Abstract
Aging is associated with diminished muscle mass, reductions in muscle stem cell functions, and increased muscle fibrosis. The immune system, especially macrophages, can have important roles in modulating muscle growth and regeneration, suggesting that the immune system may also have significant influences on muscle aging. Moreover, the immune system experiences changes in function during senescence, suggesting that regulatory interaction between muscle cells and the immune system may also change during aging. In this study, we performed bone marrow transplantations between age-mismatched donor and recipient mice to test the influence of the age of the immune system on muscle aging. Transplantation of young bone marrow cells into old recipients prevented sarcopenia and prevented age-related change in muscle fiber phenotype. Transplantation of old bone marrow cells into young animals reduced satellite cell numbers and promoted satellite cells to switch toward a fibrogenic phenotype. We also demonstrated that conditioned media from young, but not old, bone marrow cells promoted myoblast proliferation in vitro, and we found that factors released by young bone marrow cells were more supportive of myotube differentiation in vitro. Together, our results demonstrate that aging of bone marrow cells promotes the age-related reduction of satellite cell number and function and contributes to sarcopenia.—Wang, Y., Wehling-Henricks, M., Welc, S. S., Fisher, A. L., Zuo, Q., Tidball, J. G. Aging of the immune system causes reductions in muscle stem cell populations, promotes their shift to a fibrogenic phenotype, and modulates sarcopenia.
Keywords: skeletal muscle, macrophage, satellite cell, bone marrow transplantation, muscle fibrosis
Skeletal muscle undergoes morphologic and functional changes during aging, which include a progressive loss of muscle mass and strength (1, 2). Aging muscle also experiences a reduced capacity to repair and regenerate (3–5) and increased fibrosis and muscle stiffness (6, 7). These age-related changes in muscle directly contribute to loss of functional independence and increased morbidity and mortality (8). Furthermore, with the extension of life span and the increasing number and percentage of older people in modern society (9), muscle aging can lead to great socioeconomic and health challenges.
Muscle aging is influenced by intrinsic changes in satellite cells, as well as extrinsic changes in the environment of the satellite cells. Those intrinsic and extrinsic factors work together with extensive crosstalk to determine the outcome of muscle aging (10). For example, muscle regenerative capacity is affected by the age of the systemic environment in which regeneration occurs. Previous studies using muscle transplantation experiments (11, 12) or heterochronic parabiosis (13, 14) showed that young muscles that regenerated in an aged environment experienced impaired regeneration, and old muscles experience improved regeneration when exposed to a youthful environment.
The immune system has been well-studied for its role in regulating skeletal muscle growth and regeneration in both acute and chronic muscle injury models (15, 16). Because immune cells are important sources of cytokines and other secreted factors in circulation and some of those factors can affect myogenesis (17–19), it is feasible that the influence of the age of the host environment on muscle regeneration is at least partly attributable to the aging of the immune system. Moreover, the immune system experiences changes in function during senescence, which may affect its interaction with the muscle and contribute to muscle aging. For example, the imbalance between inflammatory and anti-inflammatory networks during immunosenescence causes a low-grade, chronic systemic inflammation, termed inflammaging (20). Inflammaging has been associated with many age-related diseases (21, 22), including age-dependent muscle wasting (23, 24).
Macrophages are important members of the immune system, comprising most of the intramuscular leukocytes and having an indispensable role in regulating muscle repair and regeneration. Previous studies in our laboratory and by other groups demonstrated that depletion of myeloid cells from injured muscle slows muscle growth and regeneration (25–28), whereas boosting macrophage numbers can enhance regeneration (25). However, aging muscle shows a progressive loss of the ability to regenerate after injury despite the ∼2-fold increase in intramuscular macrophages that occurs during aging, to reach concentrations of >2000 macrophages/mm3 (29). This suggests that qualitative, age-related changes in intramuscular macrophages may influence their ability to support muscle growth and regeneration during aging.
Macrophages may also contribute to muscle aging by influencing satellite cell functions. In vitro experiments showed that the presence of macrophages or macrophage-conditioned medium (CM) in satellite cell cultures increases muscle cell numbers and elevates expression of MyoD, a transcription factor expressed by activated, proliferative satellite cells (30). In addition, exposing old satellite cells to young serum increased satellite cell proliferation after acute injury (13). Although untested, some of the rejuvenating effects of the young serum may be attributable to the factors generated by the immune system, especially macrophages, suggesting that the age-related decrease in the number and myogenic capacity of satellite cells may be partly attributed to the aging of macrophages.
Although an influence of macrophages on satellite cell aging has not been explored previously, we have shown that aging of the immune system affects muscle fibrosis during aging. Transplantation of bone marrow cells (BMCs) from young donors into old recipients reduced muscle fibrosis (29). Previous investigations have identified mechanisms through which macrophages facilitate fibrosis in injured and dystrophic muscles, including elevated secretion of TGF-β by macrophages (31, 32) and increased arginine metabolism by arginase expressed by M2 macrophages (33). However, the mechanisms through which aging of immune cells contributes to fibrosis of aging muscle are less certain, and aging satellite cells may be a component of profibrotic processes that are influenced by macrophages. Satellite cells undergo myogenic-to-fibrogenic transdifferentiation during aging, which results in the impaired regenerative capacity and increased fibrosis of old muscle (14), although the factors that regulate that transdifferentiation are unknown. Because bone marrow–derived cells reside in muscle and have the potential to influence satellite cell numbers and function (16, 34), they are a potential source of factors that affect satellite cell shifts to a fibrogenic phenotype.
In the present study, we tested whether the age of the immune system contributes to sarcopenia and satellite cell function by heterochronic bone marrow transplantation (BMT). Our results show that transplantation of young BMCs into old recipients prevented sarcopenia and prevented age-related shifts in muscle fiber phenotype. Transplantation of old BMCs did not induce sarcopenia in young recipients but did decrease satellite cell numbers, despite the young age of the recipients. Moreover, we showed that CM collected from young BMCs promoted myoblast proliferation, whereas CM from old BMCs did not have that pro-proliferative effect. CM from young BMCs was also more supportive of differentiation of myotubes in vitro. Finally, we measured the prevalence of fibrogenic-converted satellite cells in the muscles of heterochronic BMT recipient mice and found that mice into which old BMCs were transplanted had significantly higher percentage of fibrogenic-converted satellite cells. Our results indicate that aging of the immune system has an important role in regulating muscle aging and contributes to sarcopenia through multiple mechanisms, including the regulation of satellite cell proliferation and phenotype switch.
MATERIALS AND METHODS
Mice
C57 BL/6 mice were obtained from the National Institute on Aging (Baltimore, MD, USA) mouse colony. All protocols were conducted according to the Guide for the Care and Use of Laboratory Animals (National Institutes of Health (Bethesda, MD, USA) and were approved by the Institutional Animal Care and Use Committee of the University of California, Los Angeles.
BMTs
BMCs from 2- or 18-mo-old, female, C57 BL/6 mice were aseptically flushed from femurs and tibia with Dulbecco’s phosphate-buffered saline (DPBS; MilliporeSigma, Burlington, MA, USA). The cells were then treated with BioWhitaker ACK (ammonium–chloride–potassium) Lysing Buffer (Lonza, Basel, Switzerland) to lyse red blood cells before filtering through a Falcon 70-μm cell strainer (BD, Franklin Lakes, NJ, USA). BMCs were washed 3 times with DPBS, counted, and used immediately for transplantation into 2- or 12-mo-old, male, recipient mice. Two-month-old mice are young adults, in which there are no changes in the immune system or in muscles that reflect aging; that age corresponds to about 20-yr-old humans (35). Twelve-month-old mice correspond to middle-aged humans at about 40 yr old (35), when senescent changes in the immune system and sarcopenia are at early stages (36–40). Eighteen-month-old mice are old adults, corresponding to about 56-yr-old humans, and 20-mo-old mice correspond to about 60-yr-old humans (35). Thus, our experimental design for 1 treatment group entailed transplanting young or old BMCs into young mice and then collecting muscle at the age of the onset of sarcopenia. In the second treatment, we transplanted young or old BMCs into middle-aged mice, and then collected muscle at an age when sarcopenia is more advanced.
Recipient mice were given antibiotic-treated water containing 0.5 mg/ml trimethoprim/sulfamethoxazole for 6 d before irradiation. The mice then received total body irradiation of 950–1000 rads 1 d before BMT. Freshly isolated BMCs were transplanted through tail vein injection at a dose of 107 BMCs/mouse. The recipients were maintained on antibiotic water for 2 wk after irradiation and then switched to acidified water without antibiotics for the rest of their lives. A more-detailed protocol was described in our previous article (29). Survival of irradiated and transplanted mice was >70% at 8 mo after BMT when muscles and blood were collected from recipient mice.
Chimerism assay
Engraftment of transplanted cells was assessed by fluorescence in situ hybridization (FISH) for X and Y chromosome markers (Kreatech; Leica Biosystems, Wetzlar, Germany) with leukocytes isolated from blood that was collected from each recipient mouse. In brief, after red blood cell lysis with 0.85% ammonium chloride and sample fixation with ice-cold fixation buffer (3:1 methanol to glacial acetic acid), isolated leukocytes adhered to microscope slides and were stained with the X/Y chromosomes probe, following the manufacturer protocol. XY and XX immunolabeled cells on each slide were counted. Chimerism was expressed as the number of XX cells/total cell number for each mouse. The percentage of donor-derived cells was >87% for all animals in the current investigation.
Myofiber cross-sectional area measurement
One quadriceps muscle from each mouse was dissected and rapidly frozen in isopentane cooled in liquid nitrogen. Frozen cross-sections were cut from the midbelly of each muscle at a thickness of 10 μm. Sections were then stained with hematoxylin (Vector Laboratories, Burlingame, CA, USA) for 10 min. The muscle fiber cross-sectional area (CSA) was measured for 500 fibers randomly sampled from complete cross-sections using a digital imaging system (Bioquant, Nashville, TN, USA).
Slow myosin heavy chain immunohistochemistry and counting
Frozen sections cut from the midbelly of soleus muscles were air-dried for 30 min and fixed in ice-cold acetone for 10 min. Endogenous peroxidase activity was quenched with 0.3% H2O2. Sections were then blocked in Mouse on Mouse Blocking Kit (Vector Laboratories) for 1 h and then immunolabeled with mouse anti-slow myosin heavy chain (anti-sMHC; Vector Laboratories) for 3 h at room temperature. Sections were washed with PBS and then probed with biotin-conjugated anti-mouse IgG antibody from the Mouse on Mouse Kit (1/200) for 30 min. Sections were subsequently washed with PBS and then incubated for 30 min with avidin–biotin complex reagent from the Mouse on Mouse kit. Staining was visualized with an AEC kit (3-amino-9-ethylcarbazole; Vector Laboratories). Negative control sections were treated identically, except the primary antibody was eliminated from the protocol. Images were taken from sMHC-stained sections and merged to cover the complete cross-sections on which total fiber and sMHC+ fibers were counted.
RNA isolation and quantitative PCR
One quadriceps muscle of each mouse was dissected and rapidly frozen in liquid nitrogen. Frozen muscles were homogenized in Trizol (Thermo Fisher Scientific, Waltham, MA, USA). RNA was extracted and isolated with chloroform separatory extraction and isopropyl alcohol precipitation. The RNA samples were then treated with DNase and cleaned with RNeasy spin columns, according to the manufacturer’s protocol (Qiagen, Hilden, Germany). Total RNA was reverse transcribed with SuperScript Reverse Transcriptase II using oligo deoxythymidine to prime the extension (Thermo Fisher Scientific) to produce cDNA, which was then used for quantitative PCR (qPCR) using SYBR Green qPCR Master Mix (Bio-Rad Laboratories, Hercules, CA, USA), according to the manufacturer’s protocol. qPCR was performed on an iCycler thermal cycler system (Bio-Rad Laboratories) equipped with the iQ5 optical system software (Bio-Rad Laboratories). RNPS1 (RNA binding protein with serine-rich domain 1) and SRP14 (signal recognition particle 14) were used as reference genes. The normalization factor for each sample was calculated by geometric averaging of the cycle threshold (Ct) values of both reference genes using the geNorm software. Primers used for qPCR are listed in Supplemental Table S1.
Pax7+ antibody preparation and immunohistochemistry
Pax7 (paired box 7) hybridoma cells (Developmental Studies Hybridoma Bank, Iowa City, IA, USA) were cultured in complete medium consisting of DMEM with 1% penicillin–streptomycin (Thermo Fisher Scientific) and 20% heat-inactivated fetal bovine serum (FBS). CM was collected from the cultures and used for purification of antibodies to Pax7, as previously described (29).
Pax7 antibody was used for immunohistochemistry staining of cross-sections from quadriceps muscles that had been rapidly frozen in liquid nitrogen-cooled isopentane. Sections were fixed in 2% paraformaldehyde for 10 min and then immersed in antigen retrieval buffer (10 mM sodium citrate, 0.05% Tween 20, pH 6.0) at 95–100°C for 40 min. Endogenous peroxidase activity in the sectioned tissue was quenched by immersion in 0.3% H2O2. Sections were then treated with blocking buffer from the Mouse on Mouse immunohistochemistry kit for 1 h and immunolabeled with mouse anti-Pax7 antibody overnight at 4°C. Sections were subsequently incubated with biotin-conjugated anti-mouse IgG followed by incubation with avidin-biotin complex reagents from the Mouse on Mouse kit, following manufacturer’s instructions. Staining was visualized with the AEC kit. Negative control sections were treated identically, except the primary antibody was eliminated from the protocol. The number of satellite cells/sectioned muscle fiber was determined by counting the number of Pax7+ cells in midbelly cross-sections of muscles. For Pax7+ cells/mm3, the volume of muscle tissue was determined by measuring the total volume of each section using a stereologic, point-counting technique to determine section area and then multiplying that value by the section thickness (10 μm). To express the data as Pax7+ cells/100 fibers, the total number of fibers for each section was counted on the merged image of the whole section.
Muscle cell culture with CM from BMDMs
BMCs were isolated from young (10-mo-old) and old (20-mo-old) mice, as described above in the BMT section. Freshly isolated BMCs were seeded at 5 × 106/6-cm dish in RPMI-1640 (MilliporeSigma) with 20% heat-inactivated fetal bovine serum (FBS; Omega Scientific, Tarzana, CA, USA), penicillin (100 U/ml; Thermo Fisher Scientific), streptomycin (100 µg/ml; Thermo Fisher Scientific), and 10 ng/ml M-CSF (R&D Systems, Minneapolis, MN, USA) at 37°C with 5% CO2 for 6 d. The medium was changed on d 3 and 5. On d 6, BMDMs were stimulated for 24 h with activation medium consisting of DMEM with 0.25% heat-inactivated FBS, penicillin–streptomycin, and 10 ng/ml M-CSF. CM was collected and spun at 500 g for 5 min to remove floating cells and then kept frozen at −20°C.
C2C12 cells were obtained from ATCC (Manassas, VA, USA). For the muscle cell proliferation assay, C2C12 cells were seeded at 6 × 104 cells/well in a 6-well plate with sterile glass coverslips coated with 0.01% collagen, type I (Thermo Fisher Scientific) and 2% gelatin in DMEM with 10% FBS. Seeding medium was removed from cultures at 24 h after seeding, and the cells were washed with DPBS before adding CM from young or old BMDMs at 2 ml/well. Coverslips were collected 24 h after treatment with CM and then immunolabeled with goat anti-Ki67 (M-19; Santa Cruz Biotechnology, Dallas, TX, USA). The staining protocol was similar to that described above for sMHC immunohistochemistry, except that coverslips were fixed by immersion in cold methanol for 15 min. Ki67+ cells and total cell numbers were counted for each slide in ≥5 randomly chosen fields. Proliferation index was expressed as the percentage of Ki67+ cells/total cells.
A separate set of C2C12 cells was seeded at 6 × 104 cells/well in a 6-well plate without coverslips and maintained in DMEM with 10% FBS for 24 h. The cells then received 48 h of treatment with CM collected from young or old BMDM cultures with a medium change at 24 h. After treatment, cells were washed twice with DPBS and treated with 0.05% trypsin-EDTA (MilliporeSigma) for 3 min. Unattached cells were collected in centrifuge tubes, spun, and resuspended in 200 μl of DPBS before being diluted 1:1 with BioWhitaker trypan blue (Lonza) and counted with a hemocytometer.
For the muscle-cell differentiation assay, C2C12 cells were seeded at 1.2 × 105 cells/well in a 6-well plate without coverslips and maintained in DMEM with 10% FBS for 24 h, followed by DMEM only for 24 h. The cells then received 48 or 96 h of treatment with CM from young or old BMDM cultures with a medium changed every 48 h. After treatment, cells were washed twice with DPBS and collected in Trizol. RNA was extracted and isolated with chloroform extraction and isopropyl alcohol precipitation, followed by cleaning with an RNA Clean and Concentrator Kit (Zymo Research, Irvine, CA, USA). Total RNA was reverse transcribed and used for qPCR, as previously described. SRP14 was used as the reference gene.
Western blotting and analysis
After 24 h of treatment with CM or the control medium, C2C12 cells were collected in reducing buffer (80 mM Tris-HCl, pH 6.8, 0.1 M DTT, 70 mM SDS, and 1.0 mM glycerol). Samples were boiled for 1 min, and then centrifuged at 12,000 g for 1 min. The supernatant fraction of each sample was removed and used to determine protein concentration by filter paper assay. Homogenates containing 20 µg of total protein were separated on 12% SDS-PAGE gels. Proteins were electrophoretically transferred onto nitrocellulose membranes immersed in transfer buffer (39 mM glycine, 48 mM Tris). After transfer, membranes were stained with Ponceau S (MilliporeSigma) to validate the uniformity of protein loading and efficiency of transfer. After destaining of Ponceau S with 0.5% Tween-20 in PBS, membranes were blocked in buffer containing 0.5% Tween 20, 0.2% gelatin, and 3.0% dry milk (blocking buffer) for at least 1 h at room temperature. Membranes were probed with mouse anti-Pax7 antibody prepared as described above (1:400) for 3 h at room temperature. Subsequently, membranes were overlaid with ECL horseradish peroxidase–linked anti-mouse IgG (GE Healthcare, Chicago, IL, USA) for 1 h at room temperature. After each incubation, membranes were washed 6 times for 10 min in wash buffer (0.5% Tween-20, 0.2% gelatin, and 0.3% dry milk). Blots were developed using Chemglow West Chemiluminescence Substrate Kit (ProteinSimple, San Jose, CA, USA). The relative concentration of Pax7 protein in each sample was determined by scanning densitometry using ImageJ (NIH) software.
Double-labeling for Pax7 and ER-TR7 or HSP47
The immunofluorescence, double-staining protocol was similar to the anti-Pax7 method described above, although the anti-Pax7 antibody (1:50) was applied to sections together with rabbit anti-HSP47 (1:1000, heat shock protein 47; Abcam, Cambridge, United Kingdom) antibody or rat anti–ER-TR7 (1:1000; Santa Cruz Biotechnology) antibody for overnight incubation at 4°C. Sections were subsequently incubated with a combination of Dylight 594 anti-mouse IgG with either Dylight 488 anti-rat IgG or Dylight 488 anti-rabbit IgG (Vector Laboratories) and coverslipped with Prolong Gold antifade reagent with DAPI (Thermo Fisher Scientific). Negative control sections were treated identically, except the primary antibodies were eliminated from the protocol. The percentage of satellite cells that had switched toward the fibrogenic phenotype was expressed as the percentage of Pax7+/ER-TR7+ or Pax7+/HSP47+ cells in total Pax7+ cells.
Statistics
Data are presented as means ± sem, and 1-way ANOVA (GraphPad Software, La Jolla, CA, USA) was used to test whether differences between groups were significant at P < 0.05. Comparisons of 2 groups of values were analyzed using the unpaired, 2-tailed Student’s t test.
RESULTS
Effects of BMT on the expression of macrophage phenotypic markers and cytokines
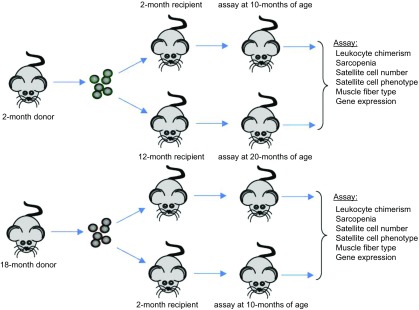
BMCs isolated from 2- and 18-mo-old, female mice were transplanted into 2- or 12-mo-old, male recipients by tail vein injection of 107 BMCs/mouse. Transplanted recipients were housed under sterile conditions for 8 mo before euthanasia and dissections (Fig. 1). fluorescence in situ hybridization for X and Y chromosomes in leukocytes isolated from blood of each recipient mouse showed that >87% of circulating leukocytes were donor-derived for all the animals used in this investigation.
Figure 1.
Diagram showing the experimental design of heterochronic BMT.
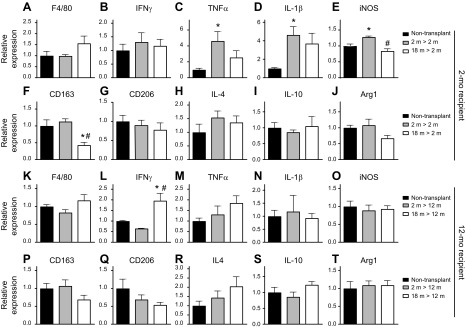
We assayed whether BMT affected the expression of macrophage phenotypic markers or cytokines by qPCR for selected transcripts in quadriceps muscles of transplant recipients or age-matched, nontransplanted controls. Notably, expression levels of all transcripts assayed were very low, reflecting the absence of muscle inflammation. We observed no effect of BMT into 2-mo-old recipients on the expression of the pan-macrophage marker, F4/80 (Fig. 2A), indicating that the numbers of resident macrophages in each treatment group were similar to controls. Assays for expression of transcripts associated with a proinflammatory environment showed variable treatment effects. Although expression of IFN-γ was unaffected by BMT, the expression of TNF-α, IL-1β, and iNOS were all elevated in recipients of 2-mo-old donor cells but not in recipients of 18-mo-old donor cells (Fig. 2B–E). In contrast, BMT had little effect on the expression of transcripts associated with an anti-inflammatory environment (Fig. 2F–J). Although 2-mo-old recipients of 18-mo-old donor BMCs showed less expression of CD163 (Fig. 2F), a marker of M2-biased macrophages, no effect was observed on the expression of CD206 (Fig. 2G) or arginase-1 (Arg1) (Fig. 2J), which are also expressed by anti-inflammatory M2 macrophages. In addition, BMT into 2-mo-old recipients had no significant effect on the expression of the anti-inflammatory cytokines IL-4 or IL-10 (Fig. 2H, I).
Figure 2.
Expression of cytokines and macrophage phenotypic markers in muscles of nontransplanted and BMC-transplanted mice. A) Expression of F4/80 did not differ significantly between transplanted and nontransplanted, 10-mo-old mice. B–E) Expression of transcripts associated with a proinflammatory environment. Transcripts were IFN-γ (B), TNF-α (C), IL-1β (D), and iNOS (E). F–J) Expression of transcripts associated with an anti-inflammatory environment. Transcripts were CD163 (F), CD206 (G), IL-4 (H), IL-10 (I), and Arg1 (J). K) Expression of F4/80 did not differ significantly between transplanted and nontransplanted, 20-mo-old mice. L–O) Expression of transcripts associated with a proinflammatory environment. Transcripts are IFN-γ (L), TNF-α (M), IL-1β (N), and iNOS (O). P–T) Expression levels of transcripts associated with an anti-inflammatory environment. Transcripts are CD163 (P), CD206 (Q), IL-4 (R), IL-10 (S), and Arg1 (T). All data in each set were normalized to expression levels in muscles of nontransplanted mice, which were set at 1.0. Error bars indicate sem. *P < 0.05 (significant difference from age-matched, nontransplanted group), #P < 0.05 (significant difference from age-matched mice that received young BMCs); n = 4–5/data set.
BMT had little effect on the expression of macrophage phenotypic markers or cytokines in 12-mo-old recipient mice. Again, F4/80 expression did not differ significantly between BMT recipients and nontransplanted controls (Fig. 2K), indicating that the numbers of resident macrophages were similar. Among all other transcripts assayed (Fig. 2L–T), only IFN-γ expression was affected by BMT, where it was significantly higher in 12-mo-old recipients of 18-mo-old BMCs (Fig. 2L).
Transplantation of young BMCs into old recipients prevents the age-related reduction in muscle fiber size and changes in fiber-type composition
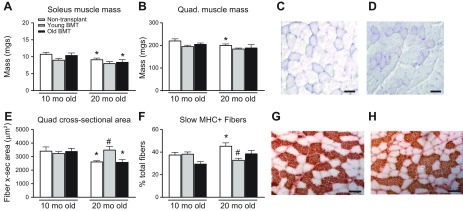
We first tested whether aging of BMCs affect sarcopenia by using changes in gross muscle mass between 10- and 20-mo-old mice as an index of sarcopenia. In nontransplanted controls, the mass of soleus muscles was significantly less in 20-mo-old mice than it was in 10-mo-old mice (Fig. 3A), indicating measurable sarcopenia during that period of aging. Similarly, the mass of soleus muscles from 20-mo-old mice that received old BMT was significantly less than the mass of soleus muscles from 10-mo-old mice that received old BMT (Fig. 3A), reflecting a magnitude of sarcopenia that was similar to nontransplanted controls. However, the mass of soleus muscles from 20-mo-old mice that received young BMTs did not differ from the mass of soleus muscles from 10-mo-old mice that received young BMTs (Fig. 3A). Thus, using changes in gross muscle mass between 10- and 20-mo-olds as an index of sarcopenia, the findings show that recipients of young BMTs did not experience detectible sarcopenia during that period.
Figure 3.
Transplantation of young BMCs into old recipients prevents age-related reduction in myofiber size and change of fiber-type composition. A) Soleus muscle mass of nontransplanted control mice decreased from 10 to 20 mo of age. Soleus muscle mass of mice that received old-BMT also decreased from 10 to 20 mo of age. Mice that received transplantation of young BMCs or old BMCs did not show significant differences in soleus muscle mass compared with age-matched, nontransplanted mice. B) Quadriceps muscle mass of 20-mo-old, nontransplanted mice was significantly less than that of 10-mo-old, nontransplanted mice. Mice that received transplantation of young BMCs or old BMCs did not show significant differences in quadriceps muscle mass compared with age-matched, nontransplanted mice. C) Representative image of a hematoxylin-stained section of a quadriceps muscle from a 10-mo-old mouse receiving transplantation of old BMCs. D) Representative image of a hematoxylin-stained section of a quadriceps muscle from a 20-mo-old mouse receiving transplantation of old BMCs. Scale bars, 50 μm. E) The average myofiber CSA measured in quadriceps muscles of transplanted and control mice. Transplantation of young, but not old, BMCs into adult recipients increased fiber CSA at 20-mo-old compared with 20-mo-old, nontransplanted mice. F) Quantification of the percentage of sMHC+ fibers in total fibers of soleus muscles. Ten-month-old nontransplanted mice had a lower proportion of sMHC+ fibers/total fibers than 20-mo-old, nontransplanted mice. Transplantation of young BMCs into old mice reduced sMHC+ fiber ratio, whereas transplantation of old BMT did not affect sMHC+ fiber percentage compared with control mice. G) Representative image of sMHC immunostaining of a soleus muscle section from 20-mo-old, nontransplanted mouse. Scale bars, 100 μm. H) Representative image of sMHC staining of a soleus muscle section from 20-mo-old mouse that had received transplantation of young BMCs, showing fewer sMHC+ fibers compared with control mice shown in G. Error bars indicate sem. *P < 0.05 (in all panels, except for images, significant difference from 10-mo-old mice that received the same treatment), #P < 0.05 (significant difference from age-matched, nontransplanted mice); n = 5/data set.
We also observed that the mass of nontransplanted, control quadriceps muscles declined between 10 and 20 mo of age (Fig. 3B). Consistent with our observations on soleus muscles, we found that the mass of quadriceps muscles from 20-mo-old mice that received young BMTs did not differ from the mass of quadriceps muscles from 10-mo-old mice that received young BMTs (Fig. 3B). However, unlike our observations on soleus muscles, the masses of quadriceps muscles from 20 and 10-mo-old mice that received old BMTs did not differ (Fig. 3B).
Changes in gross muscle mass are a relatively insensitive index of sarcopenia and the actual loss of muscle fiber mass can be masked by increases in the mass of other constituents of the muscle tissue (29). We next used changes in muscle fiber cross-section as an index of sarcopenia because that assay would not be influenced by changes in noncontractile components of the muscle tissue. Although quadriceps mass showed no differences between 10- and 20-mo-old mice that had received old BMTs, histologic observations indicated that the CSA of muscle fibers in quadriceps was less in 20-mo-old mice receiving old BMTs than it was 10-mo-old mice receiving old BMTs (Fig. 3C–E). This indicates that sarcopenia occurred in mice receiving BMTs from old donors and suggests that an increase in the mass of noncontractile components of muscle during aging of those mice could underlie the discrepancy between mass measurements vs. CSA measurements.
In nontransplanted mice, CSAs of quadriceps muscle fibers were less at 20 mo old than they were at 10 mo old (Fig. 3E). At 10 mo, transplantation of neither young nor old BMCs affected myofiber CSA (Fig. 3E). However, 20-mo-old mice that received young BMCs when the recipients were 12-mo-old showed a greater myofiber CSA compared with 20-mo-old, nontransplanted mice (Fig. 3C–E). In fact, myofiber CSA of 20-mo-old mice that received young BMTs was similar to that of 10-mo-old, transplanted and control mice, showing that transplantation of young BMCs into old recipients prevented the decrease in fiber size that normally happens during aging (Fig. 3E). Moreover, transplantation of 18-mo-old BMCs into old recipients did not have that protective effect, shown by a myofiber CSA similar to that of nontransplanted 20-mo-old mice (Fig. 3E). Myofiber CSA of 20-mo-old mice that received BMCs from old donors was less than the myofiber CSA of 10-mo-old mice (transplanted or nontransplanted) and 20-mo-old mice transplanted with young BMCs (Fig. 3E). Notably, the CSA of 20-mo-old mice that received young BMTs was greater than the CSA of nontransplanted 20-mo-old mice, although muscle masses between the groups did not differ (Fig. 3B, E). This incongruence occurs because during muscle aging, there is an increase in muscle fibrosis that can partially mask losses in muscle fiber size or mass and young BMT causes a reduction in connective tissue accumulation in aging muscle (29). Thus, young BMT prevents loss of muscle fiber size and prevents increases in muscle fibrosis during aging, so that neither muscle mass or fiber CSA change between 10 and 20 mo in young BMT recipients.
Aging muscle also undergoes a preferential loss of type-II fast-twitch fibers because of the specific loss of fast-twitch motor neurons (41). We performed sMHC staining on sections of soleus muscle (Fig. 3G, H) and found that the ratio of sMHC+ fibers/total fibers increased by 21% in 20-mo-old, nontransplanted mice compared with 10-mo-old, nontransplanted mice (Fig. 3F). Similarly, the proportion of sMHC+ fibers was 31% greater in the soleus of 20-mo-old mice that had BMT of old BMCs, compared with 10-mo-old mice that had received old BMT (Fig. 3F). However, the proportion of sMHC+ fibers in young and old recipients that received young BMTs did not differ (Fig. 3F) and the proportion of sMHC+ fibers in neither young nor old transplant recipients differed from quadriceps muscles of 10-mo-old, nontransplanted mice. Together, these data indicate that transplantation of young, but not old, BMCs into adult mice prevents changes in muscle fiber phenotype that normally occur during aging, in addition to preventing sarcopenia.
Transplantation of old BMCs decreases satellite cell numbers
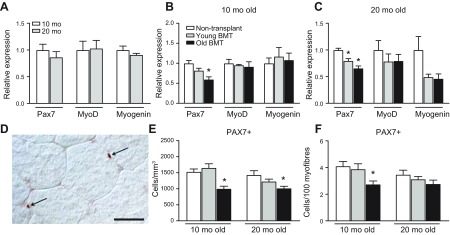
Because macrophage-derived factors affect the expression of myogenic transcription factors that regulate satellite cell proliferation and differentiation, we tested whether heterochronic BMT affected the expression of key transcription factors that control myogenesis. Our qPCR analysis of quadriceps muscles from nontransplanted mice showed that Pax7, MyoD, and myogenin expression did not differ between 10- and 20-mo-old mice (Fig. 4A); 10-mo-old mice that received transplantation of old BMCs had significantly lower expression of Pax7 compared with 10-mo-old, nontransplanted mice, whereas BMT of young BMCs did not change Pax7 mRNA level (Fig. 4B). Heterochronic BMT did not affect the expression of MyoD and myogenin in 10-mo-old mice (Fig. 4B). In 20-mo-old mice, we observed a similar decrease in Pax7 expression in the recipients that received the transplantation of old BMCs compared with nontransplanted mice (Fig. 4C). Interestingly, we also found that transplantation of BMCs from 2-mo-old donors into old recipients significantly decreased Pax7 expression, although the reduction was less than that caused by transplantation of old BMCs (Fig. 4C). Expression levels of MyoD and myogenin showed an insignificant trend for reduced expression in 20-mo-old mice that received either young or old BMT (Fig. 4C).
Figure 4.
Transplantation of old BMCs reduces Pax7 expression and the number of Pax7+ cells in young and old recipients. A) Expression of Pax7, MyoD, and myogenin did not differ in quadriceps muscles of 10- and 20-mo-old, nontransplanted mice. Values normalized to 10-mo-old mice. B, C) qPCR analysis for myogenic transcription factors in 10- (B) and 20-mo-old (C) recipients. D) Image of Pax7+ satellite cell at surface of 10-mo-old quadriceps muscle. Scale bar, 25 μm. E) Stereological counts for Pax7+ cells. Satellite cell numbers in 10- or 20-mo-old, nontransplanted mice did not differ. Transplantation of old, but not young, BMCs decreases satellite cells at both 10 and 20 mo of age. F) Quantification of satellite cell numbers per 100 myofibers. Error bars indicate sem.*P < 0.05 (significant difference from age-matched, nontransplanted mice.); n = 5/data set for all groups in all panels.
We also assayed whether age of the immune system influences satellite cell number because satellite cells are necessary for muscle growth and muscle regeneration after injury. We performed immunohistochemistry of Pax7 (Fig. 4D) and found that satellite cell numbers did not differ in nontransplanted mice between 10 and 20 mo (Fig. 4E). Negative control sections for which the primary antibody was eliminated from the treatment showed no labeling (data not shown). Transplantation of old BMCs into both young (2-mo-old) and adult (12-mo-old) recipients caused significant reductions in Pax7+ cell numbers at 10 and 20 mo of age, compared with nontransplanted mice (Fig. 4E). However, transplantation of young BMCs did not affect satellite cell numbers (Fig. 4E). These changes were in accord with changes in Pax7 mRNA levels (Fig. 4B, C). We also quantified satellite cell numbers/100 myofibers because heterochronic BMT may have influences on fiber size (Fig. 3E) and observed a similar treatment effect (Fig. 4F).
In summary, our qPCR and histologic data showed that transplantation of old BMCs decreased Pax7+ satellite cell numbers compared with control mice, whereas transplantation of young BMCs did not affect the number of Pax7+ cells.
Macrophages from young mice promote myoblast proliferation
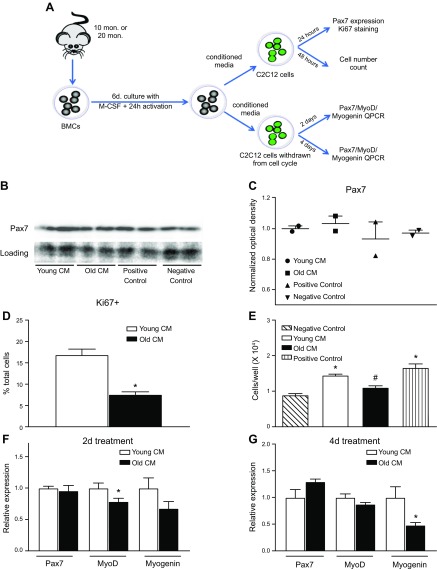
The decrease of Pax7 mRNA and decrease in Pax7+ cells in transplanted mice receiving old BMCs may reflect reductions in satellite cell proliferation or may result from decreased Pax7 expression in each satellite cell. Moreover, Pax7+ satellite cell numbers may be influenced by direct effects of the aging of BMCs or by unidentified, indirect effects. We used an in vitro model to test directly the influence on muscle cells of BMDMs isolated from young or old mice (Fig. 5A). Western blotting data from myoblast cultures treated with CM from cultures of young BMDMs (young-CM) or old BMDMs (old-CM) showed no effect on Pax7 protein levels after 24 h of treatment with the CM (Fig. 5B, C). However, proliferation of myoblasts in cultures, reflected by Ki67 expression, was elevated in cultures treated with young-CM, compared with old-CM (Fig. 5D). At 48 h after treatment, young-CM treatment increased myoblast numbers compared with myoblasts treated with non-CM (Fig. 5E). However, old-CM did not affect myoblast numbers compared with myoblasts cultured with non-CM, and myoblast cell numbers in old-CM cultures were lower from those in young-CM cultures (Fig. 5E). These data show that macrophages from young, but not old, mice, promote myoblast proliferation, indicating that aging of BMCs can contribute to reductions in satellite cell number caused by a reduction in pro-proliferative macrophage-derived factors.
Figure 5.
BMDM age affects their production of myogenic regulatory factors. A) Diagram showing the experimental design of myoblast culture with CM from young and old BMDMs. B) Western blotting for Pax7 showed that CM from either young or old BMDMs did not affect Pax7 protein levels. Positive control used medium containing 10% FBS. Negative control used unconditioned BMDM activation medium. C) Optical density of Pax7 Western blots normalized to Ponceau S staining. D) Quantification of myoblasts that entered cell cycle within 24 h of CM treatment by anti-Ki67 staining. Myoblasts cultured with CM from old BMDMs showed lower Ki67+ cell ratio compared with myoblasts treated with young BMDM CM. Error bar indicates sem.P < 0.05 (significant difference from young BMDM CM treated group); n = 24/data set. E) Counts of total cell numbers of myoblasts treated with CM or control medium showed that medium from young BMDMs increased cell numbers compared with the negative control. Medium from old BMDMs did not increase cell numbers. Error bar indicates sem *P < 0.05 (significant difference from the negative control group), #P < 0.05 (significant difference from the young BMDM CM-treated group); n = 6/data set. F) qPCR analysis of Pax7, MyoD, and myogenin expression in muscle cells differentiated in CM for 2 d. Myotubes in old-CM showed significantly lower levels of MyoD expression. *P < 0.05; n = 6/data set. Error bar indicates sem. G) qPCR analysis of Pax7, MyoD, and myogenin expression in muscle cells differentiated in CM for 4 d. Myotubes in old-CM showed significantly lower levels of myogenin expression. Error bar indicates sem. *P < 0.05; n = 5/data set.
Expression of transcription factors that regulate muscle differentiation is reduced by old BMDMs
Although heterochronic BMT did not produce changes in the expression level of myogenic transcriptions factors associated with muscle differentiation in vivo, we assayed whether macrophage age affected their influence on muscle differentiation in vitro. Muscle cells were differentiated in culture to withdraw from the cell cycle and fuse to become myotubes and then assayed at 2 or 4 d after induction of differentiation, in the presence of young-CM or old-CM. As reported above for myoblasts, there was no difference in Pax7 expression in myotubes cultured in young-CM vs. old-CM. However, at the 2-d differentiation, myotubes in old-CM showed significantly lower levels of expression of MyoD (Fig. 5F), a transcription factor that is expressed at elevated levels in early muscle differentiation. We also found that at the 4-d differentiation, myotubes in old-CM showed significantly lower levels of myogenin expression (Fig. 5G), a transcription factor that is expressed at elevated levels in later stages of muscle differentiation. Together, the data indicate that factors released by young-CM are more supportive of muscle differentiation in vitro, although the effect was not detectible in vivo.
Transplantation of old BMCs into young and adult recipients biased satellite cells toward a fibrogenic phenotype
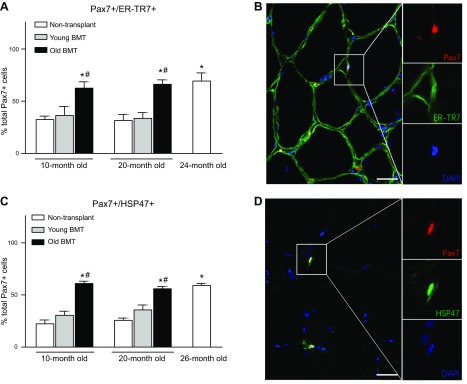
Aging of BMCs may also affect myogenic capacity and may contribute to muscle aging by promoting satellite cell shifts toward a fibrogenic, nonmyogenic phenotype. Because previous investigations showed that this phenotypic switch is reflected by the expression of ER-TR7, an extracellular matrix protein, by Pax7+ satellite cells (14), we used immunohistochemistry to assay the percentage of Pax7+ cells that expressed ER-TR7 in our treatment groups (Fig. 6A, B). Negative control sections for which the primary antibodies were eliminated from the treatment showed no labeling (data not shown). We observed that the proportion of Pax7+ cells that expressed ER-TR7 was not different in nontransplanted mice at 10 and 20 mo of age but more than doubled between 20 and 24 mo of age (Fig. 6A). We also found that transplantation of young BMCs did not influence the proportion of satellite cells that expressed the fibrogenic marker at either 10 or 20 mo (Fig. 6A). However, transplantation of old BMCs into young and adult recipients more than doubled the proportion of ER-TR7–expressing satellite cells, to levels similar to 24-mo-old, nontransplanted controls (Fig. 6A).
Figure 6.
Transplantation of old BMCs biased satellite cells toward a fibrogenic phenotype. A) Quantification of the proportion of Pax7+/ER-TR7+ cells in total Pax7+ cells. Transplantation of old, but not young, BMCs increased the ratio of Pax7+/ER-TR7+ cells in both 10- and 20-mo-old recipients. Error bar indicates sem. *P < 0.05 (significant difference from 10-mo-old, nontransplanted group), #P < 0.05 (significant difference from age-matched mice that received young BMCs); n = 5/data set. B) Immunofluorescence, double-labeling for Pax7 and ER-TR7. Quadriceps muscle from 20-mo-old, nontransplanted mouse labeled with anti-Pax7 (red) and anti-ER-TR7 (green). Double-positive cells appear yellow. Nuclei are stained blue with DAPI. Scale bar, 25 µm. Area enclosed in the box is shown in nonmerged images at higher magnification to the right. C) Quantification of the proportion of Pax7+/HSP47+ cells in total Pax7+ cells showing treatment outcomes identical to assays for Pax7+/ER-TR7+ cells in A. Error bar indicates sem. *P < 0.05 (significant difference from 10-mo-old, nontransplanted group), #P < 0.05 (significant difference from age-matched mice that received young BMCs); n = 5/data set. D) Immunofluorescence, double-labeling for Pax7 and HSP47. Quadriceps muscle from 20-mo-old, nontransplanted mouse labeled with anti-Pax7 (red) and anti-HSP47 (green). Double-positive cells appear yellow. Nuclei are stained blue with DAPI. Scale bar, 25 µm. Area enclosed in the box is shown in nonmerged images at higher magnification to the right.
Because ER-TR7 is also secreted by fibroblasts and binds to the extracellular matrix between myofibers, distinguishing between ER-TR7 within satellite cells and vs. extracellular ER-TR7 was occasionally obscured in immunolabeled sections. We chose to further validate the ER-TR7 data by assaying for HSP47, a collagen-specific molecular chaperone and marker of connective tissue–producing cells (42) (Fig. 6C, D). Data obtained by assaying HSP47+/Pax7+ (double-positive) cells were nearly identical to the data obtained for ER-TR7+/Pax7+ (double-positive) cells (Fig. 6A, C). In addition, we observed that mice that received transplantation of old BMCs had similar proportion of Pax7+/HSP47+ cells as 26-mo-old, nontransplanted mice (Fig. 6C); this is intriguing because the age of the BMCs in both the 10- and 20-mo-old recipients was 26 mo old because the donor BMCs were isolated from 18-mo-old mice and were transplanted into the recipients for 8 mo before tissue collection. Collectively, these findings show that the conversion of satellite cells toward a more-fibrogenic lineage is accelerated by the aging of BMCs, which may provide an additional mechanism through which aging of the BMCs contribute to muscle aging.
DISCUSSION
The results of the present investigation show that age-related changes in skeletal muscle are strongly influenced by aging of BMCs. In particular, we found that the rate of sarcopenia, changes in muscle fiber phenotype, and the numbers and phenotypes of satellite cells were significantly changed by perturbations that modify the age of BMCs and their progeny. One intriguing aspect of our results is the asymmetric effect on muscle aging caused by transplantation of young vs. old BMCs. For example, transplantation of young BMCs into old recipients prevented the decrease of myofiber size and prevented the increased proportion of slow-twitch fibers seen when the recipients were 20 mo old. However, the presence of old BMCs in young recipients did not induce the change in myofiber size or slow-twitch fiber percentage at 10 mo old. These data indicate that aging of the immune system alone is not sufficient to induce sarcopenia in a young host, whereas aged muscles in the old recipients also did not experience sarcopenia if a young immune system was present. Thus, an aged immune compartment and aged muscle compartment are both required for sarcopenia to occur in 20-mo-old mice.
Although aging of the immune system alone is insufficient to induce sarcopenia, it is sufficient to cause reductions in satellite cell numbers and to shift satellite cells to a fibrogenic phenotype. Transplantation of 18-mo-old BMCs into young or old recipients decreased the number of Pax7+ satellite cells, despite the lack of effect on myofiber CSA. Moreover, we also observed that 20-mo-old, nontransplanted mice did not have fewer satellite cells than 10-mo-old, nontransplanted mice, but they did have smaller myofiber CSA and an increased proportion of slow-twitch myofibers. Those data show that reductions in satellite cell numbers are not sufficient or necessary to induce sarcopenia. On the other hand, we observed that at both 10 and 20 mo of age, the presence of 26-mo-old BMCs more than doubled the proportion of satellite cells that switched to a fibrogenic phenotype compared with age-matched, nontransplanted mice, showing that aging of the BMCs may contribute to age-related muscle fibrosis by affecting satellite cell phenotype. A previous investigation showed that lifelong reduction of satellite cells in sedentary mice did not affect the rate or extent of changes in muscle mass, myofiber CSA or fiber type composition during aging, but did increase fibrosis (43). Those observations are consistent with our findings, indicating that aging of the immune system affects satellite cell numbers and phenotype switch, which then contributes to age-related muscle fibrosis but not sarcopenia.
Interestingly, controversy exists concerning whether satellite cell numbers decrease during aging (3, 44–47). Different muscles and various methods used to quantify satellite cell numbers may underlie part of the discrepancy in the results, but the age of mice chosen for comparison is also an important variable. For example, electron microscopy showed that the percentage of total myonuclei in soleus muscle that comprised satellite cell nuclei did not decrease when comparing 8–10-mo-old muscles with 19–20-mo-old muscles, but a significant reduction was seen between 19–20-mo-old muscles and 29–30-mo-old muscles (44). However, quantifying satellite cell number/myofiber in soleus muscles with Pax7 staining of freshly isolated myofibers, showed that the number of satellite cells/myofiber did not change significantly when comparing 7–10-mo-old muscles to 18–27-mo-old muscles, but there was a reduction when comparing 18–27- to 28–33-mo-old muscles (46). Those observations suggest that the reduction of satellite number does not occur during early stages of aging but is obvious when the mice reach senescence. Those results are consistent with our findings, which showed no difference between 10- and 20-mo-old mice in the number of satellite cells/unit volume of muscle or their number/100 myofibers. Although observations in previous investigations and the present study show that reducing satellite cell numbers by either genetic manipulation of Pax7 (43) or by manipulating the age of the immune system did not induce sarcopenia at 10–24-mo of age, whether reductions in satellite cell numbers contribute to sarcopenia at later, senescent stages of aging is unknown.
We also observed that young BMDMs secrete factors into the medium that increase myoblast proliferation, but CM collected from old BMDMs does not promote myoblast proliferation; this suggests that the reduction of satellite cell numbers in aging muscle may result in part from lost production of a macrophage-derived mitogen. However, the identity of that macrophage-derived factor remains unclear and is the subject of our continuing studies. A recent study identified macrophage-derived ADAMTS1 (ADAM metallopeptidase with thrombospondin type 1 motif) as an extracellular regulator that promotes satellite cell activation by inhibiting Notch signaling (48). The failure to activate Notch signaling after injury in old satellite cells led to decreased myogenic ability of satellite cells and impaired regeneration in old, injured muscle (3), which could be rescued by exposing the old satellite cells to young serum through heterochronic parabiosis (13). However, whether Notch signaling is involved in sarcopenia of sedentary mice without injury is unknown and the observation that the expression of Notch-1 is similar in satellite cells isolated from resting muscles of young and aged mice (3) argues against the possibility. Further studies are needed to determine whether the age of macrophages affects ADAMTS1 expression, what its function is in regulating Notch signaling, and whether this mechanism contributes to satellite cell proliferation in aging muscles.
Our finding that that young mice that received old BMT had a large reduction in CD163 expression (an M2 macrophage marker) although expression of F4/80 (a pan-macrophage marker) was not reduced suggests that transplantation of old BMCs produced a shift in macrophages away from the CD163+ M2-biased phenotype, without affecting total macrophages. In that case, age-related changes in macrophage phenotype may contribute to some effects of heterochronic BMT that we report. However, the reduction in CD163 expression was not accompanied by increased expression of genes associated with the proinflammatory M1 phenotype or by a decrease in other genes associated with an M2 phenotype. Although this indicates some specificity in the reduction of CD163 expression in old BMCs transplanted into young recipients, the reduction in CD163 per se is unlikely to mediate the reduction in Pax7+ cells because ablation of CD163 causes increases in satellite cell numbers after muscle injury (49). Thus, the specific functional change in old myeloid cells that contributes to reduced satellite cell numbers in 10-mo-old BMT recipients is subject to our continuing investigation.
Understanding the communication between cells derived from the bone marrow and cells in other systems is necessary to fully understand mechanisms of aging, including processes that drive sarcopenia and age-related muscle fibrosis. In addition, understanding those interactions may reveal viable new strategies to slow muscle aging because the immune system is a relatively amenable and accessible therapeutic target. Although heterochronic BMT used in this and our previous investigation (29) demonstrated that the approach significantly slowed many features of muscle aging, BMT would be a radical approach to reduce muscle aging in human populations. However, our findings do show that rejuvenating the immune system may be a feasible way to help sustain muscle health in old age.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank Mr. David Rangel and Ms. Katherine Wen for their expert technical assistance, and Ms. Christine Phan and Mr. Edward Garcia (all from University of California, Los Angeles) for performing fiber counts. Research reported in this publication was supported by the U.S. National Institutes of Health, National Institute on Aging and National Institute of Arthritis and Musculoskeletal and Skin Diseases (Grants R01AR054451 and RO1AG041147 to J.G.T.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the U.S. National Institutes of Health. The authors declare no conflicts of interest.
Glossary
- ARG1
arginase-1
- BMC
bone marrow cell
- BMDM
bone marrow–derived macrophage
- BMT
bone marrow transplantation
- CM
conditioned medium
- CSA
cross-sectional area
- DPBS
Dulbecco’s phosphate-buffered saline
- FBS
fetal bovine serum
- qPCR
quantitative PCR
- sMHC
slow myosin heavy chain
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
Y. Wang, M. Wehling-Henricks, and J. G. Tidball designed the research; Y. Wang, M. Wehling-Henricks, S. S. Welc, A. L. Fisher, Q. Zuo, and J. G. Tidball performed the research and analyzed the data; Y. Wang and J. G. Tidball wrote the manuscript; and all authors critically revised and approved the final version.
REFERENCES
- 1.Roubenoff R. (2001) Origins and clinical relevance of sarcopenia. Can. J. Appl. Physiol. 26, 78–89 [DOI] [PubMed] [Google Scholar]
- 2.Cruz-Jentoft A. J., Baeyens J. P., Bauer J. M., Boirie Y., Cederholm T., Landi F., Martin F. C., Michel J. P., Rolland Y., Schneider S. M., Topinková E., Vandewoude M., Zamboni M.; European Working Group on Sarcopenia in Older People (2010) Sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older people. Age Ageing 39, 412–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conboy I. M., Conboy M. J., Smythe G. M., Rando T. A. (2003) Notch-mediated restoration of regenerative potential to aged muscle. Science 302, 1575–1577 [DOI] [PubMed] [Google Scholar]
- 4.Cosgrove B. D., Gilbert P. M., Porpiglia E., Mourkioti F., Lee S. P., Corbel S. Y., Llewellyn M. E., Delp S. L., Blau H. M. (2014) Rejuvenation of the muscle stem cell population restores strength to injured aged muscles. Nat. Med. 20, 255–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sousa-Victor P., Gutarra S., García-Prat L., Rodriguez-Ubreva J., Ortet L., Ruiz-Bonilla V., Jardí M., Ballestar E., González S., Serrano A. L., Perdiguero E., Muñoz-Cánoves P. (2014) Geriatric muscle stem cells switch reversible quiescence into senescence. Nature 506, 316–321 [DOI] [PubMed] [Google Scholar]
- 6.Alnaqeeb M. A., Al Zaid N. S., Goldspink G. (1984) Connective tissue changes and physical properties of developing and ageing skeletal muscle. J. Anat. 139, 677–689 [PMC free article] [PubMed] [Google Scholar]
- 7.Wood L. K., Kayupov E., Gumucio J. P., Mendias C. L., Claflin D. R., Brooks S. V. (2014) Intrinsic stiffness of extracellular matrix increases with age in skeletal muscles of mice. J. Appl. Physiol. 117, 363–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marty E., Liu Y., Samuel A., Or O., Lane J. (2017) A review of sarcopenia: enhancing awareness of an increasingly prevalent disease. Bone 105, 276–286 [DOI] [PubMed] [Google Scholar]
- 9.Petterson S. M., Liaw W. R., Phillips R. L., Jr., Rabin D. L., Meyers D. S., Bazemore A. W. (2012) Projecting US primary care physician workforce needs: 2010-2025. Ann. Fam. Med. 10, 503–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dumont N. A., Wang Y. X., Rudnicki M. A. (2015) Intrinsic and extrinsic mechanisms regulating satellite cell function. Development 142, 1572–1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carlson B. M., Faulkner J. A. (1989) Muscle transplantation between young and old rats: age of host determines recovery. Am. J. Physiol. 256, C1262–C1266 [DOI] [PubMed] [Google Scholar]
- 12.Roberts P., McGeachie J. K., Grounds M. D. (1997) The host environment determines strain-specific differences in the timing of skeletal muscle regeneration: cross-transplantation studies between SJL/J and BALB/c mice. J. Anat. 191, 585–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conboy I. M., Conboy M. J., Wagers A. J., Girma E. R., Weissman I. L., Rando T. A. (2005) Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 433, 760–764 [DOI] [PubMed] [Google Scholar]
- 14.Brack A. S., Conboy M. J., Roy S., Lee M., Kuo C. J., Keller C., Rando T. A. (2007) Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science 317, 807–810 [DOI] [PubMed] [Google Scholar]
- 15.Sun D., Martinez C. O., Ochoa O., Ruiz-Willhite L., Bonilla J. R., Centonze V. E., Waite L. L., Michalek J. E., McManus L. M., Shireman P. K. (2009) Bone marrow-derived cell regulation of skeletal muscle regeneration. FASEB J. 23, 382–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tidball J. G. (2017) Regulation of muscle growth and regeneration by the immune system. Nat. Rev. Immunol. 17, 165–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zamir O., Hasselgren P. O., Higashiguchi T., Frederick J. A., Fischer J. E. (1992) Tumour necrosis factor (TNF) and interleukin-1 (IL-1) induce muscle proteolysis through different mechanisms. Mediators Inflamm. 1, 247–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng M., Nguyen M. H., Fantuzzi G., Koh T. J. (2008) Endogenous interferon-γ is required for efficient skeletal muscle regeneration. Am. J. Physiol. Cell Physiol. 294, C1183–C1191 [DOI] [PubMed] [Google Scholar]
- 19.Londhe P., Davie J. K. (2011) Gamma interferon modulates myogenesis through the major histocompatibility complex class II transactivator, CIITA. Mol. Cell. Biol. 31, 2854–2866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franceschi C., Capri M., Monti D., Giunta S., Olivieri F., Sevini F., Panourgia M. P., Invidia L., Celani L., Scurti M., Cevenini E., Castellani G. C., Salvioli S. (2007) Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech. Ageing Dev. 128, 92–105 [DOI] [PubMed] [Google Scholar]
- 21.Franceschi C., Campisi J. (2014) Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. A. Biol. Sci. Med. Sci. 69 (Suppl 1), S4–S9 [DOI] [PubMed] [Google Scholar]
- 22.Fülöp T., Dupuis G., Witkowski J. M., Larbi A. (2016) The role of immunosenescence in the development of age-related diseases. Rev. Invest. Clin. 68, 84–91 [PubMed] [Google Scholar]
- 23.Jo E., Lee S. R., Park B. S., Kim J. S. (2012) Potential mechanisms underlying the role of chronic inflammation in age-related muscle wasting. Aging Clin. Exp. Res. 24, 412–422 [DOI] [PubMed] [Google Scholar]
- 24.Wilson D., Jackson T., Sapey E., Lord J. M. (2017) Frailty and sarcopenia: the potential role of an aged immune system. Ageing Res. Rev. 36, 1–10 [DOI] [PubMed] [Google Scholar]
- 25.Lescaudron L., Peltékian E., Fontaine-Pérus J., Paulin D., Zampieri M., Garcia L., Parrish E. (1999) Blood borne macrophages are essential for the triggering of muscle regeneration following muscle transplant. Neuromuscul. Disord. 9, 72–80 [DOI] [PubMed] [Google Scholar]
- 26.Tidball J. G., Wehling-Henricks M. (2007) Macrophages promote muscle membrane repair and muscle fibre growth and regeneration during modified muscle loading in mice in vivo. J. Physiol. 578, 327–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Segawa M., Fukada S., Yamamoto Y., Yahagi H., Kanematsu M., Sato M., Ito T., Uezumi A., Hayashi S., Miyagoe-Suzuki Y., Takeda S., Tsujikawa K., Yamamoto H. (2008) Suppression of macrophage functions impairs skeletal muscle regeneration with severe fibrosis. Exp. Cell Res. 314, 3232–3244 [DOI] [PubMed] [Google Scholar]
- 28.Martinez C. O., McHale M. J., Wells J. T., Ochoa O., Michalek J. E., McManus L. M., Shireman P. K. (2010) Regulation of skeletal muscle regeneration by CCR2-activating chemokines is directly related to macrophage recruitment. Am. J. Physiol. Regul. Integr. Comp. Physiol. 299, R832–R842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y., Wehling-Henricks M., Samengo G., Tidball J. G. (2015) Increases of M2a macrophages and fibrosis in aging muscle are influenced by bone marrow aging and negatively regulated by muscle-derived nitric oxide. Aging Cell 14, 678–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Merly F., Lescaudron L., Rouaud T., Crossin F., Gardahaut M. F. (1999) Macrophages enhance muscle satellite cell proliferation and delay their differentiation. Muscle Nerve 22, 724–732 [DOI] [PubMed] [Google Scholar]
- 31.Shen W., Li Y., Zhu J., Schwendener R., Huard J. (2008) Interaction between macrophages, TGF-β1, and the COX-2 pathway during the inflammatory phase of skeletal muscle healing after injury. J. Cell. Physiol. 214, 405–412 [DOI] [PubMed] [Google Scholar]
- 32.Serrano A. L., Muñoz-Cánoves P. (2010) Regulation and dysregulation of fibrosis in skeletal muscle. Exp. Cell Res. 316, 3050–3058 [DOI] [PubMed] [Google Scholar]
- 33.Wehling-Henricks M., Jordan M. C., Gotoh T., Grody W. W., Roos K. P., Tidball J. G. (2010) Arginine metabolism by macrophages promotes cardiac and muscle fibrosis in mdx muscular dystrophy. PLoS One 5, e10763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tidball J. G., Villalta S. A. (2010) Regulatory interactions between muscle and the immune system during muscle regeneration. Am. J. Physiol. Regul. Integr. Comp. Physiol. 298, R1173–R1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Flurkey K., Currer J. M., Harrison D. E. (2007) The mouse in aging research. In The Mouse in Biomedical Research, 2nd ed., (Fox J. G., Barthold S. W., Davisson M. T., Newcomer C. E., Quimby F. W., and Smith A. L., eds.), pp. 637–672, American College Laboratory Animal Medicine (Elsevier), Burlington, MA, USA [Google Scholar]
- 36.Tzankoff S. P., Norris A. H. (1977) Effect of muscle mass decrease on age-related BMR changes. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 43, 1001–1006 [DOI] [PubMed] [Google Scholar]
- 37.Fleg J. L., Lakatta E. G. (1988) Role of muscle loss in the age-associated reduction in VO2 max. J. Appl. Physiol. 65, 1147–1151 [DOI] [PubMed] [Google Scholar]
- 38.Greenlund L. J., Nair K. S. (2003) Sarcopenia--consequences, mechanisms, and potential therapies. Mech. Ageing Dev. 124, 287–299 [DOI] [PubMed] [Google Scholar]
- 39.Haynes L., Swain S. L. (2012) Aged-related shifts in T cell homeostasis lead to intrinsic T cell defects. Semin. Immunol. 24, 350–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goronzy J. J., Weyand C. M. (2017) Successful and maladaptive T cell aging. Immunity 46, 364–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kung T. A., Cederna P. S., van der Meulen J. H., Urbanchek M. G., Kuzon W. M., Jr., Faulkner J. A. (2014) Motor unit changes seen with skeletal muscle sarcopenia in oldest old rats. J. Gerontol. A. Biol. Sci. Med. Sci. 69, 657–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ishida Y., Nagata K. (2011) Hsp47 as a collagen-specific molecular chaperone. Methods Enzymol. 499, 167–182 [DOI] [PubMed] [Google Scholar]
- 43.Fry C. S., Lee J. D., Mula J., Kirby T. J., Jackson J. R., Liu F., Yang L., Mendias C. L., Dupont-Versteegden E. E., McCarthy J. J., Peterson C. A. (2015) Inducible depletion of satellite cells in adult, sedentary mice impairs muscle regenerative capacity without affecting sarcopenia. Nat. Med. 21, 76–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Snow M. H. (1977) The effects of aging on satellite cells in skeletal muscles of mice and rats. Cell Tissue Res. 185, 399–408 [DOI] [PubMed] [Google Scholar]
- 45.Gibson M. C., Schultz E. (1983) Age-related differences in absolute numbers of skeletal muscle satellite cells. Muscle Nerve 6, 574–580 [DOI] [PubMed] [Google Scholar]
- 46.Shefer G., Van de Mark D. P., Richardson J. B., Yablonka-Reuveni Z. (2006) Satellite-cell pool size does matter: defining the myogenic potency of aging skeletal muscle. Dev. Biol. 294, 50–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Day K., Shefer G., Shearer A., Yablonka-Reuveni Z. (2010) The depletion of skeletal muscle satellite cells with age is concomitant with reduced capacity of single progenitors to produce reserve progeny. Dev. Biol. 340, 330–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Du H., Shih C. H., Wosczyna M. N., Mueller A. A., Cho J., Aggarwal A., Rando T. A., Feldman B. J. (2017) Macrophage-released ADAMTS1 promotes muscle stem cell activation. Nat. Commun. 8, 669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Akahori H., Karmali V., Polavarapu R., Lyle A. N., Weiss D., Shin E., Husain A., Naqvi N., Van Dam R., Habib A., Choi C. U., King A. L., Pachura K., Taylor W. R., Lefer D. J., Finn A. V. (2015) CD163 interacts with TWEAK to regulate tissue regeneration after ischaemic injury. Nat. Commun. 6, 7792 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.