Abstract
Exosomes derived from chondroitin sulfate proteoglycan (CSPG) 4 type neural precursor cells (CSPG4Es) were purified from human plasma by sequential immunoabsorption with anti-CSPG4 and anti–platelet growth factor receptor α mAb to characterize the potential in vivo roles of CSPG4 cells in neuronal repair. Hepatocyte growth factor, fibroblast growth factors (FGFs)-2 and -13, and type 1 insulin-like growth factor (IGF-1), which enhance neuronal survival and functions, were quantified in CSPG4E extracts. For CSPG4Es of 24 healthy control subjects, mean levels of hepatocyte growth factor, FGF-13, and IGF-1, but not FGF-2, were significantly higher by up to 7-fold than in their neuronal-derived exosomes, and mean levels of all 4 growth factors were significantly higher by up to 8-fold than in their astrocyte-derived exosomes. Mean CSPG4E levels of all growth factors were significantly lower in patients with mild Alzheimer disease (AD) (n = 24) than in age- and sex-matched cognitively normal control subjects (n = 24). Mean CSPG4E levels of all growth factors were also significantly lower in 15 patients at the stage of moderate dementia from AD (AD2) and at their preclinical stage 3 to 8 yr earlier (AD1), with no differences between values at stages AD1 and AD2. Current findings suggest that CSPG4 cells export in exosomes higher levels of neurotrophic factors than neurons or astrocytes and that CSPG4E neurotrophic factors are diminished early in AD, with no significant progression of decreases later in the course.—Goetzl, E. J., Nogueras-Ortiz, C., Mustapic, M., Mullins, R. J., Abner, E. L., Schwartz, J. B., Kapogiannis, D. Deficient neurotrophic factors of CSPG4-type neural cell exosomes in Alzheimer disease.
Keywords: neurodegeneration, dementia, growth factors
Accumulated evidence strongly suggests that all CNS cells are adversely affected by Alzheimer disease (AD) (1–4). The resultant abnormalities of astroglial cells and damaged neurons, as well as their interactions, are being unraveled, but it is not clear if any type of CNS cell is responsible for the protection and improved survival of neurons in proteinopathic neurodegenerative diseases and whether such functions are altered by AD.
One subtype of CNS neural precursor cells, termed CSPG4 cells in humans, which expresses melanoma-associated membrane chondroitin sulfate proteoglycan (CSPG), representing 5% to 6% of CNS neural cells, are distributed diffusely in the brain throughout human life and differentiate into oligodendrocytes in several developmental waves, as well as into distinct subsets of astrocytes and neurons (5, 6). In addition to their extreme developmental plasticity, CSPG4 cells generate and secrete several proteins that may play roles in both repair and regeneration of neurons (7–9).
Hepatocyte growth factor (HGF) is the most characteristic neuronal precursor growth and survival protein of CSPG4 cells, and promotes mature neuron extension and dendrite maturation with preferential effects on hippocampal and some cortical neurons (9–11). Positive effects of HGF on neuronal precursor growth, morphogenesis, and survival have been demonstrated in rodent models of acute neural injury, neural ischemia, and neurodegeneration. As for HGF, IGF-1 is produced at functional levels by CSPG4 cells but has more pleiotropic effects, including enhanced mobility, differentiation, maturation, and regeneration of glia as well as neurons, and more pronounced promotion of synapse formation (12–14). CNS levels of receptors for IGF-1 and HGF remain high throughout life, and levels of both growth factors also are maintained with age in some areas of the CNS, such as the hippocampus (13). Support of neuronal survival by IGF-1 and HGF involves signaling through the serine–threonine protein kinase Akt (15). At least 2 types of secreted fibroblast growth factor (FGF), acidic FGF (aFGF or FGF-1) and basic FGF (bFGF or FGF-2), have been identified in CSPG4 cells (9). FGF-1 also is found immunochemically in ependymal cells of the third ventricle and some reactive astrocytes, whereas FGF-2 is more broadly distributed in astrocytes and neurons (16, 17). Both types of FGF enhance differentiation, proliferation, and survival of neuron precursors (18–20).
Each of these growth factors is found in a different distribution in brain tissues of AD patients than in control subjects, with the highest levels in proteinopathic lesions of AD brain tissues (16, 21, 22). Further, levels of HGF and FGF-2 are higher and those of FGF-1 and IGF-1 are lower overall in brain tissues of AD patients than control subjects. Plasma and cerebrospinal fluid levels of the growth factors have been less consistently related than tissue levels to the risk or stage of AD (16, 21, 22). Analyses of concentration dependence of neuronal effects of the growth factors have shown major changes in AD only for IGF-1, to which responses are decreased compared to control subjects, much like the observed resistance to insulin (23). AD-specific neuron-protective mechanisms of growth factors have been partially elucidated for IGF-1. Both IGF-1 and insulin protect neurons from proteinopathic damage in AD by evoking release of amyloid β-peptide (Aβ) oligomers bound to neurons, increasing clearance of Aβ oligomers from the CNS and concurrently suppressing phosphorylation of tau (23, 24).
Exosomes derived from neurons, astrocytes, and some other types of cells have been separately enriched from the total exosome population in plasma using immunochemical absorption methods (25, 26). In the present study, enrichment of CSPG4 cell–derived exosomes (CSPG4Es) from human plasma with 2 sequential immunochemical absorption steps using anti-CSPG4 antibody and then anti–platelet growth factor receptor α (PDGFRα) antibody has permitted demonstration of cargo levels of several growth factors that are higher than those in exosomes from neurons or astrocytes. The present finding of distinctively lower levels of such growth and survival factors in CSPG4Es of patients with AD than in those of matched control subjects now has allowed initial direct ex vivo analyses of the abnormalities of CSPG4 cells in proteinopathic neurodegenerative diseases.
MATERIALS AND METHODS
Experimental design and patient evaluation
For cross-sectional studies, we retrospectively identified 24 patients with mild cognitive impairment or mild dementia due to AD who had been evaluated extensively in the Clinical Research Unit of the National Institute on Aging (NIA; Baltimore, MD, USA) and 24 age- and sex-matched cognitively normal control subjects who had donated blood at the Jewish Home of San Francisco in the same time period as the patients (Table 1). For longitudinal studies, we selected 15 participants at the University of Kentucky, Lexington, AD Center who had moderate AD and who had provided blood first when cognitively intact (AD1, Table 1) and again 3 to 8 yr after diagnosis of dementia due to AD (AD2, Table 1). Fifteen cognitively normal control subjects at the Jewish Home of San Francisco who were age and sex matched with the AD1 group had plasma samples available for analysis from the same time period. One investigator (E.J.G.) supervised identification and storage of all plasma samples by the same methods and processed all plasma samples together by the same procedures. Plasma samples from patients in the longitudinal studies were analyzed by personnel without knowledge of the clinical data.
TABLE 1.
Characteristics of AD patients and control subjects
| Diagnosis | N | Sex (M/F) | Age (mean ± sem) | MMSE (mean ± sem) | ADAS-cog (mean ± sem) |
|---|---|---|---|---|---|
| Cross-sectional sets | |||||
| C | 24 | 9/15 | 73.1 ± 1.82 | 29.3 ± 0.19 | 3.33 ± 0.24 |
| AD | 24 | 9/15 | 73.1 ± 1.60 | 26.1 ± 0.90* | 13.2 ± 1.39* |
| Longitudinal sets | |||||
| C | 15 | 7/8 | 80.2 ± 1.78 | 29.4 ± 0.62 | ND |
| AD1 | 15 | 7/8 | 80.7 ± 1.84 | 28.3 ± 0.46 | ND |
| AD2 | 15 | 7/8 | 84.5 ± 1.70 | 24.3 ± 0.90* | ND |
AD and C are patients and control subjects in cross-sectional study of AD. AD1 and AD2 are groups of AD patients evaluated 2 times in longitudinal study, at preclinical phase (AD1) and after conversion to moderate dementia (AD2); C refers to control subjects matched to AD1 patients. ND, not done. Significance of differences between cognitive state (MMSE, ADAS-cog) values of groups were calculated by an unpaired Student’s t test for C vs. AD and for C vs. AD1, and by a paired Student’s t test for AD1 vs. AD2. *P < 0.001.
Patients with AD and control subjects had mental status testing at the time of each blood sampling. The Mini-Mental State Examination (MMSE) and the AD Assessment Scale–Cognitive Subscale (ADAS-cog) were administered as previously described (27). Cross-sectional study patients from the NIA had amnestic mild cognitive impairment or mild dementia with high probability of AD and a Clinical Dementia Rating global score of 0.5 or 1.0 according to NIA–Alzheimer Association and International Working Group 2 criteria (28, 29). All NIA cross-sectional patients with AD had abnormal cerebrospinal fluid levels of Aβ 1-42 and P-T181-tau that supported their diagnosis (30). AD1 and AD2 patients from University of Kentucky had probable AD and mild to moderate dementia at the AD2 stage according to National Institute of Neurologic and Communicative Disorders and Stroke–Alzheimer Disease and Related Disorders Association criteria and had a Clinical Dementia Rating global score of 1.0 at the time of the second blood collection (31). The protocol and procedures of this study received prior approval by the institutional review boards of the University of California, San Francisco, the University of Kentucky, and the NIA Intramural Program. Informed consent was obtained from each participant.
Blood and cerebrospinal fluid from patients and control participants
Ten milliliters of venous blood were drawn by syringe into 0.5 ml of saline with EDTA, incubated for 10 min at room temperature, and centrifuged for 15 min at 2500 g.
Plasma samples were stored in 0.25 ml aliquots at −80°C. Cerebrospinal fluid levels of P-T181-tau and Aβ 1-42 were quantified by xMAP Technology (Luminex, Austin, TX, USA) using Inno-Bia AlzBio3 Kits (Innogenetics, Ghent, Belgium).
Spatial covariance analysis
Correlations were performed using the Allen Human Brain Atlas website (AHBA; http://human.brain-map.org/). The AHBA consists of numerous well-distributed microarray samples (∼500 per brain) from brain specimens of 6 cognitively normal humans. Detailed methods, case qualification, and donor profiles are available online (http://help.brain-map.org/display/humanbrain/documentation). The CSPG4 gene was compared to other genes of interest using the correlative search function to evaluate similarity of spatial expression profiles. R coefficient values quantitatively represent CSPG4 gene spatial coexpression with ligands and receptors of interest in relation to our findings. Positive R coefficients ranging from 0.400 to close to 1.00 indicated spatial expression in an increasingly similar pattern to CSPG4, while lower and negative R coefficients indicated less similar and reversed patterns, respectively.
Enrichment of plasma CSPG4Es, neuron-derived exosomes, and astrocyte-derived exosomes for extraction and ELISA quantification of cargo proteins
Aliquots of 0.25 ml of plasma were incubated with 0.10 ml of thromboplastin-D (Thermo Fisher Scientific, Waltham, MA, USA) followed by addition of 0.15 ml of Dulbecco’s balanced salt solution (DBS) with protease inhibitor cocktail (Roche Applied Sciences, Indianapolis, IN, USA) and phosphatase inhibitor cocktail (Pierce Halt; Thermo Fisher Scientific) as previously described (25). After centrifugation at 3000 g for 30 min at 4°C, supernatants were incubated with 126 µl of ExoQuick exosome precipitation solution (EXOQ; System Biosciences, Mountain View, CA, USA), and the resultant suspensions were centrifuged at 1500 g for 30 min at 4°C. Each pellet was resuspended in 350 μl of distilled water with inhibitor cocktails for immunochemical enrichment of CSPG4Es.
Each total plasma exosome suspension was mixed and incubated with 1.5 µg of rat monoclonal IgG1 anti-human CSPG4 biotinylated antibody (clone 1E6.4; Miltenyi Biotec, San Diego, CA, USA) in 50 µl of 3% bovine serum albumin (BSA) for 90 min, followed by incubation with 10 μl of Streptavidin-Plus UltraLink resin (Pierce; Thermo Fisher Scientific) in 40 µl of 3% BSA for 60 min with continuous mixing at room temperature. After centrifugation at 800 g and removal of the supernatant, each pellet was resuspended in 100 µl cold 0.05 M acetic acid, incubated at 4°C for 10 min, and centrifuged at 4°C for 10 min at 4000 g. These supernatants were transferred to new prechilled Eppendorf 1.5 ml tubes containing 265 µl DBS, 10 µl 1 M Tris-HCl (pH 8.0), and 25 µl of 10% BSA, and mixed. Each of these exosome suspensions was mixed with 1.5 µg of mouse monoclonal IgG1κ anti-human platelet growth factor receptor α (PDGFRα)/CD140a biotinylated antibody (BioLegend, San Diego, CA, USA) in 50 µl of 3% BSA and incubated for 90 min, followed by incubation with 10 μl of Streptavidin-Plus UltraLink resin (Pierce; Thermo Fisher Scientific) in 40 µl of 3% BSA for 60 min, all at room temperature. After centrifugation at 800 g and removal of the supernatant, each pellet was resuspended in 100 µl of cold 0.05 M acetic acid, incubated at 4°C for 10 min, and centrifuged at 4°C for 10 min at 4000 g. These supernatants were transferred to new prechilled Eppendorf tubes containing 10 µl of 1 M Tris-HCl (pH 8.0) and 25 µl of 10% BSA, and mixed. Five percent of each suspension was transferred to 300 µl Eppendorf tubes for counting before addition of 365 µl of M-PER mammalian protein extraction reagent (Thermo Fisher Scientific) containing the protease and phosphatase inhibitor cocktails and storage at −80°C. Neuron-derived exosomes (NDEs) and astrocyte-derived exosomes (ADEs) were enriched immunochemically from plasmas of the same patients and control subjects as previously described (25, 26).
For counting of exosomes, each suspension was diluted 1:50 in PBS. The mean diameter (nanometers) and concentration (particles per milliliter) of exosomes in each suspension were determined by nanoparticle tracking analysis (NTA) using the Nanosight NS500 system with a G532nm laser module and NTA 3.1 nanoparticle tracking software (Malvern Instruments, Malvern, United Kingdom). Camera settings were as follows: gain 366; shutter 31.48; and frame rate 24.9825 frames/s. Brownian motion was captured by 5 repeated 60 s video recordings.
Exosome cargo proteins were quantified by ELISA kits for human myelin–oligodendrocyte glycoprotein (MOG), FGF-13, GluA4-containing glutamate receptors (AMPA4), neuronal pentraxin 2 (NPTX2), neurofilament light chain (NF-Lch) and the tetraspanning exosome marker CD81 (Cusabio; American Research Products, Waltham, MA, USA), glutamine synthetase (GluSyn) (Cloud-Clone, Katy, TX, USA; American Research Products), IGF-1, hepatocyte growth factor (HGF) (Abcam, Cambridge, MA, USA), and FGF-2 (Thermo Fisher Scientific), according to the manufacturer’s instructions. The mean value for all determinations of CD81 in each assay group was set at 1.00, and the relative individual values of CD81 for each sample were used to normalize recovery.
Statistical analyses
A Shapiro-Wilks test showed that data in all sets were distributed normally. The statistical significance of differences between means for each patient group and their respective control group was determined by an unpaired Student’s t test including a Bonferroni correction (GraphPad Prism 6; GraphPad Software, La Jolla, CA, USA). The statistical significance of differences between mean values for groups AD1 and AD2 were calculated with a paired Student’s t test (GraphPad Prism 6). Reproducibility assessed by repeating isolation of CSPG4Es from plasma of 5 AD patients and 5 control subjects, and ELISAs for 3 analytes gave results within ± 14% of the first quantification.
RESULTS
Participant demographics
In this cross-sectional series, patients with probable AD with mild dementia showed significantly lower mean MMSE and higher ADAS-cog than matched control subjects (Table 1). Participants in the longitudinal sets were older than those in the cross-sectional sets at the time of entry into the study (C and AD1) and had moderate dementia after conversion to AD (AD2), as documented by significantly lower mean MMSE scores (Table 1).
Spatial genetic covariance of CSPG4 with growth factors and growth factor receptors
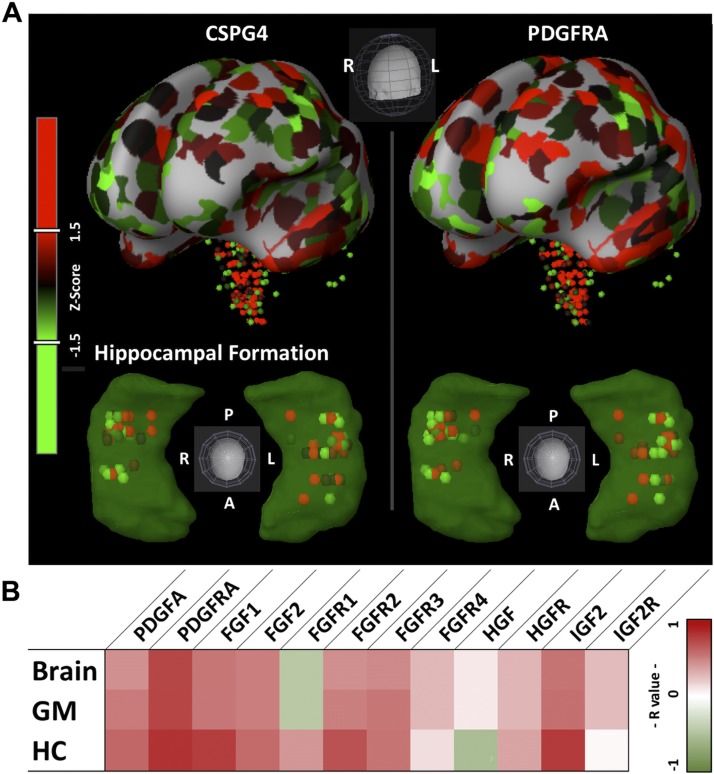
The correlative search function of the AHBA showed highly similar distribution of the CSPG4 gene with the PDGFRα gene and moderately similar distribution of the CSPG4 gene with genes encoding FGF1, FGF2, FGF receptor (FGFR) 2, and FGFR3 widely in whole brain, as well as in gray matter and hippocampus (Fig. 1 and Table 2). These findings support the previously observed high levels of expression of PDGFRα by CSPG4 cells and suggest the possibility that CSPG4 cells have high levels of FGFs and some FGFRs.
Figure 1.
CSPG4 spatial expression correlation. A) Brain Explorer 2 (v.2.3.5) images of microarray sample locations and z-score (patches or globes; red high, green low) intensities for representative specimen H0351.2001. Whole brain is shown in the top row; hippocampal formation of the above whole brain and genes shaded in green are shown in the lower row. Color bar indicates z score from Brain Explorer 2. B) Heat map (red high, green low) showing R values for gene probes of interest within the brain, gray matter (GM), and hippocampus (HC). Color bar and color map are from Microsoft Excel conditional formatting tool with 3 colors (r = 1 dark red, r = 0 white, r = −1 dark green).
TABLE 2.
Brain CSPG4 gene spatial expression correlation
| Gene |
R coefficient |
||
|---|---|---|---|
| Whole brain | Gray matter | Hippocampus | |
| PDGFRα | 0.731 | 0.734 | 0.819 |
| FGF1 | 0.537 | 0.533 | 0.769 |
| FGF2 | 0.503 | 0.503 | 0.586 |
| FGFR1 | −0.437 | −0.438 | 0.41 |
| FGFR2 | 0.438 | 0.501 | 0.683 |
| FGFR3 | 0.461 | 0.534 | 0.543 |
| FGFR4 | 0.281 | 0.28 | 0.134 |
| HGF | 0.091 | 0.092 | −0.527 |
| HGFR | 0.293 | 0.287 | 0.355 |
| IGF-2 | 0.547 | 0.561 | 0.784 |
| IGF-2R | 0.264 | 0.264 | 0.034 |
R coefficient values derived from AHBA correlative search function for genes of interest. HGFR, HGF receptor; IGFR, IGF receptor.
Prevalence and properties of CSPG4Es
The mean number of CSPG4Es recovered from total plasma exosomes by sequential immunoabsorption with anti-human CSPG4 and then anti-human CD140a/PDGFRα mAb were 50% of those of ADEs and 15% of those of NDEs, with no difference between the levels for AD patients and matched control subjects (Table 3). The size distributions of CSPG4Es were the same as for NDEs and ADEs at 184 ± 11.3 nm for controls and 168 ± 20.7 nm for AD patients. When quantified by amounts of extracted CD81 exosome marker protein, the relative plasma levels of the 3 types of exosomes were the same as those estimated by direct counting. Using the ratio of mean level of CD81 protein in CSPG4Es to that in total plasma exosomes, CSPG4Es represent ∼2% of the total pool (Table 3).
TABLE 3.
Frequency and analyte levels of neural cell–derived exosomes in plasma
| Total |
NDE |
ADE |
CSPG4E |
||
|---|---|---|---|---|---|
| Exosome type | Control | Control | Control | Control | AD |
| Count (×109/ml) | ND | 265 ± 47.8* | 85.6 ± 17.3* | 41.5 ± 10.6 | 38.3 ± 10.2 |
| CD81 (pg/ml) | 38,454 ± 3978 | 4363 ± 204* | 1453 ± 110* | 875 ± 59.2 | 702 ± 51.7 |
| GluSyn (pg/ml) | ND | 337 ± 15.5† | 2277 ± 131* | 207 ± 19.0 | 127 ± 13.1† |
| NF-Lch (pg/ml) | ND | 8645 ± 277* | 834 ± 111* | 1319 ± 52.4 | 1310 ± 82.9 |
| MOG (pg/ml) | ND | 19.9 ± 5.18* | 21.0 ± 2.36* | 157 ± 10.1 | 20.0 ± 5.18* |
| NPTX2 (pg/ml) | ND | 2321 ± 280* | 148 ± 16.3† | 509 ± 61.2 | 269 ± 26.3† |
| AMPA4 (pg/ml) | ND | 5195 ± 162* | 794 ± 33.6† | 2084 ± 218 | 1012 ± 92.7* |
All values are expressed as means ± sem. Statistical significance of differences between levels for control CSPG4Es (n = 24) and those for AD CSPG4Es (n = 24), control NDEs (n = 10), or control ADEs (n = 10) was calculated by an unpaired Student’s t test. Total plasma exosomes were from 10 control participants. ND, not done. *P < 0.0001, †P ≤ 0.001.
Marker proteins characteristic of astroglial cells (GluSyn), neurons (NF-Lch), and oligodrendrocytes (MOG) were quantified and normalized by CD81 level in extracts of the 3 types of enriched exosomes from control subjects. CSPG4Es and NDEs had far lower levels of GluSyn than ADEs, CSPG4Es had levels of NF-Lch significantly higher than ADEs and significantly lower than NDEs, and CSPG4Es had significantly higher levels of MOG than ADEs or NDEs (Table 3). Thus, by neural cell–type marker protein levels in their exosomes, CSPG4 cells appear to partially resemble oligodendrocytes and neurons, but not astroglial cells. CSPG4 cell similarity to neurons was supported by levels of the neuronal synaptic proteins NPTX2 and AMPA4 in CSPG4Es, which were significantly higher than in ADEs but lower than in NDEs (Table 3). This finding is consistent with previous suggestions that CSPG4 cells may form synapses with and receive signals from neurons (6). Contrasting CSPG4E levels of neural cell-type marker proteins for AD patients with those of control subjects showed significantly lower values in the AD patients for GluSyn, MOG, NPTX2, and AMPA4, but not NF-Lch (Table 3).
CSPG4E growth factors
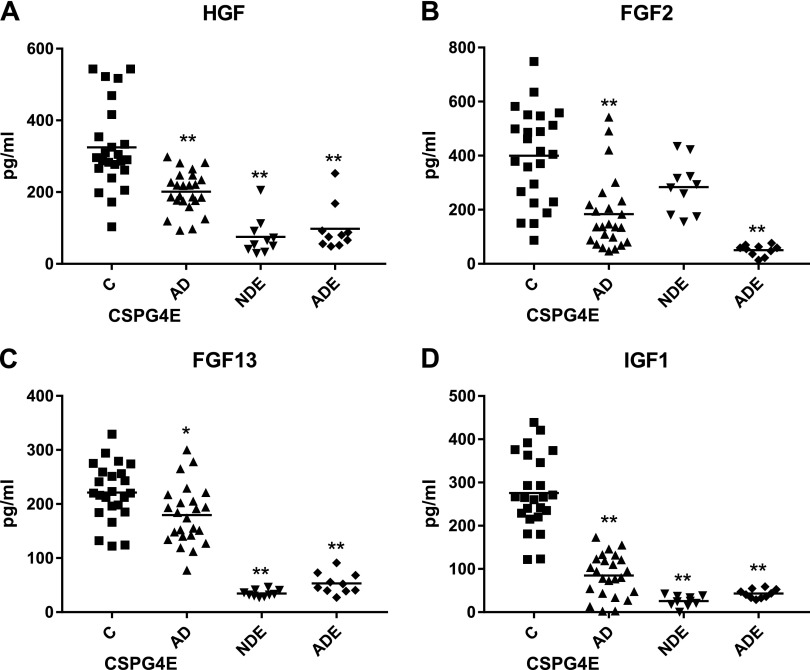
Quantification and normalization by CD81 content of control subjects’ CSPG4E levels of several growth and survival factors known to be present in intact CSPG4 cells by immunocytochemical staining (7, 9) showed significantly higher levels than in NDEs and ADEs for all but FGF-2, where CSPG4E and NDE values were indistinguishable (Fig. 2). For these 4 growth factors of CSPG4Es, levels of control subjects were significantly higher than in AD patients.
Figure 2.
CSPG4 cell–derived exosome levels of growth factors in cross-sectional control and AD groups. Each point represents value for 1 subject, and horizontal line in point clusters depicts mean level for that group. Data depicted are for HGF (A), FGF-2 (B), FGF-13 (C), and IGF-1 (D). Mean ± sem values for control subject and AD patient CSPG4Es, and for NDEs and ADEs of same control subjects, respectively, are 325 ± 24.4 and 201 ± 11.5 pg/ml, and 75.1 ± 16.6 and 97.8 ± 20.3 pg/ml for HGF; 399 ± 35.1 and 184 ± 27.7 pg/ml, and 284 ± 30.6 and 50.3 ± 6.41 pg/ml for FGF-2; 222 ± 10.8 and 180 ± 11.1 pg/ml, and 34.6 ± 1.97 and 53.2 ± 6.07 pg/ml for FGF-13; 136 ± 13.1 and 84.9 ± 9.98 pg/ml, and 25.5 ± 4.05 and 42.9 ± 3.22 pg/ml for IGF-1. Significance of differences between levels for control subjects and AD patients was calculated by unpaired Student’s t test; *P ≤ 0.01, **P < 0.0001.
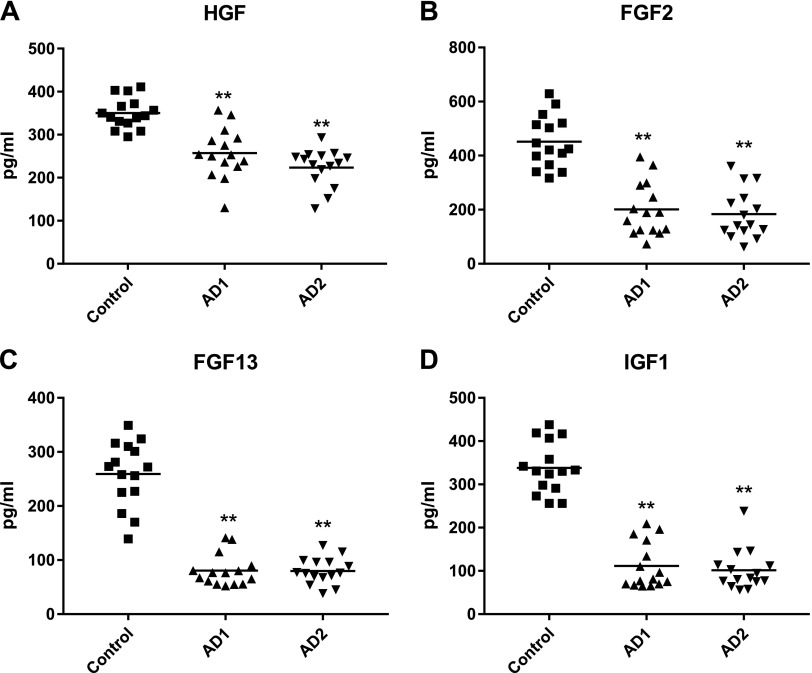
A longitudinal study of CSPG4E levels of growth factors was conducted in 15 AD patients and in 15 age- and sex-matched control subjects to examine changes that occurred with the progression of AD over 3 to 8 yr from the preclinical phase (AD1) to that of mild to moderate dementia (AD2). At the AD1 preclinical stage, mean CSPG4E levels of all 4 growth and survival factors were significantly lower in patients than in matched control subjects, and there was no progression of any decreases in the ensuing 3 to 8 yr (Fig. 3).
Figure 3.
CSPG4 cell–derived exosome levels of growth factors in longitudinal study. Data depicted are for HGF (A), FGF-2 (B), FGF-13 (C), and IGF-1 (D). Mean ± sem values for CSPG4Es of control subjects, AD patients at preclinical stage (AD1), and same AD patients later in time with mild to moderate dementia (AD2), respectively, are 350 ± 9.19, 224 ± 11.2, and 257 ± 15.0 pg/ml for HGF; 451 ± 24.8, 201 ± 25.3, and 184 ± 23.4 pg/ml for FGF-2; 259 ± 15.5, 80.5 ± 7.50, and 79.8 ± 6.33 pg/ml for FGF-13; and 338 ± 15.4, 111 ± 13.7, and 101 ± 12.1 pg/ml for IGF-1. Significance of differences between levels for control subjects and AD1 patients were calculated by unpaired Student’s t test, whereas that between levels for AD1 and AD2 patients were calculated by paired Student’s t test; **P < 0.0001.
DISCUSSION
CSPG4 oligodendrocyte neuronal precursor cells have the distinctive characteristics of widespread CNS distribution throughout life (5–9) and a unique profile of exosomal proteins that differs quantitatively from that of neurons and astrocytes (Table 3). The likely high level of coexpression by CSPG4 cells of PDGFRα with CSPG4 (Fig. 1 and Table 2) presumably explains the success of enrichment of CSPG4Es by sequential immunoabsorption with antibodies to these 2 membrane surface proteins.
A functionally relevant aspect of the CSPG4E protein mediator profile is significantly higher levels than NDEs and ADEs of at least 4 neurotrophic factors (Fig. 2). Each of these neurotrophic factors has a different range of effects on neuronal differentiation, proliferation, survival, and other neural functions, thus suggesting the possibility that combinations may have complementary beneficial actions. Proliferation and differentiation of mammalian embryonic neuronal precursors are enhanced by HGF, IGF-1, and secreted FGF-2, but not intracellular FGF-13, with CNS regional and stage-specific differences, but much less or no such effects on most mature neural cells (13, 32–36). FGF-2 also stimulates proliferation of astrocytes and promotes organization of spinal anatomy (37). IGF-1 has the broadest range of neuronal functions, including facilitation of synaptic development, adaptation to stress, enhancement of survival, and repair of injuries (36). Intracellular FGF-13 binds to and stabilizes neuronal microtubules and thereby augments neuronal migration and polarization, as well as axonal branching and leading process development (38, 39). The array of neuronal mediators contained in CSPG4Es as a potential vehicle for delivery to injured neurons thus suggests a major role for this cell type in neuronal survival and repair.
Because AD alters many constituents and functions of all CNS cells, it is not surprising that CSPG4E cargo levels of marker proteins and neurotrophic factors are significantly different in AD patients than in matched control subjects. Several protein markers of neural cell identity showed lower levels in CSPG4Es of AD patients than control subjects (Table 3). Mean CD81-normalized levels of all 4 neurotrophic factors also are decreased in a cross-sectional analysis of CSPG4Es from AD patients, in contrast to those in CSPG4Es of matched control subjects without a difference between the plasma concentrations of CSPG4Es of the 2 groups (Fig. 2 and Table 3). Further, CSPG4E levels of all 4 neurotrophic factors had declined significantly from those of matched control subjects in AD1 and remained depressed at the same levels 3 to 8 yr later, when patients had developed moderate dementia (AD2) (Fig. 3).
The course of changes in levels of AD-relevant protein constituents of CSPG4Es resembles those of NDEs and ADEs in becoming abnormal at preclinical stages up to 12 yr before any aspect of dementia is clinically detectable (26, 40, 41). However, CSPG4Es represent only a mean of about 12 to 16% of the total plasma exosomes derived from CNS cells, CSPG4E levels of neurotrophic factors for AD patients and control subjects have substantial overlap, and there is no change in CSPG4E levels of neurotrophic factors for AD patients between AD1 and AD2. Thus, it is unlikely that these constituent proteins will be useful biomarkers for the prediction of onset or assessment of severity or estimation of likely responsiveness to treatment of AD. However, the finding of depressed levels of mediators of endogenous neuronal repair in AD may inform new approaches to therapies involving administration or greater local generation of protein mediators of neuronal repair in neurodegenerative diseases.
ACKNOWLEDGMENTS
The authors thank J. H. Goetzl (Jewish Home of San Francisco) for expert preparation of the illustrations. D.K., C.N.-O., and M.M. were supported by the Intramural Research Program of the U.S. National Institutes of Health/National Institute on Aging. E.L.A. was supported by NIA Grant P30 AG028383. E.J.G. has filed an application with the U.S. Patent Office for the platform and methodologies described in this report. All other authors declare no conflicts of interest.
Glossary
- AD
Alzheimer disease
- AD1
preclinical stage of Alzheimer disease
- AD2
later stage of Alzheimer disease with dementia
- ADAS-cog
AD Assessment Scale–Cognitive Subscale
- ADE
astrocyte-derived exosome
- AHBA
Allen Human Brain Atlas
- AMPA4
GluA4-containing glutamate receptors
- Aβ
amyloid β-peptide
- BSA
bovine serum albumin
- CSPG
chondroitin sulfate proteoglycan
- CSPG4 cells
subset of CNS cells that express melanoma-associated membrane chondroitin sulfate proteoglycan
- CSPG4E
CSPG4 cell–derived exosome
- FGF
fibroblast growth factor
- FGFR
fibroblast growth factor receptor
- GluSyn
glutamine synthetase
- HGF
hepatocyte growth factor
- MMSE
Mini-Mental State Examination
- MOG
myelin–oligodendrocyte glycoprotein
- NDE
neuron-derived exosome
- NF-Lch
neurofilament light chain
- NIA
U.S. National Institute on Aging
- NPTX2
neuronal pentraxin 2
- NTA
nanoparticle tracking analysis
- PDGFRα
platelet growth factor receptor α
AUTHOR CONTRIBUTIONS
E. J. Goetzl developed the initial concept and approach; E. J. Goetzl, C. Nogueras-Ortiz, M. Mustapic, and D. Kapogiannis designed the study; E. J. Goetzl performed the exosome isolations and ELISAs; R. J. Mullins conducted the spatial genetic covariance analyses; E. L. Abner, J. B. Schwartz, and D. Kapogiannis selected and evaluated the patients and control participants; and E. J. Goetzl, C. Nogueras-Ortiz, M. Mustapic, E. L. Abner, J. B. Schwartz, and D. Kapogiannis prepared and edited the report.
REFERENCES
- 1.Vinters H. V. (2015) Emerging concepts in Alzheimer’s disease. Annu. Rev. Pathol. 10, 291–319 [DOI] [PubMed] [Google Scholar]
- 2.Aron L., Yankner B. A. (2016) Neurodegenerative disorders: neural synchronization in Alzheimer’s disease. Nature 540, 207–208 [DOI] [PubMed] [Google Scholar]
- 3.Palop J. J., Mucke L. (2016) Network abnormalities and interneuron dysfunction in Alzheimer disease. Nat. Rev. Neurosci. 17, 777–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goetzl E. J., Miller B. L. (2017) Multicellular hypothesis for the pathogenesis of Alzheimer’s disease. FASEB J. 31, 1792–1795 [DOI] [PubMed] [Google Scholar]
- 5.Nishiyama A. (2001) NG2 cells in the brain: a novel glial cell population. Hum. Cell 14, 77–82 [PubMed] [Google Scholar]
- 6.Nishiyama A., Komitova M., Suzuki R., Zhu X. (2009) Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity. Nat. Rev. Neurosci. 10, 9–22 [DOI] [PubMed] [Google Scholar]
- 7.Eugenín-von Bernhardi J., Dimou L. (2016) NG2-glia, more than progenitor cells. Adv. Exp. Med. Biol. 949, 27–45 [DOI] [PubMed] [Google Scholar]
- 8.Sun W., Matthews E. A., Nicolas V., Schoch S., Dietrich D. (2016) NG2 glial cells integrate synaptic input in global and dendritic calcium signals. Elife 5, e16262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakano M., Tamura Y., Yamato M., Kume S., Eguchi A., Takata K., Watanabe Y., Kataoka Y. (2017) NG2 glial cells regulate neuroimmunological responses to maintain neuronal function and survival. Sci. Rep. 7, 42041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lim C. S., Walikonis R. S. (2008) Hepatocyte growth factor and c-Met promote dendritic maturation during hippocampal neuron differentiation via the Akt pathway. Cell. Signal. 20, 825–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsumoto K., Funakoshi H., Takahashi H., Sakai K. (2014) HGF-met pathway in regeneration and drug discovery. Biomedicines 2, 275–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guan J., Williams C. E., Skinner S. J., Mallard E. C., Gluckman P. D. (1996) The effects of insulin-like growth factor (IGF)-1, IGF-2, and des-IGF-1 on neuronal loss after hypoxic-ischemic brain injury in adult rats: evidence for a role for IGF binding proteins. Endocrinology 137, 893–898 [DOI] [PubMed] [Google Scholar]
- 13.Nieto-Estévez V., Defterali Ç., Vicario-Abejón C. (2016) IGF-I: a key growth factor that regulates neurogenesis and synaptogenesis from embryonic to adult stages of the brain. Front. Neurosci. 10, 52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bianchi V. E., Locatelli V., Rizzi L. (2017) Neurotrophic and neuroregenerative effects of GH/IGF1. Int. J. Mol. Sci. 18, E2441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dudek H., Datta S. R., Franke T. F., Birnbaum M. J., Yao R., Cooper G. M., Segal R. A., Kaplan D. R., Greenberg M. E. (1997) Regulation of neuronal survival by the serine–threonine protein kinase Akt. Science 275, 661–665 [DOI] [PubMed] [Google Scholar]
- 16.Stopa E. G., Gonzalez A. M., Chorsky R., Corona R. J., Alvarez J., Bird E. D., Baird A. (1990) Basic fibroblast growth factor in Alzheimer’s disease. Biochem. Biophys. Res. Commun. 171, 690–696 [DOI] [PubMed] [Google Scholar]
- 17.Mashayekhi F., Hadavi M., Vaziri H. R., Naji M. (2010) Increased acidic fibroblast growth factor concentrations in the serum and cerebrospinal fluid of patients with Alzheimer’s disease. J. Clin. Neurosci. 17, 357–359 [DOI] [PubMed] [Google Scholar]
- 18.Walicke P., Cowan W. M., Ueno N., Baird A., Guillemin R. (1986) Fibroblast growth factor promotes survival of dissociated hippocampal neurons and enhances neurite extension. Proc. Natl. Acad. Sci. USA 83, 3012–3016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Unsicker K., Reichert-Preibsch H., Schmidt R., Pettmann B., Labourdette G., Sensenbrenner M. (1987) Astroglial and fibroblast growth factors have neurotrophic functions for cultured peripheral and central nervous system neurons. Proc. Natl. Acad. Sci. USA 84, 5459–5463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stemple D. L., Mahanthappa N. K., Anderson D. J. (1988) Basic FGF induces neuronal differentiation, cell division, and NGF dependence in chromaffin cells: a sequence of events in sympathetic development. Neuron 1, 517–525 [DOI] [PubMed] [Google Scholar]
- 21.Fenton H., Finch P. W., Rubin J. S., Rosenberg J. M., Taylor W. G., Kuo-Leblanc V., Rodriguez-Wolf M., Baird A., Schipper H. M., Stopa E. G. (1998) Hepatocyte growth factor (HGF/SF) in Alzheimer’s disease. Brain Res. 779, 262–270 [DOI] [PubMed] [Google Scholar]
- 22.Thorns V., Licastro F., Masliah E. (2001) Locally reduced levels of acidic FGF lead to decreased expression of 28-kda calbindin and contribute to the selective vulnerability of the neurons in the entorhinal cortex in Alzheimer’s disease. Neuropathology 21, 203–211 [DOI] [PubMed] [Google Scholar]
- 23.Carro E., Torres-Aleman I. (2004) The role of insulin and insulin-like growth factor I in the molecular and cellular mechanisms underlying the pathology of Alzheimer’s disease. Eur. J. Pharmacol. 490, 127–133 [DOI] [PubMed] [Google Scholar]
- 24.Pitt J., Wilcox K. C., Tortelli V., Diniz L. P., Oliveira M. S., Dobbins C., Yu X. W., Nandamuri S., Gomes F. C. A., DiNunno N., Viola K. L., De Felice F. G., Ferreira S. T., Klein W. L. (2017) Neuroprotective astrocyte-derived insulin/insulin-like growth factor 1 stimulates endocytic processing and extracellular release of neuron-bound Aβ oligomers. Mol. Biol. Cell 28, 2623–2636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goetzl E. J., Kapogiannis D., Schwartz J. B., Lobach I. V., Goetzl L., Abner E. L., Jicha G. A., Karydas A. M., Boxer A., Miller B. L. (2016) Decreased synaptic proteins in neuronal exosomes of frontotemporal dementia and Alzheimer’s disease. FASEB J. 30, 4141–4148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goetzl E. J., Mustapic M., Kapogiannis D., Eitan E., Lobach I. V., Goetzl L., Schwartz J. B., Miller B. L. (2016) Cargo proteins of plasma astrocyte-derived exosomes in Alzheimer’s disease. FASEB J. 30, 3853–3859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cano S. J., Posner H. B., Moline M. L., Hurt S. W., Swartz J., Hsu T., Hobart J. C. (2010) The ADAS-cog in Alzheimer’s disease clinical trials: psychometric evaluation of the sum and its parts. J. Neurol. Neurosurg. Psychiatry 81, 1363–1368 [DOI] [PubMed] [Google Scholar]
- 28.Petersen R. C. (2004) Mild cognitive impairment as a diagnostic entity. J. Intern. Med. 256, 183–194 [DOI] [PubMed] [Google Scholar]
- 29.Sperling R. A., Aisen P. S., Beckett L. A., Bennett D. A., Craft S., Fagan A. M., Iwatsubo T., Jack C. R., Jr., Kaye J., Montine T. J., Park D. C., Reiman E. M., Rowe C. C., Siemers E., Stern Y., Yaffe K., Carrillo M. C., Thies B., Morrison-Bogorad M., Wagster M. V., Phelps C. H. (2011) Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging–Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 7, 280–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shaw L. M., Vanderstichele H., Knapik-Czajka M., Clark C. M., Aisen P. S., Petersen R. C., Blennow K., Soares H., Simon A., Lewczuk P., Dean R., Siemers E., Potter W., Lee V. M., Trojanowski J. Q.; Alzheimer’s Disease Neuroimaging Initiative (2009) Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann. Neurol. 65, 403–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dubois B., Feldman H. H., Jacova C., Dekosky S. T., Barberger-Gateau P., Cummings J., Delacourte A., Galasko D., Gauthier S., Jicha G., Meguro K., O’brien J., Pasquier F., Robert P., Rossor M., Salloway S., Stern Y., Visser P. J., Scheltens P. (2007) Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 6, 734–746 [DOI] [PubMed] [Google Scholar]
- 32.Maina F., Hilton M. C., Andres R., Wyatt S., Klein R., Davies A. M. (1998) Multiple roles for hepatocyte growth factor in sympathetic neuron development. Neuron 20, 835–846 [DOI] [PubMed] [Google Scholar]
- 33.Kokuzawa J., Yoshimura S., Kitajima H., Shinoda J., Kaku Y., Iwama T., Morishita R., Shimazaki T., Okano H., Kunisada T., Sakai N. (2003) Hepatocyte growth factor promotes proliferation and neuronal differentiation of neural stem cells from mouse embryos. Mol. Cell. Neurosci. 24, 190–197 [DOI] [PubMed] [Google Scholar]
- 34.Kelly C. M., Zietlow R., Dunnett S. B., Rosser A. E. (2003) The effects of various concentrations of FGF-2 on the proliferation and neuronal yield of murine embryonic neural precursor cells in vitro. Cell Transplant. 12, 215–223 [DOI] [PubMed] [Google Scholar]
- 35.Tropepe V., Sibilia M., Ciruna B. G., Rossant J., Wagner E. F., van der Kooy D. (1999) Distinct neural stem cells proliferate in response to EGF and FGF in the developing mouse telencephalon. Dev. Biol. 208, 166–188 [DOI] [PubMed] [Google Scholar]
- 36.Mattson M. P., Maudsley S., Martin B. (2004) A neural signaling triumvirate that influences ageing and age-related disease: insulin/IGF-1, BDNF and serotonin. Ageing Res. Rev. 3, 445–464 [DOI] [PubMed] [Google Scholar]
- 37.Diez Del Corral R., Morales A. V. (2017) The multiple roles of FGF signaling in the developing spinal cord. Front. Cell Dev. Biol. 5, 58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu Q. F., Yang L., Li S., Wang Q., Yuan X. B., Gao X., Bao L., Zhang X. (2012) Fibroblast growth factor 13 is a microtubule-stabilizing protein regulating neuronal polarization and migration. Cell 149, 1549–1564 [DOI] [PubMed] [Google Scholar]
- 39.Zhang X., Bao L., Yang L., Wu Q., Li S. (2012) Roles of intracellular fibroblast growth factors in neural development and functions. Sci. China Life Sci. 55, 1038–1044 [DOI] [PubMed] [Google Scholar]
- 40.Fiandaca M. S., Kapogiannis D., Mapstone M., Boxer A., Eitan E., Schwartz J. B., Abner E. L., Petersen R. C., Federoff H. J., Miller B. L., Goetzl E. J. (2015) Identification of preclinical Alzheimer's disease by a profile of pathogenic proteins in neurally derived blood exosomes: a case–control study. Alzheimers Dement 11, 600–607.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goetzl E. J., Abner E. L., Jicha G. A., Kapogiannis D., Schwartz J. B. (2018) Declining levels of functionally specialized synaptic proteins in plasma neuronal exosomes with progression of Alzheimer’s disease. FASEB J. 32, 888–893 [DOI] [PMC free article] [PubMed] [Google Scholar]