Abstract
The transition zone (TZ) is a domain at the base of the cilium that is involved in maintaining ciliary compartment-specific sensory and signaling activity by regulating cilia protein composition. Mutations in TZ proteins result in cilia dysfunction, often causing pleiotropic effects observed in a group of human diseases classified as ciliopathies. The purpose of this study is to describe the importance of the TZ component Meckel-Grüber syndrome 6 (Mks6) in several organ systems and tissues regarding ciliogenesis and cilia maintenance using congenital and conditional mutant mouse models. Similar to MKS, congenital loss of Mks6 is embryonic lethal, displaying cilia loss and altered cytoskeletal microtubule modifications but only in specific cell types. Conditional Mks6 mutants have a variable cystic kidney phenotype along with severe retinal degeneration with mislocalization of phototransduction cascade proteins. However, other phenotypes, such as anosmia and obesity, which are typically associated with cilia and TZ dysfunction, were not evident. These data indicate that despite Mks6 being a core TZ component, it has tissue- or cell type-specific functions important for cilia formation and cilia sensory and signaling activities. Lewis, W. R., Bales, K. L., Revell, D. Z., Croyle, M. J., Engle, S. E., Song, C. J., Malarkey, E. B., Uytingco, C. R., Shan, D., Antonellis, P. J., Nagy, T. R., Kesterson, R. A., Mrug, M. M., Martens, J. R., Berbari, N. F., Gross, A. K., Yoder, B. K. Mks6 mutations reveal tissue- and cell type-specific roles for the cilia transition zone.
Keywords: ciliopathy, cystic kidney disease, retinal degeneration, obesity
The primary cilium is an immotile, microtubule-based cellular appendage, found on most mammalian cell types, where they regulate several signaling and sensory cascades. Defects in cilia function are associated with human genetic disorders and syndromes collectively called ciliopathies (1, 2). These include Meckel-Grüber syndrome (MKS), nephronophthisis (NPHP), and Bardet-Biedl syndrome (BBS). MKS is a midgestational lethal disorder with fetuses displaying enlarged cystic kidneys, liver fibrosis, microphthalmia, occipital encephalocele, polydactyly, and neural tube closure defects (3). NPHP patients exhibit renal cysts and variable penetrance of retinal degeneration. BBS patients display a range of cognitive deficits, retinal degeneration, anosmia, and pediatric obesity. MKS and NPHP are frequently caused by mutations in proteins that localize to the transition zone (TZ), a multifaceted macromolecular complex at the base of the cilium. The TZ is a structural component thought to regulate entry into and exit of proteins from the cilium (4–8), whereas the BBS proteins mediate ciliary membrane protein transport across the TZ (9). Data also support a possible role for proteins in the TZ as docking sites for ciliary-targeted vesicles (10). As such, the TZ is a critical regulator needed for cilia to carry out their normal sensory and signaling activities.
MKS6 (coiled-coil and C2 domains containing protein 2A) is a core component of the TZ that was identified in a genetic screen for autosomal-recessive mental retardation with associated retinitis pigmentosa (11). Subsequently, mutations in MKS6 have been identified in NPHP patients and in ∼10% of all Joubert syndrome patients (11–14). MKS6 is highly conserved across ciliated eukaryotes and has 3 coiled-coil domains and a calcium-dependent lipid-binding C2 domain similar to that found in several other TZ proteins, such as MKS5, MKSR1, and MKSR2 (11, 13). Several animal models containing congenital Mks6 mutations have been described in mice and zebrafish that exhibit a wide spectrum of phenotypes (13, 15–17). These include midgestational lethality with severe neural tube and left-right body axis defects, microphthalmia, rod-cone dystrophy, and ciliary protein mislocalization. This constellation of phenotypes is consistent with multiple other models of cilia dysfunction or loss (18, 19). However, there is marked variability in the phenotypes among the Mks6 congenital mutants in mice, zebrafish, as well as in human patients with MKS6 mutations. For example, in one of the Mks6 congenital null mutant mouse lines, cilia formation was reported to be abrogated, whereas in the other null Mks6 mutant model, ciliogenesis is largely unaffected, but proteins normally present in the cilium are mislocalized. The cause of phenotypic pleiotropy is poorly understood but suggests that the TZ may not function uniformly across all cells types.
The early lethality and the severe developmental abnormalities in the null congenital Mks6 mouse mutants preclude the analysis of Mks6 and its role in TZ function in specific cell types or in adult tissues. To analyze the postnatal roles of Mks6 and the TZ, we report here the generation of the first conditional Mks6 mutant mouse line. Analysis of the phenotype in the conditional mutants, as reported in the mutant embryos, revealed that some tissues/cells maintain their cilia following Mks6 disruption, whereas others do not. Additionally, the expressivity of some phenotypes, such as the cysts in the kidney, is highly variable, whereas others, such as retinal phenotypes involving rod-cone dystrophy, are fully penetrant and severe in all mutants analyzed. Intriguingly, multiple phenotypes that are typically associated with cilia dysfunction, such as anosmia and obesity, were not present in the conditional mutants. In contrast to the results with Mks6, disruption of Mks5, another core TZ protein, did lead to onset of severe weight gain similar to that observed in conditional cilia mutant mice [e.g., intraflagellar transport 88 (Ift88)]. These data, along with previous studies in zebrafish models and in congenital null Mks6 mutant mouse embryos and cell lines, argue that the function of the TZ is not uniform across tissues/cell types and needs to be analyzed further. These data raise the possibility that different TZ components have distinct roles in regulating ciliogenesis and the cilia function.
MATERIALS AND METHODS
Animals
All animal studies were conducted in compliance with the Guide for the Care and Use of Laboratory Animals [National Institutes of Health (NIH), Bethesda, MD, USA] and approved by the Institutional Animal Care and Use Committee at the University of Alabama at Birmingham and Indiana University–Purdue University Indianapolis.
The congenital Mks6 gene trap line (Mks6GT) was generated using embryonic stem cell line AA0274 (Mutant Mouse Regional Resource Centers, University of California Davis, Davis, CA, USA; Resource Identification Initiative: MMRRC_021518-UCD) in which a β-galactosidase neomycin-resistance fusion cassette was inserted into intron 11 of Mks6. The insertion site was confirmed by genomic PCR and sequence analysis. PCR primers for genotyping were designed based on the insertion site 5′-CCTGCTCAAACCTGAACCCC-3′, 5′-GTCAATATGGAGAGTAATCTCAAC-3′, and 5′-CTGGTTAGTGATGGCCTTGAGGGAAACG-3′. The Mks6GT embryonic stem cells were injected into C57BL/6 blastocysts using standard procedures. Chimeras were bred with albino C57BL/6 females, and germline transmission was confirmed by PCR genotyping.
The conditional Mks6 line (Mks6FL) was generated using embryonic stem cell line EPD0381_1_C03 [European Conditional Mouse Mutagenesis allele coiled-coil and C2 domains containing protein 2Atm1A(European Conditional Mouse Mutagenesis)Wtsi; Resource Identification Initiative: MGI:5450403] in which a targeted knockout (KO)-first construct (Mks6KO) containing a β-galactosidase neomycin-resistance fusion cassette flanked by flippase (FLP) recognition target recombination sites and exons 6 and 7 flanked by LoxP recombination sites. The Mks6KO embryonic stem cells were injected into C57BL/6 blastocysts using standard procedures. Chimeras were bred with albino C57BL/6 females, and germline transmission was confirmed by PCR genotyping. The β-geo cassette was removed by breeding the allele to FLP recombinase mice. Recombination was confirmed by genotyping for the β-geo cassette. Once the absence of the β-geo was confirmed, mice were bred off of the FLP recombinase, and the Mks6FL line was established. Genotyping for the Mks6FL and Mks6Δ alleles (after Cre-mediated deletion) were done using the following primers 5′-CAGCATCTCACTTCCTGTATCCG-3′, 5′-TGCTGGGGACTGTCATTTTGG-3′, and 5′-CTCAGTAGCCCCCAAATGG-3′.
The Mks5FL allele is as previously reported (20). Genotyping for the Mks5FL and Mks5Δ alleles were done using the following primers 5′-GTGGGTTGTACAGTTTCTGCTTCATCCAC-3′, 5′-GTCCTCTGACTTCCAGTGTCATGTGC-3′, and 5′-AAGCTCTAAAGCTGGGACTGCAGC-3′. The conditional Ift88FL and Ift88Δ alleles (Ift88tm1Bky/J, stock 022409; The Jackson Laboratory, Bar Harbor, ME, USA) and genotyping were the same as previously reported (21). The CAGCreER allele [Tg(CAG-cre/Esr1*)5Amc/J, stock 004682; The Jackson Laboratory] was used and induced as previously reported (22).
Mouse embryonic fibroblast generation
Mouse embryonic fibroblasts (MEFs) were harvested from embryonic days (E)10.5 to E11.5 and cultured in DMEM with high glucose, 0.05 mg/ml penicillin/streptomycin, 2 mM L-glutamine, 0.2 mM β-ME, and 20% fetal bovine serum, as previously described (23). Before immune labeling, MEFs were cultured to confluence and treated with reduced serum medium containing 0.5% fetal bovine serum for 48 h to induce primary cilia formation.
Cre induction
CAGCreER activity in juvenile mice was induced by a single intraperitoneal injection of 9 mg/40 g tamoxifen (T5648; MilliporeSigma, St. Louis, MO, USA) at postnatal day 7 (P7). Adult 8-wk-old mice were induced by intraperitoneal injections of 6 mg/40 g tamoxifen, given once daily for 5 consecutive days, as previously reported (22).
Quantitative RT-PCR
RNA was isolated from Mks6wild type (WT) and Mks6GT MEFs with Trizol reagent, according to the manufacturer’s protocol (15596-026; Thermo Fisher Scientific, Waltham, MA, USA). cDNA was generated using Superscript II RT-PCR kit (18064; Thermo Fisher Scientific). Real-time quantitative RT-PCR (qRT-PCR) analysis was performed using iQ Sybr Green Supermix (170888; Bio-Rad, Hercules, CA, USA) with the CFX96 real-time RT-PCR detection system (Bio-Rad). The following primers were used for Mks6: 5′-CAGGTGGAACAGGTACTGTAC-3′ and 5′-CTCAACATTAGGAAGGTC-3′. The following primers were also used as a positive control to confirm the presence of actin in all samples: 5′-ATGGGTCAGAAGGACTCCTA-3′ and 5′-GGTGTAAAACGCAGCTCA-3′. For each sample, Mks6 expression was normalized to actin expression in the sample and compared with the control Mks6 expression level. Fold change was calculated as 2−ΔΔCt.
Tissue preparation and immunofluorescence microscopy
Animals were housed with 12-h light/dark cycles. For dark-adapted studies, animals were dark adapted for at least 4 h. After euthanasia, all tissues and cells were fixed in 4% paraformaldehyde (PFA; tissues overnight at 4°C and cells for 10 min at room temperature); tissues were then cryoprotected in 30% sucrose. Olfactory tissues required a decalcification step and were placed in 0.5 M EDTA made in PBS overnight at 4°C with additional cryoprotection steps of 10% (1 h), 20% (1 h), and 30% sucrose overnight at 4°C. Tissues were embedded in optimal cutting temperature compound and sliced into 12 µm sections. All cells and tissues were postfixed in 4% PFA and permeabilized with 0.02% Triton X-100 in PBS. Incubations and washes are as follows: all kidney tissue/cell washes were in PBS with blocking and antibody incubations done in PBS with 1% normal donkey serum, 0.02% sodium azide, and 1% bovine serum albumin. Retinal section block and primary incubations were in 10% normal goat serum, in PBS with 0.01% sodium azide and 0.3% Triton X-100, and washed with PBS. Olfactory sections were blocked and incubated in 10% normal donkey serum, in PBS with 0.1% Triton X-100 and washed with PBS.
Primary antibody incubations were performed for 16–24 h at 4°C. Primary antibodies included the following: anti-acetylated α-tubulin (T-6793,1:1000; MilliporeSigma), fluorescein-conjugated Lotus lectin (LTA; FL-1321, 1:500; Vector Laboratories, Burlingame, CA, USA), rhodamine-conjugated Dolichos biflorus agglutinin (DBA; RL-1032, 1:250; Vector Laboratories), Aquaporin 1 (AQP11-A, 1:50; Alpha Diagnostics, San Antonio, TX, USA), Aquaporin 2 (sc-9882, 1:50; Santa Cruz Biotechnology, Dallas, TX, USA), ADP-ribosylation factor-like protein 13B (Arl13b; 1:2000, kind gift from Tamara Caspary; Emory University, Atlanta, GA, USA), rhodopsin [1D4 1:2000, courtesy of Robert Molday (University of British Columbia, Vancouver, BC, Canada) (24–29)], visual arrestin (sc-67130, 1:250; Santa Cruz Biotechnology), transducin (K-20, 1:250; Santa Cruz Biotechnology), olfactory marker protein (OMP; 544-10001, 1:1000; Wako Chemicals, Neuss, Germany), rabbit polyclonal antibody-adenylyl cyclase III (ACIII; 1:2000; EnCor Biotechnology, Gainesville, FL, USA), and tyrosine hydroxylase (TH; MAB318, 1:1000; MilliporeSigma, Darmstadt, Germany).
All secondary antibodies were purchased from Thermo Fisher Scientific. Incubations were performed for 1 h at room temperature and were used at a 1:1000 dilution for kidney tissues/cells and olfactory tissues and a 1:500 dilution for retinal tissues. Secondary antibodies included the following: Alexa Fluor 546-conjugated donkey anti-mouse IgG (A21203), Alexa Fluor 546-conjugated donkey anti-rabbit IgG (A21207), Alexa Fluor 488-conjugated donkey anti-mouse IgG (A21202), Alexa Fluor 488-conjugated donkey anti-rabbit IgG (A21206), Alexa Fluor 488-conjugated goat anti-mouse IgG (A32723), Alexa Fluor 488-conjugated goat anti-rabbit IgG (A-11034), Alexa Fluor 594-conjugated donkey anti-rabbit IgG (R37119), Alexa Fluor 647-conjugated donkey anti-goat IgG (A-21447), Alexa Fluor 568-conjugated donkey anti-goat IgG (A11057), and nuclear stain DAPI (1:3000). Kidney tissue/cell nuclei were visualized by Hoechst nuclear stain (33258); retinal and olfactory tissue nuclei were visualized with nuclear stain DAPI (62248). Coverslips were mounted using Immu-Mount (9990402).
Kidney and retinal tissue images were taken on a Perkin-Elmer Ultra ERS6 spinning disk confocal microscope with a 60 times Apo TIRF 1.49 numerical aperture (NA) oil-immersion objective and a Hamamatsu C9100 electron multiplying charge-coupled device or a Nikon Eclipse Ti2 spinning disc confocal with a 20 times PlanFluor MMI 0.75 NA objective, equipped with a Yokogawa CSU-X1 and a Hamamatsu Flash4 sCMOS. Olfactory tissues were imaged on a Nikon TiE-PFS-A1R confocal microscope with a CFI Apo Lamda S 60 times 1.4 NA objective. Kidney and retinal tissues, hematoxylin (72704; Thermo Fisher Scientific) and eosin (17372-87-1; Acros Organics, Morris, NJ, USA) staining, were performed and imaged on a Nikon Eclipse TE2000s with a 40 times objective with a Micropublisher 3.3 real-time viewing (QImaging, Surrey, BC, Canada).
Retinal spidergrams were constructed by plotting ONL number of nuclei as a function of position in the retina from the optic nerve (in micrometers). To assess cell death, TUNEL labeling was performed using the ApopTag Red kit (S7165; MilliporeSigma), counting the number of positively labeled apoptotic nuclei from 8 retinal sections from each animal. All images were compiled using ImageJ software (Image Processing and Analysis in Java; NIH, Bethesda, MD, USA; http://imagej.nih.gov/).
Electroretinography
Mice were dark adapted for at least 4 h and anesthetized with 2.5% isoflurane. Following general anesthesia, animals were placed on a heating pad, corneas were anesthetized with proparacaine (0.5%), and pupils were dilated with topical phenylephrine HCl (2.5%) and tropicamide (1%). Electroretinogram recordings were collected using an HMsERG unit (Ocuscience, Henderson, NV, USA) and loop electrodes of 37-gauge platinum wire (Amazon.com), as previously described (30). Protocols Scotopic 2 and Photopic (Ocuscience) were used over a range of flash intensities (0.1–25 cd ⋅ s/m2). ERGView software (Ocuscience) was used to analyze the electroretinogram (ERG) recordings and determine a- and b-wave amplitudes. Averaged recordings were exported to text file and regraphed in KalediaGraph (Synergy Software, Reading, PA, USA).
Small animal MRI
Kidneys were visualized using a 9.4 T MRI system BioSpec (Bruker BioSpin, Billerica, MA, USA) with surface coil (Bruker BioSpin). Each mouse, anesthetized with isoflurane, was loaded on a body temperature-regulating mouse bed for the surface coil imaging. Low-resolution images were used to confirm the location and orientation of the kidneys, followed by acquisition of axial images covering the entire kidney region with a T2-weighted fast spin-echo sequence (rapid acquisition with relaxation enhancement), with slice thickness = 0.5 mm. Kidney area from each axial slice was measured by tracing the outline of the kidney using ImageJ, v.1.47 (NIH). Kidney volume was determined by multiplication of the sum of the kidney area and slice thickness.
µCT analysis of kidneys
Samples were fixed with 4% PFA, washed in PBS, and incubated in Lugol solution. Kidneys were scanned using the Scanco micro computed tomography (µCT)40 desktop cone-beam µCT scanner (using µCT v.5.44; Scanco Medical, Brüttisellen, Switzerland). Scans were automatically reconstructed into 2-dimensional (2D) slices, and all slices were analyzed using the µCT Evaluation Program (v.6.5–2; Scanco Medical). Tiff and DICOM files were created for all slices for each sample. Images were generated using ImageJ (NIH).
Renal function analysis
Serum and creatinine analyses were performed on blood captured from tail vein and measured using liquid chromatography, coupled to tandem mass spectrometry, as previously described with assistance from the University of Alabama at Birmingham O’Brien Center for Acute Kidney Injury (Birmingham, AL, USA) (31).
Taqman qRT-PCR analysis
Adult female C57BL/6 mice were either allowed ad libitum access to a standard chow diet or unfed overnight. The following morning, brains were harvested and hypothalami were dissected and flash frozen. RNA was isolated using Trizol reagent and Purelink RNA mini kit (Thermo Fisher Scientific) and then reverse transcribed into cDNA using the Quantitect RT kit (Qiagen, Venlo, The Netherlands). Reactions were performed on a QuantStudio 7 Real-Time PCR System (Thermo Fisher Scientific), CT values were normalized to β-actin, and relative expression was calculated by the ΔΔCt method. Assay-on-Demand gene expression products (Thermo Fisher Scientific) were as follows: Ift88 Mm01313467_m1, MKS5 Mm00557886_m1, and MKS6 Mm00552125_m1.
In situ hybridization
Tissues from C57BL/6 mice or Mks6GT embryos were harvested and fixed as for immunofluorescence, described above. Cryosections (15 μm) were mounted directly on slides and then postfixed with 4% PFA for 16 h at 4°C. Detection of transcripts was performed using the RNAscope fluorescent multiplex reagent kit (Advanced Cell Diagnostics, San Francisco, CA, USA). Tissue pretreatment, probe hybridization, counterstaining, and mounting of slides were performed according to the manufacturer. Slides were assayed using probes to positive control, negative control, or mouse Mks6 or Mks5 transcripts [probe catalog numbers: 320881(positive control), 320871(negative control), 420191(Mks5), and 534051(Mks6); Advanced Cell Diagnostics]. The Mks6 probe binds to a region 5ʹ of the gene trap β-geo cassette. Sections were counterstained with DAPI and mounted using Prolong Gold (Thermo Fisher Scientific).
Statistical analyses
Groups were compared by the Student’s t test or in the case of multiple comparisons, by 1-way ANOVA, followed by appropriate post hoc tests. Body weight (BW) data were compared using a repeated-measures 2-way ANOVA with multiple comparisons. Kruskal-Wallis nonparametric ANOVA with post hoc Mann-Whitney was used for TUNEL quantifications. Greenhouse-Geisser correction was used for ERG analyses. A value of P < 0.05 was considered statistically significant.
RESULTS
Generation of Mks6 congenital and conditional mutants
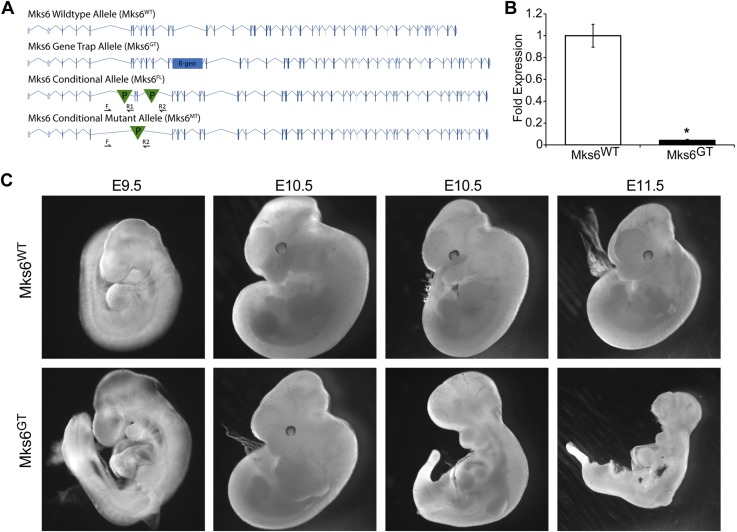
Whether the TZ is a stable structure with uniform function across all cilia and whether there are differential roles for the TZ components in specific cell types or stages of development or postnatal periods are largely unknown. To address these types of questions, we generated 2 mutant mouse alleles of Mks6: first, a congenital gene trap null allele (Mks6GT) that has a β-geo cassette between exons 11 and 12 (blue rectangle; Fig. 1A) that disrupts Mks6 function during early development; the second, a conditional allele with LoxP sites (green triangles; Fig. 1A) flanking exons 6 and 7 (Mks6FL) for cell type-specific and later-stage disruption of Mks6. Cre-mediated deletion of these exons (Mks6Δ) results in a frameshift and a predicted truncation of the protein (Fig. 1A). qRT-PCR analysis showed loss of Mks6 message in Mks6GT cells (Fig. 1B).
Figure 1.
Mouse Mks6 mutant alleles. A) Allele schematics drawn to scale: WT (Mks6WT), congenital gene trap allele with β-galactosidase cassette indicated (β-geo) in intron 11 (Mks6GT), conditional allele with LoxP sites indicated (P) flanking exons 6 and 7 (Mks6FL), and conditional mutant allele (Mks6Δ). Genotyping primers for the conditional and conditional mutant alleles are indicated (F, R1, and R2 arrows). B) qRT-PCR indicates loss of transcript in Mks6GT MEFs. Fold change was calculated as 2−ΔΔCt. C) Mks6GT embryos presented with variable phenotypes at different embryonic stages. *P < 0.05 (Student’s t test).
Congenital disruption of Mks6 (Mks6GT) results in embryonic lethality associated with variable ciliogenesis defects and hyperacetylation of cytoplasmic tubulin
As reported in previous Mks6 null mouse alleles, we detected no viable homozygous Mks6GT offspring from heterozygous matings (15). With the use of timed pregnancies, homozygous Mks6GT mutant embryos could routinely be isolated between E10.5 and 11.5 but not at E13.5, indicating that congenital loss of Mks6 causes midgestational lethality (Fig. 1C). Whereas there is variability in the phenotype, mutants frequently exhibited severe developmental delay and morphologic defects by E10.5.
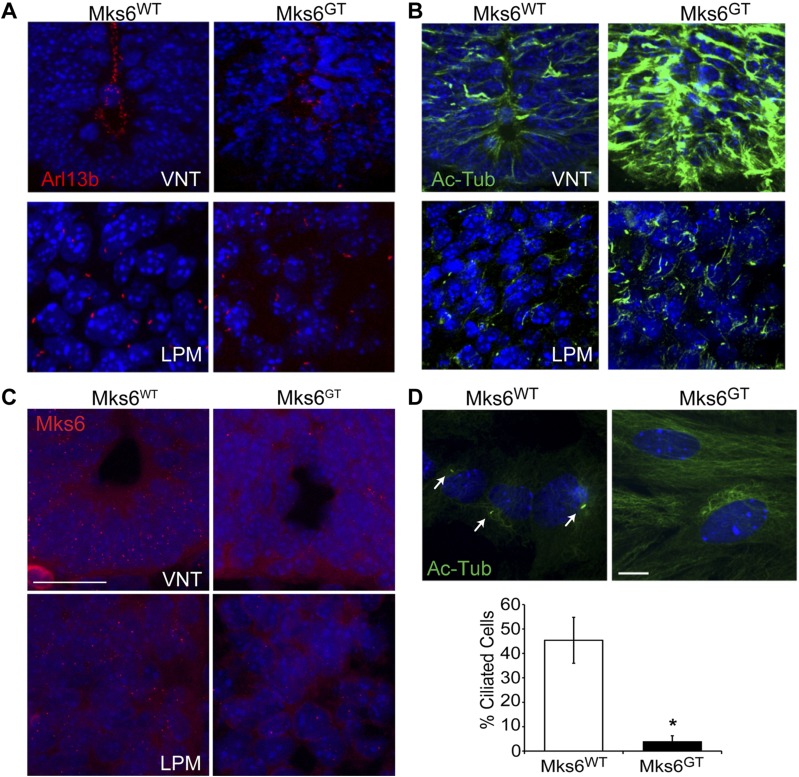
To assess cilia in mutant embryos, immunofluorescence microscopy analysis was done on Mks6GT mutants using the ciliary membrane-associated protein Arl13b. This revealed reduced numbers of primary cilia on cells in the ventral neural tube (VNT) compared with Mks6WT controls (Fig. 2A). In contrast, cilia were present on most cells in the lateral plate mesoderm (LPM) and other regions of the Mks6GT mutants (Fig. 2A).
Figure 2.
Analysis of Mks6GT mutant embryos and cells. A–C) Analysis of VNT and LPM at E10.5 from Mks6WT and Mks6GT embryos. A) Immunofluorescence microscopy analysis using cilia marker Arl13b (red) shows cilia lining the neural tube lumen and LPM. Note the overall disorganized morphology in Mks6GT VNT. B) Immunofluorescence microscopy analysis for cilia marker acetylated α-tubulin (Ac-Tub; green) shows hyperacetylation in both the VNT and LPM of Mks6GT embryos compared with control Mks6WT embryos. C) Fluorescent in situ hybridization for Mks6 (red) mRNA on Mks6WT and Mks6GT mutant embryos. Note the reduction in labeling in Mks6GT embryos. Original scale bar, 20 µm. D) MEF analysis. Immunofluorescence microscopy analysis in MEFs using anti-Ac-Tub (green) antibodies after 24 h of serum starvation. Mks6GT cells lack cilia and display more acetylated cytoplasmic microtubules. Cilia are indicated with arrows. Original scale bar, 32 µm. Graph of Ac-Tub-positive cilia frequency in MEFs between Mks6WT and Mks6GT cells. *P < 0.05 for Student’s t test; means ± sem, n = 7 Mks6GT and 4 Mks6WT MEF embryo isolations per group. DAPI and Hoechst-stained nuclei are blue in all images.
To determine if cilia were, in fact, present but unable to localize Arl13b properly in the VNT of Mks6GT mutants, we used the cilia cytoskeletal marker acetylated α-tubulin. Unexpectedly, this staining revealed increased levels of cytoskeletal acetylated α-tubulin in Mks6GT mutants compared with the readily detected cilia in the VNT and LPM of Mks6WT control embryos (Fig. 2B). The increased levels of cytoskeletal acetylated α-tubulin were to such an extent that it precluded assessing cilia staining in Mks6GT mutants using this marker (Fig. 2B). The hyperacetylation of the tubulin phenotype was previously reported in multiple mouse models of disrupted cilia formation (32–35), lending further support to the Arl13b data, indicating defects in cilia formation in the VNT of Mks6GT mutants.
To address further the potential for variations in Mks6 expression and alternative splicing to be involved in the variable ciliogenesis phenotype, we performed in situ hybridization analysis for Mks6 on both control and mutant embryo VNT and LPM (Fig. 2C and Supplemental Fig. 1). Consistent with a loss-of-function allele, Mks6GT embryos displayed reduced in situ labeling throughout. We also isolated MEFs from the Mks6GT mutant and Mks6WT controls to analyze directly their ability to form cilia. Multiple MEF isolations revealed a marked reduction in the presence of cilia in Mks6GT cells compared with control embryos using either Arl13b or acetylated α-tubulin as cilia markers (Fig. 2D). These data are in contrast to those from Garcia-Gonzalo et al. (15) but in line with those from the Mks6 mutant mice reported by Veleri et al. (16) and in human MKS6 patients (36), Additionally, as seen in vivo in the VNT, the Mks6GT MEFs in culture had increased acetylation of their cytoskeletal tubulin (Fig. 2D). Collectively, these data indicate that despite Mks6 being a core component of the TZ, the function of Mks6 is not required for ciliogenesis in all cell types during development.
Generation of a conditional (Mks6FL) Mks6 mutant mouse line
To determine if Mks6 is required for maintenance of cilia in adult and differentiated tissues, we initiated studies using a conditional Mks6 allele (Mks6FL; Fig. 1A). Mice with the conditional allele were crossed with the near-ubiquitously expressed and inducible CAGCreER line to delete Mks6 upon tamoxifen injection (22). Efficient deletion of Mks6 (Mks6Δ) was observed in ear biopsies and in the tissues analyzed when tamoxifen was injected at P7 (Fig. 3).
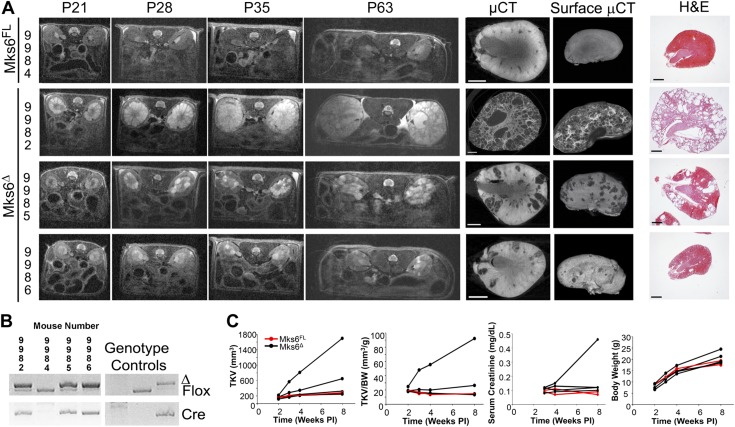
Figure 3.
Variable cystic kidney phenotype in Mks6Δ mutants. A) Longitudinal MRI, terminal µCT, and histology studies of Mks6Δ mutant kidneys. Mks6Δ mice (9982, 9985, 9986 littermates) show variability in cyst progression, kidney size, and cyst growth over time after Cre induction on P7 compared with control littermates (9984). Terminal µCT and histology [hematoxylin and eosin (H&E)] analysis was performed on kidneys at P63. Original scale bars, 1 mm. B) PCR genotyping for Mks6FL (Flox), Mks6Δ (Δ), and Cre alleles was performed to evaluate potential correlations between cyst severity and the extent of Cre-mediated deletion. C) Kidney function and phenotypic severity were also assessed by measuring TKV, TKV/BW, serum creatinine levels (mg/dl), and BWs post-Cre induction (Weeks PI).
Conditional loss of Mks6 results in a variable cystic kidney disease phenotype
A hallmark shared by multiple human ciliopathies, including MKS, is the formation of cysts in the kidney. As observed in human MKS patients, loss of Mks6 (induced at P7) in Mks6FL mice also causes renal cyst formation. A remarkable outcome from this analysis is that the expressivity of the cystic phenotype was highly variable, both histologically and in relation to renal function (Fig. 3A, C and Supplemental Movies 1 and 2). This variability did not correlate with the extent of deletion, as near-complete loss of the Mks6 was detected in each animal (Fig. 3B). In some mutants, the kidneys become greatly enlarged, with cyst formation occurring uniformly throughout most nephrons (e.g., mouse 9982; Supplemental Movies 1 and 2), whereas others only had a few cysts that developed in focal regions (e.g., mouse 9985 and 9986; Supplemental Movies 1 and 2).
We assessed the variability and progression of the renal phenotype in longitudinal studies with the use of MRI to quantify cyst size, growth, and number; total kidney volume (TKV); and TKV/BW ratio and with the use of analyzing changes in serum creatinine levels to track renal function decline. At the end point of the study, kidney pathology was analyzed by histology and Lugol-enhanced µCT (Fig. 3A, C and Supplemental Movies 1 and 2). Based on these data, mice, in which cysts began developing earlier, resulted in more severe pathology and showed greater rates of renal decline. The phenotype variability and reasons for differences in onset in the conditional mutants are unknown. The 3D analysis conducted by MRI and µCT, along with the analysis of individual sections from these image reconstructions, reveals the focal nature of the cysts that can develop in this model (Fig. 3A and Supplemental Movies). These analyses further highlight the importance of these 3D imaging approaches in evaluating cyst severity, as conventional data could be misinterpreted, depending on where the histologic section sampling was performed (see sections in Supplemental Movies 1 and 2).
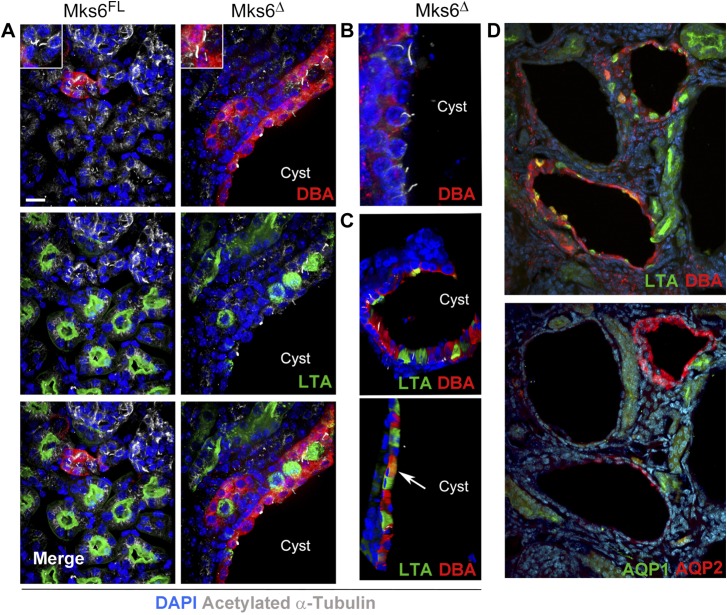
To determine if the cysts that develop in the Mks6 mutants are associated with cilia loss on tubule epithelium, we stained sections of the kidney with anti-acetylated α-tubulin antibodies and proximal and distal tubule markers LTA and DBA, respectively. Similar to the LPM of the Mks6GT mutant embryos, cilia can be detected on many epithelial cells in the tubules of Mks6Δ mutants (Fig. 4A, B and Table 1). This includes the epithelium lining cystic tubules (Fig. 4B), suggesting that whereas the cilia are present, they may not be functioning normally. In contrast to what is observed in the Mks6GT mutant embryo neural tube and LPM or in Mks6 mutant MEFs in culture, we did not observe a significant increase in cytoskeletal acetylated α-tubulin levels in the renal epithelium.
Figure 4.
Analysis of cystic tubules in Mks6Δ mutants. A, B) Immunofluorescence microscopy analysis for the presence of cilia, collecting and proximal tubules markers (acetylated α-tubulin in white, DBA in red, LTA in green). Original scale bar, 15 µm. A insets, B) Note the presence of cilia within a cyst in the Mks6Δ sample. C) Confocal microscopy-extended projections of mutant Mks6Δ tubules showing individual cysts lined by cells that express LTA, DBA, or both (arrow) within the same tubule. D) Immunofluorescence microscopy analysis for tubule markers (DBA and LTA) shows mixed cells in a Mks6Δ cyst, but Aquaporin 1 and 2 immunofluorescence (AQP1 and AQP2) shows cysts with uniform staining. Hoechst-stained nuclei are blue in all panels.
TABLE 1.
Percent ciliated cells in the kidney
| Genotype | Nuclei analyzed (n) | Cilia (n) | Ciliated (%) |
|---|---|---|---|
| Mks6F/F; Cre− | 621 | 83 | 13.4 |
| Mks6Δ/Δ; Cre+ | 2116 | 113 | 5.3 |
We further evaluated the effect of the disruption of Mks6 on ciliation using primary renal epithelial cells isolated from the CAGCreER;Mks6FL/FL conditional mutant mice. This allowed us to disrupt Mks6 before or after cilia have formed, based on when the cells were treated with 4-OH tamoxifen to activate Cre. With the use of this approach, we can determine if there is a different requirement for Mks6 in the renal epithelia for reciliogenesis in subconfluent, proliferative conditions or for cilia maintenance once cilia have formed in nonproliferative, confluent cells cultured in low-serum conditions. As observed in vivo, we note that there is an overall reduction in the number of cilia present but not a complete absence in the Mks6Δ mutant cells compared with that in the control cells (Table 2). This occurred regardless of whether Cre activity was induced before or after serum removal. Additionally, there were roughly twice as many ciliated cells present in the Mks6Δ mutants when Cre induction occurred after cilia had already formed compared with inducing Mks6 loss before serum starvation and cilia formation. There was no difference in the number of ciliated cells present in controls under these 2 conditions. This suggests, in the case of renal epithelial cells, that Mks6 has a more significant role in ciliogenesis than in maintenance of the existing cilia.
TABLE 2.
Cilia frequency
| Induction | Cilia (%) | Arl13b+ (%) | ACIII+ (%) |
|---|---|---|---|
| Preciliation | |||
| WT | 61.5 | 90 | 81.5 |
| Mks6 mutant | 20.2 | 81 | 73.9 |
| Postciliation | |||
| WT | 57.1 | 93.2 | 78.9 |
| Mks6 mutant | 36.3 | 91.5 | 72.1 |
We also used the conditional Mks6 primary renal epithelial cell lines to evaluate whether the cilia that are present have changes in their protein composition by analyzing localization of cilia membrane proteins ACIII and Arl13b. Based on these markers, we did not observe significant differences in the localization or retention of ACIII or Arl13b in the cilia that do form in the Mks6Δ mutant epithelium, regardless of whether the deletion of Mks6 occurred pre- or postciliation (Table 2 and Supplemental Fig. 2). This is in direct contrast to data by Garcia-Gonzalo et al. (15), who reported that ACIII and Arl13b largely fail to localize in cilia that form on the Mks6 MEFs isolated from congenital mutants. Additionally, the studies by Garcia-Gonzalo et al. (15) indicated that polycystin-2 does not localize in the cilium of Mks6 mutant MEFs. We made multiple attempts, which were ultimately unsuccessful, with independent antibodies to analyze localization of polycystin-2 (data not shown).
An intriguing observation made from the analysis in the kidney is that within the cystic tubules of the Mks6Δ mutants, there are frequently neighboring cells that stain positive for proximal tubule (LTA, green) and collecting (DBA, red) lectin markers or that are positive for both markers (Fig. 4A–C). Mixed expression of proximal tubule and collecting duct markers within a single tubule was also reported in WT mice during embryonic stages and following injury, as well as in the polycystin 1 kidney disease conditional mutants (37). However, in cysts of older polycystin 1 kidney disease animals, none was found to be double LTA-DBA labeled. These data raised the possibility that the differentiation state of cells within the tubules is altered following loss of Mks6. We analyzed this possibility further by costaining serial sections with LTA and DBA and with antibodies against Aquaporin 1 and Aquaporin 2 water channels, which are expressed in proximal tubule and collecting tubule nephron segments, respectively. Whereas we could detect many tubules with mixed LTA and DBA staining, none of these tubules also had mixed or coexpression of the aquaporin proteins (Fig. 4D). These data indicate that it is unlikely to result from trans- or dedifferentiation of the tubule segments, as functional proteins of the segment appear to be maintained, at least based on the markers used.
Conditional loss of Mks6 results in progressive retinal degeneration
Loss of Mks6 in zebrafish causes rod-cone dystrophy, but as a result of early lethality in congenital Mks6GT mouse mutants, the assessment of the roles of mammalian Mks6 in the retina is not possible (17). To investigate the role of Mks6 in both the development and maintenance of photoreceptors, CAGCreER activity was induced in juveniles (P7) and adults (8 wk). Juvenile-induced retinas of cre-negative Mks6FL control and Mks6Δ mutant mice were analyzed at P21, 14 d postinduction (P.I.). Adult-induced retinas were analyzed at 20 wk of age, 12 wk P.I.
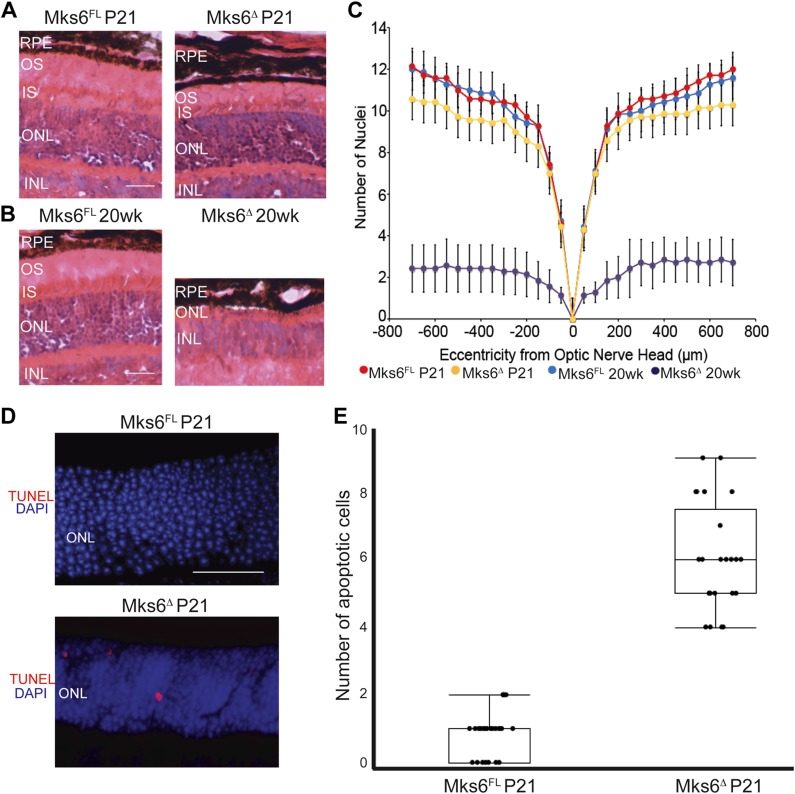
In juvenile-induced Mks6Δ mutant retinas, there was a slight reduction in the number of nuclei in the outer nuclear layer (ONL) but a significant increase in apoptotic nuclei (Fig. 5A, C–E). In contrast, adult-induced Mks6Δ mutant retinas contained only 2–3 rows of nuclei remaining in the ONL (Fig. 5B, C). Thus, in the case of photoreceptor cells, Mks6 function is essential to maintain outer segments (OSs), even after they have been established in adult mice.
Figure 5.
Retinal degeneration phenotype in Mks6Δ mutants. A, B) Hematoxylin and eosin staining of retina sections from Mks6FL and Mks6Δ mutants from juveniles (P21) and adults (20 wk). INL, inner nuclear retinal layer; ONL, outer nuclear retinal layer; RPE, retinal pigmented epithelia retina layer. Original scale bars, 50 µm. C) Morphometric analysis of nuclei counts at different distances from the optic nerve head using Student’s t test reveals only slight retinal degeneration in juveniles (P21) compared with statistically significant retinal degeneration in adults (20 wk). D) TUNEL staining images for apoptosis (red) in P21 retinas. DAPI-stained nuclei are blue. Original scale bar, 50 µm. E) Graph of TUNEL quantification in P21 retinas. Kruskal-Wallis nonparametric ANOVA with post hoc Mann-Whitney comparisons yielded P < 0.05; means ± sem, n = 6 per group.
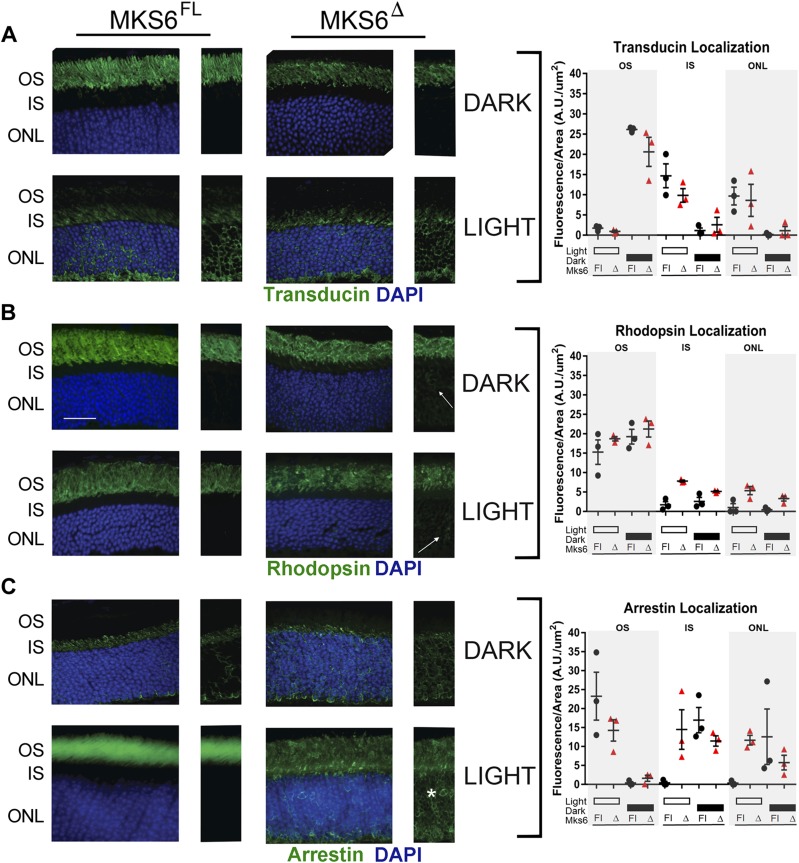
One proposed function of the TZ is to regulate protein movement into and out of the cilium. To evaluate whether the retinal phenotypes are associated with altered regulation of phototransduction protein transport, we analyzed the localization of the GPCR rhodopsin, its G-protein transducin, and arrestin in juvenile-induced Mks6Δ mutant and Mks6FL control mice under light- or dark-adapted conditions (Fig. 6).
Figure 6.
Analysis of phototransduction protein localization in Mks6Δ retinas. A) Transducin staining (green) is similar between Mks6Δ mutants and Mks6FL controls in both the light and dark conditions. B) Rhodopsin staining (green) in both light- and dark-adapted conditions reveals slight mislocalization in the IS (arrows) in juvenile Mks6Δ mutants compared with Mks6FL controls. C) Arrestin staining (green) is present in the OS and is mislocalized to the IS and ONL in the light-adapted Mks6Δ mutant retinas (asterisk). One-way ANOVA was used for quantification of the average fluorescence intensity distribution indicated for each protein in the graphs (right). DAPI-stained nuclei are blue. Original scale bar, 25 µm.
In both light- and dark-adapted conditions, transducin localizes normally to the OS and inner segment (IS), respectively (Fig. 6A). In contrast, in Mks6Δ mutants, rhodopsin is not only found in the OS, but it is also ectopically observed at low levels in the ONL and synapse in both light- and dark-adapted conditions (Fig. 6B). In dark conditions, arrestin is restricted from the OS and is properly localized to the IS and ONL of both mutant and control retinas. However, the loss of Mks6 did have a marked impact on arrestin localization in the response to light (Fig. 6C). In control retinas, light induces the near-complete movement of arrestin into the OS. Whereas arrestin does accumulate in the OS of the juvenile-induced Mks6Δ mutant retina following light adaptation, there remains a large fraction of arrestin in the IS and ONL and at the synapse that is not observed in the control.
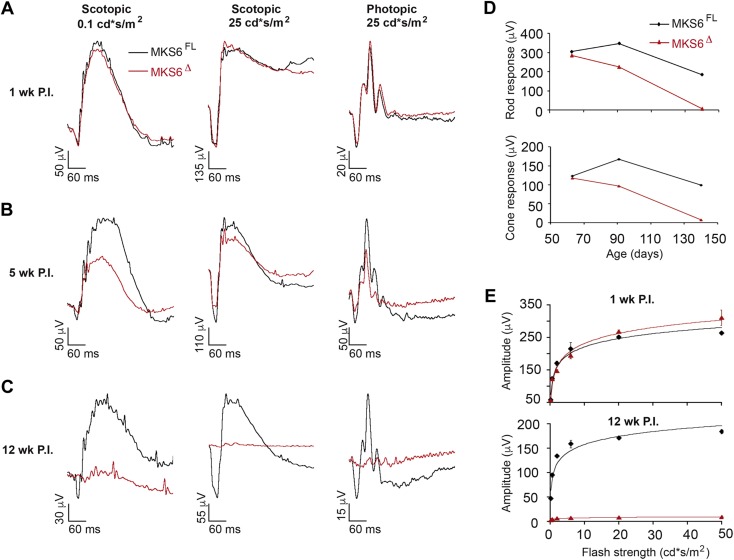
To assess the effects of the absence of Mks6 in mature photoreceptors, rod and cone photoreceptor function was monitored using ERG in adult Mks6Δ mutant and Mks6FL controls (Fig. 7). Both scotopic and photopic ERG responses revealed no major differences at 1 wk P.I. (8 wk old; Fig. 7A). However, by 5 wk P.I. (13 wk old), there is a significant decrease of a- and b-wave responses (Fig. 7B). At 12 wk P.I. (20 wk old), there is a complete loss of a- and b-wave signal (Fig. 7C–E), indicating a progressive and severe loss of rod and cone function in the absence of Mks6.
Figure 7.
Longitudinal functional analysis of Mks6Δ retinas. A–C) Conditional loss of Mks6 results in visual deficits. Overlaid representative scotopic and photopic ERG traces at the lowest and highest flash intensities (0.1 and 25 cd ⋅ s/m2) from dark-adapted Mks6FL control (black) and Mks6Δ mutant (red) animals. Scotopic and photopic ERGs were recorded in the same cohort of animals. A) One week P.I. does not display a phenotype. B, C) Five week (B) and 12 wk (C) P.I. both display visual deficits. D) Rod and cone response summary data averaged across Mks6FL (black circles) and Mks6Δ (red circles) animals are shown over a period of 12 wk. E) Scotopic a-wave summary data averaged across Mks6FL (black circles) and Mks6Δ (red circles) at 1 wk and 12 wk P.I. a-wave amplitude was measured as baseline to trough, whereas b-wave amplitude was measured baseline to peak at each flash intensity. Mks6Δ a- and b-wave amplitudes were significantly decreased at 12 wk of age compared with Mks6FL controls. Repeated measures of ANOVA were performed, means ± sd, n = 3 per group.
Loss of Mks6 does not result in olfactory deficits
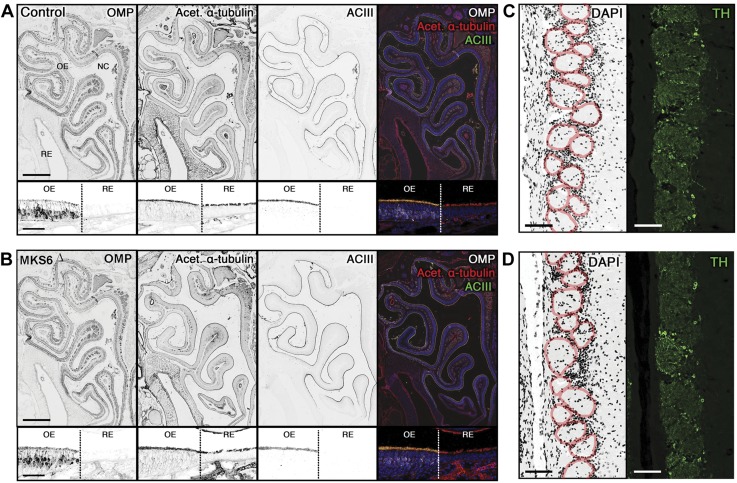
In multiple ciliopathy mouse models, loss of ciliary proteins causes reduced ciliation within the olfactory sensory neurons (OSNs) of the olfactory epithelium (OE), leading to anosmia (23, 38–40). Olfactory cilia project from the apical dendrites of mature OSNs, which are critical for normal odorant detection. To assess the phenotypic penetrance of mutant Mks6Δ within the olfactory system, snouts from juvenile-induced Mks6Δ mice were analyzed. Coronal sections from Mks6Δ mutant and Mks6FL controls were immunostained for a marker for mature OSNs, an OSN cilia marker (ACIII) and cilia marker acetylated α-tubulin (Fig. 8A, B). Surprisingly, Mks6Δ mutant mice demonstrated no overt differences in anatomy and ciliation compared with Mks6FL control mice. Much like Mks6FL control mice, Mks6Δ mutant mice exhibited uniform acetylated α-tubulin labeling along the apical surfaces of the OE and respiratory epithelium (RE), whereas OMP and ACIII immunostaining was normal and highly restricted within the surface of the OE. In other ciliopathy models with impaired ciliation, the loss of olfactory function manifests as reduced TH expression in postsynaptic dopaminergic juxtaglomerular interneurons of the olfactory bulb (OB) and reduced glomerular size as a result of axonal pathfinding defects (23, 39, 40). Consistent with normal OSN ciliation, we observed no difference in glomerular sizes and in TH expression in Mks6Δ mutant mice (Fig. 8B, D). Together, these observations indicate that the loss of Mks6 has no effect on the ciliation of mature OSNs in the OE or on olfactory activity within the OB.
Figure 8.
Analysis of the olfactory system in Mks6Δ mutants. A, B) Global coronal section of the nasal cavity with the OE and RE from Mks6FL (Control) and mutant (Mks6Δ). Immunofluorescence for a marker for mature OMPs, and cilia markers (acetylated α-tubulin and ACIII). Closer examination of the OE and RE border (demarcated with dashed line) from Mks6FL and Mks6Δ mutant mice demonstrates the presence of olfactory sensory cilia and motile respiratory cilia in both mice. C, D) Coronal section of the OB medial surface from Mks6FL (Control) and mutant (Mks6Δ). DAPI stained with individual glomeruli (red outlines) and TH immunolabeling demonstrated no difference in glomerular size and trans-synaptic activation, respectively. Original scale bars, 500 µm (A, B, upper), 50 µm (A, B, lower); 100 µm (C, D), n = 3 per group.
Adult loss of Mks6 does not result in weight gain
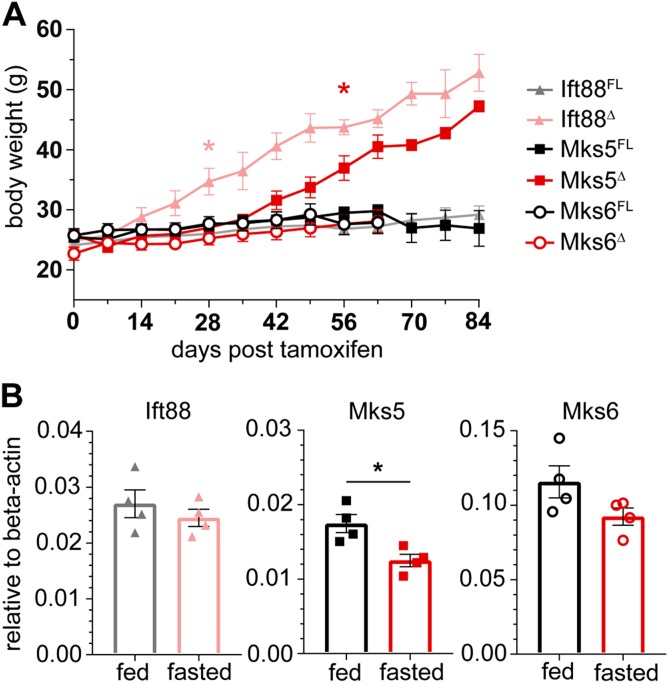
Human ciliopathies, such as BBS and Alstrom syndrome, and adult-induced Ift88 mutant mice exhibit hyperphagia-associated obesity. Furthermore, haploinsufficiency and conditional loss of the TZ protein Mks5 (Rpgrip1l) also result in increases in feeding behavior, leading to obesity (20, 28). To evaluate whether Mks6/TZ function is similarly needed for normal satiation responses, we assessed BW in adult-induced Mks6Δ mutants and compared their weight gain with both Ift88 and Mks5 conditional mutant mice using the same Cre line (CAGCreER). BW was tracked in male mutant and control mice (Fig. 9). In contrast to the obesity phenotype evident in the Ift88 and Mks5 conditional mutants, there were no overt differences in weight gain between the control or Mks6Δ mice (Fig. 9A).
Figure 9.
Adult loss of Mks6 does not result in increased weight gain. A) Eight-week-old CAGCreER;Mks6FL, CAGCreER;Ift88FL, and CAGCreER;Mks5FL conditional mutants were induced with tamoxifen injections, and BWs were tracked weekly post-tamoxifen injection. Whereas Ift88Δ and Mks5Δ mutants become obese, Mks6Δ mice did not weigh significantly more than their controls. *P < 0.05 (first time point), repeated-measures 2-way ANOVA with multiple comparisons; means ± sem, n = 3–9 per group. B) qRT-PCR on whole hypothalamic RNA from unfed and fed WT animals for Ift88, Mks5, and Mks6. *P < 0.05, Student’s t test; means ± sem.
Dynamic decreases in Mks5 gene expression in the hypothalamus of unfed mice have been reported (29). To determine if Mks6 expression is similarly dynamic, we performed qRT-PCR analysis on adult C57BL/6 mice that were unfed and compared the expression levels of Ift88, Mks5, and Mks6 with ad libitum-fed controls. Interestingly, we observed a statistically significant change in Mks5, similar to reported observations, but no changes in either Ift88 or Mks6 in RNA from whole hypothalami (Fig. 9B). These data suggest the need for some TZ members in normal adult feeding behavior and energy homeostasis (Mks5), whereas others are not required (Mks6). To determine if there are differential patterns of TZ component expression in regions of the adult brain implicated in ciliopathy-associated hyperphagia (27), we performed in situ hybridization. Dual FISH for both Mks5 and Mks6 in the paraventricular nucleus and arcuate nucleus of the hypothalamus revealed no overt differences in gene expression in C57BL/6 mice (Supplemental Figs. 3 and 4).
DISCUSSION
MKS is a severe human ciliopathy associated with mutations in several genes; nearly all of these gene products are required for formation or function of the TZ at the base of the cilium. In several model systems, as well as in humans, Mks6 has been implicated as a central component of the TZ protein complex (4, 8, 15, 17). The early lethality and severe developmental defects resulting from congenital loss of Mks6 (Mks6GT) or in human MKS patients have been observed for other alleles affecting the TZ, as well as for genes associated with cilia assembly (e.g., Ift genes). Thus, it was surprising to learn that mutant embryos possessed cilia in several regions, including the LPM, and had no or very few cilia in other regions, such as the neural tube. Analysis of MEFs isolated from mutant embryos suggested a defect in ciliogenesis in vitro. These observations raised several questions about the cellular role for mammalian Mks6 in vivo. Is Mks6 required for cilia formation or maintenance? Does loss of Mks6 preferentially affect certain cell types? Or even more fundamentally, do all adult mammalian TZs contain Mks6?
Data from both the congenital Mks6 mutant as well as systemic conditional deletion of Mks6 revealed its importance for ciliogenesis/cilia maintenance in select cell types and resulted in mice exhibiting a subset of the ciliopathy phenotypes that are commonly observed in mice with cilia deficiencies. These results suggest a need for Mks6 in cilia formation or maintenance in some cell lineages and not others.
We also evaluated whether the effects on cilia formation and variability in the expressivity of the phenotype are related to whether the TZ had already been established when deletion of Mks6 occurred. With the use of conditional renal epithelium, the data indicate that there is an overall reduction in the number of cilia that will form when Mks6 deletion occurs before ciliogenesis. Thus, Mks6 does have a role in formation of the cilium, at least in renal epithelium in culture. Deletion of Mks6 after ciliogenesis has occurred did reduce the number of cilia on these cells compared with the control, also indicating that Mks6 function is needed for cilia maintenance. This is also observed in the retina, where induction of Mks6 loss before and after OS formation causes cone and rod cell degeneration. As the confluent kidney cells in culture and rods/cones in vivo are nondividing cells, the data indicate that the TZ, once formed, does undergo remodeling with exchange of new protein.
Surprisingly, the cilia that form on the Mks6 mutant cells have overtly normal localization of Arl13b and ACIII. This result is in contrast to the data reported by Garcia-Gonzalo et al. (15), showing that in Mks6 mutant MEFs, these proteins fail to localize in the cilium. Whether this is a difference in the cell type (renal epithelium compared with MEFs) is not known. Furthermore, in contrast to Garcia-Gonzalo et al. (15), none of the independently isolated MEF cell lines generated from our Mks6 mutants produce cilia (whereas control cells did), and thus, we could not directly compare outcomes of our data with that of Garcia-Gonzalo et al. (15).
Another major difference between the data presented here and that of Garcia-Gonzalo et al. (15) is that in our model, loss of Mks6 in MEFs and in the neural tube resulted in hyperacetylation of cytosolic microtubules. The contribution of the hyperacetylation to the Mks6 phenotype is not understood but has been reported in several other mutant models in which cilia formation is disrupted (32–35).
The conditional Mks6 allele (Mks6Δ) was generated to analyze TZ function in adult tissues. To our knowledge, the only other MKS conditional allele is in Mks5, which was used to analyze the importance of the TZ in the obesity phenotype and adipose development. As observed in several other conditional cilia mutants, such as Ift88, Kif3a, and Ift20, loss of Mks6 during juvenile stages results in rapid renal cystogenesis and slow disease progression when induced in adults (41–43). The expressivity of the cystic kidney disease in the juvenile Mks6Δ mutants was highly variable, of which we concluded was not a result of differences in Cre-mediated deletion efficiency. We used MRI in longitudinal studies within individual mice to assess disease progression and μCT to visualize and quantify cyst severity in 3D imaging. As a result of the renal injury resulting from contrast agents used in μCT, along with the known impact that renal injury has on cyst formation (41), we were not able to use μCT for in vivo longitudinal studies. 3D analysis and surface projections, along with analysis of individual sections of the µCT images, show that in some of the mutant kidneys, the cysts form in very focal domains or clusters in a subset of nephrons, whereas in others, nearly every nephron appears to be affected. These focal cysts form in kidneys, despite genotyping data, which indicate that the kidneys are largely devoid of Mks6.
In addition to kidney cysts, the Mks6Δ mutants developed retinal degeneration. In the juvenile Mks6Δ mutant mice, the photoreceptors exhibited rhodopsin mislocalization, typical of some forms of retinitis pigmentosa in humans, as well as light-dependent arrestin mislocalization, although transducin had proper localization. These data imply that the loss of Mks6 induces a slight change in the TZ or its gating properties, such that select proteins can enter and exit without issue, whereas others are hindered. Simultaneous with this, with the use of ERG, we detected a significant decrease in the electrical conductivity of the neural retina over time in the Mks6 adult-induced mutants, which had minimal OS present. Thus, even after the TZ and cilia are established in the retina, MKS6 function is necessary for select phototransduction proteins gating properties and overall retinal function and homeostasis.
In contrast to conditional IFT cilia loss models, BBS mouse models, and even more intriguing, the recent Mks5 conditional mutant mouse, we found that loss of Mks6 in adult animals did not result in hyperphagic behavior or increased weight gain (20, 27, 28, 44, 45).
Likewise, we found that the Mks6Δ mutants fail to develop any overt defects in olfactory ciliation that was different from BBS and other ciliopathy mouse models (23, 38–40). The window of observation in juvenile mice was chosen, as this is a period of maturation and expansion of the OE. If Mks6 was necessary for ciliogenesis, it should manifest as a decline in ciliation during this period. However, it is important to note that this does not eliminate the involvement of Mks6 in maintenance of cilia during aging. A disease phenotype may take time to manifest, given that olfactory neurons undergo continuous regeneration, and this homeostatic replacement is heterogeneous. This plasticity may lead to the delayed onset or even the circumvention of a disease phenotype. In addition, it is possible that the loss of Mks6 could disrupt olfactory function without impacting the anatomy. Likewise, hypomorphic mutation of the Cep290/NPHP6 exhibited loss of olfactory function as a result of the specific mislocalization of G-proteins, with no change in ciliation status (46, 47). This observation does not exclude a role of Mks6 in OE regeneration following chemical ablation or injury, where primary cilia signaling from the olfactory stem cell population participates. Nevertheless, it does not detract from the conclusion that conditional deletion of Mks6 does not disrupt cilia formation and maintenance in mature-differentiated olfactory neurons.
The differential effects of Mks6 disruption on ciliogenesis and cilia function are intriguing, as most proteins associated with MKS disorders are thought to be involved in general formation of a common structure in the TZ that collectively functions to regulate cilia protein composition. These contrasting data between the different mutants affecting the TZ may reflect differential requirements for these proteins in the cilium on specific cell types. Alternatively, it may indicate that there are multiple specific modes of transport into and out of the cilium, such that TZ components may have distinct roles in regulating ciliary entry, retention, or exit of specific proteins. Finally, once the function of the TZ is fulfilled in development and in ciliogenesis, then proteins, such as MKS6, may no longer be required in some cell types, or other TZ proteins are able to compensate to perform its function, resulting in normal olfaction or satiation signaling that does not occur correctly in the context of the kidney and retina. The genetic analysis of other conditional mutant alleles affecting the TZ are needed to determine how in mammalian systems, the TZ is involved in regulating specific activities of the cilium across multiple cell types.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank Mary Ann Accavitti-Loper (University of Alabama at Birmingham) and Logan S. Whitehouse (Indiana University Purdue University Indianapolis, Indianapolis, IN, USA) for technical assistance on the project. This work was supported by U.S. National Institutes of Health (NIH) National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grants R01DK065655, R01DK115752 (to B.K.Y.), and R01DK114008 (to N.F.B.); NIH National Institute on Deafness and Other Communication Disorders Grant R01DC009606 (to J.R.M.); and NIH National Eye Institute (NEI) R01EY019311 (to A.K.G.). Additional support was from the E. Matilda Ziegler Foundation (to A.K.G.) and Pilot and Feasibility Award from the Center for Diabetes and Metabolic Diseases (P30 DK097512; to N.F.B.). Center support for the project was provided by NIH NIDDK Grants P30DK074038, P30DK079337, P30DK079626, and NIH NEI Grant P30EY003039, and the NIH Instrumentation Grant S10RR026887. Services provided in this publication through the University of Alabama at Birmingham Transgenic & Genetically Engineered Models (TGEMS) facility (R.A.K.) are supported by NIH National Cancer Institute Grant P30CA13148, NIH National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant P30AR048311, and NIH NIDDK Grants P30 DK074038, P30 DK05336, and P60 DK079626. The authors declare no conflicts of interest.
Glossary
- 2D
2-dimensional
- 3D
3-dimensional
- µCT
micro computed tomography
- ACIII
adenylyl cyclase III
- Arl13b
ADP-ribosylation factor-like protein 13B
- BBS
Bardet-Biedl syndrome
- BW
body weight
- DBA
Dolichos biflorus agglutinin
- ERG
electroretinography
- FLP
flippase
- Ift88
intraflagellar transport 88
- IS
inner segment
- KO
knockout
- LPM
lateral plate mesoderm
- LTA
Lotus lectin
- MEF
mouse embryonic fibroblast
- MKS
Meckel-Grüber syndrome
- NA
numerical aperture
- NPHP
nephronophthisis
- OB
olfactory bulb
- OE
olfactory epithelium
- OMP
olfactory marker protein
- ONL
outer nuclear layer
- OS
outer segment
- OSN
olfactory sensory neuron
- P.I.
postinduction
- PFA
paraformaldehyde
- qRT-PCR
quantitative RT-PCR
- RE
respiratory epithelium
- TH
tyrosine hydroxylase
- TKV
total kidney volume
- TZ
transition zone
- VNT
ventral neural tube
- WT
wild type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
Mouse lines were generated by R. A. Kesterson and maintained by M. J. Croyle; congenital allele and KO work was performed by W. R. Lewis and N. F. Berbari; eye phenotypes were analyzed by K. L. Bales and A. K. Gross; renal phenotypes were analyzed by W. R. Lewis, D. Z. Revell, M. J. Croyle, C. J. Song, E. B. Malarkey, and B. K. Yoder; D. Z. Revell, M. J. Croyle, E. B. Malarkey, and T. R. Nagy conducted µCT analysis; W. R. Lewis, E. B. Malarkey, D. Shan, and M. M. Mrug conducted MRI studies; analysis of olfactory phenotypes was done by C. R. Uytingco and J. R. Martens; obesity studies were performed by W. R. Lewis, S. E. Engle, P. J. Antonellis, and N. F. Berbari; reagent and other resources were provided by M. M. Mrug, J. R. Martens, N. F. Berbari, A. K. Gross, and B. K. Yoder; and all authors helped design experiments and write the manuscript.
REFERENCES
- 1.Berbari N. F., O’Connor A. K., Haycraft C. J., Yoder B. K. (2009) The primary cilium as a complex signaling center. Curr. Biol. 19, R526–R535 10.1016/j.cub.2009.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharma N., Berbari N. F., Yoder B. K. (2008) Ciliary dysfunction in developmental abnormalities and diseases. Curr. Top. Dev. Biol. 85, 371–427 10.1016/S0070-2153(08)00813-2 [DOI] [PubMed] [Google Scholar]
- 3.Barker A. R., Thomas R., Dawe H. R. (2014) Meckel-Gruber syndrome and the role of primary cilia in kidney, skeleton, and central nervous system development. Organogenesis 10, 96–107 10.4161/org.27375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chih B., Liu P., Chinn Y., Chalouni C., Komuves L. G., Hass P. E., Sandoval W., Peterson A. S. (2011) A ciliopathy complex at the transition zone protects the cilia as a privileged membrane domain. Nat. Cell Biol. 14, 61–72 10.1038/ncb2410 [DOI] [PubMed] [Google Scholar]
- 5.Kee H. L., Dishinger J. F., Blasius T. L., Liu C. J., Margolis B., Verhey K. J. (2012) A size-exclusion permeability barrier and nucleoporins characterize a ciliary pore complex that regulates transport into cilia. Nat. Cell Biol. 14, 431–437 10.1038/ncb2450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Awata J., Takada S., Standley C., Lechtreck K. F., Bellvé K. D., Pazour G. J., Fogarty K. E., Witman G. B. (2014) NPHP4 controls ciliary trafficking of membrane proteins and large soluble proteins at the transition zone. J. Cell Sci. 127, 4714–4727 10.1242/jcs.155275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jensen V. L., Li C., Bowie R. V., Clarke L., Mohan S., Blacque O. E., Leroux M. R. (2015) Formation of the transition zone by Mks5/Rpgrip1L establishes a ciliary zone of exclusion (CIZE) that compartmentalises ciliary signalling proteins and controls PIP2 ciliary abundance. EMBO J. 34, 2537–2556 10.15252/embj.201488044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams C. L., Li C., Kida K., Inglis P. N., Mohan S., Semenec L., Bialas N. J., Stupay R. M., Chen N., Blacque O. E., Yoder B. K., Leroux M. R. (2011) MKS and NPHP modules cooperate to establish basal body/transition zone membrane associations and ciliary gate function during ciliogenesis. J. Cell Biol. 192, 1023–1041 10.1083/jcb.201012116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klink B. U., Zent E., Juneja P., Kuhlee A., Raunser S., Wittinghofer A. (2017) A recombinant BBSome core complex and how it interacts with ciliary cargo. eLife 6, e27434 10.7554/eLife.27434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nachury M. V., Seeley E. S., Jin H. (2010) Trafficking to the ciliary membrane: how to get across the periciliary diffusion barrier? Annu. Rev. Cell Dev. Biol. 26, 59–87 10.1146/annurev.cellbio.042308.113337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Noor A., Windpassinger C., Patel M., Stachowiak B., Mikhailov A., Azam M., Irfan M., Siddiqui Z. K., Naeem F., Paterson A. D., Lutfullah M., Vincent J. B., Ayub M. (2008) CC2D2A, encoding a coiled-coil and C2 domain protein, causes autosomal-recessive mental retardation with retinitis pigmentosa. Am. J. Hum. Genet. 82, 1011–1018 10.1016/j.ajhg.2008.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chaki M., Hoefele J., Allen S. J., Ramaswami G., Janssen S., Bergmann C., Heckenlively J. R., Otto E. A., Hildebrandt F. (2011) Genotype-phenotype correlation in 440 patients with NPHP-related ciliopathies. Kidney Int. 80, 1239–1245 10.1038/ki.2011.284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gorden N. T., Arts H. H., Parisi M. A., Coene K. L., Letteboer S. J., van Beersum S. E., Mans D. A., Hikida A., Eckert M., Knutzen D., Alswaid A. F., Ozyurek H., Dibooglu S., Otto E. A., Liu Y., Davis E. E., Hutter C. M., Bammler T. K., Farin F. M., Dorschner M., Topçu M., Zackai E. H., Rosenthal P., Owens K. N., Katsanis N., Vincent J. B., Hildebrandt F., Rubel E. W., Raible D. W., Knoers N. V., Chance P. F., Roepman R., Moens C. B., Glass I. A., Doherty D. (2008) CC2D2A is mutated in Joubert syndrome and interacts with the ciliopathy-associated basal body protein CEP290. Am. J. Hum. Genet. 83, 559–571 10.1016/j.ajhg.2008.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bachmann-Gagescu R., Dona M., Hetterschijt L., Tonnaer E., Peters T., de Vrieze E., Mans D. A., van Beersum S. E., Phelps I. G., Arts H. H., Keunen J. E., Ueffing M., Roepman R., Boldt K., Doherty D., Moens C. B., Neuhauss S. C., Kremer H., van Wijk E. (2015) The ciliopathy protein CC2D2A associates with NINL and functions in RAB8-MICAL3-regulated vesicle trafficking. PLoS Genet. 11, e1005575 10.1371/journal.pgen.1005575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcia-Gonzalo F. R., Corbit K. C., Sirerol-Piquer M. S., Ramaswami G., Otto E. A., Noriega T. R., Seol A. D., Robinson J. F., Bennett C. L., Josifova D. J., García-Verdugo J. M., Katsanis N., Hildebrandt F., Reiter J. F. (2011) A transition zone complex regulates mammalian ciliogenesis and ciliary membrane composition. Nat. Genet. 43, 776–784 10.1038/ng.891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Veleri S., Manjunath S. H., Fariss R. N., May-Simera H., Brooks M., Foskett T. A., Gao C., Longo T. A., Liu P., Nagashima K., Rachel R. A., Li T., Dong L., Swaroop A. (2014) Ciliopathy-associated gene Cc2d2a promotes assembly of subdistal appendages on the mother centriole during cilia biogenesis. Nat. Commun. 5, 4207 10.1038/ncomms5207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bachmann-Gagescu R., Phelps I. G., Stearns G., Link B. A., Brockerhoff S. E., Moens C. B., Doherty D. (2011) The ciliopathy gene cc2d2a controls zebrafish photoreceptor outer segment development through a role in Rab8-dependent vesicle trafficking. Hum. Mol. Genet. 20, 4041–4055 10.1093/hmg/ddr332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waters A. M., Beales P. L. (2011) Ciliopathies: an expanding disease spectrum. Pediatr. Nephrol. 26, 1039–1056 10.1007/s00467-010-1731-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Braun D. A., Hildebrandt F. (2017) Ciliopathies. Cold Spring Harb. Perspect. Biol. 9, a028191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stratigopoulos G., Burnett L. C., Rausch R., Gill R., Penn D. B., Skowronski A. A., LeDuc C. A., Lanzano A. J., Zhang P., Storm D. R., Egli D., Leibel R. L. (2016) Hypomorphism of Fto and Rpgrip1l causes obesity in mice. J. Clin. Invest. 126, 1897–1910 10.1172/JCI85526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haycraft C. J., Zhang Q., Song B., Jackson W. S., Detloff P. J., Serra R., Yoder B. K. (2007) Intraflagellar transport is essential for endochondral bone formation. Development 134, 307–316 10.1242/dev.02732 [DOI] [PubMed] [Google Scholar]
- 22.Hayashi S., McMahon A. P. (2002) Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse. Dev. Biol. 244, 305–318 10.1006/dbio.2002.0597 [DOI] [PubMed] [Google Scholar]
- 23.McIntyre J. C., Davis E. E., Joiner A., Williams C. L., Tsai I. C., Jenkins P. M., McEwen D. P., Zhang L., Escobado J., Thomas S., Szymanska K., Johnson C. A., Beales P. L., Green E. D., Mullikin J. C., Sabo A., Muzny D. M., Gibbs R. A., Attié-Bitach T., Yoder B. K., Reed R. R., Katsanis N., Martens J. R.; NISC Comparative Sequencing Program (2012) Gene therapy rescues cilia defects and restores olfactory function in a mammalian ciliopathy model. Nat. Med. 18, 1423–1428 10.1038/nm.2860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Molday R. S., MacKenzie D. (1983) Monoclonal antibodies to rhodopsin: characterization, cross-reactivity, and application as structural probes. Biochemistry 22, 653–660 10.1021/bi00272a020 [DOI] [PubMed] [Google Scholar]
- 25.MacKenzie D., Arendt A., Hargrave P., McDowell J. H., Molday R. S. (1984) Localization of binding sites for carboxyl terminal specific anti-rhodopsin monoclonal antibodies using synthetic peptides. Biochemistry 23, 6544–6549 10.1021/bi00321a041 [DOI] [PubMed] [Google Scholar]
- 26.Gross A. K., Rao V. R., Oprian D. D. (2003) Characterization of rhodopsin congenital night blindness mutant T94I. Biochemistry 42, 2009–2015 10.1021/bi020613j [DOI] [PubMed] [Google Scholar]
- 27.Davenport J. R., Watts A. J., Roper V. C., Croyle M. J., van Groen T., Wyss J. M., Nagy T. R., Kesterson R. A., Yoder B. K. (2007) Disruption of intraflagellar transport in adult mice leads to obesity and slow-onset cystic kidney disease. Curr. Biol. 17, 1586–1594 10.1016/j.cub.2007.08.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stratigopoulos G., Martin Carli J. F., O’Day D. R., Wang L., Leduc C. A., Lanzano P., Chung W. K., Rosenbaum M., Egli D., Doherty D. A., Leibel R. L. (2014) Hypomorphism for RPGRIP1L, a ciliary gene vicinal to the FTO locus, causes increased adiposity in mice. Cell Metab. 19, 767–779 10.1016/j.cmet.2014.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stratigopoulos G., LeDuc C. A., Cremona M. L., Chung W. K., Leibel R. L. (2011) Cut-like homeobox 1 (CUX1) regulates expression of the fat mass and obesity-associated and retinitis pigmentosa GTPase regulator-interacting protein-1-like (RPGRIP1L) genes and coordinates leptin receptor signaling. J. Biol. Chem. 286, 2155–2170 10.1074/jbc.M110.188482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reish N. J., Maltare A., McKeown A. S., Laszczyk A. M., Kraft T. W., Gross A. K., King G. D. (2013) The age-regulating protein klotho is vital to sustain retinal function. Invest. Ophthalmol. Vis. Sci. 54, 6675–6685 10.1167/iovs.13-12550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takahashi N., Boysen G., Li F., Li Y., Swenberg J. A. (2007) Tandem mass spectrometry measurements of creatinine in mouse plasma and urine for determining glomerular filtration rate. Kidney Int. 71, 266–271 10.1038/sj.ki.5002033 [DOI] [PubMed] [Google Scholar]
- 32.Berbari N. F., Sharma N., Malarkey E. B., Pieczynski J. N., Boddu R., Gaertig J., Guay-Woodford L., Yoder B. K. (2013) Microtubule modifications and stability are altered by cilia perturbation and in cystic kidney disease. Cytoskeleton (Hoboken) 70, 24–31 10.1002/cm.21088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bizet A. A., Becker-Heck A., Ryan R., Weber K., Filhol E., Krug P., Halbritter J., Delous M., Lasbennes M. C., Linghu B., Oakeley E. J., Zarhrate M., Nitschké P., Garfa-Traore M., Serluca F., Yang F., Bouwmeester T., Pinson L., Cassuto E., Dubot P., Elshakhs N. A., Sahel J. A., Salomon R., Drummond I. A., Gubler M. C., Antignac C., Chibout S., Szustakowski J. D., Hildebrandt F., Lorentzen E., Sailer A. W., Benmerah A., Saint-Mezard P., Saunier S. (2015) Mutations in TRAF3IP1/IFT54 reveal a new role for IFT proteins in microtubule stabilization. Nat. Commun. 6, 8666 10.1038/ncomms9666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berbari N. F., Kin N. W., Sharma N., Michaud E. J., Kesterson R. A., Yoder B. K. (2011) Mutations in Traf3ip1 reveal defects in ciliogenesis, embryonic development, and altered cell size regulation. Dev. Biol. 360, 66–76 10.1016/j.ydbio.2011.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dafinger C., Liebau M. C., Elsayed S. M., Hellenbroich Y., Boltshauser E., Korenke G. C., Fabretti F., Janecke A. R., Ebermann I., Nürnberg G., Nürnberg P., Zentgraf H., Koerber F., Addicks K., Elsobky E., Benzing T., Schermer B., Bolz H. J. (2011) Mutations in KIF7 link Joubert syndrome with sonic hedgehog signaling and microtubule dynamics. J. Clin. Invest. 121, 2662–2667 10.1172/JCI43639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tallila J., Jakkula E., Peltonen L., Salonen R., Kestilä M. (2008) Identification of CC2D2A as a Meckel syndrome gene adds an important piece to the ciliopathy puzzle. Am. J. Hum. Genet. 82, 1361–1367 10.1016/j.ajhg.2008.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Starremans P. G., Li X., Finnerty P. E., Guo L., Takakura A., Neilson E. G., Zhou J. (2008) A mouse model for polycystic kidney disease through a somatic in-frame deletion in the 5′ end of Pkd1. Kidney Int. 73, 1394–1405 10.1038/ki.2008.111 [DOI] [PubMed] [Google Scholar]
- 38.Kulaga H. M., Leitch C. C., Eichers E. R., Badano J. L., Lesemann A., Hoskins B. E., Lupski J. R., Beales P. L., Reed R. R., Katsanis N. (2004) Loss of BBS proteins causes anosmia in humans and defects in olfactory cilia structure and function in the mouse. Nat. Genet. 36, 994–998 10.1038/ng1418 [DOI] [PubMed] [Google Scholar]
- 39.Tadenev A. L., Kulaga H. M., May-Simera H. L., Kelley M. W., Katsanis N., Reed R. R. (2011) Loss of Bardet-Biedl syndrome protein-8 (BBS8) perturbs olfactory function, protein localization, and axon targeting. Proc. Natl. Acad. Sci. USA 108, 10320–10325 10.1073/pnas.1016531108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Williams C. L., Uytingco C. R., Green W. W., McIntyre J. C., Ukhanov K., Zimmerman A. D., Shively D. T., Zhang L., Nishimura D. Y., Sheffield V. C., Martens J. R. (2017) Gene therapeutic reversal of peripheral olfactory impairment in Bardet-Biedl syndrome. Mol. Ther. 25, 904–916 10.1016/j.ymthe.2017.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sharma N., Malarkey E. B., Berbari N. F., O’Connor A. K., Vanden Heuvel G. B., Mrug M., Yoder B. K. (2013) Proximal tubule proliferation is insufficient to induce rapid cyst formation after cilia disruption. J. Am. Soc. Nephrol. 24, 456–464 10.1681/ASN.2012020154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Piontek K., Menezes L. F., Garcia-Gonzalez M. A., Huso D. L., Germino G. G. (2007) A critical developmental switch defines the kinetics of kidney cyst formation after loss of Pkd1. Nat. Med. 13, 1490–1495 10.1038/nm1675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patel V., Li L., Cobo-Stark P., Shao X., Somlo S., Lin F., Igarashi P. (2008) Acute kidney injury and aberrant planar cell polarity induce cyst formation in mice lacking renal cilia. Hum. Mol. Genet. 17, 1578–1590 10.1093/hmg/ddn045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guo D. F., Rahmouni K. (2011) Molecular basis of the obesity associated with Bardet-Biedl syndrome. Trends Endocrinol. Metab. 22, 286–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seo S., Guo D. F., Bugge K., Morgan D. A., Rahmouni K., Sheffield V. C. (2009) Requirement of Bardet-Biedl syndrome proteins for leptin receptor signaling. Hum. Mol. Genet. 18, 1323–1331 10.1093/hmg/ddp031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McEwen D. P., Koenekoop R. K., Khanna H., Jenkins P. M., Lopez I., Swaroop A., Martens J. R. (2007) Hypomorphic CEP290/NPHP6 mutations result in anosmia caused by the selective loss of G proteins in cilia of olfactory sensory neurons. Proc. Natl. Acad. Sci. USA 104, 15917–15922 10.1073/pnas.0704140104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Joiner A. M., Green W. W., McIntyre J. C., Allen B. L., Schwob J. E., Martens J. R. (2015) Primary cilia on horizontal basal cells regulate regeneration of the olfactory epithelium. J. Neurosci. 35, 13761–13772 10.1523/JNEUROSCI.1708-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.