype 1 replicon model (19).
ATP production measurement
The ATP content in cultured cells was measured with the ATP Bioluminescence Assay Kit HS II according to the manufacturer’s instructions (Roche Life Science, Penzberg, Germany). In brief, cells were harvested and suspended in dilution buffer at a concentration of 105 per milliliter. The same volume of cell lysis reagent was added to the above cell suspension and incubated at 15°C for 5 min and for an extra 2 min at 100°C. Then, it was centrifuged at 10,000 g for 60 s, and the supernatant was transferred to a fresh tube. Fifty microliters of the supernatant was mixed with luciferase reagent and subjected to luciferase measurement with LumiStar Optima Luminescence Counter (BMG Labtech, Offenburg, Germany). The supernatant was kept on ice until measurement.
Flow cytometry
Intracellular ROS was measured by using a general oxidative stress indicator according to the manufacturer’s instructions (chloromethyl-H2-2′,7′-dichlorodihydrofluorescein diacetate; Thermo Fisher Scientific). In brief, cells were seeded into a 12-well plate at 5000 cells per well. After treatment with the appropriate reagents, the cells were harvested and resuspended in prewarmed PBS containing 10 μM chloromethyl-H2-2′,7′-dichlorodihydrofluorescein diacetate and incubated at 37°C for 20 min. After incubation, the cells were resuspended and returned to prewarmed growth medium. Then, the cells were subjected to flow cytometry analysis with excitation of 488 nm and emission of 530 nm.
An MPTP assay was performed according to the manufacturer’s instructions (BioVision, Milpitas, CA, USA). In brief, cells were harvested and resuspended in prewarmed MPTP wash buffer at a final concentration of 106 cells per milliliter. The cell suspension was then incubated with the appropriate reagents at 37°C for 15 min and centrifuged at 1000 g for 5 min to pellet cells and remove excess staining and quenching reagents. Cells were then resuspended in 1 ml of MPTP wash buffer and were subjected to flow cytometry with a 488 nm excitation filter. The cell suspension was kept on ice and analyzed within 1 h.
Western blot assay
Whole-cell lysates were heated at 95°C for 8 min, followed by loading onto a 15% SDS-polyacrylamide gel and separating by electrophoresis. After separation on a 12% SDS-PAGE gel, the proteins were transferred onto a PVDF membrane (Thermo Fisher Scientific). Subsequently, the membrane was blocked for 1 h at room temperature followed by incubation with mouse anti-CypD (1:500) antibody overnight at 4°C. The membrane was washed 3 times followed by incubation for 1.5 h with an anti-mouse peroxidase–conjugated secondary antibody (1:10,000). After the membrane was washed 3 times, the protein bands were detected with the Odyssey 3.0 Infrared Imaging System (Li-Cor, Lincoln, NE, USA).
Quantification of HEV replication
For the HEV luciferase model (p6-Luc), the activity of secreted Gaussia luciferase in the cell culture medium was measured with the BioLux Luciferase Flex Assay Kit (New England Biolabs, Ipswich, MA, USA), to quantify viral replication. Luciferase activity was quantified with a LumiStar Optima Luminescence Counter. All presented HEV luciferase values were normalized by MTT assay to exclude the effect of cell toxicity on HEV replication (Supplemental Fig. 1). For the p6 infectious model, SYBR-green–based real-time quantitative PCR (qPCR) was used to quantify genomic RNA. All the other RNA levels in this study were also quantified by real-time qPCR assay, with the primer sequences provided as follows: HEV: 5′-ATCGGCCAGAAGTTGGTTTTTAC-3′ (sense) and 5′-CCGTGGCTATAACTGTGGTCT-3′ (antisense); GAPDH: 5′-GTCTCCTCTGACTTCAACAGCG-3′ (sense) and 5′-ACCACCCTGTTGCTGTAGCCAA-3′ (antisense); CYTB: 5′-CCCTAACAAACTAGGAGGCG-3′ (sense) and 5′-TCTGCGGCTAGGAGTCAATA-3′ (antisense); COX I: 5′-CCTGACTGGCATTGTATTAG-3′ (sense) and 5′-GATAGGATGTTTCATGTGGTG-3′ (antisense); COX II: 5′-CATCCCTACGCATCCTTTAC-3′ (sense) and 5′-GGTTTGCTCCACAGATTTCAG-3′ (antisense); COX IV: 5′-CAGAAGGCACTGAAGGAGAAG-3′ (sense) and 5′-TCATGTCCAGCATCCTCTTG-3′ (antisense); and CypD: 5′-CGACTTCACCAACCACAATGGC-3′ (sense) and 5′-GGTGTTAGGACCAGCATTAGCC-3′ (antisense).
MTT assay
Ten millimolar MTT (MilliporeSigma) was added to the cells seeded in a 96-well plate, and cells were maintained at 37°C with 5% CO2 for 3 h. The medium was removed and 100 μl DMSO was added to each well. The absorbance of each well was read on the microplate absorbance reader (Bio-Rad, Hercules, CA, USA) at a wavelength of 490 nm. All measurements were performed in triplicate.
Gene knockdown by lentiviral vectors
The pLKO.1-based short hairpin RNA (shRNA) lentiviral vectors (Erasmus Center for Biomics) targeting CypD (shCypD) was used to knockdown CypD gene expression and scrambled control vector (shSCR) was used as the control. Lentiviral pseudoparticles were generated as described by Wang et al. (18). After the pilot study, the shRNA vectors exerting optimal gene knockdown were selected. To obtain a stable gene knockdown cell line, cells were transduced with shRNA lentiviral particles for 3 d and selected by puromycin (MilliporeSigma) at a concentration of 2.5 μg/ml. The shRNA sequence was: CypD, 5′-CCGGGCTCTAAGAGTGGGAGGACATCTCGAGATGTCCTCCCACTCTTAGAGCTTTTTG-3′.
Oxygen consumption rate measurement
Mitochondrial respiration was measured with the High Resolution Oxygraph (Oxygraph-2k; Oroboros Instruments, Innsbruck, Austria). Respiration was measured at 37°C in 2 ml chambers, and the cells were suspended in 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid–Tris buffer (HT). HT buffer contained 4.2 mM KCl, 132 mM NaCl, 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, 1.2 mM MgCl2, and 1 mM CaCl2 and was adjusted to pH = 7.4 with Tris. Equilibration with the ambient air was achieved when the oxygen concentration in each chamber reached a constant level. After calibrating the chambers for volume, cells were introduced to each chamber to measure the mitochondrial basal respiration. After 10 min stabilization of the oxygen flux, the basal respiration was measured for 10 min. The corresponding average oxygen consumption was calculated with Dat-Lab5 software (Oroboros Instruments).
Statistical analysis
Statistical analysis was performed with the nonpaired, nonparametric test with the Mann-Whitney U test and 1-way ANOVA with Bonferroni post hoc test (Prism v.5.01; GraphPad Software, La Jolla, CA, USA). Values of P < 0.05 were considered statistically significant.
RESULTS
Profiling ETC inhibitors identifies an essential role of complex III in sustaining HEV replication
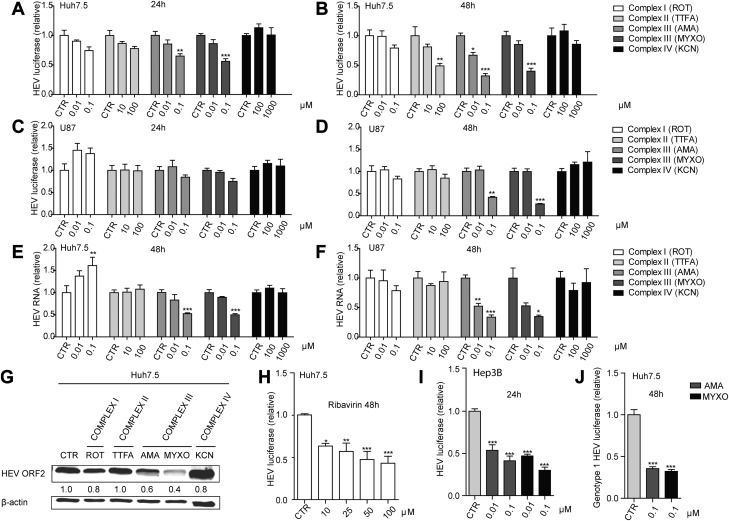
To study the potential role of ETC in HEV infection, we profiled the effects of targeting different complexes of ETC by pharmacological inhibitors. The Huh7.5 liver cell line and the U87 neuronal cell line were used to model HEV infection (2). We transfected Huh7.5 and U87 cells with an HEV subgenomic replicon (p6 luciferase model) and treated the cells with various inhibitors targeting complex I, II, III or IV. In the Huh7.5-p6 luciferase model, 24-h treatment with complex III inhibitors AMA and MYXO significantly inhibited HEV replication (Fig. 1A) and these effects were further enhanced after 48 h of treatment (Fig. 1B). Similar results were observed in U87 cells harboring the HEV subgenomic replicon (Fig. 1D). We next evaluated this effect in both cell lines harboring the full-length infectious HEV genome (the p6 infectious model). Consistently, 48-h treatment resulted in significant inhibition of viral RNA determined by real-time qPCR (Fig. 1E, F). The anti-HEV effect was further confirmed by Western blot analysis at the viral protein level (Fig. 1G). Of note, the complex II inhibitor TTFA exerted a significant inhibitory effect with high concentrations after 48-h treatment in the Huh7.5 HEV subgenomic replicon cells (Fig. 1B). However, this effect was not seen in other models. We found that the complex III inhibitors at 0.1 μM showed a comparable inhibitory effect on HEV replication compared with ribavirin at 100 μM (Fig. 1H). Besides, complex III inhibitors also showed strong inhibition of HEV in the Hep3B cell model (Fig. 1I) and effectively restricted genotype 1 HEV-related luciferase activity in the Huh7.5-based genotype 1 replicon model, Sar55/S17/luc (Fig. 1J). Taken together, complex III is essential in supporting HEV replication and can be targeted by pharmacological inhibitors to inhibit viral replication.
Figure 1.
Mitochondrial complex III inhibitors specifically inhibit HEV replication. A–F) Analysis of HEV-related Gaussia luciferase activity in an Huh7.5-p6 luciferase model treated with the indicated concentrations of different complex inhibitors for 24 h (A) and 48 h (B) and in a U87-p6 luciferase model treated with indicated concentrations of different complex inhibitors for 24 h (C) and 48 h (D). real-time qPCR analysis of HEV viral RNA level in Huh7.5 (E) and U87 (F) cell-based p6 infectious models treated with indicated concentrations of different complex inhibitors for 48 h. G) Western-blot analysis of HEV ORF-2 expression in Huh7.5-p6 infectious model after treatment with indicated concentrations of different complex inhibitors for 48 h (Ctr, nontreatment control; 0.1 μM ROT, 100 μM TTFA, 0.1 μM AMA, 0.1 μM MYXO, and 1000 μM KCN). H) Real-time qPCR analysis of HEV viral RNA level in Huh7.5 cell–based p6 luciferase model treated with the indicated concentrations of ribavirin. I) Analysis of HEV-related Gaussia luciferase activity in an Hep3B-p6 luciferase model treated with the indicated concentrations of complex III inhibitors for 24 h. J) Analysis of genotype 1 HEV (Sar55/S17/luc) viral replication-related Gaussia luciferase activity in Huh7.5-Sar/S17/luc model treated with the indicated concentrations of complex III inhibitors for 48 h. The data are means ± sd of 4 independent experiments normalized to GAPDH. *P < 0.05, **P < 0.01, ***P < 0.001 vs. DMSO CTR (set as 1).
The anti-HEV effects of complex III inhibition require the integrity of ETC
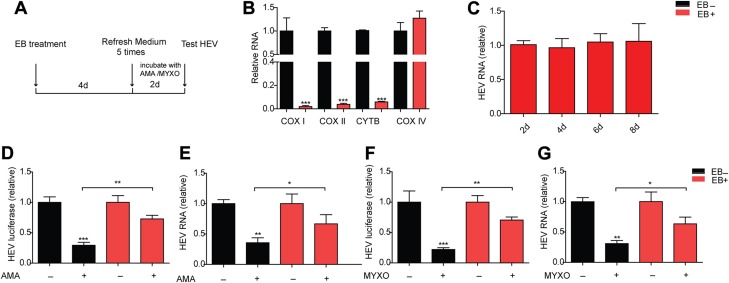
To further validate the specificity, we depleted ETC by using EB and then tested the HEV RNA level (Fig. 2A). EB is widely used to establish an ETC-deficient cell model by depleting mitochondrial DNA (15, 20). Cytochrome c oxidase (COX) I, cytochrome c oxidase (COX) II, and cytochrome b (CYTB) are encoded by mitochondrial DNA (21). Cytochrome c oxidase IV (COX IV) is encoded by nuclear gene and was used as a control (22). We found that, compared with Huh7 cells, U87 cells were more sensitive to EB treatment and showed less toxicity when treated with complex inhibitors (Supplemental Fig. 1I–K). Thus, U87 cells were used to establish an ETC-deficient cell model. After 4 d treatment, the mRNA level of COX I, COX II, and CYTB showed remarkable reduction with unchanged mRNA level of COX IV (Fig. 2B). These data demonstrate the successful establishment of an ETC-deficient cell model. EB treatment had no effect on the HEV RNA level (Fig. 2C). We then tested the anti-HEV effect of AMA and MYXO in this model. Lack of ETC strongly attenuated the anti-HEV effect of AMA and MYXO, as determined by real-time qPCR (Fig. 2D–G). These data suggest that ETC is necessary for complex III inhibitors to restrict HEV replication.
Figure 2.
ETC deficiency reverses the anti-HEV effects of complex III inhibitors. A) U87-p6 infectious cells were treated with EB to establish an ETC-deficient cell model and were maintained in a medium supplemented with uridine and pyruvate to complement the metabolic deficiency connected with the defective ETC. B) mRNA levels of COX I, II, and IV and CYTB in the EB-treated or untreated group were measured by real-time qPCR. Data are means ± sd of 4 independent experiments, normalized to GAPDH. *P < 0.05, **P < 0.01, ***P < 0.001 vs. EB-untreated group. C) U87-p6 infectious cells were treated with EB for the indicated time before measurement of HEV RNA. D, E) EB-treated and -untreated groups were incubated with 0.1 μM AMA for 2 d before measurement of HEV luciferase activity (D) and HEV RNA (E). F, G) The EB-treated or -untreated group was incubated with 0.1 μM MYXO for 2 d before measurement of HEV luciferase activity (F) and HEV RNA (G). Data in the EB-treated group are presented relative to EB-treated CTR (set as 1). The data are means ± sd of 4 independent experiments, normalized to GAPDH. *P < 0.05, **P < 0.01, ***P < 0.001 vs. EB-untreated CTR (set as 1).
The anti-HEV effect of complex III inhibition is independent of ATP and ROS production or pyrimidine depletion
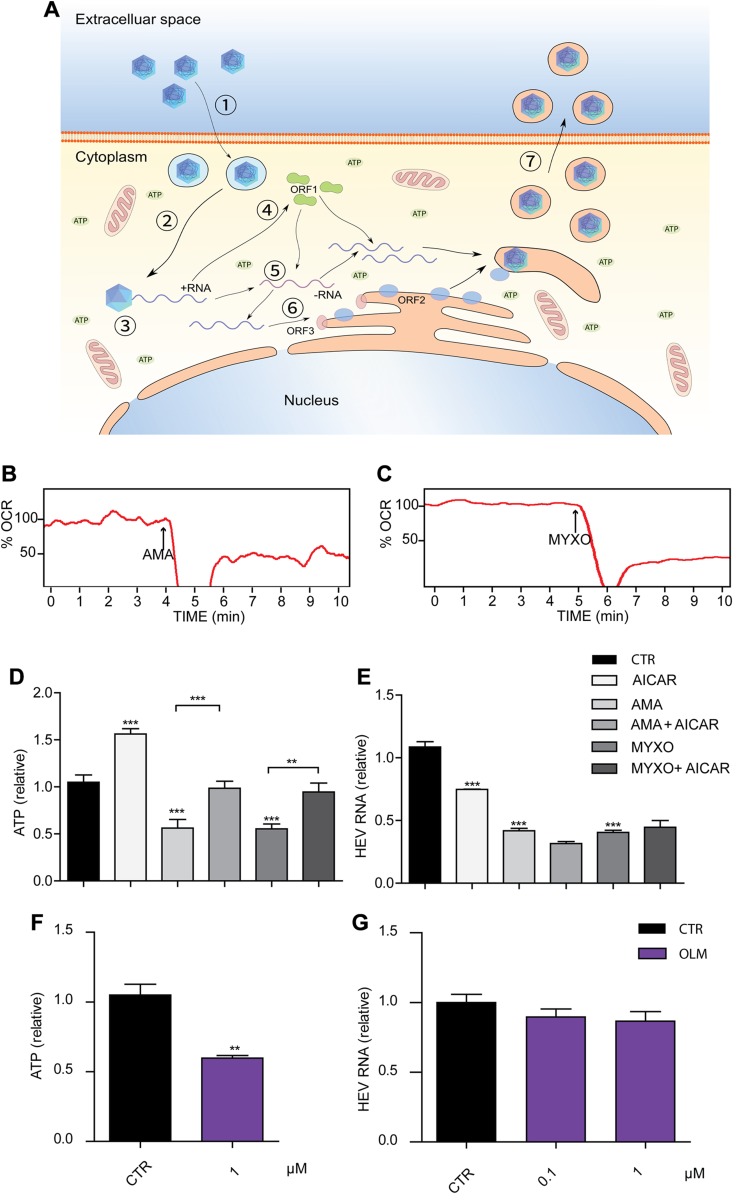
Up to 90% of ATP is produced in ETC. AMA and MYXO are well-known complex III inhibitors that specifically block the transportation of electrons from cytochrome b to c, resulting in loss of intracellular ATP (15, 23, 24). We further calculated the energy cost for each step of the HEV life cycle (Fig. 3A; see details in Supporting Information), using the method described for influenza virus (25). The energy consumption of HEV was lower than influenza virus; whereas influenza virus consumed only 1% of the cell energy budget. This suggests that HEV may not be sensitive to the change in intracellular ATP abundance.
Figure 3.
Inhibition of HEV by complex III inhibitors is independent of ATP level reduction. A) Energetic cost of each step of HEV life cycle. Cell entry (1; ATP < 103); intracellular transportation (2; ATP < 103); uncoating of capsid (3); translation (from parental genome (4); transcription (5; ATP < 3 × 105); translation (6; ATP ≈ 1.1 × 107); and budding (7; ATP ≈ 3.9 × 106). B, C) Oxygen consumption rate of Huh7.5 cells in the absence and presence of 0.1 μM AMA (B) and 0.1 μM MYXO (C) at the indicated time points. D) Huh7.5 cells were treated with 200 μM AICAR, 0.1 μM AMA, or 0.1 μM MYXO, alone or in combination for 3 h. After treatment, the cell lysates were subjected to ATP assay. E) The Huh7.5-p6 infectious model was treated with 200 μM AICAR, 0.1 μM AMA, or 0.1 μM MYXO, alone or in combination for 48 h before being subjected to real-time qPCR analysis of HEV RNA. F) Huh7.5 cells were treated with 0.1 μM OLM for 3 h. After treatment, the cell lysates were subjected to ATP assay. G) The Huh7.5-p6 infectious model was treated with 0.1 μM OLM for 48 h before real-time qPCR analysis of HEV RNA. Data are means ± sd of results in 4 independent experiments, normalized to GAPDH. **P < 0.01, ***P < 0.001 vs. DMSO nontreated control group (Ctr) (set as 1).
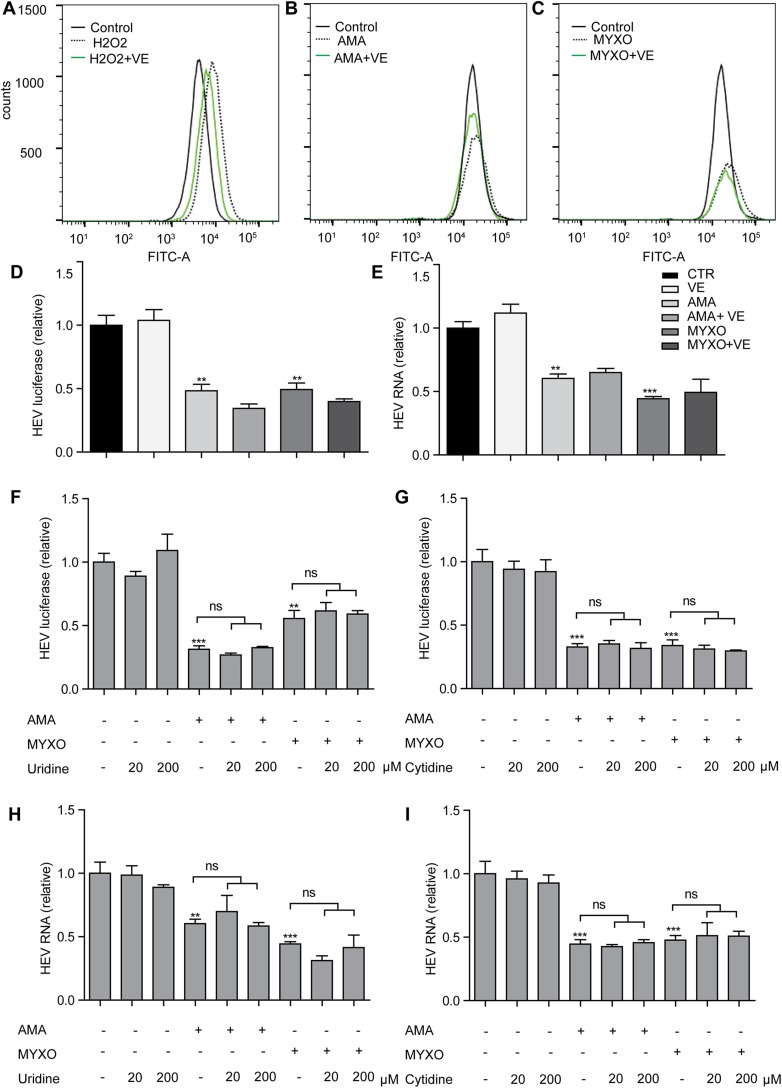
The dramatic reduction of the oxygen consumption rate was observed in Huh7.5 cell treated with AMA and MYXO (Fig. 3B, C). To investigate whether ATP production is involved in the anti-HEV effect, we coincubated AMA or MYXO with AICAR, a 5′-AMPK activator that is capable of accumulating ATP. AICAR effectively increased the ATP level and significantly reversed the reduction of ATP caused by AMA and MYXO in Huh7.5 cells (Fig. 3D). However, AICAR neither increased the HEV RNA level nor attenuated the anti-HEV effects of AMA or MYXO (Fig. 3E). To further confirm this, we treated the Huh7.5 cells with OLM, which binds to ATP synthase and inhibits ATP production. OLM showed inhibition of ATP production comparable to AMA and MYXO, but not on the HEV RNA level (Fig. 3F, G). The complex III is also the major site to produce ROS. ROS has been implicated in the innate immune response against pathogen infections (26). Inhibition of complex III has been shown to increase the cellular ROS level. Both AMA and MYXO increased ROS production. H2O2 induced marked ROS production serving as a positive control (Fig. 4A–C). To investigate the potential role of ROS in the anti-HEV activity of AMA and MYXO, vitamin E was added to scavenge ROS (Fig. 4A–C). However, the anti-HEV effects of AMA and MYXO were not affected by supplementation of vitamin E (Fig. 4D, E). Thus, the anti-HEV effects of AMA and MYXO are most likely independent of ATP and ROS production.
Figure 4.
Inhibition of HEV by complex III inhibitors is independent of ROS production. A–C) Flow cytometry of ROS level in Huh7.5 cells treated as indicated for 3 h. VE (100 mM) was added 1 h before treatment with 100 μM H2O2 (A), 0.1 μM AMA (B), or 0.1 μM MYXO (C). Ctr, nontreated control group. D, E) Coincubation of 100 μM VE with 0.1 μM AMA or 0.1 μM MYXO for 48 h before measurement of HEV luciferase activity (D) or HEV RNA (E). F, G) The Huh7.5-p6 luciferase model was treated with 0.1 μM MYXO, 0.1 μM AMA, and uridine (20 or 200 μM; F) or cytidine (20 or 200 μM; G), alone or in combination for 48 h before measurement of HEV luciferase activity. H, I) The Huh7.5-p6 infectious model was treated with 0.1 μM MYXO, 0.1 μM AMA, and uridine (20 or 200 μM; H) or cytidine (20 or 200 μM; I), alone or in combination for 48 h before measurement of HEV RNA by real-time qPCR assay. The data are means ± sd of results in 4 independent experiments, normalized to GAPDH. **P < 0.01, ***P < 0.001, vs. DMSO Ctr (set as 1).
Strong inhibition of pyrimidine biosynthesis has been observed after treatment with AMA or MYXO (20, 27). We have shown that inhibition of the pyrimidine pathway exerts a potent anti-HEV effect (7). Besides, 2′-C-methylcytidine, a cytidine nucleotide analog, also has a strong anti-HEV effect (28). However, supplementation of uridine or cytidine did not reverse the anti-HEV effects of AMA or MYXO, excluding pyrimidine depletion, as a principal mechanism of their anti-HEV effects (Fig. 4F–I).
HEV blocks MPTP opening and genetic silencing of the MPTP regulator enhances viral replication
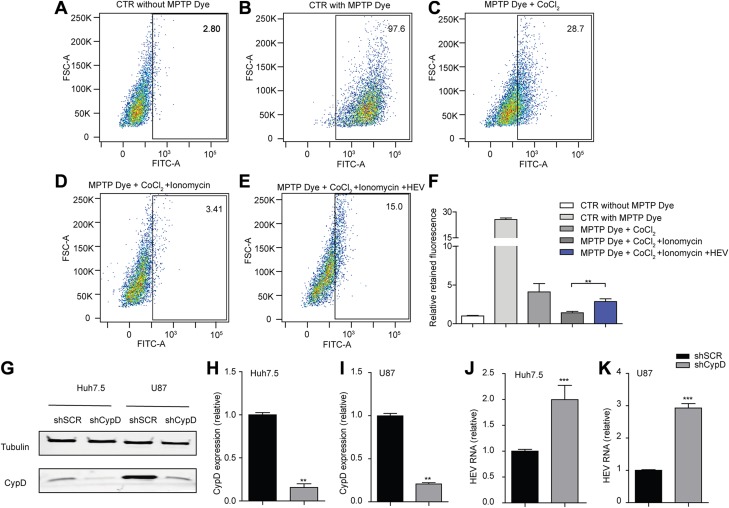
MPTP, a newly identified component of ETC, is a nonselective channel through which mitochondria constantly exchange metabolites with the cytoplasm (11, 12). Opening of MPTP may lead to leakage of ETC respiratory substrates to cytoplasm (29). We investigated whether HEV interacts with the pore opening. The CoCl2 quenching assay has been widely used to assess the opening of MPTP (30–32). In Huh7.5 cells, in the absence of CoCl2 and ionomycin, MPTP dye is present in the cytosol as well as in the mitochondria (Fig. 5B), resulting in a bright signal compared to the negative control without treatment of MPTP dye (Fig. 5A). In the presence of CoCl2 alone, MPTP dye in the mitochondria emits fluorescence, but the cytosolic fluorescence is reduced by CoCl2 quenching (Fig. 5C). Opening of MPTP induced by ionomycin leads to further reduction of mitochondrial fluorescence signal in comparison to the cells treated with CoCl2 only (Fig. 5D). In the p6 infectious model, ionomycin-induced reduction of fluorescence was significantly reversed (Fig. 5E, F), indicating that HEV blocks the MPTP opening.
Figure 5.
HEV blocks the MPTP opening, and genetic silencing of the MPTP regulator enhances viral replication. A–E) Flow cytometry analysis of MPTP opening in Huh7.5 cells (A–D) and in Huh7.5-p6 infectious cells (E) by measuring retained fluorescence after treatment with the indicated molecules for 15 min. F) Mean retained fluorescence ± sem, from 4 independent experiments. **P < 0.01 vs. PBS control. G) Western-blot analysis of CypD expression in Huh7.5- and U87-based p6 infectious cells transduced with lentiviral shRNA vector targeting CypD (shCypD) or scrambled control (shSCR). H–K) The CypD expression level (H, I) and HEV viral RNA level (J, K) were analyzed by real-time qPCR in stable CypD knockdown or SCR control cells. The data are means ± sem, of results in 4 independent experiments, normalized to GAPDH. **P < 0.01, ***P < 0.001 vs. shSCR (set as 1).
Genetic ablation of CypD in hepatocytes and neurons has been shown to reduce MPTP opening and to be effective in correcting pathologic conditions that are involved in mitochondrial dysfunction, oxidative stress, and cell necrosis (29, 32). To test this effect on HEV, we silenced CypD expression in Huh7.5- and U87-based p6 infectious models, which is confirmed by real-time qPCR and Western-blot assays (Fig. 5G–I). This silencing resulted in a mean 2.0 ± 0.27- and 2.9 ± 0.14-fold increase of HEV RNA levels, respectively (Fig. 5J, K). These data indicate a basal defense role of MPTP against HEV infection.
MPTP mediates the anti-HEV action of the complex III inhibitors
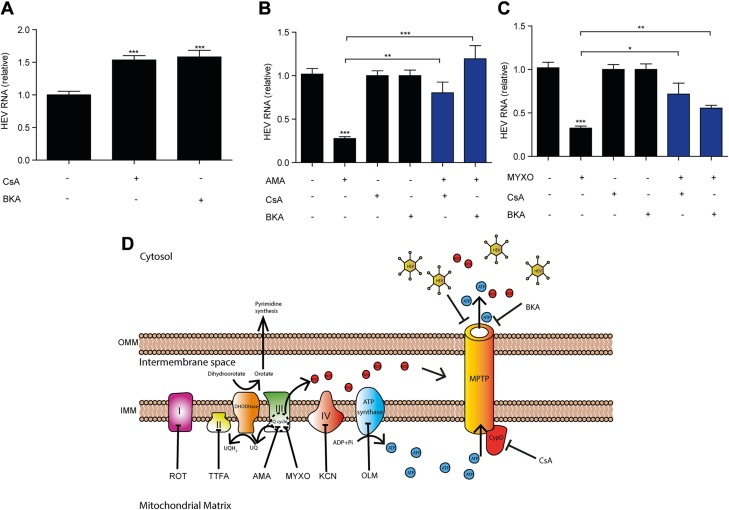
We next examined whether the anti-HEV effect of inhibition of complex III is dependent on MPTP. CsA potently prevents MPTP opening through binding and inhibiting CypD (11). CsA alone significantly increased the HEV RNA level (Fig. 6A), which is consistent with our previous findings (18). Adding CsA significantly reversed the anti-HEV effects of AMA and MYXO (Fig. 6B, C). This result was further confirmed by adding another MPTP inhibitor, BKA, which favors the closed conformation of adenine nucleotide translocase (Fig. 6A). Thus, the anti-HEV effects of complex III inhibitors require MPTP opening.
Figure 6.
AMA and MYXO inhibit HEV through MPTP. A) Huh7.5-p6 infectious model was treated with MPTP inhibitors (5 μg/ml CsA and 50 μM BKA). HEV RNA was analyzed by real-time qPCR assay. The data are means ± sd of 4 independent experiments, normalized to GAPDH. ***P < 0.001 vs. DMSO control. B, C) Coincubation of 0.1 μM AMA (B), 0.1 μM MYXO (C), 5 μg/ml CsA, and 50 μM BKA, alone or in combination for 48 h. HEV RNA was analyzed by real-time qPCR assay. The data are means ± sd of 4 independent experiments. Data in AMA- or MYXO-treated groups were presented relative to the nontreated group (set as 1). Data in combination group of AMA with CsA or BKA are presented relative to the CsA- or BK-treated group, respectively (set as 1). Data in combination group of MYXO with CsA or BKA are presented relative to CsA- or BKA-treated groups, respectively (both groups set as 1). Data were normalized to GAPDH. *P < 0.05, **P < 0.01, ***P < 0.001. D) Summary diagram. HEV, BKA, and CsA inhibit MPTP opening to block the release of metabolites from mitochondrial matrix to cytosol. CypD is located in mitochondrial matrix and specifically targeted by CsA. Inhibition of complex III by AMA and MYXO leads to an increase in ROS level, a decrease in ATP level, and inhibition of pyrimidine synthesis. UQ, ubiquinone; UQH2, ubiquinol.
DISCUSSION
The liver is a metabolically active organ and contains abundant mitochondria. Mitochondria are the major energy source for the cell and act as a central hub for multiple signal transduction, including dictating the immune response (33). Metabolites from mitochondria, such as succinate and citrate, are engaged in the process related to immunity and inflammation, which is essential to maintaining liver homeostasis and preventing pathogen invasion (34, 35). Alteration in mitochondrial metabolic states has been associated with various liver diseases, including chronic hepatitis C (36). However, the interactions of mitochondrial metabolism and HEV remain largely unexplored.
Viruses rely highly on their host for energy production, reproduction, and survival. Meanwhile, the virus strategically interferes with the host metabolism to establish persistent infection. Hepatitis B virus infection has been shown to affect hepatic metabolic responses, including glucose, lipid, nucleic acid, bile acid, and vitamin metabolism (37). HCV leads to increased expression of many glycolytic enzymes and change of intracellular ATP level during replication (38, 39). In turn, the host has developed various ways to combat viral infection. In this study, we roughly calculated ATP consumption of the HEV life cycle, and the total energy cost is lower than that of influenza virus. Of note, influenza viral infection costs only 1% of the total energetic budget of a eukaryotic cell (25), suggesting that HEV consumes an extremely low percentage of the host energy budget, which may explain why the reduction of intracellular ATP is not responsible for the anti-HEV effect of the complex III inhibitors. Release of mitochondrial ROS into the cytoplasm leads to activation of transcription factors, specifically NF-κB and hypoxia-inducible factor-1α, which coordinate the function of cells during virus infection (33). ROS has been shown to be involved in oligomerization of mitochondrial antiviral-signaling protein, an adaptor for transcription and production of IFN, by remodeling the mitochondrial outer membrane property (40). HEV has recently been shown to induce mitochondrial antiviral-signaling protein oligomerization, suggesting a potential role of mitochondrial signaling in HEV infection (41). Most of the ATP and ROS are produced from the ETC, which is closely linked to the mitochondrial metabolic state. The ETC consists of series of enzyme complexes (I, II, III, and IV). The important role of mitochondrial ETC in viral infection has been demonstrated by using ETC inhibitors and uncouplers (42). The hepatitis B virus-X protein has been shown to down-regulate ETC complex activity. Expression of the HCV polyprotein inhibited complex I activity (43, 44). These findings suggest the specific role of different complexes in the setting of particular viral infection. Complex III has diverse biologic functions (15, 20). For example, CD4+ T cells with a deficient ETC complex III fail to induce the translocation of nuclear factor to the nucleus, leading to decreased transcription of IL-2 mRNA. Loss of mitochondrial complex III results in impaired antigen-driven T-cell responses in vivo (33). Targeting complex III is the mode of action of a currently used antimalarial drug (45). In this study, we found that inhibition of complex III inhibits HEV replication. One of the features that differentiates complex III from other ETC complexes is the Q cycle by which complex III moves protons and transfers electrons to cytochrome c (46). Inhibition of the Qi site by MYXO showed comparable inhibitory effect on HEV with inhibition of the Q0 site by AMA at the same concentration. We also found that deficiency of ETC abolished the anti-HEV effect of these inhibitors. However, the anti-HEV effect is independent of ATP production, ROS level, and pyridine depletion.
HEV completes its life cycle solely in cytosol, which is separated from ETC by the mitochondrial inner membrane. MPTP is a nonselective channel that allows the communication of molecules between the mitochondrial matrix to the cytosol to convey their signals. Most recently, MPTP has been identified as a vital component of ETC (11, 12). Inhibition of CypD, an MPTP channel regulator, decreases the channel opening (47). HCV has been reported to open the MPTP to facilitate its replication (29). In this study, silencing of CypD promoted HEV replication. This effect was further demonstrated by adding the MPTP inhibitors CsA and BKA. Consistent with our previous study (18), both inhibitors increase HEV replication. In contrast, CsA inhibited HCV replication. Whether these opposing effects are attributed to the specific role of MPTP on these 2 viruses remains an intriguing question to be further investigated.
Recent evidence showed that the HEV ORF-3 protein could form an ion channel in the plasma membrane to facilitate viral replication (48). Moreover, it has been shown that ORF-3 prevents the release of cytochrome c (14). In line with these findings, we demonstrated that HEV robustly blocked the MPTP opening. The MPTP is involved in several neurologic diseases and is a potential therapeutic target for neurodegenerative diseases, including Alzheimer’s disease and Parkinson’s disease (49). HEV has recently been associated with several types of neurologic diseases. Thus, it would be interesting to examine whether HEV-associated neurologic diseases are related to its effect on MPTP.
In summary, we have identified mitochondrial ETC complex III in sustaining HEV infection through the MPTP. Pharmacological inhibition of complex III effectively inhibits HEV replication. Because therapeutic targeting of complex III has been widely explored for treating different diseases, repurposing or optimizing these existing U.S. Food and Drug Administration–approved or upcoming drugs represents a viable option for therapeutic development against HEV infection.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank Dr. Suzanne U. Emerson (U.S. National Institutes of Health, National Institute of Allergy and Infectious Diseases) for kindly providing the plasmids to generate subgenomic and full-length HEV genotype 3 cell culture systems. This work was supported by Dutch Digestive Foundation (MLDS) for Career Development Grant CDG 1304 (to Q.P.); Dutch Cancer Society (KWF) Young Investigator Grant 10140 (to Q.P.); National Natural Science Foundation of China Grant 31770186 (to Y.W.); and the China Scholarship Council for funding Ph.D. Fellowship Grants 201509110121 (to C.Q.), 201303250056 (to W.W.), and 201506100033 (to M.L.). The authors declare no conflicts of interest.
Glossary
- AICAR
5-aminoimidazole-4-carboxamide ribonucleotide
- AMA
antimycin A
- BKA
bongkrekic acid
- COX
cytochrome c oxidase
- CsA
cyclosporin A
- CypD
cyclophilin D
- CYTB
cytochrome b
- EB
ethidium bromide
- ETC
electron transport chain
- H2O2
hydrogen peroxide
- HCV
hepatitis C virus
- HEV
hepatitis E virus
- MPTP
mitochondrial permeability transition pore
- MYXO
myxothiazol
- OLM
oligomycin A
- ORF
open reading frame
- qPCR
quantitative PCR
- ROS
reactive oxygen species
- ROT
rotenone
- SCR
scrambled
- shRNA
short hairpin RNA
- VE
vitamin E acetate, TTFA, thenoyltrifluoroacetone
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
C. Qu and Q. Pan designed the research; C. Qu, S. Zhang, M. S. Hakim, N. J. H. Raat, M. P. Peppelenbosch, and Q. Pan analyzed the data; C. Qu, S. Zhang, W. Wang, M. Li, Y. Wang, M. van der Heijde-Mulder, and E. Shokrollahi performed the research; S. Zhang and N. J. H. Raat contributed reagents and analytic tools; and C. Qu and Q. Pan wrote the paper.
REFERENCES
- 1.Debing Y., Emerson S. U., Wang Y., Pan Q., Balzarini J., Dallmeier K., Neyts J. (2014) Ribavirin inhibits in vitro hepatitis E virus replication through depletion of cellular GTP pools and is moderately synergistic with alpha interferon. Antimicrob. Agents Chemother. 58, 267–273 10.1128/AAC.01795-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou X., Huang F., Xu L., Lin Z., de Vrij F. M. S., Ayo-Martin A. C., van der Kroeg M., Zhao M., Yin Y., Wang W., Cao W., Wang Y., Kushner S. A., Marie Peron J., Alric L., de Man R. A., Jacobs B. C., van Eijk J. J., Aronica E. M. A., Sprengers D., Metselaar H. J., de Zeeuw C. I., Dalton H. R., Kamar N., Peppelenbosch M. P., Pan Q. (2017) Hepatitis E virus infects neurons and brains. J. Infect. Dis. 215, 1197–1206 10.1093/infdis/jix079 [DOI] [PubMed] [Google Scholar]
- 3.Debing Y., Gisa A., Dallmeier K., Pischke S., Bremer B., Manns M., Wedemeyer H., Suneetha P. V., Neyts J. (2014) A mutation in the hepatitis E virus RNA polymerase promotes its replication and associates with ribavirin treatment failure in organ transplant recipients. Gastroenterology 147, 1008–1011 [DOI] [PubMed] [Google Scholar]
- 4.Todt D., Gisa A., Radonic A., Nitsche A., Behrendt P., Suneetha P. V., Pischke S., Bremer B., Brown R. J., Manns M. P., Cornberg M., Bock C. T., Steinmann E., Wedemeyer H. (2016) In vivo evidence for ribavirin-induced mutagenesis of the hepatitis E virus genome. Gut 65, 1733–1743 10.1136/gutjnl-2015-311000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Debing Y., Neyts J. (2014) Antiviral strategies for hepatitis E virus. Antiviral Res. 102, 106–118 10.1016/j.antiviral.2013.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Degli Esposti D., Hamelin J., Bosselut N., Saffroy R., Sebagh M., Pommier A., Martel C., Lemoine A. (2012) Mitochondrial roles and cytoprotection in chronic liver injury. Biochem. Res. Int. 2012, 387626 10.1155/2012/387626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y., Wang W., Xu L., Zhou X., Shokrollahi E., Felczak K., van der Laan L. J., Pankiewicz K. W., Sprengers D., Raat N. J., Metselaar H. J., Peppelenbosch M. P., Pan Q. (2016) Cross talk between nucleotide synthesis pathways with cellular immunity in constraining hepatitis E virus replication. Antimicrob. Agents Chemother. 60, 2834–2848 10.1128/AAC.02700-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Acín-Pérez R., Bayona-Bafaluy M. P., Fernández-Silva P., Moreno-Loshuertos R., Pérez-Martos A., Bruno C., Moraes C. T., Enríquez J. A. (2004) Respiratory complex III is required to maintain complex I in mammalian mitochondria. Mol. Cell 13, 805–815 10.1016/S1097-2765(04)00124-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Birsoy K., Wang T., Chen W. W., Freinkman E., Abu-Remaileh M., Sabatini D. M. (2015) An essential role of the mitochondrial electron transport chain in cell proliferation is to enable aspartate synthesis. Cell 162, 540–551 10.1016/j.cell.2015.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim S. J., Ahn D. G., Syed G. H., Siddiqui A. (2018) The essential role of mitochondrial dynamics in antiviral immunity. Mitochondrion 41, 21–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alavian K. N., Beutner G., Lazrove E., Sacchetti S., Park H. A., Licznerski P., Li H., Nabili P., Hockensmith K., Graham M., Porter G. A., Jr., Jonas E. A. (2014) An uncoupling channel within the c-subunit ring of the F1FO ATP synthase is the mitochondrial permeability transition pore. Proc. Natl. Acad. Sci. USA 111, 10580–10585 10.1073/pnas.1401591111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giorgio V., von Stockum S., Antoniel M., Fabbro A., Fogolari F., Forte M., Glick G. D., Petronilli V., Zoratti M., Szabó I., Lippe G., Bernardi P. (2013) Dimers of mitochondrial ATP synthase form the permeability transition pore. Proc. Natl. Acad. Sci. USA 110, 5887–5892 10.1073/pnas.1217823110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Machida K., Cheng K. T., Lai C. K., Jeng K. S., Sung V. M., Lai M. M. (2006) Hepatitis C virus triggers mitochondrial permeability transition with production of reactive oxygen species, leading to DNA damage and STAT3 activation. J. Virol. 80, 7199–7207 10.1128/JVI.00321-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moin S. M., Panteva M., Jameel S. (2007) The hepatitis E virus Orf3 protein protects cells from mitochondrial depolarization and death. J. Biol. Chem. 282, 21124–21133 10.1074/jbc.M701696200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma X., Jin M., Cai Y., Xia H., Long K., Liu J., Yu Q., Yuan J. (2011) Mitochondrial electron transport chain complex III is required for antimycin A to inhibit autophagy. Chem. Biol. 18, 1474–1481 10.1016/j.chembiol.2011.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ichinohe T., Yamazaki T., Koshiba T., Yanagi Y. (2013) Mitochondrial protein mitofusin 2 is required for NLRP3 inflammasome activation after RNA virus infection. Proc. Natl. Acad. Sci. USA 110, 17963–17968 10.1073/pnas.1312571110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou X., Wang Y., Metselaar H. J., Janssen H. L., Peppelenbosch M. P., Pan Q. (2014) Rapamycin and everolimus facilitate hepatitis E virus replication: revealing a basal defense mechanism of PI3K-PKB-mTOR pathway. J. Hepatol. 61, 746–754 10.1016/j.jhep.2014.05.026 [DOI] [PubMed] [Google Scholar]
- 18.Wang Y., Zhou X., Debing Y., Chen K., Van Der Laan L. J., Neyts J., Janssen H. L., Metselaar H. J., Peppelenbosch M. P., Pan Q. (2014) Calcineurin inhibitors stimulate and mycophenolic acid inhibits replication of hepatitis E virus. Gastroenterology 146, 1775–1783 10.1053/j.gastro.2014.02.036 [DOI] [PubMed] [Google Scholar]
- 19.Shukla P., Nguyen H. T., Faulk K., Mather K., Torian U., Engle R. E., Emerson S. U. (2012) Adaptation of a genotype 3 hepatitis E virus to efficient growth in cell culture depends on an inserted human gene segment acquired by recombination. J. Virol. 86, 5697–5707 10.1128/JVI.00146-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khutornenko A. A., Roudko V. V., Chernyak B. V., Vartapetian A. B., Chumakov P. M., Evstafieva A. G. (2010) Pyrimidine biosynthesis links mitochondrial respiration to the p53 pathway. Proc. Natl. Acad. Sci. USA 107, 12828–12833 10.1073/pnas.0910885107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khare S., Roach S. L., Barnes S. W., Hoepfner D., Walker J. R., Chatterjee A. K., Neitz R. J., Arkin M. R., McNamara C. W., Ballard J., Lai Y., Fu Y., Molteni V., Yeh V., McKerrow J. H., Glynne R. J., Supek F. (2015) Utilizing chemical genomics to identify cytochrome b as a novel drug target for Chagas disease. PLoS Pathog. 11, e1005058 10.1371/journal.ppat.1005058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carelli V., Chan D. C. (2014) Mitochondrial DNA: impacting central and peripheral nervous systems. Neuron 84, 1126–1142 10.1016/j.neuron.2014.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pham N. A., Robinson B. H., Hedley D. W. (2000) Simultaneous detection of mitochondrial respiratory chain activity and reactive oxygen in digitonin-permeabilized cells using flow cytometry. Cytometry 41, 245–251 [DOI] [PubMed] [Google Scholar]
- 24.Maguire J. J., Kagan V. E., Packer L. (1992) Electron transport between cytochrome c and alpha tocopherol. Biochem. Biophys. Res. Commun. 188, 190–197 10.1016/0006-291X(92)92368-8 [DOI] [PubMed] [Google Scholar]
- 25.Mahmoudabadi G., Milo R., Phillips R. (2017) Energetic cost of building a virus. Proc. Natl. Acad. Sci. USA 114, E4324–E4333 10.1073/pnas.1701670114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buskiewicz I. A., Montgomery T., Yasewicz E. C., Huber S. A., Murphy M. P., Hartley R. C., Kelly R., Crow M. K., Perl A., Budd R. C., Koenig A. (2016) Reactive oxygen species induce virus-independent MAVS oligomerization in systemic lupus erythematosus [published correction in Sci. Signal 2017, 10, eean5765. Sci. Signal. 9, ra115 10.1126/scisignal.aaf1933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gattermann N., Dadak M., Hofhaus G., Wulfert M., Berneburg M., Loeffler M. L., Simmonds H. A. (2004) Severe impairment of nucleotide synthesis through inhibition of mitochondrial respiration. Nucleosides Nucleotides Nucleic Acids 23, 1275–1279 10.1081/NCN-200027545 [DOI] [PubMed] [Google Scholar]
- 28.Qu C., Xu L., Yin Y., Peppelenbosch M. P., Pan Q., Wang W. (2017) Nucleoside analogue 2'-C-methylcytidine inhibits hepatitis E virus replication but antagonizes ribavirin. Arch. Virol. 162, 2989–2996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quarato G., D’Aprile A., Gavillet B., Vuagniaux G., Moradpour D., Capitanio N., Piccoli C. (2012) The cyclophilin inhibitor alisporivir prevents hepatitis C virus-mediated mitochondrial dysfunction. Hepatology 55, 1333–1343 10.1002/hep.25514 [DOI] [PubMed] [Google Scholar]
- 30.Vaseva A. V., Marchenko N. D., Ji K., Tsirka S. E., Holzmann S., Moll U. M. (2012) p53 opens the mitochondrial permeability transition pore to trigger necrosis. Cell 149, 1536–1548 10.1016/j.cell.2012.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Marchi E., Bonora M., Giorgi C., Pinton P. (2014) The mitochondrial permeability transition pore is a dispensable element for mitochondrial calcium efflux. Cell Calcium 56, 1–13 10.1016/j.ceca.2014.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakagawa T., Shimizu S., Watanabe T., Yamaguchi O., Otsu K., Yamagata H., Inohara H., Kubo T., Tsujimoto Y. (2005) Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature 434, 652–658 10.1038/nature03317 [DOI] [PubMed] [Google Scholar]
- 33.Mehta M. M., Weinberg S. E., Chandel N. S. (2017) Mitochondrial control of immunity: beyond ATP. Nat. Rev. Immunol. 17, 608–620 10.1038/nri.2017.66 [DOI] [PubMed] [Google Scholar]
- 34.Wang T., Weinman S. A. (2013) Interactions between hepatitis C virus and mitochondria: impact on pathogenesis and innate immunity. Curr. Pathobiol. Rep. 1, 179–187 10.1007/s40139-013-0024-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mills E. L., Kelly B., O’Neill L. A. J. (2017) Mitochondria are the powerhouses of immunity. Nat. Immunol. 18, 488–498 10.1038/ni.3704 [DOI] [PubMed] [Google Scholar]
- 36.Khan M., Syed G. H., Kim S. J., Siddiqui A. (2015) Mitochondrial dynamics and viral infections: a close nexus. Biochim. Biophys. Acta 1853(10 Pt B), 2822–2833 10.1016/j.bbamcr.2014.12.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shi Y. X., Huang C. J., Yang Z. G. (2016) Impact of hepatitis B virus infection on hepatic metabolic signaling pathway. World J. Gastroenterol. 22, 8161–8167 10.3748/wjg.v22.i36.8161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanchez E. L., Lagunoff M. (2015) Viral activation of cellular metabolism. Virology 479-480, 609–618 10.1016/j.virol.2015.02.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ando T., Imamura H., Suzuki R., Aizaki H., Watanabe T., Wakita T., Suzuki T. (2012) Visualization and measurement of ATP levels in living cells replicating hepatitis C virus genome RNA. PLoS Pathog. 8, e1002561 10.1371/journal.ppat.1002561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nobre L., Wise D., Ron D., Volmer R. (2015) Modulation of innate immune signalling by lipid-mediated MAVS transmembrane domain oligomerization. PLoS One 10, e0136883 10.1371/journal.pone.0136883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yin X., Li X., Ambardekar C., Hu Z., Lhomme S., Feng Z. (2017) Hepatitis E virus persists in the presence of a type III interferon response. PLoS Pathog. 13, e1006417 10.1371/journal.ppat.1006417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.El-Bacha T., Da Poian A. T. (2013) Virus-induced changes in mitochondrial bioenergetics as potential targets for therapy. Int. J. Biochem. Cell Biol. 45, 41–46 10.1016/j.biocel.2012.09.021 [DOI] [PubMed] [Google Scholar]
- 43.Brault C., Levy P. L., Bartosch B. (2013) Hepatitis C virus-induced mitochondrial dysfunctions. Viruses 5, 954–980 10.3390/v5030954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Claus C., Schönefeld K., Hübner D., Chey S., Reibetanz U., Liebert U. G. (2013) Activity increase in respiratory chain complexes by rubella virus with marginal induction of oxidative stress. J. Virol. 87, 8481–8492 10.1128/JVI.00533-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Painter H. J., Morrisey J. M., Mather M. W., Vaidya A. B. (2007) Specific role of mitochondrial electron transport in blood-stage Plasmodium falciparum. Nature 446, 88–91 10.1038/nature05572 [DOI] [PubMed] [Google Scholar]
- 46.Quinlan C. L., Gerencser A. A., Treberg J. R., Brand M. D. (2011) The mechanism of superoxide production by the antimycin-inhibited mitochondrial Q-cycle. J. Biol. Chem. 286, 31361–31372 10.1074/jbc.M111.267898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taddeo E. P., Laker R. C., Breen D. S., Akhtar Y. N., Kenwood B. M., Liao J. A., Zhang M., Fazakerley D. J., Tomsig J. L., Harris T. E., Keller S. R., Chow J. D., Lynch K. R., Chokki M., Molkentin J. D., Turner N., James D. E., Yan Z., Hoehn K. L. (2013) Opening of the mitochondrial permeability transition pore links mitochondrial dysfunction to insulin resistance in skeletal muscle. Mol. Metab. 3, 124–134 10.1016/j.molmet.2013.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ding Q., Heller B., Capuccino J. M., Song B., Nimgaonkar I., Hrebikova G., Contreras J. E., Ploss A. (2017) Hepatitis E virus ORF3 is a functional ion channel required for release of infectious particles [published correction in Proc. Natl. Acad. Sci. USA 2017, 114, E4897]. Proc. Natl. Acad. Sci. USA 114, 1147–1152 10.1073/pnas.1614955114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Norenberg M. D., Rao K. V. (2007) The mitochondrial permeability transition in neurologic disease. Neurochem. Int. 50, 983–997 10.1016/j.neuint.2007.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.