Abstract
Although convincing in genetic models, the relevance of β-cell insulin resistance in diet-induced type 2 diabetes (T2DM) remains unclear. Exemplified by diabetes-prone, male, C57B1/6J mice being fed different combinations of Western-style diet, we show that β-cell insulin resistance occurs early during T2DM progression and is due to a combination of lipotoxicity and increased β-cell workload. Within 8 wk of being fed a high-fat, high-sucrose diet, mice became obese, developed impaired insulin and glucose tolerances, and displayed noncompensatory insulin release, due, at least in part, to reduced expression of syntaxin-1A. Through reporter islets transplanted to the anterior chamber of the eye, we demonstrated a concomitant loss of functional β-cell mass. When mice were changed from diabetogenic diet to normal chow diet, the diabetes phenotype was reversed, suggesting a remarkable plasticity of functional β-cell mass in the early phase of T2DM development. Our data reinforce the relevance of diet composition as an environmental factor determining different routes of diabetes progression in a given genetic background. Employing the in vivo reporter islet–monitoring approach will allow researchers to define key times in the dynamics of reversible loss of functional β-cell mass and, thus, to investigate the underlying, molecular mechanisms involved in the progression toward T2DM manifestation.—Paschen, M., Moede, T., Valladolid-Acebes, I., Leibiger, B., Moruzzi, N., Jacob, S., García-Prieto, C. F., Brismar, K., Leibiger, I. B., Berggren, P.-O. Diet-induced β-cell insulin resistance results in reversible loss of functional β-cell mass.
Keywords: in vivo imaging, diabetes mellitus, fluorescence microscopy, biosensor, diet intervention
Type 2 diabetes (T2DM) is a heterogeneous disease that has reached pandemic dimensions. The development of T2DM is influenced by genetic and environmental factors; hence, each patient has unique genetics and susceptibility to environmental factors. Consequently, there is a huge variety in the underlying mechanisms leading to manifestation of the disease. Therefore, there is an absolute need to understand the interface between genetics and environment in T2DM at the individual level and, based on that understanding, to establish well-defined and personalized programs for prevention and treatment of the disease.
Genome-wide association studies revealed that most identified, potential genes associated with T2DM development are linked to pancreatic islet/β-cell function (1). To that end, pancreatic islet/β-cell insulin signaling has been proposed to be of importance for appropriate β-cell function and survival. Consequently, β-cell insulin resistance is likely to constitute an important factor causing and/or contributing to β-cell dysfunction and T2DM development (2–4). However, because most of the data have been obtained from genetic models, including both β-cell–targeted manipulation of candidate genes (5–11) and models of insulin resistance and obesity (12–15), the pathophysiologic relevance of β-cell insulin resistance in diet-induced T2DM development remains unclear.
Diabetes manifests when β cells cannot compensate for the increasing demand in insulin to maintain euglycemia. Although extensive efforts have been made to monitor the dynamics of total β-cell mass during diabetes development (16), there is a lack of techniques that allow longitudinal monitoring of functional β-cell mass. We have previously shown that pancreatic islets transplanted to the anterior chamber of the eye (ACE) can be used to monitor islet cell function and survival in vivo noninvasively and longitudinally with cellular resolution by confocal laser scanning microscopy (17, 18). Importantly, islets engrafted in the ACE report on the function of in situ pancreatic islets in the same animal (13, 14, 19–24).
In the present study, we wanted to test the hypothesis that diet-induced β-cell insulin resistance intersects with genetics and specifically affects functional β-cell mass and, thereby, the development of T2DM. We reproduced Western-style, fast-food consumption by treating diabetes-prone, C57Bl/6J mice with a combination diet consisting of solid high fat and liquid sucrose (HFHSD) or fructose (HFHFrD) and monitored in vivo β-cell insulin sensitivity noninvasively with reporter islets in the ACE expressing a β-cell–specific, insulin-resistance biosensor (13). To evaluate the consequences for functional β-cell mass, we employed a combination of reporter islets that allowed in vivo monitoring of glucose-induced β-cell reporter gene transcription as well as Ca2+ handling during the course of the diet-intervention study.
MATERIALS AND METHODS
Animals and diet
Male, C57Bl/6J (B6) mice were purchased at 2 mo old from Charles River Laboratories (Wilmington, MA, USA). After delivery, the mice were allowed to adapt to the animal core facility for 1 wk before the start of the experiment. All mice were group-housed on a 12/12-h dark/light cycle with free access to food and water. If not otherwise stated, the mice received a normal chow diet (R70; Lantmännen, Stockholm, Sweden). The following special diets were fed to 3-mo-old B6 mice for 8 wk: a high-sucrose diet (HSD; 32% sucrose dissolved in tap water); a high-fat diet (HFD; 60% kcal from fat, TD.06414; Envigo, Huntingdon, United Kingdom); a HFHSD (HFD + HSD); a HFHFrD [HFD + high-fructose diet (HFrD; 32% fructose in tap water)]; and a control diet [managed formulation–purified ingredient diet (5P76; LabDiet, St. Louis, MO, USA)]; with tap water. The control diet given to the control group differed from the R70 diet all animals received before and some mice received up to 8 wk after the diet intervention. Consequently, all the animals underwent the same stress provoked by diet change. All experiments were performed in accordance with the Karolinska Institutet’s guidelines for the care and use of animals in research and were approved by the institute’s Animal Ethics Committee.
Expression constructs
The adenovirus assembly encoding the β-cell insulin resistance biosensor (βIRB) was previously described in Paschen et al. (13). The adenovirus encoding the β-cell fluorescent metabolic transcriptional-response indicator (βFLUOMETRI) was generated as follows: pENTR1A.RIP1.DsRed2/rbGK.EGFP/CMV.Cerulean was generated by inserting the RIP1.dsRed2/rbGK.EGFP cassette from pd2.RIP1.dsRed2/rbGK.EGFP (25) into pENTR1A (Thermo Fisher Scientific, Waltham, MA, USA) and adding a CMV.Cerulean cassette downstream of the rbGK.EGFP cassette. The 3 individual expression cassettes are separated by transcription blocker sequences from the pd2EGFP-Promoter (Takara Bio, Kusatsu, Japan). All constructs were verified by DNA sequencing. The RIP1.DsRed2/rbGK.EGFP/CMV.Cerulean cassette was transferred into the promoterless adenovirus plasmid pAd/PL-DEST (Thermo Fisher Scientific) by the Gateway technique. The ViraPower Adenoviral Expression System (Thermo Fisher Scientific) was used to generate a replication-deficient adenovirus, which was used for transduction of cells and islets.
Isolation and transduction of pancreatic islets
Islets were prepared from mice by duct injection of collagenase (F. Hoffmann-La Roche, Basel, Switzerland) and were handpicked under a stereomicroscope MZ6 (Leica Microsystems, Wetzlar, Germany) after digestion (18). Thereafter islets were cultured in RPMI-1640 medium, with a final concentration of 10% heat-inactivated fetal bovine serum, 2 mM glutamine, 100 U/ml penicillin, and 100 µg/ml streptomycin at 5% CO2 and 37°C. Islets were transduced with 107 plaque-forming units/ml of the respective expression construct encoding the adenovirus.
Transplantation of pancreatic islets into the ACE
If not otherwise stated, 2–3 d after transduction, islets were transplanted into the ACE of syngeneic, age-matched, littermate recipients, using a technique previously described by Speier et al. (18). Briefly, under anesthesia, islets of Langerhans were transplanted into the ACE with a glass cannula after generating a puncture in the cornea with a 27-gauge needle. Great care was taken to avoid bleeding and damage to the iris. Mice were injected s.c. with Temgesic (0.1 ml/kg; RB Pharmaceuticals, Berkshire, United Kingdom) for postoperative analgesia. The first transplantation was performed at 2 mo of age and, if necessary, was retransplanted after 2 mo.
In vivo imaging of intraocular islet grafts
Islet grafts were imaged in vivo beginning 1 mo after transplantation (17, 18) and at 0, 4, and 8 wk after the start of diet intervention, as well as up to 8 wk after refeeding a chow diet. An upright laser scanning confocal microscope (TCSSP5; Leica Microsystems), equipped with a long-distance, water-dipping objective (HXC-APO10×/0.30 numerical aperture; Leica Microsystems) and a custom-built stereotaxic head holder, allowing positioning of the mouse eye containing the engrafted islets toward the objective used. Viscotears (Théa Nordic, Örebro, Sweden) was used as an immersion liquid between the eye and the objective, and isoflurane was used to anesthetize the mice during in vivo imaging. Islets were imaged as 3-dimensional stacks with 3-µm step size. After imaging, the mice were allowed to recover from anesthesia, and an i.p. glucose tolerance test (IPGTT) was performed; 4 h after glucose injection, a second imaging was performed using the same settings as described above to obtain the 4-h image set.
Pancreatic βIRB
Enhanced green fluorescent protein (EGFP) was excited at 488 nm, and the fluorescence was detected at 505–535 nm. Tomato was excited at 561 nm, and fluorescence was detected at 580–650 nm. Backscatter signal (26) from the 561-nm excitation was collected at 555–565 nm.
βFLUOMETRI
Cerulean fluorescence was excited at 405 nm and detected at 460–490 nm. EGFP fluorescence was excited at a 488 nm and detected at 505–535 nm. Red fluorescent protein (DsRed2) was excited at 561 nm and detected at 580–650 nm. Backscatter signal (26) from the 561 nm excitation was collected at 555–565 nm. Channel crosstalk was avoided by using in-between lines sequential imaging, combining Cerulean, backscatter, and dsRed2 signals and separating the EGFP signal.
Image analysis
βIRB
In vitro and in vivo imaging were analyzed with ImageJ (U.S. National Institutes of Health, Bethesda, MD, USA) and the Leica Application Suite software (Leica Microsystems), respectively. For each cell, the central plane was determined with information from each detection channel (EGFP, Tomato, and backscatter); 3 regions of interest were drawn manually for each cell: nucleus, cytoplasm, and background, respectively (13). Only the intensity values of the EGFP channel were considered for the calculation of the relative βIRI (rβIRI): for each cell, a ratio was calculated based on the obtained fluorescence intensity values: ratio r = (Nucleus − Background)/(Cytoplasm − Background). For each experimental in vitro and in vivo condition, the ratio was calculated. For in vivo experiments, the mean ratio of the experimental starting point (t = 0; correction value c) was used to further calculate the rβIRI for each time point and experimental group: rβIRI = r/c. More precisely, in the diet-intervention study, the mean ratio of all mice at 0 wk of treatment was the correction value. The rβIRI was considered a measure of β-cell insulin resistance and was used for further statistical analysis. For in vitro experiments, the mean ratio of the control group for the respective time point (correction value c) was used to further calculate the rβIRI for each time point and experimental group: rβIRI = r/c.
βFLUOMETRI
Image analysis for in vitro experiments was performed as described in (27–30) using ImageJ. Average fluorescence intensities for each cell was determined for t = 60 min (start) and t = 240 min. In vivo imaging results were analyzed with Leica Application Suite software. For each analyzed cell, fluorescence intensity for all 3 fluorescent dyes (Cerulean, EGFP, and DsRed2) was determined before glucose stimulation and 4 h after glucose stimulation. To calculate the change in promoter activity after glucose stimulation, as a readout for β-cell functionality, fluorescence intensity for each cell was determined at the beginning of the experiment and 4 h after glucose stimulation. Cells were identified with the DsRed2-fluorescence signal, thereby ensuring the analysis of β cells. Promoter activation was calculated as follows: [(EGFP/DsRed24-h − Background4-h)/(Cerulean4-h − Background4-h)]/[(EGFP/DsRed2start − Backgroundstart)/(Ceruleanstart − Backgroundstart)]. To determine functional β-cell mass as a percentage of glucose-responsive cells, cells with promoter activation >1.15 were considered responsive.
Backscatter intensity
Backscatter intensity was analyzed in the backscatter images obtained during the imaging of the βFLUOMETRI biosensor with Image J. Average signal intensity of the islet was normalized to the average signal intensity of the surrounding iris and the ratio was used as backscatter intensity.
Islet size
Islet size was determined based on the backscatter images obtained during the imaging of the βFLUOMETRI biosensor, as described in Van Krieken et al. (31). Relative islet size was calculated by dividing the size at 8 wk by the size at the start of the experiment for each individual islet.
Calcium imaging
For Ca2+ measurements, islets from C57Bl/6J mice, heterozygous for Ins-CRE and floxed genetically encoded calcium indicator 3 (GCaMP3) (32, 33), were isolated and used for transplantation. From 4 wk after transplantation, the imaging was performed as follows: unfed mice were anesthetized with midazolam/fentanyl/fluanisone (i.p.), and during imaging, they were supplied with oxygen via a nose mask. The whole microscope was enclosed to allow heating to 35°C, with the microscope equipped with a ×25/0.95 numerical aperture objective. GCaMP3 was excited at 480 nm, and fluorescence detected at 500–550 nm. A tail vein catheter containing 14 µl heparin (100 IU/ml) was inserted in the mouse’s tail, and heparin was injected; 2 min after the start of imaging, glucose (0.4 g/kg) was carefully injected. Images were acquired with μManager microscopy software (34). Image processing was performed in MatLab (MathWorks, Natick, MA, USA). Every z-stack was denoised (35), and consecutive planes were aligned (36). z-Stacks were deconvolved (37) and registered to a reference z-stack (38). Individual β cells were identified based on their temporal [Ca2+]i profile. Single-cell [Ca2+]i traces were normalized to their baseline. β cells were considered as having responded when their glucose-induced [Ca2+]i increase was >3 times the sd before glucose injection.
IPGTT
To determine glucose tolerance, blood glucose levels were measured in mice that were unfed for 6 h at basal state (0 min) and at 5 (not always measured), 10, 30, 60, and 120 min after glucose injection (2 g/kg body weight i.p., dissolved in PBS). The results were depicted as the area under the curve (AUC) of the IPGTT. Glucose concentrations were measured with the Accu-Chek Aviva monitoring system (F. Hoffmann-La Roche).
Intraperitoneal insulin tolerance test
To measure whole-body insulin resistance, an intraperitoneal insulin tolerance test (IPITT) was performed to determine the rate of glucose blood levels after an insulin challenge. Blood glucose concentration was measured in mice that were unfed for 6 h at basal state (0 min). Then, mice were injected with insulin (0.25 U/kg body weight i.p., diluted in PBS; Novo Nordisk, Bagsværd, Denmark), followed by glucose administration (1 g/kg body weight i.p.), and blood-glucose concentrations were determined at 15, 30, 60, 90, and 120 min after glucose injection. The results are depicted as the AUC of the IPITT.
Intraperitoneal pyruvate tolerance test
To determine liver insulin tolerance, blood glucose levels were measured in mice unfed for 12 h overnight at basal state (0 min) and at 30, 60, 90, 120, and 180 min after pyruvate injection (2 g/kg body weight i.p., dissolved in PBS). The results are depicted as the AUC of the intraperitoneal pyruvate tolerance test (IPPTT).
Body weight and fasting blood glucose
Body weight and fasting blood glucose were measured after 6 h denial of food.
Serum biology
Blood samples were obtained at all time points during the IPGTT, centrifuged to gain blood serum, and preserved at −20°C until use. Ultrasensitive mouse ELISA kits (Crystal Chem, Elk Grove Village, IL, USA) were used to analyze different factors in the serum.
β-cell proliferation measurement with 5-bromo-2′-deoxyuridine
To assess β-cell proliferation in vivo, mice were treated with 0.8 mg/ml of the synthetic nucleoside and thymidine analog 5-bromo-2′-deoxyuridine (BrdU; Thermo Fisher Scientific) 1 wk during the second month of diet treatment. After isolation and sectioning, pancreases were stained for BrdU and insulin to determine the amount of proliferating β cells. The images obtained with the Pathway 855 imaging system (BD Biosciences, San Jose, CA, USA) were analyzed with the Cell Profiler (http://cellprofiler.org/). Nuclei were identified by DAPI staining; the intensities of the insulin and BrdU staining were measured in the nucleus and displayed as histogram. Insulin-positive objects were defined as β cells. BrdU-positive and -negative cells were identified and used for calculating the percentage of BrdU-positive β cells.
Tissue extraction and sectioning
Pancreas tissue was obtained and sectioned to verify and complement data obtained in vivo. Mice were anesthetized with isoflurane and transcardially perfused with PBS, followed by freshly prepared 4% (wt/vol) paraformaldehyde in PBS. Pancreases were dissected and postfixed for 2 h. Before cryopreservation, the tissues were processed with a sucrose gradient [10–30% (wt/vol) sucrose in PBS containing 0.01% (wt/vol) sodium azide and 0.02% (wt/vol) bacitracin], frozen in dry ice, and preserved at −80°C until use. Then, 20-µm-thick pancreas and liver cryosections were collected on SuperFrost Plus microscope slides (VWR International, Radnor, PA, USA) and kept at −20°C until use.
Immunofluorescence in pancreas tissue
For immunostaining, pancreas tissue sections were equilibrated to room temperature, washed, blocked, and then, incubated with the primary antibodies (anti-FoxO1 and anti-insulin; Cell Signaling Technology, Danvers, MA, USA) in the presence of 0.1% Triton X-100 and 10% serum. After washing, secondary antibodies were applied, and mounting with medium containing DAPI for nuclear counterstaining (Thermo Fisher Scientific) was performed after repeated washing.
Western blotting
Islets were lysed immediately after isolation in lysis buffer [50 mM Tris (pH 7.5); 1 mM EDTA; 1 mM EGTA; 0.5 mM Na3VO4; 0.1% (v/v) 2-mercaptoethanol; 1% Triton X-100; 50 mM NaF; 5 nM sodium pyrophosphate; 10 mM sodium β-glycerol phosphate; 0.1 mM PMSF; 1 μg/ml of aprotinin, pepstatin, and leupeptin; and 1 μM microcystin]. The proteins (45 μg) were separated over a 7.5% SDS-polyacrylamide gel (Laemmli buffering system) and electrotransferred to a PVDF membrane. In the cases of phospho-specific antibodies, the membranes were probed with the respective phospho-specific antibodies, then stripped and reprobed with antibodies recognizing the respective total protein levels. The following antibodies were used: rabbit pAb phospho-FoxO1 (Ser256), rabbit mAb FoxO1, rabbit mAb phospho-insulin receptor β (Tyr 1150/1151), rabbit mAb insulin receptor β, rabbit mAb phospho-Akt (Ser 473), rabbit pAb pan-Akt, and rabbit mAb Syntaxin-1A, which were all purchased from Cell Signaling Technology; and mouse mAb α-tubulin (MilliporeSigma, Billerica, MA, USA). Immunoreactivity was detected with horseradish peroxidase–conjugated secondary antibodies using Clarity Western ECL substrate and the Chemi Doc Touch imaging system (Bio-Rad Laboratories, Hercules, CA, USA). Band intensities were quantified with Image Lab (v.5.2.1) software from Bio-Rad Laboratories.
Gene expression analysis in islets of Langerhans
For expression analysis, 100–200 freshly isolated islets were processed with RNeasy Mini Kit (Qiagen, Hilden, Germany) to isolate mRNA. cDNA was synthesized with an RT2 First Strand Kit (Qiagen) and used in a SYBR Green–based custom PCR array. Analysis was performed as previously described by Schmittgen et al. (39), where the cycle threshold (2−ΔCt) was calculated for experimental groups and used for further statistical analysis.
Statistics
The values are expressed as means ± sem. A 2-sided, unpaired t test was used to determine statistical significance among different treatment groups. Statistical significance was used as follows: *P < 0.05, **P < 0.01, ***P < 0.001. Origin 2015 64-bit (OriginLab, Northampton, MA, USA) and Excel (Microsoft, Redmond, WA, USA) were used for statistical analyses. The 2-sided, unpaired t test was used to determine statistical significance between treatment groups and the control group.
RESULTS
The combination of HFD and HSD, but not HFD and HFrD, leads to β-cell insulin resistance
We transplanted reporter islets expressing the β-cell insulin resistance biosensor βIRB (13) to the ACE into 2-mo old male C57Bl/6J (B6) mice, let the islets engraft for 1 mo, and obtained fluorescence from the biosensor by confocal microscopy. We determined β-cell insulin sensitivity by calculating the relative β-cell insulin resistance index (rβIRI; see Materials and Methods). We administrated a solid HFD in combination with either an HSD (HFHSD) or an HFrD (HFHFrD). Throughout the study, we compared the diet intervention groups with mice fed a control diet and tap water, monitoring the dynamics of insulin sensitivity and additional physiologic parameters. Whole-body insulin resistance, representing primarily insulin resistance in muscle and fat, was assessed by an IPITT. To measure liver insulin sensitivity in vivo an IPPTT was used, which is a measurement of insulin-sensitive gluconeogenesis of the liver (40). In vivo glucose handling was assessed by an IPGTT.
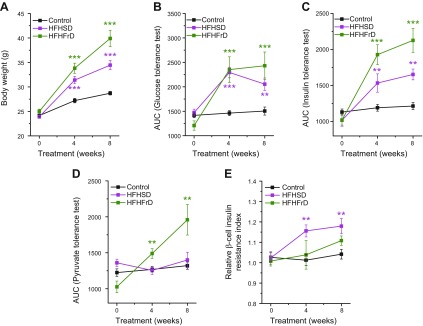
Mice fed an HFHSD had an increased body weight (Fig. 1A) and showed an impaired glucose tolerance (Fig. 1B and Supplemental Fig. S1) 4 and 8 wk after start of diet intervention. This was paralleled by whole-body insulin resistance (Fig. 1C and Supplemental Fig. S1) and β-cell insulin resistance (Fig. 1E). Impaired liver insulin sensitivity (Fig. 1D and Supplemental Fig. S1) did not develop during the duration of the diet intervention study of 8 wk. Mice that were administered an HFHFrD showed an increased body weight (Fig. 1A) and impaired glucose tolerance (Fig. 1B and Supplemental Fig. S1). Whole-body insulin resistance was observed 4 and 8 wk after the start of the diet (Fig. 1C and Supplemental Fig. S1). In contrast to HFHSD, HFHFrD did not lead to β-cell insulin resistance (Fig. 1E) but led to impaired liver insulin sensitivity (Fig. 1D and Supplemental Fig. S1).
Figure 1.
The combination of HFD and HSD, but not HFD and HFrD, leads to β-cell insulin resistance. A) Body weight of mice fed a control diet, HFHSD, or HFHFrD (n = 5–11). B) Glucose tolerance obtained by IPGTT and depicted as AUC of the IPGTT in mice fed a control diet, HFHSD, or HFHFrD (n = 5–10). C) Whole-body insulin tolerance obtained by IPITT and depicted as AUC of the IPITT in mice fed a control diet, HFHSD, or HFHFrD (n = 5–9). D) Liver insulin tolerance obtained by IPPTT and depicted as AUC of the IPPTT in mice fed a control diet, HFHSD, or HFHFrD (n = 5–8). E) Relative β-cell insulin resistance measured using the β-cell insulin resistance biosensor (βIRB) in islets transplanted into the ACE in mice fed a control diet, HFHSD, or HFHFrD (n = 4–14). *P < 0.05,**P < 0.01, ***P < 0.001. Data are expressed as means ± sem. See also Supplemental Fig. S1.
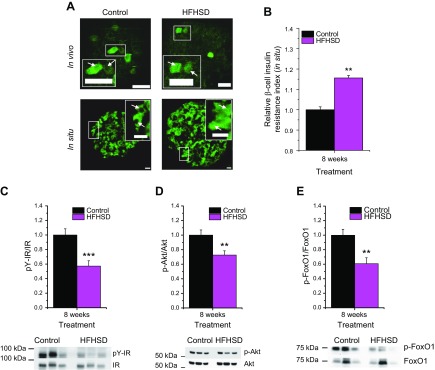
To verify that the reporter islets for β-cell insulin resistance in the ACE did, indeed, report the status of the endogenous islets of the animals, we analyzed FoxO1 localization by immunofluorescence in pancreatic islet sections from mice fed an HFHSD for 8 wk (Fig. 2A, B). The rβIRI was determined in the same way as for the images obtained from in vivo monitoring of reporter islets in the ACE, but, in this case, were normalized to the control animals at 8 wk. Our data show that HFHSD treatment led to β-cell insulin resistance in the endogenous islets of these animals (Fig. 2B). Moreover, Western blot analysis of islets from mice treated for 8 wk with HFHSD showed decreased tyrosine phosphorylation of the insulin receptor at Tyr 1150/1151 (Fig. 2C and Supplemental Fig. S2A, B), reduced phosphorylation of protein kinase B (Akt) at Ser 473 (Fig. 2D and Supplemental Fig. S2C, D), and reduced phosphorylation of FoxO1 at Ser 256 (Fig. 2E and Supplemental Fig. S2E, F), all indicative of impaired insulin sensitivity.
Figure 2.
Verification of β-cell insulin resistance in HFHSD-fed mice. A) In vivo representative image of single-focal planes of engrafted islets transduced with βIRB in mice fed a control diet or HFHSD at 8 wk of diet treatment. In situ representative image of immunostaining of endogenous FoxO1 in situ in islets from pancreas sections from mice fed a control diet or HFHSD for 8 wk. Scale bars, 30 µm. B) Relative β-cell insulin resistance indicated by immunostaining of endogenous FoxO1 in situ in islets from pancreas sections from mice fed a control diet or HFHSD for 8 wk. C–E) Western blot quantification of pY-IR/IR, p-Akt/Akt, and p-FoxO1/FoxO1 in isolated islets from mice fed a control diet or HFHSD for 8 wk (n = 11–12). Representative Western blots for 3 control diet and HFHSD mice are shown. **P < 0.01, ***P < 0.001. Data are expressed as means ± sem. See also Supplemental Fig. S2.
The combination of lipotoxicity and sucrose-induced high β-cell workload leads to β-cell insulin resistance
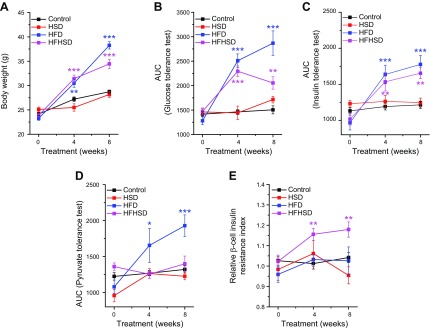
Because the HFHSD provoked β-cell insulin resistance, we examined whether the combination of fat and sucrose was necessary or whether one of those components alone was able to reproduce the same effect. To address that question, we administrated either a solid HFD or a liquid HSD to mice for 8 wk. Mice that received an HSD did not change in any of the measured physiologic parameters (Fig. 3A–E and Supplemental Fig. S1). Mice that received an HFD showed an increase in body weight (Fig. 3A), impaired glucose tolerance (Fig. 3B and Supplemental Fig. S1), whole-body insulin resistance (Fig. 3C and Supplemental Fig. S1), and impaired pyruvate tolerance (Fig. 3D and Supplemental Fig. S1). However, those mice did not develop β-cell insulin resistance (Fig. 3E) at 4 and 8 wk of diet intervention. These data suggest that the combination of lipotoxicity provided by the high-fat component and the high workload enforced by the high-glucose component of the diet were needed to develop β-cell insulin resistance. Consequently, we focused our further work on the HFHSD.
Figure 3.
The combination of lipotoxicity and high β-cell workload leads to β-cell insulin resistance. A) Body weight of mice fed a control diet, HFHSD, HSD, or HFD (n = 4–12). B) Glucose tolerance obtained by IPGTT and depicted as AUC of the IPGTT in mice fed a control diet, HFHSD, HSD, or HFD (n = 4–12). C) Whole-body insulin tolerance obtained by IPITT and depicted as AUC of the IPITT in mice fed a control diet, HFHSD, HSD, or HFD (n = 4–9). D) Liver insulin tolerance obtained by IPPTT and depicted as AUC of the IPPTT in mice fed a control diet, HFHSD, HSD, or HFD (n = 5–8). E) Relative β-cell insulin resistance measured by βIRB in islets transplanted into the ACE in mice fed a control diet, HFHSD, HSD, or HFD (n = 3–14). *P < 0.05, **P < 0.01, ***P < 0.001. Data are expressed as means ± sem. See also Supplemental Fig. S1.
Generation of a βFLUOMETRI for in vivo monitoring of glucose-stimulated β-cell function
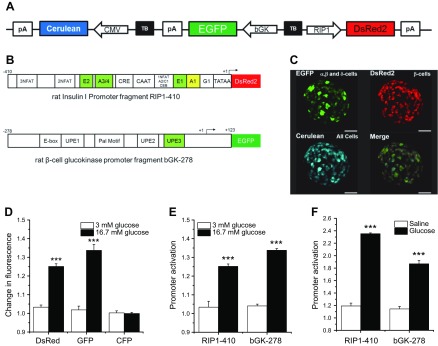
To monitor the consequences of HFHSD longitudinally, and thus the β-cell insulin resistance, on β-cell function in vivo, we again used the ACE in vivo imaging approach. We developed a biosensor that allowed us to monitor β-cell function in response to glucose stimulation by changes in fluorescence intensity. As we have previously shown (27–29, 41, 42), the rat insulin gene-1 promoter (RIP1) and the rat β-cell–active glucokinase gene promoter contain metabolic response elements that allow an increase in promoter activity upon stimulation with glucose or insulin in vitro. To generate βFLUOMETRI for in vivo monitoring, we combined 3 expression cassettes in a single adenoviral vector (Fig. 4A–C). The first cassette comprises a DsRed2 gene driven by a fragment of the rat insulin-1 gene promoter (−410/+1 bp; RIP1-410), containing transcriptional metabolic response elements for glucose, insulin, Ca2+, and cAMP as well as cis-elements allowing β-cell–selective expression (27, 43–45). The second cassette comprises the EGFP gene driven by a fragment of the rat β-cell–active glucokinase gene promoter (−278/+123 bp; bGK-278) containing transcriptional metabolic response elements for glucose and insulin, the latter being activated by a signaling cascade that is different from the one activating the RIP1-410 fragment (Supplemental Fig. S3A) (28, 46). As a third expression cassette, we chose cytomegalovirus (CMV)-promoter–driven Cerulean, which does not respond to the metabolic stimuli glucose or insulin (27).
Figure 4.
Measurement of functional β-cell mass. A) Schematic illustration of βFLUOMETRI. Although all cells express Cerulean under the CMV promoter, all endocrine cells express EGFP under the rat β-cell-active glucokinase promoter fragment bGK-278, and only β cells express DsRed2 under the insulin promoter fragment RIP1-410. Cerulean under the CMV promoter was used for normalization of fluorescence changes in EGFP under bGK-278 and DsRed2 under RIP1-410. B) Schematic illustration of the metabolic response elements within RIP1-410 and bGK-278. Elements highlighted in green are glucose and insulin responsive; that in yellow is glucose responsive, and NFAT and CRE are Ca2+ responsive. C) Representative image of an islet expressing βFLUOMETRI (n = 6). Scale bars, 50 µm. D) Changes in EGFP, DsRed2, and Cerulean (CFP) fluorescence after glucose stimulation in islets in vitro (n = 6). E) Promoter activation after glucose stimulation in vitro. DsRed2 and EGFP fluorescence intensities were normalized to CFP fluorescence intensity (n = 6). F) Activation of RIP1-410 and bGK-278 after glucose and saline injection in vivo (n = 5). ***P < 0.001. Data are expressed as means ± sem. See also Supplemental Fig. S3.
To verify the functionality of βFLUOMETRI, we transduced mouse islets with an adenovirus encoding the biosensor and first analyzed changes of fluorescence intensities of DsRed2, EGFP, and Cerulean upon glucose stimulation in vitro. Fluorescence intensities were analyzed only in DsRed2-positive cells, that is, β cells. Glucose stimulation led to a significant increase in both EGFP and DsRed2 fluorescence, whereas fluorescence of CMV-promoter–driven Cerulean remained unchanged (Fig. 4D). Hence, fluorescence intensity generated by CMV-promoter–driven Cerulean could be used to normalize changes in RIP1-410 and bGK-278 activities (Fig. 4E). To test βFLUOMETRI in vivo, we transduced isolated islets from B6 mice with an adenovirus encoding the biosensor and transplanted those islets to the ACE of littermates. We let the islets engraft for 1 mo before we performed the experiment, as illustrated in Supplemental Fig. S3B. Briefly, we measured fluorescence intensities of EGFP, DsRed2, and Cerulean by confocal microscopy in anesthetized mice that were unfed overnight. After the first recording, we i.p. injected awake mice with glucose or saline and performed a second recording 4 h after the injection in anesthetized mice, maintaining the previous microscopic settings. Mice injected with glucose showed a significant increase in bGK-278 and RIP1-410 activity, whereas mice injected with saline did not (Fig. 4F). These data showed the feasibility of using βFLUOMETRI to monitor β-cell function upon glucose stimulation in vivo.
βFLUOMETRI reveals loss of functional β-cell mass in response to HFHSD and recovery after reintroduction of normal chow diet
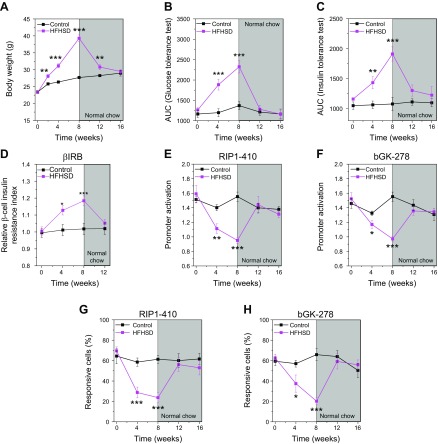
To investigate the effect of HFHSD on β-cell function in vivo, we transduced isolated islets from B6 mice with an adenovirus encoding βFLUOMETRI, transplanted them to the ACE of male littermates at 2 mo old, and followed the experimental settings as above (Supplemental Fig. S3B). Within 4 wk of HFHSD the mice increased their body weight (Fig. 5A) and developed impaired glucose tolerance (Fig. 5B), whole-body insulin resistance (Fig. 5C), and β-cell insulin resistance (Fig. 5D). All parameters became more pronounced by 8 wk of diet. Four weeks of feeding an HFHSD led to a significant reduction in glucose-stimulated activities of RIP1-410 and bGK-278 in β cells, which became more pronounced 8 wk after the start of the diet intervention (Fig. 5E, F). Because we intended to measure functional β-cell mass, we took advantage of the high amount of analyzable β cells/islets to calculate the percentage of glucose-responsive β cells (Fig. 5G, H). The data show that, under normal conditions, 64 ± 2% of the β cells were responsive to glucose stimulation. During the diet intervention with an HFHSD, the amount of glucose-responsive β cells decreased to 22 ± 3%.
Figure 5.
βFLUOMETRI reveals loss of functional β-cell mass in response to HFHSD and the regaining of function upon recovery from the diet. A) Body weight of B6 mice before, during, and after feeding of a control diet or HFHSD (n = 5–10). B) Glucose tolerance obtained by IPGTT and depicted as the AUC of the IPGTT in B6 mice before, during, and after feeding of a control diet or HFHSD (n = 5–10). C) Whole-body insulin resistance obtained by IPITT and depicted as the AUC of the IPITT in B6 mice before, during, and after feeding of a control diet or HFHSD (n = 5–10). D) Relative β-cell insulin resistance indicated by βIRB in islets transplanted into the ACE in mice before, during, and after feeding of a control diet or HFHSD (n = 12–20). E) RIP1-410 promoter activation indicated by the βFLUOMETRI and obtained in vivo in transduced islets transplanted into the ACE of B6 mice before, during, and after feeding of a control diet or HFHSD (n = 5–10). F) bGK-278 promoter activation indicated by the βFLUOMETRI and obtained in vivo in islets transplanted into the ACE of B6 mice before, during, and after feeding of a control diet or HFHSD (n = 5–10). G) Proportion of glucose-responsive cells indicated by RIP1-410 of the βFLUOMETRI and obtained in vivo in islets transplanted into the ACE of B6 mice before, during, and after feeding of a control diet or HFHSD (n = 5–10). H) Proportion of glucose-responsive cells indicated by bGK-278 of the βFLUOMETRI and obtained in vivo in islets transplanted into the ACE of B6 mice before, during, and after feeding of a control diet or HFHSD (n = 5–10). *P < 0.05, **P < 0.01, ***P < 0.001. Data are expressed as means ± sem.
To test whether β cells can recover their functionality after 8 wk of HFHSD feeding, we changed the diet to normal chow (Fig. 5). Interestingly, all measured parameters, except the body weight, recovered to normal within 4 wk of feeding normal chow diet, that is, they were not different from the control group.
HFHSD leads to noncompensatory, glucose-stimulated insulin secretion because of a defect downstream of glucose-stimulated Ca2+ influx
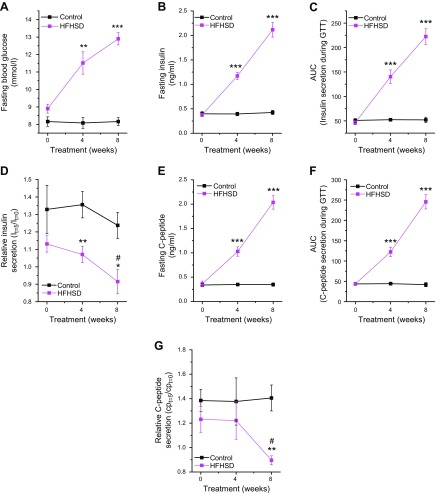
To analyze the consequences of the HFHSD, we next analyzed the metabolic state of the animals. Mice treated with an HFHSD became overweight, had impaired glucose tolerance, were whole-body and β-cell insulin resistant (Figs. 1 and 3 and Supplemental Fig. S1) after 4 wk of diet intervention. That was paralleled by increased fasting blood glucose levels (Fig. 6A), increased fasting insulin levels (Fig. 6B), and increased fasting C-peptide levels (Fig. 6E). Impairment of all parameters was more pronounced after 8 wk of HFHSD. Although the total amounts of secreted insulin (Fig. 6C and Supplemental Fig. S4) and C-peptide (Fig. 6F and Supplemental Fig. S4) were increased, the levels of insulin were not sufficient to compensate for hyperglycemia. The relative secretory response of β cells, in terms of insulin and C-peptide release during the first 5 min of an IPGTT, was significantly reduced 8 wk after the start of the diet intervention (Fig. 6D, G and Supplemental Fig. S4).
Figure 6.
HFHSD leads to noncompensatory insulin secretion. A) Fasting blood glucose in mice fed a control diet or HFHSD. B) Fasting insulin in mice fed a control diet or HFHSD. C) Insulin secretion during an IPGTT in mice fed a control diet or HFHSD. D) Relative insulin secretion during the first 5 min of an IPGTT in mice fed a control diet or HFHSD. E) Fasting C-peptide results in mice fed a control diet or HFHSD. F) C-peptide secretion during an IPGTT in mice fed a control diet or HFHSD. G) Relative C-peptide secretion during the first 5 min of an IPGTT in mice fed a control diet or HFHSD. n = 5–6. *P < 0.05, **P < 0.01, ***P < 0.001 for control diet vs. HFHSD; #P < 0.01 for HFHSD at 8 wk of treatment vs. 0 wk of treatment. Data are expressed as means ± sem. See also Supplemental Fig. S4.
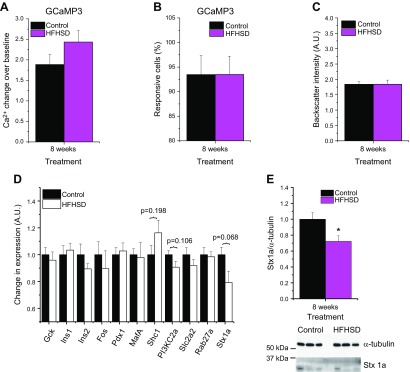
To better understand where in the glucose-stimulation/insulin-secretion coupling the β cells failed to secrete sufficient amounts of insulin to compensate for a glucose challenge, we next measured the glucose-stimulated β-cell Ca2+ handling by means of glucose-stimulated Ca2+ excursions in reporter islets in the ACE in vivo. We isolated islets from Ins-Cre:GCaMP3 mice, which express the Ca2+ biosensor GCaMP3 in β cells, and transplanted those islets into the ACE of male B6 mice. Eight weeks after feeding an HFHSD or control diet, Ca2+ excursions upon glucose stimulation were monitored in anesthetized animals by confocal microscopy. Ca2+ excursions were not different between the groups (Fig. 7A). Similarly, the amount of responding β cells in the HFHSD group did not differ from the control group (Fig. 7B), suggesting a defect downstream of glucose-stimulated Ca2+ handling. Analysis of the backscatter intensity of the engrafted islets, indicative for insulin content (26), revealed no differences between mice fed HFHSD and the control group (Fig. 7C). Employing a biased transcriptomics analysis (real-time quantitative PCR) in islets obtained from male B6 mice treated for 8 wk with either a control diet or an HFHSD, we found a trend toward decreased expression of the soluble N-ethylmaleimide attachment receptor protein syntaxin-1A among different candidate genes implicated in β-cell function (Fig. 7D). The decrease in expression of syntaxin-1A was confirmed at the protein level (Fig. 7E and Supplemental Fig. S5).
Figure 7.
Impaired insulin secretion of HFHSD mice is due to a defect downstream of glucose-stimulated Ca2+ influx. A) Ca2+ excursions upon glucose stimulus, as indicated by the GCaMP3 Ca2+ biosensor, obtained in vivo in islets from Ins-Cre:GCaMP3-mice transplanted into the ACE of B6 mice at 8 wk of an HFHSD (n = 6–9). B) Amount of glucose-responsive cells indicated by the GCaMP3 Ca2+ biosensor obtained in vivo in islets from Ins-Cre:GCaMP3-mice transplanted to the ACE of B6 mice at 8 wk of an HFHSD (n = 6–9). C) Backscatter intensity of islets transplanted to the ACE of B6 mice at 8 wk of an HFHSD shown in arbitrary units (A.U.) (n = 10). D) Gene expression analysis of isolated islets from mice fed a control diet or HFHSD for 8 wk shown in A.U. (n = 6 mice). E) Western blot quantification of syntaxin-1A in isolated islets from mice fed a control diet or HFHSD for 8 wk (n = 11–12). Representative Western blot of 3 control diet and HFHSD mice is shown. *P < 0.05, ***P < 0.001. Data are expressed as means ± sem. See also Supplemental Figs. S5 and S6.
Finally, we tested whether the HFHSD led to an increase in β-cell proliferation to compensate for inadequate function by supplementing the liquid food in both control and HFHSD mice with BrdU during wk 8 of the diet treatment. In this case, we found that HFHSD treatment led to an increase in β-cell proliferation during the last week of diet intervention (Supplemental Fig. S6A, B), which was mirrored by an increase in the relative size of the reporter islets in the ACE (Supplemental Fig. S6C).
DISCUSSION
Relevance of β-cell insulin resistance in dietary models of T2DM development
Sugar and fat are discussed in the context of macronutrient (over) consumption, leading to obesity, insulin resistance, and subsequent development of T2DM (47, 48). Fast food consists of food with high-fat contents and beverages with high-sugar contents, primarily sucrose or fructose. To reproduce fast-food consumption in animal models with a diabetic-prone genetic background, we performed an 8-wk diet intervention, with a combination of a solid diet high in fat and drinking water containing either sucrose (HFHSD) or fructose (HFHFrD) (49, 50) in male C57Bl/6J mice, which develop a T2DM-like phenotype when metabolically challenged (51, 52). Although both types of diet led to the development of a diabetic phenotype, including elevated fasting blood glucose levels, increased body weight, impaired glucose tolerance, and whole-body insulin resistance, only the HFHSD-treated animals developed β-cell insulin resistance in the metabolic branch of insulin signaling. Moreover, HFHFrD led to impaired pyruvate tolerance, whereas HFHSD did not. It is remarkable that none of the diets alone, that is, HFD or HSD, were able to cause β-cell insulin resistance. Even if an HFD caused functional damage to glucose and insulin handling, lipotoxicity alone was insufficient to provoke β-cell insulin resistance within 8 wk of diet treatment. In fact, the combination of lipotoxicity with the high workload of the β cell because of additional dietary glucose was required to cause β-cell insulin resistance. These data reinforce the relevance of diet composition as an environmental factor determining different routes of diabetes progression.
Western blot analysis of pancreatic islets from mice fed an HFHSD for 8 wk revealed reduced phosphorylation of Akt and FoxO1, together with reduced Tyr phosphorylation within the activation loop of the insulin receptor (Tyr 1150/1151), reflecting decreased activity by the receptor. Future work will have to show whether that is the result of enhanced activity by the phosphotyrosine phosphatases, as recently discussed for T-cell protein-tyrosine phosphatase-45 (53), or of increased binding of members of the Grb7 family to the receptor (54).
Consequences of HFHSD and β-cell insulin resistance on functional β-cell mass
To understand how an HFHSD, and thus β-cell insulin resistance, affects β-cell function in vivo, we established an approach for longitudinal, noninvasive monitoring of functional β-cell mass using the reporter-islet concept. That allowed us to combine the assessment of robust parameters reflecting the metabolic state of the mice during the diet intervention, such as IPGTT, IPITT, IPPTT, fasting blood glucose, and insulin levels, with data on β-cell function by monitoring reporter islets engrafted into the ACE. These islets reported on β-cell insulin sensitivity (βIRB) and β-cell responsiveness to glucose stimulation by employing a βFLUOMETRI and a fluorescent Ca2+ indicator (GCaMP3). T2DM development is generally discussed as a chronic process, which, in humans, takes several years or even decades to complete. It was astonishing to see that impairments in β-cell insulin sensitivity and glucose responsiveness were already observed 4 wk after start of diet intervention and that all parameters measured became more deranged by 8 wk of HFHSD. These data support recent observations that changes in β cells occur early in response to extreme metabolic challenges (55). The many β cells expressing either βFLUOMETRI or GCaMP3, allowed us to monitor functional β-cell mass. Interestingly, although there was no change in functional β-cell mass in terms of glucose-stimulated Ca2+ excursions, a 65% decrease in glucose-responsive β-cell mass was observed when using the βFLUOMETRI. Remarkably, replacing the HFHSD after 8 wk of diet intervention with a normal chow diet allowed recovery of all measured parameters to within reference range. Because β cells in the reporter islets were color-coded by the biosensor, that recovery was achieved, at least in part, by the mice regaining function in the β cells that were previously malfunctioning. This highlights the plasticity of β-cell functionality, at least during the early phase of diabetes development and may have important implications for intervention of T2DM progression.
Finally, when starting to explore the molecular mechanism(s) involved in HFHSD-evoked β-cell dysfunction, we speculated that there might be a defect in insulin exocytosis based on the inability of the β cells to increase serum C-peptide levels within the first 5 min of an IPGTT. Results of a biased-transcriptomics analysis revealed no changes in the expression of genes responsible for β-cell identity (MafA, Pdx1) or genes involved in β-cell glucose handling (glucose transporter 2, glucokinase), indicating no β-cell dedifferentiation at that stage of diet intervention. This is in agreement with earlier findings by Kitamura et al. (56), showing that MafA may replace Pdx1 as a master regulator of β-cell identity and function when FoxO1 is enriched in the β-cell nucleus. Loss of nuclear Pdx1 and reduced binding of MafA to cis-elements with the promoters of respective target genes may, in addition to impaired insulin signaling, explain the loss of glucose responsiveness indicated by βFLUOMETRI under glucotoxic/lipotoxic conditions (57). Moreover, we identified reduced expression of the soluble N-ethylmaleimide attachment receptor protein syntaxin-1A, a key protein in insulin exocytosis (58, 59), in islets from mice fed an HFHSD for 8 wk. This is in agreement with earlier observations showing that impaired insulin signaling in β cells results in reduced expression of syntaxin-1A (8, 60). Because syntaxin-1A acts in the final steps of insulin granule docking/fusion in glucose-stimulated insulin secretion, that is, downstream of glucose-stimulated Ca2+ entry, these data are not in conflict with the unchanged glucose-stimulated Ca2+ excursions obtained by GCaMP3-reporter islets. Syntaxin-1A has been shown to bind both insulin granules and L-type voltage-gated Ca2+ channels (61), thus allowing glucose-stimulated Ca2+ influx close to the release site of the insulin granule. Previous data have demonstrated that, under conditions of lipotoxicity, the association between insulin granules and L-type voltage-gated Ca2+ channels is reduced, resulting in decreased insulin exocytosis despite normal glucose-evoked Ca2+ excursions (62, 63).
Limitations of study
One major objective of this study was to address the pathophysiologic relevance of β-cell insulin resistance in dietary-based models of T2DM progression. To characterize the metabolic state of the mice, we used a set of techniques that were least invasive, such as excluding additional anesthetic episodes, to match them with the noninvasive character of our in vivo reporter-islet monitoring approach. We are well aware that for proper metabolic characterization of HFHSD-fed animals, appropriate clamp studies, as well as analysis involving metabolic cages, are necessary (40, 64). Moreover, as clearly stated, we performed a biased transcriptomic/proteomic analysis that aimed for genes that were documented to be compromised under conditions of β-cell insulin resistance. Future analyses need to be based on a nonbiased approach, including whole-RNA sequencing analysis, microRNA profiling, epigenetic characterization, and proteomic analysis, including enzyme kinetic profiling, to disclose the molecular mechanisms involved in HFHSD-based β-cell dysfunction.
CONCLUSIONS
In summary, our data reveal that β-cell insulin resistance develops when specific dietary conditions (HFHSD) are brought onto a specific genetic background (C57Bl/6J). This highlights the relevance of diet composition as an environmental factor determining different routes of diabetes progression in a given individual with genetic predisposition for the disease. Exemplified by the HFHSD, we show that β-cell insulin resistance occurs early during T2DM progression and is provoked by the combination of lipotoxicity provided by the high-fat component and increased β-cell workload caused by the additional glucose in the diet. Within 8 wk, mice became obese, developed impaired insulin and glucose tolerances, and displayed noncompensatory insulin release, at least in part, because of reduced expression of syntaxin-1A. Reporter islets transplanted into the ACE revealed a concomitant loss of functional β-cell mass. Of note, when mice were changed from the diabetogenic diet to a normal diet, the diabetes phenotype was reversed, suggesting a remarkable plasticity of functional β-cell mass in the early phase of T2DM development. This is likely to be explained by a still functional glucose metabolism, adequate Ca2+ handling, and the expression of markers of β-cell identity at that stage of the disease. These findings have major implications, offering a time window within which the progression to full-blown T2DM can be halted. Finally, employing the in vivo reporter-islet monitoring approach will not only allow researchers to define key time points in the dynamics of reversible loss of functional β-cell mass and, thus, to investigate the underlying molecular mechanisms involved in the progression toward T2DM manifestation but also will represent a monitoring approach for a personalized medicine treatment of the disease.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
This work was supported by funds from the Karolinska Institutet (KID programme), the Swedish Research Council, the Family Erling-Persson Foundation, the Novo Nordisk Foundation, Novo Nordisk A/S, the Stichting af Jochnick Foundation, the Swedish Diabetes Association, Scandia Insurance Company Limited, the Diabetes Research and Wellness Foundation, the Berth von Kantzow’s Foundation, the Strategic Research Program in Diabetes at Karolinska Institutet, the ERC-2013-AdG 338936 BetaImage, the ERC-2017-PoC 727306 BETASCREEN, the Swedish Foundation for Strategic Research, and the Knut and Alice Wallenberg Foundation. N.M. was supported by a Novo Nordisk postdoctoral fellowship run in partnership with the Karolinska Institutet. P.-O.B. is cofounder of Biocrine AB, I.B.L. and B.L. are consultants for Biocrine AB, and S.J. is employed by Biocrine AB.
Glossary
- ACE
anterior chamber of the eye
- Akt
protein kinase B
- AUC
area under the curve
- B6 mice
C57Bl/6J mice
- BrdU
5-bromo-2′-deoxyuridine
- CMV
cytomegalovirus
- DsRed
red fluorescent protein
- EGFP
enhanced green fluorescent protein
- βFLUOMETRI
β-cell fluorescent metabolic transcriptional response indicator
- GCaMP
genetically encoded calcium indicator
- HFD
high-fat diet
- HFHFrD
high-fat–high-fructose diet
- HFHSD
high-fat–high-sucrose diet
- HFrD
high-fructose diet
- HSD
high-sucrose diet
- IPGTT
i.p. glucose tolerance test
- IPITT
i.p. insulin tolerance test
- IPPTT
i.p. pyruvate tolerance test
- βIRB
β-cell insulin-resistance biosensor
- RIP1
rat insulin-1 gene promoter
- rβIRI
relative β-cell insulin-resistance biosensor index
- T2DM
type 2 diabetes mellitus
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
M. Paschen, T. Moede, B. Leibiger, I. B. Leibiger, and P.-O. Berggren conceptualized the project; M. Paschen and T. Moede conceptualized new analysis methods; M. Paschen, T. Moede, I. Valladolid-Acebes, B. Leibiger, N. Moruzzi, S. Jacob, and C. F. García-Prieto performed experiments; B. Leibiger, I. B. Leibiger, and P.-O. Berggren provided resources; M. Paschen and I. B. Leibiger wrote the original draft of the article; M. Paschen, T. Moede, I. Valladolid-Acebes, B. Leibiger, N. Moruzzi, S. Jacob, C. F. García-Prieto, K. Brismar, I. B. Leibiger, and P.-O. Berggren reviewed and edited the article; M. Paschen visualized the data; B. Leibiger, I. B. Leibiger, and P.-O. Berggren supervised the project; and I. B. Leibiger and P.-O. Berggren acquired funding.
REFERENCES
- 1.McCarthy M. I. (2010) Genomics, type 2 diabetes, and obesity. N. Engl. J. Med. 363, 2339–2350 10.1056/NEJMra0906948 [DOI] [PubMed] [Google Scholar]
- 2.Goldfine A. B., Kulkarni R. N. (2012) Modulation of β-cell function: a translational journey from the bench to the bedside. Diabetes Obes. Metab. 14(Suppl 3), 152–160 10.1111/j.1463-1326.2012.01647.x [DOI] [PubMed] [Google Scholar]
- 3.Kubota T., Kubota N., Kadowaki T. (2017) Imbalanced insulin actions in obesity and type 2 diabetes: key mouse models of insulin signaling pathway. Cell Metab. 25, 797–810 10.1016/j.cmet.2017.03.004 [DOI] [PubMed] [Google Scholar]
- 4.Leibiger I. B., Leibiger B., Berggren P. O. (2008) Insulin signaling in the pancreatic β-cell. Annu. Rev. Nutr. 28, 233–251 10.1146/annurev.nutr.28.061807.155530 [DOI] [PubMed] [Google Scholar]
- 5.Kulkarni R. N., Brüning J. C., Winnay J. N., Postic C., Magnuson M. A., Kahn C. R. (1999) Tissue-specific knockout of the insulin receptor in pancreatic β cells creates an insulin secretory defect similar to that in type 2 diabetes. Cell 96, 329–339 10.1016/S0092-8674(00)80546-2 [DOI] [PubMed] [Google Scholar]
- 6.Bernal-Mizrachi E., Wen W., Stahlhut S., Welling C. M., Permutt M. A. (2001) Islet β cell expression of constitutively active Akt1/PKB α induces striking hypertrophy, hyperplasia, and hyperinsulinemia. J. Clin. Invest. 108, 1631–1638 10.1172/JCI200113785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hashimoto N., Kido Y., Uchida T., Asahara S., Shigeyama Y., Matsuda T., Takeda A., Tsuchihashi D., Nishizawa A., Ogawa W., Fujimoto Y., Okamura H., Arden K. C., Herrera P. L., Noda T., Kasuga M. (2006) Ablation of PDK1 in pancreatic β cells induces diabetes as a result of loss of β cell mass. Nat. Genet. 38, 589–593 10.1038/ng1774 [DOI] [PubMed] [Google Scholar]
- 8.Kaneko K., Ueki K., Takahashi N., Hashimoto S., Okamoto M., Awazawa M., Okazaki Y., Ohsugi M., Inabe K., Umehara T., Yoshida M., Kakei M., Kitamura T., Luo J., Kulkarni R. N., Kahn C. R., Kasai H., Cantley L. C., Kadowaki T. (2010) Class IA phosphatidylinositol 3-kinase in pancreatic β cells controls insulin secretion by multiple mechanisms. Cell Metab. 12, 619–632 10.1016/j.cmet.2010.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okada T., Liew C. W., Hu J., Hinault C., Michael M. D., Krtzfeldt J., Yin C., Holzenberger M., Stoffel M., Kulkarni R. N. (2007) Insulin receptors in β-cells are critical for islet compensatory growth response to insulin resistance. Proc. Natl. Acad. Sci. USA 104, 8977–8982 10.1073/pnas.0608703104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cantley J., Choudhury A. I., Asare-Anane H., Selman C., Lingard S., Heffron H., Herrera P., Persaud S. J., Withers D. J. (2007) Pancreatic deletion of insulin receptor substrate 2 reduces β and α cell mass and impairs glucose homeostasis in mice. Diabetologia 50, 1248–1256 10.1007/s00125-007-0637-9 [DOI] [PubMed] [Google Scholar]
- 11.Bernal-Mizrachi E., Fatrai S., Johnson J. D., Ohsugi M., Otani K., Han Z., Polonsky K. S., Permutt M. A. (2004) Defective insulin secretion and increased susceptibility to experimental diabetes are induced by reduced Akt activity in pancreatic islet β cells. J. Clin. Invest. 114, 928–936 10.1172/JCI200420016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nandi A., Kitamura Y., Kahn C. R., Accili D. (2004) Mouse models of insulin resistance. Physiol. Rev. 84, 623–647 10.1152/physrev.00032.2003 [DOI] [PubMed] [Google Scholar]
- 13.Paschen M., Moede T., Leibiger B., Jacob S., Bryzgalova G., Leibiger I. B., Berggren P. O. (2016) Non-invasive cell type selective in vivo monitoring of insulin resistance dynamics. Sci. Rep. 6, 21448 10.1038/srep21448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Åvall K., Ali Y., Leibiger I. B., Leibiger B., Moede T., Paschen M., Dicker A., Daré E., Köhler M., Ilegems E., Abdulreda M. H., Graham M., Crooke R. M., Tay V. S., Refai E., Nilsson S. K., Jacob S., Selander L., Berggren P. O., Juntti-Berggren L. (2015) Apolipoprotein CIII links islet insulin resistance to β-cell failure in diabetes. Proc. Natl. Acad. Sci. USA 112, E2611–E2619 10.1073/pnas.1423849112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kluth O., Mirhashemi F., Scherneck S., Kaiser D., Kluge R., Neschen S., Joost H. G., Schürmann A. (2011) Dissociation of lipotoxicity and glucotoxicity in a mouse model of obesity associated diabetes: role of forkhead box O1 (FOXO1) in glucose-induced β cell failure. Diabetologia 54, 605–616 10.1007/s00125-010-1973-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laurent D., Vinet L., Lamprianou S., Daval M., Filhoulaud G., Ktorza A., Wang H., Sewing S., Juretschke H. P., Glombik H., Meda P., Boisgard R., Nguyen D. L., Stasiuk G. J., Long N. J., Montet X., Hecht P., Kramer W., Rutter G. A., Hecksher-Sørensen J. (2016) Pancreatic β-cell imaging in humans: fiction or option? Diabetes Obes. Metab. 18, 6–15 10.1111/dom.12544 [DOI] [PubMed] [Google Scholar]
- 17.Speier S., Nyqvist D., Cabrera O., Yu J., Molano R. D., Pileggi A., Moede T., Köhler M., Wilbertz J., Leibiger B., Ricordi C., Leibiger I. B., Caicedo A., Berggren P. O. (2008) Noninvasive in vivo imaging of pancreatic islet cell biology. Nat. Med. 14, 574–578 10.1038/nm1701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Speier S., Nyqvist D., Köhler M., Caicedo A., Leibiger I. B., Berggren P. O. (2008) Noninvasive high-resolution in vivo imaging of cell biology in the anterior chamber of the mouse eye. Nat. Protoc. 3, 1278–1286 10.1038/nprot.2008.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ilegems E., Dicker A., Speier S., Sharma A., Bahow A., Edlund P. K., Leibiger I. B., Berggren P. O. (2013) Reporter islets in the eye reveal the plasticity of the endocrine pancreas. Proc. Natl. Acad. Sci. USA 110, 20581–20586 10.1073/pnas.1313696110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abdulreda M. H., Faleo G., Molano R. D., Lopez-Cabezas M., Molina J., Tan Y., Echeverria O. A., Zahr-Akrawi E., Rodriguez-Diaz R., Edlund P. K., Leibiger I., Bayer A. L., Perez V., Ricordi C., Caicedo A., Pileggi A., Berggren P. O. (2011) High-resolution, noninvasive longitudinal live imaging of immune responses. Proc. Natl. Acad. Sci. USA 108, 12863–12868 10.1073/pnas.1105002108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chmelova H., Cohrs C. M., Chouinard J. A., Petzold C., Kuhn M., Chen C., Roeder I., Kretschmer K., Speier S. (2015) Distinct roles of β-cell mass and function during type 1 diabetes onset and remission. Diabetes 64, 2148–2160 10.2337/db14-1055 [DOI] [PubMed] [Google Scholar]
- 22.Chen C., Chmelova H., Cohrs C. M., Chouinard J. A., Jahn S. R., Stertmann J., Uphues I., Speier S. (2016) Alterations in β-cell calcium dynamics and efficacy outweigh islet mass adaptation in compensation of insulin resistance and prediabetes onset. Diabetes 65, 2676–2685 10.2337/db15-1718 [DOI] [PubMed] [Google Scholar]
- 23.Leibiger I. B., Berggren P. O. (2017) Intraocular in vivo imaging of pancreatic islet cell physiology/pathology. Mol. Metab. 6, 1002–1009 10.1016/j.molmet.2017.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diez J. A., Arrojo E Drigo R., Zheng X., Stelmashenko O. V., Chua M., Rodriguez-Diaz R., Fukuda M., Köhler M., Leibiger I., Tun S. B. B., Ali Y., Augustine G. J., Barathi V. A., Berggren P. O. (2017) Pancreatic islet blood flow dynamics in primates. Cell Rep. 20, 1490–1501 10.1016/j.celrep.2017.07.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uhles S., Moede T., Leibiger B., Berggren P. O., Leibiger I. B. (2003) Isoform-specific insulin receptor signaling involves different plasma membrane domains. J. Cell Biol. 163, 1327–1337 10.1083/jcb.200306093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ilegems E., van Krieken P. P., Edlund P. K., Dicker A., Alanentalo T., Eriksson M., Mandic S., Ahlgren U., Berggren P. O. (2015) Light scattering as an intrinsic indicator for pancreatic islet cell mass and secretion. Sci. Rep. 5, 10740 10.1038/srep10740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leibiger I. B., Leibiger B., Moede T., Berggren P. O. (1998) Exocytosis of insulin promotes insulin gene transcription via the insulin receptor/PI-3 kinase/p70 s6 kinase and CaM kinase pathways. Mol. Cell 1, 933–938 10.1016/S1097-2765(00)80093-3 [DOI] [PubMed] [Google Scholar]
- 28.Leibiger B., Leibiger I. B., Moede T., Kemper S., Kulkarni R. N., Kahn C. R., de Vargas L. M., Berggren P. O. (2001) Selective insulin signaling through A and B insulin receptors regulates transcription of insulin and glucokinase genes in pancreatic β cells. Mol. Cell 7, 559–570 10.1016/S1097-2765(01)00203-9 [DOI] [PubMed] [Google Scholar]
- 29.Moede T., Leibiger B., Berggren P. O., Leibiger I. B. (2001) Online monitoring of stimulus-induced gene expression in pancreatic β-cells. Diabetes 50 Suppl 1, S15–S19 10.2337/diabetes.50.2007.S15 [DOI] [PubMed] [Google Scholar]
- 30.Leibiger B., Moede T., Uhles S., Barker C. J., Creveaux M., Domin J., Berggren P. O., Leibiger I. B. (2010) Insulin-feedback via PI3K-C2α activated PKBα/Akt1 is required for glucose-stimulated insulin secretion. FASEB J. 24, 1824–1837 10.1096/fj.09-148072 [DOI] [PubMed] [Google Scholar]
- 31.Van Krieken P. P., Dicker A., Eriksson M., Herrera P. L., Ahlgren U., Berggren P. O., Ilegems E. (2017) Kinetics of functional β cell mass decay in a diphtheria toxin receptor mouse model of diabetes. Sci. Rep. 7, 12440 10.1038/s41598-017-12124-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thorens B., Tarussio D., Maestro M. A., Rovira M., Heikkilä E., Ferrer J. (2015) Ins1(Cre) knock-in mice for β cell-specific gene recombination. Diabetologia 58, 558–565 10.1007/s00125-014-3468-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zariwala H. A., Borghuis B. G., Hoogland T. M., Madisen L., Tian L., De Zeeuw C. I., Zeng H., Looger L. L., Svoboda K., Chen T. W. (2012) A Cre-dependent GCaMP3 reporter mouse for neuronal imaging in vivo. J. Neurosci. 32, 3131–3141 10.1523/JNEUROSCI.4469-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Edelstein A., Amodaj N., Hoover K., Vale R., Stuurman N. (2010) Computer control of microscopes using µManager. Curr. Protoc. Mol. Biol. Chapter 14, Unit 14.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boutet de Monvel J., Le Calvez S., Ulfendahl M. (2001) Image restoration for confocal microscopy: improving the limits of deconvolution, with application to the visualization of the mammalian hearing organ. Biophys. J. 80, 2455–2470 10.1016/S0006-3495(01)76214-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guizar-Sicairos M., Thurman S. T., Fienup J. R. (2008) Efficient subpixel image registration algorithms. Opt. Lett. 33, 156–158 10.1364/OL.33.000156 [DOI] [PubMed] [Google Scholar]
- 37.Zanella R., Zanghirati G., Cavicchioli R., Zanni L., Boccacci P., Bertero M., Vicidomini G. (2013) Towards real-time image deconvolution: application to confocal and STED microscopy. Sci. Rep. 3, 2523 10.1038/srep02523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kroon D. J. (2011) Segmentation of the Mandibular Canal in Cone-Beam CT Data, University of Twente, Enschede, The Netherlands: 10.3990/1.9789036532808 [DOI] [Google Scholar]
- 39.Schmittgen T. D., Livak K. J. (2008) Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 3, 1101–1108 10.1038/nprot.2008.73 [DOI] [PubMed] [Google Scholar]
- 40.Hughey C. C., Wasserman D. H., Lee-Young R. S., Lantier L. (2014) Approach to assessing determinants of glucose homeostasis in the conscious mouse. Mamm. Genome 25, 522–538 10.1007/s00335-014-9533-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leibiger B., Walther R., Zuhlke H., Leibiger I. B. (1994) Characterization of the 5′-flanking region of the pancreatic B-cell specific transcription unit of the rat glucokinase gene. In Insulin Secretion and Pancreatic B-Cell Research (Flatt P. R., and Lenzen S., eds.), pp. 15–24, Smith-Gordon and Company Limited, London [Google Scholar]
- 42.Leibiger B., Berggren P. O., Leibiger I. B. (2004) Regulation of β-cell GK gene transcrition by insulin. In Glucokinase and Glycemic Disease: From Basics to Novel Therapeutics (Matschinsky F. M., and Magnuson M. A., eds.), pp. 249–261, Karger Publishers, Basel, Switzerland [Google Scholar]
- 43.Melloul D., Marshak S., Cerasi E. (2002) Regulation of insulin gene transcription. Diabetologia 45, 309–326 10.1007/s00125-001-0728-y [DOI] [PubMed] [Google Scholar]
- 44.Lawrence M. C., Bhatt H. S., Watterson J. M., Easom R. A. (2001) Regulation of insulin gene transcription by a Ca2+-responsive pathway involving calcineurin and nuclear factor of activated T cells. Mol. Endocrinol. 15, 1758–1767 10.1210/mend.15.10.0702 [DOI] [PubMed] [Google Scholar]
- 45.Hay C. W., Docherty K. (2006) Comparative analysis of insulin gene promoters: implications for diabetes research. Diabetes 55, 3201–3213 10.2337/db06-0788 [DOI] [PubMed] [Google Scholar]
- 46.Uhles S., Moede T., Leibiger B., Berggren P. O., Leibiger I. B. (2007) Selective gene activation by spatial segregation of insulin receptor B signaling. FASEB J. 21, 1609–1621 10.1096/fj.06-7589com [DOI] [PubMed] [Google Scholar]
- 47.Drewnowski A. (1998) Energy density, palatability, and satiety: implications for weight control. Nutr. Rev. 56, 347–353 10.1111/j.1753-4887.1998.tb01677.x [DOI] [PubMed] [Google Scholar]
- 48.Lindqvist A., de la Cour C. D., Stegmark A., Håkanson R., Erlanson-Albertsson C. (2005) Overeating of palatable food is associated with blunted leptin and ghrelin responses. Regul. Pept. 130, 123–132 10.1016/j.regpep.2005.05.002 [DOI] [PubMed] [Google Scholar]
- 49.Sellmann C., Priebs J., Landmann M., Degen C., Engstler A. J., Jin C. J., Gärttner S., Spruss A., Huber O., Bergheim I. (2015) Diets rich in fructose, fat or fructose and fat alter intestinal barrier function and lead to the development of nonalcoholic fatty liver disease over time. J. Nutr. Biochem. 26, 1183–1192 10.1016/j.jnutbio.2015.05.011 [DOI] [PubMed] [Google Scholar]
- 50.Softic S., Gupta M. K., Wang G. X., Fujisaka S., O’Neill B. T., Rao T. N., Willoughby J., Harbison C., Fitzgerald K., Ilkayeva O., Newgard C. B., Cohen D. E., Kahn C. R. (2017) Divergent effects of glucose and fructose on hepatic lipogenesis and insulin signaling. J. Clin. Invest. 127, 4059–4074 10.1172/JCI94585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Surwit R. S., Kuhn C. M., Cochrane C., McCubbin J. A., Feinglos M. N. (1988) Diet-induced type II diabetes in C57BL/6J mice. Diabetes 37, 1163–1167 10.2337/diab.37.9.1163 [DOI] [PubMed] [Google Scholar]
- 52.Kulkarni R. N., Almind K., Goren H. J., Winnay J. N., Ueki K., Okada T., Kahn C. R. (2003) Impact of genetic background on development of hyperinsulinemia and diabetes in insulin receptor/insulin receptor substrate-1 double heterozygous mice. Diabetes 52, 1528–1534 10.2337/diabetes.52.6.1528 [DOI] [PubMed] [Google Scholar]
- 53.Yoo T., Ham S. A., Lee W. J., Hwang S. I., Park J. A., Hwang J. S., Hur J., Shin H. C., Han S. G., Lee C. H., Han D. W., Paek K. S., Seo H. G. (2018) Ligand-dependent interaction of PPARδ with T-cell protein tyrosine phosphatase 45 enhances insulin signaling. Diabetes 67, 360–371 10.2337/db17-0499 [DOI] [PubMed] [Google Scholar]
- 54.Li L., Li X., Zhu Y., Zhang M., Yin D., Lu J., Liu F., Wang C., Jia W. (2013) Growth receptor binding protein 10 inhibits glucose-stimulated insulin release from pancreatic β-cells associated with suppression of the insulin/insulin-like growth factor-1 signalling pathway. Clin. Exp. Pharmacol. Physiol. 40, 841–847 10.1111/1440-1681.12160 [DOI] [PubMed] [Google Scholar]
- 55.Mosser R. E., Maulis M. F., Moullé V. S., Dunn J. C., Carboneau B. A., Arasi K., Pappan K., Poitout V., Gannon M. (2015) High-fat diet-induced β-cell proliferation occurs prior to insulin resistance in C57Bl/6J male mice. Am. J. Physiol. Endocrinol. Metab. 308, E573–E582 10.1152/ajpendo.00460.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kitamura Y. I., Kitamura T., Kruse J. P., Raum J. C., Stein R., Gu W., Accili D. (2005) FoxO1 protects against pancreatic β cell failure through NeuroD and MafA induction. Cell Metab. 2, 153–163 10.1016/j.cmet.2005.08.004 [DOI] [PubMed] [Google Scholar]
- 57.Kaneto H., Matsuoka T. A. (2015) Role of pancreatic transcription factors in maintenance of mature β-cell function. Int. J. Mol. Sci. 16, 6281–6297 10.3390/ijms16036281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rorsman P., Ashcroft F. M. (2018) Pancreatic β-cell electrical activity and insulin secretion: of mice and men. Physiol. Rev. 98, 117–214 10.1152/physrev.00008.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lam P. P., Leung Y. M., Sheu L., Ellis J., Tsushima R. G., Osborne L. R., Gaisano H. Y. (2005) Transgenic mouse overexpressing syntaxin-1A as a diabetes model. Diabetes 54, 2744–2754 10.2337/diabetes.54.9.2744 [DOI] [PubMed] [Google Scholar]
- 60.Gerdes J. M., Christou-Savina S., Xiong Y., Moede T., Moruzzi N., Karlsson-Edlund P., Leibiger B., Leibiger I. B., Östenson C. G., Beales P. L., Berggren P. O. (2014) Ciliary dysfunction impairs β-cell insulin secretion and promotes development of type 2 diabetes in rodents. Nat. Commun. 5, 5308 10.1038/ncomms6308 [DOI] [PubMed] [Google Scholar]
- 61.Yang S. N., Larsson O., Bränström R., Bertorello A. M., Leibiger B., Leibiger I. B., Moede T., Köhler M., Meister B., Berggren P. O. (1999) Syntaxin 1 interacts with the LD subtype of voltage-gated Ca2+ channels in pancreatic β cells. Proc. Natl. Acad. Sci. USA 96, 10164–10169 10.1073/pnas.96.18.10164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hoppa M. B., Collins S., Ramracheya R., Hodson L., Amisten S., Zhang Q., Johnson P., Ashcroft F. M., Rorsman P. (2009) Chronic palmitate exposure inhibits insulin secretion by dissociation of Ca2+ channels from secretory granules. Cell Metab. 10, 455–465 10.1016/j.cmet.2009.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Collins S. C., Hoppa M. B., Walker J. N., Amisten S., Abdulkader F., Bengtsson M., Fearnside J., Ramracheya R., Toye A. A., Zhang Q., Clark A., Gauguier D., Rorsman P. (2010) Progression of diet-induced diabetes in C57BL6J mice involves functional dissociation of Ca2+ channels from secretory vesicles. Diabetes 59, 1192–1201 10.2337/db09-0791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alquier T., Poitout V. (2018) Considerations and guidelines for mouse metabolic phenotyping in diabetes research. Diabetologia 61, 526–538 10.1007/s00125-017-4495-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.