Abstract
Prostate cancer remains a significant cause of cancer-related deaths in men in the United States. Significant advances in the treatment of metastatic castration-resistant prostate cancer have been made in recent years with the arrival of new therapeutic targets and options. The definition of progression of disease must be thought of in the context of clinical symptoms and radiographic evidence rather than as changes in prostate-specific antigen (PSA). Ultimately, the use of PSA criteria alone should not be used to determine the progression of disease; instead, PSA should be evaluated in combination with other clinical data.
Keywords: prostate-specific antigen, androgen receptor, metastatic castration resistant, PSAWG1, PSAWG2
Prostate cancer is the second-leading cause of cancer-related deaths in men in the United States, with an estimated 233,000 new cases diagnosed and 29,490 deaths in 2014.1 In some subgroups of patients, early-stage prostate cancer can be cured with initial definitive therapy modalities, with survival rates of more than 90% at 15 years.2 Primary androgen ablation is the mainstay of treatment in metastatic disease; however, tumors that are sensitive to androgen blockade eventually progress to become castration resistant.3,4
Castration-resistant prostate cancer (CRPC) occurs when patients’ disease progresses despite castrate levels of testosterone (<50 ng/dL). For many years, cytotoxic chemotherapy with docetaxel was the standard treatment for metastatic castration-resistant prostate cancer (mCRPC) in patients with symptomatic or rapidly progressing disease. New developments in treatment have occurred in the last several years, as modern agents have permeated the landscape.
Prostate-Specific Antigen
Prostate-specific antigen (PSA) is a protein, the transcription of which is positively regulated by the androgen receptor (AR).5 PSA may be elevated for various reasons, including infection of the prostate gland (prostatitis), benign prostatic hypertrophy, inflammation, or prostate cancer.6 In 1979, Wang et al published data suggesting the potential significance of PSA in the serum as a marker in the detection of prostate cancer.7 In the 1980s, further investigation through the National Cancer Institute–sponsored National Prostatic Cancer Project detected the prognostic value of PSA.8 PSA as a tumor marker to monitor men with prostate cancer was approved by the US Food and Drug Administration (FDA) in 1986. PSA can be used to detect biochemical recurrence, which is defined as increasing PSA in men who have undergone definitive treatment for prostate cancer.9 It is important to consider that PSA values may be affected by treatments such as 5-α reductase inhibitors, which can decrease PSA secretion from benign and malignant tissue, leading to a reduction in serum PSA levels without an effect on cell growth.6,10 In addition, in patients who receive radiation therapy for the treatment of prostate cancer, a phenomenon known as PSA bounce has been documented whereby temporary increases in PSA are seen as the result of a possible delayed radiation effect or bacterial prostatitis, which do not correlate with tumor growth.11 Understanding the role of PSA is an important consideration in the treatment of men with mCPRC.
PSA as a Means to Monitor Response to Therapy in mCRPC
The role of PSA in evaluating patients with mCRPC has been clarified. Many early trials in prostate cancer used PSA criteria to define progression, thus allowing discontinuation of treatment based on an increasing PSA. In the landmark phase III TAX 327 study, docetaxel given every 3 weeks or weekly plus prednisone was compared with mitoxantrone plus prednisone in men with mCRPC.12 The median survival was 16.5 months in the mitoxantrone group compared with 17.4 months in the weekly docetaxel group and 18.9 months in the every-3-weeks docetaxel group. PSA was measured every 3 weeks and progression was defined as an increase from the nadir of at least 25% in men who had no PSA response or at least 50% in all other men. Interestingly, the PSA response rate was highest in the weekly docetaxel cohort; however, overall survival was not extended in this group. In an analysis of the study, 80 patients who had an increasing PSA, which did not fulfill the criteria of progressive disease, achieved a PSA response at a later time.13 Twenty-three patients were identified who initially fulfilled the criteria PSA progression but later experienced a decrease in serum PSA of at least 50% when compared with baseline. These findings suggest that patients with PSA progression may have benefited from additional treatment.
An increase in PSA after treatment with docetaxel in patients with CRPC has been identified as a PSA flare phenomenon and can affect treatment courses. In a retrospective study of 44 patients who were treated with docetaxel-based regimens, reversible PSA surges were seen in 8 patients.14 In that study, the flare phenomenon was defined as a PSA increase after initiation of docetaxel-based therapy, followed by a decrease to values below baseline (a decrease of ≤50% of the maximal PSA value).14 PSA values rose to a maximum of 107% to 180% between weeks 1 and 7 of treatment and then decreased by 21% to 67% of baseline values in weeks 7 to 14. In this group of patients, the PSA flare did not negatively affect overall survival. In the absence of signs of clinical progression, PSA alone is not a reliable marker for progression of disease. Findings such as these affected the design of clinical trials in defining progression of disease and discontinuation of therapy.
As the landscape for treatment in mCRPC continues to evolve, it is important to realize that patients will benefit from multiple individual therapies sequentially, and that PSA should not be used as a marker to discontinue therapy in the absence of other criteria. Of note, in no other oncologic disease state is a marker such as serum PSA used alone to discontinue therapy.
In a phase II trial of bevacizumab, thalidomide, docetaxel, and prednisone in mCRPC, Ning et al initially determined disease progression by PSA criteria, tumor progression as determined by Response Evaluation Criteria in Solid Tumors (RECIST), or clinical status.15 After the enrollment of 22 patients, the protocol was amended to eliminate the use of PSA criteria alone to determine study discontinuation. It was found that changes in clinical status were not reflected by the PSA criteria. Patients then continued in the study until clinical progression or radiographic progression was determined. Twelve patients had been discontinued from the trial based on PSA criteria alone, which were based on the Prostate-Specific Antigen Working Group (PCWG1) criteria. The estimated time to progression was 18.3 months, including 12 patients who had been taken off the trial. This study demonstrates the limitations of using PSA responses to determine progression and prematurely removing patients from studies who can benefit from further therapy.
In addition, Dahut et al conducted a phase II clinical trial of sorafenib in patients with CRPC. This was an open-label, single-arm study that enrolled 22 patients in the first stage with progressive CRPC who had been treated with no more than one previous cytotoxic chemotherapy regimen.16 Sorafenib is an oral multikinase inhibitor that exerts an antiangiogenic effect. Patients received 400 mg of sorafenib twice daily on a 28-day cycle. Response and progression were evaluated using RECIST. Progression was defined on bone scan as the appearance of a new lesion. The first radiographic assessment was done within 1 month of enrollment and then every 2 cycles. The median progression-free survival was 1.8 months. Two patients in the study who had an increasing PSA while taking sorafenib had improvement in metastatic lesions on bone scan.
PCWG1
In 1999, the PCWG1 developed recommendations for the conduct of clinical trials, which included standards for the use of PSA.17 Through this consensus conference, eligibility and response guidelines were published for use in phase II clinical trials in castration-resistant patients.17 At that time, four patient groups were defined: progressive measurable disease, progressive bone metastasis, stable metastases and increasing PSA, and increasing PSA with no other evidence of metastatic disease. PSA progression was defined as a 25% increase over baseline and an increase in the absolute PSA level by at least 5 ng/mL, which is confirmed by a second value, obtained a minimum of 1 week from the reference value. For patients who did not experience a decrease in PSA, progressive disease was defined at the time when the PSA increased 25% over the nadir, confirmed by a second value, when the increase was a minimum of 5 ng/mL.
In 2000, researchers introduced the New Guidelines to Evaluate the Response to Treatment in Solid Tumors.18 Measurable lesions (nodal and visceral) are not required for entry into a trial, but if present, RECIST criteria should be used to record the lesions as target or nontarget. To be considered a target lesion, the diameter of a lymph node should be at least 2 cm as measured on computed tomography (CT) imaging.19,20 Imaging plays a crucial role in the authentication of disease progression in cancer, particularly in prostate cancer.
In mCRPC, bone metastases can occur in upwards of 90% of patients.21 Bone scan is used to assess metastases and changes in bone; however, it remains difficult to objectively demonstrate the response of the disease to therapy on this imaging modality.22 Bone scan is limited by low specificity because benign pathologies, such as degenerative disease, which is prevalent in men with mCRPC, may resemble metastatic disease.23 Early skeletal metastases and low-volume disease can remain undetected. In addition, for bone scan, there are no validated criteria for response to treatment. After starting systemic therapy, there may be an increase in isotope uptake by metastatic lesions or the appearance of new lesions that were previously unseen.23–26 This is referred to as bone scan flare phenomenon and can manifest itself as a worsening bone scan on the first follow-up. The bone scan flare phenomenon can be seen up to 3 months after beginning systemic therapy and can be seen when there is an osteoblastic healing response in a skeletal metastasis that causes an increase in the uptake of bone-specific radiotracers.23 As such, the Prostate Cancer Clinical Trials Working Group (PCWG2) recommends performing a follow-up bone scan at least 12 weeks after treatment has begun, as long as the patient is clinically stable. Progressive disease on bone scan is defined as when two or more new lesions are identified. PCWG2 recommends confirming progression with a second scan performed at least 6 weeks later, as long as the patient is clinically stable and there is no worsening of soft-tissue disease.
In 2007, at the urging of the FDA, the PCWG2 published guidelines to define new census criteria.19 In terms of disease progression, PSA testing should be obtained at a minimum of 1 -week intervals and the threshold PSA level should be 2.0 ng/mL because of the availability of sensitive assays. PSA values during the first 12 weeks of treatment should not be used as the sole criterion for clinical decision making.26 Decreases in PSA values may not be seen for several weeks and no PSA surrogate for clinical benefit has been identified.19,27 Reporting PSA response rates is not advised because the significance of decline from baseline is uncertain. Instead, PCWG2 recommends that the percentage of change in the PSA value from baseline to 12 weeks (or earlier if a patient progresses or discontinues treatment) be reported. PSA progression is documented on the day when there is a ≥25% increase and an absolute increase of ≥2 ng/mL from the PSA nadir. This needs to be confirmed with a second PSA value obtained ≥3 weeks later. When there is no decrease in the PSA value from baseline, PSA progression is defined as a 25% increase from the baseline value with an increase in absolute value of 2 ng/mL after 12 weeks of treatment. Although the PCWG2 has made these PSA recommendations, emphasis should be placed on keeping patients in trials until there is radiographic or symptomatic progression. Progression of disease in a nodal or visceral site should be defined using RECIST criteria, and progression at the first assessment should be confirmed with a second scan ≥6 weeks later because with some treatments, lesions may increase in size before decreasing in size. Even with treatment that is effective, PSA values may increase before decreasing.28 Therapy is not to be discontinued solely on the basis of an increasing PSA.
Figg et al conducted an analysis that demonstrated a lack of correlation between PSA and the presence of measurable soft tissue metastases. This retrospective analysis evaluated 177 patients with newly diagnosed CRPC treated at the National Cancer Institute from 1990 to 1994.29 Abdominal/pelvic CT scan, bone scan, and PSA results were evaluated in these patients. Thirty-four patients had soft tissue involvement based on CT imaging that was compatible with metastatic disease. One hundred sixty-five patients had bone lesions compatible with metastatic disease. The results of the imaging scans were compared with the serum PSA concentration. In these castrate-resistant patients, there was no correlation between the mean serum PSA concentration and the presence or absence of measurable soft tissue disease. In addition, the mean PSA concentration was not different in patients with soft tissue disease compared with patients without soft tissue disease.
Another trial that demonstrated that discontinuation of therapy should not be based on PSA progression was the Immunotherapy for Prostate Adenocarcinoma Treatment (IMPACT) study. Sipuleucel-T is an autologous vaccine approved by the FDA for the treatment of minimally symptomatic CRPC.30 The vaccine combines granulocyte-macrophage colony-stimulating factor with prostatic acid phosphatase fusion protein and a patient’s own dendritic cells, which are harvested via leukapheresis. In the double-blind placebo-controlled IMPACT study, 512 men with mCRPC were randomly assigned to receive sipuleucel-T or placebo with OS as the primary endpoint.31 An improvement in OS was seen in the sipuleucel-T arm (25.8 months) versus placebo (21.7 months). The 3-year survival was 30% with sipuleucel-T compared with 23% in the placebo arm. As observed in trials such as IMPACT, PSA-related changes are not always reliable in predicting efficacy of the therapies rendered.32 Analyzing the efficacy of therapy remains a challenge and no validated biomarkers exist that can do so reliably. Again, caution needs to be exercised when using PSA as a surrogate marker for efficacy.
A challenging issue that remains in the realm of mCRPC and clinical trials and clinical practice is the anxiety that both a physician and patient can experience when PSA levels rise. In a phase III trial of atrasentan in patients with nonmetastatic CRPC, 941 patients had adequate androgen suppression and no radiographic evidence of metastases but had increasing PSA levels.33 A total of 467 patients were randomized to receive atrasentan, which is an oral, selective endothelin-A receptor antagonist, and 474 patients were randomized to receive placebo. The primary endpoint was time to disease progression (TTP), where disease progression was defined as the onset of metastases. Secondary endpoints included time to PSA progression, PSA doubling time, and overall survival. On subset analysis, large regional differences in TTP were noted because TTP was prolonged among patients outside the United States; however, no TTP delay was seen among the US patients. US patients were twice as likely to discontinue therapy as non-US patients (40.8% vs 21.9%; P < 0.001).34 A shorter median duration of treatment was observed for both treatment groups in the United States. The data suggested that physicians or patients were more inclined to discontinue treatment prematurely because of an increasing PSA level.
PSA does not always accurately reflect clinical benefit, particularly when the administered treatment does not target the AR pathway.35 An increasing PSA may be attributable to a dysfunctional AR with increased expression at low androgen levels, gene mutations that broaden the function of the receptor, or activation of the AR through androgen-independent pathways.36,37 Through these mechanisms, expression of PSA and cell growth may not correlate. Decreases in PSA may not accurately reflect tumor responses, as in the case of secondary hormonal agents, which can inhibit AR-stimulated PSA expression without suppression of tumor growth.5 Antiandrogen therapies such as enzalutamide and abiraterone target extragonadal activation of the AR,38 which also can affect PSA production. A systematic, individualized approach must be taken in evaluating patients in both a clinical trial and nonclinical trial setting.
In addition to other parameters, PSA doubling time, which reflects the amount of time it takes for PSA to double in quantity over time, can be used to aid in clinical decision making. A standardized method to calculate it has not been established in mCRPC, however.39,40
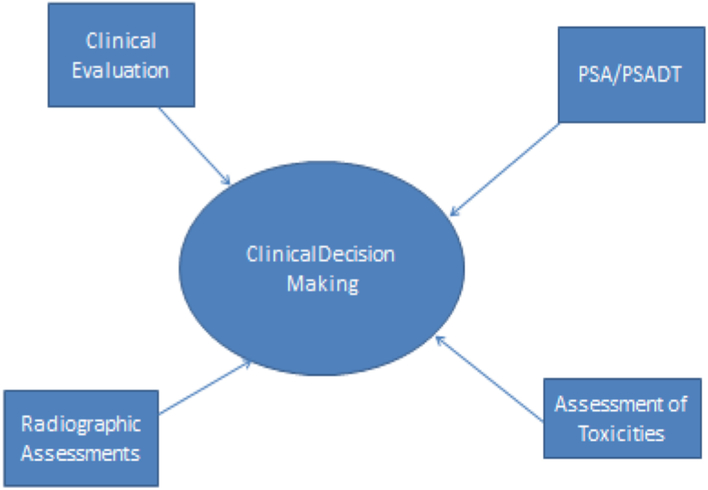
Although using only PSA criteria can negatively affect clinical management greatly, using PSA in combination with other factors can lead to better treatment practices. Physicians and patients will need to openly discuss PSA and attempt to deal with any anxiety associated with rising levels (Fig.). Assessment of patients requires a thorough evaluation for toxicities related to treatment; clinical evaluation for signs or symptoms of progression; and objective measures of progression of disease, including imaging studies per PCWG2 guidelines. Changes in therapy cannot be fueled by changes in PSA alone, but rapidly rising levels or a rapid PSA doubling time may lead to more active evaluation for signs or symptoms of progressive disease.
Fig.
A multidimensional approach to clinical decision making. PSA, prostate-specific antigen; PSADT, PSA doubling time.
Future Perspectives
Although PSA is easy and inexpensive to obtain, it must be used prudently for clinical decision making in prostate cancer. In mCRPC, using the information provided by PSA levels in clinical practice is more clearly defined than in the past. PSA should not be used as a sole criterion to discontinue treatment for a patient. Studies have shown that PSA secretion is a poor marker of disease progression and should be treated as such. The PCWG2 has recommendations for the design and endpoints of clinical trials for patients with mCRPC that include definition of progression of disease and radiographic assessments. Although significant strides have been made during the past several years in terms of treatment options for patients with mCRPC, there remain areas in which further elucidation is required, and the role of PSA is one of them. For now, effort needs to be placed in exploring suitable biomarkers to determine the efficacy of treatments (cytotoxic and noncytotoxic). This also will have added importance as the horizon of immunotherapeutic agents continues to evolve.
Key Points.
In metastatic castration-resistant prostate cancer, prostate-specific antigen secretion is a poor marker of disease progression and should be treated as such.
Clinical decision making should take into account the patient’s clinical status and imaging results in addition to laboratory values.
Effort needs to be placed into exploring suitable biomarkers to determine efficacy of treatments (cytotoxic and noncytotoxic) in metastatic prostate cancer.
Footnotes
The authors have no financial relationships to disclose and no conflicts of interest to report.
References
- 1.Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9–29. [DOI] [PubMed] [Google Scholar]
- 2.Han M, Partin AW, Pound CR, et al. Long-term biochemical disease-free and cancer-specific survival following anatomic radical retropubic prostatectomy. The 15-year Johns Hopkins experience. Urol Clin North Am 2001;28:555–565. [DOI] [PubMed] [Google Scholar]
- 3.Mohler ML, Coss CC, Duke CB 3rd, et al. Androgen receptor antagonists: a patent review (2008–2011). Expert Opin Ther Pat 2012;22:541–565. [DOI] [PubMed] [Google Scholar]
- 4.Papalia MA, Davis SR. What it the rationale for androgen therapy for women? Treat Endocrinol 2003;2:77–84. [DOI] [PubMed] [Google Scholar]
- 5.Balk SP, Ko YJ, Bubley GJ. Biology of prostate-specific antigen. J Clin Oncol 2003;21:383–391. [DOI] [PubMed] [Google Scholar]
- 6.Haythorn MR, Ablin RJ. Prostate-specific antigen testing across the spectmm of prostate cancer. Biomark Med 2011. ;5:515–526. [DOI] [PubMed] [Google Scholar]
- 7.Wang MC, Valenzuela LA, Murphy TM, et al. Purification of a human prostate specific antigen. Invest Urol 1979; 17:159–163. [PubMed] [Google Scholar]
- 8.Chu TM. Prostate specific antigen (PSA): the historical perspective, http://www.medicine.mcgill.ca/mjm/v02n02/psa.html Published 1996. Accessed July 3, 2014.
- 9.Kuriyama M, Wang MC, Lee CI, et al. Use of human prostate-specific antigen in monitoring prostate cancer. Cancer Res 1981;41:3874–3876. [PubMed] [Google Scholar]
- 10.Marberger M, Freedland SJ, Andriole GL, et al. Usefulness of prostate-specific antigen (PSA) rise as a marker of prostate cancer in men treated with dutasteride: lessons from the REDUCE study. BJU Int 2012; 109:1162–1169. [DOI] [PubMed] [Google Scholar]
- 11.Naghavi AO, Strom TJ, Nethers K, et al. Clinical implications of a prostate-specific antigen bounce after radiation therapy for prostate cancer. Int J Clin Oncol 2014. September 6 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 12.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 2004;351:1502–1512. [DOI] [PubMed] [Google Scholar]
- 13.Roessner M, de Wit R, Tannock IF, et al. Prostate-specific antigen (PSA) response as a surrogate endpoint for overall survival (OS): analysis of TAX327 study comparing docetaxel plus prednisone with mitoxantrone plus prednisone in advanced prostate cancer. 2005 ASCO Annual Meeting Proceedings J Clin Oncol 2005;23(Suppl 4554). [Google Scholar]
- 14.Olbert PJ, Hegele A, Kraeuter P, et al. Clinical significance of a prostate-specific antigen flare phenomenon in patients with hormone-refractory prostate cancer receiving docetaxel. Anticancer Drugs 2006; 17:993–996. [DOI] [PubMed] [Google Scholar]
- 15.Ning YM, Gulley JL, Arlen PM, et al. Phase II trial of bevacizumab, thalidomide, docetaxel, and prednisone in patients with metastatic castration-resistant prostate cancer. J Clin Oncol 2010;28:2070–2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dahut WL, Scripture C, Posadas E, et al. A phase II clinical trial of sorafenib in androgen-independent prostate cancer. Clin Cancer Res 2008; 14:209–214. [DOI] [PubMed] [Google Scholar]
- 17.Bubley GJ, Carducci M, Dahut W, et al. Eligibility and response guidelines for phase II clinical trials in androgen-independent prostate cancer: recommendations from the Prostate-Specific Antigen Working Group. J Clin Oncol 1999; 17:3461–3467. [DOI] [PubMed] [Google Scholar]
- 18.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205–216. [DOI] [PubMed] [Google Scholar]
- 19.Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol 2008;26:1148–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scher HI, Morris MJ, Kelly WK, et al. Prostate cancer clinical trial endpoints: “RECIST”ing a step backwards. Clin Cancer Res 2005; 11: 5223–5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Halabi S, Small EJ, Kantoff PW, et al. Prognostic model for predicting survival in men with hormone-refractory metastatic prostate cancer. J Clin Oncol 2003;21:1232–1237. [DOI] [PubMed] [Google Scholar]
- 22.Levenson RM, Sauerbrunn BJ, Bates HR, et al. Comparative value of bone scintigraphy and radiology in monitoring tumor response in systemically treated prostate carcinoma. Radiology 1983; 146:513–518. [DOI] [PubMed] [Google Scholar]
- 23.Cook GJ, Venkitaraman R, Sohaib AS, et al. The diagnostic utility of the flare phenomenon on bone scintigraphy in staging prostate cancer. Eur J Nucl Med Mol Imag 2011;38:7–13. [DOI] [PubMed] [Google Scholar]
- 24.Gillespie PJ, Alexander JL, Edelstyn GA. Changes in 87mSR concentrations in skeletal metastases in patients responding to cyclical combination chemotherapy for advanced breast cancer. J Nucl Med 1975; 16:191–193. [PubMed] [Google Scholar]
- 25.Alexander J, Gillespie PJ, Edelstyn GA. Serial bone scanning using technetium 99 m diphosphonate in patients undergoing cyclical combination chemotherapy for advanced breast cancer. Clin Nucl Med 1976; 1:13–17. [Google Scholar]
- 26.Rossleigh MA, Lovegrove FT, Reynolds PM, et al. The assessment of response to therapy in bone metastases in breast cancer. Aust N Z J Med 1984; 14:19–22. [DOI] [PubMed] [Google Scholar]
- 27.Fleming MT, Morris MJ, Heller G, et al. Post-therapy changes in PSA as an outcome measure in prostate cancer clinical trials. Nat Clin Pract Oncol 2006;3:658–667. [DOI] [PubMed] [Google Scholar]
- 28.Mukherji D, Pezaro CJ, De-Bono JS. MDV3100 for the treatment of prostate cancer. Expert Opin Investig Drugs 2012;21:227–233. [DOI] [PubMed] [Google Scholar]
- 29.Figg WD, Ammerman K, Patronas N, et al. Lack of correlation between prostate-specific antigen and the presence of measurable soft tissue metastases in hormone-refractory prostate cancer. Cancer Invest 1996; 14:513–517. [DOI] [PubMed] [Google Scholar]
- 30.Gardner TA, Elzey BD, Hahn NM. Sipuleucel-T (Provenge) autologous vaccine approved for treatment of men with asymptomatic or minimally symptomatic castrate-resistant metastatic prostate cancer. Hum Vaccin Immunother 2012;8:534–539. [DOI] [PubMed] [Google Scholar]
- 31.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 2010;363:411–422. [DOI] [PubMed] [Google Scholar]
- 32.Karan D, Holzbeierlein JM, Van Veldhuizen P, et al. Cancer immunotherapy: a paradigm shift for prostate cancer treatment. Nat Rev Urol 2012;9:376–385. [DOI] [PubMed] [Google Scholar]
- 33.Nelson JB, Love W, Chin JL, et al. Phase 3, randomized, controlled trial of atrasentan in patients with nonmetastatic, hormone-refractory prostate cancer. Cancer 2008; 113:2478–2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dawson NA. Targeted therapy in prostate cancer—are we our own worst enemy? Cancer 2008; 113:2376–2378. [DOI] [PubMed] [Google Scholar]
- 35.Pezaro C, Woo HH, Davis ID. Prostate cancer: measuring PSA. Intern Med J 2014;44:433–440. [DOI] [PubMed] [Google Scholar]
- 36.Crawford ED, Bennett CL, Andriole GL, et al. The utility of prostate-specific antigen in the management of advanced prostate cancer. BJU Int 2013; 112:548–560. [DOI] [PubMed] [Google Scholar]
- 37.Bonkhoff H, Berges R. From pathogenesis to prevention of castration resistant prostate cancer. Prostate 2010;70:100–112. [DOI] [PubMed] [Google Scholar]
- 38.Grist E, de Bono JS, Attard G. Targeting extra-gonadal androgens in castration-resistant prostate cancer. J Steroid Biochem Mol Biol 2015; 145:157–163. [DOI] [PubMed] [Google Scholar]
- 39.Arlen PM, Bianco F, Dahut WL, et al. Prostate Specific Antigen Working Group guidelines on prostate specific antigen doubling time. J Urol 2008; 179:2181–2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Colloca G Prostate-specific antigen kinetics as a surrogate endpoint in clinical trials of metastatic castration-resistant prostate cancer: a review. Cancer Treat Rev 2012;38:1020–1026. [DOI] [PubMed] [Google Scholar]