Abstract
Prenatal stress is associated with altered behavioral, cognitive, and psychiatric outcomes in offspring. Due to the importance of GABAergic systems in normal development and in psychiatric disorders, prenatal stress effects on these neurons have been investigated in animal models. Prenatal stress delays GABAergic progenitor migration, but the significance of these early developmental disruptions for the continued development of GABAergic cells in the juvenile brain is unclear. Here, we examined effects of prenatal stress on populations of GABAergic neurons in juvenile and adult medial frontal cortex (mFC) and hippocampus through stereological counting, gene expression, and relevant anxiety-like and social behaviors. Postnatally, total GABAergic populations showed altered trajectories in mFC and hippocampus. Parvalbumin neuron proportion in juvenile brain was altered by prenatal stress, but parvalbumin gene expression showed no differences. In adult brain, parvalbumin neuron proportions were altered by prenatal stress with opposite changes in gene expression. Adult prenatally-stressed offspring showed a lack of social preference on a 3-chambered task, increased anxiety-like behavior on the elevated plus maze, and reduced time in the center of an open field. Despite a lack of significant group differences in adult total GABAergic cell populations, performance on these tasks was correlated with GABAErgic populations in mFC and hippocampus. In conclusion, prenatal stress resulted in a delay in GABAergic cell number and maturation of the parvalbumin subtype. Influences of prenatal stress on GABAergic populations during developmentally dynamic periods and during adulthood may be relevant to the anxiety-like behaviors that occur after prenatal stress.
Keywords: prenatal stress, gaba, anxiety, medial frontal cortex, parvalbumin
INTRODUCTION
Prenatal stress and maternal anxiety and depression are risk factors for some child behavioral disruptions and psychiatric disorders. Maternal depression during pregnancy has been linked to negative affect, ADHD, and conduct disorder in offspring (Huot et al., 2004; Martini et al., 2010). Maternal anxiety during the prenatal period has been correlated with an increased risk for behavioral and emotional deficits during childhood (O'Connor et al., 2003). In addition, prenatal stress has been associated with psychiatric diseases such as schizophrenia, autism, childhood anxiety, and Tourette syndrome (Leckman et al., 1990; King et al., 2005; Bergman et al., 2007; Ronald et al., 2010). In order to better understand the cellular and molecular mechanisms of outcomes after prenatal maternal stress, animal models have been used to study offspring brain and behavior (Stevens and Vaccarino, 2015).
Animal models have implicated multiple neural systems as mechanisms for prenatal stress effects. Among these, inhibitory neurons may be of particular interest due to their importance in processes of brain development and associations with the pathology of schizophrenia, autism, and anxiety (Fine et al., 2014). In adult rats, prenatal stress decreased levels of benzodiazepine receptors in hippocampus and increased GABAergic synapses in the hypothalamus (Barros et al., 2006; Viltart et al., 2006). Although mature neural systems after prenatal stress have been studied, developmental trajectories are less understood. The protracted nature of GABAergic system development (Le Magueresse and Monyer, 2013) and the lengthy process by which subtypes such as parvalbumin cells mature makes these particularly important processes to evaluate after early stress. Interestingly, early developmental processes including methylation in cortical interneurons (Matrisciano et al., 2013), the migration of GABAergic progenitors to the cerebral cortex (Stevens et al., 2013), and embryonic neurogenesis of GABAergic progenitors (Uchida et al., 2014) are altered by prenatal stress. How GABAergic cells then develop through vulnerable juvenile stages is a question of paramount interest to understanding mechanisms of disease, treatments, and preventive measures.
Long term changes in brain function after prenatal stress have been in part evaluated by behavioral changes in animal models. Prenatal stress causes most consistently an array of inhibited behaviors in offspring with reduced activity in an open field, reduced social preference, and increased anxiety-like behavior in the elevated plus maze and light-dark box (Harmon et al., 2009; Laloux et al., 2012; Marrocco et al., 2012; Grigoryan and Segal, 2013). How altered neural development after early stress relates to these behavioral changes is not well understood.
In our previous work on prenatal stress, we have demonstrated changes in GABAergic cell migration into the cerebral cortex and hippocampus as well as changes in molecular aspects of these processes (Stevens et al., 2013). Other models in which GABAergic cell migration is affected during embryonic brain development show long-lasting alterations in cortical inhibitory neuron populations, particularly those of the parvalbumin subtype (Flames et al., 2004; Meechan et al., 2012; Lee et al., 2013; McCarthy et al., 2014; Nakai et al., 2014). Any changes due to prenatal stress in postnatal populations of cortical or hippocampal GABAergic neurons following altered progenitor migration have not been described. Here, we tracked how early GABAergic population changes progressed through juvenile and adult development. We also evaluated animal behavior for profiles of behavioral inhibition and whether this correlated with altered GABAergic populations.
METHODS
Mice
GAD67GFP(Δneo) mice (Tamamaki et al., 2003) were bred on a CD1 background. GAD67GFP+/− male mice were used for mating with wildtype CD1 females. Timed pregnancies were monitored following detection of vaginal plug on embryonic day 0 (E0) and pregnant females were singly housed from E12. All experimental procedures involving animals were performed in accordance with the Yale and University of Iowa Animal Resources Center/Office of Animal Resources and Institutional Animal Care and Use Committee (IACUC) policies.
Prenatal stress
Beginning on E12, half of the pregnant female mice were subjected to acute stress within a plexiglass restraint for 45 min under bright lights, three times daily during the daytime light cycle (at approximately 9 am, 12:30 pm, and 4 pm). The plexiglass restraints allowed pregnant females to change positions throughout each restraint period. Litters were reduced to 7-9 pups at postnatal day 0 (P0), left with their mother until P24, and then weaned to single-sex grouphousing. All evaluations here were performed on male offspring.
Brain collection
Offspring brains were collected at four different time points: P0, P24, P48, and P150. At P0, animals were anesthetized with ice, rapidly decapitated, and the brain was immersion postfixed in 4% paraformadelhyde (PFA) for at least 4 hours. At all other time points, deeply anesthetized animals were intracardiacally perfused with phosphate buffered saline (PBS) followed by 4% PFA perfusion and immersion post-fixation for at least 12 hours.
Immunohistochemistry
Brains from offspring were rinsed with PBS and transferred to 20% sucrose for at least 15 hours. Tissue was embedded and cryo-sectioned (Leica, CM1900, Bannockburn, Illinois) at 25 μm (P0) or 50 μm (P24, P48, and P150). Slide mounted (P0) or free-floating (all other ages) sections were processed for staining first by blocking for 1 hour with 10% goat serum in PBS with 0.025% TritonX-100, 0.0125% Tween20 (PBS++) and then incubating for 24–48 hours at 4°C with 5% goat serum/PBS++ containing primary antibodies as follows: parvalbumin (PV) (1:4000; Sigma, SAB4200545) and green fluorescent protein (GFP) (1:1000; Abcam, AB13970, #660556). Brain sections were then washed three times in PBS followed by an incubation in 5% goat serum/PBS++ containing Alexa dye-conjugated secondary antibodies (1:500-1000; Molecular Probes). Fluorescently-labeled sections were coverslipped using mounting medium with DAPI (Vector Laboratories, #H-1200).
Cell Counting
Stereological counting was utilized in order to obtain reliable estimates of cell number as done previously (Stevens et al., 2010). Stereological, unbiased estimates of GAD67GFP+ and Parvalbumin+ cells within the neonatal, adolescent, and adult medial frontal cortex (mFC) and hippocampal cornus ammonis (hippocampal CA) were obtained with a computer running the StereoInvestigator software (Microbrightfield, Colchester, VT) and coupled to a Zeiss Axioskope 2 Mot Plus (Carl Zeiss, Oberkochen, Germany) equipped with a digital camera and calibrated motorized stage controller that allows precise control of y-, x-, and z-axes. Using the optical fractionator, nuclear profiles were counted in 3-dimensional counting boxes. Every 10th coronal section was used that contained any portion of mFC or hippocampal CA. Variations of counts were assessed by the Gunderson coefficients of error and data were only used when cell count error values were below 0.15.
Quantitative PCR
To quantify the expression of inhibitory neuron genes, brain tissue was collected at P27 and P150. Deeply anesthetized animals were rapidly decapitated and brain tissue was dissected out, sectioned on ice at 100 micrometers, and flash frozen on dry ice. Samples of mFC and hippocampal CA were collected using a micro-punch from 2 sections bilaterally and processed for total mRNA using an RNeasy Mini Kit (Qiagen). RNA concentrations were determined using a Nanodrop Spectrophotometer (Thermo Scientific). cDNA was synthesized using the Transcriptor First-Strand cDNA Synthesis kit (Roche). Quantitative PCR was carried out using Taqman Gene Assays (Applied Biosystems) for GAD1 (ID Mm00725661_s1) and parvalbumin (ID Mm00443100_m1). Beta-actin (predeveloped) was used as an endogenous control in all cases. qPCR was run using GeneAmp PCR Mastermix (Applied Biosystems) in a 7900HT or StepOneTM Instrument (Applied Biosystems). The cycle number threshold for signal detection for each gene of interest for each sample was normalized to that sample’s cycle number threshold for beta-actin and the difference in cycle number (ΔCT) was converted to gene expression values using the formula: [expression= 2 −ΔCT].
Graphs depict gene expression values for all averaged prenatal stress individual samples normalized to average gene expression values for control samples in the same region. Gene expression levels were grouped for analysis at each age across brain regions.
Behavioral Tests
All behavior assessments were performed on 2-3 month old male mice during the light cycle in a dedicated testing room with only one behavior assessment performed per day, allowing mice to habituate to the testing room for 60 minutes prior to testing. Unless otherwise noted, mice remained in their home cage with cage-mates immediately before and after assessments. At least 3 weeks elapsed after testing before brain tissue was collected.
Social Approach:
In a three-chamber social approach apparatus (Nadler et al., 2004), mice were tested for social preference and recognition in a well-lit room on a single day. Two male, non-experimental “stranger” mice of the same strain and age were habituated for five minutes to small cylinders in one end of each side chamber. Individual experimental mice were habituated to the center chamber for five minutes. Subsequently, the experimental mouse underwent a ten minute trial moving through all 3 chambers with only one stranger present and then a second, ten minute trial in which the first stranger mouse and the second stranger mouse were present.
Movement was recorded using an overhead camera and Anymaze software (Stoelting, Wood Dale, Illinois) and evaluated for the amount of time the experimental mouse spent around the cylinder on each side. Social discrimination index was calculated from behavior during the first trial taking the quotient of the time spent with the first stranger and the time spent with both cylinders overall. Social learning coefficient was calculated from the time spent with the second stranger divided by the time spent with both cylinders overall.
Elevated Plus Maze:
In a Stoelting (Wood Dale, Illinois) Elevated Plus Maze, mice were tested for anxiety-like behavior in a well-lit room for 5 minutes on a single day. Mouse movement throughout the maze was recorded using an overhead camera. Anymaze software coded and assessed the amount of time spent in the closed arms, open arms, and the center of the maze.
Open Field:
In a rectangular plastic arena, approximately 1800 cm2 in area, mice were tested for locomotor activity in a well-lit room for 30 minutes on two consecutive days. Test mice were placed in the corner of the arena and their movements recorded using an overhead camera and Anymaze software. The amount of time spent in the center 70% of the arena was measured for each day to assess behavioral inhibition.
Statistical Analysis
Analysis of variance (ANOVA) approaches were used to compare the number of GAD67GFP+ cells across different ages within each region in each condition and the proportion of GAD67GFP+ cells that were parvalbumin+ across each region within each age. Post-hoc two-tailed Student’s t-tests were used to compare the number of GAD67GFP+ cells at P0 and P24. GAD67GFP+ cell densities, qPCR gene expression values within each region in each condition and differences on behavioral tasks were compared using two-tailed Student's t-tests.
Pearson’s coefficient was calculated across all individuals in both conditions to determine the correlation between inhibited behavior and GAD67GFP+ cells populations and two-tailed significance was tested.
RESULTS
Developmental Alterations in GABAergic Cell Populations
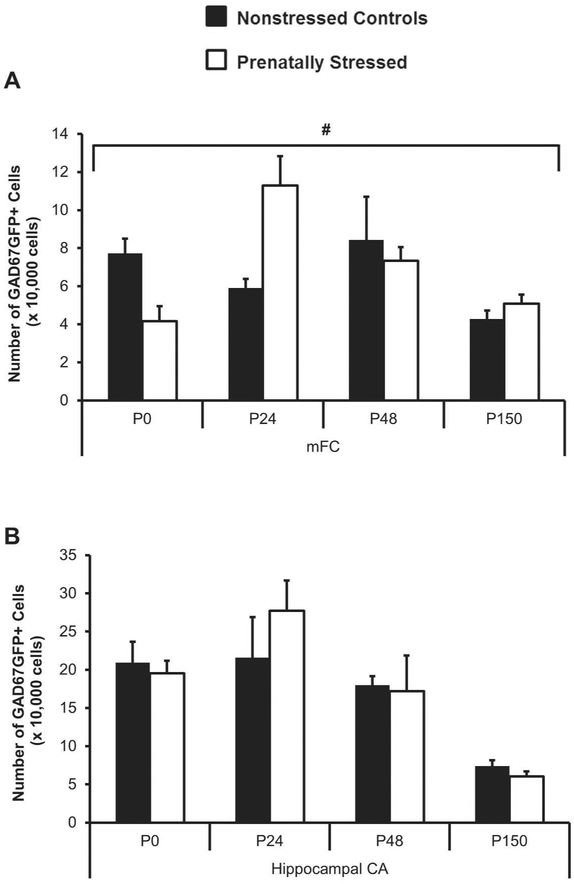
GAD67GFP+ cell populations were examined at multiple time points from birth through adulthood to understand the trajectory of neural development. An altered developmental time course was observed in the medial frontal cortex (mFC); prenatally-stressed mice had significantly less GAD67GFP+ neurons at postnatal day 0 (P0) and significantly more GAD67GFP+ neurons at P24 (Figure 1A, ANOVA for condition by age p=0.001 and post-hoc t-tests p<0.01 for P0 and p<0.05 for P24). At P48 and P150, differences in mFC GABAergic cell number were no longer present after prenatal stress. In hippocampal CA, no significant differences in the trajectory of GAD67GFP+ cell number were observed after prenatal stress, but a similar tendency was seen for increased cell number at P24 and no differences at later ages (Figure 1B). Similar differences were shown for GAD67GFP+ cell densities in these same regions, with the normal drop in density as the brain grows after birth apparent in both groups, but on a different time course after prenatal stress (Table 1).
Figure 1.
Changes in GABAergic cell number in dorsal regions from birth through adulthood after prenatal stress. Stereological cell counts of GAD67GFP+ cells in mFC were altered after prenatal stress (A) while cell number in hippocampal CA (B) was not significantly changed (#p<0.05 for interaction of age by stress by ANOVA).
Table 1.
GAD67GFP+ cell density in dorsal forebrain regions from day of birth, adolescence, and adulthood in non-stressed (NS) and prenatally-stressed (PS) offspring. At P0, a decrease in GAD67+ cell density was seen in medial frontal cortex (mFC) and a trend decrease was seen in hippocampal CA. A trend increase in cell density was observed at P24 in mFC.
| Age | NS GAD67GFP+ Cell Density (+/− SEM) |
PS GAD67GFP+ Cell Density (+/− SEM) |
%Change | p-value | |
|---|---|---|---|---|---|
| mFC | P0 | 1.23E-04 (+/− 1.3E-05) |
8.34E-05 (+/− 8.0E-06) |
−32.4 | 0.03 |
| P24 | 1.94E-05 (+/− 1.5E-06) |
2.47E-05 (+/− 1.5E-06) |
26.9 | 0.07 | |
| P48 | 2.55E-05 (+/− 3.4E-06) |
2.42E-05 (+/− 1.9E-06) |
−5.2 | 0.75 | |
| P150 | 2.09E-05 (+/− 2.4E-06) |
1.68E-05 (+/− 1.6E-06) |
−19.8 | 0.18 | |
| Hippocampal CA | P0 | 1.24E-04 (+/− 1.8E-05) |
8.47E-05 (+/− 9.4E-06) |
−31.5 | 0.10 |
| P24 | 1.75E-05 (+/− 2.3E-06) |
2.32E-05 (+/− 4.4E-06) |
32.4 | 0.31 | |
| P48 | 1.27E-05 (+/− 3.7E-06) |
1.30E-05 (+/− 2.9E-06) |
2.8 | 0.91 | |
| P150 | 8.22E-06 (+/− 2.6E-06) |
6.75E-06 (+/− 4.5E-07) |
−17.8 | 0.14 |
Effects of Prenatal Stress on Parvalbumin Cell Maturation
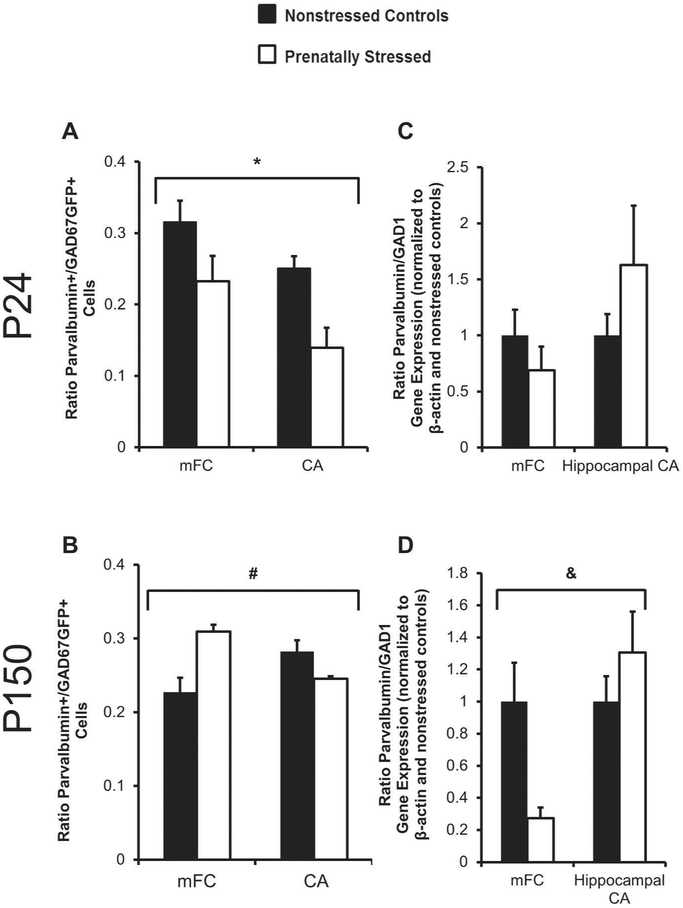
Parvalbumin is a calcium binding protein in one sub-class of interneurons of the mFC and hippocampus that begins to be expressed two to three weeks after birth. At P24, when an increase in GAD67GFP+ neurons was observed in the mFC, we also observed changes in the ration of parvalbumin cells or gene expression to total GAD67 cells or gene expression. After exposure to prenatal stress, the ratio of parvalbumin+ (PV) to GAD67GFP+ cells was lower in both the mFC and hippocampus in juvenile brain (Figure 2A, ANOVA for main effect of condition p=0.01). At postnatal day 150, prenatally-stressed mice continued to show a decreased ratio of PV+ to GAD67GFP+ cells in hippocampal CA, but this ratio was increased in mFC (Figure 2B, ANOVA for condition by region p=.001). This suggested an impairment in maturation of this cell type after prenatal stress that was over-corrected by adulthood only in mFC. The pattern of PV and GAD1 gene expression in juvenile and adult time points after prenatal stress did not follow the same pattern as cell density. At P24, when PV+/GAD67+ cell density was decreased, there were no significant differences in PV to GAD1 ratio of gene expression (Fig 2C). However in adulthood, the ratio of PV to GAD1 gene expression was opposite that seen for cell number ratios at this same time point (Figure 2D; ANOVA for condition by region p<0.05). These findings show that the developmental trajectory of a specific GABAergic subtype is altered by prenatal stress; cell number changes after prenatal stress may be accompanied by gene expression compensation.
Figure 2.
Ratios of Paravalbumin (PV) to GAD cell density and gene expression after prenatal stress in adolescence and adulthood. Prenatal stress decreased the ratio of PV+ to GAD67+ cells in mFC and hippocampal CA at P24 (A) and in hippocampal CA at P150 (B). The ratio was increased after prenatal stress at P150 in mFC (B). No significant changes were found in the gene expression at P24 (C), but PS did affect PV/GAD1 expression at P150 (D) (*p<0.01 for stress by ANOVA, #p=.001 for interaction of condition by region by ANOVA, &p<0.05 for interaction of condition by region by ANOVA).
Prenatal Stress, Adult Behavior and GAD67GFP+ Correlations
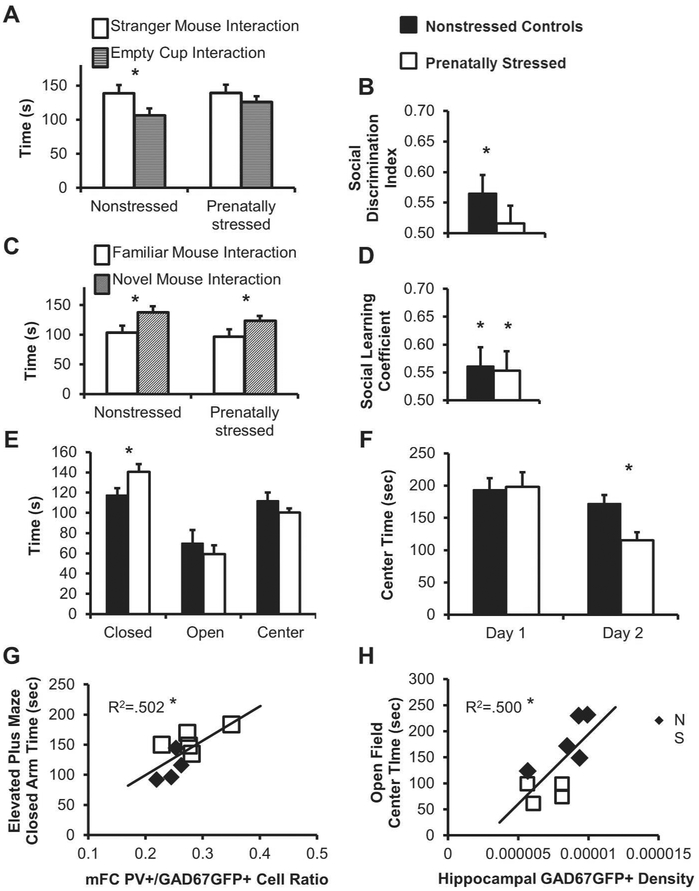
While there were no significant differences in GAD67GFP+ cell number after prenatal stress in adult mFC or hippocampal CA (Figure 1A,B), changes in adult behavior were observed. In order to understand the long-term effects of prenatal stress and potential connections to neuropsychiatric disorders, mice underwent testing in the elevated plus maze, open field, and 3-chamber social behavior task in adulthood. Non-stressed control mice showed a normal preference for spending more time with a stranger mouse than an empty cylinder while prenatally-stressed mice did not (Figure 3A,B). Social preference index was significantly above chance for non-stressed mice, but not for their prenatally-stressed counterparts (Figure 3B, non-stressed controls p<0.05, prenatally-stressed n.s.). However, both non-stressed and prenatally-stressed mice showed normal social recognition, spending more time with a novel than a familiar mouse (Figure 3C,D, p<0.05 for both).
Figure 3.
Effect of prenatal stress on behavioral outcomes and the relationship of behavior with neurobiological changes. Both non-stressed and prenatally-stressed mice spent more time with a “stranger” mouse than an empty cup (A), but only non-stressed controls had a significantly higher social discrimination index than chance (B). Non-stressed and prenatally-stressed mice spent more time with a novel than familiar mice (C) and had higher social learning coefficient than chance (D). Prenatally-stressed mice spent more time in the closed arm of the elevated plus maze (E) and less time in the center of the open field on Day 2 of testing (F). A postive correlation was found between the mFC PV+/GAD67GFP+ cell ratio and the time spent in the closed arm of the elevated plus maze (G) and a positive correlation was found between hippocampal GAD67GFP+ cell density and the time spent in the center of the open field (H) (*p<0.05 by two-tailed student’s t-test or for two-tailed significance of Pearson’s correlation).
After prenatal stress, mice spent more time in the closed arm of the elevated plus maze compared to non-stressed controls (Figure 3E, p<0.05). In the open field task, prenatally-stressed mice spent less time in the exposed center of the apparatus on the second day of testing (Figure 3F, p<0.05).
Interestingly, correlations were found between cortical interneuron populations and behavior of individual animals. The amount of time spent in the closed arm of the elevated plus maze correlated significantly with the density of GAD67GFP+ cells in mFC (R= −0.508, p<0.05), hippocampal GAD67GFP+ density (R=−0.707, p<0.05), and the ratio of PV+/GAD67GFP+ cells in mFC and hippocampal CA (Figure 3G, R=0.709, R=−0.739; p<0.05). There were also correlations between the amount of time spent in the center of the open field apparatus and the density of GAD67GFP+ cells in the hippocampal CA (Figure 3H, R=0.707, p<0.05) and the hippocampal ratio of PV+/GAD67GFP+ cells (R=0.636, trend at p=0.06). Social behavior only significantly correlated with hippocampal PV+/GAD67GFP+ ratio (R=0.688, p<0.05). These results show that prenatal stress resulted in alterations of behavior in adult mice that are related to the inhibitory neurons of mFC and hippocampus.
DISCUSSION
In this study, we have shown that prenatal stress altered GABAergic populations, GABAergic gene expression, and behavioral outcomes of mice through multiple postnatal stages of development. Our findings are consistent with previous work from our lab showing delayed migration of GABAergic progenitors (Stevens et al., 2013). The current findings support a continued delayed time course for GABAergic development after birth and in adolescence that eventually equilibrates in adulthood. Although we found here no significant differences in total GABAergic populations in adulthood, the proportion of parvalbumin+ cells was altered. Lastly, prenatally-stressed adult mice displayed more anxious-like behaviors across multiple tasks, which were correlated with GABAergic cell densities and the proportion of PV+/GAD67GFP+ cells in medial frontal cortex (mFC) and hippocampus.
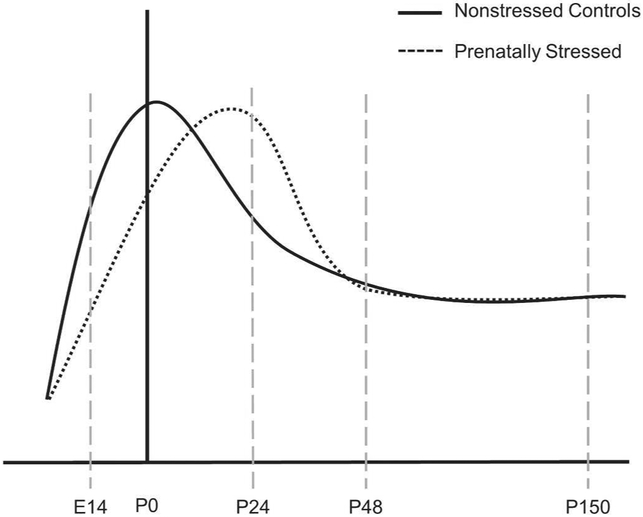
In the normal mouse dorsal forebrain, inhibitory neuron populations increase in early postnatal life until reaching a peak and undergoing pruning by apoptotic processes (Southwell et al., 2012). The changes we found suggest that the normal peak of inhibitory neuron populations in the cortex was delayed by prenatal stress from its normal time of occurrence during postnatal days 5-10 leading to a temporary and atypically high number of GABAergic cells at the time of weaning. Based on these findings, we propose a model of GABAergic neuronal population developmental trajectory in prenatally-stressed versus non-stressed mice (Figure 4). Our model suggests a delay after prenatal stress in the cellular processes that lead to populating, pruning, and molecular differentiation of GABAergic cells in mFC and other brain regions (Patz et al., 2003; Southwell et al., 2012) and a normalization of the total population by adulthood. While we found no significant effect of stress in hippocampal GABAergic cell numbers, a similar pattern of atypically high GABAergic cell number was present at weaning (Fig 1B). Thereafter, at postnatal day 48 and 150, there were no significant differences between prenatally-stressed and non-stressed animals in their mFC or hippocampal GABAergic populations suggesting that this delay of cellular pruning equalizes by adulthood. The importance of these populations and the developmental changes they undergo after prenatal stress may be reflected by the correlations of behavior with small range of individual variability in total GABAergic populations in adult brain.
Figure 4.
Proposed model of the altered trajectory of GABAergic cell populations after prenatal stress.
While the molecular and cellular mechanisms responsible for the temporary delay in GABAergic cell development are not clear, the early impact of maternal stress beginning at day E12 on these cells is likely important given the lack of change found in GABAergic migration and total populations when stress is begun later in embryonic development (Uchida et al., 2014). Our previous work showed that transcription factors responsible for migration and other processes of GABAergic cell development like dlx2 and nkx2.1 were affected as early as one day after prenatal stress began, embryonic day 13 (Stevens et al., 2013). Alterations in these cell-intrinsic regulators of GABAergic cell development and others such as NPAS1, KCC2, and Sox6 (Batista-Brito et al., 2009; Bortone and Polleux, 2009; Stanco et al., 2014) could arise in early development, persist, and be responsible for multiple developmental changes. Because of the importance of GABAergic network activity for GABA neuron development (Manent et al., 2005; Baho and Di Cristo, 2012), altered network characteristics of developing GABAergic cells may cause a more protracted maturation of the same cell populations. Alternately, indirect mechanisms such as through alterations in glial cells that are important for cortical interneuron development postnatally (Barros et al., 2006; Smith et al., 2014) may also be involved.
In order to better understand the maturation of GABAergic systems, the parvalbumin subtype of cortical interneurons was evaluated as one assessment of how prenatal stress influences GABAergic cell differentiation during development. In both the mFC and CA at P24, prenatal stress decreased the ratio of parvalbumin+ among all GAD67GFP+ cells with no significant differences in gene expression. Because parvalbumin is a late onset protein, the decrease in ratio of cell number suggests that prenatally-stressed inhibitory neurons in dorsal regions are less mature at P24. These findings are similar to effects of later embryonic stress (Uchida et al., 2014) and of early postnatal stress on parvalbumin interneurons in mFC and hippocampus (Brenhouse and Andersen, 2011; Giovanoli et al., 2014), the latter implicating altered maternal postnatal care (Smith et al., 2004) in the effects seen here after prenatal stress. Alterations in these populations in the juvenile mFC may implicate prenatal stress in the sensitive periods in cortical plasticity that rely on GABAergic activity and the mechanisms of parvalbumin neuron development (Hensch, 2005) and related factors including Otx2 and perineuronal nets that may be influenced by stress (Beurdeley et al., 2012; Morishita et al., 2015). One form of prenatal stress, prenatal exposure to maternal depression, has been shown to alter developmentally sensitive periods in a related way (Weikum et al., 2012).
In adulthood, the decrease in PV+ to GAD67GFP+ cell ratio was still present in hippocampal CA, but an “over-correction” had occurred in mFC. We also examined PV and Gad1 gene expression which may parallel PV+ cell numbers, but may alternatively become up or down regulated in response to a system with abnormal cell numbers (Saji et al., 1994). We found a decreased PV to Gad1 gene expression ratio after prenatal stress in mFC and an increased ratio in hippocampal CA. These changes were in the opposite direction of changes in PV+ cell number and may reflect gene expression levels compensating for the imbalance in differentiated PV+ cells in these regions or the opposite, with abnormal levels of gene expression becoming compensated for post-transcriptionally and resulting in altered protein labeling with PV. These results contribute to growing evidence that prenatal or early-life stress alters the parvalbumin subtype of cortical interneurons in multiple ways (Helmeke et al., 2008; Zohar et al., 2015). Differences in the timing of early life stress effects may result from different types and timing of stress. In general, these changes suggest that the cellular composition of GABAergic cells is disrupted by prenatal stress and remains altered in adulthood, affecting both gene and protein expression.
At P150, when mice had reached adulthood, an impact of prenatal stress was no longer seen in the total number of GAD67+ cells in mFC or hippocampal CA. However, changes in behavior of P150 mice were observed. Prenatally-stressed mice exhibit decreased sociability, shown through the first phase of the 3-chambered social task, and increased anxiety, shown through elevated plus maze and open field task. These behaviors correlated with mFC and hippocampal CA GAD67+ cell density and PV+/GAD67GFP+ cell ratio, which suggest the importance of inhibitory cell densities and the developmental processes that influence them in dorsal forebrain regions. Although significant cell count changes diminish by P150, correlations suggest that the GABAergic and PV+ cell density in dorsal regions may impact behavior. The importance of acute local GABAergic activity in these regions has been demonstrated for anxiety-like behaviors in rodents (Bi et al., 2013).
The behavioral findings here contribute to the relevance of the prenatal stress model for neuropsychiatric disorders (Fine et al., 2014). Inhibited sociability is a central symptom of autism and increased anxiety has been observed in generalized anxiety disorder and schizophrenia. The association of these behaviors with dorsal forebrain GABAergic populations after prenatal stress provides a possible mechanism for how exposure to prenatal stress becomes a risk factor for neuropsychiatric disorders, in which GABAergic systems have been shown to differ postmortem (Fatemi et al., 2002; Lewis, 2012). The links between neural mechanism and behavioral outcomes in this model suggest that one component of effective intervention may be to normalize either acute GABAergic neuron function or altered development in at-risk populations (Benham et al., 2014; Cellot and Cherubini, 2014).
REFERENCES
- Baho E, Di Cristo G. 2012. Neural activity and neurotransmission regulate the maturation of the innervation field of cortical GABAergic interneurons in an age-dependent manner. J Neurosci 32:911–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros VG, Rodriguez P, Martijena ID, Perez A, Molina VA, Antonelli MC. 2006. Prenatal stress and early adoption effects on benzodiazepine receptors and anxiogenic behavior in the adult rat brain. Synapse 60:609–618. [DOI] [PubMed] [Google Scholar]
- Batista-Brito R, Rossignol E, Hjerling-Leffler J, Denaxa M, Wegner M, Lefebvre V, Pachnis V, Fishell G. 2009. The cell-intrinsic requirement of Sox6 for cortical interneuron development. Neuron 63:466–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham RS, Engin E, Rudolph U. 2014. Diversity of neuronal inhibition: a path to novel treatments for neuropsychiatric disorders. JAMA Psychiatry 71:91–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman K, Sarkar P, O'Connor TG, Modi N, Glover V. 2007. Maternal stress during pregnancy predicts cognitive ability and fearfulness in infancy. J Am Acad Child Adolesc Psychiatry 46:1454–1463. [DOI] [PubMed] [Google Scholar]
- Beurdeley M, Spatazza J, Lee HH, Sugiyama S, Bernard C, Di Nardo AA, Hensch TK, Prochiantz A. 2012. Otx2 binding to perineuronal nets persistently regulates plasticity in the mature visual cortex. J Neurosci 32:9429–9437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi LL, Wang J, Luo ZY, Chen SP, Geng F, Chen YH, Li SJ, Yuan CH, Lin S, Gao TM. 2013. Enhanced excitability in the infralimbic cortex produces anxiety-like behaviors. Neuropharmacology 72:148–156. [DOI] [PubMed] [Google Scholar]
- Bortone D, Polleux F. 2009. KCC2 expression promotes the termination of cortical interneuron migration in a voltage-sensitive calcium-dependent manner. Neuron 62:53–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenhouse HC, Andersen SL. 2011. Developmental trajectories during adolescence in males and females: a cross-species understanding of underlying brain changes. Neurosci Biobehav Rev 35:1687–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cellot G, Cherubini E. 2014. GABAergic signaling as therapeutic target for autism spectrum disorders. Front Pediatr 2:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Halt AR, Stary JM, Kanodia R, Schulz SC, Realmuto GR. 2002. Glutamic acid decarboxylase 65 and 67 kDa proteins are reduced in autistic parietal and cerebellar cortices. Biol Psychiatry 52:805–810. [DOI] [PubMed] [Google Scholar]
- Fine R, Zhang J, Stevens HE. 2014. Prenatal stress and inhibitory neuron systems: implications for neuropsychiatric disorders. Mol Psychiatry 19:641–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flames N, Long JE, Garratt AN, Fischer TM, Gassmann M, Birchmeier C, Lai C, Rubenstein JL, Marin O. 2004. Short- and long-range attraction of cortical GABAergic interneurons by neuregulin-1. Neuron 44:251–261. [DOI] [PubMed] [Google Scholar]
- Giovanoli S, Weber L, Meyer U. 2014. Single and combined effects of prenatal immune activation and peripubertal stress on parvalbumin and reelin expression in the hippocampal formation. Brain Behav Immun 40:48–54. [DOI] [PubMed] [Google Scholar]
- Grigoryan G, Segal M. 2013. Prenatal stress affects network properties of rat hippocampal neurons. Biol Psychiatry 73:1095–1102. [DOI] [PubMed] [Google Scholar]
- Harmon KM, Greenwald ML, McFarland A, Beckwith T, Cromwell HC. 2009. The effects of prenatal stress on motivation in the rat pup. Stress 12:250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmeke C, Ovtscharoff W Jr., Poeggel G, Braun K. 2008. Imbalance of immunohistochemically characterized interneuron populations in the adolescent and adult rodent medial prefrontal cortex after repeated exposure to neonatal separation stress. Neuroscience 152:18–28. [DOI] [PubMed] [Google Scholar]
- Hensch TK. 2005. Critical period mechanisms in developing visual cortex. Curr Top Dev Biol 69:215–237. [DOI] [PubMed] [Google Scholar]
- Huot RL, Brennan PA, Stowe ZN, Plotsky PM, Walker EF. 2004. Negative affect in offspring of depressed mothers is predicted by infant cortisol levels at 6 months and maternal depression during pregnancy, but not postpartum. Ann N Y Acad Sci 1032:234–236. [DOI] [PubMed] [Google Scholar]
- King S, Laplante D, Joober R. 2005. Understanding putative risk factors for schizophrenia: retrospective and prospective studies. J Psychiatry Neurosci 30:342–348. [PMC free article] [PubMed] [Google Scholar]
- Laloux C, Mairesse J, Van Camp G, Giovine A, Branchi I, Bouret S, Morley-Fletcher S, Bergonzelli G, Malagodi M, Gradini R, Nicoletti F, Darnaudery M, Maccari S. 2012. Anxiety-like behaviour and associated neurochemical and endocrinological alterations in male pups exposed to prenatal stress. Psychoneuroendocrinology 37:1646–1658. [DOI] [PubMed] [Google Scholar]
- Le Magueresse C, Monyer H. 2013. GABAergic interneurons shape the functional maturation of the cortex. Neuron 77:388–405. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Dolnansky ES, Hardin MT, Clubb M, Walkup JT, Stevenson J, Pauls DL. 1990. Perinatal factors in the expression of Tourette's syndrome: an exploratory study. J Am Acad Child Adolesc Psychiatry 29:220–226. [DOI] [PubMed] [Google Scholar]
- Lee FH, Zai CC, Cordes SP, Roder JC, Wong AH. 2013. Abnormal interneuron development in disrupted-in-schizophrenia-1 L100P mutant mice. Mol Brain 6:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA. 2012. Cortical circuit dysfunction and cognitive deficits in schizophrenia--implications for preemptive interventions. Eur J Neurosci 35:1871–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manent JB, Demarque M, Jorquera I, Pellegrino C, Ben-Ari Y, Aniksztejn L, Represa A. 2005. A noncanonical release of GABA and glutamate modulates neuronal migration. J Neurosci 25:4755–4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrocco J, Mairesse J, Ngomba RT, Silletti V, Van Camp G, Bouwalerh H, Summa M, Pittaluga A, Nicoletti F, Maccari S, Morley-Fletcher S. 2012. Anxiety-like behavior of prenatally stressed rats is associated with a selective reduction of glutamate release in the ventral hippocampus. J Neurosci 32:17143–17154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini J, Knappe S, Beesdo-Baum K, Lieb R, Wittchen HU. 2010. Anxiety disorders before birth and self-perceived distress during pregnancy: associations with maternal depression and obstetric, neonatal and early childhood outcomes. Early Hum Dev 86:305–310. [DOI] [PubMed] [Google Scholar]
- Matrisciano F, Tueting P, Dalal I, Kadriu B, Grayson DR, Davis JM, Nicoletti F, Guidotti A. 2013. Epigenetic modifications of GABAergic interneurons are associated with the schizophrenia-like phenotype induced by prenatal stress in mice. Neuropharmacology 68:184–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy DM, Kabir ZD, Bhide PG, Kosofsky BE. 2014. Effects of prenatal exposure to cocaine on brain structure and function. Prog Brain Res 211:277–289. [DOI] [PubMed] [Google Scholar]
- Meechan DW, Tucker ES, Maynard TM, LaMantia AS. 2012. Cxcr4 regulation of interneuron migration is disrupted in 22q11.2 deletion syndrome. Proc Natl Acad Sci U S A 109:18601–18606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishita H, Cabungcal JH, Chen Y, Do KQ, Hensch TK. 2015. Prolonged Period of Cortical Plasticity upon Redox Dysregulation in Fast-Spiking Interneurons. Biol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadler JJ, Moy SS, Dold G, Trang D, Simmons N, Perez A, Young NB, Barbaro RP, Piven J, Magnuson TR, Crawley JN. 2004. Automated apparatus for quantitation of social approach behaviors in mice. Genes Brain Behav 3:303–314. [DOI] [PubMed] [Google Scholar]
- Nakai T, Nagai T, Wang R, Yamada S, Kuroda K, Kaibuchi K, Yamada K. 2014. Alterations of GABAergic and dopaminergic systems in mutant mice with disruption of exons 2 and 3 of the Disc1 gene. Neurochem Int 74:74–83. [DOI] [PubMed] [Google Scholar]
- O'Connor TG, Heron J, Golding J, Glover V, Team AS. 2003. Maternal antenatal anxiety and behavioural/emotional problems in children: a test of a programming hypothesis. J Child Psychol Psychiatry 44:1025–1036. [DOI] [PubMed] [Google Scholar]
- Patz S, Wirth MJ, Gorba T, Klostermann O, Wahle P. 2003. Neuronal activity and neurotrophic factors regulate GAD-65/67 mRNA and protein expression in organotypic cultures of rat visual cortex. Eur J Neurosci 18:1–12. [DOI] [PubMed] [Google Scholar]
- Ronald A, Pennell CE, Whitehouse AJ. 2010. Prenatal Maternal Stress Associated with ADHD and Autistic Traits in early Childhood. Front Psychol 1:223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saji M, Cohen M, Blau AD, Wessel TC, Volpe BT. 1994. Transient forebrain ischemia induces delayed injury in the substantia nigra reticulata: degeneration of GABA neurons, compensatory expression of GAD mRNA. Brain Res 643:234–244. [DOI] [PubMed] [Google Scholar]
- Smith JW, Seckl JR, Evans AT, Costall B, Smythe JW. 2004. Gestational stress induces postpartum depression-like behaviour and alters maternal care in rats. Psychoneuroendocrinology 29:227–244. [DOI] [PubMed] [Google Scholar]
- Smith KM, Maragnoli ME, Phull PM, Tran KM, Choubey L, Vaccarino FM. 2014. Fgfr1 inactivation in the mouse telencephalon results in impaired maturation of interneurons expressing parvalbumin. PLoS One 9:e103696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southwell DG, Paredes MF, Galvao RP, Jones DL, Froemke RC, Sebe JY, Alfaro-Cervello C, Tang Y, Garcia-Verdugo JM, Rubenstein JL, Baraban SC, Alvarez-Buylla A. 2012. Intrinsically determined cell death of developing cortical interneurons. Nature 491:109–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanco A, Pla R, Vogt D, Chen Y, Mandal S, Walker J, Hunt RF, Lindtner S, Erdman CA, Pieper AA, Hamilton SP, Xu D, Baraban SC, Rubenstein JL. 2014. NPAS1 represses the generation of specific subtypes of cortical interneurons. Neuron 84:940–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens HE, Smith KM, Maragnoli ME, Fagel D, Borok E, Shanabrough M, Horvath TL, Vaccarino FM. 2010. Fgfr2 is required for the development of the medial prefrontal cortex and its connections with limbic circuits. J Neurosci 30:5590–5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens HE, Su T, Yanagawa Y, Vaccarino FM. 2013. Prenatal stress delays inhibitory neuron progenitor migration in the developing neocortex. Psychoneuroendocrinology 38:509–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens HE, Vaccarino FM. 2015. How animal models inform child and adolescent psychiatry. J Am Acad Child Adolesc Psychiatry 54:352–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamamaki N, Yanagawa Y, Tomioka R, Miyazaki J, Obata K, Kaneko T. 2003. Green fluorescent protein expression and colocalization with calretinin, parvalbumin, and somatostatin in the GAD67-GFP knock-in mouse. J Comp Neurol 467:60–79. [DOI] [PubMed] [Google Scholar]
- Uchida T, Furukawa T, Iwata S, Yanagawa Y, Fukuda A. 2014. Selective loss of parvalbumin-positive GABAergic interneurons in the cerebral cortex of maternally stressed Gad1-heterozygous mouse offspring. Transl Psychiatry 4:e371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viltart O, Mairesse J, Darnaudery M, Louvart H, Vanbesien-Mailliot C, Catalani A, Maccari S. 2006. Prenatal stress alters Fos protein expression in hippocampus and locus coeruleus stress-related brain structures. Psychoneuroendocrinology 31:769–780. [DOI] [PubMed] [Google Scholar]
- Weikum WM, Oberlander TF, Hensch TK, Werker JF. 2012. Prenatal exposure to antidepressants and depressed maternal mood alter trajectory of infant speech perception. Proc Natl Acad Sci U S A 109 Suppl 2:17221–17227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zohar I, Dosoretz-Abittan L, Shoham S, Weinstock M. 2015. Sex dependent reduction by prenatal stress of the expression of 5HT1A receptors in the prefrontal cortex and CRF type 2 receptors in the raphe nucleus in rats: reversal by citalopram. Psychopharmacology (Berl) 232:1643–1653. [DOI] [PubMed] [Google Scholar]