Abstract
The chemokine CXC motif ligand 12 (CXCL12) and its cognate receptor, CXCR4, have been implicated in the ovulatory process in various animal models. However, little is known about the expression and regulation of CXCL12 and CXCR4 and their functions during the ovulatory period in the human ovary. In this study, we characterized the expression patterns of CXCL12 and CXCR4 in preovulatory follicles collected before the luteinizing hormone (LH) surge and at defined hours after hCG administration in women with the regular menstrual cycle. The levels of mRNA and protein for CXCR4 were increased in granulosa cells of late ovulatory follicles, whereas CXCL12 expression was constant in follicles throughout the ovulatory period. Both CXCR4 and CXCL12 were localized to a subset of leukocytes around and inside the vasculature of human preovulatory follicles. Using a human granulosa cell culture model, the regulatory mechanisms and functions of CXCL12 and CXCR4 expression were investigated. Human chorionic gonadotropin (hCG) stimulated CXCR4 expression, whereas CXCL12 expression was not affected, mimicking in vivo expression patterns. Both RU486 (progesterone receptor antagonist) and CoCl2 (HIFs activator) blocked the hCG-induced increase in CXCR4 expression, whereas AG1478 (EGFR inhibitor) had no effect. The treatment with CXCL12 had no effect on granulosa cell viability but decreased hCG-stimulated CXCR4 expression.
Keywords: CXCL12, CXCR4, granulosa cells, progesterone receptor, human
Summary Sentence
In conclusion, these results suggest that the CXCL12/CXCR4 system plays a role(s) in the LH surge-induced follicular changes and infiltration of leukocytes in dominant follicles during the ovulatory period in humans.
Introduction
In humans, the mid-cycle preovulatory gonadotropin surge sets in motion the complex cellular events in preovulatory follicular cells, leading to physio-morphological changes necessary for ovulation and subsequent formation of a corpus luteum. These changes include extensive tissue remodeling, angiogenesis, and a massive influx of leukocytes (reviewed in [1–3]), which share characteristic elements of an inflammatory-like response. These diverse, yet highly synchronized processes are accomplished by coordinated actions of a variety of autocrine or paracrine factors that are expressed in and secreted by preovulatory follicular cells. Along with follicular cells, accumulating evidence has demonstrated that leukocytes also play a key role in ovulation and luteinization by secreting various factors (e.g. cytokines and proteases) that in turn act on follicular cells and stromal tissues to facilitate luteinization and the breakdown of extracellular matrix at the apex of ovulatory follicles, respectively [4].
The findings that the number of leukocytes entering the ovary is markedly increased during the ovulatory period suggest that preovulatory follicles produce chemotactic substances in response to the luteinizing hormone (LH) surge [5, 6]. Chemokines are a diverse group of small secretory proteins that exert potent chemotactic activity to attract a certain type of motile cells. According to structural differences, chemokines are divided into four groups; C-, CC-, CXC- and CX3C chemokines. In the preovulatory ovary, the increase in the levels of chemokines such as CCL2 (a.k.a. MCP-1) and CXCL8 (a.k.a. IL-8) has been reported in the follicular fluid of ovulatory follicles in humans [4]. More recently, we have demonstrated that ovulatory human chorionic gonadotropin (hCG) stimulation rapidly and dramatically upregulates the expression of CCL20 in both granulosa and theca cells of human preovulatory follicles [7]. Similarly, the expression of CXCL12 (SDF-1) was detected in both granulosa and theca cells of equine preovulatory follicles [8], although its expression was predominant in the theca cell layer.
Chemokines exert their biological effects by binding to their respective chemokine receptors on the surface of target cells. At least 20 different chemokine receptors have been identified to date [9] and have been found to be highly, yet differentially expressed on leukocytes [10]. In addition, chemokine receptors are expressed in various cell types, such as embryonic stem cells, endothelial cells, and neuronal cells [10]. In the ovary, recent studies have documented the expression of CXC chemokine receptor 4 (CXCR4) in the preovulatory ovary of various species [8, 11]. Importantly, the expression of CXCR4 was highly upregulated by hCG in granulosa and cumulus cells of ovulatory follicles [8, 11–13], suggesting that CXCR4 plays a role in the ovulatory process and luteinization. Initially, CXCR4 was thought to be a monogamous receptor for CXCL12, but recent studies have revealed that ubiquitin and macrophage migration inhibitory factor (MIF) also act as noncognate ligands for CXCR4 [14, 15]. Moreover, a previous study has reported the expression of MIF in human granulosa cells isolated from in vitro fertilization (IVF) patients [16] and suggested a role of this protein in ovulation [17]. Functionally, CXCR4 is well known for its role in the migration of hematopoietic stem cells, immune cells, and CXCR4-positive cancer cells triggered by its chemotactic ligand, CXCL12 [18]. In addition, both CXCL12 and CXCR4 have been found to be expressed in a wide variety of tissues and implicated in various biological functions, including cell differentiation and survival/apoptosis, tissue remodeling, and angiogenesis (reviewed in [19]).
In the human ovary, the expression of CXCR4 and CXCL12 has been reported in granulosa cells of follicular aspirates from patients undergoing IVF procedures [20–22]. In addition, CXCR4 mRNA levels were reported to be differentially regulated between hCG- and gonadotropin-releasing hormone (GnRH)-triggered ovulation [22]. CXCL12 caused migration of T lymphocytes isolated from follicular aspirates of IVF patients and reduced the early apoptotic, but not late apoptotic process in human granulosa cells in vitro, suggesting that CXCL12 may play a role in granulosa cell survival [20]. However, there is no report on whether the expression of CXCR4 and CXCL12 is induced and regulated by hormones (e.g. ovulatory LH or progesterone) during the preovulatory period in the human ovary.
Based on previous findings of Cxcr4 and Cxcl12 expression in preovulatory follicles of mice, cattle, and horses, and their well-known function in leukocyte migration, we hypothesized that the expression of CXCL12 and CXCR4 is upregulated in preovulatory follicles of human ovaries and the CXCL12/CXCR4 system is involved in the ovulatory process. This hypothesis was tested by examining the expression of CXCR4 and CXCL12 in dominant follicles obtained before the LH surge and at defined times after hCG administration which mimics the natural LH surge. Furthermore, the regulatory mechanisms involved in the expression of CXCR4 and CXCL12 were investigated using a human primary granulosa cell culture model. Lastly, we explored the potential function of CXCL12/CXCR4 in human granulosa cells in vitro.
Materials and methods
Materials
Unless otherwise noted, all chemicals and reagents were purchased from Sigma Chemical Co. (St. Louis, MO). Mifepristone (RU486) was purchased from Cayman Chemical. (Ann Arbor, MI). Molecular biological enzymes, culture media, antibiotic-antimycotic, fetal bovine serum, deoxynucleotide triphosphates, superscript III reverse transcriptase, oligodeoxythymidine, RNaseOUT, and Trizol were purchased from Invitrogen Life Technologies, Inc. (Carlsbad, CA). Oligonucleotide primers were purchased from Eurofins MWG operon (Huntsville, AL).
Human tissue collection: in vivo ovulatory follicles
The protocol using human tissues is approved by the Human Ethics Committee of the Sahlgrenska Academy at the University of Gothenburg, and all patients had given their informed written consent before participating. Granulosa and theca cells of whole follicles were collected from patients across the ovulatory period as previously described [23]. Briefly, women (age 30–38 yr) exhibiting regular menstrual cycles and no hormonal contraceptives for at least 3 months prior to their enrollment in the study underwent laparoscopic sterilization.
Women were monitored by transvaginal ultrasound for two to three menstrual cycles before surgery to ascertain cycle regularity and monitor the size of the dominant follicles during the follicular phase. These patients were divided into three groups: pre-, early-, and late ovulatory phases. In the preovulatory group, surgery was performed when the dominant follicle reached more than 14 mm and no more than 17.5 mm in diameter prior to the spontaneous LH surge. These patients were not given hCG. To mimic the endogenous LH surge, the remaining women were administrated with 250 μg of recombinant hCG (Ovitrelle; Merck Serono). These women were divided into two groups: early (surgery between 12 to <18 h post-hCG) and late ovulatory phase (surgery between 18 to 34 h post-hCG). To confirm that these patients followed a normal hormonal pattern before the LH surge or after hCG administration, blood samples were taken at surgery and measured for serum progesterone and estradiol. The patient characteristics including steroid levels are shown in Supplemental Table S1 [7].
The whole intact follicle was removed using laparoscopic scissors, and then placed on ice and brought to the laboratory for dissection. For gene expression analysis, the follicle was bisected with scissors and then the mural granulosa cells were gently scraped off from the interior of the follicle by a small tissue forceps. The follicular fluid and cell suspension were combined and centrifuged at 500 × g to pellet granulosa cells. After removal of the granulosa cells, the theca interna layer was separated from the underlying theca externa layer by gently pulling the layer using watchmaker's forceps. The isolated granulosa cells and theca interna layer were frozen at –70°C and stored until further analysis of mRNA. For immunohistochemical analysis, whole intact follicles (n = 2/pre, n = 4/early, n = 4/late) were processed as described below.
Isolation and culture of human granulosa/lutein cells
Human granulosa/lutein cells were obtained from patients undergoing IVF procedure. The granulosa cell collection protocol was approved by the Institutional Review Board of the University of Kentucky Office of Research Integrity. Ovarian hyperstimulation was induced by the administration of recombinant human follicle-stimulating hormone (FSH) in individualized doses to IVF patients at the Bluegrass Fertility Center (Lexington, Kentucky). IVF patients were administered with hCG on days 9 to 11 after initial FSH administration and dominant follicles were aspirated at 36 h after hCG administration. Human granulosa cell culture experiments were carried out as described previously [7]. Briefly, immediately following retrieval of the cumulus–oocyte complexes, the remaining cells in aspirates were subjected to a Percoll gradient centrifugation (1400 × g for 30 min) to remove red blood cells. The isolated cells were resuspended in OptiMEM media supplemented with 10% fetal bovine serum and antibiotic-antimycotic and plated onto 6, 24, or 96-well plates (2.5 × 105 cells/ml). The cells were cultured at 37°C in 5% CO2 for 6 days, with changing media every 24 h. At the end of the acclimation period, the cells were changed with OptiMEM media without fetal bovine serum. One hour later, the cells were treated with various reagents in the absence or presence of hCG and further cultured for defined hours before collecting the cells or conditioned media for subsequent analyses of transcripts, proteins, and progesterone. We have previously documented that this 6-day acclimation period allows the cells to regain responsiveness to hCG [7]. For instance, hCG increased the levels of mRNA for key ovulatory genes such as AREG, PTGS2, and PGR in our culture model [24]. In addition, the expression of the leukocyte marker CD45 dramatically decreased during the acclimation period [7], indicating that the majority of leukocytes have been removed from our granulosa/lutein cell cultures by changing media every 24 h.
Analysis of gene expression
Total RNA was isolated from follicular cells of human ovaries using a TRIzol reagent and from granulosa cells using an RNeasy mini kit (Qiagen, Inc.) according to the manufacturer's instructions. The synthesis of first-strand cDNA was performed by reverse transcription of 500 ng total RNA using superscript III with Oligo(dt)20 primer. The levels of mRNAs for CXCR4, CXCL12, and PGR were measured by real-time PCR. Real-time PCR was performed using Brilliant 3 Ultra-Fast SYBR green according to the manufacturer's protocol (Stratagene, La Jolla, CA). Oligonucleotide primers corresponding to each gene were designed using Primer3 software and listed in Table 1. The specificity for each primer set was confirmed by both running the PCR products on a 2% agarose gel and analyzing the melting (dissociation) curve using the MxPro real-time PCR analysis program after each PCR reaction. The relative abundance of the target transcript was normalized to the endogenous reference genes, GADPH or RNA18S5, and calculated according to the 2−ΔΔCT method [25].
Table 1.
List of primers used for real-time PCR.
| Gene name | Accession no | Primer sequence, 5'-3' |
|---|---|---|
| CXCL12 | NM_199 168.3 | CACTTTCACTCTCCGTCAGG |
| TTGAGATGCTTGACGTTGGC | ||
| CXCR4 | NM_0 010 08540.1 | ACTGAGAAGCATGACGGACA |
| GATGAAGGCCAGGATGAGGA | ||
| PGR | NM_0 012 02474.3 | ATCAACTAGGCGAGAGGCAA |
| TGCCACATGGTAAGGCATAA | ||
| ADAMTS1 | NM_0 06988.4 | ACGAGTGCGCTACAGATCCT |
| TCCTTGCACACAGACAGAGG | ||
| TNFAIP6 | NM_0 07115.3 | TCACATTTCAGCCACTGCTC |
| AGACCGTGCTTCTCTGTGGT | ||
| CTSV | NM_0 012 01575.1 | TCCGTGAGCCTCTGTTTCTT |
| TTCCGGAACATCTGTCCTTC | ||
| VEGFA | NM_0 010 25366.2 | CTTGCTGCTCTACCTCCACC |
| ATGTTGGACTCCTCAGTGGG | ||
| RNA18S5 | NR_04 6235.1 | GTAACCCGTTGAACCCCATT |
| CCATCCAATCGGTAGTAGGG |
Immunohistochemical and immunocytochemical analyses
Follicles were fixed in 4% formaldehyde, embedded in paraffin, sectioned (4 μm), and processed for immunostaining as previously described [26]. Heat-induced epitope retrieval was performed in a Biocare Medical Decloaking chamber utilizing Dako's low pH Target Retrieval Solution. Primary antibody incubation was carried out at 4°C overnight for CXCL12 (Santa Cruz, sc-28 876, 1:50) and at room temperature for 2 h for CXCR4 (Abcam, ab124824, 1:10). The antibody was detected using an Immpress alkaline phosphatase Anti-Rabbit kit and Vector Red AP chromogen (Vector Laboratories) according to the manufacturer's instructions. Slides were counterstained with hematoxylin. The negative control slides were prepared in the identical manner and processed without primary antibody.
Human granulosa/lutein cells were plated onto poly-L-lysin-coated coverslips (Corning) in 24-well culture dishes and treated without or with hCG for 12 h. The cells were fixed in 4% formaldehyde and permeabilized with 0.3% Triton X-100 in phosphate-buffered saline (PBS) and then blocked with 1% BSA and 5% normal serum before incubating them with the primary PGR antibody (Cell Signaling Technology, #8767, 1:800, ). Next day, the cells were washed with PBS and then incubated with Donkey anti-Rabbit IgG (H+L) secondary antibody, Alexa Fluor 488 conjugate (Invitrogen, 1: 2000 dilution) for 1 h at room temperature and mounted with Fluoroshield containing propidium iodide. The sections were washed with PBS and then incubated with anti-goat biotin-conjugated secondary antibody for 30 min.
Progesterone assay
Concentrations of progesterone in conditioned media were measured using an Immulite kit as described previously [27]. Assay sensitivity for the Immulite was 0.02 ng/ml. The intraassay and interassay coefficients of variation were 7% and 12%, respectively.
Cell viability assay
Cell viability was measured using CellTiter 96 Aqueous One Solution cell proliferation assay (MTS) according to the manufacturer's protocol (Promega, Madison, WI). Briefly, the cells cultured on 96-well plates were treated with or without CXCL12 in the presence or absence of hCG for 24 h. At the end of culture, 20 μl of reagent were pipetted into each well and then further incubated for an additional 2 h. The absorbance was measured at 492 nm in the Infinite F200 plate reader (Tecan USA) to determine the formazan concentration, which is proportional to the number of live cells.
Statistical analyses
All data are presented as means ± SEM. One-way analysis of variance (ANOVA) was used to test differences in levels of mRNA for each gene across time of tissue collection, time of culture, or among treatments in vitro, as appropriate. If ANOVA revealed significant effects, the means were compared by the Duncan test, with P < 0.05 considered significant.
Results
The expression of CXCR4 and CXCL12 mRNA in human preovulatory follicles
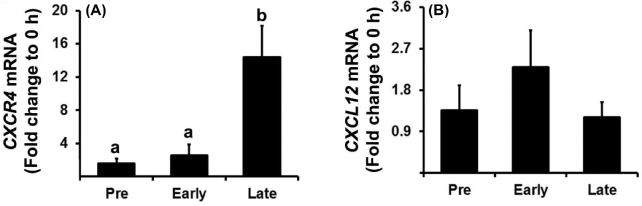
To determine whether the expression of CXCR4 and CXCL12 is regulated in human preovulatory follicles, granulosa and theca cells were isolated from dominant follicles obtained during the pre-, early, and late ovulatory period. The levels of CXCR4 mRNA were significantly increased in granulosa cells during the late ovulatory phase (Figure 1A), whereas levels in theca cells did not change throughout the ovulatory period (data not shown). The levels of CXCL12 mRNA in granulosa cells were constant during the preovulatory period (Figure 1B).
Figure 1.
The expression of CXCR4 and CXCL12 mRNA in human preovulatory follicles. Human ovarian follicles were collected at three preovulatory phases: pre-, early, and late ovulatory phases (mean ± SEM; n = 5–6 women/phase). The levels of CXCR4 (A) and CXCL12 (B) mRNA in granulosa cells were measured using real-time PCR and normalized to the GAPDH in each sample. Bars with no common superscripts are significantly different (P < 0.05).
Localization of CXCR4 and CXCL12 proteins in human preovulatory follicles
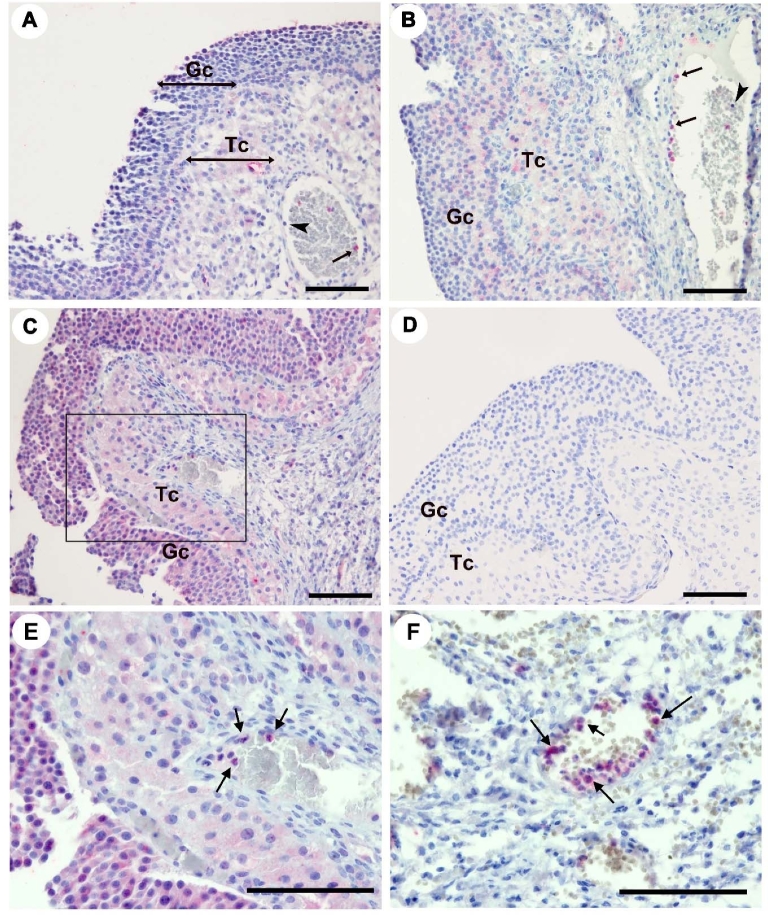
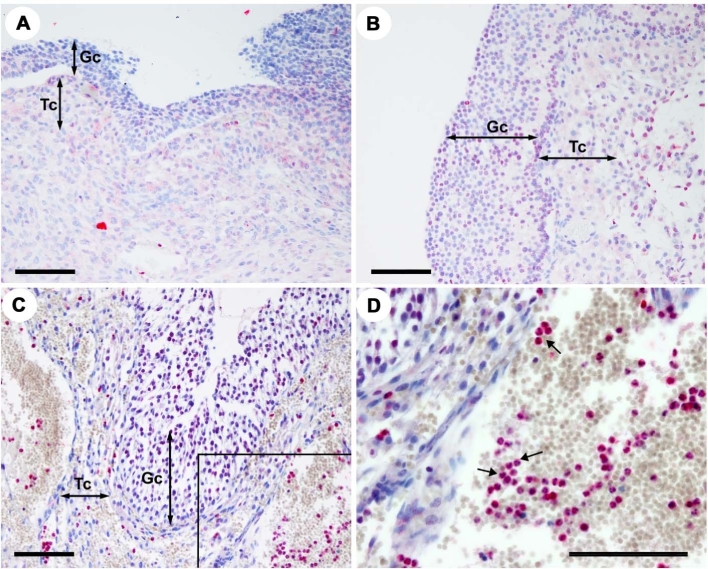
Tissue localization of proteins for CXCR4 and its ligand, CXCL12, was determined in dominant follicles obtained during pre-, early, and late ovulatory period. Immunostaining for CXCR4 protein was localized to granulosa and theca cell compartment of dominant follicles and to leukocytes inside the vasculature (Figure 2). Comparable to the CXCR4 mRNA profile (Figure 1A), staining intensity for CXCR4 protein appeared to increase in granulosa cells of preovulatory follicles obtained during the late ovulatory phase (Figure 2C) compared to that obtained before the LH surge. CXCL12 protein was localized to both granulosa and theca layers of preovulatory follicles of all phases (Figure 3). Of note, intensive staining for CXCL12 and CXCR4 was detected in leukocytes, notably in a subset of leukocytes inside and around the vasculature (Figures 2F and 3D).
Figure 2.
Tissue localization of CXCR4 protein in human preovulatory follicles. Sections of human follicles were obtained at pre- (A), early (B), and late (C, D, E, F) ovulatory phases and immunostained with primary antibodies for CXCR4 (A–C, E, F) or primary antibody omitted as a negative control (D). Panel E was a magnified image of the box in panel C. Panel F showed a subset of leukocytes inside or around the vasculature of ovulatory follicles. Arrows indicate leukocytes expressing CXCR4. Scale bar, 500 μm; Gc, granulosa cell layer; Tc, theca cell layer.
Figure 3.
Tissue localization of CXCL12 protein in human preovulatory follicles. Sections of human follicles were obtained at pre- (A), early (B), and late (C and D) ovulatory phases and immunostained with primary antibodies for CXCL12. Arrows indicate leukocytes expressing CXCL12. Panel D was a magnified image of the box in panel C. Scale bar, 500 μm; Gc, granulosa cell layer; Tc, theca cell layer.
The expression of CXCR4 and CXCL12 in human granulosa cells
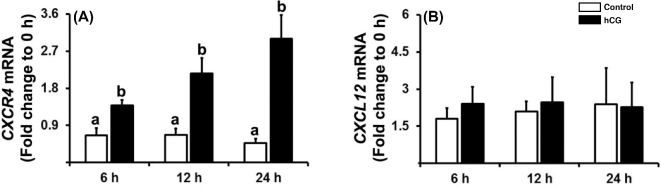
To determine whether the expression patterns of CXCR4 and CXCL12 in human granulosa cells after hCG administration in vivo can be mimicked in vitro, we utilized a primary human granulosa cell culture model. Human CG treatment increased the levels of mRNA for CXCR4 in human granulosa cells compared to control at 12 and 24 h (Figure 4A). In contrast, hCG had no effect on the levels of CXCL12 mRNA in cultured human granulosa cells (Figure 4B).
Figure 4.
The expression of CXCR4 and CXCL12 in human granulosa cell cultures. Human granulosa cells isolated from follicle aspirates of IVF patients were cultured for 6 days and then treated without (Control) or with hCG (1 IU/ml) for indicated hours. The levels of mRNAs for CXCR4 (A) and CXCL12 (B) were measured by real-time PCR and normalized to the RNA18S5 in each sample. Experiments were repeated five times, each with different cell samples. Bars with no common superscripts are significantly different (P < 0.05).
Regulation of CXCR4 mRNA expression in human granulosa cells
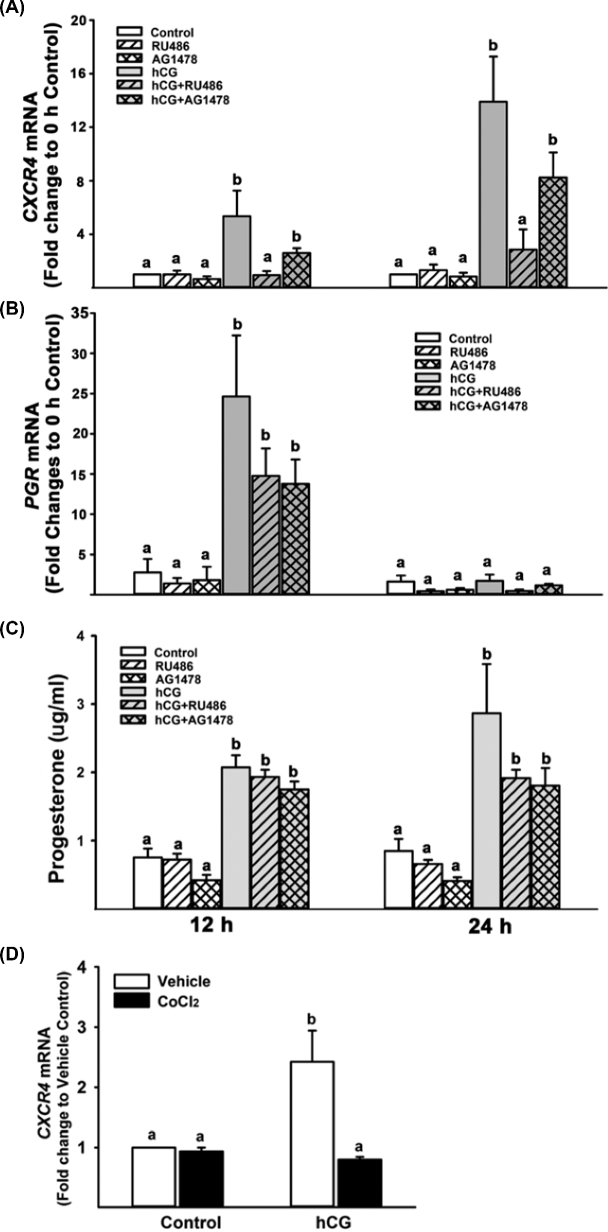
It is well known that progesterone (P4) and progesterone receptor (PGR) play an essential role in ovulation by regulating the expression of specific genes in ovulatory follicles (reviewed in [28]). Previous studies have shown that P4/PGR plays a role in the upregulation of Cxcr4 mRNA expression in granulosa cells of rats and cows [8, 29]. To verify that our cultured cells increase P4 production and PGR expression in response to hCG treatment, we measured the concentration of P4 in culture media and the levels of mRNA for PGR. As shown in Figure 5, hCG increased the level of mRNA for PGR. Consistent with the mRNA data, strong staining for PGR protein was localized to the nucleus of human granulosa cells treated with hCG. The levels of P4 were also increased by hCG (Figure 6C). Next, we have tested whether P4/PGR is involved in the hCG-induced increase in CXCR4 expression by treating human granulosa cells with RU486 (PGR antagonist, 10 μM) in the absence or presence of hCG (1 IU/ml) for 12 or 24 h. The dosage of RU486 was chosen based on P4 levels in these cells (∼3–9 μM). As expected, hCG increased the levels of CXCR4 mRNA (Figure 6A) and protein (Supplemental Figure S1) at both 12 and 24 h, but the stimulatory effect of hCG was completely inhibited by RU486 (Figure 6A).
Figure 5.
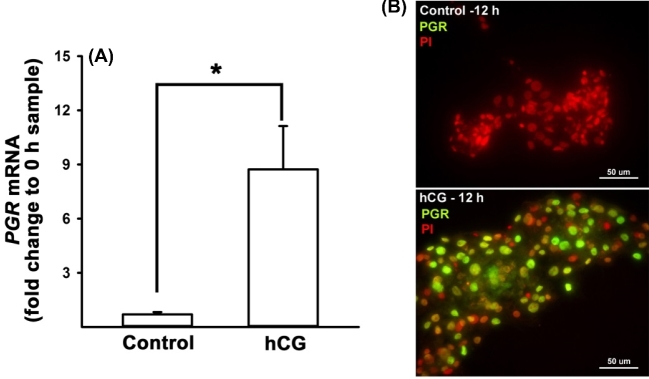
Regulation of PGR expression in human granulosa cells. Cultured human granulosa cells were treated with or without hCG (1 IU/ml) for 12 h. (A) Real-time PCR analyses were performed to measure the levels of PGR mRNA and their levels were normalized to the RNA18S5 in each sample. (B) Immunocytochemistry analysis showed strong positive staining for PGR protein (green/yellow) in the nucleus of granulosa cells stimulated with hCG. Propidium iodide (red) was used to stain the nucleus of cells. Experiments were repeated four times, each with different cell samples. Bars with no common superscripts are significantly different (P < 0.05).
Figure 6.
Effects of inhibitors on the level of mRNA for CXCR4 and PGR and progesterone production in human granulosa cells. Cultured human granulosa cells were treated with or without hCG (1 IU/ml) in the absence or presence of inhibitors or activator (RU486; 10 μM, AG1478; 5 μM and CoCl2; 100 μM) and cultured for 12 or 24 h. Real-time PCR analyses were performed to measure the levels of mRNA for CXCR4 (A) and PGR (B), and their levels were normalized to the RNA18S5 in each sample. The concentration of progesterone (C) was measured in the conditioned media of human granulosa cell cultures. Experiments were repeated three to four times, each with different cell samples. Bars with no common superscripts in each time point are significantly different (P < 0.05).
A previous study showed that EGF signaling is involved in the forskolin-induced increase CXCR4 mRNA levels in bovine granulosa cells [8]. Moreover, the expression of EGF-like factors (e.g. AREG and EREG) was induced by hCG in human granulosa cell cultures, suggesting that EGF signaling is functional [24]. To test whether the expression of CXCR4 is regulated by the activation of EGF signaling, the cultured cells were treated with AG1478 (5 μM, an inhibitor of EGF receptor activation) in the absence or presence of hCG. As shown in Figure 6A, AG1478 had no effect on hCG-stimulated CXCR4 expression.
We also determined whether blocking PGR by RU486 and EGFR by AG1478 affects P4 production and PGR expression in our granulosa culture model. As expected, hCG stimulated P4 production and PGR expression, but these inhibitors at the dosage used had no effect on hCG-induced increases in P4 and PGR expression (Figure 6B and C).
Recent studies have shown that the expression of Cxcr4 mRNA is regulated by hypoxia-inducible factors (HIFs) in murine and bovine granulosa cells [8, 11]. We determined whether HIFs affect the expression of CXCR4 by treating with CoCl2, an HIF activator, in the absence or presence of hCG in human granulosa cells. The hCG-induced increase in CXCR4 mRNA levels was completely blocked by CoCl2 in human granulosa cells (Figure 6D).
Effects of CXCL12 and AMD3100 on gene expression in human granulosa cells
A previous study reported that CXCL12 reduced the early apoptotic but not late apoptotic process in human granulosa/lutein cells in vitro [20]. To determine whether CXCL12 has a similar effect on cell survival, cultured human granulosa cells were treated with CXCL12 in the absence or presence of hCG for 24 h. Human CG increased cell viability, whereas CXCL12 treatment had no effect on basal as well hCG-induced cell viability (Figure 7A). In bovine granulosa cell cultures, AMD3100 (an inhibitor of CXCR4) has been shown to completely block forskolin-stimulated increases in ovulatory gene expression including PGR, ADAMTS1, TNFAIP6, VEGFA, CTSV, MMP1, and MMP9 [8]. To determine whether the expression of these genes is regulated by the CXCL12/CXCR4-mediated signaling in humans, cultured granulosa cells were treated with or without hCG in the presence of CXCL12 or AMD3100 for 24 h. Real-time PCR data revealed that neither CXCL12 nor AMD3100 had an effect on the levels of mRNA for PGR, CTSV, ADAMTS, TNFIAP5, and VEGFA (Supplemental Figure S2). The only gene which was affected by CXCL12 was CXCR4 (Figure 7B).
Figure 7.
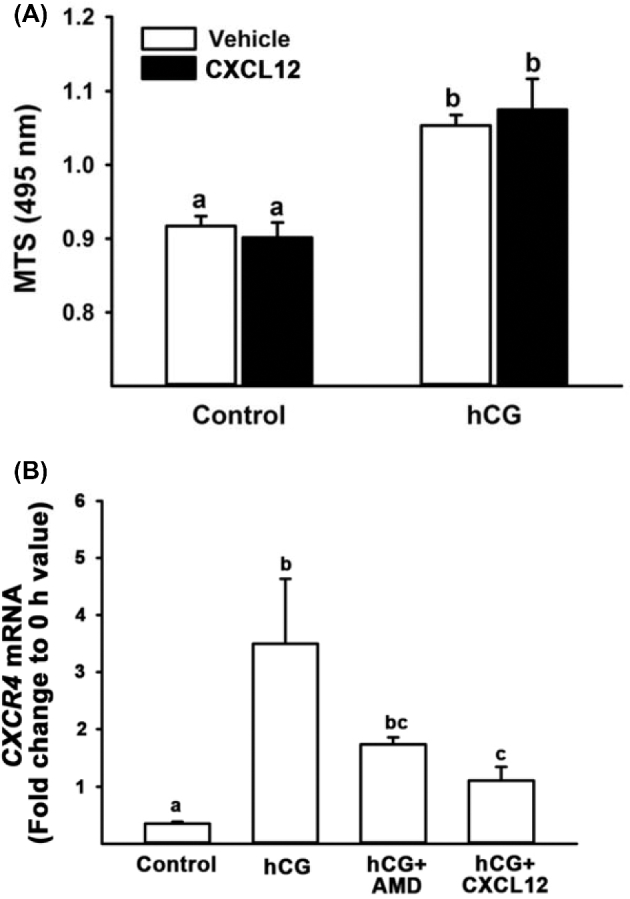
Effects of CXCL12 and AMD3100 in human granulosa cells. (A) Human granulosa cells were treated with CXCL12 (250 ng/ml) in the absence or presence of hCG (1 IU/ml) and cultured for 24 h. At the end of culture, the cells were subjected to MTS assay which measures cell viability. (B) Human granulosa cells were treated with CXCL12 (250 ng/ml) or AMD3100 (50 nM) in the absence or presence of hCG (1 IU/ml). Real-time PCR analyses were performed to measure the levels of CXCR4 (A and B) mRNAs, and their levels were normalized to the RNA18S5 in each sample. Experiments were repeated four times, each with different cell samples. Bars with no common superscripts are significantly different (P < 0.05).
Discussion
This is the first study to report the spatiotemporal-specific induction of CXCR4 expression in human preovulatory follicles. The expression of CXCR4 is upregulated by hCG administration in granulosa cells of late ovulatory follicles. Meanwhile, its cognate ligand, CXCL12, is constitutively expressed in dominant follicles during the preovulatory period. In addition, both CXCL12 and CXCR4 proteins are localized to a subset of leukocytes inside or nearby the vasculature of ovulatory follicles. These data documented the presence of CXCL12/CXCR4 system in both follicular cells and infiltrating leukocytes in human ovulatory follicles, suggesting that this system is involved in the ovulatory process via acting on follicular cells as well as leukocytes in humans. Using a human granulosa cell culture model, we have also demonstrated that hCG increased the expression of CXCR4, but not CXCL12, mimicking the in vivo upregulation of CXCR4 expression following hCG administration in granulosa cells of ovulatory follicles. Further in vitro studies revealed a progesterone/PGR-specific regulation of CXCR4 expression in human granulosa cells.
This study of CXCR4 and CXCL12 expression in the human ovary revealed a species-specific expression of these genes. For instance, in cattle and horse, CXCR4 expression increased in both granulosa and theca cells of preovulatory follicles after hCG administration [8]. However, in this study, the hCG-stimulated increase in CXCR4 expression was observed in granulosa cells, but not in the theca layer of human ovulatory follicles. In the mouse ovary, the level of Cxcr4 mRNA was increased in both cumulus and granulosa cells after hCG injection [12]. But, CXCR4 expression was much higher (∼20-fold) in granulosa cells compared to that in cumulus cells isolated from IVF patients (microarray data [30]), indicating the predominant expression of CXCR4 in granulosa cells of human preovulatory follicles. Meanwhile, in nonhuman primates, granulosa cell levels of CXCR4 mRNA were not changed throughout the preovulatory period, whereas the levels of CXCL12 mRNA were slightly decreased after hCG injection (microarray data, Supplemental Figure S3 [31]). In horses, CXCL12 expression was predominant in the theca layer of follicles [8]. In the human ovary, we found that the expression of CXCL12 is ubiquitous in both granulosa and theca cells throughout the preovulatory period (summarized in Supplemental Table S2). Nonetheless, these studies all point to the upregulation of CXCR4 expression by hCG, except in monkeys and the presence of CXCL12 in ovulatory follicles, indicating that the CXCL12/CXCR4 axis is stimulated by the upregulation of CXCR4 expression and constitutively expressing CXCL12 in ovulatory follicular cells [8, 12, 30, 31]. Along with follicular cells, this study also documented the positive staining for CXCR4 and CXCL12 in a subset of leukocytes inside or around the vasculature of ovulatory follicles in the human ovary, indicating the presence and possible function of the CXCL12/CXCR4 axis in a subpopulation of leukocytes. These findings also suggest that CXCL12 from leukocytes can act on follicular cells to enhance CXCR4 signaling in granulosa and theca cells of ovulatory follicles, while CXCL12 from follicular cells acts on leukocytes to facilitate infiltration into the ovulatory follicle.
Since CXCR4 expression was increased in human granulosa cells of preovulatory follicles following hCG administration in vivo, the next logical step was to determine whether hCG can increase the expression of CXCR4 in granulosa cells in vitro. Indeed, we found that the levels of both mRNA and protein for CXCR4 were increased by hCG treatment in cultured cells, indicating that hCG-initiated signaling pathways are involved in the upregulation of CXCR4 expression in granulosa cells of human preovulatory follicles. These data are in agreement with previous findings in other species; hCG or forskolin (an activator of adenylate cyclase) increased the levels of mRNA for Cxcr4 in rodent as well as bovine granulosa cell cultures [8, 11, 29], respectively. Among the LH-induced mediators in preovulatory granulosa cells, the PGR is known to play a critical role in ovulation by regulating the expression of specific ovulatory genes [28]. Particularly, previous studies have shown that treatment with RU486, an antagonist of PGR, reduced the hCG- or forskolin-induced increase in the level of Cxcr4 mRNA in rat and bovine granulosa cell cultures [8, 29]. In this study, we first confirmed that cultured human granulosa cells increased the production of progesterone and PGR expression in response to hCG stimulation. Based on these findings, we further demonstrated that antagonizing PGR with RU486 completely blocked the expression of CXCR4, suggesting that PGR mediates the hCG-induced increase in CXCR4 expression in granulosa cells of preovulatory follicles in humans.
In addition to PGR, a growing body of evidence indicates that the activation of EGF-mediated signaling pathways plays an important role in ovulation and is involved in regulating the expression of ovulatory genes in the ovary [32]. In a previous study using bovine granulosa cells in vitro, Sayasith and Sirois [8] showed that treatment with EGF increased the levels of CXCR4 mRNA, while the EGFR inhibitor PD153035 reduced forskolin-induced CXCR4 expression, indicating the involvement of EGF-mediated signaling in the upregulation of CXCR4 expression. More recently, we reported the increase in the levels of mRNA for AREG and EREG (EGF-like peptides) by hCG in our human granulosa cell cultures [24], indicating that the EGF-mediated signaling pathway is active and may be involved in ovulatory gene expression in human preovulatory granulosa cells. In contrast to the data from bovine granulosa cells, we found that AG1478 (an inhibitor of EGFR) had no effect on hCG-stimulated increases in CXCR4 expression in human granulosa cell cultures, suggesting that the upregulation of CXCR4 expression is independent on EGF signaling in human granulosa cells.
A number of studies have shown that hypoxia, though the action of HIF-1a, induces CXCR4 expression in various cell types [33]. Moreover, a recent study in mice showed that HIFs, mainly Hif-1a, Hif-2a, and Hif-1b, play critical roles in ovulation by regulating the expression of ovulatory genes [11]. In that study, injection of echinomycin (an inhibitor of HIF-1a) blocked ovulation and reduced the levels of Cxcr4 mRNA. In support of the concept that Hifs regulate the expression of Cxcr4, treatment of mouse and bovine granulosa cells with CoCl2 (an inducer of HIF-1) increased the levels of Cxcr4 mRNA [8, 11]. In contrast, we found that CoCl2 treatment had no effect on the basal expression of CXCR4, yet completely abolished the hCG-stimulated increase in the levels of CXCR4 mRNA, suggesting that HIF-1 may act as a negative regulator of CXCR4 expression in cultured human granulosa cells, and the exact mechanism of HIFs’ action remains to be further determined.
To date, there are two reports on the function of CXCL12/CXCR4 in granulosa cells of ovulatory follicles. Kryczek et al. [20] showed that T lymphocytes from follicular aspirates of IVF patients migrate toward CXCL12 in transwell migration assays, indicating that the CXCL12 contributes to the migration of T lymphocytes into human ovulatory follicles. Furthermore, CXCL12 treatment reduced the number of early apoptotic cells [20]. In contrast, we found that CXCL12 has no effect on both basal and hCG-induced increases in cell viability in our culture model. This discrepancy could be due to the difference in culture models and the presence of leukocytes in the cell preparation used in the previous study. For instance, the previous study used the cells immediately after isolation, and the anti-apoptotic effect of CXCL12 was significantly diminished when the cells from follicular aspirates were pre-incubated for 24 h, and then the nonadherent cells were removed. Based on the observation that the adherent cells were largely granulosa cells and contained less than 1% lymphocytes, Kryczek et al. [20] suggested that the presence of lymphocytes is required for the anti-apoptotic effect of CXCL12. Similarly, in our culture model, the cells were pre-incubated for 6 days before treatment and the majority of leukocytes were also removed from our granulosa/lutein cell cultures by changing media every 24 h for 6 days. Therefore, we concluded that hCG increased granulosa cell viability, but this increase was not mediated by CXCL12/CXCR4-induced downstream signaling in cultured human granulosa cells.
Second, in bovine granulosa cells, AMD3100 (a blocker of CXCR4) inhibited forskolin-induced increases in the level of transcripts for several ovulatory genes, such as Pgr, Adamts1, and Vegfa [8]. However, in human granulosa cell cultures, we found that AMD3100 treatment had no effect on the levels of transcripts for most of the genes regulated in bovine granulosa cells (Supplemental Figure S2). To make sure that the lack of effect by AMD3100 was not due to the low levels of CXCL12, we treated the cells with CXCL12 but found no changes in any of those genes. Interestingly, the only gene whose level of the transcript was reduced by CXCL12 was its receptor CXCR4. These data, taken together, indicated that CXCL12/CXCR4 might exert different functions in granulosa cells of different species, and the exact function of CXCL12/CXCR4 in human granulosa cells needs to be determined.
In conclusion, this study demonstrated the hCG-dependent, progesterone/PGR-regulated increase in CXCR4 in granulosa cells of human ovulatory follicles. Considering the evidence that both CXCL12 and CXCR4 are expressed in follicular cells of ovulatory follicles and a subset of leukocytes inside or around the vasculature of ovulatory follicles, these data implicate the role(s) of the CXCL12/CXCR4 system in follicular cells as well as leukocytes during the ovulatory process in the human ovary.
Supplementary data
Supplementary data are available at BIOLRE online.
Supplemental Figure S1. Effect of RU486 on CXCR4 expression in human granulosa cell cultures. Human granulosa cells isolated from follicle aspirates of IVF patients were cultured for 6 to 7 days and then treated without (control, Ct) or with hCG (1 IU/ml) in the presence of RU486 for indicated hours. CXCR4 protein was detected by western blot analyses. Each lane was loaded with 40 μg of whole‐cell extracts isolated from cultured human granulosa cells. The membrane was re‐probed with a monoclonal antibody against β‐actin to assess the loading of protein in each lane. Band intensity was calculated by dividing the semiquantitative intensity value of CXCR4 by that of ACTB in each lane. Experiments were repeated with two different samples.
Supplemental Figure S2. Effects of CXCL12 or AMD3100 in human granulosa cells. Human granulosa cells were treated with CXCL12 (250 ng/ml) or AMD3100 (50 nM) in the absence or presence of hCG (1 IU/ml) for 24 h. Real‐time PCR analyses were performed to measure the levels of PGR, ADAMTS1,TNFAIP6, CTSV, and VEGFA mRNAs, and their levels were normalized to the RNA18S5 in each sample. Experiments were repeated three or four times, each with different cell samples. Bars with no common superscripts are significantly different (mean ± SEM; P < 0.05).
Supplemental Figure S3. The expression profile of CXCR4 and CXCL12 mRNA in primate ovulatory follicles. Microarray data were extracted from NIH GEO (Submission number: GSE22776) submitted by Xu et al. [31]. Dominant follicles collected before (0 h) or indicated time points after rhCG administration in rhesus monkeys were used for microarray analyses.
Supplemental Table S1. Characteristics for follicle size, time of surgery in relation to hCG, preoperative hormone values of peripheral blood (serum), menstrual cycle day of surgery, menstrual cycle length, and age of the study population. All data are given as means ± SEM.
Supplemental Table S2. Species-specific expression of CXCR4 and CXCL12.
Supplementary data are available at BIOLRE online.
Acknowledgments
We thank Dr Patrick Hannon for critical reading of the manuscript. We also thank Ms Georgia Bryant for technical help with steroid assays.
Conflict of Interest: The authors have declared that no conflict of interest exists.
References
- 1. Curry TE Jr, Osteen KG. The matrix metalloproteinase system: changes, regulation, and impact throughout the ovarian and uterine reproductive cycle. Endocr Rev 2003; 24:428–465. [DOI] [PubMed] [Google Scholar]
- 2. Oakley OR, Frazer ML, Ko C. Pituitary-ovary-spleen axis in ovulation. Trends Endocrinol Metab 2011; 22:345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stouffer RL, Xu F, Duffy DM. Molecular control of ovulation and luteinization in the primate follicle. Front Biosci 2007; 12:297–307. [DOI] [PubMed] [Google Scholar]
- 4. Brannstrom M, Enskog A. Leukocyte networks and ovulation. J Reprod Immunol 2002; 57:47–60. [DOI] [PubMed] [Google Scholar]
- 5. Oakley OR, Kim H, El-Amouri I, Lin PC, Cho J, Bani-Ahmad M, Ko C. Periovulatory leukocyte infiltration in the rat ovary. Endocrinology 2010; 151:4551–4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang LJ, Brannstrom M, Pascoe V, Norman RJ. Cellular composition of primary cultures of human granulosa-lutein cells and the effect of cytokines on cell proliferation. Reprod Fertil Dev 1995; 7:21–26. [DOI] [PubMed] [Google Scholar]
- 7. Al-Alem L, Puttabyatappa M, Rosewell K, Brannstrom M, Akin J, Boldt J, Muse K, Curry TE Jr.. Chemokine ligand 20: a signal for leukocyte recruitment during human ovulation? Endocrinology 2015; 156:3358–3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sayasith K, Sirois J. Expression and regulation of stromal cell-derived factor-1 (SDF1) and chemokine CXC motif receptor 4 (CXCR4) in equine and bovine preovulatory follicles. Mol Cell Endocrinol 2014; 391:10–21. [DOI] [PubMed] [Google Scholar]
- 9. Sun X, Cheng G, Hao M, Zheng J, Zhou X, Zhang J, Taichman RS, Pienta KJ, Wang J. CXCL12 / CXCR4 / CXCR7 chemokine axis and cancer progression. Cancer Metastasis Rev 2010; 29:709–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Griffith JW, Sokol CL, Luster AD. Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu Rev Immunol 2014; 32:659–702. [DOI] [PubMed] [Google Scholar]
- 11. Kim J, Bagchi IC, Bagchi MK. Signaling by hypoxia-inducible factors is critical for ovulation in mice. Endocrinology 2009; 150:3392–3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hernandez-Gonzalez I, Gonzalez-Robayna I, Shimada M, Wayne CM, Ochsner SA, White L, Richards JS. Gene expression profiles of cumulus cell oocyte complexes during ovulation reveal cumulus cells express neuronal and immune-related genes: does this expand their role in the ovulation process? Mol Endocrinol 2006; 20:1300–1321. [DOI] [PubMed] [Google Scholar]
- 13. Segers I, Adriaenssens T, Wathlet S, Smitz J. Gene expression differences induced by equimolar low doses of LH or hCG in combination with FSH in cultured mouse antral follicles. J Endocrinol 2012; 215:269–280. [DOI] [PubMed] [Google Scholar]
- 14. Bernhagen J, Krohn R, Lue H, Gregory JL, Zernecke A, Koenen RR, Dewor M, Georgiev I, Schober A, Leng L, Kooistra T, Fingerle-Rowson G et al. . MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment. Nat Med 2007; 13:587–596. [DOI] [PubMed] [Google Scholar]
- 15. Saini V, Staren DM, Ziarek JJ, Nashaat ZN, Campbell EM, Volkman BF, Marchese A, Majetschak M. The CXC chemokine receptor 4 ligands ubiquitin and stromal cell-derived factor-1alpha function through distinct receptor interactions. J Biol Chem 2011; 286:33466–33477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wada S, Fujimoto S, Mizue Y, Nishihira J. Macrophage migration inhibitory factor in the human ovary: presence in the follicular fluids and production by granulosa cells. Biochem Mol Biol Int 1997; 41:805–814. [DOI] [PubMed] [Google Scholar]
- 17. Wada S, Kudo T, Kudo M, Sakuragi N, Hareyama H, Nishihira J, Fujimoto S. Induction of macrophage migration inhibitory factor in human ovary by human chorionic gonadotrophin. Hum Reprod 1999; 14:395–399. [DOI] [PubMed] [Google Scholar]
- 18. Nagasawa T. CXC chemokine ligand 12 (CXCL12) and its receptor CXCR4. J Mol Med 2014; 92:433–439. [DOI] [PubMed] [Google Scholar]
- 19. Juarez J, Bendall L, Bradstock K. Chemokines and their receptors as therapeutic targets: the role of the SDF-1/CXCR4 axis. Curr Pharm Des 2004; 10:1245–1259. [DOI] [PubMed] [Google Scholar]
- 20. Kryczek I, Frydman N, Gaudin F, Krzysiek R, Fanchin R, Emilie D, Chouaib S, Zou W, Machelon V. The chemokine SDF-1/CXCL12 contributes to T lymphocyte recruitment in human pre-ovulatory follicles and coordinates with lymphocytes to increase granulosa cell survival and embryo quality. Am J Reprod Immunol 2005; 54:270–283. [DOI] [PubMed] [Google Scholar]
- 21. Nishigaki A, Okada H, Okamoto R, Shimoi K, Miyashiro H, Yasuda K, Kanzaki H. The concentration of human follicular fluid stromal cell-derived factor-1 is correlated with luteinization in follicles. Gynecol Endocrinol 2013; 29:230–234. [DOI] [PubMed] [Google Scholar]
- 22. Borgbo T, Povlsen BB, Andersen CY, Borup R, Humaidan P, Grondahl ML. Comparison of gene expression profiles in granulosa and cumulus cells after ovulation induction with either human chorionic gonadotropin or a gonadotropin-releasing hormone agonist trigger. Fertil Steril 2013; 100:994–1001. [DOI] [PubMed] [Google Scholar]
- 23. Thoroddsen A, Dahm-Kahler P, Lind AK, Weijdegard B, Lindenthal B, Muller J, Brannstrom M. The water permeability channels aquaporins 1–4 are differentially expressed in granulosa and theca cells of the preovulatory follicle during precise stages of human ovulation. J Clin Endocrinol Metab 2011; 96:1021–1028. [DOI] [PubMed] [Google Scholar]
- 24. Choi Y, Wilson K, Hannon PR, Rosewell KL, Brannstrom M, Akin JW, Curry TE Jr, Jo M. Coordinated regulation among progesterone, prostaglandins, and EGF-like factors in human ovulatory follicles. J Clin Endocrinol Metab 2017March09 doi: 10.1210/jc.2016-3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001; 25:402–408. [DOI] [PubMed] [Google Scholar]
- 26. Rosewell KL, Li F, Puttabyatappa M, Akin JW, Brannstrom M, Curry TE Jr.. Ovarian expression, localization, and function of tissue inhibitor of metalloproteinase 3 (TIMP3) during the periovulatory period of the human menstrual cycle. Biol Reprod 2013; 89:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Park ES, Choi S, Muse KN, Curry TE Jr, Jo M. Response gene to complement 32 expression is induced by the luteinizing hormone (LH) surge and regulated by LH-induced mediators in the rodent ovary. Endocrinology 2008; 149:3025–3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Robker RL, Akison LK, Russell DL. Control of oocyte release by progesterone receptor-regulated gene expression. Nucl Recept Signal 2009; 7:e012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mishra B, Park JY, Wilson K, Jo M. X-linked lymphocyte regulated gene 5c-like (Xlr5c-like) is a novel target of progesterone action in granulosa cells of periovulatory rat ovaries. Mol Cell Endocrinol 2015; 412:226–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Grondahl ML, Andersen CY, Bogstad J, Borgbo T, Boujida VH, Borup R. Specific genes are selectively expressed between cumulus and granulosa cells from individual human pre-ovulatory follicles. Mol Hum Reprod 2012; 18:572–584. [DOI] [PubMed] [Google Scholar]
- 31. Xu F, Stouffer RL, Muller J, Hennebold JD, Wright JW, Bahar A, Leder G, Peters M, Thorne M, Sims M, Wintermantel T, Lindenthal B. Dynamics of the transcriptome in the primate ovulatory follicle. Mol Hum Reprod 2011; 17:152–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Conti M, Hsieh M, Park JY, Su YQ. Role of the EGF network in ovarian follicles. Mol Endocrinol 2005; 20:715–723. [DOI] [PubMed] [Google Scholar]
- 33. Wang X, Li C, Chen Y, Hao Y, Zhou W, Chen C, Yu Z. Hypoxia enhances CXCR4 expression favoring microglia migration via HIF-1alpha activation. Biochem Biophys Res Commun 2008; 371:283–288. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data are available at BIOLRE online.