Abstract
Tumor vasculature is known to be more permeable than the vasculature found in healthy tissue, which in turn can lead to a more aggressive tumor phenotype and impair drug delivery into tumors. While the stiffening of the stroma surrounding solid tumors has been reported to increase vascular permeability, the mechanism of this process remains unclear. Here, we utilize an in vitro model of tumor stiffening, ex ovo culture, and a mouse model to investigate the molecular mechanism by which matrix stiffening alters endothelial barrier function. Our data indicate that the increased endothelial permeability caused by heightened matrix stiffness can be prevented by pharmaceutical inhibition of focal adhesion kinase (FAK) both in vitro and ex ovo. Matrix stiffness–mediated FAK activation determines Src localization to cell–cell junctions, which then induces increased vascular endothelial cadherin phosphorylation both in vitro and in vivo. Endothelial cells in stiff tumors have more activated Src and higher levels of phosphorylated vascular endothelial cadherin at adherens junctions compared to endothelial cells in more compliant tumors. Altogether, our data indicate that matrix stiffness regulates endothelial barrier integrity through FAK activity, providing one mechanism by which extracellular matrix stiffness regulates endothelial barrier function. Additionally, our work also provides further evidence that FAK is a promising potential target for cancer therapy because FAK plays a critical role in the regulation of endothelial barrier integrity.—Wang, W., Lollis, E. M., Bordeleau, F., Reinhart-King, C. A. Matrix stiffness regulates vascular integrity through focal adhesion kinase activity.
Keywords: substrate compliance, endothelial barrier function, VE-cadherin
Solid tumors are typically stiffer than healthy tissue (1, 2). Heightened matrix stiffness of tumor tissue is correlated with poor prognosis (3), and it is associated with increased tumor metastatic potential and an abnormal tumor vascular phenotype (4). The tumor vasculature present in tumors tends to be more permeable compared to that found in healthy tissues (5). This increased leakiness of the tumor vessels has important consequences, notably impaired drug delivery (6). It was recently shown that increased matrix stiffness of tumor tissue disrupts vessel integrity and increases endothelial permeability (4, 7, 8). However, it is still mechanistically unclear how heightened matrix stiffness leads to increased endothelial permeability.
Endothelial adherens junctions play an important role in regulating vessel integrity and endothelial barrier function (9). Among the transmembrane adhesion proteins involved in adherens junctions, vascular endothelial cadherin (VE-cadherin) is a crucial cell–cell adhesion molecule (9, 10). VE-cadherin binds to p120-catenin (p120), β-catenin, and plakoglobin (11). This VE-cadherin complex connects adherens junctions to actin-binding proteins and signaling partners, and it influences and is influenced by the actin cytoskeleton (12). In turn, posttranslational VE-cadherin modifications are critical for adherens junction formation and regulation (9, 13–16). Both Src and focal adhesion kinase (FAK) have been reported as mediators of vascular permeability in multiple studies (17–19). Together with FAK, Src mediates tyrosine phosphorylation of VE-cadherin at Y658, Y685, and Y731, which disrupts p120 and β-catenin binding and results in decreased endothelial integrity (9, 20, 21).
Interestingly, FAK is also a key focal adhesion protein and is involved in cell mechanical sensing (22, 23). FAK phosphorylation has been correlated to tumor stiffening and progression (17). Of note, within various cancer types, FAK overexpression is linked to poor patient prognosis (24–26). When cells attach onto stiff extracellular matrix (ECM), FAK is recruited to focal adhesions and gets phosphorylated (25). FAK phosphorylation on Tyr 379 creates a high affinity binding site for Src and triggers the activation of Src (25). The FAK:Src complex phosphorylates many components of focal adhesions, resulting in the initiation of downstream signaling cascades (7, 16, 25, 27). Given the involvement of FAK and Src in endothelial barrier integrity, we hypothesized that stiffness-mediated FAK activation plays a major role in regulating endothelial integrity.
Here, we show that matrix stiffness regulates endothelial permeability through FAK activation. Stiffened matrix disrupts endothelial integrity and increases permeability, while pharmaceutical inhibition of FAK prevents the increased permeability induced by matrix stiffness both in vitro and ex ovo. In addition, stiffness-mediated FAK activation triggers Src translocation and VE-cadherin phosphorylation both in vitro and in vivo. FAK inhibition prevents Src translocation and VE-cadherin phosphorylation induced by heightened matrix stiffness. Our results support the key role for FAK in the matrix stiffness regulation of endothelial integrity through tyrosine phosphorylation of VE-cadherin.
MATERIALS AND METHODS
Antibodies and reagents
HUVECs, endothelial basal medium (EBM), EBM-Plus, and endothelium growth medium (EGM) SingleQuot Kit were purchased from Lonza (Basel, Switzerland). Antibodies used in Western blot analysis were as follows: phospho-FAK (Tyr397) antibody (3283S; Cell Signaling Technology, Danvers, MA, USA), FAK antibody (3285S; Cell Signaling Technology), phospho-Src family (Tyr416) antibody (2101S; Cell Signaling Technology), Src antibody (2108S; Cell Signaling Technology), β-catenin antibody (SC-7199; Santa Cruz Biotechnology, Dallas, TX, USA), anti–glyceraldehyde-3-phosphate dehydrogenase antibody (GAPDH, MAB374; MilliporeSigma, Burlington, MA, USA), and anti-GAPDH (Poly6314; BioLegend, San Diego, CA, USA). Primary and secondary antibodies used in immunofluorescence staining were as follows: VE-cadherin antibody (SC-9989; Santa Cruz Biotechnology), phospho-VE-cadherin (Tyr685) antibody (ABT1760; MilliporeSigma), β-catenin antibody (SC-7199; Santa Cruz Biotechnology), preconjugated anti-Src (phospho Y418) antibody (Alexa Fluor 488, ab200824; Abcam, Cambridge, United Kingdom), rat monoclonal VE-cadherin antibody (eBioBV13; Thermo Fisher Scientific, Waltham, MA, USA), phospho-FAK (Tyr397) antibody (44-624G; Thermo Fisher Scientific), Alexa Fluor 488 goat anti-mouse antibody (A11029; Invitrogen; Thermo Fisher Scientific), Alexa Fluor 568 donkey anti-rabbit antibody (A10042; Thermo Fisher Scientific), Alexa Fluor 594 donkey anti-rat antibody (A21209; Thermo Fisher Scientific), and Alexa Fluor 488 goat anti-rabbit antibody (A11008; Thermo Fisher Scientific). FAK inhibitor (PF573228) was purchased from MilliporeSigma. Acrylamide [40% (w/v) solution], bis-acrylamide [2% (w/v) solution], ammonium persulfate, and tetramethylethylenediamine were purchased from Bio-Rad (Hercules, CA, USA). All other reagents used were purchased from Thermo Fisher Scientific.
Cell culture
HUVECs between p3 and p5 were cultured in EBM supplemented with the EGM SingleQuot Kit, EBM-Plus with EGM SingleQuot Kit, and 10 mM GlutaMax (Thermo Fisher Scientific). Medium was replaced every 48 h. Cells were maintained in a 37°C humidified atmosphere of 5% (v/v) CO2 in air. Subculture was conducted using trypsin and trypsin neutralizer before 100% confluence.
Collagen-coated polyacrylamide gels
Polyacrylamide (PA) hydrogel substrates were prepared as previously described (7, 28). PA gels with different stiffness (E = 2.5 or 10 kPa) were synthesized by mixing varying amount of acrylamide [40% (w/v) solution] and bis-acrylamide [2% (w/v) solution] in Milli-Q water (MilliporeSigma) with HEPES and tetramethylethylenediamine (TEMED; Bio-Rad) at pH 6. Ammonium persulfate dissolved in Milli-Q water was added to the solution to initiate polymerization. Substrates were functionalized with N-6-[(acryloyl)amido]hexanoic acid, succinimidyl ester, and subsequently covalently bound to 0.1 mg/ml type I rat tail collagen (Corning, Corning, NY, USA). Unreacted N-6-[(acryloyl)amido]hexanoic acid, succinimidyl ester was quenched using 1:1000 ethanolamine in 50 mM HEPES solution at pH 8. PA gels were washed in PBS and stored at 4°C in a PBS solution containing 4% penicillin/streptomycin until cell seeding.
Monolayer permeability assay
Square glass coverslips coated with PA gels functionalized with collagen were moved to a sterile laminar flow hood, placed in wells of uncovered 6-well plates containing sterile PBS, and exposed to UV light for 1 h. Each PA gel was seeded with 2 ml of HUVECs suspended in M199 medium supplied with EGM SingleQuot Kit prepared at a concentration of 50,000 to 100,000 cells/ml. HUVECs were allowed to form confluent monolayers over a period of 5 to 7 d, with the medium replaced every 48 h. Addition of pharmacologic treatments and permeability assays were performed 2 d after cells formed a confluent monolayer. For the dose–response curve, EGM containing PF573228 at 50, 100, 200, or 400 nM or DMSO were added 3 h before the assay. In other cases, EGM containing 200 nM PF573228 or DMSO was added 3 h before the assay. FITC-dextran solution (0.4 mg/ml 40 kDa) was prepared in medium. For each seeded PA gel, 4 ml of this solution was added to a MatTek glass-bottomed dish, all of which were stored in an incubator at 37°C at 5% CO2. Seeded PA gels were transferred to filled MatTek (Ashland, MA, USA) dishes and visualized using a Zeiss LSM800 microscope equipped with a Plan-Apochromat ×20/0.8 objective (Carl Zeiss GmbH, Jena, Germany). Five minutes after submersion in FITC-dextran solution, 8 vertical line scans were captured on each gel spanning from 200 μm below the monolayer to 200 μm above the monolayer (n = 28). Relative permeability of HUVEC monolayers was quantified by calculating the normalized intensity ratio (NIR) of each sample. Using ImageJ software (Image Processing and Analysis in Java; National Institutes of Health, Bethesda, MD, USA; https://imagej.nih.gov/ij/), 2 rectangular boxes with the same size were drawn directly above and below the HUVEC monolayer. The average signal intensity within these 2 boxes was measured separately. The signal intensity measured from the top box represented the relative concentration of FITC-dextran in solution, and the intensity measured from the bottom box represented the relative concentration of FITC-dextran that had permeated through the cell monolayer into the gel. The intensity ratio was calculated by dividing the average intensity within the gel by the average intensity above the gel. To produce NIR values, the intensity ratio values of seeded PA gels were divided by the intensity ratio values of unseeded control PA gels. NIR values of each sample were calculated by averaging the NIR of 8 vertical line scans that were captured on the same gel, as previously described by our lab (7).
Chicken embryo culture
White Leghorn chicken eggs supplied by the Cornell poultry farm were cleaned of debris using a dry cloth and stored in a wine cooler at 12.8°C on embryonic day (ED) 0 before transfer to a GQF 1500 Professional rocking egg incubator maintained at 37.8°C, 60% relative humidity. On ED3, eggs were cracked and plied open within a laminar flow cabinet using a C-clamp and a hacksaw. Egg contents were transferred into ex ovo culture platforms, which were constructed by slinging a single layer of plastic wrap over a 5 oz Fineline Savvi Serve cup partially filled with warm water. A small scoop of autoclaved ground eggshell was distributed around the albumin, and culture platforms were capped with a Petri dish lid. Chicks within culture platforms were incubated in Hova-Bator circulated air incubators (GQF, Savannah, GA, USA) maintained at 37.8°C, with 55% relative humidity.
Collagen construct formation and grafting
The collagen solutions described above were infused with recombinant human VEGF 165 protein (R&D Systems, Minneapolis, MN, USA) and basic fibroblast growth factor (PeproTech, Rocky Hill, NJ, USA) to generate concentrations of 5 and 16.7 μg/ml, respectively, which have previously been shown to induce angiogenic ingrowth into collagen gels (29). Thirty microliters of collagen was polymerized for 30 min via incubation at 37°C, 5% CO2, onto 5-mm autoclaved nylon mesh squares. The collagen stiffness was modulated using glycation to produce collagen gels of similar density and pore size at all stiffnesses tested (30). Constructs were placed on the chick chorioallantoic membrane of ED10 chicken embryos in a laminar flow cabinet and immediately returned to incubators.
Ex ovo neovessel permeability assay
On ED15, chicken embryos cultured ex ovo were transferred from hammock-style platforms to Petri dishes. Proximal vessels were injected with 100 μl of dye solution containing 2.5 mg/ml 65–85 kDa tetramethylrhodamine (TRITC)-dextran (T1162; MilliporeSigma) and 2.5 mg/ml 2 MDa FITC-dextran (FD2000S; MilliporeSigma) in PBS. One hundred nanomoles of PF573228 was applied to collagen constructs 24 h before data collection. Neovessels within collagen constructs were visualized using 2-photon microscopy on a Zeiss LSM 880 upright equipped with an EC Plan-Neofluar ×10/0.3 objective and a Mai Tai laser (Spectra-Physics, Santa Clara, CA, USA) with a wavelength of 900 nm. Fluorescent z stacks of neovessels, which were identified using the FITC-dextran, were taken approximately every 15 min for 1 h (ntotal = 86). The method for quantification of neovessel permeability was adapted from others (4). Briefly, the fluorescent intensity of the TRITC signal across a 20-pixel-thick line perpendicular to the neovessel of interest at each time point was plotted. Intensity values were normalized to the peak value per plot, and peaks were aligned across all time points. The integrals under each peak were calculated and plotted as a function of time. Neovessel permeability was defined as the rate of increase of the integral of the normalized fluorescent intensity signal.
Western blot analysis
HUVECs were seeded on PA substrates with 2.5 or 10 kPa stiffness at 100,000 cells/ml and allowed to grow for 5 to 7 d to reach confluence. Pharmacologic treatments and cell lysis were performed 2 d after cells made a confluent monolayer. For dose–response curve, cells were treated with EGM containing PF573228 of varying concentrations or DMSO for 3 h before cell lysis. In other cases, cells were treated with EGM containing 200 nM PF573228 (31) to inhibit FAK activation or the same amount of DMSO as vehicle control for 3 h before the lysis. Confluent HUVECs on substrates with different stiffnesses and treated with or without PF573228 were lysed with Laemmli buffer and stored at −80°C until use. The protein concentration of samples was measured with the DC Assay Kit (Bio-Rad) and subjected to gel electrophoresis [8% (w/v) acrylamide gel] and Western blot analysis (n = 7 for dose–response study, n = 6 for other study). Membranes were blocked with either 5% milk or 5% bovine serum albumin (MilliporeSigma) in Tris-buffered saline. Primary antibodies were prepared at 1:40,000 dilution in 5% milk in the case of GAPDH MAB374 antibody, or at 1:1000 dilution in 5% bovine serum albumin in all other cases. Secondary antibodies conjugated to horseradish peroxidase were prepared at 1:2000 dilution. Membranes were imaged with West Pico, Dura, or Femto (Thermo Fisher Scientific) per their respective protocols, using an ImageQuant LAS-4000 system.
VE-cadherin junction gap width measurement
Two days after confluence, endothelial monolayers on PA gels of different stiffnesses and treated with 200 nM PF573228 or DMSO were fixed and permeabilized with 4% paraformaldehyde and 1% Triton X-100 (J. T. Baker, Center Valley, PA, USA) in PBS, respectively. VE-cadherin was stained using a mouse monoclonal VE-cadherin primary antibody (SC 9989; Santa Cruz Biotechnology) at 1:100 dilution and Alexa Fluor 488 goat anti-mouse antibody (A11029; Thermo Fisher Scientific) at 1:200 dilution. Fluorescent images were acquired on a Zeiss Axio Observer.Z1m microscope equipped with a Hamamatsu Orca-ER camera using a Plan-Apochromat ×20/0.8 objective. Adherens junction width was quantified using ImageJ software and a custom-written MatLab (MathWorks, Natick, MA, USA) algorithm as previously described (7).
Immunofluorescence
HUVECs and FVB/N-Tg(MMTV-PyVT)634Mul/J (MMTV-PyMT) mouse tumor sections were fixed with 4% (v/v) paraformaldehyde in PBS for 10 min at room temperature. After fixation, samples were washed with PBS, permeabilized with 1% (v/v) Triton X-100 (J. T. Baker) in PBS, and blocked with 10% (v/v) fetal bovine serum (FBS) and 5% (v/v) goat serum. VE-cadherin was stained as an endothelial adherens junction marker. Cells were stained with primary antibodies at 1:100 diluted in PBS with 10% (v/v) FBS and 5% (v/v) goat serum overnight at 4°C, washed with PBS, and incubated with secondary antibodies at 1:200 diluted in PBS with 10% (v/v) FBS for 1 h at room temperature in the dark. In cases of phosphorylated Src (p-Src) staining, cells were stained with VE-cadherin primary antibody (SC-9989 for HUVECs, eBioBV13 for mouse tumors) at 1:100 diluted in PBS with 10% (v/v) FBS and 5% (v/v) goat serum overnight at 4°C, washed with PBS, incubated with secondary antibody (A11031 for HUVECs, A21209 for mouse tumors) at 1:200 diluted in PBS with 10% (v/v) FBS for 1 h at room temperature in the dark, then stained with anti-Src (phospho Y418) antibody (Alexa Fluor 488) at 1:50 diluted in PBS with 10% (v/v) FBS overnight at 4°C in the dark. Immunofluorescent images were taken with a Zeiss LSM700 microscope using a ×40/1.1 NA water immersion objective and 488 and 568 nm excitation laser lines. MMTV-PyMT mouse tissue samples were imaged with the same microscope and z-stack imaging. Protein colocalization analysis was performed with a customized Matlab code as previously described (7).
MMTV-PyMT transgenic mice studies
All mice were maintained following a protocol approved by the Cornell University Institutional Animal Care and Use Committee. Female MMTV-PyMT transgenic mice on the FVB strain background (obtained from The Jackson Laboratory, Bar Harbor, ME, USA) were treated with β-aminopropionitrile (BAPN; MilliporeSigma) at 3 mg/kg body weight in drinking water (n = 3) beginning at 4 wk of age (4). BAPN is an inhibitor of the matrix cross-linking enzyme lysyl oxidase. BAPN treatment inhibits lysyl oxidase activity, which results in more compliant tumors compared to those in untreated mice (32–34). Mice between 10 and 12 wk old were humanely killed by CO2 asphyxiation and necropsied. Mammary tumors were collected and snap frozen in liquid nitrogen. Frozen mouse tumor samples were sectioned at 15 μm thickness and kept at −80°C until fixation and staining.
Statistical analysis
Statistical analysis was performed with GraphPad Prism 7.0a (GraphPad Software, La Jolla, CA, USA). All data are presented as means ± sem. Parametric 1- or 2-way ANOVA followed by Tukey’s post hoc test were used where appropriate. A value of P < 0.05 was considered statistically significant.
RESULTS
Matrix stiffness alters FAK activation and regulates endothelial permeability both in vitro and ex ovo
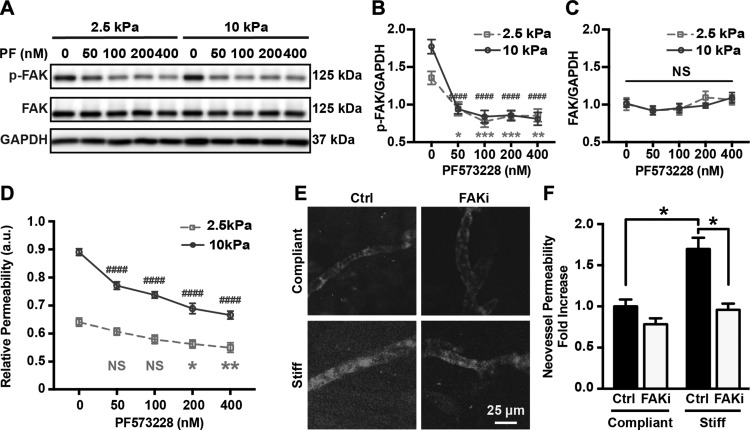
Our prior work showed that matrix stiffening increases endothelial permeability (4). Because matrix stiffening has also been reported to up-regulate FAK activation in both endothelial cells and tumor cells (7, 17, 19), we thus investigated the effect of FAK activity on matrix stiffness–mediated endothelial permeability. The FAK activation and overall expression in HUVEC monolayer were measured with Western blot analysis. Endothelial permeability was addressed with 2-dimensional permeability assays, which were performed based on diffusion of FITC-dextran through the monolayer. PF573228, a small molecule inhibitor of phosphorylation of FAK at Tyr397 currently in preclinical trial, was utilized (35). We tested the effect of PF573228 treatment by generating a dose–response curve showing changes in FAK phosphorylation in confluent HUVECs on PA gel substrate with different stiffnesses treated with DSMO or PF573228 at 50, 100, 200, and 400 nM. We observed that PF573228 treatment inhibited FAK activation but not its expression in HUVECs in a dose-dependent manner (Fig. 1A–C). We then performed permeability assays, where permeability of a monolayer on a 2-dimensional PA system is measured on the basis of diffusion of FITC-dextran through the monolayer, to investigate how stiffness-mediated FAK activation was related to endothelial permeability. As expected, HUVEC monolayers on stiff substrates (E = 10 kPa) exhibited higher permeability compared to monolayers on more compliant substrates (E = 2.5 kPa) (Fig. 1D). Notably, FAK inhibition leads to significantly less dye leaking through the monolayers (Fig. 1D) at all concentrations of PF573228 tested on stiff substrates and above 100 nM on compliant substrates.
Figure 1.
FAK activity regulates matrix stiffness–mediated endothelial permeability both in vitro and ex ovo. A) Protein bands generated by Western blot showing p-FAK and FAK expression of HUVEC monolayer seeded on compliant or stiff PA gel substrate and treated with PF573228 with various concentrations. GAPDH was utilized as loading control; 2.5 and 10 kPa indicate stiffness of PA gel substrate. Ctrl indicates samples treated with vehicle solution; PF, samples treated with FAK inhibitor PF573228. B) Corresponding quantification of FAK phosphorylation normalized over GAPDH expression (n = 7). C) Corresponding quantification of overall FAK expression normalized against GAPDH (n = 7). D) Relative permeability of HUVEC monolayers seeded on 2.5 and 10 kPa PA gels. E) Fluorescent images of ex ovo neovessels in cross-linked collagen gels glycated with 0 (compliant) or 100 mM (stiff) ribose 1 h after injection of permeable TRITC dye and with or without topical PF573228 (100 nM) application. F) Corresponding quantification of neovessel permeability as function of collagen cross-linking and FAK inhibition. Data are presented as means ± se. NS, no statistical difference. *2.5 kPa groups; #10 kPa groups. *P < 0.05, **P = 0.0015, ***P = 0.0001, ####P < 0.0001.
To further test how matrix stiffness influences endothelial permeability, we expanded our analysis to an ex ovo system using glycated collagen matrix to increase stiffness (4). The glycated collagen matrices have similar density and pore size but different stiffness based on the extent of glycation as previously described (30). PF573228 or vehicle solution was applied to cell-seeded collagen constructs ex ovo 24 h before data collection. PF573228 treatment reduced dye extravasation from neovessels within stiff constructs to levels exhibited by those within compliant constructs, but had no significant effect on the permeability of neovessels within compliant matrices (Fig. 1E, F), similar to our results found in vitro (Fig. 1D). Inhibition of FAK activity restores endothelial barrier integrity of HUVECs induced by stiffer matrices to the level of the compliant conditions. These results suggest that matrix stiffness mediates endothelial permeability through FAK activation.
Matrix stiffening–induced FAK activation regulates cell–cell junction size
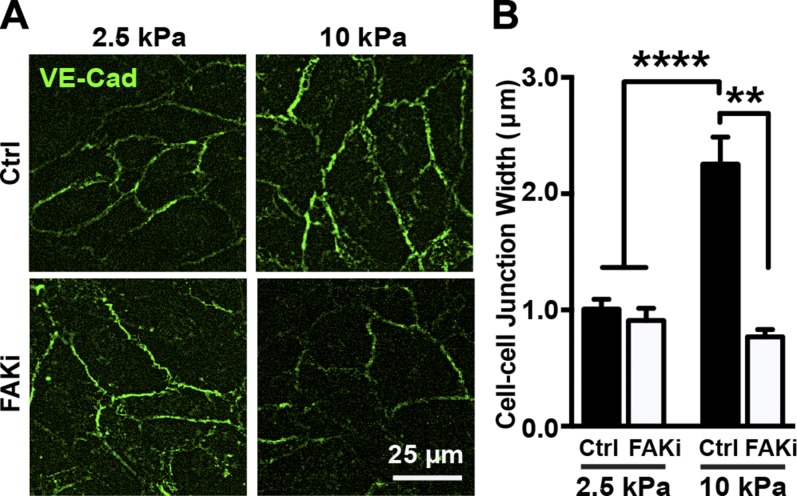
Noting that VE-cadherin is critical for adherens junction integrity and endothelial barrier function (9), we extended our investigation to endothelial junction integrity as a function of stiffness in response to FAK inhibition. Endothelial monolayers on PA gel substrates of 2.5 or 10 kPa were treated with PF573228 or the vehicle control, DMSO, and stained for VE-cadherin (Fig. 2A). While adherens junction width increased as a function of substrate stiffness, PF573228 treatment was able to significantly decrease the junction width observed on the 10 kPa substrates (Fig. 2). These data suggest that matrix stiffening–induced FAK activation is involved in regulation of endothelial barrier integrity at cell–cell junctions.
Figure 2.
FAK activation regulates matrix stiffness–mediated cell–cell junction integrity. A) Representative fluorescence images of VE-cadherin (VE-Cad)-stained endothelial cell–cell junction of endothelial cell monolayers on compliant (2.5 kPa) and stiff (10 kPa) substrate treated with (FAKi) or without [control (Ctrl)] PF573228. B) Corresponding quantification of cell–cell junction width as function of substrate stiffness and FAK inhibition (n = 3; 180–200 junctions included per condition in each experiment). Data are presented as means ± se. NS, no statistical difference. ****P < 0.0001, **P = 0.0015.
FAK activation is involved in matrix stiffness regulation of VE-cadherin phosphorylation and adherens junction architecture
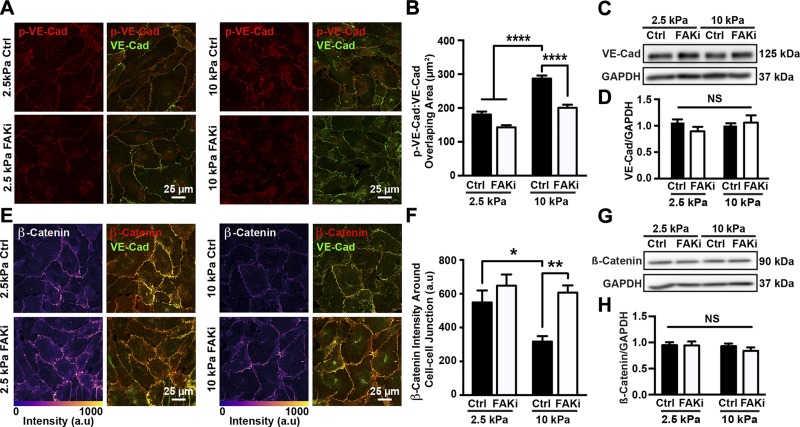
Phosphorylation of VE-cadherin has been shown to down-regulate endothelial integrity by either disrupting p120 and β-catenin binding or triggering VE-cadherin internalization (9, 20, 21). Noting that matrix stiffening disrupts endothelial integrity and increases permeability, we investigated whether matrix stiffening influenced VE-cadherin phosphorylation and adherens junction architecture in a FAK-dependent manner. HUVEC monolayers were seeded on 2.5 and 10 kPa substrates and treated with PF573228 or the vehicle control, DMSO. Cells were stained for phosphorylated VE-cadherin and VE-cadherin as an adherens junction marker (Fig. 3A). Quantification of the overlapping area between the phosphorylated form and total VE-cadherin showed that HUVECs on stiff substrates presented adherens junctions that are richer in phosphorylated VE-cadherin compared to cells on more compliant substrates (Fig. 3B). In contrast, the amount of phosphorylated VE-cadherin present in adherens junctions decreased when cells were treated with PF573228 (Fig. 3B). At the same time, neither substrate stiffness nor PF573228 treatment influenced VE-cadherin expression (Fig. 3C, D). On that basis, we tested whether this aggregation of phosphorylated VE-cadherin induces changes in the presence of the adherens junction–associated protein β-catenin. HUVEC monolayers seeded on PA gel substrates of different stiffness were treated with PF573228 or the vehicle solution, DMSO, and stained for VE-cadherin and β-catenin (Fig. 3E). The amount of β-catenin around adherens junctions was quantified. HUVECs on stiff substrates showed less β-catenin around adherens junctions compared to cells on soft substrates (Fig. 3F). When cells were treated with PF573228, more β-catenin was observed around junctions (Fig. 3F), but the overall expression of β-catenin was not influenced by substrate stiffness or FAK inhibition (Fig. 3G, H). These results suggest that matrix stiffness, through FAK activity, regulates phosphorylation of VE-cadherin and adherens junction architecture.
Figure 3.
Matrix stiffness regulates VE-cadherin phosphorylation and adherens junction architecture through FAK activation. A) Representative confocal microscopy images of HUVECs seeded on compliant (2.5 kPa) or stiff (10 kPa) substrates, treated with (FAKi) or without (Ctrl) PF573228. Cells were stained for VE-cadherin (VE-Cad) and phosphorylated VE-cadherin (p-VE-Cad). B) Corresponding quantification of overlapping area of phosphorylated VE-cadherin and VE-cadherin showing that matrix stiffening increased amount of phosphorylated VE-cadherin around adherens junctions, while FAK inhibition restored phosphorylated VE-cadherin to levels on compliant substrates (n = 3; 25–35 cells were included in each condition for each experiment). C) Protein bands generated by Western blot showing VE-cadherin. GAPDH was utilized as loading control; 2.5 and 10 kPa indicate stiffness of PA gel substrate. Ctrl indicates samples treated with vehicle solution; FAKi, samples treated with FAK inhibitor PF573228. D) Corresponding quantification of VE-cadherin normalized over GAPDH (n = 6). E) Confocal microscopy images showing HUVECs seeded on compliant (2.5 kPa) or stiff (10 kPa) PA gel substrates, treated with or without PF573228. Cells were stained for VE-cadherin and β-catenin. F) Corresponding quantification of β-catenin at adherens junctions showing increased substrate stiffness increased amount of β-catenin around junctions (n = 3; 20–30 cells were included in each condition during each experiment). G) Protein bands generated with Western blot showing β-catenin expression. H) Corresponding quantification of β-catenin normalized over GAPDH (n = 6). Data are presented as means ± se. NS, no statistical difference. ****P < 0.0001, **P = 0.0011, *P < 0.005.
Matrix stiffness regulates endothelial integrity through modulating Src translocation to adherens junctions
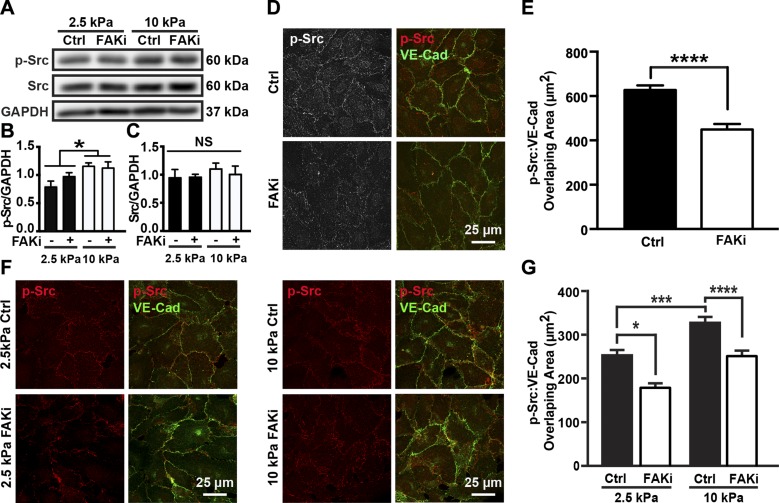
Previous knockout and pharmacologic inhibition studies show that Src activation is important in VE-cadherin phosphorylation regulation (36, 37). While FAK inhibition decreased VE-cadherin phosphorylation induced by heightened matrix stiffness (Fig. 3A, B), it did not alter stiffness-mediated Src activation or Src expression (Fig. 4A–C). Other than through overall activation of Src, Src can also regulate cell behaviors, like the formation of new focal adhesions (38), through translocation (39). As such, we addressed whether Src translocation is affected by FAK inhibition. HUVEC monolayers seeded on a glass slide were treated with PF573228 or DMSO and stained for p-Src (Fig. 4D). HUVECs treated with PF573228 showed less p-Src at the adherens junctions compared to the control group (Fig. 4D, E). We then investigated whether increased matrix stiffness also affected Src targeting to adherens junctions and whether it involved FAK activity. We observed that HUVEC monolayers on stiff substrates had more p-Src around cell–cell junctions. However, when cells were treated with PF573228, the amount of p-Src present at adherens junctions decreased to a level comparable to cells on compliant substrates (Fig. 4F, G). Together, these results indicate that stiffness regulates endothelial integrity through mediating Src translocation in a FAK-dependent manner.
Figure 4.
FAK activation regulates matrix stiffness–mediated Src translocation but not Src activation. A) Protein bands generated by Western blot showing p-Src and Src from cells seeded on compliant (2.5 kPa) or stiff (10 kPa) substrates, treated with (FAKi) or without [control (Ctrl)] PF573228. GAPDH was utilized as loading control. B) Corresponding quantification of Src phosphorylation normalized to loading control, GAPDH (n = 6). C) Corresponding quantification of overall Src expression normalized to GAPDH (n = 6). D) Confocal microscopy images showing HUVECs seeded on collagen-coated glass slides treated with or without PF573228. E) Corresponding quantification of overlapping area of p-Src and VE-cadherin (VE-Cad) showing FAK inhibition decreased amount of p-Src around adherens junctions (n = 3; 20–30 imaging fields included in each condition for each experiment). F) Confocal microscopy images showing HUVECs seeded on compliant (2.5 kPa) or stiff (10 kPa) PA gel substrates, treated with or without PF573228. Cells were stained for VE-cadherin and p-Src. G) Corresponding quantification of overlapping area of p-Src and VE-cadherin showing that substrate stiffness increased amount of p-Src around junctions (n = 3; 20–30 imaging fields included in each condition during each experiment). Data are presented as means ± se. ****P < 0.0001, *P < 0.005.
Matrix stiffness regulates VE-cadherin phosphorylation through translocation of p-Src in vivo
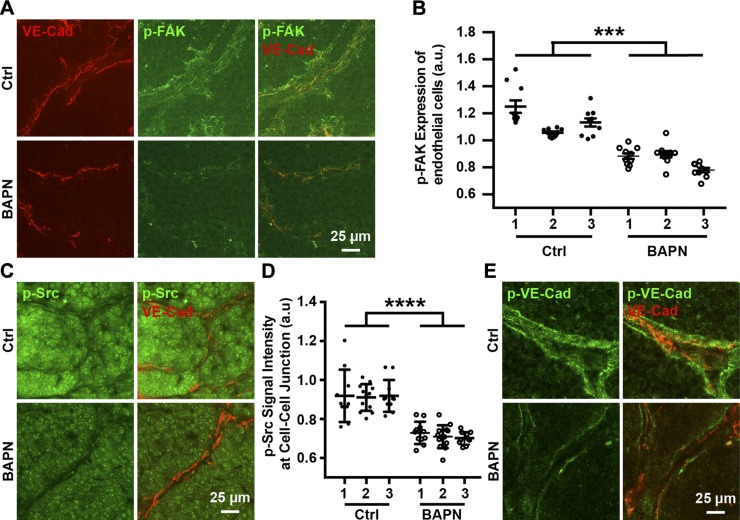
Noting that matrix stiffening increases FAK activation and endothelial permeability in vivo (4, 25), we extended our analysis of adherens junctions to the MMTV-PyMT breast tumor mouse model. Compliant tumors were obtained by treating mice with BAPN, a commonly used inhibitor of the matrix cross-linking enzyme lysyl oxidase involved in tumor ECM stiffening (32–34). Consistent with data previously published by other groups (4, 32), endothelial cells within tumor sections from MMTV-PyMT mice treated with BAPN had lower FAK activation due to decreased stiffness compared to the control group (Fig. 5A, B). Imaging of histologic tumor sections revealed overall higher Src activation in stiff tumors (Fig. 5C). Quantification of p-Src intensity around VE-cadherin–positive areas indicated that stiff tumors have more p-Src around endothelial adherens junctions (Fig. 5D; n = 3, each tumor includes 10–15 imaging fields), which is consistent with our in vitro data. Similarly, stiff tumors showed higher levels of phosphorylated VE-cadherin around adherens junctions compared to compliant tumors (Fig. 5E). Together, these results indicate that matrix stiffness–mediated Src targeting at adherens junctions and subsequent phosphorylation of VE-cadherin are also involved in the regulation of endothelial barrier function in vivo.
Figure 5.
Matrix stiffness regulates endothelial integrity in vivo. A) Confocal microscopy images showing tumor sections of MMTV-PyMT mice treated with or without (Ctrl) BAPN. Tumor sections were stained for p-Src and VE-cadherin (VE-Cad). B) Corresponding quantification of p-Src signal around endothelial adherens junctions showing matrix stiffening increased amount of p-Src around adherens junctions (n = 3; 10–15 imaging fields included in each condition for each experiment). C) Confocal microscopy images showing p-FAK expression of tumor sections derived from MMTV-PyMT mice with or without BAPN treatment. D) Corresponding quantification of p-FAK expression of tumor endothelial cells showing endothelial cells of stiffer tumors maintain higher FAK activation. E) Confocal microscopy images showing tumor sections of MMTV-PyMT mice treated with or without BAPN. Tumor sections were stained for VE-cadherin and phosphorylated VE-cadherin (n = 3; 10–15 imaging fields included in each condition during each experiment). Data are presented as means ± se. ****P < 0.0001.
DISCUSSION
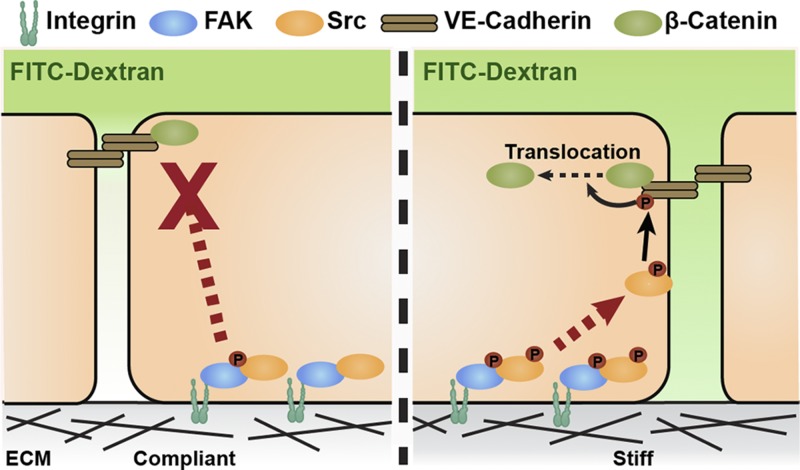
Matrix stiffening that accompanies the progression of tumor development has been shown to disrupt endothelial integrity and regulate permeability (4, 7, 8); however, the mechanism of how matrix stiffness regulates endothelial integrity is still relatively unclear. Here, we demonstrate that matrix stiffness regulates endothelial integrity through FAK activity. Our results show that pharmaceutical inhibition of FAK activity can prevent matrix stiffness–mediated disruption of endothelial integrity and can decrease permeability both in vitro and ex ovo. Src translocation toward adherens junctions, rather than its overall activation level, regulates adherens junction integrity. We observe that stiffness-mediated FAK activation causes Src translocation to adherens junctions and stimulates VE-cadherin phosphorylation both in vitro and in vivo. FAK inhibition prevented matrix stiffening–induced Src translocation and VE-cadherin phosphorylation (Fig. 6). Our results support our prior data showing that matrix stiffness affects endothelial integrity and also highlights the key role of FAK activity in this regulation (4).
Figure 6.
Proposed mechanism of matrix stiffness–mediated FAK activation regulation of endothelial barrier function. Schematic showing involvement of matrix stiffness–mediated FAK activity in regulation of endothelial barrier function. Compared to cells on compliant ECM (left), cells on stiff ECM (right) have heightened endothelial FAK phosphorylation, promoting p-Src translocation to cell–cell junctions (red arrow), and increased VE-cadherin phosphorylation, which triggers dissociation of β-catenin. These cellular events further widen cell–cell junction, disrupts endothelial integrity, and induce increased permeability (shown as FITC-dextran diffusion gradient).
We have previously shown that matrix stiffening induces higher cell contractility, which results in physical separation of adherens junctions and increased permeability (7). Cells sense the matrix stiffness mainly through focal adhesions (40), which are integrin-rich sites that physically interact with ECM (41). FAK is a major component of focal adhesions, and its activation is known to be sensitive to matrix stiffness (22). Integrin clustering around focal adhesions stimulates FAK autophosphorylation on Tyr397 and binding of Src family proteins, which promotes formation of an activated FAK-Src complex and Src-mediated phosphorylation of the FAK kinase domain activation loop (on Y576 and Y577). Src that is recruited to activated FAK can then initiate downstream signaling (25). FAK inhibition prevents its autophosphorylation (35), which would prevent Src binding and initiation of downstream signaling. Because both FAK and Src are important regulators of cell contractility (42), matrix stiffness–triggered FAK and Src activation is one potential downstream pathway that could up-regulate contractility, resulting in wider adherens junctions and increased permeability. Interestingly, while our current results show increased Src activation with stiffness, FAK inhibition only affected Src targeting to adherens junctions. This suggests that while cell contractility may certainly play a critical role in promoting endothelial permeability, other signaling events are occurring to destabilize adherens junctions.
VE-cadherin internalization controls permeability both in vitro and in vivo (43). VE-cadherin phosphorylation disrupts the binding of p120 and β-catenin, resulting in junction disassembly and a decrease in endothelial integrity and increased permeability (9, 20). Our results show that matrix stiffening decreases the amount of β-catenin at adherens junctions while FAK inhibition restored this decrease to levels comparable to cells on compliant substrates. Additionally, Tyr685 residue of VE-cadherin was identified as a unique target site for Src in vitro (44), and phosphorylation of VE-cadherin at Y685 is associated with enhanced VE-cadherin internalization (45). However, other studies have shown that Src-mediated phosphorylation of VE-cadherin was not sufficient to lower endothelium barrier function (46). Interestingly, our recent results indicate that matrix stiffness and cell contractility also influence endocytosis and downstream signaling of the transmembrane receptor vascular endothelial growth factor receptor 2 (47). However, VEGF stimulation induces Src-mediated phosphorylation of VE-cadherin and its subsequent internalization (48), suggesting the possible involvement of growth factors in the stiffness-mediated behavior we observe here. Together, these observations suggest that matrix stiffness, together with FAK, may also modulate endothelial permeability through regulating VE-cadherin internalization.
Our in vivo data indicate that endothelial cells in stiffer tumors maintain overall higher FAK activation, more p-Src around cell–cell junctions, and higher VE-cadherin phosphorylation around cell–cell junctions. Previous studies have also shown that increased FAK activity is correlated to tumor progression and plays critical roles in cancer cell growth, survival, motility, transformation, and angiogenesis. As such, it has been considered a potential therapeutic target for over a decade (17, 49). Over a dozen FAK inhibitors, many of which disrupt phosphorylation and some of which disrupt scaffolding function, have been developed and are currently undergoing clinical trials (49). For example, PF562261, an analog of PF573228, has already passed a phase 1 clinical trial, and it has even been shown to be capable of preventing VE-cadherin phosphorylation, resulting in improved endothelial-cell barrier function (16, 50). Our data show that inhibition of FAK activity can prevent the disruption of endothelial integrity and barrier function caused by matrix stiffening. While matrix stiffening increases endothelial permeability both in vitro and ex ovo, inhibiting FAK activity helped restore permeability to similar levels of that measured from compliant matrix. These data suggest that FAK inhibition may potentially be helpful in restoring vascular integrity and repairing impaired drug delivery in tumor tissue. Additionally, reestablishing endothelial integrity could also be a good target to prevent tumor metastasis. A recent study showed that Src was activated in endothelial cells within minutes of the contact with metastatic melanoma cells (51). Src activation then initiates downstream VE-cadherin phosphorylation, followed by endothelial integrity disruption (51). Inhibition of endothelial FAK activity has also been show to prevent tumor metastasis by enhancing endothelial integrity (16). Given that endothelial integrity plays such a key role in tumor progression, our work also provides an evidence indicating that FAK inhibitors may have greater utility in cancer therapy than previously realized.
ACKNOWLEDGMENTS
The authors gratefully acknowledge support from the U.S. National Institutes of Health (NIH) National Heart, Lung, and Blood Institute (R01HL127499 to C.A.R.-K.), the Scholarship for the Next Generation of Scientists from the Cancer Research Society, and the NIH National Cancer Institute (K99CA212270 to F.B.). The authors declare no conflicts of interest.
Glossary
- BAPN
β-aminopropionitrile
- EBM
endothelial basal medium
- ECM
extracellular matrix
- ED
embryonic day
- EGM
endothelium growth medium
- FAK
focal adhesion kinase
- FBS
fetal bovine serum
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- MMTV-PyMT
FVB/N-Tg(MMTV-PyVT)634Mul/J
- NIR
normalized intensity ratio
- PA
polyacrylamide
- p-Src
phosphorylated Src
- TRITC
tetramethylrhodamine
- VE-cadherin
vascular endothelial cadherin
AUTHOR CONTRIBUTIONS
W. Wang, E. M. Lollis, F. Bordeleau, and C. A. Reinhart-King designed research; W. Wang and E. M. Lollis performed the experiments; W. Wang and E. M. Lollis analyzed data; F. Bordeleau and C. A. Reinhart-King aided in experimental design; and all authors contributed to writing the report.
REFERENCES
- 1.Kim J. H., Asthagiri A. R. (2011) Matrix stiffening sensitizes epithelial cells to EGF and enables the loss of contact inhibition of proliferation. J. Cell Sci. 124, 1280–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bordeleau F., Alcoser T. A., Reinhart-King C. A. (2014) Physical biology in cancer. 5. The rocky road of metastasis: the role of cytoskeletal mechanics in cell migratory response to 3D matrix topography. Am. J. Physiol. Cell Physiol. 306, C110–C120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maskarinec G., Pagano I. S., Little M. A., Conroy S. M., Park S. Y., Kolonel L. N. (2013) Mammographic density as a predictor of breast cancer survival: the multiethnic cohort. Breast Cancer Res. 15, R7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bordeleau F., Mason B. N., Lollis E. M., Mazzola M., Zanotelli M. R., Somasegar S., Califano J. P., Montague C., LaValley D. J., Huynh J., Mencia-Trinchant N., Negrón Abril Y. L., Hassane D. C., Bonassar L. J., Butcher J. T., Weiss R. S., Reinhart-King C. A. (2017) Matrix stiffening promotes a tumor vasculature phenotype. Proc. Natl. Acad. Sci. USA 114, 492–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Azzi S., Hebda J. K., Gavard J. (2013) Vascular permeability and drug delivery in cancers. Front. Oncol. 3, 211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maeda H., Wu J., Sawa T., Matsumura Y., Hori K. (2000) Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J. Control. Release 65, 271–284 [DOI] [PubMed] [Google Scholar]
- 7.Huynh J., Nishimura N., Rana K., Peloquin J. M., Califano J. P., Montague C. R., King M. R., Schaffer C. B., Reinhart-King C. A. (2011) Age-related intimal stiffening enhances endothelial permeability and leukocyte transmigration. Sci. Transl. Med. 3, 112ra122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Califano J. P., Reinhart-King C. A. (2008) A balance of substrate mechanics and matrix chemistry regulates endothelial cell network assembly. Cell. Mol. Bioeng. 1, 122–132 [Google Scholar]
- 9.Dejana E., Orsenigo F., Lampugnani M. G. (2008) The role of adherens junctions and VE-cadherin in the control of vascular permeability. J. Cell Sci. 121, 2115–2122 [DOI] [PubMed] [Google Scholar]
- 10.Dejana E., Vestweber D. (2013) The role of VE-cadherin in vascular morphogenesis and permeability control. Prog. Mol. Biol. Transl. Sci. 116, 119–144 [DOI] [PubMed] [Google Scholar]
- 11.Weis W. I., Nelson W. J. (2006) Re-solving the cadherin–catenin–actin conundrum. J. Biol. Chem. 281, 35593–35597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartsock A., Nelson W. J. (2008) Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim. Biophys. Acta 1778, 660–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Le Guelte A., Dwyer J., Gavard J. (2011) Jumping the barrier: VE-cadherin, VEGF and other angiogenic modifiers in cancer. Biol. Cell 103, 593–605 [DOI] [PubMed] [Google Scholar]
- 14.Belvitch P., Dudek S. M. (2012) Role of FAK in S1P-regulated endothelial permeability. Microvasc. Res. 83, 22–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen X. L., Nam J. O., Jean C., Lawson C., Walsh C. T., Goka E., Lim S. T., Tomar A., Tancioni I., Uryu S., Guan J. L., Acevedo L. M., Weis S. M., Cheresh D. A., Schlaepfer D. D. (2012) VEGF-induced vascular permeability is mediated by FAK. Dev. Cell 22, 146–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jean C., Chen X. L., Nam J. O., Tancioni I., Uryu S., Lawson C., Ward K. K., Walsh C. T., Miller N. L., Ghassemian M., Turowski P., Dejana E., Weis S., Cheresh D. A., Schlaepfer D. D. (2014) Inhibition of endothelial FAK activity prevents tumor metastasis by enhancing barrier function. J. Cell Biol. 204, 247–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sulzmaier F. J., Jean C., Schlaepfer D. D. (2014) FAK in cancer: mechanistic findings and clinical applications. Nat. Rev. Cancer 14, 598–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas S. M., Brugge J. S. (1997) Cellular functions regulated by Src family kinases. Annu. Rev. Cell Dev. Biol. 13, 513–609 [DOI] [PubMed] [Google Scholar]
- 19.Zebda N., Dubrovskyi O., Birukov K. G. (2012) Focal adhesion kinase regulation of mechanotransduction and its impact on endothelial cell functions. Microvasc. Res. 83, 71–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lampugnani M. G., Dejana E., Giampietro C. (2017) Vascular endothelial (VE)-cadherin, endothelial adherens junctions, and vascular disease. [E-pub ahead of print] Cold Spring Harb. Perspect. Biol. doi: 10.1101/cshperspect.a029322; erratum: 9, a029322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Potter M. D., Barbero S., Cheresh D. A. (2005) Tyrosine phosphorylation of VE-cadherin prevents binding of p120- and β-catenin and maintains the cellular mesenchymal state. J. Biol. Chem. 280, 31906–31912 [DOI] [PubMed] [Google Scholar]
- 22.Bae Y. H., Mui K. L., Hsu B. Y., Liu S. L., Cretu A., Razinia Z., Xu T., Puré E., Assoian R. K. (2014) A FAK-Cas-Rac-lamellipodin signaling module transduces extracellular matrix stiffness into mechanosensitive cell cycling. Sci. Signal. 7, ra57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwartz M. A. (2010) Integrins and extracellular matrix in mechanotransduction. Cold Spring Harb. Perspect. Biol. 2, a005066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyazaki T., Kato H., Nakajima M., Sohda M., Fukai Y., Masuda N., Manda R., Fukuchi M., Tsukada K., Kuwano H. (2003) FAK overexpression is correlated with tumour invasiveness and lymph node metastasis in oesophageal squamous cell carcinoma. Br. J. Cancer 89, 140–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitra S. K., Schlaepfer D. D. (2006) Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr. Opin. Cell Biol. 18, 516–523 [DOI] [PubMed] [Google Scholar]
- 26.Chen J. S., Huang X. H., Wang Q., Chen X. L., Fu X. H., Tan H. X., Zhang L. J., Li W., Bi J. (2010) FAK is involved in invasion and metastasis of hepatocellular carcinoma. Clin. Exp. Metastasis 27, 71–82 [DOI] [PubMed] [Google Scholar]
- 27.Urbano R. L., Furia C., Basehore S., Clyne A. M. (2017) Stiff substrates increase inflammation-induced endothelial monolayer tension and permeability. Biophys. J. 113, 645–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Califano J. P., Reinhart-King C. A. (2010) Substrate stiffness and cell area predict cellular traction stresses in single cells and cells in contact. Cell. Mol. Bioeng. 3, 68–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zijlstra A., Seandel M., Kupriyanova T. A., Partridge J. J., Madsen M. A., Hahn-Dantona E. A., Quigley J. P., Deryugina E. I. (2006) Proangiogenic role of neutrophil-like inflammatory heterophils during neovascularization induced by growth factors and human tumor cells. Blood 107, 317–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mason B. N., Starchenko A., Williams R. M., Bonassar L. J., Reinhart-King C. A. (2013) Tuning three-dimensional collagen matrix stiffness independently of collagen concentration modulates endothelial cell behavior. Acta Biomater. 9, 4635–4644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bryant P. W., Zheng Q., Pumiglia K. M. (2012) Focal adhesion kinase is a phospho-regulated repressor of Rac and proliferation in human endothelial cells. Biol. Open 1, 723–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levental K. R., Yu H., Kass L., Lakins J. N., Egeblad M., Erler J. T., Fong S. F., Csiszar K., Giaccia A., Weninger W., Yamauchi M., Gasser D. L., Weaver V. M. (2009) Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 139, 891–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kagan H. M., Li W. (2003) Lysyl oxidase: properties, specificity, and biological roles inside and outside of the cell. J. Cell. Biochem. 88, 660–672 [DOI] [PubMed] [Google Scholar]
- 34.Bordeleau F., Califano J. P., Negrón Abril Y. L., Mason B. N., LaValley D. J., Shin S. J., Weiss R. S., Reinhart-King C. A. (2015) Tissue stiffness regulates serine/arginine-rich protein-mediated splicing of the extra domain B-fibronectin isoform in tumors. Proc. Natl. Acad. Sci. USA 112, 8314–8319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Slack-Davis J. K., Martin K. H., Tilghman R. W., Iwanicki M., Ung E. J., Autry C., Luzzio M. J., Cooper B., Kath J. C., Roberts W. G., Parsons J. T. (2007) Cellular characterization of a novel focal adhesion kinase inhibitor. J. Biol. Chem. 282, 14845–14852 [DOI] [PubMed] [Google Scholar]
- 36.Weis S., Cui J., Barnes L., Cheresh D. (2004) Endothelial barrier disruption by VEGF-mediated Src activity potentiates tumor cell extravasation and metastasis. J. Cell Biol. 167, 223–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Orsenigo F., Giampietro C., Ferrari A., Corada M., Galaup A., Sigismund S., Ristagno G., Maddaluno L., Koh G. Y., Franco D., Kurtcuoglu V., Poulikakos D., Baluk P., McDonald D., Grazia Lampugnani M., Dejana E. (2012) Phosphorylation of VE-cadherin is modulated by haemodynamic forces and contributes to the regulation of vascular permeability in vivo. Nat. Commun. 3, 1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hamadi A., Deramaudt T. B., Takeda K., Rondé P. (2009) Src activation and translocation from focal adhesions to membrane ruffles contribute to formation of new adhesion sites. Cell. Mol. Life Sci. 66, 324–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Y., Botvinick E. L., Zhao Y., Berns M. W., Usami S., Tsien R. Y., Chien S. (2005) Visualizing the mechanical activation of Src. Nature 434, 1040–1045 [DOI] [PubMed] [Google Scholar]
- 40.Provenzano P. P., Inman D. R., Eliceiri K. W., Keely P. J. (2009) Matrix density–induced mechanoregulation of breast cell phenotype, signaling and gene expression through a FAK-ERK linkage. Oncogene 28, 4326–4343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arold S. T., Hoellerer M. K., Noble M. E. (2002) The structural basis of localization and signaling by the focal adhesion targeting domain. Structure 10, 319–327 [DOI] [PubMed] [Google Scholar]
- 42.Tilghman R. W., Parsons J. T. (2008) Focal adhesion kinase as a regulator of cell tension in the progression of cancer. Semin. Cancer Biol. 18, 45–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wessel F., Winderlich M., Holm M., Frye M., Rivera-Galdos R., Vockel M., Linnepe R., Ipe U., Stadtmann A., Zarbock A., Nottebaum A. F., Vestweber D. (2014) Leukocyte extravasation and vascular permeability are each controlled in vivo by different tyrosine residues of VE-cadherin. Nat. Immunol. 15, 223–230 [DOI] [PubMed] [Google Scholar]
- 44.Wallez Y., Cand F., Cruzalegui F., Wernstedt C., Souchelnytskyi S., Vilgrain I., Huber P. (2007) Src kinase phosphorylates vascular endothelial–cadherin in response to vascular endothelial growth factor: identification of tyrosine 685 as the unique target site. Oncogene 26, 1067–1077 [DOI] [PubMed] [Google Scholar]
- 45.Hatanaka K., Simons M., Murakami M. (2011) Phosphorylation of VE-cadherin controls endothelial phenotypes via p120-catenin coupling and Rac1 activation. Am. J. Physiol. Heart Circ. Physiol. 300, H162–H172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Adam A. P., Sharenko A. L., Pumiglia K., Vincent P. A. (2010) Src-induced tyrosine phosphorylation of VE-cadherin is not sufficient to decrease barrier function of endothelial monolayers. J. Biol. Chem. 285, 7045–7055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.LaValley D. J., Zanotelli M. R., Bordeleau F., Wang W., Schwager S. C., Reinhart-King C. A. (2017) Matrix stiffness enhances VEGFR-2 internalization, signaling, and proliferation in endothelial cells. Converg. Sci. Phys. Oncol. 3, 044001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gavard J., Gutkind J. S. (2006) VEGF controls endothelial-cell permeability by promoting the β-arrestin–dependent endocytosis of VE-cadherin. Nat. Cell Biol. 8, 1223–1234 [DOI] [PubMed] [Google Scholar]
- 49.Golubovskaya V. M. (2014) Targeting FAK in human cancer: from finding to first clinical trials. Front. Biosci. 19, 687–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Infante J. R., Camidge D. R., Mileshkin L. R., Chen E. X., Hicks R. J., Rischin D., Fingert H., Pierce K. J., Xu H., Roberts W. G., Shreeve S. M., Burris H. A., Siu L. L. (2012) Safety, pharmacokinetic, and pharmacodynamic phase I dose-escalation trial of PF-00562271, an inhibitor of focal adhesion kinase, in advanced solid tumors. J. Clin. Oncol. 30, 1527–1533 [DOI] [PubMed] [Google Scholar]
- 51.Aragon-Sanabria V., Pohler S. E., Eswar V. J., Bierowski M., Gomez E. W., Dong C. (2017) VE-cadherin disassembly and cell contractility in the endothelium are necessary for barrier disruption induced by tumor cells. Sci. Rep. 7, 45835 [DOI] [PMC free article] [PubMed] [Google Scholar]