Abstract
Objectives
Tobacco exposure is an established risk factor for pancreatic cancer and chronic pancreatitis; however, its role in pancreatic insufficiency is not clear.
Methods
This controlled, cross-sectional study examined smokers and non-smokers with no history of pancreatic disease. Histories and validated inventories of alcohol and tobacco use were obtained, and pancreatic insufficiency was assessed using the fecal elastase-1 assay.
Results
Of 7,854 patients approached, 226 were interviewed and 200 enrolled. The rate of pancreatic insufficiency [18% (18/100)], and severe pancreatic insufficiency [10% (10/100)] was significantly higher in smokers than in controls [6% (6/100) (p =0.009)] and [1% (1/100) p =0.010], respectively. On multivariate logistic regression, the risk of pancreatic insufficiency in smokers was significantly increased (Odds Ratio, 4.34 [1.37 – 13.75] p =0.012), controlling for alcohol use and relevant covariates. Tobacco exposure was associated with the highest odds ratio for pancreatic insufficiency. Alcohol consumption was strongly associated with tobacco exposure (p <0.001); but not with pancreatic insufficiency by multivariate analysis (p =0.792).
Conclusions
This study suggests that tobacco exposure is independently associated with pancreatic exocrine insufficiency in patients without a prior diagnosis of pancreatic disease. Tobacco exposure appears to have greater detrimental effects on pancreatic function than alcohol in this population.
Keywords: pancreatic insufficiency, tobacco, smoking, fecal elastase-1
INTRODUCTION
Tobacco exposure is recognized as a cause of chronic disease in multiple organ systems through mechanisms that involve DNA damage, inflammation, and oxidative stress 1,2. In the pancreas, the impact of tobacco exposure has not been as well characterized as alcohol; however, there is increasing evidence that smoking is toxic to the pancreas and may have significant clinical implications. The pancreatic parenchyma manifests histologic changes in a dose dependent manner with tobacco exposure 3, and the amount of cigarette smoking per day correlates with advanced hyaline thickening in pancreatic arterioles 3. There is a 75% increase in the risk of pancreatic cancer in smokers as compared to non-smokers 4,5.
Though not as widely recognized, smoking is emerging as an important risk factor in the etiology of chronic pancreatitis 6. Multiple studies have demonstrated that smoking may potentiate the effects of alcohol 7,8, and may also be an independent risk factor for chronic pancreatitis 7,9,10 in a dose-dependent manner 11. In patients with alcoholic chronic pancreatitis, exocrine pancreatic insufficiency was only found in patients with at least 15 pack-years, and patients with ≥30 pack-years had exocrine pancreatic insufficiency at a significantly earlier age than those with less than 30 pack-years, suggesting a dose-response 11. A large population screening study assessed patients for pancreatic insufficiency at enrollment and several risk factors emerged. Among these, patients with a self-reported history of smoking had a significantly increased odds of developing exocrine pancreatic insufficiency as compared to subjects who did not currently smoke 12.
These studies and clinical observations suggest that smoking is more toxic to the pancreas than is widely recognized and led to the hypothesis that pancreatic exocrine function may be significantly impacted by tobacco exposure prior to a diagnosis of pancreatic disease. This study enrolled patients with and without a history of heavy tobacco exposure to examine the relationship between smoking and pancreatic insufficiency in patients with no prior history of pancreatic disease. As frequent covariates, we further sought to distinguish the contributions of smoking and alcohol exposure on pancreatic exocrine function in this population.
MATERIALS AND METHODS
Design Overview
This study was reviewed and approved by the Institutional Review Board of the Emory University School of Medicine. All patients in the study provided written informed consent for participation. We performed a controlled cross-sectional analysis of patients recruited from primary care and subspecialty outpatient clinics between April and August of 2013 (clinicaltrials.gov NCT01988350). Participants completed validated questionnaires and interviews to obtain a focused medical, smoking, and alcohol consumption history. The focused medical history included patients’ self-reported demographic information, medical diagnoses, medication list, and five-year changes in weight. Heights, weights, and Body Mass Index (BMI) from prior visits were also abstracted from the patients’ electronic medical record when available. Smoking history was obtained via the validated National Health And Nutrition Examination Survey (NHANES) of Smoking and Cigarette Use13, and additionally a total lifetime smoking pack-year history was calculated. Alcohol consumption history was obtained via the validated Lifetime Drinking History Questionnaire 14.
Setting and Participants
Patients were offered enrollment if they were at least 30 years of age, able to read, and could understand and sign the informed consent. Smokers and non-smokers were recruited. Patients were excluded if they had a prior diagnosis of pancreatic insufficiency, a current or remote history of acute or chronic pancreatitis, any other pancreatic disease or malignancy, a history of pancreatic surgery, or a prior diagnosis of small bowel malabsorption or Celiac disease.
Exposure Definitions
Non-smokers were defined as patients who had at least 7 years of abstinence from smoking as well as less than a 5 cumulative pack-year smoking history. Smokers were defined as patients who had a heavy smoking history of at least 20 pack-years. These definitions were based on the presence of changes in the pancreatic parenchyma which became significant with a 20 pack-year smoking history.11 The 30-year age limit for all patients enrolled was determined based on the 20 pack-year requirement for the tobacco exposure cohort (the control cohort also needed to be old enough to potentially have had a 20 pack-year smoking history). Alcohol consumption was measured using the standard definition of 0.6 ounces of pure alcohol per drink (12 ounces of beer, 5 ounces of wine, or 1.5 ounces of hard liquor). Alcohol categories were defined as: abstainer <0.25 drinks per week; light drinker 0.25–3 drinks per week; moderate 3–14 drinks per week; and heavy >14 drinks per week 15.
Outcomes and Follow-up
All participants provided a stool specimen that was assayed for the fecal elastase-1 (FE-1) level via a quantitative sandwich Enzyme-Linked Immunosorbent Assay 16 (ARUP Laboratories, Salt Lake City, Utah). Pancreatic insufficiency was defined as a FE-1 concentration ≤200μg/g. Mild to moderate pancreatic insufficiency was defined as a FE-1 level between 100–200μg/g, and severe pancreatic insufficiency was defined as a FE-1 level of 99μg/g or less 17. FE-1 values above 200μg/g were considered to indicate normal pancreatic function and no insufficiency. The results of the FE-1 testing were reported back to patients. All patients with abnormal FE-1 levels were offered follow-up with the pancreatology service outside of the study for clinical evaluation and management.
Statistical Analysis
Pancreatic glandular changes may be seen in approximately 5 percent of the general population and in 30% or more with a heavy smoking history (>20 cigarettes/day) 3. We hypothesized at least a 15 percent difference in the proportion of patients with low FE-1 levels in the tobacco exposure arm as compared to the non-smoking control arm. Using a p-value of 0.05 and a power of 80 percent, we estimated our sample size in each arm to be 88 subjects. Assuming a 10 percent dropout rate, the target enrollment cohort was 100 patients per arm. Univariate associations of smoking status and pancreatic insufficiency with covariates were examined with analysis of variance (ANOVA) or Wilcoxon rank-sum tests for numerical covariates and chi-square or Fisher’s exact test for categorical covariates. Independent t-tests were used for continuous variables. A logistic regression model was further employed to identify independent predictors of pancreatic insufficiency. A backwards variable selection method with an alpha level of removal of 0.1 was used. Data was stored behind the institution’s firewall and analyzed using SAS 9.3 software (SAS Institute, Inc., Cary, North Carolina). Significance was defined as a p-value <0.05.
RESULTS
Enrollment
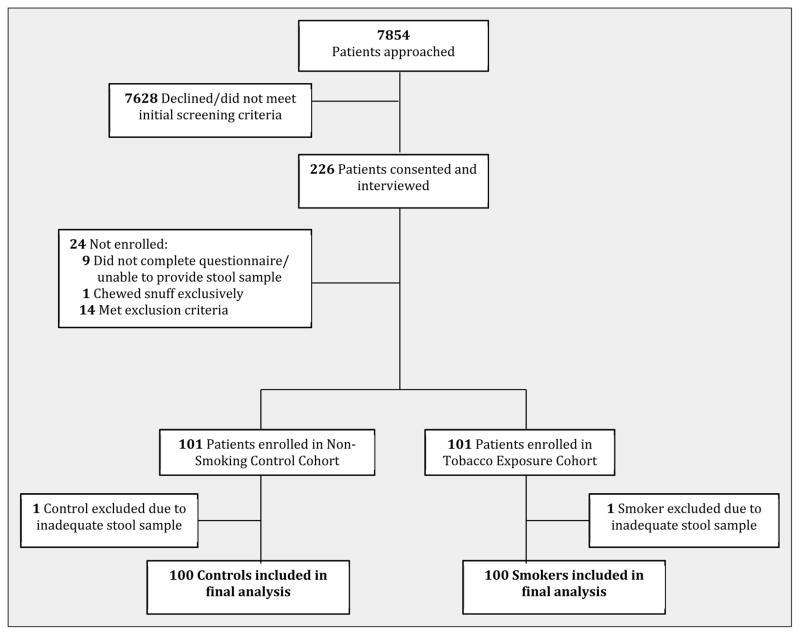
Between April 2013 and August 2013, 7,854 outpatients were approached. 226 patients signed informed consent and agreed to further screening and interview. 24 were subsequently excluded: 9 did not complete the questionnaire, 14 met exclusion criteria, and 1 patient was excluded due to tobacco use exclusively in the form of snuff. 202 patients completed the screening process. 2 of these provided inadequate stool specimens. The final cohort met the predetermined target enrollment and consisted of 100 smokers and 100 controls who were included in the final analysis (Figure 1).
Figure 1. Study Enrollment.
100 smokers and 100 non-smoker controls were ultimately enrolled in the study.
Characteristics of Study Participants
Baseline demographic and clinical characteristics in each cohort are outlined in Table 1. As compared with non-smoking controls, smokers were slightly older (64.5 years vs. 60.5 years, p=0.020) and had a higher rate of diabetes (24 percent vs. 13 percent, p=0.045), and dyslipidemia by history (58 percent vs. 43 percent, p=0.034). The tobacco exposure cohort also had a higher prevalence of marijuana use (6 percent vs. 0 percent, p=0.029) as well as greater alcohol consumption as described below.
Table 1.
Baseline Characteristics of Study Participants
| Characteristic | Nonsmoker Controls (N=100) | Tobacco Exposure (N=100) | P-value |
|---|---|---|---|
| Demographics | |||
| Mean Age in yrs (SD) | 60.5 (2.6) | 64.5 (11.5) | 0.020 |
| Male – no. | 40 | 47 | 0.318 |
| Caucasian race – no. | 61 | 67 | 0.377 |
| Diabetes – no. | 13 | 24 | 0.045 |
| Hypertension – no. | 49 | 60 | 0.118 |
| Hyperlipidemia – no. | 43 | 58 | 0.034 |
| Hypothyroidism – no. | 14 | 19 | 0.341 |
| Marijuana User – no. | 0 | 6 | 0.029 |
| Weight | |||
| Median Body Mass Index ❖(range) | 27.8 (11.8–55.3) | 27.7 (15.4 – 46.3) | 0.798 |
| Mean perceived 5yr weight change§ (SD) | −2.9 (6.8) | −4.8 (10.4) | 0.123 |
| Mean actual weight change†(SD) | 0.6 (8.3) | −1.4 (10.0) | 0.086 |
| Alcohol Use¶ | |||
| Heavy Drinker – no. | 2 | 9 | <0.001 |
| Moderate Drinker – no. | 14 | 27 | |
| Light Drinker – no. | 45 | 50 | |
| Abstainer – no. | 39 | 14 | |
The body-mass index is the weight in kilograms divided by the square of the height in meters.
Perceived 5yr weight change is the patient’s self-reported change in weight over the five years prior to the study, reported in kilograms.
Actual weight change is the patient’s recorded weight in kilograms at the time of the interview minus the recorded weight in kilograms either five years earlier, or the earliest recorded weight within the five years prior to the study.
The reference category for all levels of alcohol use was abstainers.
Pancreatic Insufficiency
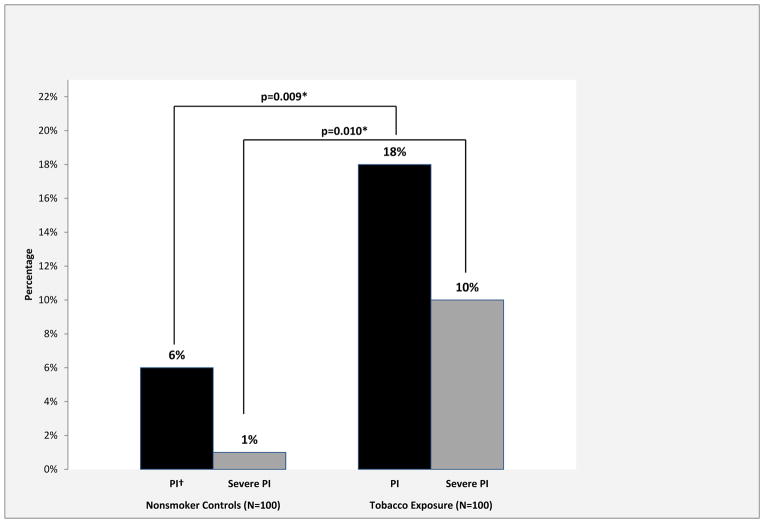
On univariate analysis, several characteristics were significantly associated with pancreatic insufficiency: tobacco exposure (p=0.009), current smoker (p=0.002), pack-years of smoking (p=0.008), the type of tobacco product used (p=0.021), diabetes (p=0.046), and marijuana use (p=0.024) (Table 2). Alcohol use, ace-inhibitor use, age, race, and measures of weight change were not significantly associated with pancreatic insufficiency. The prevalence of pancreatic insufficiency (FE-1 ≤200μg/g) was 18 percent (18/100 patients) in the tobacco exposure cohort, significantly greater than in the non-smoking control group with 6 percent (6/100 patients) (p=0.009). Additionally, 10 percent of the tobacco exposure cohort had severe pancreatic exocrine insufficiency (FE-1 < 100μg/g) as compared to 1 percent in the non-smoker cohort (p=0.010) (Figure 2).
Table 2.
Variables Associated with Pancreatic Insufficiency by Univariate Analysis
| Characteristic | No Pancreatic Insufficiency (N=176) | Pancreatic Insufficiency (N=24) | P-value |
|---|---|---|---|
| Demographics | |||
| Mean Age (SD) | 62.5 (12.4) | 62.3 (10.8) | 0.943 |
| Caucasian race – no. (%) | 111 (63.1) | 17 (70.8) | 0.457 |
| Diabetes – no. (%) | 29 (16.5) | 8 (33.3) | 0.046 |
| Ace-Inhibitor Use – no. (%) | 45 (25.6) | 8 (33.3) | 0.419 |
| Marijuana User – no. (%) | 3 (1.7) | 3 (12.5) | 0.024 |
| Smoking Status | |||
| Tobacco Exposure – no. (%) | 82 (46.6) | 18 (75.0) | 0.009 |
| Current Smoker – no. (%) | 19 (10.8) | 9 (37.5) | 0.002 |
| Median Smoking Pack Years (range) | 4 (0–132) | 27 (0–106) | 0.008 |
| Type of Tobacco Product | |||
| Cigarettes, cigars, other smokable Tobacco products – no. (%)^ | 14 (8.0) | 4 (16.7) | 0.021 |
| Cigarettes only – no. (%) | 82 (46.6) | 16 (66.7) | |
| No tobacco products – no. (%) | 80 (45.4) | 4 (16.7) | |
| Alcohol Use | |||
| Heavy Drinker – no. (%) | 10 (5.7) | 1 (4.2) | 0.671 |
| Moderate Drinker – no. (%) | 35 (19.9) | 6 (25.0) | |
| Light Drinker – no. (%) | 82 (46.6) | 13 (54.2) | |
| Abstainer – no. (%) | 49 (27.8) | 4 (16.7) | |
| Weight ¶ | |||
| Median Body Mass Index ❖(range) | 27.8 (11.8 – 49.9) | 29.1 (15.4–55.3) | 0.993 |
| Mean Perceived 5yr Wt Change§ (SD) | −3.1 (6.3) | −9.8 (18.2) | 0.084 |
| Mean Actual Weight Change† (SD) | −0.57 (8.9) | −1.50 (11.5) | 0.705 |
Other smokable tobacco products include cigarillos, pipes, and water pipes.
The body-mass index is the weight in kilograms divided by the square of the height in meters.
Perceived 5yr weight change is the patient’s self-reported change in weight over the five years prior to the study, reported in kilograms.
Actual weight change is the patient’s recorded weight in kilograms at the time of the interview minus the recorded weight in kilograms either five years earlier, or the earliest recorded weight within the five years prior to the study.
The reference category for all levels of alcohol use was abstainers.
Figure 2. Prevalence of Pancreatic Insufficiency and Severe Pancreatic Insufficiency in the Nonsmoker Control and Tobacco Exposure Cohorts.
* Denotes statistical significance, p<0.050
†Pancreatic insu ciency, abbreviated as PI
The prevalence of both pancreatic insufficiency and severe pancreatic insufficiency were significantly greater in the tobacco exposure cohort as compared to the nonsmoker controls.
Pancreatic Insufficiency and Weight Loss
The smokers with pancreatic insufficiency reported a mean weight loss of 12.7 kg during the five years prior to this study, or a loss of 2.54kg/year, significantly greater than the self-reported weight loss in smokers without pancreatic insufficiency (3.1 kg weight loss, 0.62kg/year loss, p<0.001). Within the non-smoker cohort, neither patients with pancreatic insufficiency nor patients without pancreatic insufficiency reported a significant five-year weight change (−1.1kg (−0.22kg/year) and −3.0kg (−0.60kg/year), p=0.512). To corroborate patient’s self-reported weight change, the medical records was reviewed; however, historical weight data was not available for all patients. From the available data, smokers with pancreatic insufficiency averaged a weight loss of 0.51kg/year and smokers without pancreatic insufficiency averaged a weight loss of 0.43kg/year. In the non-smoking group, patients with pancreatic insufficiency averaged a weight loss of 0.41kg/year while patients without pancreatic insufficiency had an average weight gain of 0.38kg/year.
Outcome Analysis
On multivariate logistic regression, the risk of pancreatic insufficiency in smokers, when controlled for covariates, was significantly increased (p=0.012, OR=4.34 [1.37–13.75]) (Table 3). In the multivariate model, tobacco exposure was associated with the highest odds ratio for pancreatic insufficiency, with smokers having greater than four-fold increased risk. Hyperlipidemia was significantly and inversely associated with the presence of pancreatic insufficiency (p=0.009, OR=0.20 [0.06–0.67]). The association of diabetes mellitus and pancreatic insufficiency was borderline in the multivariate model (p=0.056, OR=3.27 [0.97–11.02]). Age, gender, BMI, ace-inhibitor use, and alcohol use were also included in the final multivariable model; however, none were independently or significantly associated with pancreatic insufficiency.
Table 3.
Multivariate Logistic Regression Model of Independent Risk Factors and Covariates for Pancreatic Insufficiency
| Covariate | Level | Presence of Pancreatic Insufficiency | ||||
|---|---|---|---|---|---|---|
| Odds Ratio | 95% CI Low | 95% CI High | OR P-value | Type III P-value | ||
| Tobacco Exposure | Yes | 4.34 | 1.37 | 13.75 | 0.012* | 0.012 |
| No | 1 (Ref) | |||||
| Hyperlipidemia | Yes | 0.20 | 0.06 | 0.67 | 0.009* | 0.009 |
| No | 1 (Ref) | |||||
| Diabetes | Yes | 3.27 | 0.97 | 11.02 | 0.056 | 0.056 |
| No | 1 (Ref) | |||||
| Gender | Male | 2.74 | 0.95 | 7.94 | 0.063 | 0.063 |
| Female | 1 (Ref) | |||||
| Vitamin D Deficiency | Yes | 2.62 | 0.92 | 11.02 | 0.070 | 0.070 |
| No | 1 (Ref) | |||||
| Ace Inhibitor Use | Yes | 1.55 | 0.47 | 5.13 | 0.476 | 0.476 |
| No | 1 (Ref) | |||||
| Age | - | 1.00 | 0.96 | 1.05 | 0.862 | 0.862 |
| BMI❖ | - | 1.02 | 0.95 | 1.10 | 0.517 | 0.517 |
| Abstainer | 1 (Ref) | 0.792 | ||||
| Light Drinker | 1.07 | 0.27 | 4.22 | 0.918 | ||
| Alcohol Use Category | Moderate Drinker | 1.26 | 0.26 | 6.11 | 0.774 | |
| Heavy Drinker | 0.37 | 0.03 | 4.81 | 0.444 | ||
The body-mass index (BMI) is the weight in kilograms divided by the square of the height in meters.
Effects of Alcohol Consumption
Smokers had a higher prevalence of alcohol consumption at any level than nonsmokers (86 percent versus 61 percent (p<0.001) (Table 1). Alcohol use was associated with smoking; however, on both univariate analysis and multivariate logistic regression, alcohol consumption at any level was not significantly associated with pancreatic insufficiency (p=0.671 and p=0.792 respectively) (Tables 2 and 3).
DISCUSSION
The impact of tobacco exposure on the pancreas is being increasingly recognized. Tobacco exposure appears to damage the pancreatic parenchyma3, to increase the risk of pancreatic cancer 3–5, and has a clear though underappreciated impact on the development of chronic pancreatitis 7,11,18. This study identified pancreatic insufficiency in a high proportion of smokers with no prior history of pancreatic disease. The prevalence rate in smokers (18%) significantly exceeded the rate found in the nonsmoking control population (6%) (p=0.009). The rate of severe pancreatic insufficiency was also significantly increased in the tobacco exposure cohort (p=0.010). The relationship appeared to be independent of alcohol consumption, and tobacco exposure was the strongest predictor of pancreatic insufficiency in the multivariate model (OR, 4.34 [CI 1.37 – 13.75] p=0.012). Tobacco exposure appears to be detrimental to pancreatic exocrine function prior to the development of chronic pancreatitis and appears to be an independent risk factor for the development of pancreatic insufficiency in the absence of previously established or known morphologic disease of the pancreas.
Numerous nutritional deficiencies occur in smokers. Recent trials evaluating nutritional markers in smokers and non-smokers have revealed decreases in plasma levels of Vitamin C, Vitamin B-12, red-cell folate, and albumin in smokers 19–21 and tobacco exposure has been associated with significant reductions in body weight, BMI, and other anthropometric markers of nutritional status 19. In our study, smokers self-reported weight loss, and this relationship was corroborated in the subset with prior weight data. While the etiology for compromised nutritional status is not well understood, several mechanisms have been proposed. One hypothesis suggests an impaired oxidation state as a result of free radical production by cigarette smoking, leading to a depletion of the intracellular antioxidants Vitamin E, Vitamin C, and beta-carotene 22. Other postulated mechanisms include poor dietary intake of protein, fruits, vegetables, fiber, and essential nutrients 23. An increased work of breathing combined with difficulty eating while experiencing dyspnea has also been hypothesized 24. In our study, hyperlipidemia by history was more common in the smoker cohort; however it was inversely related to pancreatic insufficiency in the multivariate analysis. We believe that the relationship is complex – smokers may have more dyslipidemia overall due to lifestyle factors; however, dyslipidemia may be decreased in patients with pancreatic insufficiency due to fat malabsorption. Overall, pancreatic insufficiency may be involved in the nutritional deficiencies seen in smokers and may well be a contributor to the weight loss seen in patients with heavy tobacco exposure.
Alcohol has been considered to be the primary toxic exposure leading to disease of the pancreas and is the clear established risk factor for the development of chronic and calcific pancreatitis. In this study, alcohol was associated with smoking, with more smokers reporting higher prior levels of alcohol consumption. However, tobacco appeared to be the primary toxic exposure in terms of pancreatic insufficiency in the cohort of patients without established pancreatic disease. Tobacco exposure may also have been an under-recognized comorbid exposure in previous studies examining the impact of alcohol on disease of the pancreas. Several prior studies analyzing the relationship between alcohol and the etiology, course, and sequelae of chronic pancreatitis did not report on tobacco exposure in the study population25–27. When included, greater cigarette smoke and nicotine exposure have had a direct relationship with chronic pancreatitis in humans 28.
While this study focused on the exocrine manifestations of pancreatic disease, there was also an association with endocrine dysfunction. We found a relationship between diabetes and both smoking and pancreatic insufficiency. On univariate analysis, smokers had a significantly higher prevalence of diabetes as compared to the control cohort. Diabetes was borderline in the multivariate model (p=0.056). These findings are in line with previous reports which have found a pooled relative risk of diabetes in smokers of 1.44 with a dose-response relationship 29. There are several potential limitations in this trial. The study population was obtained from a single center. Many patients were not willing or able to provide a stool sample, leading to the need to approach a large number of patients to enroll the target cohort. This could have introduced an element of volunteer bias; which if present would hopefully have been similar in each arm of the study. The historic data in the area of weight were not available for all patients, as some patients who enrolled were new to the clinics. Additionally, Fecal Elastase-1 is not a perfect marker of pancreatic insufficiency, with variability and the potential for false positives and negatives. However, Fecal Elastase-1 is the best non-invasive marker of pancreatic insufficiency with a sensitivity and specificity of 93 percent16,17; presumably, variations in test results would be equally distributed between the groups. Direct pancreatic function testing with timed duodenoscopic measurement of secretin or CCK has been previously identified as the gold-standard, however, it would be difficult to justify invasive testing for pancreatic function in patients without known pancreatic disease.
This study suggests that tobacco exposure may be independently associated with decreased pancreatic exocrine function. Tobacco exposure had the strongest association with pancreatic insufficiency in multivariate modeling, persisting with control for covariates, most importantly alcohol exposure. 18 percent of patients with heavy tobacco exposure had a low fecal elastase without a history of chronic pancreatitis or known pancreatic disease. These results agree with prior studies which implicate tobacco exposure in histologic changes, chronic pancreatitis, and pancreatic cancer. The results also raise questions regarding the mechanism of weight loss in heavy smokers. Tobacco exposure appears to be associated with the development of pancreatic insufficiency, and the relationship may manifest before patients have come to clinical recognition or have developed symptoms of pancreatic disease.
Acknowledgments
Support: This manuscript was supported in part by a development grant from the Department of Medicine at Emory University School of Medicine. Research reported in this publication was supported in part by the Biostatistics and Bioinformatics Shared Resource of Winship Cancer Institute of Emory University and NIH/NCI under award number P30CA138292. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Presentation: The data contained in this manuscript was presented in part at Digestive Disease Week in Chicago, IL, on May 4, 2014.
There are no conflicts of interest.
References
- 1.How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. Atlanta (GA): 2010. [PubMed] [Google Scholar]
- 2.Barreto SG. How does cigarette smoking cause acute pancreatitis? Pancreatology. 2016;16:157–163. doi: 10.1016/j.pan.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Auerbach O, Garfinkel L. Histologic changes in pancreas in relation to smoking and coffee-drinking habits. Dig Dis Sci. 1986;31:1014–1020. doi: 10.1007/BF01300252. [DOI] [PubMed] [Google Scholar]
- 4.Iodice S, Gandini S, Maisonneuve P, et al. Tobacco and the risk of pancreatic cancer: a review and meta-analysis. Langenbecks Arch Surg. 2008;393:535–545. doi: 10.1007/s00423-007-0266-2. [DOI] [PubMed] [Google Scholar]
- 5.Duell EJ. Epidemiology and potential mechanisms of tobacco smoking and heavy alcohol consumption in pancreatic cancer. Mol Carcinog. 2012;51:40–52. doi: 10.1002/mc.20786. [DOI] [PubMed] [Google Scholar]
- 6.Andriulli A, Botteri E, Almasio PL, et al. Smoking as a cofactor for causation of chronic pancreatitis: a meta-analysis. Pancreas. 2010;39:1205–1210. doi: 10.1097/MPA.0b013e3181df27c0. [DOI] [PubMed] [Google Scholar]
- 7.Cote GA, Yadav D, Slivka A, et al. Alcohol and smoking as risk factors in an epidemiology study of patients with chronic pancreatitis. Clin Gastroenterol Hepatol. 2011;9:266–273. doi: 10.1016/j.cgh.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whitcomb DC, Yadav D, Adam S, et al. Multicenter approach to recurrent acute and chronic pancreatitis in the United States: the North American Pancreatitis Study 2 (NAPS2) Pancreatology. 2008;8:520–531. doi: 10.1159/000152001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yadav D, Hawes RH, Brand RE, et al. Alcohol consumption, cigarette smoking, and the risk of recurrent acute and chronic pancreatitis. Arch Intern Med. 2009;169:1035–1045. doi: 10.1001/archinternmed.2009.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tolstrup JS, Kristiansen L, Becker U, et al. Smoking and risk of acute and chronic pancreatitis among women and men: a population-based cohort study. Arch Intern Med. 2009;169:603–609. doi: 10.1001/archinternmed.2008.601. [DOI] [PubMed] [Google Scholar]
- 11.Rebours V, Vullierme MP, Hentic O, et al. Smoking and the course of recurrent acute and chronic alcoholic pancreatitis: a dose-dependent relationship. Pancreas. 2012;41:1219–1224. doi: 10.1097/MPA.0b013e31825de97d. [DOI] [PubMed] [Google Scholar]
- 12.Rothenbacher D, Low M, Hardt PD, et al. Prevalence and determinants of exocrine pancreatic insufficiency among older adults: results of a population-based study. Scand J Gastroenterol. 2005;40:697–704. doi: 10.1080/00365520510023116. [DOI] [PubMed] [Google Scholar]
- 13.Caraballo RS, Giovino GA, Pechacek TF, et al. Factors associated with discrepancies between self-reports on cigarette smoking and measured serum cotinine levels among persons aged 17 years or older: Third National Health and Nutrition Examination Survey, 1988–1994. Am J Epidemiol. 2001;153:807–814. doi: 10.1093/aje/153.8.807. [DOI] [PubMed] [Google Scholar]
- 14.Koenig LB, Jacob T, Haber JR. Validity of the lifetime drinking history: a comparison of retrospective and prospective quantity-frequency measures. J Stud Alcohol Drugs. 2009;70:296–303. doi: 10.15288/jsad.2009.70.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dawson DA, Grant BF, Chou PS. Gender differences in alcohol intake. In: Hunt WA, SZ, editors. Stress, Gender, and Alcohol-Seeking Behavior. Bethesda, MD: U.S. Dept. of Health and Human Services, Public Health Service, National Institutes of Health, National Institute on Alcohol Abuse and Alcoholism; 1995. [Google Scholar]
- 16.Loser C, Mollgaard A, Folsch UR. Faecal elastase 1: a novel, highly sensitive, and specific tubeless pancreatic function test. Gut. 1996;39:580–586. doi: 10.1136/gut.39.4.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dominici R, Franzini C. Fecal elastase-1 as a test for pancreatic function: a review. Clin Chem Lab Med. 2002;40:325–332. doi: 10.1515/CCLM.2002.051. [DOI] [PubMed] [Google Scholar]
- 18.Yadav D, Slivka A, Sherman S, et al. Smoking is underrecognized as a risk factor for chronic pancreatitis. Pancreatology. 2010;10:713–719. doi: 10.1159/000320708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gariballa S, Forster S. Effects of smoking on nutrition status and response to dietary supplements during acute illness. Nutr Clin Pract. 2009;24:84–90. doi: 10.1177/0884533608329441. [DOI] [PubMed] [Google Scholar]
- 20.Shaper AG, Wannamethee SG, Whincup PH. Serum albumin and risk of stroke, coronary heart disease, and mortality: the role of cigarette smoking. J Clin Epidemiol. 2004;57:195–202. doi: 10.1016/j.jclinepi.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 21.Pfeiffer C, Sternberg MR, Schleicher RL, et al. Dietary supplement use and smoking are important correlates of biomarkers of water-soluble vitamin status after adjusting for sociodemographic and lifestyle variables in a representative sample of U.S. adults. J Nutr. 2013;143:957S–965S. doi: 10.3945/jn.112.173021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diana JN. Tobacco smoking and nutrition. Ann N Y Acad Sci. 1993;686:1–11. doi: 10.1111/j.1749-6632.1993.tb39147.x. [DOI] [PubMed] [Google Scholar]
- 23.Subar AF, Harlan LC, Mattson ME. Food and nutrient intake differences between smokers and non-smokers in the US. Am J Public Health. 1990;80:1323–1329. doi: 10.2105/ajph.80.11.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cochrane WJ, Afolabi OA. Investigation into the nutritional status, dietary intake and smoking habits of patients with chronic obstructive pulmonary disease. J Hum Nutr Diet. 2004;17:3–11. doi: 10.1046/j.1365-277x.2003.00478.x. [DOI] [PubMed] [Google Scholar]
- 25.Ammann RW, Buehler H, Muench R, et al. Differences in the natural history of idiopathic (nonalcoholic) and alcoholic chronic pancreatitis. A comparative long-term study of 287 patients. Pancreas. 1987;2:368–377. doi: 10.1097/00006676-198707000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Durbec JP, Sarles H. Multicenter survey of the etiology of pancreatic diseases. Relationship between the relative risk of developing chronic pancreaitis and alcohol, protein and lipid consumption. Digestion. 1978;18:337–350. doi: 10.1159/000198221. [DOI] [PubMed] [Google Scholar]
- 27.Lin Y, Tamakoshi A, Matsuno S, et al. Nationwide epidemiological survey of chronic pancreatitis in Japan. J Gastroenterol. 2000;35:136–141. doi: 10.1007/s005350050026. [DOI] [PubMed] [Google Scholar]
- 28.Alexandre M, Pandol SJ, Gorelick FS, et al. The emerging role of smoking in the development of pancreatitis. Pancreatology. 2011;11:469–474. doi: 10.1159/000332196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Willi C, Bodenmann P, Ghali WA, et al. Active smoking and the risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2007;298:2654–2664. doi: 10.1001/jama.298.22.2654. [DOI] [PubMed] [Google Scholar]