ABSTRACT
An animal model of alcoholic liver disease (ALD) that recapitulates human disease is an unmet need. An alcohol-preferring strain and WT mice were fed alcohol by different techniques and with different diet compositions. Interestingly, the greatest alcohol consumers did not develop the worst ALD. This editorial highlights how diet and the gut microbiome/metabolome may influence the development/severity of ALD.
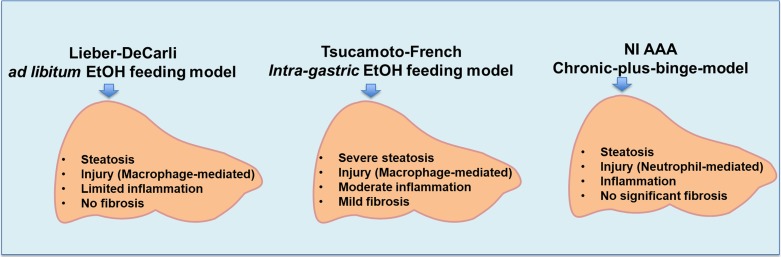
Alcoholic liver disease (ALD) is a major health problem both in the USA and worldwide. Models of ALD are important for evaluating mechanisms of liver disease development/progression, as well as efficacy of potential therapeutic agents. Rodents have a natural aversion to alcohol, and thus, creating an experimental model of ALD has been difficult. The liquid Lieber–DeCarli (LD) alcohol diet was a major advance in that the animals consumed alcohol with the liquid diet, usually for a 4-week period of time. This type of diet (even for longer periods) usually only causes hepatic steatosis with limited liver injury, inflammation and no fibrosis. The Tsukamoto-French intragastric alcohol feeding model causes severe steatosis with moderate macrophage-mediated liver injury, inflammation and mild fibrosis. However, this complicated and labor-intensive model is performed in only a few laboratories in the country. The NIAAA model of chronic-plus-binge alcohol feeding produces predominantly a neutrophil-mediated liver injury. This is a simpler, shorter model but, again, there is no significant fibrosis (Fig. 1, Mathews et al., 2014). Thus, an animal model in which the rodents voluntarily consume large amounts of alcohol to achieve sustained blood alcohol concentrations (BACs) and subsequent liver injury, which recapitulates human disease, could theoretically be an attractive experimental model of ALD.
Fig. 1.
Animal models of ALD and critical outcomes.
The crossed high alcohol preferring (cHAP) mice were developed through selective breeding of high alcohol preferring lines. The cHAP mice voluntarily consume high amounts of ethanol (EtOH, ~25 g/kg/d) and achieve mean BACs ≥ 250 mg/dl. Unfortunately, 4 weeks of voluntary 10% EtOH consumption in drinking water [EtOH-DW] failed to produce major liver pathology in cHAP mice (Matson et al., 2013), suggesting a more aggressive regimen must be required.
The study by Thompson et al. (2017) demonstrated that cHAP as well as wild-type (WT) mice developed more severe liver injury while consuming the Lieber–DeCarli alcohol containing diet for 4 weeks followed by a single EtOH binge by gavage (LD-EtOH+B mice) compared to animals maintained on a standard rodent chow and voluntary alcohol intake plus an EtOH gavage (EtOH-DW+B mice). Thus, ALT and AST levels, markers of hepatocyte damage, were significantly higher in LD-EtOH+B compared to EtOH-DW+B mice, although they were similar in cHAP and WT animals. Histological evaluation of liver tissue revealed more pronounced hepatic fat accumulation in LD-EtOH+B vs EtOH-DW+B mice, which was comparable between genotypes (cHAP and WT). A critical observation was that the cHAP mice on the EtOH-DW regimen consumed significantly more ethanol than cHAP or WT mice on the LD-EtOH diet; however, this difference did not result in more severe liver injury in these mice. In fact, greater liver injury and steatosis developed in LD-EtOH+B mice with less (compared to EtOH-DW+B animals) ethanol consumption. Intriguingly, the BACs levels were significantly elevated in LD-EtOH+B vs EtOH-DW+B animals, with higher levels in WT compared to cHAP animals. Taken together, these observations indicate that factors other than the total amount of alcohol consumed can affect ALD development and progression in experimental animals.
Only a limited number of patients who drink heavily go on to develop advanced liver disease. There are several risk factors in humans (Table 1) that are postulated to play a role in progressive ALD. Among them, diet/nutrition is highly relevant to both clinical and experimental ALD.
Table 1.
Risk factors for ALD
|
Although the authors in this study did not specify the composition of the control liquid LD diet, the levels of protein, carbohydrate and fat in commonly used LD diets vary between 15–17, 35–45 and 35–40% of total energy, respectively. In alcohol containing diets, ethanol energy is substituted for carbohydrate energy. In comparison to LD, the mice which received EtOH in drinking water were fed a standard rodent chow with 32, 54 and 14% total energy from protein, carbohydrate and fat, respectively. Therefore, the ways the animals were fed and their diet compositions were dramatically different in the Thompson et al.'s study. One group received a pelleted diet which contained much more fiber than the LD liquid diet. One group received alcohol in their water supply and the other (LD) in their liquid diet. Lastly, the diets were markedly different in macronutrient composition, especially protein and fat. The LD diet was ~40% fat and the pelleted diet 14% fat. Further, the composition of fat (e.g. the total saturated, monounsaturated and polyunsaturated fatty acids), as well as n6/n3 ratio (which was ~3-fold higher in LD compared to standard chow) in these diets were different.
Accumulating evidence suggests that dietary fat and heavy alcohol consumption interact to play critical roles in the ALD pathogenesis. Indeed, the beneficial effects of dietary saturated fat (SF, primarily rich in medium chain triglycerides and beef tallow) and the damaging effects of dietary unsaturated fat (USF, specifically corn oil/linoleic acid enriched) on experimental alcohol-induced liver injury have been documented by several research groups. We have recently demonstrated that dietary SF and USF differentially modulate the gut microbiome, intestinal barrier and liver injury in a mouse model of ALD (Kirpich et al., 2016). Thus, compared to SF+EtOH, USF+EtOH administration produced hepatic steatosis (micro- and macrovesicular), inflammation (determined by elevated levels of pro-inflammatory cytokines, including TNF-α) and injury (documented by elevated serum ALT levels). In parallel with liver injury, significantly elevated serum LPS levels, intestinal inflammation and increased gut permeability with intestinal tight junction and mucus layer alterations were observed in mice fed USF+EtOH but not SF+EtOH. Major alterations in gut microbiota, including a prominent reduction in Bacteroidetes, and an increase in Proteobacteria and Actinobacteria, were seen in USF+EtOH but not in SF+EtOH fed animals, suggesting that the types of dietary fat play a critical role in ethanol-mediated changes of the gut microbiota composition, which was linked with the ALD development and progression. Moreover, fecal metabolites were also changed by these different diets. Low fecal levels of the short chain fatty acid butyrate was seen in the USF+EtOH group. In the present study, the LD diet is high in linoleic acid compared to the chow diet, and it is low in fiber, which is a substrate for butyrate production.
A critical role of the gut microbiome and fecal metabolites is becoming increasingly appreciated in experimental and human ALD. Effects of diet on the microbiome/metabolome are also well documented (but not evaluated in this project). Marked differences in the composition of the diets used in this study may help explain why mice consuming the highest amounts of alcohol did not develop the most severe liver injury. It is critical in all experimental ALD studies that the diet composition be well defined. We suggest that diet and microbiome may be important variables in the different outcomes (and lack of reproducibility) observed in various experimental ALD models.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- Kirpich IA, Petrosino J, Ajami N, et al. (2016) Saturated and unsaturated dietary fats differentially modulate ethanol-induced changes in gut microbiome and metabolome in a mouse model of alcoholic liver disease. Am J Pathol 186:765–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews S, Xu M, Wang H, et al. (2014) Animals models of gastrointestinal and liver diseases. Animal models of alcohol-induced liver disease: pathophysiology, translational relevance, and challenges. Am J Physiol Gastrointest Liver Physiol 306:G819–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson L, Liangpunsakul S, Crabb D, et al. (2013) Chronic free-choice drinking in crossed high alcohol preferring mice leads to sustained blood ethanol levels and metabolic tolerance without evidence of liver damage. Alcohol Clin Exp Res 37:194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson KJ, et al. (2017) Use of a crossed high alcohol preferring (chap) mouse model with the NIAAA-model of chronic-binge ethanol intake to study liver injury. Alcohol Alcohol XX:XXX–XXX. [DOI] [PMC free article] [PubMed] [Google Scholar]