Abstract
Jawed vertebrates (Gnathostomes) have 4 tissue inhibitors of metalloproteinases (TIMPs), multifunctional proteins that all inhibit members of the large matrix metalloproteinase (MMP) family but differ in their other roles, including the regulation of pro-MMP activation, cell growth, apoptosis and angiogenesis, and the structure of extracellular matrices (ECMs). Molecular phylogeny analyses indicate that vertebrate TIMP genes arose from an invertebrate ancestor through 3 successive duplications, possibly including 2 whole genome duplications, during early vertebrate phylogeny. TIMPs from invertebrates also inhibit metalloproteinases, bind to pro-MMPs, and contribute to ECM structures but are not orthologs of any particular vertebrate TIMP. The most ancient vertebrate superclass, the Agnatha (jawless fish), seems to provide a snapshot of a stage in TIMP evolution preceding the third gene duplication. This review examines the structures of TIMPs from different vertebrate orders using information relating to the structural basis of their various functions. Provisional conclusions are that during their evolutionary divergence, various TIMPs lost inhibitory activity toward some metalloproteinases, specialized in effects on different pro-MMPs, and developed new interactions with discrete targets (including integrins and receptors), while recapitulating a role in ECM structure. The analysis is limited by the sparse information available regarding the functional properties of nonmammalian TIMPs.—Brew, K. Reflections on the evolution of the vertebrate tissue inhibitors of metalloproteinases.
Keywords: angiogenesis, cell growth, matrix structure, enzyme inhibition, zymogen activation
The tissue inhibitors of metalloproteinases (TIMPs) are secreted metalloproteinase inhibitors that are widely distributed in vertebrates and invertebrates and form family I35 of the MEROPS database (1). Four TIMP paralogs (TIMP1–TIMP4) are present in the Gnathostomata superclass of the vertebrate subphylum, with the exception that TIMP1 is not found in birds or teleost fishes. The 4 TIMPs have multiple functions, and their structures, activities, genomics, and roles in disease and immunity have been extensively reviewed (2–7). The goal of the present article was to attempt to understand the process through which these 4 protein lines and their specialized functions developed from a single common ancestor during vertebrate phylogeny, with the aim of better comprehending their current complex and distinct roles (7).
The primary role of the TIMPs is the inhibition of metalloproteinases, particularly the many members of the matrix metalloproteinase (MMP) family (MEROPS family M10), through tight binding with affinities reflected in Ka values between 109 and 1011 M−1. There are 23 MMPs in humans that encompass enzymes with overlapping but distinct substrate specificities and variations in noncatalytic domain structures that are classified as collagenases, gelatinases, stromelysins, matrilysins, and membrane-type MMPs (5). Among the 4 TIMPs, only TIMP1 exhibits pronounced selectivity as an inhibitor of the MMPs, being a weak inhibitor of a subgroup that includes the type-1 membrane-bound MMPs (MMP14, -15, -16, and -24) and MMP19. However, TIMP1 inhibits all other MMPs, including the 2 glycosylphosphatidylinositol-anchored membrane-type MMPs, MMP17 and -26 (3). In contrast, TIMP3 has the broadest range of metalloproteinase targets, being a potent inhibitor of multiple key disintegrin-metalloproteinases (ADAMs, members of MEROPs family M12), including ADAM-17 (TNF-α–converting enzyme, or TACE), and ADAMTS-4 and -5 (aggrecanase-1 and aggrecanase-2). TIMP2, -3, and -4 are the most similar in sequence, the human proteins having 45–50% identical residues, whereas TIMP1 is an outlier with 36–41% identity to the others. TIMP4 is an outlier in another respect: whereas the other TIMPs are ubiquitous, TIMP4 is restricted in expression, being abundant in the heart, adipose tissue, and pituitary gland, with some expression in brain, pancreas, eye, bone, and other extracellular matrices (ECMs) (7).
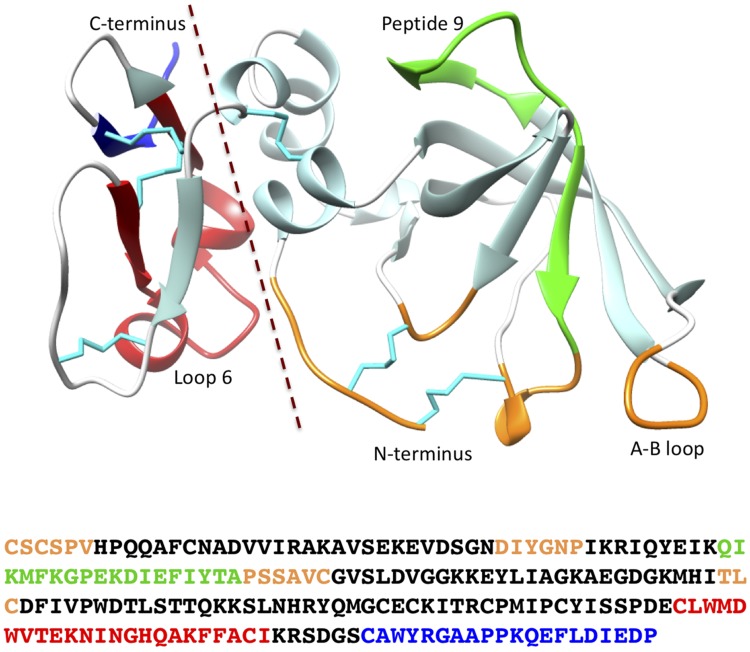
The TIMPs have 2 domains, each stabilized by 3 disulfide crosslinks. Two thirds of the mature protein (∼120 residues) constitutes the large N-terminal domain, whereas the remaining one third forms the C-terminal domain (Fig. 1). The N-domain (N-TIMP) has an oligonucleotide/oligosaccharide binding fold, a 5-stranded β-barrel structure plus 2 small helices (8), and is related to the following: C-terminal domains of netrins; complement proteins C3, C4, and C5; secreted frizzled-related proteins; and type 1 procollagen C-proteinase enhancer proteins (9). The C-terminal domain is structurally categorized into a broad group of small disulfide-rich proteins and domains but has a fold distinct from those of other polypeptides of this group, encompassing 2 β-hairpins and 2 helices (10). The 2 domains are separated by a short spacer of 1 or 2 residues (more in some invertebrate TIMPs), located between the sixth and seventh cysteines in the mature TIMP sequence, limiting flexibility in the interdomain interface.
Figure 1.
The 3-dimensional structure of human TIMP2 and amino acid sequence showing the locations of the 2 domains and other regions discussed in the text. The representation was generated from pdb 1BR9 (105) using the University of California, San Francisco (UCSF) chimera package (106). Specific sections of the structure are colored as follows: reactive site, orange; peptide 9 (interaction with α3β1 integrin), green; loop 6 (interaction with IGF-1R), red; and C-terminal tail (MMP2–Hpx domain interaction), blue. Cystines and disulfide bonds are cyan, and the broken line marks the division between the N- and C-domains.
TIMPs have multiple functions that are independent of MMP inhibition (2–6) and include binding to the inactive zymogens of MMPs (proMMPs) via the TIMP C-domain and MMP hemopexin (Hpx) domain (2, 3). An erythroid cell growth–promoting activity of TIMP1 was described shortly before its MMP inhibitory activity (11, 12), and both TIMP1 and -2 are now recognized as promoters of the growth of erythroid and other cells (13–16). The promotion of cell growth by TIMP1 results from its binding to a tetraspanin, CD63, which mediates its interaction with β1 integrin, but TIMP2 promotes cell growth by directly binding to α3β1 integrin (17, 18). TIMP2 and -3 also have antiangiogenic actions that are not linked to metalloproteinase inhibition (19, 20), and TIMP3 has a structural role in some ECMs that is mediated by its avid binding to sulfated oligosaccharides (21–23) and to fibulin-3 (EGF-containing fibulin-like extracellular matrix protein 1), an ECM structural protein (24).
GENOMICS OF THE VERTEBRATE TIMPs
Although invertebrate TIMPs generally have 2 domains, some—including the 2 TIMPs of the nematode, Caenorhabditis elegans, TIMPs from some bivalves, and TIMP homologs from some bacteria (2)—have a single domain that corresponds to the N-domain of vertebrate TIMPs (25). Because homologs of N-TIMP are found in some bacteria, single-domain invertebrate TIMPs might be regarded as evolutionary relics of an ancestral form. However, most invertebrate TIMPs, including those from Cnidaria, have 2 domains, suggesting that the single-domain invertebrate TIMPs arose by truncation of 2-domain metazoan ancestors (25). The multiple TIMP genes found in some invertebrates do not correspond to any of the 4 vertebrate TIMP lines (2, 25).
The vertebrate TIMP genes (Timp) encompass 5 exons and are located on different chromosomes (Table 1). Timp1, -3, and -4 are each nested in an intron of a gene for 1 of 3 paralogs of a neuronal phosphoprotein, synapsin (Syn) (26, 27). Timp1 is nested in Syn1, Timp3 in Syn3, and Timp4 in Syn2. The Timp2 gene lacks a Syn host, but the synteny of Timp and Syn is ancient, being conserved in a diverse array of invertebrates that includes insects, mollusks, echinoderms, annelids, and cnidarians. However, the single-domain C. elegans TIMPs do not have a Syn host (27). Synapsins are multidomain proteins associated with synaptic vesicles, and each Timp is located in a highly conserved site in an intron within the conserved Syn domain C region (27, 28). Like most nested genes, Timp and Syn are transcribed from different DNA strands (29, 30), and there is no evidence for coordination of expression or biologic function between TIMPs and synapsins (28). Although Timp2 is not associated with a Syn, it is a host to DDC8, the gene for differential display clone 8 (31). In this case, both proteins are encoded by the same DNA strand and appear to be linked with regard to expression because both are produced in testes and neurons and both exhibit enhanced transcription after traumatic brain injury. The synteny of genes can have a significant impact on their evolution and expression; the proteins encoded by nested genes have faster rates of evolution and more restricted ranges of expression than those encoded by other genes (30).
TABLE 1.
General properties of the 4 vertebrate TIMPs
| Property | TIMP1 | TIMP2 | TIMP3 | TIMP4 |
|---|---|---|---|---|
| Mean pI (range) | 7.95 (4.81–9.06) | 7.17 (5.23–8.41) | 9.14 (9.01–9.24) | 7.98 (6.86–8.84) |
| Residues Hs N-domaina | 126 | 127 | 121 | 128 |
| Hs C-domain | 58 | 67 | 67 | 67 |
| Other species | ||||
| N | Varies (123–126) | 127 | 127 | Up to 130 |
| C | Varies (55–74) | 67 | 67 | 67 |
| N-glycosylation | One or 2 sites in N- and C-domains | None | Conserved site near C-terminus | None |
| MMP inhibition range | Weak for MMP-14, -15, 16, -19, and -24 | All | All | Most |
| Inhibition of other MPs | ADAM10 | ADAM12 | ADAM10, 12, 17, 28, and 33; ADAMTS-1, -4, and -5 | ADAM17, 28 |
| ProMMP interactions | proMMP-9 | proMMP-2 | proMMP-2, proMMP-9 | proMMP-2 |
| Localization | Cell surface, secreted | Cell surface, secreted | ECM, cell surface, nucleus | Cell surface, secreted |
| Other partners | CD63, CD88 LRP-1 (MMP-9 complex) | α3β1 integrin, IGFR1, LRP1, LRP2 (proMMP-2 complex) | EFEMP1 VEGFR2 AGTR1, LRP1, SP | |
| Human chromosomal location | X11p11.23-11.4 | 17q23-25 | 22q12.1-q13.2 | 3p25 |
| Synteny | Hosted by Syn I | Host to DDC8 | Hosted by Syn III | Hosted by Syn II |
| Genetic disorders | SFD; lung and eye disease |
EFEMP1, EGF-containing fibulin-like extracellular matrix protein 1. aSequence numbers refer to the mature protein. The N-domain includes residues between the sixth and seventh Cys, and the C-domain starts at the seventh Cys.
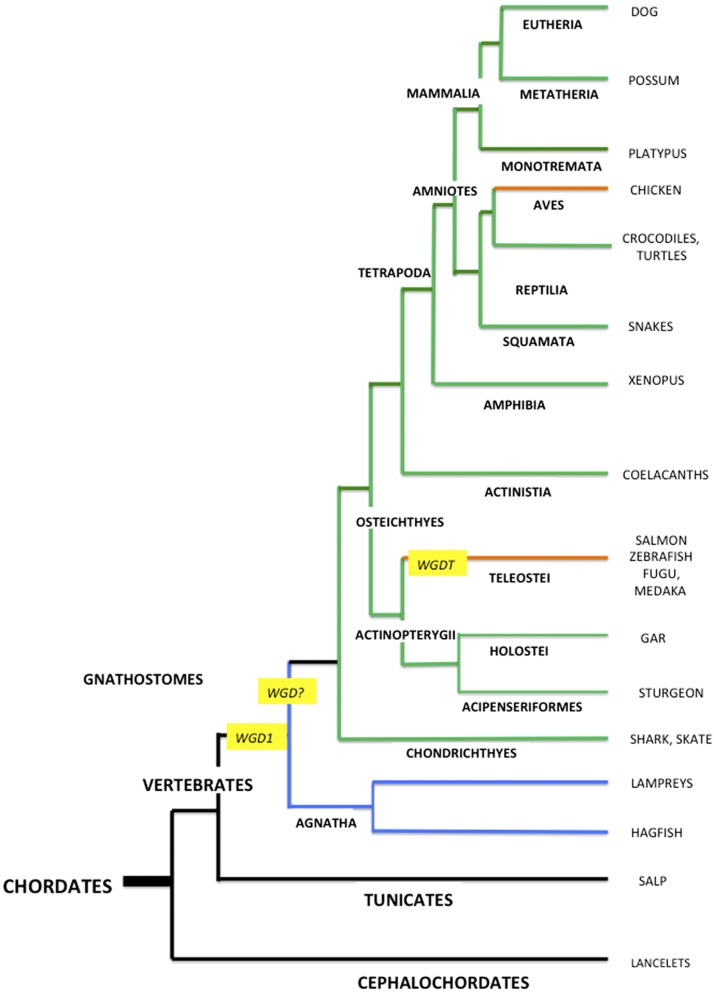
Timp1 has not been found in any avian species, suggesting that it has been eliminated from the Aves, possibly as part of a reduction in genome size, associated with their adaptation to flight (32). A whole genome duplication (WGD) occurred early in the evolutionary development of teleosts (teleost WGD), 225–333 million years ago (Mya) (Fig. 2), an event that was followed by the loss of many duplicated genes and inactivation of redundant genes (33–35). There was a more recent WGD in the Salmonid line, ∼25–100 Mya (36). Teleost fishes have 2 TIMP2 lines (27, 37–39), presumably products of the teleost WGD, and the salmon (Salmo salar) have these 2 TIMP2 lines plus additional TIMP2-like proteins that seem to be products of the salmonid-specific WGD. An examination of the pairs of TIMP2 proteins from 9 teleost species (Supplemental Table 1) shows that one, designated TIMP2a, is consistently more similar to TIMP2s from other vertebrates than the second, TIMP2b [as also discussed in Kubota et al. (37)]. Several residues that are conserved within the teleost TIMP2a and -2b lines differ between the 2 lines, suggesting that TIMP2a and -2b may provide an example of subfunctionalization, similar to that noted in teleost synapsins (27). The differences include residues 6, 44, and 87 of the N-domain and residues 136, 151, 160, and 176 of the C-domain (Supplemental Fig. 1). Some of these are within regions implicated in interactions with metalloproteinases and receptors, but no information is currently available regarding specific functional differences between teleost TIMP2a and TIMP2b.
Figure 2.
A phylogenetic tree of the Chordate phylum, showing the interrelationships of some species discussed in the text and positioning of WGDs. Black lines represent invertebrate lineages; green lines, lineages with the 4 vertebrate TIMPs; orange lines, lineages lacking TIMP1; and blue lines, the Agnatha, which appear to have undergone 1 or 2 TIMP gene duplication events. The figure is based on Fig. 6 from Nakatani et al. (33).
As discussed in Origins of the vertebrate TIMP genes, the evolution of TIMP1 in the therian mammals (placentals and marsupials) has been affected by its localization in the X chromosome (40), which has no ortholog in other vertebrates, including the most ancient mammalian order, the prototherians (monotremes). These egg-laying mammals diverged from the main mammalian line ∼177 Mya, before the marsupials (41), and have different sex-determining genes. The 5 X and 5 Y chromosomes in the platypus, Ornithorhynchus anatinus, are not orthologous with the X and Y chromosomes of therian mammals but are related to the avian Z chromosome (42, 43), which is part of a distinct WZ sex-determining system (ZZ male and WZ female). The primary structure of platypus TIMP1 is known, but the chromosomal location of its gene is unknown. However, the platypus Timp1 gene is probably located with Syn1 on chromosome 6. Chromosome 6 contains the SOX3 gene, from which the male-determining gene in therian mammals (SRY) was derived, implying that this chromosome of the platypus is the ortholog of the autosome ancestor of the therian X and Y chromosomes.
ORIGINS OF THE VERTEBRATE TIMP GENES
Vertebrates are 1 of the 3 subphyla of the Chordates; the other 2 subphyla, the Cephalochordates and Tunicates (or Urochordates), are invertebrates (Fig. 2). The primary structures of TIMPs are known for 3 cephalochordates, amphioxi, or Lancelets (Branchiostoma floridae, Branchiostoma lanceolatum, and Asymmetron lucayanum) and for 2 Tunicates (the vase tunicate, Ciona intestinalis, and a salp, Salpa thompsoni). Sequence alignments of these TIMPs with vertebrate TIMPs have multiple extended gaps in both the N- and C-domains (Supplemental Fig. 2), and in phylogenetic analyses, the invertebrate TIMPs separate as a branch preceding the divergence of the 4 vertebrate TIMPs.
The most ancient vertebrate superclass is the Agnatha (jawless fish), which diverged from the Gnathostomes (jawed vertebrates) ∼615 Mya (41). The Agnatha separated from the vertebrate stem much earlier than the next most ancient group, the Chondrichthyes (the cartilaginous fish), which diverged 473 Mya. The Chondrichthyes have all 4 vertebrate TIMPs, directing attention to the Agnatha as a possible source of clues about the early evolution of vertebrate TIMPs. The only extant Agnatha are the cyclostomes (hagfish and lampreys), representing the Myxiniformes and Petromyzontiformes orders, respectively. The sequences of 2 TIMPs from the inshore hagfish, Eptatretus burger, and 3 from the sea lamprey, Petromyzon marinus, are provided, variously, in the Uniprot database, the NCBI TSA database, the Japanese Lamprey Genome Project (jlampreygenome.imcb.a-star.edu.sg), the Vertebrate TimeCapsule database (41), and MEROPS (Supplemental Table 2). It should be noted that some of the protein sequences were assembled by automated analysis of genomic data and are classified as preliminary.
The relationship of the Agnatha TIMP sequences to those of the 4 vertebrate TIMPs is not immediately apparent. To facilitate an analysis of the sequences, 1 lamprey TIMP, which is ∼50% identical in sequence to TIMP-2, -3, and -4 but only 35% identical to TIMP1, was designated lamprey TIMPa. Also, the hagfish TIMP that is most similar to this protein (37% identical) was designated as hagfish TIMPa. The second hagfish TIMP is most similar to another lamprey TIMP (36.5% identity), and both were designated TIMPb; the remaining lamprey TIMP, which is <30% identical to its paralogs, was designated TIMPc. Figure 3 displays an alignment of these TIMPs with those from a mammal (human) and a cartilaginous fish (elephant shark), selected because of their extensive temporal divergence (471 Mya) among the Gnathostomes. An examination of this alignment and separate alignments of lamprey TIMPa with multiple TIMP2, -3, and -4 sequences from different orders shows that lamprey TIMPa is more similar to TIMP3 than -2 or -4 in overall sequence, and has residues that in TIMP3 facilitate its broad metalloproteinase inhibition spectrum. However, it does not have lysine residues corresponding to those that are crucial for TIMP3 binding to ECM (see Interactions with non-metalloproteinase targets and Structural roles in ECM) and lacks a N-glycosylation Asn-X-Ser/Thr sequence motif located near the C-terminus in all TIMP3 sequences that is absent from TIMP2 and -4 (2, 3). The substantial sequence differences between the TIMPa and TIMPb proteins from the 2 cyclostomes probably reflect the 400 million years of divergence between lampreys and hagfish that is also displayed in their large differences in morphology. Lampreys share features with jawed vertebrates that hagfish lack, including eyes, a closed vascular system, vertebral elements, and pancreas; these differences seem to result from regressive changes in the hagfish lineage (44).
Figure 3.
A comparison of the amino acid sequences of the 3 TIMPs from the sea lamprey (P. marinus) and the 2 from inshore hagfish (E. burger) with the 4 human (Homo sapiens) and elephant shark (Callorhinchus milii) TIMPs. The sequences were aligned by using T-coffee (https://www.ebi.ac.uk/Tools/msa/tcoffee/) and shaded by using Boxshade (embnet.vital-it.ch/software/BOX_form.html). Residues with black backgrounds are identical in ≥7 sequences. CALM, Callorhinchus milii; HAGF, Eptatretus burger; HU, Homo sapiens; LAMP, Petromyzon marinus; TIMPa, Ta; TIMPb, Tb; TIMPc; TcTIMP1, T1; TIMP2, T2; TIMP3, T3.
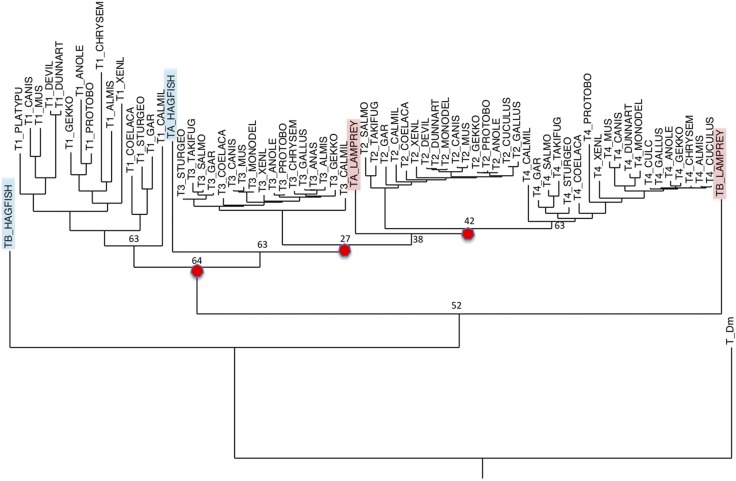
In an attempt to clarify the relationship between the TIMPs from the Agnatha and Gnathostomes, phylogenetic trees were constructed according to the Maximum Likelihood method using the PHYML and RAxML programs (45, 46), and alignments of TIMP sequences from different vertebrate groups were generated by using the Multiple Sequence Comparison by Log- Expectation (MUSCLE) computer program (47). This method was conducted through the Trex (www.trex.uqam.ca) and CIPRES Gateway (www.phylo.org) websites. The overall results are consistent with previous reports (2, 25–28, 37) in indicating that the 4 vertebrate TIMP lines were produced by 3 successive gene duplications that occurred before the separation of the Chondrichthyes (cartilaginous fish) from the vertebrate stem (Fig. 4). Two of these duplications may correspond to the 2 WGDs originally proposed by Ohno (48) that are widely considered to be key events in early vertebrate evolution (49). The first gene duplication separated the Timp1 line from the ancestor of the other 3 Timps, the second generated the Timp3 line and an ancestor of Timp2 and Timp4, and the third duplication generated Timp2 and Timp4. The first 2 duplications parallel duplications of the host Syn gene that gave rise to mammalian Syn1, Syn3, and Syn4, respectively (27). In trees that include the cyclostome TIMPs, both lamprey TIMPb and TIMPc, as well as hagfish TIMPb, diverge before the main TIMP1 group, indicating that they are orthologs of TIMP1 or represent an earlier TIMP subgroup. In the RAxML tree, hagfish TIMPa seems to be an early branch of the TIMP3 group, but in the PHYML result, it is a later branch of the TIMPb/TIMPc group. In both cases, lamprey TIMPa branches at the root of the TIMP2/4 group, following the second gene duplication. Historically, this tree implies that the 2 gene duplications that gave rise to the TIMP3, and then the TIMP2/4 line, occurred later than the divergence of the Agnatha and before the divergence of the Chondrichthyes from the main vertebrate stem. It is tentative because: 1) the support values for the hagfish TIMPa line in the PHYML tree and for lamprey TIMPa in both trees are not strong; 2) we may not know the full array of TIMPs from the Agnatha; and 3) it assumes that no intermediate gene line was lost in an Agnathan ancestor.
Figure 4.
A phylogenetic tree generated from vertebrate TIMP amino acid sequences. This tree was generated by using TIMP sequences, devoid of secretion signal sequences, from species representing therian and prototherian mammals, reptiles (including birds), amphibians, and jawless, cartilaginous, lobe-finned, and bony fish. The sequences were aligned by using the computer program MUSCLE (47), and the tree was generated by using RAxML (46). The tree was rooted with D. melanogaster TIMP, and sequence differences were scored by using the JTT matrix. The red circles identify the projected gene duplication events.
The rates of evolution of the 4 vertebrate Timp lines, based on amino acid sequence differences, vary (Table 2). The higher rate of change in the amino acid sequence of TIMP1 may be partly linked to the location of its gene on the X chromosome (50). A higher evolutionary rate in X-linked genes is believed to result from the single X chromosome in males applying greater selective pressure on its component genes, accelerating the rate of adaptive evolutionary change. Although the rate of evolution of TIMP1 in therian mammals is greater than in the vertebrates as a whole, it seems to have been fast before the development of the X chromosome in therian mammals (Table 3). TIMP2 and -3 have the slowest rates, and TIMP4 has an intermediate evolutionary rate.
TABLE 2.
Rates of evolutionary change for the vertebrate TIMPs calculated from amino acid sequence differences between placental mammals and representatives of other vertebrate groups
| Parameter | Squamataa | Amphibia | Actinopterygii | Chondrichthye | Mean (rate)b |
|---|---|---|---|---|---|
| Eutherian to divergence time (Mya)c | 312 | 353 | 435 | 473 | |
| TIMP1 | |||||
| % Change | 54.7 | 55.7 | 58.7 | 65.6 | |
| Correctedd | 79.2 | 81.3 | 88.4 | 106.6 | |
| Rateb | 25.4 | 23.0 | 20.3 | 22.5 | 22.7 |
| TIMP2 | |||||
| % Change | 11.3 | 17.0 | 22.0 | 23.0 | |
| Correctedd | 12.0 | 18.6 | 24.8 | 26.1 | |
| Rate | 3.8 | 5.2 | 5.7 | 5.5 | 5.1 |
| TIMP3 | |||||
| % Change | 14.6 | 16.8 | 26.2 | 24.8 | |
| Correctedd | 15.7 | 18.3 | 30.3 | 28.4 | |
| Rate | 5.1 | 5.2 | 6.9 | 5.9 | 5.8 |
| TIMP4 | |||||
| % Change | 26.3 | 35.2 | 41.0 | 43.5 | |
| Correctedd | 30.5 | 43.4 | 52.8 | 57.1 | |
| Rate | 9.8 | 12.2 | 12.1 | 12.1 | 11.6 |
TIMPs used in the measurement were: Actinopterygii, Lepisosteus oculatus; Amphibia, Xenopus laevis; Chondricthyes, Callorhinchus milii; Squamata, Protobothrops mucrosquamatus and Anolis carolinensi. Because the C-terminus of the Lepisosteus TIMP2 sequence was highly deviant, TIMP2 sequences for Actinopterygii were from Scleropages formosus and Clupea harengus. bAccepted change in 100 residues per 100 million years of divergence. cDivergence times were obtained from the TimeTree website (www.timetree.org) (42). dPoisson correction for multiple substitutions at the same site –Ln(1-p), where p is the fractional change.
TABLE 3.
Rates of evolutionary change for TIMP1 and TIMP2 in mammals calculated from amino acid sequence differences between human, other eutherian, marsupial, and one monotreme TIMP
| Parameter | Cana | Mus | Rat | Bos | Dun | Tas | Plat | Mean (rate) | Vert (rate) |
|---|---|---|---|---|---|---|---|---|---|
| H. sapiens to divergence time (Mya)b | 96 | 90 | 90 | 96 | 159 | 159 | 177 | ||
| TIMP1 | |||||||||
| % Change | 20.7 | 26.3 | 28.5 | 13.0 | 52.2 | 51.1 | 48.1 | ||
| Correctedc | 23.2 | 30.5 | 33.5 | 13.9 | 73.8 | 67.1 | 73.2 | ||
| Rated | 24.1 | 33.9 | 37.2 | 14.5 | 46.4 | 42.2 | 41.4 | 34.2 | 22.7 |
| TIMP2 | |||||||||
| % Change | 3.1 | 1.5 | 1.5 | 5.2 | 7.7 | 8.8 | NA | ||
| Corrected | 3.1 | 1.5 | 1.5 | 5.3 | 8.0 | 9.2 | |||
| Rated | 3.2 | 1.7 | 1.7 | 5.5 | 5.0 | 5.8 | 3.8 | 5.1 |
NA, not available. aSpecies: Bos, Bos taurus; Can, Canis familiaris; Dun (dunnart), Sminthopsis crassicaudata; Mus, Mus musculus, Rat, Ratus norvegicus, Plat (platypus) Ornithorhynchus anatinus; Tas (Tasmanian devil) Sarcophilus harrisii. bDivergence times were obtained from the TimeTree website: www.timetree.org (44). cPoisson correction for multiple substitutions at the same site –Ln(1-p), where p is the fractional change. dSubstitutions per 100 residues change per 100 million years of divergence.
STRUCTURAL BASIS OF METALLOPROTEINASE INHIBITION BY TIMPs
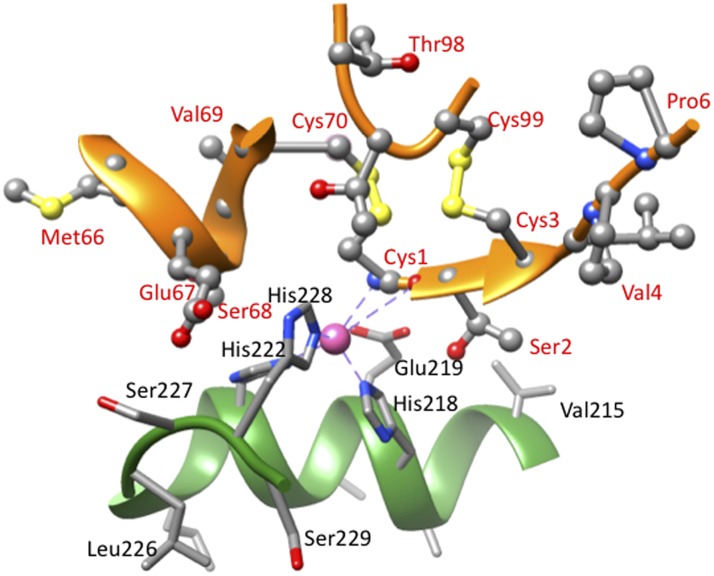
The crystallographic structures of multiple TIMP/MMP complexes have shown that the core of the TIMP interaction site is a surface ridge that engages in ∼75% of the interactions with the MMP active site (Figs. 1 and 5). A crucial component of this ridge is the N-terminal pentapeptide, which has the sequence Cys-X-Cys-Y-Z in which, in the vertebrate TIMPs, X is Ser or Thr; Y can be Ala, Val, Ser, or Met; and Z is often P. Cystines 1 and 3 are connected through disulfide bonds with cystines 70 and 99, respectively (sequence numbers for TIMP1) that clamp 2 other parts of the ridge, residues 66–70 and 98–99, to the N-terminal pentapeptide. The cysteines and covalent structure of this region are absolutely conserved in all vertebrate and invertebrate TIMPs. In complexes with MMPs, the uncommon N-terminal disulfide-bonded Cys is oriented in the protease active site so that its α-amino group and carbonyl oxygen coordinate the catalytic Zn2+ of the MMP (Fig. 5) and displace a water molecule that is normally involved in peptide bond hydrolysis (51). If the α-amino group of Cys1 is changed by chemical modification or by an N-terminal extension, the ability of the TIMP to inhibit MMPs is essentially eliminated (2, 3). An amino group is an atypical ligand for a divalent metal ion; α-amino groups can interact with Zn2+ and other divalent metal ions when unprotonated but not when protonated (52). Because the average pK of the α-amino groups of proteins is 7.7 ± 0.5 (53), the binding of TIMPs to MMPs would be predicted to be weak and pH dependent in the physiologic pH range. However, studies of cystine-containing synthetic peptides with disulfide bonds by Wyman and coworkers (54) have shown that the α-amino groups of N-terminal disulfide-bonded cystines have pK values that are ∼1.3 U lower than those of similar peptides in which the cystine is not at the N-terminus. In addition, the positive charge on the amino group is partially balanced by the negative charge on the catalytic Glu of the MMP.
Figure 5.
Interactions of the core of the TIMP1 reactive site with the active site of collagenase 1 (MMP-1). The image was generated from the structure of the N-TIMP1/MMP-1 complex, pdb 2JOT (107) using the University of California, San Francisco (UCSF) chimera package (106). Residues of N-TIMP1 are labeled in red, and those of MMP-1 in black. Atom colors are as follows: C, silver; N, blue; O, red; S, yellow; and Zn, pink.
The 5 β-strands of the TIMP N-domain are designated A through E; the residues in the MMP-binding ridge include 5 residues of the loop between β-strands C and D and a small region between β-strands D and E (2, 3) that occupy different subsites in the extended substrate-binding site of the MMP. The side chain of residue 2 (Ser or Thr in vertebrate TIMPs) interacts with the key S1′ subsite (known as the specificity pocket), and mutational changes in this residue and others in the TIMP reactive site ridge have substantial effects on their relative affinities for different MMPs. In addition to the ridge, loops between β-strands A and B (the A-B loop) (Fig. 1) and strands E and F, as well as part of β-strand D, make variable contributions in different TIMP/MMP complexes. The A-B loop of TIMP2 is longer by 7 residues than that of TIMP1 and has multiple interactions with the MMP in TIMP2 complexes with MMP14 (MT1-MMP), MMP13, and MMP10 that are absent from complexes of TIMP1 with MMP1, MMP3, or MMP10 (2, 51, 52). In the structures of complexes between full-length TIMPs and MMPs, there are also some contacts between the TIMP C-domain and the MMP; however, the fact that the TIMPs and their truncated N-domains are similar in inhibitory activity indicates that C-domain contacts are not necessary for their MMP inhibitory function (2, 3).
The persistence and wide distribution of the 4 vertebrate TIMPs suggest that they have acquired distinct functions during vertebrate evolution. To evaluate whether this evolution involved the acquisition (neofunctionalization), subdivision (subfunctionalization), or elimination of functions in the different lines, we would need to understand the full array of functions of invertebrate TIMPs. However, there is very limited information available regarding this topic. Previously, the N-domain of the single TIMP from an insect, Drosophila melanogaster (Dm N-TIMP), was expressed and characterized functionally (55). In addition to being an effective inhibitor of the 2 endogenous Drosophila MMPs, Dm N-TIMP was found to inhibit human MMP-1, -2, -3, and -14 with nanomolar Ki values. It also inhibited the ectodomain of human ADAM-17 (TACE) with a nanomolar Ki value but was a relatively weak inhibitor of human ADAM-10 (Ki > 100 nM). These properties are similar to those of human N-TIMP3, although the latter is a more potent inhibitor of MMP-1 and weaker inhibitor of MMP-3. TIMP3 is the most cationic of the vertebrate TIMPs, with an average pI of 9.1 (range: 9.0–9.2); Dm-TIMP has a pI of 9.6 and a similar charge distribution to TIMP3. The pI values for other TIMPs are lower and their ranges greater (Table 1). Also, it should be noted that variability in their N-glycans may affect the net charge on TIMP1 and -3 in biologic systems. Although caution is needed when extrapolating from the properties of a single insect TIMP to other invertebrates, TIMP3 seems to have conserved more of the properties of the ancestral TIMP than the other TIMPs.
EVOLUTIONARY CHANGES IN TIMP FUNCTIONS
Metalloproteinase inhibition
The broad inhibitory spectrum of TIMP3 includes a large number of ADAMs (disintegrin-metalloproteinases) and ADAMTS (ADAMs with thrombospondin motifs) that are very divergent from the MMPs in catalytic domain sequence and have contrasting C-terminal domains from the MMPs, including disintegrin and cysteine-rich domains (56, 57). Of the 13 catalytically active ADAMs and 19 ADAMTSs in humans, TIMP3 inhibits ADAM-10, -12, -17, -28, and -33, as well as ADAMTS-1, -2, -4, and -5 (2, 3). Other TIMPs have limited inhibitory activity for ADAMs; TIMP1 inhibits ADAM10, TIMP2 inhibits ADAM12, and TIMP4 inhibits ADAM28 (2, 7), but none is an effective inhibitor of ADAM17 (2). Human TIMP3 and its truncated N-domain inhibit ADAM17 (TACE), and the high-resolution structure of the complex of human N-TIMP3 with the TACE catalytic domain (Fig. 6) shows that their interaction is similar to that between TIMPs and MMPs (58). The N-domain of D. melanogaster TIMP shares with vertebrate TIMP3 the ability to effectively inhibit several human MMPs and ADAM17 (55), suggesting that ancestors of the vertebrate TIMPs also inhibited both MMPs and ADAMs and that during the evolution of TIMP-1, -2, and -4, their inhibitory ranges became restricted. TIMP1 (from humans) displays the largest change, being a weak inhibitor of membrane-type MMPs, MMP-19, and ADAM17.
Figure 6.
A homology-based model of lamprey TIMPa superimposed on the crystallographic structure of the N-TIMP3/TACE complex, pdb 3CKI (58). The TIMPa model was generated by using the Swiss-Model Homology Modeling Server (108) based on structures of TIMP2 (pdb 1GXD and 2E2D). The structure was superimposed on the N-TIMP3 chain of 3CKI, and the image was generated by using the University of California, San Francisco (UCSF) chimera package (106). The backbones of TIMP3 and lamprey TIMPa are shown as cyan and pink ribbons, and the backbone of TACE is blue. The side chains of residues from TIMP3 are colored dark gray, and those from lamprey TIMPa are colored red. The labels for both are red. Side chains from TACE are colored green, and their labels are purple. The catalytic Zn2+ ion is shown as a yellow sphere.
Gill Murphy’s research group has investigated how TIMP-1, -2, and -4 can be engineered to extend their inhibitory ranges to include additional MMPs and ADAMs (59–62). Their findings are particularly interesting with respect to the evolution of the vertebrate TIMPs because they identify sequence changes that seem to reverse mutational changes that narrowed the inhibitory ranges of these TIMPs during vertebrate phylogeny. In human TIMP1, substitutions of Ala for Val4 and Val for Pro6 in the N-terminal region and Leu for Thr98, in the D-E loop, were found to greatly increase the affinity for MT1-MMP (59, 60). Subsequently, the same changes in TIMP1 were found to also enhance its inhibitory activities for ADAM17, MMP19, and MT5-MMP (60, 61), implying that these sequence changes during TIMP1 evolution reduced its inhibitory activity for multiple MMPs and ADAM17. An examination of TIMP1 sequences from different vertebrate orders (Supplemental Fig. 3) suggests that residues which favor MMP14 and ADAM17 inhibition (Ala4 and Leu98) were present at the equivalent sites in TIMP1 sequences from earlier-branching vertebrate orders. The unfavorable Pro6 is found only in the TIMP1 from a subgroup of mammals and is replaced by other residues, principally Gln and Arg, in other orders. Reconstructed sequences of ancestral forms of TIMP1 at different nodes of phylogenetic trees suggest that these changes occurred progressively during vertebrate evolution, resulting in the weak inhibition of MMP14, -19, and ADAM17 by human TIMP1.
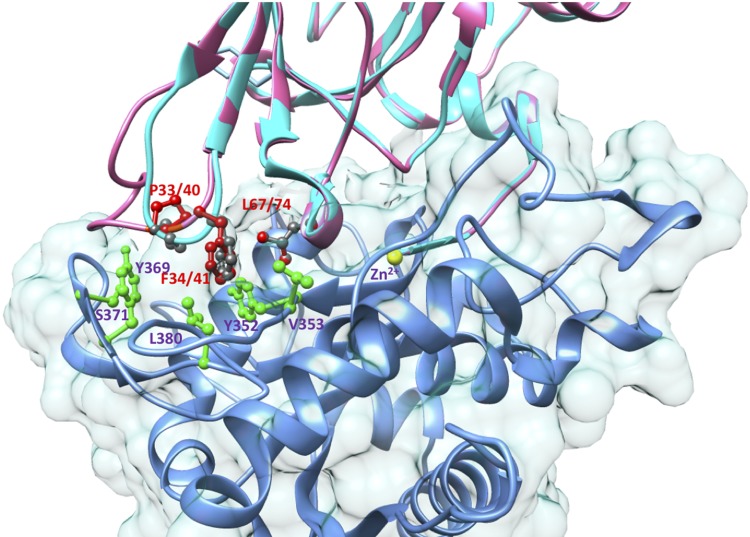
Because the vertebrate TIMP lines diverged successively in the sequence Timp1, followed by Timp3, and then Timp2 and Timp4 (Fig. 4), it is reasonable to conclude that the inhibitory activity for ADAM17 (present in Dm-TIMP) was lost independently during the evolution of the TIMP1 and the TIMP2/4 lines. The affinity of TIMP2 for ADAM17 was found to increase 40-fold, reflected in a Ki of 1.5 nM, when 6 residues are deleted from its long AB loop, a Pro33-Phe34-Gly35 sequence (from TIMP3) is substituted into this loop, and 2 substitutions made adjacent to the fourth cysteine (change from Ala-Val-Cys to Ser-Leu-Cys). With TIMP4, deletion of the C-domain, and substitution of a Pro-Phe-Gly sequence into the A-B loop, was found to increase its affinity for ADAM17 to a subnanomolar Ki (62). Human TIMP4 has a Ser-Leu sequence preceding the fourth cysteine; thus, the engineered changes in TIMP2 and TIMP4 that restore ADAM17 inhibitory activity resulted in similar sequences in these key regions. The crystallographic structure of the complex of N-TIMP3 with the catalytic domain of TACE, determined more recently, showed that the side chains of Pro33 and Phe34 of TIMP3 extend into a hydrophobic groove on the TACE surface formed by the side chains of Tyr352, Val353, Tyr369, and Leu380 (Fig. 6). The side chain of N-TIMP3 Leu67 (of the Ser-Leu-Cys sequence) also interacts with Tyr352 and Leu350 (58). The idea that these sequence changes mirror evolutionary changes is bolstered by the sequence of lamprey TIMPa, an offshoot of the TIMP3, TIMP2, and TIMP4 group that has residues Pro40, Phe41, and Leu74 corresponding to Pro33, Phe34, and Leu67 of TIMP3. Molecular modeling suggests that the A-B loop of lamprey TIMPa would be able to interact with human TACE in a similar way to TIMP3.
These observations are consistent with the hypothesis that the during gnathostome evolution, there was a loss of inhibitory activity toward subgroups of metalloproteinases in TIMP-1, -2, and -4. These functional changes may be linked to increasing their availability and adaptation to new functions, including integrin binding, pro-MMP activation, and inhibiting angiogenesis.
Interactions with progelatinases
TIMPs interact with progelatinases A and B (proMMP-2 and proMMP-9, respectively) through the TIMP C-domains and the Hpx domains of the proMMP. These interactions are more specific than those with MMP catalytic domains; TIMP2, -3, and -4 all bind to proMMP-2, whereas TIMP1 and TIMP3 interact with proMMP-9 (2–4). The C-terminal region (“tail”), following the last (sixth) Cys, in TIMP-2, -3, and -4 are similar in both length and sequence but differ from TIMP1, and appear to be crucial for binding to proMMP-2 (63). These interactions convolute the activation of the proMMPs because the TIMP molecule bound to a proMMP has a free N-terminal domain that can inhibit active MMPs, thereby preventing them from catalyzing prodomain cleavage to release an active MMP.
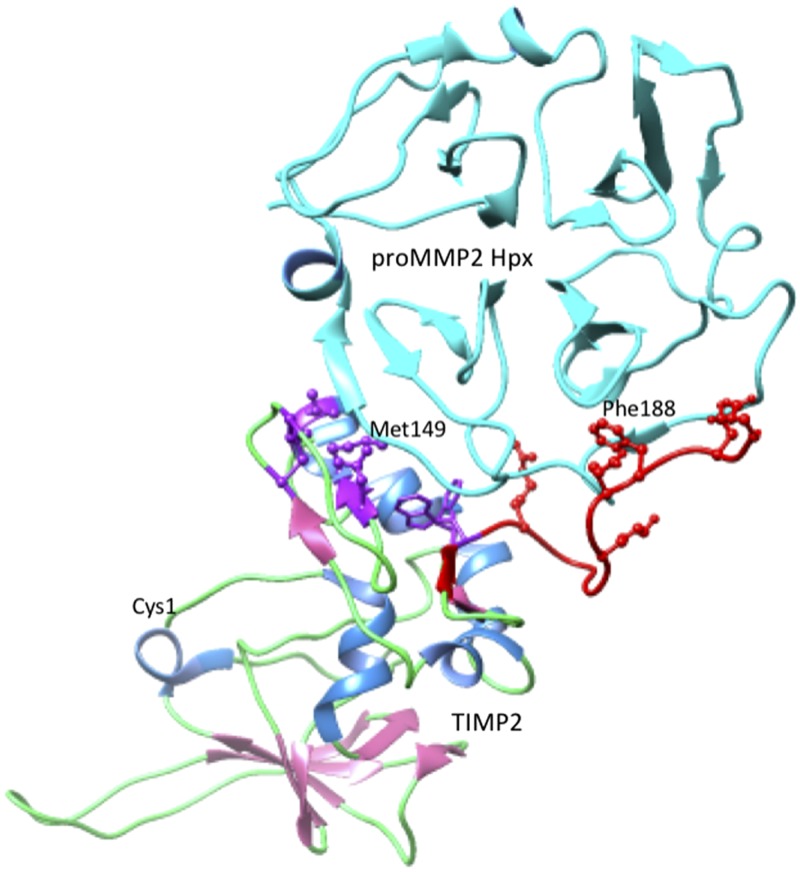
In contrast, the interaction of TIMP2 with proMMP-2 has a unique role in facilitating the activation of proMMP-2 by MT1-MMP (MMP-14). The inhibition of membrane-bound MT1-MMP by TIMP2 leaves the TIMP2 C-domain available to bind to the Hpx domain of proMMP-2, thereby anchoring proMMP-2 to the plasma membrane. Subsequently, a second MT1-MMP molecule joins the ternary complex through homodimer formation and cleaves off the prodomain of proMMP-2, releasing an active MMP-2 molecule (2–5, 64). As shown in Fig. 7, the proMMP-2/TIMP2 interaction involves 2 regions of the TIMP2 C-domain, a cluster of hydrophobic residues around Met149 (Cys133, Cys138, Leu147, Val152, Phe165, and Trp177) and a group of charged residues around Phe188 (Arg179, Lys185, Glu187, Asp190, and Glu192). The first region binds to the fourth β-propeller blade of the MMP-2 Hpx domain, and the latter inserts between the third and fourth β-propeller blades (63). Many of these residues are strictly conserved in mammalian and nonmammalian TIMP2 sequences; others are conserved in mammals but are replaced in nonmammalian vertebrates, notably Met149 (Thr in most nonmammals) and Val152 (substitutions of Ala or Met in some nonmammals). These substitutions change the hydrophobicity and size of the side chain, suggesting that nonmammalian TIMP2s may be unable to facilitate proMMP-2 activation. This hypothesis is consistent with the sequence and properties of TIMP4, which binds to proMMP-2 and inhibits MMP-14 but does not promote proMMP-2 activation. This outcome is probably because TIMP4 binds less well to proMMP-2 (7, 65, 66) as the result of Met149 and Val152 of TIMP2 being replaced by Thr and Leu, respectively, in TIMP4. Studies with chimeric proteins containing the N-domain of TIMP4 and the C-domain of TIMP2 show that the TIMP2 C-domain is necessary for the activation of proMMP-2 by MT1-MMP, but full activation requires additional changes in the TIMP4 N-domain (67). This observation implies that TIMP2 may not mediate proMMP-2 activation in all vertebrates and that this activity may be a relatively recent evolutionary development found in a limited array of species that includes the mammals.
Figure 7.
Interactions between the TIMP2 and the Hpx domain of proMMP-2. The representation was generated from pdb 1GXD (105) by using the University of California, San Francisco (UCSF) chimera package (106). Specific parts of the complex structure are colored as follows: the chains of both proteins are displayed as ribbons, TIMP2 β-strands are pink, and α-helices are cornflower blue; the proMMP-2 Hpx domain is colored cyan. The side chains of TIMP2 residues that interact with the Hpx domain are displayed in ball and stick rendering. The group surrounding Met149 are colored purple, and the C-terminal tail and residues around Phe188 are colored red. The location of the N-terminal Cys of TIMP2 is labeled to show the location of the MMP-inhibitory region.
The interactions of TIMPs with progelatinases can have other outcomes, reflecting evolutionary divergence in the functions of their C-domains. Soluble proMMP-2/TIMP2 complexes can bind to LDL receptor–related protein (LRP) 1 or LRP2 (megalin) in different cells, and undergo internalization and proteolytic degradation (68). The interactions of TIMP3 with proMMP-2 and -9 and TIMP1 with proMMP-9 have potential regulatory significance. For example, TIMP3 (like TIMP4) may also inhibit the ability of TIMP2 to mediate the activation of proMMP-2 (66, 69), and the binding of the C-domain of TIMP1 by proMMP-9 could prevent it from interacting with CD63, blocking its signaling activities (17). The interaction with either proMMP-2 or proMMP-9 could disrupt the ability of TIMP3 to interact with other binding partners such as VEGF receptor 2 (VEGFR2) and fibulin-3.
The interaction of TIMP1 and proMMP-9 has a particular significance for angiogenesis and cancer. MMP-9, produced by tumor-associated neutrophils, can promote the induction of angiogenesis, which enhances tumor progression (70–73). Most cells secrete proMMP-9 as a complex with TIMP1; this complex is resistant to activation because the free inhibitory N-domain of the TIMP molecule can inhibit activated MMP-9 directly and can also inhibit MMPs, such as MMP-3, that catalyze proMMP-9 activation (72). Neutrophils do not express TIMP1, and they secrete a TIMP1 free-form of proMMP-9 that can be readily activated by other MMPs and promote angiogenesis by catalyzing ECM degradation and releasing sequestered VEGF (73). This proangiogenic effect of proMMP-9 can be obstructed by complex formation with TIMP1 or -2, thereby preventing tumor angiogenesis.
Because invertebrate MMPs, from species ranging from insects to cnidarians, have Hpx domains and most invertebrate TIMPs have C-domains, regulatory interactions between invertebrate TIMPs and proMMPs probably paved the way to those found in vertebrates. The interaction between the TIMP C-domain and the proMMP Hpx domain produces a proMMP attached to a TIMP with an N-domain that can inhibit active MMPs. Consequently, the proMMP is resistant to activation by MMPs, reflecting a possible ancestral function of this type of interaction. This scenario could be viewed as an early step in the evolution of the complex TIMP2-mediated mechanism for the activation of proMMP-2 by MMP-14.
Interactions with nonmetalloproteinase targets
The interactions of vertebrate TIMPs with proteins other than metalloproteinases underlie their discrete roles as signaling molecules. Although the TIMP domains that mediate these interactions have been identified by using truncated TIMP constructs, to date none of the complexes have been characterized structurally. Information pertinent to the structural basis of some interactions has been obtained by using synthetic peptides with sequences corresponding to regions of the TIMP primary structure that can bind to particular receptors and block the binding of the intact TIMP molecule. Such peptides are generally needed at relatively high concentrations (micromolar as opposed to nanomolar for biologic ligands) because of the entropic cost of binding an unstructured peptide to a structured target. However, examination of the conservation of these regions in TIMPs from different vertebrates can help to provide clues about when these functions were acquired or lost during phylogeny.
The interaction of TIMP1 with CD63 mediates its association with integrin β1 (17). The TIMP1–CD63–integrin β1 complex subsequently activates the focal adhesion kinase, PI3-K, and ERK pathways, thereby stimulating cell growth and protecting cells from apoptosis and extrinsic death (74–76). Although it is known that TIMP1 interacts with CD63 through its C-domain (17), the structural basis of the interaction has not been determined. Also, it is not known if TIMP1 from all jawed vertebrates can bind CD63 or whether this activity is limited to TIMP1 from a subgroup that includes the mammals. It is reasonable to expect that the residues involved in a specific protein–protein interaction of this type will be more conserved than others; however, when the sequences of the TIMP1 C-domains from different vertebrate groups are compared, the few residues conserved in all TIMP1s are also conserved in other TIMPs and seem to be important for structure (notably disulfide bonded cystines). When TIMP1 sequences from only therian mammals are examined, more nonstructural residues are conserved, and it is possible that CD63 binding is a relatively recent evolutionary acquisition in TIMP1 that is shared only by a few species; this is not known, however. Because TIMP1 is absent from birds and teleost fishes, this signaling function must be dispensable in these species, or it may have developed after the divergence of the birds from the main vertebrate stem. Recently, it has been reported that TIMP1 also binds to a second tetraspanin, CD82 (77); this interaction is distinct from the interaction with CD63 because it involves the N-domain of TIMP1, and its effects are different, facilitating TIMP1 endocytosis and antagonizing cell migration.
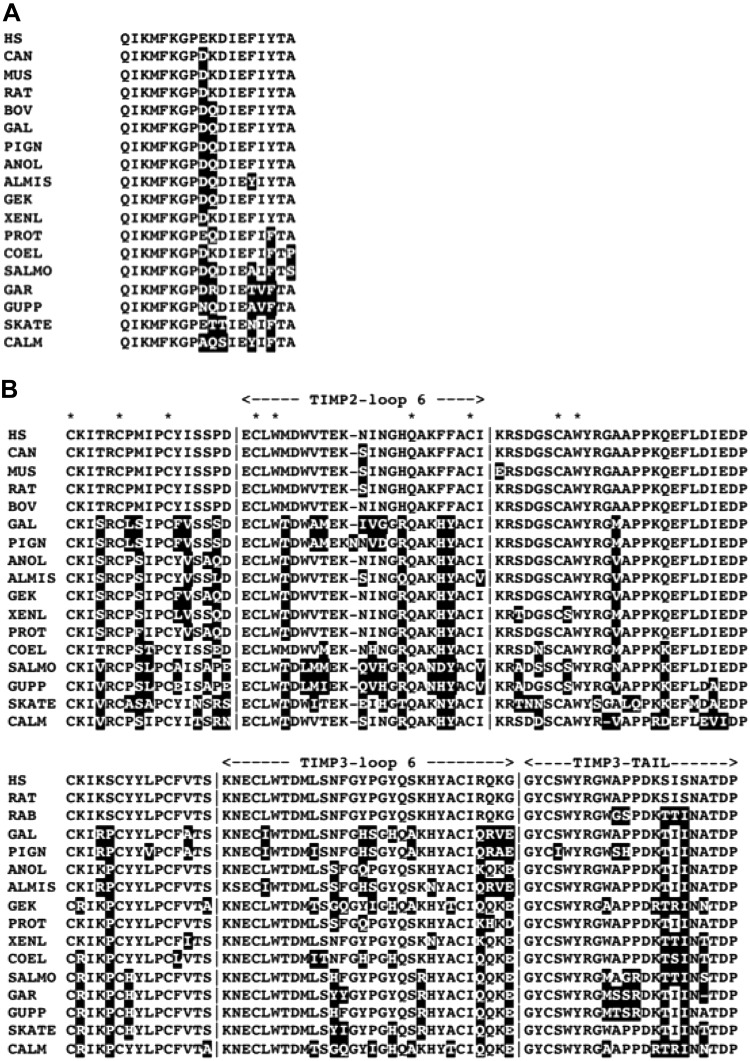
TIMP2 also interacts with an integrin, α3β1, but directly, through its N-terminal domain (18). A 24-residue peptide, corresponding to residues 43–66 of human TIMP2 (peptide 9, structural location shown in Fig. 1) binds to α3β1 integrin, competes with TIMP2, and suppresses endothelial cell growth (78). This region is similar in TIMP2 sequences from a wide array of amniotes (Fig. 8A) but is different in sequence and length from the corresponding sequences in other TIMPs, including TIMP4, the closest relative of TIMP2, and from lamprey TIMPa. Consequently, it is reasonable to hypothesize that integrin-binding activity developed in TIMP2 soon after its divergence from TIMP4.
Figure 8.
Differences in the primary structures of TIMP2 and -3 in regions implicated in different interactions. A) Integrin α3β1-binding peptide of TIMP2 (peptide 9) residues 43–66 (78). B), C-terminal domains of TIMP2 and TIMP3 showing the loop 6 peptide and C-terminal tail. Residues that differ from the human TIMP2 or -3 sequence are white with shaded backgrounds. Asterisks identify residues in the C-domain that are invariant in all TIMPs. ALMIS, Alligator mississippiensis; ANOLE, Anolis carolinensis; BOV, Bos taurus; CALM, Callorhinchus milii; CAN, Canis lupus familiaris; COEL, Latimeria chalumnae; GAL, Gallus gallus; GEK, Gekko japonicus; GUPP, Poecilia reticulata; MUS, Mus musculus; PIGN, Columba livia; HS, Homo sapiens; PROT, Protobotrhops mucrosquamatus; RAT, Rattus norvegicus; SALMO, Salmo salar; SKATE, Leucoraja ocellata; XENL, Xenopus laevis.
TIMP2, -3, and -4 all have antiangiogenic effects, some of which are linked to their inhibition of MMPs (2–4), but TIMP2 and TIMP3 have antiangiogenic activities that are mediated by interactions between their N- or C-domains and receptors for proangiogenic proteins. The antiangiogenic action of TIMP2 has been localized to a disulfide-bonded loop, designated loop 6, that corresponds to residues 146–167 of the mature human TIMP2 protein (Fig. 8B). A synthetic peptide corresponding to this region inhibits capillary endothelial cell proliferation, comparable to the complete TIMP2 C-domain (79). Subsequent studies have shown that this peptide, as with full-length TIMP2, inhibits angiogenesis by binding to the insulin-like growth factor-1 receptor (IGFR1) (80). This region of the human TIMP2 sequence includes some residues that are conserved in all TIMPs (see upper alignment in Fig. 8B) and seem to have structural roles. Most of the remaining residues are conserved in the TIMP2s of other mammals and some reptiles, but avian TIMP2 sequences display greater variation. For example, there are 9 substitutions of 16 variable sites in TIMP2 from chicken and other birds, and several avian TIMP2s have an extra residue inserted. Based on the limited conservation, it is possible that TIMP2 from birds may not bind to IGFR1. In teleost fishes, there are no insertions in this region in either TIMP2 line; teleost TIMP2b has conserved Trp 151 and generally has fewer substitutions, so that anti-angiogenic action may have become a specialized activity of this teleost TIMP2 line. However, coevolutionary changes in TIMP2 and the receptor are possible.
The multiple activities of TIMP3, which are unrelated to metalloproteinase inhibition, include both antiangiogenic effects and a role in ECM structure. Mammalian TIMP3 is a strong inhibitor of the angiogenic action of VEGF, resulting from its binding to VEGFR2 and angiotensin II type 2 receptor (AGTR2) (17, 18). AGTR2 binding is mediated by the N-domain of TIMP3 (20), whereas VEGFR2 interaction is mimicked by synthetic peptides derived from the loop 6 sequence (residues 137–166) and from the C-terminal “tail” of TIMP3 (81), the region following the last Cys (see lower section of Fig. 7B). The region of N-TIMP3 involved in the interaction of AGTR2 has not been identified. The loop 6 peptide of TIMP3 that interacts with VEGFR2 corresponds to a slightly more extended version of the loop 6 peptide of TIMP2 that binds to IGFR1. Both this and the C-terminal tail peptide of TIMP3 are derived from regions that are highly conserved in TIMP3 from mammals and show limited variation in other vertebrates. Pigeon and chicken have 9 substitutions compared with human TIMP3, but no insertions or deletions (Fig. 8B). Therefore, there is a reasonable basis for hypothesizing that the VEGFR2-binding function of TIMP3 had an early origin and has been conserved in fishes and amniotes. It should be noted that, although similar regions in the sequences of TIMP2 and TIMP3 inhibit angiogenesis by blocking the activation of the proangiogenic receptors IGFR1 and VEGFR2, these receptors have very different structures (82, 83) and the patterns of sequence conservation in loop 6 from TIMP2 and -3 differ.
There is little soluble TIMP3 in tissues or fluids because, after secretion, TIMP3 that is not bound to the ECM is reabsorbed by endocytosis via LRP1 (84). The sequestration of TIMP3 may be important for limiting its proapoptotic effects on tissues, reflecting the general homeostatic significance of LRP1 in removing degradative enzymes, inhibitors, and their complexes (85). The interaction of TIMP3 with LRP1 is reduced by substitutions of alanine for pairs of lysines in TIMP3, either Lys26 and Lys45, or Lys42 and Lys110 (86). The lysine residues that affect LRP1 binding are also conserved in all vertebrate TIMP3 sequences but not in other TIMPs, implying that the cellular uptake of TIMP3 by LRP1 has been probably conserved throughout vertebrate TIMP3 evolution. In relation to the fate of TIMP3 after endocytosis, it can be noted TIMP3 is one of >400 protein components of human sperm nuclei (87), suggesting the possibility that it may have a nuclear or other intracellular function after its cellular uptake.
Structural roles in ECM
TIMP3 binds to sulfated polysaccharides (SP) of the ECM and, in vitro, to soluble SP (including pentosan sulfate) through its N-domain (21, 22). Mutational studies have shown that 4 conserved lysine residues (26, 27, 30, and 76) of TIMP3 are crucial for ECM binding and that substituting lysine residues at the corresponding sites in TIMP1 (88) introduces ECM-binding activity. It should be noted that lamprey TIMPa (the most similar to TIMP3) does not have lysine residues corresponding to 26, 30, and 76, suggesting that it is not an ECM-binding protein. The interaction of TIMP3 with SP facilitates its matrix protective action by enhancing its ability to inhibit the aggrecan-degrading enzymes, ADAMTS-4 and -5 (22). Because it can also bind through its C-domain with fibulin-3 (EGF-containing fibulin-like extracellular matrix protein 1) (24), TIMP3 can help to also stabilize ECM by crosslinking SP and fibulin-3. Mutations in either TIMP3 or fibulin-3 cause the autosomal dominant eye diseases Sorsby fundus dystrophy (SFD) and Malattia Leventinese (or Doyne honeycomb retinal dystrophy), respectively, that result in early-onset macular degeneration (89, 90). The pathologies of these diseases are closely similar and include the accumulation of amorphous deposits (drusen) between the retinal pigmented epithelium and Bruch membrane, a basement membrane–containing structure. Most SFD mutations are missense or truncation mutations in the TIMP3 C-domain that generate proteins with an unpaired cysteine, leading to misfolding and aggregation (2, 3, 89), as well as disrupting its interaction with fibulin-3. The fibulin-3 mutation is an Arg to Trp substitution at a site adjacent to a disulfide-bonded cysteine in an EGF-like domain, suggesting that it may also affect disulfide bond formation, folding, and stability (90) and, possibly, TIMP3 binding. The similar pathologies of these diseases suggest that TIMP3 and fibulin-3 and their interactions may be important for the integrity of the Bruch membrane. The mutation in fibulin-3 leads to proteoglycan accumulation in the Bruch membrane and the disruption of diffusion, which can impair nutrient and oxygen movement to the retina (91). TIMP3 is also found in the ECM of the lung and kidney, and a unique Ser to Cys mutation in the N-domain of TIMP3 has been linked to a late-onset human disease affecting lungs and eyes (92). These findings suggest that TIMP3 may be important for ECM structure and function in both organs.
The role of TIMP3 in ECM structure is unique among the vertebrate TIMPs but is shared by some invertebrate TIMPs. At this time, TIMP homologs have been identified as components of the shells of several mollusks: Polynesian pearl oyster, Pinctada margaritifera, Akoya pearl oyster, Pinctada fucata, gold-lip oyster, Pinctada maxima, Pacific oyster, Crassostrea gigas, winged pearl oyster, Pteria penguin, and the Korean mussel Mytilus coruscus, as well as in the shell of a brachiopod, Magellania venosa (93–98). TIMPs have been found also in other mollusk ECMs, specifically in the shell hinge ligament of P. fucata and the byssus of M. coruscus and a scallop, Chlamys farreri (99–101). The byssus is a group of filaments that functions in shell adherence to underwater surfaces. The P. fucata ligament TIMP has been shown to be a functional inhibitor of human MMP-9 and MMP-13 (99).
Mollusk shells have 3 layers; the innermost nacreous layer is formed by mineralization of a matrix containing chitin and proteins (102). The TIMP from Pinctada martensii has been implicated in nacre formation; it is highly expressed in tissues involved in nacre production, and reduction of its expression by RNA interference resulted in disordered growth in the inner nacreous layer (94). Some shell TIMPs correspond to the N-terminal domains of vertebrate TIMPs, and a subgroup have 2 additional cysteines that probably form an extra disulfide crosslink (Supplemental Fig. 4). The role in ECM structures shared by TIMP3 and some invertebrate TIMPs provides a functional link between vertebrate and invertebrate TIMPs beyond metalloproteinase inhibition. However, shell matrices have highly variable proteomes, and TIMPs are not found in all mollusk shells. Also, some shell proteomes include other protease inhibitors, such as serine protease inhibitors and α2-macroglobulin homologs (103). Although the structural role in ECMs of vertebrate and invertebrate TIMPs could be regarded as an example of convergent evolution, it reflects the ability of TIMPs in shell matrices, such as TIMP3 in vertebrate ECM, to contribute to matrix stability by providing protection from unregulated proteolysis and by interacting with proteins and other molecules. Chitin-based matrices are widespread in invertebrates, and it is possible that TIMPs are matrix components in other species.
CONCLUSIONS
The 4 TIMPs found in gnathostomes display a puzzling array of overlapping, contrasting, and even antagonistic activities that this review has attempted, following Dobzhansky’s precept (104), to illuminate with “the light of evolution.” Their history has included the birth and death of gene lines and the gain and loss of functions. TIMP1, generated by the first of 3 vertebrate TIMP gene duplications, has the highest rate of evolutionary change and is unique in being absent from birds and teleost fishes. As the wild card among the 4 TIMPs, it has progressively lost inhibitory activity toward multiple metalloproteinases but has gained signaling functions, mediated by tetraspanin CD63 binding, and has a cell-specific role in regulating proMMP-9 activation. CD63 binding, mediated by the C-domain, may be a relatively recent acquisition. The Agnatha might provide a glimpse of an intermediate stage in TIMP evolution in lamprey TIMPa, a representative of the TIMP-2, -3, and -4 group, which may be derived from an ancestor of TIMP2 and -4. Consequently, it seems that the final Timp duplication that gave rise to Timp2 and Timp4 occurred after the separation of the Agnatha from the main vertebrate stem. The hagfish, another cyclostome, also has 2 TIMPs with apparently similar lineages that are more divergent in sequence than the lamprey proteins.
TIMP2 and TIMP3 have evolved at the slowest rate, probably reflecting their multiple interactions and functions. Although mammalian TIMP2 has lost most ADAM inhibitory functions, it has acquired a unique role as the mediator of the MMP14-catalyzed activation of proMMP-2, together with an antiangiogenic activity that arises from the interaction of its C-domain with IGFR1 and an ability to stimulate cell growth by binding to α3β1 integrin. TIMP3 has retained inhibitory activity for the largest array of metalloproteinases, including multiple key ADAMs and ADAMTS, and has a potent antiangiogenic activity reflecting the interactions of its C-domain with VEGFR2 and N-domain with AGTR2. TIMP3 has an apparently unique role in the stability and protection of matrices that was prefaced by some invertebrate ECM TIMPs. The VEGFR2/TIMP3 interaction seems to predate the cartilaginous fishes, the earliest diverging vertebrate class (∼473 Mya) that has all 4 TIMP genes. The antiangiogenic actions of TIMP2 and -3 involve a similar loop 6 region in their C-domains that indicates sequence conservation in TIMP2, -3, and -4 and in lamprey TIMPa, raising the question of whether it reflects a receptor-binding role in the ancestor of these TIMPs. TIMP4 can inhibit proMMP-2 activation by preventing its activation by MT1-MMP. However, TIMP4 has a narrower range of tissue expression than the other TIMPs that is limited to the heart and a few other organs, and less is known about its functional role because it has not been studied as intensively as the other TIMPs (7).
The apparently transitional character of the TIMPs from the Agnatha suggest that further investigations of their TIMPs and binding partners, including metalloproteinases and receptors, could provide valuable information relevant to the evolution of TIMP structure and function. Such information is also lacking for TIMPs from most nonmammalian species.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The author thanks the collaborators and colleagues for their helpful comments on this review. The author’s research was previously funded by Grant AR40994 from the U.S. National Institutes of Health, National Institute of Arthritis and Musculoskeletal and Skin Diseases. The author declares no conflict of interest.
Glossary
- ADAM
a disintegrin and metalloprotease
- ADAMTS
ADAM with thrombospondin motifs
- AGTR2
angiotensin II type 2 receptor
- ECM
extracellular matrix
- Hpx
hemopexin
- IGFR1
insulin-like growth factor-1 receptor
- LRP
LDL receptor–related protein
- MMP
matrix metalloproteinase
- Mya
million years ago
- SFD
Sorsby fundus dystrophy
- SP
sulfated polysaccharide
- TIMP
tissue inhibitor of metalloproteinases
- VEGFR2
VEGF receptor 2
- WGD
whole genome duplication
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Rawlings N. D., Barrett A. J., Finn R. (2016) Twenty years of the MEROPS database of proteolytic enzymes, their substrates and inhibitors. Nucleic Acids Res. 44(D1), D343–D350 10.1093/nar/gkv1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brew K., Nagase H. (2010) The tissue inhibitors of metalloproteinases (TIMPs): an ancient family with structural and functional diversity. Biochim. Biophys. Acta 1803, 55–71 10.1016/j.bbamcr.2010.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murphy G. (2011) Tissue inhibitors of metalloproteinases. Genome Biol. 12, 233 10.1186/gb-2011-12-11-233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arpino V., Brock M., Gill S. E. (2015) The role of TIMPs in regulation of extracellular matrix proteolysis. Matrix Biol. 44–46, 247–254 10.1016/j.matbio.2015.03.005 [DOI] [PubMed] [Google Scholar]
- 5.Khokha R., Murthy A., Weiss A. (2013) Metalloproteinases and their natural inhibitors in inflammation and immunity. Nat. Rev. Immunol. 13, 649–665 10.1038/nri3499 [DOI] [PubMed] [Google Scholar]
- 6.Jackson H. W., Defamie V., Waterhouse P., Khokha R. (2017) TIMPs: versatile extracellular regulators in cancer. Nat. Rev. Cancer 17, 38–53 10.1038/nrc.2016.115 [DOI] [PubMed] [Google Scholar]
- 7.Melendez-Zajgla J., Del Pozo L., Ceballos G., Maldonado V. (2008) Tissue inhibitor of metalloproteinases-4. The road less traveled. Mol. Cancer 7, 85 10.1186/1476-4598-7-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murzin A. G. (1993) OB(oligonucleotide/oligosaccharide binding)-fold: common structural and functional solution for non-homologous sequences. EMBO J. 12, 861–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bányai L., Patthy L. (1999) The NTR module: domains of netrins, secreted frizzled related proteins, and type I procollagen C-proteinase enhancer protein are homologous with tissue inhibitors of metalloproteases. Protein Sci. 8, 1636–1642 10.1110/ps.8.8.1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheek S., Krishna S. S., Grishin N. V. (2006) Structural classification of small, disulfide-rich protein domains. J. Mol. Biol. 359, 215–237 10.1016/j.jmb.2006.03.017 [DOI] [PubMed] [Google Scholar]
- 11.Gasson J. C., Golde D. W., Kaufman S. E., Westbrook C. A., Hewick R. M., Kaufman R. J., Wong G. G., Temple P. A., Leary A. C., Brown E. L. (1985) Molecular characterization and expression of the gene encoding human erythroid-potentiating activity. Nature 315, 768–771 10.1038/315768a0 [DOI] [PubMed] [Google Scholar]
- 12.Docherty A. J., Lyons A., Smith B. J., Wright E. M., Stephens P. E., Harris T. J., Murphy G., Reynolds J. J. (1985) Sequence of human tissue inhibitor of metalloproteinases and its identity to erythroid-potentiating activity. Nature 318, 66–69 10.1038/318066a0 [DOI] [PubMed] [Google Scholar]
- 13.Bertaux B., Hornebeck W., Eisen A. Z., Dubertret L. (1991) Growth stimulation of human keratinocytes by tissue inhibitor of metalloproteinases. J. Invest. Dermatol. 97, 679–685 10.1111/1523-1747.ep12483956 [DOI] [PubMed] [Google Scholar]
- 14.Hayakawa T., Yamashita K., Tanzawa K., Uchijima E., Iwata K. (1992) Growth-promoting activity of tissue inhibitor of metalloproteinases-1 (TIMP-1) for a wide range of cells. A possible new growth factor in serum. FEBS Lett. 298, 29–32 10.1016/0014-5793(92)80015-9 [DOI] [PubMed] [Google Scholar]
- 15.Stetler-Stevenson W. G., Bersch N., Golde D. W. (1992) Tissue inhibitor of metalloproteinase-2 (TIMP-2) has erythroid-potentiating activity. FEBS Lett. 296, 231–234 10.1016/0014-5793(92)80386-U [DOI] [PubMed] [Google Scholar]
- 16.Hayakawa T., Yamashita K., Ohuchi E., Shinagawa A. (1994) Cell growth-promoting activity of tissue inhibitor of metalloproteinases-2 (TIMP-2). J. Cell Sci. 107, 2373–2379 [DOI] [PubMed] [Google Scholar]
- 17.Jung K. K., Liu X. W., Chirco R., Fridman R., Kim H. R. (2006) Identification of CD63 as a tissue inhibitor of metalloproteinase-1 interacting cell surface protein. EMBO J. 25, 3934–3942 10.1038/sj.emboj.7601281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seo D. W., Li H., Guedez L., Wingfield P. T., Diaz T., Salloum R., Wei B. Y., Stetler-Stevenson W. G. (2003) TIMP-2 mediated inhibition of angiogenesis: an MMP-independent mechanism. Cell 114, 171–180 10.1016/S0092-8674(03)00551-8 [DOI] [PubMed] [Google Scholar]
- 19.Qi J. H., Ebrahem Q., Moore N., Murphy G., Claesson-Welsh L., Bond M., Baker A., Anand-Apte B. (2003) A novel function for tissue inhibitor of metalloproteinases-3 (TIMP3): inhibition of angiogenesis by blockage of VEGF binding to VEGF receptor-2. Nat. Med. 9, 407–415 10.1038/nm846 [DOI] [PubMed] [Google Scholar]
- 20.Kang K. H., Park S. Y., Rho S. B., Lee J. H. (2008) Tissue inhibitor of metalloproteinases-3 interacts with angiotensin II type 2 receptor and additively inhibits angiogenesis. Cardiovasc. Res. 79, 150–160 10.1093/cvr/cvn072 [DOI] [PubMed] [Google Scholar]
- 21.Yu W. H., Yu S., Meng Q., Brew K., Woessner J. F., Jr (2000) TIMP-3 binds to sulfated glycosaminoglycans of the extracellular matrix. J. Biol. Chem. 275, 31226–31232 10.1074/jbc.M000907200 [DOI] [PubMed] [Google Scholar]
- 22.Troeberg L., Fushimi K., Khokha R., Emonard H., Ghosh P., Nagase H. (2008) Calcium pentosan polysulfate is a multifaceted exosite inhibitor of aggrecanases. FASEB J. 22, 3515–3524 10.1096/fj.08-112680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pavloff N., Staskus P. W., Kishnani N. S., Hawkes S. P. (1992) A new inhibitor of metalloproteinases from chicken: ChIMP-3. A third member of the TIMP family. J. Biol. Chem. 267, 17321–17326 [PubMed] [Google Scholar]
- 24.Klenotic P. A., Munier F. L., Marmorstein L. Y., Anand-Apte B. (2004) Tissue inhibitor of metalloproteinases-3 (TIMP-3) is a binding partner of epithelial growth factor-containing fibulin-like extracellular matrix protein 1 (EFEMP1). Implications for macular degenerations. J. Biol. Chem. 279, 30469–30473 10.1074/jbc.M403026200 [DOI] [PubMed] [Google Scholar]
- 25.Nicosia A., Maggio T., Costa S., Salamone M., Tagliavia M., Mazzola S., Gianguzza F., Cuttitta A. (2016) Maintenance of a protein structure in the dynamic evolution of TIMPs over 600 million years. Genome Biol. Evol. 8, 1056–1071 10.1093/gbe/evw052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pohar N., Godenschwege T. A., Buchner E. (1999) Invertebrate tissue inhibitor of metalloproteinase: structure and nested gene organization within the synapsin locus is conserved from Drosophila to human. Genomics 57, 293–296 10.1006/geno.1999.5776 [DOI] [PubMed] [Google Scholar]
- 27.Yu W. P., Brenner S., Venkatesh B. (2003) Duplication, degeneration and subfunctionalization of the nested synapsin-Timp genes in Fugu. Trends Genet. 19, 180–183 10.1016/S0168-9525(03)00048-9 [DOI] [PubMed] [Google Scholar]
- 28.Candiani S., Moronti L., Pennati R., De Bernardi F., Benfenati F., Pestarino M. (2010) The synapsin gene family in basal chordates: evolutionary perspectives in metazoans. BMC Evol. Biol. 10, 32 10.1186/1471-2148-10-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar A. (2009) An overview of nested genes in eukaryotic genomes. Eukaryot. Cell 8, 1321–1329 10.1128/EC.00143-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee Y. C., Chang H. H. (2013) The evolution and functional significance of nested gene structures in Drosophila melanogaster [published corrections appear in Genome Biol. Evol. 2013 5, 2188 and 2013 5, 2072]. Genome Biol. Evol. 5, 1978–1985 10.1093/gbe/evt149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jaworski D. M., Beem-Miller M., Lluri G., Barrantes-Reynolds R. (2007) Potential regulatory relationship between the nested gene DDC8 and its host gene tissue inhibitor of metalloproteinase-2. Physiol. Genomics 28, 168–178 10.1152/physiolgenomics.00160.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Organ C. L., Shedlock A. M., Meade A., Pagel M., Edwards S. V. (2007) Origin of avian genome size and structure in non-avian dinosaurs. Nature 446, 180–184 10.1038/nature05621 [DOI] [PubMed] [Google Scholar]
- 33.Nakatani Y., Takeda H., Kohara Y., Morishita S. (2007) Reconstruction of the vertebrate ancestral genome reveals dynamic genome reorganization in early vertebrates. Genome Res. 17, 1254–1265 10.1101/gr.6316407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meyer A., Van de Peer Y. (2005) From 2R to 3R: evidence for a fish-specific genome duplication (FSGD). BioEssays 27, 937–945 10.1002/bies.20293 [DOI] [PubMed] [Google Scholar]
- 35.Glasauer S. M., Neuhauss S. C. (2014) Whole-genome duplication in teleost fishes and its evolutionary consequences. Mol. Genet. Genomics 289, 1045–1060 10.1007/s00438-014-0889-2 [DOI] [PubMed] [Google Scholar]
- 36.Berthelot C., Brunet F., Chalopin D., Juanchich A., Bernard M., Noël B., Bento P., Da Silva C., Labadie K., Alberti A., Aury J. M., Louis A., Dehais P., Bardou P., Montfort J., Klopp C., Cabau C., Gaspin C., Thorgaard G. H., Boussaha M., Quillet E., Guyomard R., Galiana D., Bobe J., Volff J. N., Genêt C., Wincker P., Jaillon O., Roest Crollius H., Guiguen Y. (2014) The rainbow trout genome provides novel insights into evolution after whole-genome duplication in vertebrates. Nat. Commun. 5, 3657 10.1038/ncomms4657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kubota S., Kinoshita M., Uji S., Yokoyama Y., Yamamoto E., Hirono I., Aoki T., Sakaguchi M., Morioka K., Itoh Y., Toyohara H. (2003) Occurrence of two distinct types of tissue inhibitor of metalloproteinases-2 in teleost fish. Biochim. Biophys. Acta 1629, 102–108 10.1016/j.bbaexp.2003.08.007 [DOI] [PubMed] [Google Scholar]
- 38.Tsukamoto H., Yokoyama Y., Suzuki T., Mizuta S., Yoshinaka R. (2007) Expression and distribution of fugu TIMP-2s (fgTIMP-2a and fgTIMP-2b) mRNAs in tissues and embryos. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 148, 225–230 10.1016/j.cbpb.2007.02.016 [DOI] [PubMed] [Google Scholar]
- 39.Xu X. Y., Shen Y. B., Yang X. M., Li J. L. (2011) Cloning and characterization of TIMP-2b gene in grass carp. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 159, 115–121 10.1016/j.cbpb.2011.02.008 [DOI] [PubMed] [Google Scholar]
- 40.Vicoso B., Charlesworth B. (2006) Evolution on the X chromosome: unusual patterns and processes. Nat. Rev. Genet. 7, 645–653 10.1038/nrg1914 [DOI] [PubMed] [Google Scholar]
- 41.Kumar S., Stecher G., Suleski M., Hedges S. B. (2017) TimeTree: a resource for timelines, timetrees, and divergence times. Mol. Biol. Evol. 34, 1812–1819 10.1093/molbev/msx116 [DOI] [PubMed] [Google Scholar]
- 42.Waters P. D., Delbridge M. L., Deakin J. E., El-Mogharbel N., Kirby P. J., Carvalho-Silva D. R., Graves J. A. (2005) Autosomal location of genes from the conserved mammalian X in the platypus (Ornithorhynchus anatinus): implications for mammalian sex chromosome evolution. Chromosome Res. 13, 401–410 10.1007/s10577-005-0978-5 [DOI] [PubMed] [Google Scholar]
- 43.Veyrunes F., Waters P. D., Miethke P., Rens W., McMillan D., Alsop A. E., Grützner F., Deakin J. E., Whittington C. M., Schatzkamer K., Kremitzki C. L., Graves T., Ferguson-Smith M. A., Warren W., Marshall Graves J. A. (2008) Bird-like sex chromosomes of platypus imply recent origin of mammal sex chromosomes. Genome Res. 18, 965–973 10.1101/gr.7101908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takechi M., Takeuchi M., Ota K. G., Nishimura O., Mochii M., Itomi K., Adachi N., Takahashi M., Fujimoto S., Tarui H., Okabe M., Aizawa S., Kuratani S. (2011) Overview of the transcriptome profiles identified in hagfish, shark, and bichir: current issues arising from some nonmodel vertebrate taxa. J. Exp. Zoolog. B Mol. Dev. Evol. 316, 526–546 10.1002/jez.b.21427 [DOI] [PubMed] [Google Scholar]
- 45.Guindon S., Gascuel O. (2003) A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 52, 696–704 10.1080/10635150390235520 [DOI] [PubMed] [Google Scholar]
- 46.Stamatakis A., Hoover P., Rougemont J. (2008) A rapid bootstrap algorithm for the RAxML web servers. Syst. Biol. 57, 758–771 10.1080/10635150802429642 [DOI] [PubMed] [Google Scholar]
- 47.Edgar R. C. (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32, 1792–1797 10.1093/nar/gkh340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ohno S. (1970) Evolution by Gene Duplication, Springer-Verlag, New York: 10.1007/978-3-642-86659-3 [DOI] [Google Scholar]
- 49.Kasahara M. (2007) The 2R hypothesis: an update. Curr. Opin. Immunol. 19, 547–552 10.1016/j.coi.2007.07.009 [DOI] [PubMed] [Google Scholar]
- 50.Singh N. D., Petrov D. A. (2007) Evolution of gene function on the X chromosome versus the autosomes. Genome Dyn. 3, 101–118 10.1159/000107606 [DOI] [PubMed] [Google Scholar]
- 51.Gomis-Rüth F. X., Maskos K., Betz M., Bergner A., Huber R., Suzuki K., Yoshida N., Nagase H., Brew K., Bourenkov G. P., Bartunik H., Bode W. (1997) Mechanism of inhibition of the human matrix metalloproteinase stromelysin-1 by TIMP-1. Nature 389, 77–81 10.1038/37995 [DOI] [PubMed] [Google Scholar]
- 52.Andersson L., Sulkowski E. (1992) Evaluation of the interaction of protein alpha-amino groups with M(II) by immobilized metal ion affinity chromatography. J. Chromatogr. 604, 13–17 10.1016/0021-9673(92)85523-V [DOI] [PubMed] [Google Scholar]
- 53.Pace C. N., Grimsley G. R., Scholtz J. M. (2009) Protein ionizable groups: pK values and their contribution to protein stability and solubility. J. Biol. Chem. 284, 13285–13289 10.1074/jbc.R800080200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Greenstein J. P., Klemperer F. W., Wyman J. (1939) Further studies on the physical chemistry of cysteine peptides. J. Biol. Chem. 129, 681–692 [Google Scholar]
- 55.Wei S., Xie Z., Filenova E., Brew K. (2003) Drosophila TIMP is a potent inhibitor of MMPs and TACE: similarities in structure and function to TIMP-3. Biochemistry 42, 12200–12207 10.1021/bi035358x [DOI] [PubMed] [Google Scholar]
- 56.Edwards D. R., Handsley M. M., Pennington C. J. (2008) The ADAM metalloproteinases. Mol. Aspects Med. 29, 258–289 10.1016/j.mam.2008.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kelwick R., Desanlis I., Wheeler G. N., Edwards D. R. (2015) The ADAMTS (a disintegrin and metalloproteinase with thrombospondin motifs) family. Genome Biol. 16, 113 10.1186/s13059-015-0676-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wisniewska M., Goettig P., Maskos K., Belouski E., Winters D., Hecht R., Black R., Bode W. (2008) Structural determinants of the ADAM inhibition by TIMP-3: crystal structure of the TACE-N-TIMP-3 complex. J. Mol. Biol. 381, 1307–1319 10.1016/j.jmb.2008.06.088 [DOI] [PubMed] [Google Scholar]
- 59.Lee M. H., Rapti M., Murphy G. (2003) Unveiling the surface epitopes that render tissue inhibitor of metalloproteinase-1 inactive against membrane type 1-matrix metalloproteinase. J. Biol. Chem. 278, 40224–40230 10.1074/jbc.M305678200 [DOI] [PubMed] [Google Scholar]
- 60.Lee M. H., Rapti M., Knaüper V., Murphy G. (2004) Threonine 98, the pivotal residue of tissue inhibitor of metalloproteinases (TIMP)-1 in metalloproteinase recognition. J. Biol. Chem. 279, 17562–17569 10.1074/jbc.M312589200 [DOI] [PubMed] [Google Scholar]
- 61.Lee M. H., Rapti M., Murphy G. (2004) Delineating the molecular basis of the inactivity of tissue inhibitor of metalloproteinase-2 against tumor necrosis factor-α-converting enzyme. J. Biol. Chem. 279, 45121–45129 10.1074/jbc.M406611200 [DOI] [PubMed] [Google Scholar]
- 62.Lee M. H., Rapti M., Murphy G. (2005) Total conversion of tissue inhibitor of metalloproteinase (TIMP) for specific metalloproteinase targeting: fine-tuning TIMP-4 for optimal inhibition of tumor necrosis factor-α-converting enzyme. J. Biol. Chem. 280, 15967–15975 10.1074/jbc.M500897200 [DOI] [PubMed] [Google Scholar]
- 63.Morgunova E., Tuuttila A., Bergmann U., Tryggvason K. (2002) Structural insight into the complex formation of latent matrix metalloproteinase 2 with tissue inhibitor of metalloproteinase 2. Proc. Natl. Acad. Sci. USA 99, 7414–7419 10.1073/pnas.102185399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Itoh Y., Takamura A., Ito N., Maru Y., Sato H., Suenaga N., Aoki T., Seiki M. (2001) Homophilic complex formation of MT1-MMP facilitates proMMP-2 activation on the cell surface and promotes tumor cell invasion. EMBO J. 20, 4782–4793 10.1093/emboj/20.17.4782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hernandez-Barrantes S., Shimura Y., Soloway P. D., Sang Q. A., Fridman R. (2001) Differential roles of TIMP-4 and TIMP-2 in pro-MMP-2 activation by MT1-MMP. Biochem. Biophys. Res. Commun. 281, 126–130 10.1006/bbrc.2001.4323 [DOI] [PubMed] [Google Scholar]
- 66.Bigg H. F., Morrison C. J., Butler G. S., Bogoyevitch M. A., Wang Z., Soloway P. D., Overall C. M. (2001) Tissue inhibitor of metalloproteinases-4 inhibits but does not support the activation of gelatinase A via efficient inhibition of membrane type 1-matrix metalloproteinase. Cancer Res. 61, 3610–3618 [PubMed] [Google Scholar]
- 67.Worley J. R., Thompkins P. B., Lee M. H., Hutton M., Soloway P., Edwards D. R., Murphy G., Knäuper V. (2003) Sequence motifs of tissue inhibitor of metalloproteinases 2 (TIMP-2) determining progelatinase A (proMMP-2) binding and activation by membrane-type metalloproteinase 1 (MT1-MMP). Biochem. J. 372, 799–809 10.1042/bj20021573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Johanns M., Lemoine P., Janssens V., Grieco G., Moestrup S. K., Nielsen R., Christensen E. I., Courtoy P. J., Emonard H., Marbaix E., Henriet P. (2017) Cellular uptake of proMMP-2:TIMP-2 complexes by the endocytic receptor megalin/LRP-2. Sci. Rep. 7, 4328 10.1038/s41598-017-04648-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.English J. L., Kassiri Z., Koskivirta I., Atkinson S. J., Di Grappa M., Soloway P. D., Nagase H., Vuorio E., Murphy G., Khokha R. (2006) Individual timp deficiencies differentially impact pro-MMP-2 activation. J. Biol. Chem. 281, 10337–10346 10.1074/jbc.M512009200 [DOI] [PubMed] [Google Scholar]
- 70.Bergers G., Brekken R., McMahon G., Vu T. H., Itoh T., Tamaki K., Tanzawa K., Thorpe P., Itohara S., Werb Z., Hanahan D. (2000) Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat. Cell Biol. 2, 737–744 10.1038/35036374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bekes E. M., Schweighofer B., Kupriyanova T. A., Zajac E., Ardi V. C., Quigley J. P., Deryugina E. I. (2011) Tumor-recruited neutrophils and neutrophil TIMP-free MMP-9 regulate coordinately the levels of tumor angiogenesis and efficiency of malignant cell intravasation. Am. J. Pathol. 179, 1455–1470 10.1016/j.ajpath.2011.05.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ogata Y., Itoh Y., Nagase H. (1995) Steps involved in activation of the pro-matrix metalloproteinase 9 (progelatinase B)-tissue inhibitor of metalloproteinases-1 complex by 4-aminophenylmercuric acetate and proteinases. J. Biol. Chem. 270, 18506–18511 10.1074/jbc.270.31.18506 [DOI] [PubMed] [Google Scholar]
- 73.Ardi V. C., Kupriyanova T. A., Deryugina E. I., Quigley J. P. (2007) Human neutrophils uniquely release TIMP-free MMP-9 to provide a potent catalytic stimulator of angiogenesis. Proc. Natl. Acad. Sci. USA 104, 20262–20267 10.1073/pnas.0706438104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Akahane T., Akahane M., Shah A., Thorgeirsson U. P. (2004) TIMP-1 stimulates proliferation of human aortic smooth muscle cells and Ras effector pathways. Biochem. Biophys. Res. Commun. 324, 440–445 10.1016/j.bbrc.2004.09.063 [DOI] [PubMed] [Google Scholar]
- 75.Liu X. W., Bernardo M. M., Fridman R., Kim H. R. (2003) Tissue inhibitor of metalloproteinase-1 protects human breast epithelial cells against intrinsic apoptotic cell death via the focal adhesion kinase/phosphatidylinositol 3-kinase and MAPK signaling pathway. J. Biol. Chem. 278, 40364–40372 10.1074/jbc.M302999200 [DOI] [PubMed] [Google Scholar]
- 76.Liu X. W., Taube M. E., Jung K. K., Dong Z., Lee Y. J., Roshy S., Sloane B. F., Fridman R., Kim H. R. (2005) Tissue inhibitor of metalloproteinase-1 protects human breast epithelial cells from extrinsic cell death: a potential oncogenic activity of tissue inhibitor of metalloproteinase-1. Cancer Res. 65, 898–906 [PubMed] [Google Scholar]
- 77.Zhang J., Wu T., Zhan S., Qiao N., Zhang X., Zhu Y., Yang N., Sun Y., Zhang X. A., Bleich D., Han X. (2017) TIMP-1 and CD82, a promising combined evaluation marker for PDAC. Oncotarget 8, 6496–6512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Seo D. W., Saxinger W. C., Guedez L., Cantelmo A. R., Albini A., Stetler-Stevenson W. G. (2011) An integrin-binding N-terminal peptide region of TIMP-2 retains potent angio-inhibitory and anti-tumorigenic activity in vivo. Peptides 32, 1840–1848 10.1016/j.peptides.2011.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fernández C. A., Butterfield C., Jackson G., Moses M. A. (2003) Structural and functional uncoupling of the enzymatic and angiogenic inhibitory activities of tissue inhibitor of metalloproteinase-2 (TIMP-2): loop 6 is a novel angiogenesis inhibitor. J. Biol. Chem. 278, 40989–40995 10.1074/jbc.M306176200 [DOI] [PubMed] [Google Scholar]
- 80.Fernandez C. A., Roy R., Lee S., Yang J., Panigrahy D., Van Vliet K. J., Moses M. A. (2010) The anti-angiogenic peptide, loop 6, binds insulin-like growth factor-1 receptor. J. Biol. Chem. 285, 41886–41895 10.1074/jbc.M110.166439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Qi J. H., Ebrahem Q., Ali M., Cutler A., Bell B., Prayson N., Sears J., Knauper V., Murphy G., Anand-Apte B. (2013) Tissue inhibitor of metalloproteinases-3 peptides inhibit angiogenesis and choroidal neovascularization in mice. PLoS One 8, e55667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stuttfeld E., Ballmer-Hofer K. (2009) Structure and function of VEGF receptors. IUBMB Life 61, 915–922 10.1002/iub.234 [DOI] [PubMed] [Google Scholar]
- 83.Menting J. G., Lawrence C. F., Kong G. K., Margetts M. B., Ward C. W., Lawrence M. C. (2015) Structural congruency of ligand binding to the insulin and insulin/type 1 insulin-like growth factor hybrid receptors. Structure 23, 1271–1282 10.1016/j.str.2015.04.016 [DOI] [PubMed] [Google Scholar]
- 84.Scilabra S. D., Troeberg L., Yamamoto K., Emonard H., Thøgersen I., Enghild J. J., Strickland D. K., Nagase H. (2013) Differential regulation of extracellular tissue inhibitor of metalloproteinases-3 levels by cell membrane-bound and shed low density lipoprotein receptor-related protein 1. J. Biol. Chem. 288, 332–342 10.1074/jbc.M112.393322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yamamoto K., Murphy G., Troeberg L. (2015) Extracellular regulation of metalloproteinases. Matrix Biol. 44–46, 255–263 10.1016/j.matbio.2015.02.007 [DOI] [PubMed] [Google Scholar]
- 86.Doherty C. M., Visse R., Dinakarpandian D., Strickland D. K., Nagase H., Troeberg L. (2016) Engineered tissue inhibitor of metalloproteinases-3 variants resistant to endocytosis have prolonged chondroprotective activity. J. Biol. Chem. 291, 22160–22172 10.1074/jbc.M116.733261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.De Mateo S., Castillo J., Estanyol J. M., Ballescà J. L., Oliva R. (2011) Proteomic characterization of the human sperm nucleus. Proteomics 11, 2714–2726 10.1002/pmic.201000799 [DOI] [PubMed] [Google Scholar]
- 88.Lee M. H., Atkinson S., Murphy G. (2007) Identification of the extracellular matrix (ECM) binding motifs of tissue inhibitor of metalloproteinases (TIMP)-3 and effective transfer to TIMP-1. J. Biol. Chem. 282, 6887–6898 10.1074/jbc.M610490200 [DOI] [PubMed] [Google Scholar]
- 89.Weber B. H., Vogt G., Pruett R. C., Stöhr H., Felbor U. (1994) Mutations in the tissue inhibitor of metalloproteinases-3 (TIMP3) in patients with Sorsby’s fundus dystrophy. Nat. Genet. 8, 352–356 10.1038/ng1294-352 [DOI] [PubMed] [Google Scholar]
- 90.Stone E. M., Lotery A. J., Munier F. L., Héon E., Piguet B., Guymer R. H., Vandenburgh K., Cousin P., Nishimura D., Swiderski R. E., Silvestri G., Mackey D. A., Hageman G. S., Bird A. C., Sheffield V. C., Schorderet D. F. (1999) A single EFEMP1 mutation associated with both Malattia Leventinese and Doyne honeycomb retinal dystrophy. Nat. Genet. 22, 199–202 10.1038/9722 [DOI] [PubMed] [Google Scholar]
- 91.Zayas-Santiago A., Cross S. D., Stanton J. B., Marmorstein A. D., Marmorstein L. Y. (2017) Mutant fibulin-3 causes proteoglycan accumulation and impaired diffusion across Bruch’s membrane. Invest. Ophthalmol. Vis. Sci. 58, 3046–3054 10.1167/iovs.17-21720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Meunier I., Bocquet B., Labesse G., Zeitz C., Defoort-Dhellemmes S., Lacroux A., Mauget-Faysse M., Drumare I., Gamez A. S., Mathieu C., Marquette V., Sagot L., Dhaenens C. M., Arndt C., Carroll P., Remy-Jardin M., Cohen S. Y., Sahel J. A., Puech B., Audo I., Mrejen S., Hamel C. P. (2016) A new autosomal dominant eye and lung syndrome linked to mutations in TIMP3 gene. Sci. Rep. 6, 32544 10.1038/srep32544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Marie B., Joubert C., Tayalé A., Zanella-Cléon I., Belliard C., Piquemal D., Cochennec-Laureau N., Marin F., Gueguen Y., Montagnani C. (2012) Different secretory repertoires control the biomineralization processes of prism and nacre deposition of the pearl oyster shell. Proc. Natl. Acad. Sci. USA 109, 20986–20991 10.1073/pnas.1210552109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yan F., Jiao Y., Deng Y., Du X., Huang R., Wang Q., Chen W. (2014) Tissue inhibitor of metalloproteinase gene from pearl oyster Pinctada martensii participates in nacre formation. Biochem. Biophys. Res. Commun. 450, 300–305 10.1016/j.bbrc.2014.05.118 [DOI] [PubMed] [Google Scholar]
- 95.Liu C., Li S., Kong J., Liu Y., Wang T., Xie L., Zhang R. (2015) In-depth proteomic analysis of shell matrix proteins of Pinctada fucata. Sci. Rep. 5, 17269 10.1038/srep17269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang G., Fang X., Guo X., Li L., Luo R., Xu F., Yang P., Zhang L., Wang X., Qi H., Xiong Z., Que H., Xie Y., Holland P. W., Paps J., Zhu Y., Wu F., Chen Y., Wang J., Peng C., Meng J., Yang L., Liu J., Wen B., Zhang N., Huang Z., Zhu Q., Feng Y., Mount A., Hedgecock D., Xu Z., Liu Y., Domazet-Lošo T., Du Y., Sun X., Zhang S., Liu B., Cheng P., Jiang X., Li J., Fan D., Wang W., Fu W., Wang T., Wang B., Zhang J., Peng Z., Li Y., Li N., Wang J., Chen M., He Y., Tan F., Song X., Zheng Q., Huang R., Yang H., Du X., Chen L., Yang M., Gaffney P. M., Wang S., Luo L., She Z., Ming Y., Huang W., Zhang S., Huang B., Zhang Y., Qu T., Ni P., Miao G., Wang J., Wang Q., Steinberg C. E., Wang H., Li N., Qian L., Zhang G., Li Y., Yang H., Liu X., Wang J., Yin Y., Wang J. (2012) The oyster genome reveals stress adaptation and complexity of shell formation. Nature 490, 49–54 10.1038/nature11413 [DOI] [PubMed] [Google Scholar]
- 97.Liao Z., Bao L. F., Fan M. H., Gao P., Wang X. X., Qin C. L., Li X. M. (2015) In-depth proteomic analysis of nacre, prism, and myostracum of Mytilus shell. J. Proteomics 122, 26–40 10.1016/j.jprot.2015.03.027 [DOI] [PubMed] [Google Scholar]
- 98.Jackson D. J., Mann K., Häussermann V., Schilhabel M. B., Lüter C., Griesshaber E., Schmahl W., Wörheide G. (2015) The Magellania venosa biomineralizing proteome: a window into brachiopod shell evolution. Genome Biol. Evol. 7, 1349–1362 10.1093/gbe/evv074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kubota K., Tsuchihashi Y., Kogure T., Maeyama K., Hattori F., Kinoshita S., Sakuda S., Nagasawa H., Yoshimura E., Suzuki M. (2017) Structural and functional analyses of a TIMP and MMP in the ligament of Pinctada fucata. J. Struct. Biol. 199, 216–224 10.1016/j.jsb.2017.07.010 [DOI] [PubMed] [Google Scholar]
- 100.Qin C. L., Pan Q. D., Qi Q., Fan M. H., Sun J. J., Li N. N., Liao Z. (2016) In-depth proteomic analysis of the byssus from marine mussel Mytilus coruscus. J. Proteomics 144, 87–98 10.1016/j.jprot.2016.06.014 [DOI] [PubMed] [Google Scholar]
- 101.Li Y., Sun X., Hu X., Xun X., Zhang J., Guo X., Jiao W., Zhang L., Liu W., Wang J., Li J., Sun Y., Miao Y., Zhang X., Cheng T., Xu G., Fu X., Wang Y., Yu X., Huang X., Lu W., Lv J., Mu C., Wang D., Li X., Xia Y., Li Y., Yang Z., Wang F., Zhang L., Xing Q., Dou H., Ning X., Dou J., Li Y., Kong D., Liu Y., Jiang Z., Li R., Wang S., Bao Z. (2017) Scallop genome reveals molecular adaptations to semi-sessile life and neurotoxins. Nat. Commun. 8, 1721 10.1038/s41467-017-01927-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Levi-Kalisman Y., Falini G., Addadi L., Weiner S. (2001) Structure of the nacreous organic matrix of a bivalve mollusk shell examined in the hydrated state using cryo-TEM. J. Struct. Biol. 135, 8–17 10.1006/jsbi.2001.4372 [DOI] [PubMed] [Google Scholar]
- 103.Aguilera F., McDougall C., Degnan B. M. (2017) Co-option and de novo gene evolution underlie molluscan shell diversity. Mol. Biol. Evol. 34, 779–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dobzhansky T. (1973) Nothing in biology makes sense except in the light of evolution. Am. Biol. Teach. 35, 125–129 10.2307/4444260 [DOI] [Google Scholar]
- 105.Tuuttila A., Morgunova E., Bergmann U., Lindqvist Y., Maskos K., Fernandez-Catalan C., Bode W., Tryggvason K., Schneider G. (1998) Three-dimensional structure of human tissue inhibitor of metalloproteinases-2 at 2.1 A resolution. J. Mol. Biol. 284, 1133–1140 10.1006/jmbi.1998.2223 [DOI] [PubMed] [Google Scholar]
- 106.Pettersen E. F., Goddard T. D., Huang C. C., Couch G. S., Greenblatt D. M., Meng E. C., Ferrin T. E. (2004) UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 10.1002/jcc.20084 [DOI] [PubMed] [Google Scholar]
- 107.Iyer S., Wei S., Brew K., Acharya K. R. (2007) Crystal structure of the catalytic domain of matrix metalloproteinase-1 in complex with the inhibitory domain of tissue inhibitor of metalloproteinase-1. J. Biol. Chem. 282, 364–371 10.1074/jbc.M607625200 [DOI] [PubMed] [Google Scholar]
- 108.Biasini M., Bienert S., Waterhouse A., Arnold K., Studer G., Schmidt T., Kiefer F., Gallo Cassarino T., Bertoni M., Bordoli L., Schwede T. (2014) SWISS-MODEL: modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res. 42, W252–W258 10.1093/nar/gku340 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.